Abstract
Endothelial caveolin-1 (cav-1) is an anchoring protein in plasma membrane caveolae where it binds endothelial nitric oxide synthase (eNOS) and limits its activation, particularly in animals fed a high salt (HS) diet. Cav-1 also interacts with steroid receptors such as the mineralocorticoid receptor (MR). To test the hypothesis that vascular reactivity is influenced by an interplay between MR and cav-1 during HS diet, we examined the effects of MR blockade on NOS-mediated vascular relaxation in normal and cav-1-deficient mice. Wild-type (WT) and cav-1 knockout mice (cav-1−/−) were fed for 14 days a HS (4% NaCl) diet with and without the MR antagonist eplerenone (Epl; 100 mg·kg−1·day−1). After systolic blood pressure (BP) was measured, the thoracic aorta was isolated for measurement of vascular reactivity, and the aorta and heart were used for measurement of eNOS and MR expression. BP was not different between WT + Epl and WT, but was higher in cav-1−/− + Epl than in cav-1−/− mice. Phenylephrine (Phe)-induced vascular contraction was less in cav-1−/− than WT, and significantly enhanced in cav-1−/− + Epl than in cav-1−/−, but not in WT + Epl compared with WT. Endothelium removal and NOS blockade by Nω-nitro-l-arginine methyl ester (l-NAME) enhanced Phe contraction in cav-1−/−, but not cav-1−/− + Epl. ACh-induced aortic relaxation was reduced in cav-1−/− + Epl versus cav-1−/−, but not in WT + Epl compared with WT. Endothelium removal, l-NAME, and the guanylate cyclase inhibitor ODQ abolished the large ACh-induced relaxation in cav-1−/− and the remaining relaxation in the cav-1−/− + Epl but had similar inhibitory effect in WT and WT + Epl. Real-time RT-PCR indicated decreased eNOS mRNA expression in the aorta and heart, and Western blots revealed decreased total eNOS in the heart of cav-1−/− + Epl compared with cav-1−/−. Vascular and cardiac MR expression was less in cav-1−/− than WT, but not in cav-1−/− + Epl compared with cav-1−/−. Plasma aldosterone (Aldo) was not different between WT and cav-1−/− mice nontreated or treated with Epl. Thus in cav-1 deficiency states and HS diet MR blockade is associated with increased BP, enhanced vasoconstriction, and decreased NOS-mediated vascular relaxation and eNOS expression. The data suggest that, in the absence of cav-1, MR activation plays a beneficial role in regulating eNOS expression/activity and, consequently, the vascular function during HS diet.
Keywords: caveolae, aldosterone, eplerenone, endothelium, endothelial nitric oxide synthase
high salt (HS) dietary intake is associated with increased vascular volume (13). The volume overload and increased renal blood flow result in inhibition of the renin-angiotensin system, leading to increased salt and water excretion and restoration of vascular volume toward normal (26, 27, 45). High dietary sodium may also affect vascular function as a result of nuclear localization of sodium and activation of nuclear mRNA expression, release of ouabain-like factor, and activation of sodium/calcium (Na+/Ca2+) exchange mechanism (4, 5, 32, 36, 54).
Aldosterone (Aldo)-induced activation of the mineralocorticoid receptor (MR) in the kidney plays a critical role in sodium reabsorption and in the control of plasma sodium levels and plasma volume during HS diet (29, 38). MR has also been identified in cardiac and vascular tissues, suggesting that Aldo-MR interaction may affect cardiac and vascular function (19, 34, 59, 66, 68). However, the role of MR in modulating vascular function, particularly during HS diet, has not been fully characterized.
Caveolin-1 (cav-1) is a transmembrane protein identified in the plasma membrane caveolae of many cell types including endothelial, vascular smooth muscle (VSM), and cardiac cells (17, 31, 47, 61, 65). In VSM, cav-1 plays a role in the coupling of α-adrenergic, ANG II, and endothelin receptors to VSM contraction and growth (63, 69, 70, 75). In the endothelium, cav-1 anchors endothelial nitric oxide synthase (eNOS) to plasma membrane caveolae, thus limiting its cytosolic translocation, and its consequent phosphorylation and full activation. A role of cav-1 in mediating the effects of steroid hormones on their receptors has also been suggested (40, 41). These studies have suggested a prominent role of cav-1 in the regulation of vascular function and growth (15, 56, 58, 72) as well as in the normal systolic and diastolic cardiac function (71).
Studies in cav-1 null mice on normal salt diet have suggested a role of cav-1 in mechanotransduction, vascular remodeling, and cardiovascular function (15, 58, 71, 72). We also have recently shown that cav-1 deficiency is associated with increased eNOS activity during HS diet and suggested potential effects of HS on cav-1 eNOS binding (56).
Despite the evidence that MR is an important regulator of the hemodynamics during HS diet, and that vascular cav-1 and eNOS binding may be influenced by HS diet, the role of MR in the regulation of cav-1 and eNOS during HS diet is unclear. Previous studies also have proposed potential interaction between steroid receptors and cav-1 and suggested that the loss of cav-1 can alter steroid receptor signaling. For instance, a recent study has shown that genetic ablation of cav-1 is associated with hypersensitivity to estrogen (46). Studies have also shown a link between the estrogen receptor (ER) and eNOS regulation (37, 53). Thus there is evidence in the literature that loss of cav-1 may dysregulate steroid signaling and the eNOS pathway. Although Aldo may activate similar signaling pathways as those induced by other steroids such as estrogen (3), whether the loss of cav-1 upregulates Aldo or affects Aldo regulation of eNOS expression/signaling has not been examined, and thus makes it important to test for potential Aldo upregulation/hypersensitivity in cav-1 deficiency states. The purpose of this study was to test the hypothesis that Aldo-MR plays a role in the regulation of the endothelial cav-1 and eNOS interaction and thereby the vascular function during HS diet. We reasoned that if MR regulates eNOS in a cav-1-dependent manner, then cav-1 deficiency should either eliminate or enhance the effects of MR blockade on eNOS function. We used wild-type (WT; cav-1+/+) and cav-1 knockout mice (KO; cav-1−/−) placed on a HS diet and nontreated or treated with the MR antagonist eplerenone (Epl) to investigate whether 1) MR blockade is associated with altered vascular contraction in cav-1−/− mice on HS diet, 2) MR blockade is associated with altered vascular relaxation in cav-1−/− mice on HS diet, and 3) the changes in vascular contraction and relaxation associated with MR blockade in cav-1−/− mice on HS diet reflect alterations in the expression/activity of eNOS.
MATERIAL AND METHODS
Animals.
