Abstract
We have generated transgenic mice that express angiotensin II (ANG II) fused downstream of enhanced cyan fluorescent protein, expression of which is regulated by the mouse metallothionein promoter. The fusion protein, which lacks a secretory signal, is retained intracellularly. In the present study, RT-PCR, immunoblot analyses, whole-animal fluorescent imaging, and fluorescent microscopy of murine embryonic fibroblasts confirm expression of the fusion protein in vivo and in vitro. The transgene is expressed in all tissues tested (including brain, heart, kidney, liver, lung, and testes), and radioimmunoassay of plasma samples obtained from transgenic mice indicate no increase in circulating ANG II over wild-type levels, consistent with intracellular retention of the transgene product. Kidneys from transgenic and corresponding wild-type littermates were histologically evaluated, and abnormalities in transgenic mice consistent with thrombotic microangiopathy were observed; microthrombosis was frequently observed within the glomerular capillaries and small vessels. In addition, systolic and diastolic blood pressures, measured by telemetry (n = 8 for each group), were significantly higher in transgenic mice compared with wild-type littermates. Blood pressure of line A male transgenic mice was 125 ± 1.7 over 97 ± 1.6 compared with 109 ± 1.7 over 83 ± 1.4 mmHg in wild-type littermates (systolic over diastolic). In summary, overexpression of an intracellular fluorescent fusion protein of ANG II correlates with elevated blood pressure and kidney pathology. This transgenic model may be useful to further explore the intracellular renin-angiotensin system and its implication in abnormal kidney function and hypertension.
Keywords: intracrine, intracellular angiotensin, transgenic, renal microangiopathy
angiotensin ii (ang ii) is a powerful vasopressor within the hemodynamic renin-angiotensin-aldosterone system (RAAS). In the classic RAAS pathway, ANG II generated in the circulation binds to G protein-coupled receptors, including the ANG type I and type II receptors (AT1R and AT2R), to stimulate G protein activation and intracellular signaling events (second and third messengers), which, in turn, regulate expression of downstream pathways involved in cellular proliferation, hypertrophy, and blood pressure homeostasis (24, 27, 49). Increasingly, however, attention is being directed toward local tissue and intracellular aspects of the system, many effects of which are independent of the circulating system.
Intracrine regulation refers to the ability of hormones, proteins, and other signaling molecules to act not only in an endocrine, autocrine, or paracrine fashion, but within the cell of production or after internalization by a target cell. While many different types of molecules can act as intracrines, components of the intracellular RAAS have become the focus of several laboratories concerned with hypertension and angiogenesis and related fields of study. Data supporting the existence of intracellular ANG II (iANG II) and, particularly, nuclear localization of iANG II and other components of the RAAS, including ANG I, angiotensin converting-enzyme, and renin, have been reported by several different groups, including ours (6, 18, 21, 23, 25, 37, 41–43, 52). Moreover, our laboratory and others have shown that a population of the cell surface AT1R, or a fragment thereof, can be transported to the nucleus in an ANG II-dependent manner (5, 7, 11, 12, 35).
Our laboratory has generated several different genetic constructs designed to investigate iANG II and its effects on different cell types. In our early studies, we showed that a mutated version of the rat angiotensinogen cDNA [pAng(-S)Exp], which lacks the signal peptide-encoding sequence, and therefore encodes a protein that is retained intracellularly (13), increases proliferation of rat liver cells through a mechanism that is insensitive to candesartan and anti-ANG II antibodies, but sensitive to intracellular renin knockdown and, in part, to anti-PDGF antibodies. Since these cells also accumulate the nonclassical alternatively spliced form of renin, renin 1b (30, 48, 54), which is retained intracellularly, we proposed that iANG II was generated intracellularly from ANG(-S)Exp and was mitogenic for rat liver cells.
Our laboratory also developed and characterized a fluorescent fusion protein of the ANG II peptide linked by a small spacer arm, upstream and in-frame, to enhanced cyan fluorescent protein (ECFP). This plasmid is referred to as pECFP/ANG II (12). By directly linking ECFP upstream of the mature octapeptide, the fusion protein is designed to be synthesized on free ribosomes and not destined for secretion. This protein was shown to alter AT1R distribution, to phosphorylate and activate cAMP response element-binding protein, and to stimulate proliferation of vascular smooth muscle cells, as well as CHO-K1 and COS-7 cells (10, 12). iANG II appears to share some signaling pathways common to extracellular ANG II, but behaves through some independent pathways as well (10).
To further investigate the biological relevance of iANG II, we used the latter construct to develop a transgenic mouse model, which expresses the fluorescent fusion protein of iANG II through the global metallothionein promoter. We postulated that iANG II would be expressed in a broad array of tissues and might increase blood pressure in these mice through mechanisms different than those of conventional ANG II acting through plasma membrane-bound angiotensin receptors. The data presented here are the first report of this unique mouse model and are focused on phenotypic changes in blood pressure and kidney histology.
MATERIALS AND METHODS
Chemicals and reagents.
Chemicals were purchased from Sigma (St. Louis, MO). Restriction enzymes were purchased from New England Biolaboratories (Ipswich, MA). All oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Isoflurane was purchased from Butler Animal Supply (Dublin, OH). Cell media, trypsin solution, and antibiotics were purchased from Gibco (Carlsbad, CA).
Generation of ECFP/ANG II transgenic mice.
