Abstract
Application of intermittent pneumatic compressions (IPC) is an extensively used therapeutic strategy in vascular medicine, but the mechanisms by which this method works are unclear. We tested the hypothesis that acute application (150 min) of cyclic leg compressions in a rat model signals upregulation of angiogenic factors in skeletal muscle. To explore the impact of different pressures and frequency of compressions, we divided rats into four groups as follows: 120 mmHg (2 s inflation/2 s deflation), 200 mmHg (2 s/2 s), 120 mmHg (4 s/16 s), and control (no intervention). Blood flow and leg oxygenation (study 1) and the mRNA expression of angiogenic mediators in the rat tibialis anterior muscle (study 2) were assessed after a single session of IPC. In all three groups exposed to the intervention, a modest hyperemia (∼37% above baseline) between compressions and a slight, nonsignificant increase in leg oxygen consumption (∼30%) were observed during IPC. Compared with values in the control group, vascular endothelial growth factor (VEGF) and monocyte chemotactic protein-1 (MCP-1) mRNA increased significantly (P < 0.05) only in rats exposed to the higher frequency of compressions (2 s on/2 s off). Endothelial nitric oxide synthase, matrix metalloproteinase-2, and hypoxia-inducible factor-1α mRNA did not change significantly following the intervention. These findings show that IPC application augments the mRNA content of key angiogenic factors in skeletal muscle. Importantly, the magnitude of changes in mRNA expression appeared to be modulated by the frequency of compressions such that a higher frequency (15 cycles/min) evoked more robust changes in VEGF and MCP-1 compared with a lower frequency (3 cycles/min).
Keywords: angiogenesis, intermittent pneumatic compression, vascular endothelial growth factor, monocyte chemotactic protein-1
intermittent pneumatic limb compression (IPC) is a widely used approach to prevent and treat a number of vascular disorders. In recent years, evidence has been provided showing that this strategy is an efficacious therapeutic option for patients with peripheral artery disease (PAD) (11, 13, 24). In a randomized controlled trial, Delis and Nicolaides (13) showed that a minimum of 2.5 h of daily IPC application for 5 mo improves walking distance, ankle brachial index, and quality of life in stable claudicants. Other studies also indicated that the efficacy of this strategy appears to be especially meaningful for patients with critical limb ischemia and tissue loss, in which surgical reconstruction is unsuitable (22, 47). In this group of patients, IPC application to the foot and calf was found to significantly improve leg pulse volume amplitude (47) and promote limb salvage (i.e., reduce the number of major amputations) (22, 47). Interestingly, despite the growing acceptance and accumulating favorable evidence, the mechanisms by which IPC promotes its remarkable clinical benefits remain elusive.
IPC application evokes a number of acute hemodynamic and extravascular mechanical effects in the leg. For example, when applied to the calf, IPC has been shown to 1) temporarily increase flow velocity, mean volume flow (by up to ∼105%), and wall shear stress in the popliteal artery in a pressure-dependent manner (48), 2) increase wall strain, exerted by the sudden increase in volume flow between compressions (10), and 3) externally compress the vasculature embedded within the tissue, due to the rise in intramuscular pressure (5, 41). These mechanical forces are well-known stimuli for angiogenic growth factors in skeletal muscle (4, 19), and accordingly, it is conceivable that repeated exposure to enhanced shear stress, strain, and deformation/stretch during IPC therapy can induce the synthesis and release of these factors. For instance, it is known that increased shear stress and cyclic strain imposed on cultured endothelial cells can increase the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF) (1, 53, 54), endothelial nitric oxide synthase (eNOS) (2, 55), and monocyte chemotactic protein-1 (MCP-1) (43, 50). Likewise, evidence derived from animal models suggests that muscle stretch modulates the expression of matrix metalloproteinases (e.g., MMP-2) and upregulates hypoxia-inducible factor (HIF)-1α, both of which are also thought to be important in the angiogenic process (30, 31). Along this line, it has been demonstrated that IPC application in a rat model can rapidly upregulate eNOS expression in skeletal muscle exposed to compressions (44). Whether the same holds true for other factors known to trigger capillary formation in skeletal muscle remains to be determined.
This study was designed to examine mRNA expression patterns of several angiogenic factors following acute IPC application. Aiming to gain insights into the mechanistic basis of how this therapeutic strategy works, we first characterized leg hemodynamic and oxygenation responses to an acute bout of IPC application. We also determined the potential impact of varying the stimulation characteristics, in this case the pressure and frequency of compressions on the expression of angiogenic mediators. Given the fact that the magnitude of flow enhancement and vascular deformation during external muscle compression appears to be proportional to the pressure and frequency of compressions (7, 12, 48), we anticipated that the effect of IPC on angiogenic factor expression would be directly dependent on both the pressure and frequency.
METHODS
Animals.
Male Sprague-Dawley rats (280–350 g; n = 65) were used in the present study. The animals were housed in a temperature-controlled room (24°C) with a 12:12-h light-dark cycle. Food and water were provided ad libitum. The Institutional Animal Care and Use Committee of the University of Missouri approved the experimental protocol.
Experimental design.
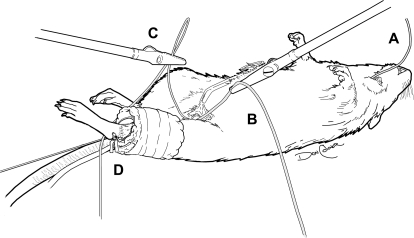
The experimental setup is shown in Fig. 1. Briefly, under anesthesia, the animals were placed supine on an acrylic platform, and a small blood pressure cuff (UPC3.3, 3.3 × 12 cm; D.E. Hokanson, Bellevue, WA) was firmly wrapped around the left leg. To stabilize the leg and prevent movement of the cuff during compressions, umbilical tape was passed in the ankle region between the bone and the Achilles tendon and tied tightly to a metal rod that was connected to a force transducer. The cuff was also secured to the leg with umbilical tape.