Twelve-week-old KO (cav-1−/−) and genetically-matched WT (cav-1+/+) male mice (stock numbers 004585 and 101045, respectively) were purchased from Jackson Laboratories (Bar Harbor, ME). The genotypes were confirmed by PCR according to Jackson Laboratories′ guidelines. Mice were housed in the animal facility in 12-h:12-h light/dark cycle at 22 ± 1°C ambient temperature and maintained on ad libitum normal Purina Rodent Chow (5053, 0.8% NaCl; Purina, St. Louis, MO) and tap water. After 3 days of acclimatization, mice from each genotype were placed on HS diet (4% NaCl) for 5 days to achieve sodium balance (28, 52) and maintained on the HS diet for an additional 14 days. A subgroup of mice received 100 mg·kg−1·day−1 Epl in the HS diet for 14 days. The Epl dose was adjusted to a food content of 0.4 mg Epl/gram chow, based on mice food consumption (Table 1). Epl has higher selectivity for MR over other steroid receptors and is >250 times more specific for MR than for the ER (9). Epl also has lower affinity for progesterone, androgen, and glucocorticoid receptors, compared with spironolactone. This is exemplified by the high incidence of progesterone-like and antiandrogenic adverse effects in spironolactone-treated patients, possibly due to nonspecific binding to steroid receptors (44, 67). Thus the effects of Epl treatment are more likely due to interference with MR. However, an interaction between MR and other steroid receptor-mediated signaling pathways cannot be excluded.
Table 1.
Food consumption, body weight, Aldo levels, Phe contraction, and ACh relaxation in aortic rings of WT and cav-1−/− mice on high salt diet and nontreated or treated with Epl
| Group | WT | WT + Epl | Cav-1−/− | Cav-1−/− + Epl |
|---|---|---|---|---|
| Food consumption, g/day | 5.29 ± 0.77 (7) | 6.13 ± 0.75 (8) | 6.15 ± 0.71 (7) | 6.38 ± 0.78 (8) |
| Body weight, g | ||||
| Day 0 | 30.1 ± 0.9 (7) | 30.9 ± 0.6 (8) | 27.1 ± 0.9 (7)† | 25.8 ± 1.5 (8)† |
| Day 14 | 30.9 ± 1.2 (7) | 31.0 ± 0.7 (8) | 29.3 ± 0.9 (7) | 26.7 ± 1.2 (8)† |
| Plasma Aldo, ng/dL | 20.3 ± 4.7 (7) | 24.9 ± 8.0 (8) | 30.5 ± 5.3 (7) | 33.2 ± 8.3 (8) |
| Phe | ||||
| Max (10−5 M) contraction, g | 0.28 ± 0.03 (15) | 0.36 ± 0.06 (16) | 0.13 ± 0.02 (12)† | 0.25 ± 0.04 (19)* |
| pED50, − log M | 6.627 ± 0.082 (15) | 6.846 ± 0.073 (16) | 6.486 ± 0.131 (12) | 6.817 ± 0.064 (19)* |
| Max Phe-Endo, g | 0.24 ± 0.04 (14) | 0.41 ± 0.09 (10) | 0.24 ± 0.05 (9)‡ | 0.25 ± 0.03 (15) |
| +l-NAME, g | 0.45 ± 0.11 (8) | 0.45 ± 0.09 (8) | 0.30 ± 0.05 (6)‡ | 0.37 ± 0.06 (9) |
| ACh | ||||
| Max (10−5 M) relaxation, % | 16.5 ± 4.5 (16) | 22.4 ± 6.0 (16) | 87.9 ± 5.0 (11)† | 60.8 ± 6.9 (20)* |
| SNP | ||||
| Max (10−5 M) relaxation, % | 97.2 ± 1.8 (15) | 98.7 ± 1.3 (13) | 98.6 ± 1.3 (12) | 96.6 ± 1.9 (17) |
Values are means ± SE; (n), number of mice or experiments in group. Aldo, aldosterone; SNP, sodium nitroprusside.
Measurements in eplerenone (Epl)-treated wild-type (WT) or Cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated WT or Cav-1−/−;
measurements in Cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT;
phenylephrine (Phe) contraction in endothelium (Endo)-denuded or Nω-nitro-l-arginine methyl ester (l-NAME)-treated aortic rings is significantly greater (P < 0.05) than Phe contraction in endothelium-intact nontreated tissues.
The body weight was significantly less in cav-1−/− compared with WT nice. However, no significant change in body weight was observed in either genotype when treated with Epl (Table 1). All experimental procedures followed the guidelines of, and were approved by, the Institutional Animal Care and Use Committee at Harvard Medical School.
Blood pressure.
Systolic blood pressure (BP) was measured in conscious mice after reaching sodium balance on days 0 and 13 using tail-cuff plethysmography (BP analyzer, model 179; IITC Life Science, Woodland Hills, CA). Mice were warmed at 30°C for 10 min and allowed to rest quietly before BP measurement. BP measurements were taken in a quiet room, and the mice were kept calm and handled by the same person. No sedation was used.
Plasma Aldo.
Mice were euthanized under deep anesthesia with isoflurane. Blood was collected in purple-top BD Microtainer tubes (EDTA). The plasma was separated by centrifugation, and Aldo levels were determined in duplicates (200 μl each) using a solid-phase RIA kit (Diagnostic Products, Los Angeles, CA).
Tissue preparation.
In euthanized mice, the thoracic cavity was opened, and the aorta and the heart were rapidly excised. The thoracic aorta was placed in oxygenated Krebs solution, carefully dissected and cleaned of connective tissue under microscopic visualization, and cut into 2-mm-wide rings. Sections of the aorta and the heart were placed in liquid nitrogen immediately after collection in preparation for mRNA and protein analysis.
Isometric contraction.
Aortic segments were suspended between two tungsten wire hooks; one hook is fixed at the bottom of a tissue bath and the other hook is connected to a Grass force transducer (FT03; Astro-Med, West Warwick, RI). Aortic segments were stretched under 0.5 g of resting tension and allowed to equilibrate for 45 min in a temperature-controlled, water-jacketed tissue bath, filled with 50 ml Krebs solution continuously bubbled with 95% O2-5% CO2 at 37°C. The changes in isometric contraction were recorded on a Grass polygraph (Model 7D; Astro-Med).
After tissue equilibration, a control contraction to 96 mM KCl was elicited. Once maximum KCl contraction was reached, the tissue was rinsed with Krebs three times for 10 min each. The control KCl-induced contraction followed by rinsing in Krebs was repeated twice. Aortic segments were stimulated with increasing concentrations of phenylephrine (Phe; 10−9 to 10−5 M), concentration-contraction curves were constructed, and the maximal Phe contraction was measured. The individual Phe concentration-response curves were further analyzed using a nonlinear regression curve (best-fit sigmoidal dose-response curve; Sigmaplot), and the effective concentration that produced half the maximal contraction (ED50) was measured and presented as pED50 (−log M). In other experiments, the tissues were precontracted with Phe (10−5 M), increasing concentrations (10−9 to 10−5 M) of ACh were added, and the percent relaxation of Phe contraction was measured. Parallel contraction and relaxation experiments were performed in endothelium-intact aortic rings pretreated with the nitric oxide (NO) synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10−5 M) for 10 min. The contraction to Phe and the relaxation to ACh and sodium nitroprusside (SNP) were also measured in endothelium-denuded aortic rings. In these experiments, the endothelium was removed by rubbing the vessel interior five times around the tip of a forceps.
Nitrite/nitrate measurements.