A transgenic construct, pmMTpro/ECFP/ANG II/3.7SPC/SV40, was designed containing a metallothionein promoter, sequences encoding the fusion protein, ECFP/ANG II, and a SV40-derived polyadenylation tail. pECFP/ANG II was prepared as previously described (12). This construct lacks a secretory signal; thus the protein remains intracellular. ECFP/ANG II was released from pECFP/AII by digestion with NheI and BamHI. The ∼800-bp fragment was end-filled and ligated to 3.7SPC/SV40 (gift of Dr. Jawed Alam, Ochsner Clinic Foundation), which had been digested with EcoRI and end-filled. The mouse MT-1 gene (from nucleotide residue −750 to +1,240) cloned in the vector pBX322 was kindly provided by Dr. R. Palmiter (University of Washington, Seattle, WA). The promoter sequence (775 bp) was amplified by PCR from using primers MT-1U (5′-ACG TAC CTC GAC CAG CAG ACT CTA ATG TTA CTC-3′) and MT-1D (5′-CAT GCA CTC GAG GAA GCT GGA GCT ACG GAG TAA-3′). The amplicon was then digested with SalI and XhoI, and ligated to SalI-digested pECFP/ANGII/3.7SPC/SV40. Release of the transgene fragment by SalI/NotI digestion produces the 775-bp promoter + 800-bp transgene cassette + 400-bp SV40 poly A/T intron region; the full length transgene (mMTpro/ECFP/ANG II/3.7SPC/SV40) is ∼1,975-bp long. Transgenic mice were generated at the Transgenic Animal Facility of the University of Iowa. Transgene DNA was microinjected into B6SJL F1 one-cell fertilized mouse eggs using standard procedures (47). Founder mice were successively backcrossed to C57BL/6J wild-type mice, and the resulting offspring were genotyped by PCR and Southern blot. Animals were bred and housed at the Ochsner Clinic Foundation Animal Services Department under controlled light, temperature, and humidity conditions. All animal protocols were approved by the in-house Institutional Animal Care and Use Committee.
Southern blotting.
Genomic DNA was extracted using previously described methods (50). DNA (5 μg) was digested overnight using BamHI. The following day, the digested DNA was electrophoresed on a 0.8% agarose gel. Depurination, denaturing, and neutralizing of the gel, as well as prehybridization, hybridization, and washing steps of the membrane, were carried out as previously described (36). The DNA was transferred to a NitroPure (GE Osmonics, Minnetonka, MN) supported nitrocellulose membrane by standard capillary blotting procedure. As a probe, a 748-bp fragment containing most of the ECFP sequence was obtained by digestion of pECFP-C1 (nt 592–1340) (Clontech, Mountain View, CA) with NheI and BglII. The fragment was electroeluted and precipitated using standard isopropanol preparation. The probe was labeled using the Prime-It II kit (Stratagene, La Jolla, CA) and [α-32P]dCTP (Perkin Elmer, Waltham, MA). The specific activity of the probe was consistently >109 cpm/μg. The hybridized blots were placed on a phosphor screen in a standard film cassette and stored at room temperature in the dark overnight. Screens were read using a Cyclone PhosphorImager (Packard, Waltham, MA). Opti-Quant software (Packard) was used to quantify the bands.
Reverse-transcription polymerase chain reaction.
Mice were anesthetized and killed, and the brain, heart, kidney, liver, lung, spleen, and testes were removed, weighed, and kept on ice. Total RNA was extracted from the various mouse organs using a standard guanidine isothiocyanate-water-saturated phenol method (9). RNA was treated with DNase I (Sigma) at room temperature for 15 min. cDNA was prepared by annealing random hexamers to the RNA and treating with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) in the presence of RNasin, an RNase inhibitor (Promega, Madison, WI). PCR was performed using exon spanning primers specific for an internal segment of ECFP/ANG II (F: 5′-ACA TGG TCC TGC TGG AGT TC-3′, R: 5′-ACA GAC TGT GAG GAC TGA GG-3′) and GAPDH (F: 5′-ACC ACA GTC CAT GCC ATC AC-3′, R: 5′-TCC ACC ACC CTG TTG CTG TA-3′).
Immunoblot analyses.
Protein was isolated from the same tissues as indicated above. Tissues were homogenized in 10 volumes of buffer A (20 mM Tris·HCl, pH 6.5, 100 mM NaCl, 5 mM EDTA, 10 mM MgCl2, 5% glycerol), supplemented with protease (Sigma, St. Louis, MO) and phosphatase (CalBiochem, San Diego, CA) inhibitors. Homogenates were strained through cheese cloth, and cells were lysed in the presence of 1% Triton X-100 and 0.5% Nonidet P-40. Lysates was centrifuged at 15 K rpm for 15 min, and supernatants removed. Lithium dodecyl sulfate (×4, for composition see http://invitrogen.custhelp.com/cgi-bin/invitrogen.cfg/php/enduser/std_adp.php?p_faqid=746) was added to ×1 concentration, and the solution was centrifuged once again. Samples were then loaded and subjected to SDS-PAGE (NuPage Gels, Invitrogen). Proteins were transferred to PVDF membrane (GE Osmonics, Minnetonka, MN) and blocked with 5% blotto for 1 h at room temperature. Primary antibodies, anti-green fluorescent protein (GFP) (FL, Santa Cruz Biotechnology, San Carlos, CA) and anti-ANG II [rabbit anti-ANG II (human), Peninsula Laboratories] were added in 2% blocking buffer and incubated on a shaker at 4°C overnight. The following day, membranes were washed with Tris-buffered saline-Tween, and secondary antibody was added for 1 h at room temperature. Chemiluminescence was detected using ECL Plus (GE Healthcare, Pittsburgh, PA) on a Chem-Doc documentation system (Bio-Rad, Hercules, CA).
Blood was collected from the retroorbital venous plexus or heart and immediately centrifuged with EDTA at 2,000 g for 20 min to obtain plasma (8). Plasma (3 μl) from wild-type and transgenic mice was subjected to electrophoresis, as indicated above. Anti-osteopontin antibody (goat anti-mouse, Abcam ab11503) was used as an internal control to standardize for loading differences.
To avoid contamination by food, water, and wastes, which can occur with metabolic cage use, mouse urine was obtained by gentle bladder massage and collected on a parafilm strip (46). Protease inhibitors (Sigma P8340) were added immediately. Urine (5 μl) from wild-type and transgenic mice was subjected to electrophoresis, as indicated above. Anti-transferrin antibody (rabbit anti-human, ab82411) was used as internal positive control.
Isolation and culture of murine embryonic fibroblasts.