Fig. 1.
Schematic illustration of intermittent pneumatic limb compression (IPC) application in the rat leg. A blood pressure cuff was wrapped around the left leg and firmly secured with umbilical tape. To prevent cuff displacement during the intermittent compressions, the leg was secured in place. A, catheter on the carotid artery; B, blood flow meter placed on the femoral artery, C, catheter placed on a branch of the femoral vein; D, cuff for application of compressions. See methods for details.
To characterize and validate our model of IPC application in the rat, we first determined leg hemodynamics and oxygenation responses to an acute bout of compressions (study 1, n = 33). Rats were divided into four groups to evaluate the impact of pressure and rate of compression on these variables: group 1, 120 mmHg (2 s inflation/2 s deflation); group 2, 200 mmHg (2 s/2 s); group 3, 120 mmHg (4 s/16 s); and group 4, no intervention performed except cuff placement. These compression rates and pressures were selected on the basis of compression characteristics of the devices used in the clinical scenario for PAD treatment (34). In preliminary experiments, we determined that the pressures set in the compression unit were accurately transferred to the calf, as measured by a catheter pressure transducer positioned underneath the cuff (Supplemental Fig. 1). (Supplemental data for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.) In the first set of experiments (n = 17), blood pressure, femoral blood flow, and oxygen consumption (see below) of the compressed leg were measured before and after a 6-min bout of compressions. In additional animals (n = 16), we measured blood flow to a superficial and a deep muscle of the hindlimb throughout a 1-h session of compressions.
In study 2 (n = 32), the impact of 150 min of compressions on mRNA expression of angiogenic factors in whole muscle extracts was investigated. The right tibialis anterior (TA) muscle was surgically removed initially (as described in Surgical preparation) to serve as a baseline expression control for the left TA, harvested in the end of the intervention period.
Surgical preparation.
Animals were anesthetized with a combination of ketamine (100 mg/kg) and acepromazine (0.5 mg/kg ip). An adequate level of anesthesia was verified by the lack of response to foot pinch, stable ventilation, and stable blood pressure. Additional doses were given throughout the intervention period if and when necessary. During the surgical interventions, supplemental oxygen was given through a mask. Core temperature was maintained at 37°C with an external heat source. Initially, a catheter was placed in the left carotid artery and advanced to the aortic arch for blood pressure determination, withdrawal of arterial blood for blood gas measurement, and infusion of microspheres. In study 1, the anteromedial portion of the left hindlimb was exposed, and the left femoral artery and veins were exposed using blunt dissection. A Transonic flow probe (0.5 V; Transonic Systems, Ithaca, NY) was placed around the proximal portion of the left femoral artery for blood flow determination. A catheter was placed in a branch of the femoral vein for venous blood withdrawal for blood gas measurement and calculation of oxygen consumption to the calf. In some animals, a third catheter was inserted into the caudal (tail) artery for withdrawal of the reference blood sample and for monitoring blood pressure during microsphere infusion. In study 2, animals had only one catheter placed on the carotid artery as described above. In these animals, after both legs were shaved and cleaned, an incision was made in the anterior part of the right leg and the TA muscle was removed and stored for analysis as described previously (44). The wound was then closed with surgical suture. At the end of intervention, the same procedure was repeated in the left leg. After completion of the experiment, the rats were euthanized with an overdose of ketamine and acepromazine (intra-arterial).
IPC device.
An automatic cuff inflator (E-20 rapid cuff inflator; D.E. Hokanson) was used for IPC application. With the cuff used in the present study (UPC3.3, 3.3 × 12 cm; D.E. Hokanson), the inflation/deflation time is <0.1 s. A computer-generated signal (PowerLab 4/S system; ADInstruments) allowed for application of the compression rates specified above.
Blood pressure and limb hemodynamics.
Mean arterial blood pressure (BP) was measured from the carotid artery catheter or tail artery by using a pressure transducer positioned at the height of each respective measurement site. Femoral blood flow (FBF) was measured with a Transonic AT206 small animal blood flow meter, and femoral vascular conductance (FVC) was calculated by dividing femoral blood flow by BP. During the 6-min IPC bout, BP, FBF, and FVC were determined by averaging the responses during the 1st and 5th minutes of compressions. These variables were determined separately during (cuff inflation) and in between compressions (deflation). The average of both periods represented the overall hemodynamic responses.
In a different set of rats, blood flow to a superficial (TA) and deep (soleus) muscle was determined using the microsphere technique (52). Stable isotope-labeled microspheres [Rhenium, Iridium, Holmium; BioPhysics Assay Laboratory (BioPAL), Wellesley, MA] of 15 μm in diameter were used for this study. A suspension of microspheres (∼0.5 ml) was vortexed for 45–60 s and infused into the carotid catheter, followed by a saline flush over ∼20 s. About 10 s before the infusion of microspheres, withdrawal of the reference blood sample began at a rate of 500 μl/min via the caudal artery catheter. Adequate mixing of the microspheres in the circulation was verified by comparing flows from the left and right kidney (52). On completion of the protocol, rats were euthanized with an overdose of ketamine and acepromazine, and the left and right soleus, TA, and kidneys were removed, weighed, and placed immediately into sample vials. The samples were dried overnight (at 37°C) and shipped to BioPAL for analysis. Blood flows (ml·min−1·100 g−1) were calculated as
where RBS is reference blood sample and CPM is counts per minute (52).
Leg oxygenation.