Aortic segments were placed in 2 ml Krebs aerated with 95% O2-5% CO2 at 37°C, and the solution was changed every 30 min for 1 h. Samples for basal accumulation of nitrite (NO2−) formed from released NO were first taken. Vascular segments were stimulated with ACh (10−5 M) for 10 min, then rapidly removed, dabbed dry with filter paper, and weighed. The incubation solutions were assayed for the stable end product of NO, NO2−. Briefly, samples of the incubation solution (50 μl, in triplicate) were mixed in 96-well microplate with 100 μl Griess reagent. The chromophore generated from the reaction with NO2− was detected spectrophotometrically (535 nm) using a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA). The concentration of NO2− was calculated using a calibration curve with known concentrations of NaNO2 (54).
Real time RT-PCR.
Total mRNA was extracted from the hearts and aortas using the RNeasy mini kit (Qiagen Sciences, Germantown, MD). cDNA was synthesized from 1.5 μg RNA with the first-strand cDNA synthesis kit (GE Healthcare, Piscataway, NJ). PCR amplification reactions were performed in duplicate using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) and using the ΔΔCT method to determine mRNA levels. Gene expression was normalized to 18S rRNA levels. PCR amplification to detect endothelial NOS (eNOS), MR, and the housekeeping 18S rRNA was performed with TaqMan gene expression assays (proprietary primers and probes designed and synthesized by Applied Biosystems). Data are presented as fold increase relative to the measurement in WT mice on HS diet.
Western blot analysis.
Protein was extracted by homogenizing cardiac and aorta tissue with radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Protein extracts (40 μg) were combined with an equal volume of 2× Laemmli loading buffer, boiled for 5 min, and size-fractionated by electrophoresis on 7.5% SDS-polyacrylamide gels. Proteins were transferred from the gel to a nitrocellulose membrane by electroblotting. Membranes were incubated with 5% nonfat dried milk, in Tris-buffered saline-Tween (USB, Cleveland, OH) for 1 h, and then overnight at 4°C with mouse anti-eNOS antibody (1:2,500; BD Transduction Laboratories, San Diego, CA), rabbit anti p-eNOS antibody (1:1,000; Cell Signaling Technology, Danvers, MA), or rabbit anti-ENaC antibody (1:200; Novus Biologicals, Littleton, CO). After incubation, samples were washed, incubated with peroxidase-conjugated secondary antibody, and analyzed using enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA). The blots were subsequently reprobed for β-actin (1:5,000 dilution), and the results were normalized to β-actin, to correct for loading. Data are presented as fold increase relative to the measurement in WT mice on HS diet.
Solutions and drugs.
Krebs solution contained (in mM) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, 1.2 MgCl2, at pH 7.4, and bubbled with 95% O2-5% CO2. KCl (96 mM) was prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, ACh, and l-NAME (10−1 M; Sigma) were prepared in distilled water. Stock solution of ODQ (10−1 M) was prepared in DMSO. Final concentration of DMSO in experimental solution was <0.1%. All other chemicals were of reagent grade or better.
Statistical analysis.
The data were analyzed using two-way ANOVA (cav-1 status, Epl treatment) and presented as means ± SE. Scheffe's F test was used for comparison of multiple means. Student's t-test for unpaired data was used for comparison of two means. Differences were considered statistically significant if P < 0.05. All studies were accomplished with the individual performing the study blinded as to the genotype of the animal and the treatment group from which the tissues were obtained.
RESULTS
Measurement of body weight demonstrated a trend for less weight gain in cav-1−/− + Epl (0.05%) compared with cav-1−/− (8.2%; P < 0.001) mice, but not in WT + Epl (0.5%) compared with WT [2.7%;P = not significant (ns); Table 1]. Epl treatment also was associated with a nonsignificant trend for more water consumption in cav-1−/− (6.4 ± 1.1 ml) compared with WT (5.5 ± 1.1 ml; P = 0.57) mice.
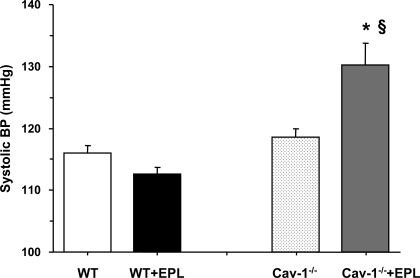
The systolic BP was not significantly different between cav-1−/− and WT mice on HS diet (Fig. 1). Treatment with Epl was not associated with significant changes in BP in WT mice. In contrast, in cav-1−/− mice treatment with Epl significantly increased BP (P < 0.05; Fig. 1).
Fig. 1.
Systolic blood pressure (BP) in wild-type (WT) and cav-1−/− mice on high salt (HS) diet and nontreated or treated with eplerenone (Epl). Data represent means ± SE (n = 8 to 23). *Measurements in Epl-treated cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated cav-1−/−; §measurements in cav-1−/− + Epl are significantly different (P < 0.05) from corresponding measurements in WT + Epl.
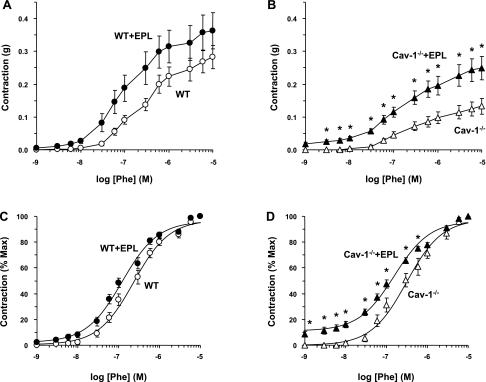
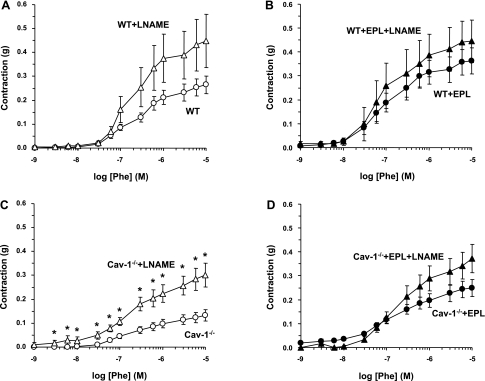
In aortic segments of WT mice, the α-adrenergic agonist Phe caused concentration-dependent contraction that reached a maximum of 0.28 ± 0.04 g at 10−5 M. The maximum Phe contraction in cav-1−/− mice was significantly reduced (P < 0.05) compared with WT animals (Fig. 2, A and B, and Table 1). The Phe-induced aortic contraction was not significantly different between WT + Epl and WT mice (Fig. 2A). In contrast, the Phe-induced aortic contraction was significantly enhanced in cav-1−/− + Epl compared with cav-1−/− mice on HS diet (Fig. 2B and Table 1). When the Phe-induced aortic contraction also was presented as a percentage of maximum and the Phe ED50 was calculated, no significant difference was observed between WT + Epl and WT mice (Fig. 2C and Table 1). In contrast, the Phe concentration-percent response curve was significantly shifted to the left, and calculation of the Phe ED50 indicated that Phe was significantly more potent in the aorta of cav-1−/− + Epl compared with cav-1−/− mice (Fig. 2D and Table 1), suggesting that Epl treatment enhances the sensitivity of the α-adrenergic receptors to Phe in the aorta of cav-1-deficient mice.