Homozygous ECFP/ANG II transgenic male and genotype-matched female mice were isolated in breeding cages. The following day, females were checked for vaginal plugs. After mating was established, females were isolated and routinely weighed to confirm pregnancy. At 13.5 days postcoitus, the female was killed, and the uterine horns were removed. Embryos were separated from the placenta and membranes, eviscerated, and decapitated. The remaining tissue was finely minced and treated with 1 ml of trypsin-EDTA/2 embryos (see these websites for more information and instruction: http://www.jove.com/index/details.stp?ID=160, http://www.molgen.mpg.de/∼soldatov/protocols/protocol/02/02_03.htm). The cell suspensions were rocked at 37°C for 15 min and neutralized with two volumes of murine embryonic fibroblast (MEF) media (DMEM, 10% FBS, antimycotic-antibiotic, and gentamycin). The remaining pieces of tissue were allowed to settle out, and the supernatant was removed and centrifuged to pellet the cells. Cells were resuspended in MEF media and plated at 2 embryos/100 mm dish. For imaging, 2 × 105 cells were plated on a 35-mm glass bottom dish (MatTek, Ashland, MA), and images were captured using a Zeiss Axiovert 200M 3D deconvolution fluorescence microscope (Intelligent Imaging Innovations, Denver, CO).
Whole mouse in vivo fluorescence imaging.
ECFP/ANG II transgenic mice and wild-type littermates were anesthetized (isoflurane) and imaged using the Xenogen IVIS Lumina (Caliper LS, Hopkinton, MA). Data were analyzed and quantified using accompanying Living Image 3.0 software.
Radioimmunoassays of ANG II.
Mice (4–6 mo old) were decapitated, and trunk blood samples were collected into an inhibitor cocktail for measurement of ANG II concentrations. Blood samples were centrifuged at 4°C for 10 min at 1,000 g, and plasma was separated. Plasma for peptide radioimmunoassay was denatured in methanol and centrifuged at 4,000 rpm for 30 min. The supernatants were dried and stored at −20°C until assayed. Plasma for the analysis of angiotensin peptides was extracted by adsorption to and elution from a phenyl-bonded, solid-phase extraction column (Bond-Elut; Varian, Harbor City, CA). The eluants were collected and stored at −20°C. Before radioimmunoassay, the eluants were evaporated to dryness under vacuum and reconstituted in assay diluent. For angiotensin peptide analysis, the dried residue was reconstituted in 0.4 ml of sodium phosphate buffer, pH 7.4, containing 1.0 mg human serum albumin per milliliter.
Plasma ANG II levels were quantified by radioimmunoassay using rabbit anti-ANG II antisera (Peninsula Laboratories, San Carlos, CA). Briefly, the reconstituted plasma samples were incubated with antiserum and 125I-radiolabeled ANG II for 48 h at 4°C. Bound and free ANG II were separated by dextran-coated charcoal, and the supernatants were counted by a computer-linked gamma counter for 3 min. Results are reported in femtomoles per milliliter plasma.
Kidney histology.
Kidneys were harvested from recently culled animals, weighed, and then submerged in a solution of 10% formalin (Ochsner Clinic Foundation, New Orleans, LA). After fixation, kidneys were embedded in paraffin and sectioned using a microtome. The 4-μm sections were then stained with either hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) reagents. Slides were examined by a trained pathologist blinded to the study identifiers. Slides were also imaged by three-dimensional deconvolution fluorescence microscopy to evaluate ECFP/ANG II distribution (51).
Telemetric blood pressure studies.
Animals were subjected to 5% isoflurane until unconscious and continuously maintained on 2% isoflurane. Surgeries were performed on warming pads. A telemetric catheter [PA-C10, Data Sciences International (DSI), St. Paul, MN] was placed in the left carotid artery and extended down to the aortic arch. The catheter was then sutured in place. The body of the telemetric unit was placed subcutaneously on the right ventral flank of the animal. Mice were permitted to recover for 7 days before measurements were collected. Systolic and diastolic blood pressure, locomotor activity, and heart rate were all measured. Data were collected for 30 s every 30 min for 72 h. During measurement, mice were permitted free mobility and maintained on a 12:12-h light-dark cycle (6 AM: lights on, 6 PM: lights off) and given food and water ad libitum. Data was analyzed using Dataquest A.R.T. software (DSI).
Statistical analyses.
Data from telemetry readings were compiled from corresponding animals and plotted, with each point representing 4-h moving average. Day and night blood pressures were calculated according to the light cycle mentioned above. Statistical probability values associated with the circadian data (blood pressure, locomotor activity, and heart rate), as well as day/night blood pressures, were calculated using the two-sample t-test and unpaired t-test. Statistical P values associated with the 72 h (combined day and night values) were calculated using ANOVA with the Bonferroni post hoc test.
RESULTS
Transgenic mice express ECFP/ANG II mRNA and protein.
To investigate phenotypic changes that accompany iANG II expression, we generated several lines of transgenic mice that express a fluorescent fusion protein of iANG II from pmMTpro/ECFP/ANG II. Since variation in the integration site and transgene copy number can affect protein expression, pilot studies were performed on five transgenic mouse lines, arbitrarily designated A–E. Lines A and D, which demonstrate relatively high copy number and robust expression of the transgene, were selected for further study. Using transgene dilution controls, we determined that line D (150 copies) possesses 10 times the copy number of incorporated transgene as that present in line A (15 copies) (Fig. 1A).
Fig. 1.
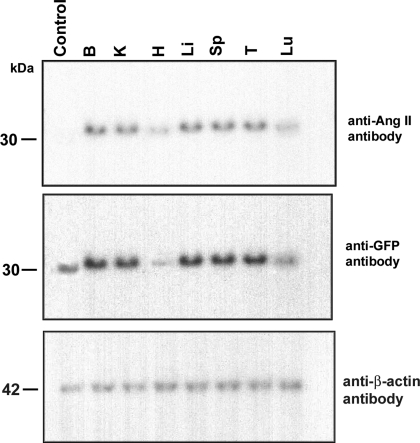
Transgenic (TG) mice possess enhanced cyan fluorescent protein (ECFP)/angiotensin II (ANG II) DNA and mRNA. A: Southern blot showing the relative copy number of the transgene in lines A (15 copies) and D (150 copies) using dilution controls. The blot shown was hybridized with pECFP-C1 probe and detects a 1-kb fragment in BamHI genomic DNA digests. WT, wild-type mice. B: 1% agarose gel showing RT-PCR products generated from total RNA collected from various tissues (B, brain; H, heart; K, kidney; Li, liver; Lu, lung; Sp, spleen; T, testes) (shown here, line A). Upstream primer 5′-ACA TGG TCC TGC TGG AGT TC-3′ and downstream primer 5′-ACA GAC TGT GAG GAC TGA GG-3′ were used to amplify a 211-bp fragment of transgene mRNA. ECFP/ANG II TG mice possess transgene mRNA in all tissues tested; RNAs derived from WT tissues produce no amplification product. Forward primer 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse primer 5′-TCC ACC ACC CTG TTG CTG TA-3′ were used to amplify a fragment of the endogenous internal control GAPDH (452 bp), which was present in all tissues. M (marker) denotes the 100-bp ladder.