Arterial and femoral venous blood samples (∼0.3 ml) were withdrawn at baseline and during the 6th minute of compressions using heparinized syringes. Within 10–15 min, blood gases (Po2 and Pco2), oxygen saturation (So2), pH, and hemoglobin concentration were determined using the ABL720 analyzer (Radiometer, Copenhagen, Denmark). Blood O2 content was calculated according to the following formula (17):
Leg O2 delivery was determined from the product of FBF and arterial O2 content. Oxygen uptake (V̇o2) was calculated as the product of FBF and the systemic arterial-venous (a-v) O2 difference and expressed relative to body weight (14).
Tissue processing and RT-PCR analysis.
Muscle samples harvested before (right TA) and after (left TA) IPC application were quickly placed in RNA stabilization reagent (RNAlater; Ambion) and kept at 4°C for up to a week until further processing. Approximately 30 mg of tissue were homogenized in a lysing solution (Buffer RLT; Qiagen, Valencia, CA) containing 14.3 M β-mercaptoethanol (β-ME) using a tissue homogenizer (Fisher Scientific, Waltham, MA). Total RNA was isolated using an RNeasy fibrous tissue mini kit (Qiagen, Valencia, CA) and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA by reverse transcription primed by a mixture of random hexamer and oligo(dT) primers (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA). The reactions were incubated in a PCR Express Hybaid thermal cycler (Hybaid, Franklin, MA). Quantitative real-time PCR was performed using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers for each target were purchased from IDT (Coralville, IA). Sequences were as following: VEGF, forward primer 5′-TTC AAG CCG TCC TGT GTG C-3′ and reverse primer 5′-TCC AGG GCT TCA TCA TTG C-3′ (28); eNOS, forward primer 5′-GTG CTG GCA TAC AGA ACC CA-3′ and reverse primer 5′-CCA TGT GGA ACA GAC CCC A-3′ (28); MMP-2, forward-primer 5′-CCA TGA AGC CTT GTT TAC CA-3′ and reverse primer 5′-CTG GAA GCG GAA CGG AAA-3′ (20); HIF-1α, forward primer 5′-AAC AAA CAG AAT CTG TCC TCA AAC C-3′ and reverse primer 5′-CAG GTA ATG GAG ACA TTG CCA G-3′ (29); MCP-1, forward primer 5′-CTG TCT CAG CCA GAT GCA GTT AA-3′ and reverse primer 5′-AGC CGA CTC ATT GGG ATC AT-3′ (38); and GAPDH, forward primer 5′-ACT CTA CCC ACG GCA AGT TC-3′ and reverse primer 5′-TAC TCA GCA CCA GCA TCA CC-3′. A 25-μl reaction mixture containing 24 μl of Power SYBR Green PCR Master Mix (Applied Biosystems) and the appropriate concentrations of gene-specific primers plus 1 μl of cDNA template was loaded in each well of a 96-well plate (duplicate samples). PCR was performed with thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A dissociation curve analysis was performed after each run to verify the identity of the PCR products. The comparative cycle threshold (Ct) method was utilized to calculate the changes in expression of each target mRNA (26).
Statistical analysis.
Statistical analysis was performed by employing SigmaStat Statistical Software (Jandel Scientific, San Rafael, CA). Limb hemodynamics and leg oxygenation responses were compared using ANOVA for repeated measures. Changes in mRNA expression between groups were compared using one-way ANOVA. Intergroup comparisons were performed by employing Tukey's procedure, when appropriate. P < 0.05 was considered to be significant. Data are means ± SE.
RESULTS
Limb hemodynamics during IPC application.
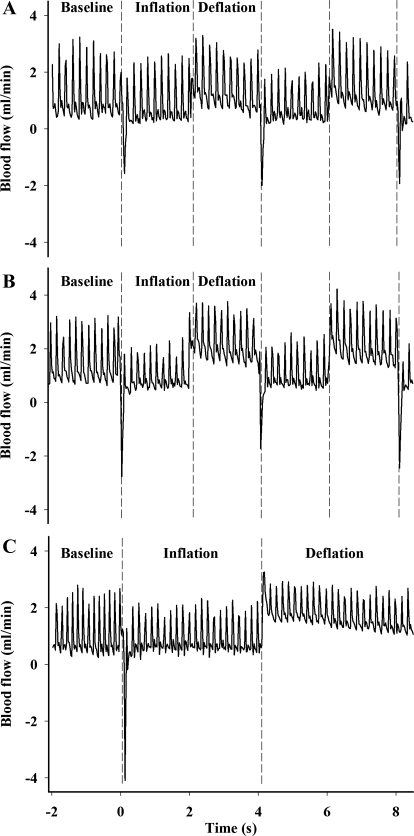
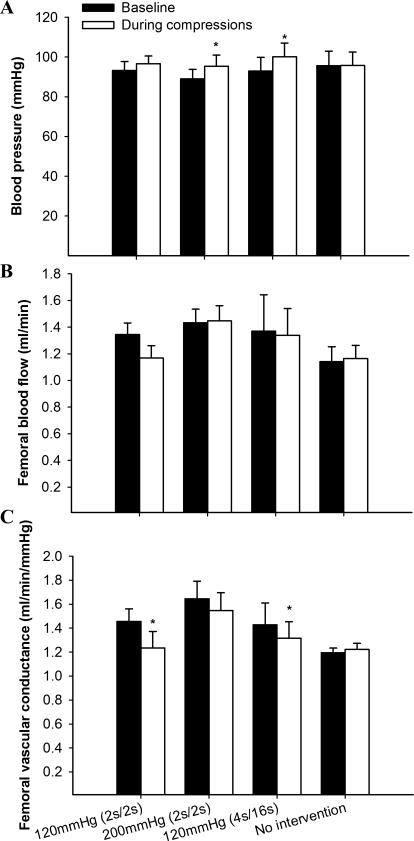
Typical examples of the profile of FBF during and in between compressions are shown in Fig. 2. Rapid cuff inflation and the consequent flow impediment generated a retrograde blood flow component in all three compression protocols. As expected, FBF remained significantly reduced compared with the baseline values throughout the compression period (Table 1). During cuff release, blood flow immediately increased above baseline levels (by an average of 37%), with the group subjected to the 200 mmHg (2 s/2 s) showing the most robust changes (∼43% increase) (Table 1). Blood pressure also increased slightly but significantly (overall mean increase of ∼5%), except in the 120 mmHg (2 s/2 s) group, thus resulting in a modest increase (∼27%) in femoral vascular conductance in all groups studied (Table 