Fig. 2.
Phenylephrine (Phe)-induced contraction in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented in grams (A and B) or as percentage of maximum Phe contraction (C and D). Data represent means ± SE (n = 12 to 19). *Measurements in Epl-treated WT or cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated WT or cav-1−/−.
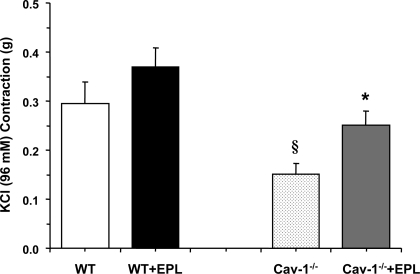
Measurement of aortic contraction to 96 mM KCl, a membrane depolarization-dependent and receptor-independent stimulant of Ca2+ influx from the extracellular space, indicated that it was reduced in cav-1−/− compared with WT mice on HS diet (Fig. 3). The KCl-induced aortic contraction was not significantly different between WT + Epl and WT mice but was significantly enhanced (P < 0.05) in cav-1−/− + Epl compared with cav-1−/− mice. Thus the enhanced vascular contraction in the cav-1 KO mice on HS and Epl is not limited to receptor-mediated responses and may involve a common postreceptor downstream signaling pathway that is disrupted in the absence of cav-1.
Fig. 3.
KCl-induced contraction in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings were stimulated with high 96-mM KCl depolarizing solution, and the contractile response was measured and presented in grams. Data represent means ± SE (n = 11 to 19). §Measurements in cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT; *measurements in Epl-treated cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated cav-1−/−.
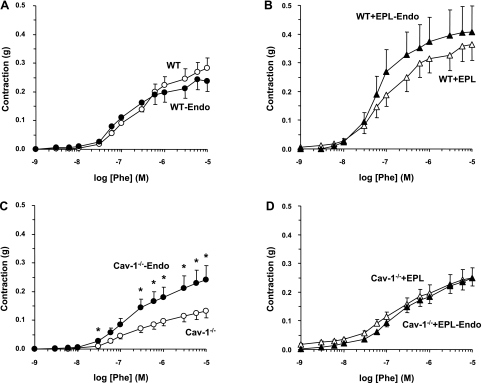
Removal of the endothelium was associated with insignificant change of the Phe contraction in aortic segments of WT (Fig. 4A) or WT + Epl mice (Fig. 4B). In contrast, removal of the endothelium was associated with significant enhancement of Phe contraction in the aorta of cav-1−/− mice (Fig. 4C and Table 1). On the other hand, Phe-induced contraction was not significantly different in endothelium-denuded and intact aortic segments of cav-1−/− + Epl mice (Fig. 4D), suggesting that an endothelium-dependent relaxation pathway is already inhibited in the aorta of Epl-treated cav-1-deficient mice.
Fig. 4.
Effect of endothelium (Endo) removal on Phe-induced contraction in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Endothelium intact (open symbols) and denuded aortic rings (closed symbols) of WT (A), WT + Epl (B), cav-1−/− (C), and cav-1−/− + Epl (D) mice were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented in grams. Data represent means ± SE (n = 9 to 19). *Measurements in endothelium-denuded vessels are significantly different (P < 0.05) from corresponding measurements in intact vessels.
In aortic segments of WT and cav-1−/− mice nontreated or treated with Epl, the NOS inhibitor l-NAME (3 × 10−4 M) alone did not cause any detectable constriction. Phe-induced contraction also was not significantly different in l-NAME-treated and nontreated aortic rings from WT (Fig. 5A) or WT + Epl mice (Fig. 5B). l-NAME treatment significantly enhanced (P < 0.05) Phe contraction in aortic rings from cav-1−/− mice (Fig. 5C and Table 1). In contrast, l-NAME treatment did not significantly enhance Phe contraction in aortic rings of cav-1−/− + Epl mice (Fig. 5D), suggesting that eNOS is already inhibited in the Epl-treated cav-1-deficient mice.
Fig. 5.
Effect of nitric oxide synthase (NOS) blockade on Phe-induced contraction in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings of WT (A), WT + Epl (B), cav-1−/− (C), and cav-1−/− + Epl (D) mice were either nontreated (circles) or pretreated with the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M; triangles) for 15 min. The tissues were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented in grams. Data represent means ± SE (n = 6 to 19). *Measurements in l-NAME-treated aortic segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
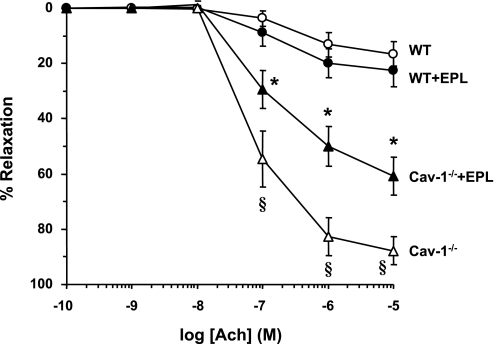
In Phe precontracted aortic rings of WT mice, ACh caused concentration-dependent relaxation that reached a maximum at 10−5 M. ACh-induced relaxation was significantly enhanced (P < 0.05) in cav-1−/− compared with WT mice (Fig. 6 and Table 1). ACh-induced aortic relaxation was significantly reduced in cav-1−/− + Epl compared with cav-1−/− mice but was not significantly different between WT + Epl and WT mice (Fig. 6 and Table 1). We should note that the aortic rings were bubbled with 95% O2. Although we observed ∼90% relaxation to ACh in the aorta of cav-1−/− mice, it is possible that 95% O2 concentration could still inhibit NO-dependent relaxation, and more so in arteries with impaired antioxidant capacity. Comparison of the results in aortic rings bubbled with 21% O2 should be examined in future studies.
Fig. 6.
ACh-induced relaxation in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings were precontracted with Phe (10−5 M), increasing concentrations of ACh were added, and the percent relaxation of Phe contraction was measured. Data represent means ± SE (n = 11 to 20). §Measurements in cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT; *measurements in Epl-treated cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated cav-1−/−.
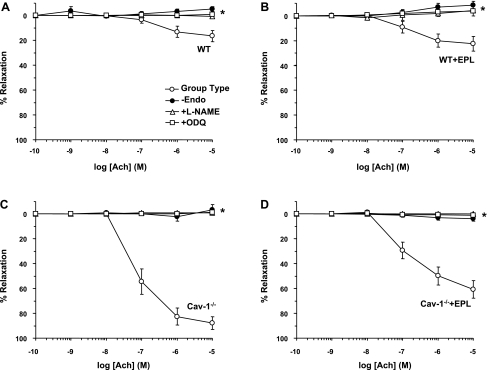
Removal of the endothelium caused similar inhibition of ACh-induced aortic relaxation in WT (Fig. 7A) and WT + Epl (Fig. 7B) mice. In contrast, endothelium removal abolished the large ACh-induced aortic relaxation in cav-1−/− mice (Fig. 7C) and the remaining smaller relaxation in the cav-1−/− + Epl mice (Fig. 7D). NO produced by eNOS is known to diffuse into VSM, activate guanylate cyclase, and increase cGMP production, which in turn promotes vascular relaxation (20, 30, 48). Treatment of aortic rings with the NOS inhibitor l-NAME or the guanylate cyclase inhibitor ODQ caused similar inhibition of ACh-induced relaxation in the aorta of WT (Fig. 7A) and WT + Epl (Fig. 7B) mice but abolished the large ACh-induced aortic relaxation in cav-1−/− mice (Fig. 7C) and the remaining smaller relaxation in the cav-1−/− + Epl mice (Fig. 7D).