Due to the permissive nature of the metallothionein promoter, ECFP/ANG II is expressed in a broad spectrum of tissues. Reverse transcription-PCR of total RNA was used to test for transgene expression. All of the line A tissues tested (brain, heart, kidney, liver, lung, spleen, and testes) were positive for ECFP transgene mRNA and, as expected, for the internal control mRNA, GAPDH (Fig. 1B). PCR analysis of line D (not shown) was indistinguishable from line A.
Several methods were employed to confirm that the fusion protein is, indeed, properly translated. Immunoblot analyses of proteins extracted from the same tissue types previously evaluated for mRNA expression also tested positive for ECFP. There was a small but detectable difference in the size of the fusion protein and the control ECFP (due to the addition of ANG II and the spacer arm) (Fig. 2 shows immunoblots from line A). The same blot was subjected to anti-ANG II antibody, and bands of the same size were detected in all of the samples, except, of course, in the pECFP-C1 transfected cell lysate (control). The absence of any distinct smaller immunoreactive fragments suggests that the fusion protein is quite stable. Line D immunoblots (not shown) are indistinguishable from those of line A, despite the difference in copy number. It has been reported that level of expression driven by the metallothionein promoter is rarely related to transgene copy number (39) and, indeed, our results support this observation.
Fig. 2.
TG mice express ECFP/ANG II protein. Immunoblots were performed with proteins from various tissues (B, K, H, Li, Sp, T, Lu) and with both anti-ANG II and anti-green fluorescent protein (GFP) antibodies (shown here, line A). The fusion protein was detected with both antibodies. The ECFP positive control, however, was detected only with anti-GFP antibody, and not with anti-ANG II antibody (as expected). Anti-β-actin antibody was used as a loading control probe.
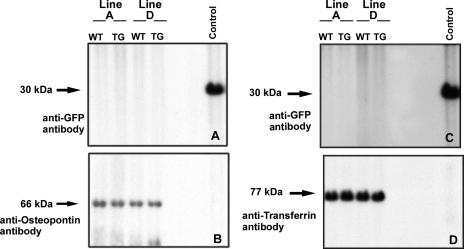
Despite the fact that our previously published studies show that ECFP/ANG II accumulates in transfected cells in cytoplasm and particularly in nucleus, and despite the fact that ECFP/ANG II is designed without a secretory signal peptide, there is a possibility that a small quantity could escape from cells and alter blood pressure through a circulatory mechanism. To assay for the presence of extracellular transgene product, we performed immunoblots of urine and plasma proteins from transgenic and wild-type mice of lines A and D (Fig. 3). No GFP-immunoreactive material was observed in urine or plasma of either mouse line.
Fig. 3.
Transgene product is not detected in circulation or urine. A: urine samples (5 μl) from lines A and D were examined by immunoblot for the presence of GFP immunoreactive proteins. B: filter was stripped and rehybridized with control antibodies. C: plasmas (3 μl) from lines A and D were assayed for the presence of GFP immunoreactive proteins. D: filter was stripped and rehybridized with control antibodies. Control represents extracts of CHO-K1 cells transfected with pECFP-C1.
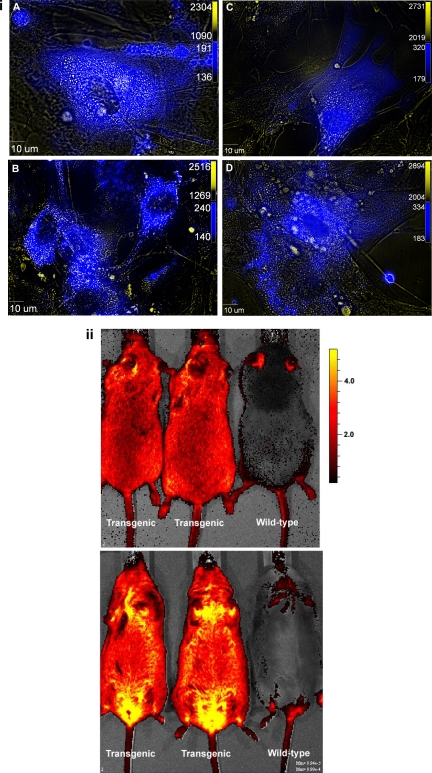
Several lines of MEFs were isolated and cultured from the ECFP/ANG II transgenic mice. More than 70% of MEFs from both lines A and D visibly fluoresce using the cyan fluorescent protein (CFP) filter set (Fig. 4i). CFP images are merged with the corresponding differential interference contrast images to provide some structural detail. To obtain a macroscopic perspective of the transgenic mice, whole animal fluorescence imaging was performed using the Caliper LS Xenogen system with Living Image software. Transgenic animals, both homozygous and heterozygous, from both lines A and D, show remarkable fluorescence intensity, although there are low levels of autofluorescence in the wild-type mouse tail and ears (Fig. 4ii). The sensitivity of the system, however, does not permit differentiation of homozygotes from heterozygotes.
Fig. 4.
ECFP/ANG II TG mice visibly fluoresce. i: Murine embryonic fibroblasts were isolated at 13.5 days postcoitus. The cells were cultured and visualized using fluorescence imaging. Cells were derived from line A (A and C) and line D (B and D). Cyan fluorescence is superimposed on differential interference contrast images for improved structural detail. ii: In vivo images of line A (left) and line D (middle) mice captured using the Caliper LS (Xenogen IVIS Lumina) with Living Image 3.0 Software. Top: dorsal view; bottom: ventral view.
The fact that the transgene product is stable (Fig. 2) suggests that the fluorescence observed in MEFs and whole-animal images reflects the distribution of the fusion protein and not a cleaved product.