1). When the overall average of the intervention period is considered (i.e., averaging the compression period with the period in between compressions), the hyperemic response is no longer evident (Fig. 3). In all the groups, FBF was either unchanged or slightly reduced compared with baseline (Fig. 3B). This fact, combined with aforementioned increase in BP, resulted in a slight drop in FVC in all three experimental groups, with the response in the 120 mmHg (2 s/2 s) and 120 mmHg (4 s/16 s) groups reaching statistical significance compared with baseline (P < 0.05) (Fig. 3C). We also measured tissue blood flow to superficial (TA) and deep (soleus) muscles during IPC application with the microsphere technique. Proper mixing of microspheres into cardiac output was confirmed by the excellent agreement between blood flows to the left and right kidneys (left/right kidney ratio of 1.04). In parallel to the trend observed during the bulk flow measurements, there was a modest, nonsignificant increase in blood flow to both muscles of the left leg at 30 min, followed by a return to baseline levels at 60 min (Table 2). An exception was found in the 200 mmHg (2 s/2 s) group, in which blood flow to the soleus muscle increased at both time points, reaching statistical significance at 60 min (P < 0.05).
Fig. 2.
Representative recordings of femoral blood flow (FBF) responses to IPC. Blood flow increased temporarily above baseline values following cuff deflation. A: 120 mmHg (2 s on/2 s off). B: 200 mmHg (2 s on/2 s off). C: 120 mmHg (4 s on/16 s off).
Table 1.
Acute systemic and leg hemodynamic responses to IPC application
| 120 mmHg (2 s/2 s) |
200 mmHg (2 s/2 s) |
120 mmHg (4 s/16 s) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | During | After | Baseline | During | After | Baseline | During | After | |
| BP, mmHg | 93.2 ± 4.4 | 96.9 ± 3.9 | 96.3 ± 3.8 | 88.9 ± 4.7 | 95.5 ± 5.4* | 95.1 ± 5.6* | 92.8 ± 6.9 | 99.5 ± 6.7* | 100 ± 7.2* |
| FBF, ml/min | 1.3 ± 0.08 | 0.6 ± 0.1* | 1.7 ± 0.1* | 1.4 ± 0.1 | 0.8 ± 0.05* | 2.0 ± 0.1* | 1.3 ± 0.2 | 0.8 ± 0.1* | 1.8 ± 0.3* |
| FVC, ml•min−1•mmHg−1 | 1.4 ± 0.1 | 0.6 ± 0.1* | 1.8 ± 0.1* | 1.6 ± 0.1 | 0.9 ± 0.09* | 2.1 ± 0.2* | 1.4 ± 0.1 | 0.8 ± 0.1* | 1.7 ± 0.1* |
| PAF, ml/min | 4.2 ± 0.3 | 3.2 ± 0.3 | 4.4 ± 0.4 | 3.5 ± 0.3 | 2.8 ± 0.2 | 4.1 ± 0.3 | 3.5 ± 0.6 | 2.8 ± 0.5 | 4.0 ± 0.7 |
| PRF, ml/min | −4.1 ± 0.7 | −2.8 ± 0.3 | −3.1 ± 0.5 | ||||||
Values are means ± SE in each group [n= 6 for 120 mmHg (2 s/2 s) and 120 mmHg (4 s/16 s) groups and n= 5 for 200 mmHg (2 s/2 s) group] before, during, and after inermittent pneumatic limb compression (IPC) application.
P < 0.05 vs. baseline. BP, blood pressure; FBF, femoral blood flow; FVC, femoral vascular conductance; PAF, peak anterograde flow; PRF, peak retrograde flow.
Fig. 3.
Net changes in blood pressure (A), FBF (B), and femoral conductance (C) during an acute bout of IPC application. *P < 0.05, baseline is different from compression.
Table 2.
Blood flow to soleus and TA muscles during a 1-h bout of IPC application
| Left Leg (IPC) |
Right Leg (Control) |
|||||
|---|---|---|---|---|---|---|
| Baseline | 30 min | 60 min | Baseline | 30 min | 60 min | |
| Soleus | ||||||
| 120 mmHg (2 s/2 s) | 17.8 ± 8.3 | 29.1 ± 3.6 | 19.4 ± 4.5 | 36.1 ± 3.5 | 31.0 ± 0.9 | 17.5 ± 1.2 |
| 200 mmHg (2 s/2 s) | 20.3 ± 6.3 | 44.4 ± 6.7 | 89.4 ± 18.6* | 30.3 ± 5.7 | 31.4 ± 3.0 | 68.9 ± 21.7 |
| 120 mmHg (4 s/16 s) | 29.5 ± 15.5 | 36.8 ± 6.0 | 31.0 ± 12.2 | 22.6 ± 9.2 | 48.9 ± 31.8 | 41.5 ± 10.3 |
| No intervention | 23.1 ± 6.1 | 10.2 ± 1.2 | 31.3 ± 22.3 | 17.3 ± 4.2 | 29.5 ± 10.8 | 28.2 ± 17.9 |
| Tibialis anterior | ||||||
| 120 mmHg (2 s/2 s) | 39.0 ± 8.1 | 39.4 ± 11.3 | 25.5 ± 4.3 | 50.9 ± 26.3 | 52.4 ± 18.6 | 25.7 ± 2.1 |
| 200 mmHg (2 s/2 s) | 19.4 ± 3.6 | 37.2 ± 3.9 | 36.1 ± 3.4 | 43.3 ± 5.9 | 41.8 ± 4.8 | 47.8 ± 11.8 |
| 120 mmHg (4 s/16 s) | 29.7 ± 6.8 | 37.7 ± 10.7 | 25.7 ± 6.7 | 61.2 ± 11.8 | 67.2 ± 22.7 | 37.0 ± 6.7 |
| No intervention | 20.5 ± 5.5 | 28.6 ± 5.0 | 22.9 ± 13 | 40.7 ± 5.1 | 57.6 ± 12.1 | 24.8 ± 8.4 |
Values are means ± SE of blood flow (ml•min−1•100 g−1) in each group [n= 4 for 120 mmHg (2 s/2 s) and 200 mmHg (2 s/2 s) groups, n = 5 for 120 mmHg (4 s/16 s) group, and n = 3 for no intervention group] in soleus and tibialis anterior muscles.