Fig. 7.
Effect of endothelium removal and blockade of the NO-cGMP pathway on ACh-induced relaxation in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings of WT (A), WT + Epl (B), cav-1−/− (C), and cav-1−/− + Epl (D) mice were either intact nontreated (○), endothelium denuded (●), pretreated with the NOS inhibitor l-NAME (3 × 10−4 M; ▵), or pretreated with the gunaylate cyclase inhibitor ODQ (10−5 M; □) for 15 min. The tissues were precontracted with Phe (10−5 M), increasing concentrations of ACh were added, and the percent relaxation of Phe contraction was measured. Data represent means ± SE (n = 5 to 20). *Measurements in endothelium denuded or l-NAME- or ODQ-treated aortic rings are significantly different (P < 0.05) from corresponding measurements in intact nontreated segments.
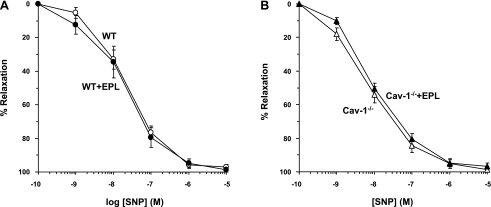
To test for the responsiveness of VSM to NO, the effects of the exogenous NO donor SNP were examined. In Phe precontracted aortic rings of WT mice, SNP caused concentration-dependent relaxation that reached a maximum at 10−5 M. SNP-induced aortic relaxation was not significantly different between WT + Epl and WT mice (Fig. 8A) or between cav-1−/− + Epl and cav-1−/− mice (Fig. 8B and Table 1).
Fig. 8.
Sodium nitroprusside (SNP)-induced relaxation in aortic rings of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Aortic rings of WT and WT + Epl (A) or cav-1−/− and cav-1−/− + Epl (B) were precontracted with Phe (10−5 M), increasing concentrations of SNP were added, and the percent relaxation of Phe contraction was measured. Data represent means ± SE (n = 12 to 17).
Although the mouse aorta is very small, we attempted to measure nitrite/nitrate (NOx) production in aortic segments of WT and cav-1 KO mice using the Griess reagent technique. The results suggested that ACh (10−5 M) caused an increase in NO production in both WT (basal, 2,366 ± 204; ACh, 3,920 ± 392 pmol/mg; n = 21) and cav-1−/− (basal, 2,158 ± 400; ACh, 3,800 ± 841 pmol/mg tissue; n = 15) mice. However, the background signal was very high, making it difficult to discern differences in basal or ACh-stimulated NO production between the aortas of cav-1−/− and WT mice. The inability to measure a significant difference in aortic NO production between cav-1−/− and WT mice made it even more difficult to determine with confidence any potential effects of Epl treatment on vascular NO production, and therefore these measurements were not performed.
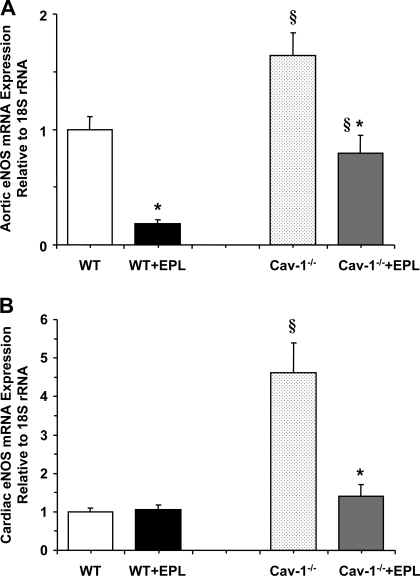
RT-PCR analysis revealed that eNOS mRNA expression was significantly increased in the aorta and heart of cav-1−/− compared with WT (Fig. 9). The eNOS mRNA expression was significantly reduced in the aorta and heart of cav-1−/− + Epl compared with cav-1−/− mice. The expression of eNOS mRNA was significantly reduced in the aorta but not in the heart of WT + Epl compared with WT mice (Fig. 9).
Fig. 9.
RT-PCR analysis of endothelial NOS (eNOS) mRNA expression in the aorta (A) and heart (B) of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Data represent means ± SE (n = 5 to 11). §Measurements in cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT; *measurements in Epl-treated WT or cav-1−/− are significantly different (P < 0.05) from corresponding measurements in nontreated WT or cav-1−/−.
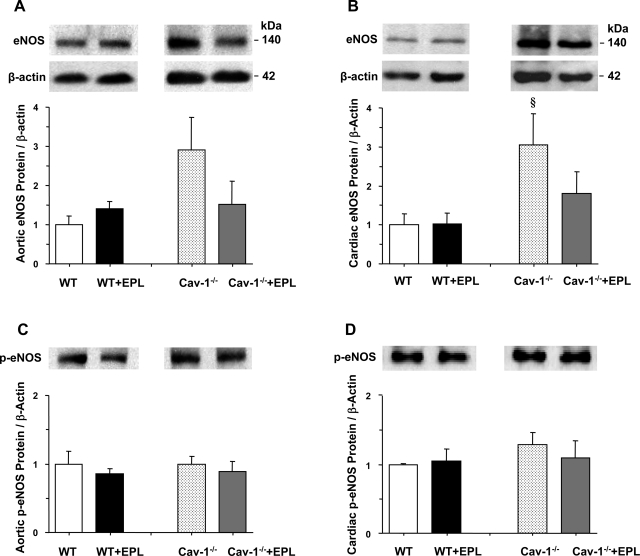
Western blot analysis revealed that the amount of eNOS protein was insignificantly increased in the aorta (P = 0.06) and significantly increased in the heart of cav-1−/− compared with WT (Fig. 10). The amount of eNOS protein was insignificantly reduced in the aorta and heart of cav-1−/− + Epl compared with cav-1−/− mice (Fig. 10). Our measurements of activated p-eNOS were less conclusive, with no discernable difference in the aorta or heart of cav-1−/− and WT mice nontreated or treated with Epl (Fig. 10).
Fig. 10.
Western blot analysis of total eNOS and p-eNOS in the aorta (A and C) and heart (B and D) of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Data represent means ± SE (n = 3 to 8). §Measurements in cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT.
Plasma Aldo levels were not significantly different between cav-1−/− and WT mice nontreated or pretreated with Epl (Table 1). Although some increase in Aldo levels was observed in the Epl-treated mice, this difference did not reach statistical significance, suggesting that the dose used minimally modified the renin-angiotensin-aldosterone-volume feedback loop. RT-PCR revealed that the mRNA expression of MR was reduced significantly (P < 0.05) in the aorta but insignificantly in the heart of cav-1−/− mice compared with WT mice (Fig. 11). MR expression was significantly reduced in WT + Epl compared with WT mice but was not significantly different in cav-1−/− + Epl compared with cav-1−/− mice (Fig. 11).