ECFP/ANG II transgenic mice have normal levels of circulating ANG II.
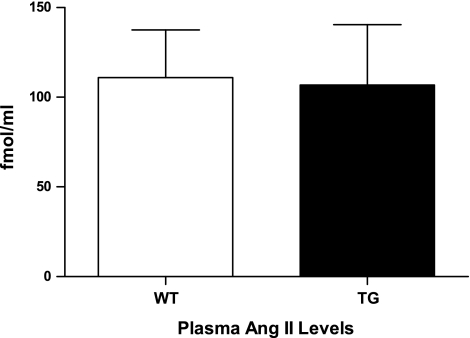
ANG II was measured in plasma of 6 mo male line A wild-type mice (mean = 111.0, SD = 65.2, n = 8) and ECFP/ANG II transgenic littermates (mean = 106.7, SD = 82.4, n = 8), and no significant difference in the level of circulating ANG II was observed between the two populations (Fig. 5). The fluorescent fusion protein does not possess a signal peptide and has been demonstrated to remain within cells of synthesis (10, 12). Moreover, this suggests that ECFP/ANG II does not provoke effects (such as blood pressure outcomes) through upregulation of ANG II created through a transgene-mediated increase in synthesis or secretion of angiotensinogen.
Fig. 5.
ECFP/ANG II TG mice have normal circulating levels of ANG II. Radioimmunoassays show no significant difference (n = 6) between plasma ANG II levels in WT and TG ECFP/ANG II (line A) mice. Values are means ± SE.
ECFP/ANG II transgenic mice possess abnormal kidneys.
Organ weights were not significantly different between wild-type and transgenic mice. To evaluate phenotypic effects secondary to expression of the transgene, histological examination of kidneys and hearts from ECFP/ANG II transgenic and wild-type animals was performed on paraffin-embedded tissues stained with either H&E or PAS reagent. While no significant histological changes were observed in heart, the kidney sections demonstrated pathology consistent with thrombotic microangiopathy.
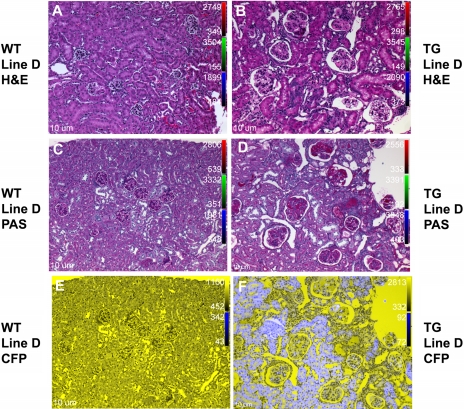
Kidneys from the wild-type littermates of both lines A and D appeared healthy and normal (Fig. 6, A, C, and E, and 7, A, C, and E). Sections from the ECFP/ANG II transgenic mice, however, exhibited many large glomeruli with dilated Bowman's capsules and evidence for thrombi within the glomeruli (Figs. 6B and 7B). PAS staining (40) corroborated the existence of fibrin thrombi in the transgenic kidneys (Figs. 6D and 7D). Sections were further analyzed by fluorescence microscopy; robust ECFP/ANG II expression was observed primarily in the proximal and distal tubules, with lower expression levels in the glomeruli (Figs. 6F and 7F). No cyan fluorescence was detected in kidneys of wild-type mice (Figs. 6E and 7E).
Fig. 6.
ECFP/ANG II line A TG mice demonstrate abnormal kidney histology. Kidney sections obtained from male mice, 4–6 mo of age, were stained with hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) to evaluate TG kidney phenotype. A: a WT kidney section stained with H&E shows normal kidney structure. B: kidney section from a TG mouse, H&E stained. Glomeruli (Bowman's capsule and intraglomerular space) are enlarged in TG mice. C and D: kidney sections of WT and TG kidneys, respectively, stained with PAS. Note glomerular blood clots consistent with thrombotic microangiopathy. E and F: corresponding sections (C and D, respectively) imaged using fluorescence microscopy. The fusion protein, ECFP/ANG II (ECFP fluorescence in blue) appears primarily in proximal and distal tubules, with less robust expression in glomeruli. All images captured at ×10 magnification. CFP, cyan fluorescent protein.
Fig. 7.
ECFP/ANG II line D TG mice demonstrate abnormal kidney histology. See Fig. 6 legend for details.
ECFP/ANG II transgenic mice demonstrate elevated blood pressure.
While elevated blood pressure is characteristic of transgenic animals overexpressing circulating angiotensin (1, 2, 15, 28), effects of the intracellular renin-angiotensin system (iRAS) on blood pressure have not been previously reported. Mice were implanted with telemetric catheters, and blood pressure, heart rate, and locomotor activity were measured for 30 s every 30 min over a 72-h period. Both lines A and D were evaluated (see Figs. 8–11).
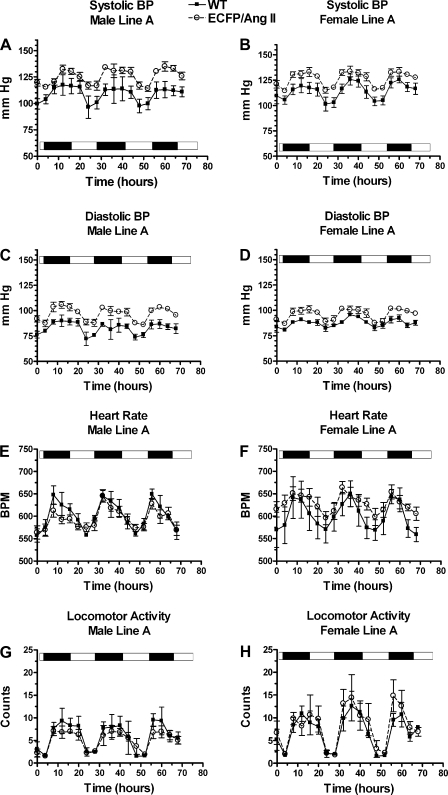
Fig. 8.