P < 0.05 vs. baseline.
Leg oxygenation.
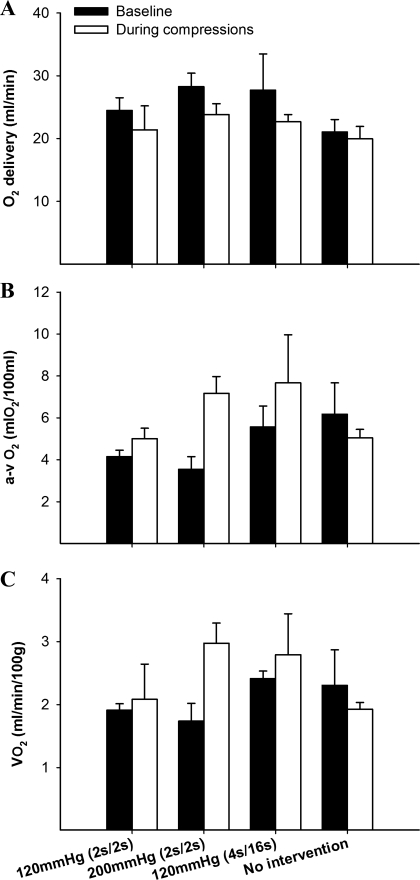
Successful leg O2 consumption measurement was possible in 13 animals only (of a total of 17) due to technical difficulties during blood sampling and analysis. Blood variables at baseline and during the compressions are shown in Table 3. Oxygen delivery, a-v O2 content, and leg V̇o2 are shown in Fig. 4. IPC application evoked a modest decrease in both venous Po2 and So2 in the experimental groups, resulting in an overall decrease in venous O2 content (by ∼17%). These changes were especially marked in the 200 mmHg (2 s/2 s) group, in which venous O2 content dropped by 26% from baseline. As a consequence, there was a modest, nonsignificant increase the a-v O2 difference and leg O2 consumption in all three compression groups, whereas no detectable changes was seen in the control group (Fig. 4).
Table 3.
Blood variables at rest and during cyclic compressions
| 120 mmHg (2 s on/2 s off) |
120 mmHg (4 s on/ 16 s off) |
200 mmHg (2 s on/2 s off) |
No Intervention |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6th min | Baseline | 6th min | Baseline | 6th min | Baseline | 6th min | |
| Hemoglobin, g/dl | ||||||||
| a | 12.4 ± 0.3 | 12.1 ± 0.3 | 13.3 ± 0.2 | 13.0 ± 0.6 | 13.0 ± 0.5 | 12.6 ± 0.5 | 13.3 ± 0.7 | 12.1 ± 0.1 |
| v | 11.4 ± 0.7 | 12.6 ± 0.3 | 12.2 ± 0.2 | 11.9 ± 0.2 | 13.2 ± 0.5 | 12.5 ± 0.5 | 11.7 ± 0.1 | 12.0 ± 0.5 |
| Po2, mmHg | ||||||||
| a | 78.3 ± 2.6 | 89.0 ± 1.2 | 94.5 ± 3.0 | 96.3 ± 2.2 | 75.3 ± 2.6 | 89.6 ± 3.7* | 77.4 ± 3.8 | 77.0 ± 5.7 |
| v | 55.7 ± 3.3 | 48.6 ± 1.7* | 53.0 ± 5.2 | 44.4 ± 6.8* | 51.9 ± 2.4 | 42.3 ± 3.2* | 47.5 ± 2.8 | 44.1 ± 0.8 |
| So2, % | ||||||||
| a | 92.4 ± 0.2 | 96.0 ± 1.8 | 98.6 ± 0.4 | 98.4 ± 1.0 | 92.1 ± 1.1 | 96.1 ± 1.7 | 93.8 ± 2.1 | 94.2 ± 2.6 |
| v | 74.1 ± 2.2 | 63.8 ± 2.0* | 74.7 ± 5.1 | 61.6 ± 10.9* | 72.0 ± 1.5 | 56.0 ± 2.5* | 68.6 ± 6.0 | 65 ± 4.3 |
| O2 content, ml/100 ml | ||||||||
| a | 15.9 ± 0.3 | 16.2 ± 0.7 | 18.2 ± 0.4 | 17.8 ± 0.9 | 16.7 ± 0.8 | 16.9 ± 1.0 | 17.3 ± 0.5 | 15.8 ± 0.3 |
| v | 11.7 ± 0.7 | 11.2 ± 0.6 | 12.7 ± 0.6 | 10.1 ± 1.6* | 13.2 ± 0.3 | 9.7 ± 0.6* | 11.1 ± 0.9 | 10.8 ± 0.3 |
| Pco2, mmHg | ||||||||
| a | 36.1 ± 0.6 | 32.8 ± 3.7 | 35.6 ± 2.8 | 32.1 ± 4.2 | 37.3 ± 2.3 | 35.3 ± 1.6 | 38.2 ± 2.5 | 33.7 ± 2.5 |
| v | 38.3 ± 3 | 42.6 ± 2.4 | 39.4 ± 0.7 | 39.3 ± 1 | 42.2 ± 1.2 | 42.8 ± 1.5 | 40.0 ± 2.1 | 39.7 ± 4.1 |
| pH | ||||||||
| a | 7.3 ± 0.01 | 7.4 ± 0.03 | 7.4 ± 0.01 | 7.42 ± 0.02 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.4 ± 0.04 |
| v | 7.3 ± 0.02 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.01 | 7.3 ± 0.03 | 7.3 ± 0.03 |
Values are means ± SE in each group [n = 3 for 120 mmHg (2 s/2 s), 120 mmHg (4 s/16 s), and no intervention groups and n = 4 for 200 mmHg (2 s/2 s) group] at rest (baseline) and during compressions (6th min).