Fig. 11.
RT-PCR analysis of mineralocorticoid receptor (MR) mRNA expression in the aorta (A) and heart (B) of WT and cav-1−/− mice on HS diet and nontreated or treated with Epl. Data represent means ± SE (n = 6 to 11). §Measurements in cav-1−/− are significantly different (P < 0.05) from corresponding measurements in WT; *measurements in Epl-treated WT are significantly different (P < 0.05) from corresponding measurements in nontreated WT.
DISCUSSION
The present study demonstrates that MR blockade in cav-1−/− mice on HS diet is associated with 1) significant increase in BP, 2) enhanced vascular contraction, 3) reduced endothelium-dependent relaxation, and 4) reduced expression of vascular and cardiac eNOS.
BP was greater in cav-1−/− + Epl compared with cav-1−/− mice on HS. The increased BP does not appear to be solely due to cav-1 deficiency, because BP was similar in cav-1−/− and WT mice. The increased BP also may not be solely due to MR blockade, because the BP was not significantly different in Epl-treated and nontreated WT mice. Thus the BP data suggest a potential link between MR and cav-1 in the regulation of the BP control mechanisms.
BP is controlled by renal, hormonal, and cardiovascular mechanisms (57). The renin-angiotensin system and ANG II stimulate the release of Aldo from the adrenal cortex, which in turn activates MR in the collecting ducts and increases sodium absorption and plasma volume (1, 27, 36, 45). Studies have shown an increase in plasma volume in cav-1−/− mice (2, 56), and hypersensitivity to low levels of Aldo might explain this volume expansion. Consequently, Epl treatment is expected to block the effects of Aldo on renal MR, and thereby decrease sodium absorption, plasma volume expansion, and BP in cav-1−/− mice. With regard to volume expansion, we did not observe significant changes in body weight or water intake in the different groups during the 2-wk experiment. The increase in BP in cav-1−/− + Epl mice also may not be due to changes in circulating Aldo levels since they were not different between cav-1−/− and WT mice nontreated or treated with Epl. These data suggest that the increased BP in cav-1−/− + Epl mice on HS diet is not related to Aldo/MR regulation of renal function and could be related to changes in the cardiovascular control mechanisms of BP.
We have previously shown that HS diet alone in mice or rats is not associated with significant cardiac tissue damage (42, 51, 59, 68). In rats, dietary salt intake alone also is not associated with significant increase in BP or vascular reactivity, because the vasoconstrictive effects of HS are normally counterbalanced by compensatory increases in NO production and vascular relaxation (24, 64). This is supported by report that in rats chronically treated with l-NAME or endothelin B receptor antagonist, either of which could inhibit NO production, HS diet is associated with increased BP and vasoconstriction (24). Therefore, it is possible that the BP sensitivity to HS diet in Epl-treated cav-1−/− mice could be due to an interaction between cav-1 and the vascular NO-dependent control mechanisms of BP.
Studies in cav-1 null mice on normal rodent chow containing ∼0.8% NaCl have suggested a role of cav-1 in mechanotransduction, vascular remodeling, and cardiovascular function (15, 58, 71, 72). We also have previously examined whether cav-1 could be affected during HS diet and demonstrated that Phe, ANG II, and KCl contraction is reduced and ACh relaxation is enhanced in the aorta of cav-1−/− versus WT mice on HS diet (56). Some studies have shown that cav-1−/− mice have impaired constriction in resistance mesenteric arteries but not the aorta (2). Other studies have reported that in the presence of NOS inhibition, α1-adrenergic stimulation produces greater contraction in femoral arteries of cav-1−/− than WT mice (63). These data are consistent with the previously reported greater pressor effect of NOS inhibition in the cav-1−/− mice from our laboratory (56), but somewhat different from the present observation that NOS inhibition enhanced the aortic Phe response in cav-1−/− mice but only to levels similar to those observed in the WT. The difference in the findings could be related to the contractile signaling mechanisms activated in large conduit arteries versus small resistance vessels, making it important to investigate the effects of cav-1 deficiency in more than one vascular bed. On the other hand, the observations that the aortic contraction to Phe and KCl is reduced and that ACh-induced aortic relaxation is enhanced in cav-1−/− compared with WT mice on HS diet are consistent with a previous report from our laboratory (56) and suggest an increased amount/activity of endothelium-derived relaxing factor(s) in cav-1−/− mice on HS diet.
In endothelial cells, the NO produced by eNOS diffuses into VSM where it activates guanylate cyclase, increases cGMP production, and inhibits VSM contraction (20, 30, 48). Under basal conditions eNOS is bound to cav-1 at the endothelial cell caveolae. An increase in endothelial cell Ca2+ enhances the release of eNOS from cav-1 to the cytosol where it becomes phosphorylated and fully activated (6, 17, 18, 47, 62). The observation that endothelium removal or treatment with the NOS inhibitor l-NAME or the guanylate cyclase inhibitor ODQ caused greater enhancement of Phe contraction and/or greater reduction of ACh relaxation in the aorta of cav-1−/− compared with WT mice is consistent with a previous report from our laboratory that the endothelium-dependent NO-cGMP pathway is enhanced in cav-1−/− mice, and that by binding to eNOS cav-1 plays a role in the control of vascular function during HS diet (56).
MR has been identified in cardiac and vascular tissues, and Aldo-MR interaction may affect cardiac and vascular function during HS diet (19, 34, 59, 66, 68). The present MR expression data demonstrate that cav-1 deficiency is associated with reduced MR expression in the aorta and to a less extent in the heart. Epl treatment was associated with downregulation of MR in the aorta and heart of WT but not cav-1−/− mice, likely due to differences in MR sensitivity to blockade. This is in agreement with our recent observation that the MR sensitivity to Aldo is altered in cav-1−/− mice (data not shown). Nevertheless, the differential effects of Epl on MR expression in WT and cav-1−/− mice suggest potential interaction of MR and cav-1. This is supported by reports that cav-1 may be involved in mediating the effects of steroid hormones on their receptors (40, 41). These observations have led us to hypothesize a potential interaction between vascular MR, cav-1, and eNOS during HS diet. We reasoned that if MR, cav-1, and eNOS function independently, then MR blockade should have no, or at least similar, vascular effects in WT and cav-1−/− mice. On the other hand, if MR and cav-1 are functionally linked, then MR blockade should be associated with changes in eNOS expression/activity, NOS-mediated vascular relaxation, and the magnitude of vascular contraction, and these vascular effects would be different in WT and cav-1−/− mice.