ECFP/ANG II (line A, 15 transgene copies) TG mice have increased blood pressure (BP) compared with WT littermates. Mice (4–6 mo) were permitted to recover for 1 wk postsurgery before radiotelemetry data acquisition. Each point is a 4-h moving average of data collected for 30 s every 30 min over a 72-h period; the capture periods begin at 10 AM. Note the circadian pattern by day-night indicator bar. Systolic BP (A and B), diastolic BP (C and D), heart rate (E and F), and locomotor activity (G and H) for males and females, respectively, are shown. Values are means ± SE; n = 8 for all groups. A–D: P < 0.0001 for TG vs. WT, systolic and diastolic, male and female BPs (two-sample t-test). F: P < 0.0001 for TG vs. WT heart rate, female only; P = 0.0120 for TG vs. WT locomoter activity, female only (two-sample t-test). BPM, beats/min.
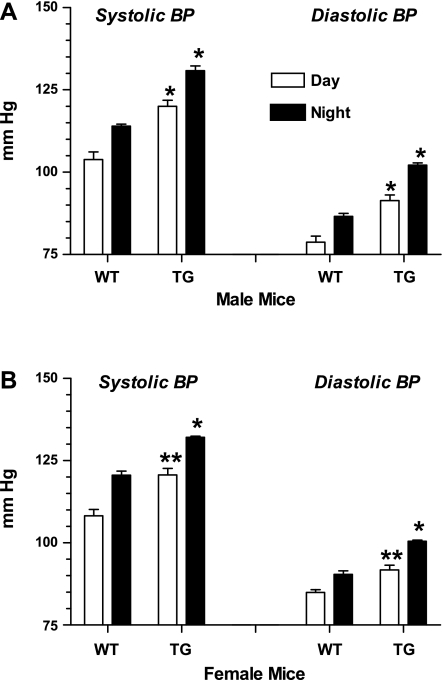
Fig. 11.
ECFP/ANG II (line D) TG mice show increased average day and night BPs compared with WT littermates. Bars represent combined 4-h moving averages for day (6 AM–6 PM) and night (6:01 PM–5:59 AM). A: males; B: females. Values are means ± SE; n = 9 for all groups. *P < 0.0001 for TG vs. corresponding WT day or night value. **P < 0.01 for TG vs. corresponding WT value (unpaired t-test).
Four-hour moving-average graphs showed distinctive circadian patterns, with highest blood pressure and locomotor activities observed during the evening hours (Fig. 8, line A, and Fig. 10, line D). Daytime and nighttime (systolic and diastolic) blood pressures were significantly higher in transgenic compared with wild-type mice for both lines A and D (Figs. 9 and 11).
Fig. 10.
ECFP/ANG II (line D, 150 transgene copies) TG mice have increased BP compared with WT littermates. Legend is the same as for Fig. 8, with the exception of the reference to locomotor activity, for which there is no significant difference in line D.
Fig. 9.
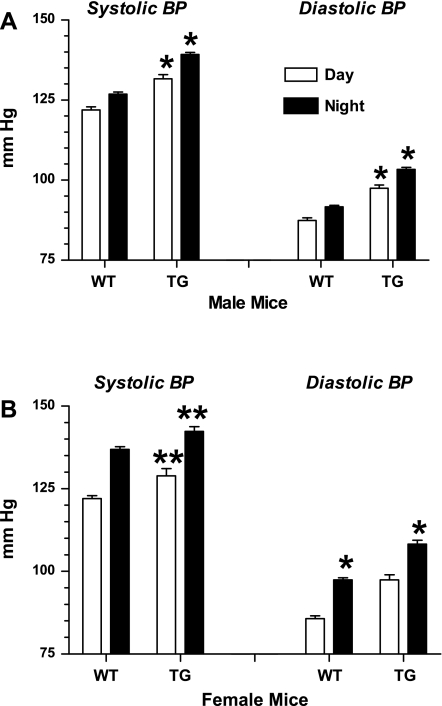
ECFP/ANG II (line A) TG mice show increased average day and night BPs compared with WT littermates. Bars represent combined 4-h moving averages for day (6 AM–6 PM) and night (6:01 PM–5:59 AM). A: males; B: females. Values are means ± SE; n = 9 for all groups. *P < 0.0001 for TG vs. corresponding WT day or night value. **P ≤ 0.001 for TG vs. corresponding WT value (unpaired t-test).
The 72-h average ± SE (day and night values combined) for line A male transgenic systolic/diastolic values was 125 ± 1.7/97 ± 1.6 compared with wild-type littermate values of 109 ± 1.7/83 ± 1.4 mmHg (P < 0.001). Line A female transgenic systolic/diastolic values measured at 126 ± 1.7/96 ± 1.3 vs. wild-type littermate values of 114 ± 1.9/87 ± 1.0 mmHg (P < 0.001).
The 72-h averages for line D male transgenic systolic/diastolic values were 135 ± 1.4/100 ± 1.2 compared with 124 ± 1.2/90 ± 1.3 mmHg for wild-type littermate values (P < 0.001). Corresponding transgenic females measured 136 ± 2.0/103 ± 1.6 compared with wild-type values of 126 ± 1.1/92 ± 1.5 mmHg (P < 0.001).
DISCUSSION
In our laboratory's prior published studies, we demonstrated that ECFP/ANG II possesses intracellular biological functions in vascular smooth muscle cells, as well as standard laboratory cell lines (such as COS-7 and CHO-K1) (10, 12). GFP (from which the CFP variant is derived) expression has been used in various transgenic mouse models and in numerous tissue culture studies. It has been established that the GFP protein itself does not interfere with biological performance of proteins under study, including such functions as protein binding, trafficking and localization, and blood pressure regulation (22, 33, 38, 44, 45, 53, 55). ANG II within the context of the fusion protein also maintains immunoreactivity, as determined by anti-ANG II enzyme-linked immunosorbent assays, as well as immunoblot analyses (10, 12). ECFP/ANG II possesses no signal peptide, is maintained within cells, and accumulates significantly within nuclei and specifically, nucleosol (as determined by three-dimensional deconvolution analyses, as well as Western blots of fractionated cells) (10). iANG II, in the context of ECFP/ANG II, also stimulates proliferation of cultured vascular smooth muscle cells and stimulates cAMP response element-binding phosphorylation activation through the p38 MAPK pathway (10, 12). A corresponding control protein, ECFP/ANG IIC (which encodes a scrambled version of ANG II fused to CFP) has no such effects.