P < 0.05 vs. baseline. So2, O2 saturation.
Fig. 4.
Leg O2 delivery (A), arterial-venous (a-v) O2 content difference (B), and oxygen consumption (V̇o2; C) before and after an acute bout of IPC application.
Gene expression changes after 150 min of IPC.
A total of 32 rats were used in this experiment (n = 8 in each group). One rat in the 120 mmHg (2 s/2 s) group died during the procedure and therefore was excluded from the analysis. VEGF mRNA in the experimental leg (left) increased significantly in both the 120 mmHg (2 s/2 s) (1.08-fold) and 200 mmHg (2 s/2 s) (1.02-fold) groups compared with the control group (no intervention) following IPC application (Fig. 5). MCP-1 mRNA content decreased in all four groups following the intervention, but the magnitude of changes differed substantially between groups. Indeed, whereas in the nonintervention group the mRNA expression of this gene decreased by ∼10-fold, only a modest downregulation was seen in both the 120 mmHg (2 s/2 s) (∼−0.9-fold) and 200 mmHg (2 s/2 s) (∼−0.5-fold) groups. eNOS [fold change: 120 mmHg (2 s/2 s), 0.2 ± 0.5; 200 mmHg (2 s/2 s), 1.6 ± 0.1; 120 mmHg (4 s/16 s), 0.1 ± 0.5; no intervention, 1.0 ± 0.4], MMP-2 [fold change: 120 mmHg (2 s/2 s), −0.2 ± 0.7; 200 mmHg (2 s/2 s), −0.2 ± 0.6; 120 mmHg (4 s/16 s), 0.3 ± 0.6; no intervention, 0.1 ± 0.5], and HIF-1α mRNA [fold change: 120 mmHg (2 s/2 s), −0.06 ± 0.7; 200 mmHg (2 s/2 s), 0.1 ± 0.6; 120 mmHg (4 s/16 s), −0.09 ± 0.5; no intervention, −0.4 ± 0.6] did not change significantly following IPC application in all experimental groups.
Fig. 5.
Fold changes in mRNA of vascular endothelial growth factor (VEGF; A) and monocyte chemoattractant protein-1 (MCP-1; B) following 150 min of IPC application. The right leg, harvested before the intervention, served as a baseline expression control. Values are means ± SE; n = 8 for 200 mmHg (2 s on/2 s off), 120 mmHg (4 s on/16 s off), and no intervention groups and n = 7 in 120 mmHg (2 s on/2 s off) group. *P < 0.05, different from no intervention group.
DISCUSSION
The aim of the present study was to test the hypothesis that IPC application in the leg acutely upregulates angiogenic mediators. The main novel findings of this investigation were that 1) 150 min of cyclic leg compressions significantly increases the mRNA expression of VEGF and MCP-1 in skeletal muscle, and 2) the magnitude of these effects appears to be dependent on the frequency but not the absolute amount of pressure during compressions.
Experimental considerations.
The search for the biological basis of the beneficial effects of IPC application through the use of animal models (25, 46) requires that the protocols used and, most importantly, the effects evoked by the compressions mimic the ones obtained in the clinical scenario. Accordingly, the protocol employed in the present investigation was based on the most commonly used stimulation characteristics utilized for treatment of patients with PAD, i.e., 120 mmHg, with cycles of 4 s of inflation and 16 s of deflation and a minimum time of exposure of 150 min (13, 47). We also manipulated the pressure (120 and 200 mmHg) and frequency (3 and 15 cycles/min) of compressions to understand whether these factors play a role in the observed adaptations. To characterize the limb hemodynamic responses, we measured FBF during the application of IPC (Figs. 2 and 3 and Tables 1 and 2). Notably, the overall magnitude of change in blood flow in between compressions observed (post/precompression blood flow ratio of 1.36) (Table 1) is remarkably similar to those seen in healthy humans under the same conditions (i.e., calf compressions in the supine position) (48). Likewise, the blood flow reduction during the compressions and the consequent unaltered net change in flow (when combining the pre- and postcompression periods) (Fig. 3) resembles the responses seen in calf plus thigh IPC in humans (35). Thus, apart from the inherent limitations associated with the experimental setup (e.g., anesthesia and leg restraint), the aforementioned observations suggest that the current model appears to be reliable for the study of the mechanistic basis of IPC therapy.
IPC-induced changes in mRNA expression of angiogenic factors.