Epl treatment of cav-1−/− mice was associated with enhanced Phe-induced aortic contraction, suggesting a role for MR in the regulation of vascular reactivity. The enhanced Phe-induced aortic contraction cav-1−/− + Epl mice was partly due to enhanced sensitivity of the α-adrenergic receptors because the Phe ED50 was less in the aorta of Epl-treated than nontreated cav-1−/− mice. KCl-induced aortic contraction, a receptor-independent response due to Ca2+ entry from the extracellular space (35, 49), also was greater in Epl-treated than nontreated cav-1−/− mice, suggesting that the enhanced vascular contraction in Epl-treated cav-1−/− mice could be due to activation of a common postreceptor contraction mechanism and/or inhibition of a common vascular relaxation mechanism.
The increase in vascular contraction associated with MR blockade in cav-1 null mice is likely due to reduced endothelium-dependent NO-cGMP relaxation pathway because 1) ACh-induced relaxation was reduced in the aorta of Epl-treated compared with nontreated cav-1−/− mice; 2) Removal of the endothelium, NOS inhibition by l-NAME, or guanylate cyclase inhibition by ODQ abolished the prominent ACh-induced aortic relaxation in cav-1−/− mice and the remaining smaller aortic relaxation in the cav-1−/− + Epl mice, but caused similar inhibition in the aorta of WT + Epl and WT mice; 3) the reduced aortic relaxation in cav-1−/− + Epl is not due to decreased responsiveness of VSM to vasodilators, since vascular relaxation to the exogenous NO donor SNP was not significantly different in the aorta of cav-1−/− or WT mice nontreated or treated with Epl; 4) consistent with a previous report from our laboratory (56), RT-PCR analysis revealed an increase in eNOS mRNA expression in the aorta and heart of cav-1−/− compared with WT mice on HS diet; and 5) Western blot analysis of eNOS protein amount revealed an insignificant increase in the aorta and significant increase in the heart of cav-1−/− compared with WT mice. Since cav-1−/− mice have intact cav-3 expression in the cardiac myocytes, the effects in the heart likely represent changes of eNOS in the vessels of the heart rather than in the cardiac myocytes. The prominent effects of MR blockade on eNOS mRNA expression and protein amount and on the NO-cGMP relaxation pathway in the aorta of cav-1−/− mice support a regulatory role of MR on the interaction between cav-1 and eNOS.
The molecular interaction between MR and the vascular cav-1 and NO pathway is unclear at the present time. Although MR has been identified in the vasculature and endothelial cells, and has been linked to vascular hypertrophy, vasospasm, and cell adhesion (10, 21–23, 60), the physiological significance of vascular MR has not been fully examined. The nature of activators of MR also is unclear, and the vascular MR can be activated by not only Aldo but also by other steroid and nonsteroid molecules (14, 21–23). One possibility is that MR and eNOS are both normally inhibited by cav-1 and that cav-1 disruption would lead to increased activity of both, so that MR can now act to stabilize or stimulate eNOS. The cellular mechanism via which vascular Aldo-MR may promote eNOS expression/activity in cav-1 deficiency states may involve genomic and nongenomic pathways. Studies have shown that the renal MR are coupled to nongenomic activation of the sodium channel ENaC in the collecting tubules (39, 43, 74). Aldo also may stimulate the activity and surface expression of Na+-H+ exchanger (NHE3) in human proximal tubule epithelial cells (16). ENaC, NHE, and Na+-Ca2+ exchanger have been identified in VSM and endothelial cells (25, 33, 57). Our Western blot experiments also revealed an increase in ENaC protein amount in the aorta of cav-1−/− (2.49 ± 0.37) compared with WT (1.00 ± 0.11; P = 0.017) mice. MR-mediated signaling may also regulate the Na+ channel in endothelial cells via a mechanism involving the cytoskeleton (25). It is tempting to suggest that in the cav-1 deficiency states and during HS diet, MR activation may promote Na+ entry into endothelial cells. In the presence of fully activated Na+-K+ pump, the increased cellular Na+ load is extruded via reverse-mode Na+-Ca2+ exchanger, leading to increased intracellular Ca2+, increased eNOS expression, and activation of Ca2+-dependent eNOS.
In support of the possibility that MR and eNOS are both normally inhibited by cav-1, and that cav-1 disruption allows MR to stabilize or stimulate eNOS, we observed that Epl treatment did not decrease eNOS mRNA expression in the heart or eNOS protein amount in the aorta or heart of WT mice, but decreased eNOS mRNA expression and protein amount in the aorta and heart of cav-1−/− mice. Although we could not discern differences in vascular NO production, other studies have shown that the plasma NOx levels are fivefold higher in cav-1−/− than WT mice (73), raising the possibility that the observed effects of Epl treatment could be due to blockade of the actions of Aldo on eNOS expression and NO production. Therefore, it is important to measure the effects of Epl treatment on plasma and vascular NOx in cav-1−/− and WT mice using more sensitive assays. Another way to determine eNOS activity is to measure activated p-eNOS. Our assays of p-eNOS were not sensitive enough to detect significant changes in activated p-eNOS in the aorta or heart of WT or cav-1−/− mice nontreated or treated with Epl. On the other hand, activated p-ERK is known to increase p-eNOS and enhance eNOS activity (7, 8). We have recently examined the effects of Aldo and canrenoate, the active ingredient in Epl, on p-ERK in cultured endothelial cells from cav-1−/− and WT mice and found that Aldo treatment for 24 h enhanced p-ERK expression and that canrenoate treatment blocked these effects (55). Other studies have shown that short-term exposure to Aldo enhances ATP-induced NO production in endothelial cells and augments ACh-induced relaxation in rat aorta and that these effects are blocked by Epl (50). Together, these data support the possibility that Epl could block the effects of Aldo on eNOS expression/activity and NO production. In this respect, it would be important to measure the direct effects of Aldo and MR antagonists on eNOS expression/activity and NO production in cultured endothelial cells from cav-1−/− and WT mice in future investigations.
In conclusion, in cav-1 deficiency states and HS diet, MR blockade is associated with increased BP, enhanced vasoconstriction, and decreased NOS-mediated vascular relaxation and eNOS expression. The salt sensitivity of the vascular mechanisms controlling BP in Epl-treated cav-1−/− mice suggests a role of MR in the regulation of endothelial cav-1 and eNOS expression and vascular remodeling during HS diet. During sodium overload the decrease in MR-mediated renal sodium reabsorption reduces untoward increases in plasma volume and BP. In cav-1 deficiency states, these renal control mechanisms could be reinforced by MR enhancement of eNOS expression/activity and reduction of vasoconstriction and vascular resistance to maintain BP toward normal.
Perspective
The observed increase in BP in the cav-1−/− mice treated with Epl raises a note of caution regarding the clinical use of MR antagonists. It has been reported that the forearm blood flow deteriorates following spironolactone treatment in type II diabetic subjects (12). Studies have also shown that the cav-1−/− mice present an insulin resistant phenotype (11), a feature in common with type II diabetes mellitus. The present results suggest that the use of MR blockers in cardiovascular disease may need to be carefully evaluated particularly in the presence of phenotypic changes in cav-1 expression.
GRANTS
This work was supported by grants from National Heart, Lung, and Blood Institute HL-69208 (to G. H. Williams) and HL-65998 and HL-70659 (to R. A. Khalil) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development HD-60702 (to R. A. Khalil). Support to L. H. Pojoga was provided by Scientist Development Grant 0735609T (American Heart Association).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the helpful technical assistance of Tham Yao and Paul Loutraris.