In addition, when ECFP/ANG II is cotransfected with a yellow fluorescent fusion protein of the AT1R (AT1R/EYFP), it triggers translocation of yellow fluorescence from the plasma membrane to the nucleus (10). Our recent published studies indicate that only the carboxy terminus of the AT1R is transferred, together with the yellow fluor, into the nucleus. A population of the receptor is cleaved, and the carboxy-terminal fragment, attached to the fluorescent moiety, is transported to the nucleus in a manner dependent on the presence of either extracellular ANG II or iANG II (11). The functional consequences that follow transport of the receptor fragment into the nucleus represent one class of iRAS signaling.
ANG II binding sites have been identified on liver and kidney nuclei and appear to be AT1R like (blocked by ARBs) (6, 19, 20, 26, 32, 34, 43, 52, 56). In our laboratory's recent published work, we addressed the source of these nuclear membrane receptors and presented a model for ANG II transport to, and accumulation within, the nuclear intramembrane space (14). The nuclear membrane receptor interaction with ANG II represents a second class of iRAS signaling and one that is sensitive to conventional AT1R blockers.
In other studies, several laboratories have reported dramatic effects related to AT1R-mediated uptake of ANG II into cells and single-cell microinjection of ANG II. Li et al. (31) have shown that AT1R-mediated ANG II uptake modifies cAMP signaling in proximal tubule cells. Moreover, they have shown that microinjected ANG II causes an increase in cytoplasmic mobilization of intracellular calcium in proximal tubule cells with no detectable influx of calcium (56). By comparison, intracellular injection of ANG II into cardiac myocytes increases the inward calcium ion current, alters junctional conductance, and modifies myocardial contractility (16, 17). Similarly, ANG II injection into VSMCs has been correlated with an increase in cytosolic and nuclear-free calcium due to an influx of extracellular calcium ions, both in the target cell, as well as, oddly enough, those cells in direct contact with the microinjected cell, suggesting a cell-cell, contact-mediated effect (23). These studies, and those describing effects of intracellular ECFP/ANG II on cultured cells and transgenic mice, appear to represent a distinct third class of iRAS signaling.
Since short peptides are generally proteolytically processed from precursors (e.g., opioids, endothelins, neuropeptide Y, substance P, neurokinin A), we have been reluctant to utilize expression constructs with direct short open-reading frames encoding ANG II, under the assumption that they will be inefficiently translated. Our recombinant peptide is designed as a fusion both to stabilize the product and to enhance translation. Direct production of the ANG II peptide from a sequence encoding the short octapeptide (instead of a precursor) has, however, been reported by the Baker laboratory (3, 4). Their data suggest that pcDNA/TO-iANG II stimulates CHO-K1 cell proliferation independent of the AT1R. Moreover, their (similar) constructs (small open-reading frame-encoding expression plasmids) were found to cause heart enlargement as early as 48 h postinjection, following tail-vein delivery of 100 μg DNA. In these systems, ANG II, like our fusion ANG II, is cytoplasmic and should have access to the nucleosol by diffusion through nucleopores. The naturally occurring ANG II correlate for cytoplasmic/nucleosolic ANG II in these experimental systems is peptide that is either generated from intracellular angiotensinogen or peptide that escapes endosomes after receptor-mediated internalization. This, once again, represents the third genre of iRAS signaling, and, clearly, significant data support this model as well as the preceding two models.
The transgenic mouse model presented here demonstrates the importance of iANG II in blood pressure regulation and kidney physiology. iANG II in this model is expressed in a broad range of tissues and is both cytoplasmic and nuclear. Moreover, we propose that naturally occurring iANG II peptides, that either escape endosomes following internalization or are generated from intracellular angiotensinogen, modify cellular functions, leading to both kidney damage and increased blood pressure.
Recently, other transgenic models have been developed to study the renin component of the iRAS, including transgenic mice that express human icRen (intracellular renin) via the GFAP glial-specific promoter (29). In this model, icRen is translated from the ATG start site inside exon II, generating a truncated prorenin protein lacking the signal peptide, and thus retained intracellularly. These mice were directly compared with mice expressing GFAP-regulated soluble renin. Double-transgenic mice expressing either secreted renin or icRen with angiotensinogen exhibited higher blood pressure and increased drinking response compared with control littermates. Intracellular renin, therefore, has potential to raise blood pressure. More recently, studies using the Cre-LoxP strategy to specifically remove exon I (leaving exon II intact), thus ablating secreted renin while preserving intracellular renin, showed that, while intracellular renin clearly serves an important role in blood pressure regulation, it is insufficient to compensate for a genetic loss of secreted renin (54). Secreted renin clearly plays important roles in renal development and function, blood pressure regulation, and viability, which cannot be substituted by icRen.
Interestingly, while there is evidence for a local RAS in the heart and several elements, including renin and angiotensinogen, are expressed within cardiomyocytes, there appears to be no ECFP/ANG II transgene effect on heart histology in our model. This may be the result of lower expression of the fusion protein in the heart relative to other tissues. Further studies will be required to determine whether kidney pathology precedes elevated blood pressure, or, indeed, increased blood pressure causes and/or contributes to kidney damage, and to quantify the impact of the noted kidney damage on kidney function.