The process of angiogenesis is initiated and controlled by a variety of growth factors and cytokines that promote endothelial cell proliferation and migration (9, 15, 39). Among the angiogenic mediators, VEGF has received special attention and is known to participate in both the maintenance of basal capillarization (37, 45) and the formation of new capillaries in skeletal muscle (e.g., following exercise training) (27, 39). In the present study, we have demonstrated for the first time that application of IPC evokes upregulation of VEGF mRNA in rat skeletal muscle. Notably, these changes were only significant in the groups exposed to the higher frequency of compressions (2 s on/2 s off) and appeared not to be importantly affected by pressure, at least in the range tested (Fig. 5). Compared with the control group, IPC application at 120 mmHg (2 s on/2 s off) and 200 mmHg (2 s on/2 s off) increased VEGF mRNA by 52 and 49%, respectively.
Accompanying the changes in VEGF mRNA, the expression of MCP-1 was altered by IPC only in the groups exposed to the higher frequency of compressions compared with the control group. MCP-1 is a potent chemokine that has been shown to modulate angiogenesis by promoting endothelial cell migration and sprouting in endothelial cells (18, 42, 51). Importantly, the angiogenic effects of MCP-1 appear to be mediated by VEGF, as shown in rat aortic rings (18). If true, the selective effect on both factors in the groups exposed to compressions at the higher frequency appears to indicate that their activity might be coupled in the skeletal muscle vasculature as well (36). Besides acting as angiogenic mediators, VEGF and MCP-1 also have been implicated in the process of arteriogenesis (21, 27). For example, it has been demonstrated that VEGF receptor blockade abolishes collateral arteriogenesis in a rat model of hindlimb ischemia following exercise training (27). These facts are especially relevant given the recent observation that 10-wk of IPC application increased the number of collaterals in rabbits with bilateral femoral artery ligation (46). It is conceivable, therefore, that the observed acute upregulation of these genes following IPC might also mediate the process of arterial remodeling seen during prolonged exposure to this therapy.
The lack of change in eNOS mRNA expression is at odds with the results of previous investigations that employed a rat model of IPC (6, 44). Tan et al. (44) reported that application of compressions rapidly increased eNOS mRNA by up to 180% in the rat TA. The reasons for this apparent discrepancy are not entirely clear but could possibly reflect fundamental differences in the protocol and type of IPC. As opposed to the circumferential cuffs used in this study, Tan et al. (44) applied an “asymmetrical” cuff in which pressure is applied only in the lateral and medial side of the leg. Since those authors were interested in the prophylactic use of IPC for deep vein thrombosis, a much lower pressure (55 mmHg) than those used in the present was applied. These differences imply that a different mechanical stimulus was given, which prevents a thorough comparison between these two studies. It can be speculated, however, that the eNOS mRNA response to the effects evoked by IPC application (i.e., increased shear stress, stretch) might be pressure specific such that lower muscle compression pressures, e.g., as employed by Tan et al. (44) or induced by simple limb movements (16), cause the most optimal stimuli for upregulation of this factor.
MMP-2 and HIF-1α mRNA are increased in chronic models of muscle stretch and during acute application of strain in endothelial cells (30–32). We reasoned that the mechanical perturbations induced by IPC application would alter the expressions of these factors, but contrary to our expectations, no changes were detected. Although unclear, a potential explanation for the absence of increased expression of these molecules is the short time of exposure to IPC therapy. In cultured endothelial cells, HIF-1α mRNA was found to be upregulated after 24 h but not after 6 h of exposure to static stretch (30). Likewise, changes in MMP-2 mRNA are not significant until 6 h of static strain application in microvascular endothelial cells (32). In humans, Rullman et al. (40) also failed to find changes in the expression of MMP-2 following an acute bout (65 min) of exercise. Thus, although our data suggest that these two factors are not acutely activated by IPC application, it is possible that repeated, prolonged exposure to this therapy and its associated effects could promote changes in the expression of these genes.
Potential mechanisms.
The exact mechanisms driving the changes in gene expression following IPC application are unknown, but the examination of the acute responses to this therapy offers some insights. Angiogenesis in skeletal muscle evolves from a complex interaction of a number of stimuli, including hypoxia, release of metabolic mediators, and mechanical forces (15, 39, 49). In the case of VEGF, for example, it is known that hypoxia appreciably increases both mRNA and protein in skeletal muscle (3), and accordingly, it has been suggested that this mechanism might be the cornerstone for the changes in capillarization in situations where the muscle experiences increased metabolic rate and decreased oxygen tension (e.g., exercise) (49). To verify whether increased muscle metabolism plays a role in the observed changes in gene expression following IPC application, we performed measurements of leg oxygenation in the rat during an acute bout of compressions (6 min). We found that cyclic limb compressions do invoke minor, nonsignificant increases in leg a-v O2 difference and V̇o2 (see Fig. 4). Although these changes are negligible compared with those observed during exercise, it is impossible to exclude a potential influence of increased muscle metabolism and its associated effects on the changes in the expression of angiogenic factors. Nonetheless, it is fair to assume that mechanical forces altered by IPC play a pivotal role in inducing the expression of these mediators in this condition.
The multitude of physical forces possibly altered during forceful external limb compressions (e.g., stretch, compression, strain, tension, and stress) makes the task of partitioning the contributors especially challenging. Most authors have suggested that increased blood flow and shear stress is potentially the most important single factor responsible for the clinical effects of IPC (5). In our study, changes in VEGF and MCP-1 mRNA appeared to be unrelated to the overall increases in blood flow during IPC. Thus, although the magnitude of flow increase was comparable across all three groups (Table 1), upregulation of these factors was only detectable in muscles of rats exposed to the higher frequency of compressions (Fig. 5). It can be argued that a high frequency of repeated exposure to brief periods of increased blood flow and wall shear stress is necessary to trigger these adaptations. As described above, however, the lack of changes in eNOS mRNA expression points toward a minor contribution of increased shear stress as the driving force in these conditions.
IPC stimulation characteristics: potential clinical implications.