REFERENCES
- 1.Adler GK, Williams GH. Aldosterone: villain or protector? Hypertension 50: 31–32, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Albinsson S, Shakirova Y, Rippe A, Baumgarten M, Rosengren BI, Rippe C, Hallmann R, Hellstrand P, Rippe B, Swärd K. Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol Regul Integr Comp Physiol 293: R1222–R1231, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alzamora R, Brown LR, Harvey BJ. Direct binding and activation of protein kinase C isoforms by aldosterone and 17beta-estradiol. Mol Endocrinol 21: 2637–2650, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol 278: C163–C173, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Barron LA, Green GM, Khalil RA. Gender differences in vascular smooth muscle reactivity to increases in extracellular sodium salt. Hypertension 39: 425–432, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Batova S, DeWever J, Godfraind T, Balligand JL, Dessy C, Feron O. The calcium channel blocker amlodipine promotes the unclamping of eNOS from caveolin in endothelial cells. Cardiovasc Res 71: 478–485, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem 275: 30707–30715, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Boo YC, Kim HJ, Song H, Fulton D, Sessa W, Jo H. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic Biol Med 41: 144–153, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Brown NJ. Eplerenone: cardiovascular protection. Circulation 107: 2512–2518, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 102: 1359–1367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Davies JI, Band M, Morris A, Struthers AD. Spironolactone impairs endothelial function and heart rate variability in patients with type 2 diabetes. Diabetologia 47: 1687–1694, 2004 [DOI] [PubMed] [Google Scholar]
- 13.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int 66: 2454–2466, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Di Zhang A, Cat AN, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, Delcayre C, Samuel JL, Jaisser F. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension 52: 1060–1067, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Drumm K, Kress TR, Gassner B, Krug AW, Gekle M. Aldosterone stimulates activity and surface expression of NHE3 in human primary proximal tubule epithelial cells (RPTEC). Cell Physiol Biochem 17: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 271: 22810–22814, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem 273: 3125–3128, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 98: 121–128, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol 31: 5–14, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Funder JW. Mineralocorticoid receptors and cardiovascular damage: it's not just aldosterone. Hypertension 47: 634–635, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension 53: 286–290, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Funder JW. Why are mineralocorticoid receptors so nonselective? Curr Hypertens Rep 9: 112–116, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension 37: 516–523, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Golestaneh N, Klein C, Valamanesh F, Suarez G, Agarwal MK, Mirshahi M. Mineralocorticoid receptor-mediated signaling regulates the ion gated sodium channel in vascular endothelial cells and requires an intact cytoskeleton. Biochem Biophys Res Commun 280: 1300–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 250: R960–R972, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Holtzman EJ, Braley LM, Williams GH, Hollenberg NK. Kinetics of sodium homeostasis in rats: rapid excretion and equilibration rates. Am J Physiol Regul Integr Comp Physiol 254: R1001–R1006, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens 21: 951–959, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53: 503–514, 2002 [PubMed] [Google Scholar]
- 31.Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension 32: 459–466, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β- and γ-ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev 10: 31–37, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Khalil RA, van Breemen C. Intracellular free calcium concentration/force relationship in rabbit inferior vena cava activated by norepinephrine and high K+. Pflügers Arch 416: 727–734, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Khalil RA. Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol Regul Integr Comp Physiol 290: R509–R513, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol 308: 3–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosachunhanun N, Hunt SC, Hopkins PN, Williams RR, Jeunemaitre X, Corvol P, Ferri C, Mortensen RM, Hollenberg NK, Williams GH. Genetic determinants of nonmodulating hypertension. Hypertension 42: 901–908, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lee IH, Campbell CR, Cook DI, Dinudom A. Regulation of epithelial Na+ channels by aldosterone: role of Sgk1. Clin Exp Pharmacol Physiol 35: 235–241, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, Ren C, Wang J, Tahir SA, Thompson TC. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res 61: 4386–4392, 2001 [PubMed] [Google Scholar]
- 41.Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol 168: 1998–2013, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension 39: 614–618, 2002 [PubMed] [Google Scholar]
- 43.McEneaney V, Harvey BJ, Thomas W. Aldosterone regulates rapid trafficking of epithelial sodium channel subunits in renal cortical collecting duct cells via protein kinase D activation. Mol Endocrinol 22: 881–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard J. The 45-year story of the development of an antialdosterone more specific than spironolactone. Mol Cell Endocrinol 217: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Mercier I, Casimiro MC, Zhou J, Wang C, Plymire C, Bryant KG, Daumer KM, Sotgia F, Bonuccelli G, Witkiewicz AK, Lin J, Tran TH, Milliman J, Frank PG, Jasmin JF, Rui H, Pestell RG, Lisanti MP. Genetic ablation of caveolin-1 drives estrogen-hypersensitivity and the development of DCIS-like mammary lesions. Am J Pathol 174: 1172–1190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285: L1179–L1183, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Murad Shattuck Lecture F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 2006; 355(19): 2003. –11. [DOI] [PubMed] [Google Scholar]
- 49.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834–C844, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Mutoh A, Isshiki M, Fujita T. Aldosterone enhances ligand-stimulated nitric oxide production in endothelial cells. Hypertens Res 31: 1811–1820, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-l-arginine methyl ester-induced myocardial injury. Circulation 108: 2517–2523, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT1A receptor for angiotensin II. Hypertension 35: 550–554, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension 43: 420–427, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of l-NAME and angiotensin II. Endocrinology 151: 1236–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294: H1258–H1265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ponnuchamy B, Khalil RA. Cellular mediators of renal vascular dysfunction in hypertension. Am J Physiol Regul Integr Comp Physiol 296: R1001–R1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 141: 3871–3878, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension 47: 312–318, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca2+-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci USA 92: 1759–1763, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol Heart Circ Physiol 277: H1167–H1177, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Shakirova Y, Bonnevier J, Albinsson S, Adner M, Rippe B, Broman J, Arner A, Swärd K. Increased Rho activation and PKC-mediated smooth muscle contractility in the absence of caveolin-1. Am J Physiol Cell Physiol 291: C1326–C1335, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Smith L, Payne JA, Sedeek MH, Granger JP, Khalil RA. Endothelin-induced increases in Ca2+ entry mechanisms of vascular contraction are enhanced during high-salt diet. Hypertension 41: 787–793, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Stan RV, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae). Mol Biol Cell 8: 595–605, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strawn WB. Eplerenone antagonizes atherosclerosis, but what is the agonist? Hypertension 46: 1093–1094, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Struthers A, Krum H, Williams GH. A comparison of the aldosterone blocking agents eplerenone and spironolactone. Clin Cardiol 31: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology 147: 3183–3189, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48: 797–803, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 97: 829–836, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun 340: 702–708, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116: 1284–1291, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou ZH, Bubien JK. Nongenomic regulation of ENaC by aldosterone. Am J Physiol Cell Physiol 281: C1118–C1130, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol 25: 1824–1830, 2005 [DOI] [PubMed] [Google Scholar]