GRANTS
This work was supported by the Ochsner Clinic Foundation and National Heart, Lung, and Blood Institute Grants HL072795 (J. L. Cook, R. N. Re) and HL084207 (C. D. Sigmund). The Core Analytical Facility at Tulane is supported by a CoBRE grant from National Center for Research Resources, P20RR017659 (L. G. Navar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Bader M, Ganten D. Transgenic rats: tools to study the function of the renin-angiotensin system. Clin Exp Pharmacol Physiol Suppl 3: S81–S87, 1996 [PubMed] [Google Scholar]
- 2.Bader M, Zhao Y, Sander M, Lee MA, Bachmann J, Bohm M, Djavidani B, Peters J, Mullins JJ, Ganten D. Role of tissue renin in the pathophysiology of hypertension in TGR(mREN2)27 rats. Hypertension 19: 681–686, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120: 5–13, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol 291: C995–C1001, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F, Jr, D'Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol 81: 274–287, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology 130: 3641–3649, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, Raymond JR, Ullian ME, Paul RV. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol 279: F440–F448, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Choi-Miura NH, Otsuyama K, Sano Y, Saito K, Takahashi K, Tomita M. Hepatic injury-specific conversion of mouse plasma hyaluronan binding protein to the active hetero-dimer form. Biol Pharm Bull 24: 892–896, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 40: 696–707, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cook JL, Mills SJ, Naquin RT, Alam J, Re RN. Cleavage of the angiotensin II type 1 receptor and nuclear accumulation of the cytoplasmic carboxy-terminal fragment. Am J Physiol Cell Physiol 292: C1313–C1322, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol 36: 75–90, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res 89: 1138–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Cook JL, Re RN. Intracellular accumulation and nuclear trafficking of angiotensin II and the angiotensin II type I receptor. In: The Local Cardiac Renin-Angiotensin Aldosterone System (2nd Ed.), edited by Frohlich ED, Re RN. New York: Springer, 2009, p. 29–42 [Google Scholar]
- 15.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics 1: 3–9, 1999 [DOI] [PubMed] [Google Scholar]
- 16.De Mello WC. Further studies on the effect of intracellular angiotensins on heart cell communication: on the role of endogenous angiotensin II. Regul Pept 115: 31–36, 2003 [DOI] [PubMed] [Google Scholar]
- 17.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension 32: 976–982, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol Cell Physiol 263: C851–C863, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension 22: 496–501, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Eggena P, Zhu JH, Sereevinyayut S, Giordani M, Clegg K, Andersen PC, Hyun P, Barrett JD. Hepatic angiotensin II nuclear receptors and transcription of growth-related factors. J Hypertens 14: 961–968, 1996 [PubMed] [Google Scholar]
- 21.Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension 28: 818–824, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Guan H, Wang P, Hui R, Edin ML, Zeldin DC, Wang DW. Adeno-associated virus-mediated human C-reactive protein gene delivery causes endothelial dysfunction and hypertension in rats. Clin Chem 55: 274–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res 79: 765–772, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Inagami T, Mizuno K, Higashimori K. Juxtaglomerular cells as a source of intrarenal angiotensin II production. Kidney Int Suppl 32: S20–S22, 1991 [PubMed] [Google Scholar]
- 26.Jimenez E, Vinson GP, Montiel M. Angiotensin II (AII)-binding sites in nuclei from rat liver: partial characterization of the mechanism of AII accumulation in nuclei. J Endocrinol 143: 449–453, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J 11: 821–827, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension 47: 461–466, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res 84: 240–246, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Carretero OA, Navar LG, Zhuo JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol 291: F375–F383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XG, Yan JT, Xu XZ, Wang JN, Cheng LM, Wang T, Zuo P, Wang DW. Recombinant adeno-associated virus-mediated delivery of antisense angiotensin II receptor 1 gene attenuates hypertension development. Acta Pharmacol Sin 28: 1737–1745, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology 139: 365–375, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1982 [Google Scholar]
- 37.Mercure C, Ramla D, Garcia R, Thibault G, Deschepper CF, Reudelhuber TL. Evidence for intracellular generation of angiotensin II in rat juxtaglomerular cells. FEBS Lett 422: 395–399, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Nisancioglu MH, Mahoney WM, Jr, Kimmel DD, Schwartz SM, Betsholtz C, Genove G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol 28: 2324–2331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmiter RD, Brinster RL. Germ-line transformation of mice. Annu Rev Genet 20: 465–499, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastural M, Barrou B, Delcourt A, Bitker MO, Ourahma S, Richard F. Successful kidney transplantation using organs from a donor with disseminated intravascular coagulation and impaired renal function: case report and review of the literature. Nephrol Dial Transplant 16: 412–415, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Re R, Fintel D, Bryan S, Haber E, Labiche R, Parab M. Studies on two novel angiotensin II actions. Clin Exp Hypertens A 4: 1649–1660, 1982 [DOI] [PubMed] [Google Scholar]
- 42.Re R, Parab M. Effect of angiotensin II on RNA synthesis by isolated nuclei. Life Sci 34: 647–651, 1984 [DOI] [PubMed] [Google Scholar]
- 43.Re RN, MacPhee AA, Fallon JT. Specific nuclear binding of angiotensin II by rat liver and spleen nuclei. Clin Sci (Lond) 61, Suppl 7: 245s–247s, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakata S, Liang L, Sakata N, Sakata Y, Chemaly ER, Lebeche D, Takewa Y, Chen J, Park WJ, Kawase Y, Hajjar RJ. Preservation of mechanical and energetic function after adenoviral gene transfer in normal rat hearts. Clin Exp Pharmacol Physiol 34: 1300–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Shui HA, Huang TH, Ka SM, Chen PH, Lin YF, Chen A. Urinary proteome and potential biomarkers associated with serial pathogenesis steps of focal segmental glomerulosclerosis. Nephrol Dial Transplant 23: 176–185, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Sigmund CD, Jones CA, Kane CM, Wu C, Lang JA, Gross KW. Regulated tissue- and cell-specific expression of the human renin gene in transgenic mice. Circ Res 70: 1070–1079, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Sinn PL, Sigmund CD. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol Genomics 3: 25–31, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Steckelings UM, Kaschina E, Unger T. The AT2 receptor–a matter of love and hate. Peptides 26: 1401–1409, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Strauss MS. Preparation of genomic DNA from mammalian tissue. Curr Protoc Mol Biol chapt. 2: unit 2.2, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells 25: 2593–2600, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Tang SS, Rogg H, Schumacher R, Dzau VJ. Characterization of nuclear angiotensin-II-binding sites in rat liver and comparison with plasma membrane receptors. Endocrinology 131: 374–380, 1992 [DOI] [PubMed] [Google Scholar]
- 53.van Haperen R, Cheng C, Mees BM, van Deel E, de Waard M, van Damme LC, van Gent T, van Aken T, Krams R, Duncker DJ, de Crom R. Functional expression of endothelial nitric oxide synthase fused to green fluorescent protein in transgenic mice. Am J Pathol 163: 1677–1686, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension 54: 1240–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926–931, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]