A particularly striking finding of the present study was that the aforementioned changes in gene expression were evident only in the protocols in which a high frequency of compressions (15 per minute) was applied. Indeed, in the group exposed to the protocol commonly employed in clinical settings for PAD treatment (3 cycles/min at a pressure of 120 mmHg), no significant changes were seen in VEGF and/or MCP-1 mRNA compared with the control group. Notwithstanding the already mentioned limitations of using animal models, this finding brings up the possibility that the stimulation characteristics routinely used clinically are less than optimal. This is a contentious issue, and although some efforts have been made to determine the most favorable rates and timing of compressions (12, 48), long-term studies comparing the efficacy of different protocols of compression are still lacking. It has been argued that a prolonged interval between compressions is necessary for promoting venous refilling and therefore maximizing the hyperemic responses triggered by the subsequent cycle (12). First, this line of reasoning neglects the fact that a significant portion of blood flow response following limb compression is independent of the arterial-venous pressure gradient (23, 48). There is now compelling evidence that a rapid and significant arteriolar dilation ensues following external mechanical compression of the skeletal muscle (7, 23, 33). In isolated arterioles, this mechanically induced vasodilation has been shown to be magnified by repeated compressions (i.e., increased frequency) as opposed to sustained compressions of the same time duration (7). Thus, as exemplified by our findings (Table 1), even in conditions where the arterial-venous pressure gradient is minimized or absent (e.g., supine position), there still is a robust hyperemic response following external compression (35). Second and most important, the rationale for using lower frequencies ignores the fact that mechanisms other than increased blood flow might mediate and/or contribute to the observed clinical benefits of IPC (see Potential mechanisms above). If, for instance, cyclic mechanical deformations and/or wall strain also participate in the IPC-induced vascular remodeling, it is desirable to design a protocol in which the action of these forces is maximized. From the standpoint addressed in this investigation, i.e., expression of angiogenic mediators, it seems that a higher frequency of compressions might be more efficient than a more sporadic stimulus.
Limitations.
One limitation of the present study is that we did not examine whether pressures applied externally to the limb are accurately transmitted to the vasculature embedded within the tissue. Indeed, although in preliminary experiments we have shown that the pressure underneath the cuff closely resembles the one set in the compression unit (Supplemental Fig. 1), it is not known how the intramuscular pressure varies with tissue depth and between muscles in this experimental scenario. In previous studies it has been shown that during application of external pressure to human cadaver limbs, there seems to be a linear relationship between external and intramuscular pressure at various tissue depths (8). Indeed, Crenshaw et al. (8) have demonstrated that the effectiveness of pressure transmission to the tissue can be higher than 95% depending on the cuff size. Whether the same holds true for rat skeletal muscle in the aforementioned conditions is not known. Future experiments are needed to ascertain how the mRNA expression of muscles located in different anatomical locations respond to this type of therapy.
Our model has two potential weaknesses that deserve consideration. The first is evidenced by the observation that mean blood flows were slightly different between limbs at baseline (Table 2). Especially for the TA muscle, it seems that elevation of the leg, cuff placement, and leg restraint caused blood flow to be reduced in the left leg compared with the right limb in the baseline conditions. As mentioned previously, however, this small reduction in mean blood flow at baseline did not affect the hyperemic response to compressions, and the magnitude of changes in FBF is in accordance with what has been reported in humans under the same conditions. Most importantly, the reduction in flow was uniformly observed in all groups, including the control group, which suggests that this isolated effect does not explain the detected changes in mRNA expression of angiogenic factors. The second striking finding was the pronounced change in VEGF and MCP-1 mRNA expression in the control (no intervention) group (Fig. 5). The reasons for this apparent time effect are not entirely clear, but several factors could possibly influence the expression of these genes under these conditions: first, there was a trauma associated with removing the muscle from the other limb and securing the left leg in place as described in methods; second, the animals were anesthetized for at least 3 h and maintained in the supine position, and expectedly, there could be changes in the magnitude and patterns of blood distribution within the limb; and finally, it is possible that some minor baseline differences exist in the expression of these genes between limbs. These results highlight the importance of including a control group to properly address changes in gene expression in future studies.
Conclusion and perspectives.
In conclusion, the present study demonstrates for the first time that acute IPC application in the leg increases the mRNA expression of angiogenic factors in skeletal muscle. These novel findings provide some valuable information for the understanding of the molecular basis of IPC-mediated acute effects and raise the possibility that repeated exposures to this therapy can influence capillary growth in skeletal muscle. Furthermore, the observation that the magnitude of mRNA changes in VEGF and MCP-1 mRNA was only significantly altered in the groups exposed to higher frequency (2 s on/2 s off) strongly encourages future studies to examine the impact of varying the stimulation characteristics of IPC on hemodynamics and functional variables in clinical populations.
GRANTS
This research was supported by National Institutes of Health Grants RR-18276 and HL-36088 and a Doctoral Student Research Grant from the American College of Sports Medicine Foundation (to B. Roseguini). B. Roseguini is a Fulbright/Brazilian Ministry of Education (Capes) Fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Ann Melloh and Jane Chen for invaluable technical assistance and Donald O'Connor for preparing the illustration.
REFERENCES
- 1.Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler Thromb Vasc Biol 22: 907–913, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest 96: 1449–1454, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices—physiological mechanisms of action. Eur J Vasc Endovasc Surg 21: 383–392, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chen LE, Liu K, Qi WN, Joneschild E, Tan X, Seaber AV, Stamler JS, Urbaniak JR. Role of nitric oxide in vasodilation in upstream muscle during intermittent pneumatic compression. J Appl Physiol 92: 559–566, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crenshaw AG, Hargens AR, Gershuni DH, Rydevik B. Wide tourniquet cuffs more effective at lower inflation pressures. Acta Orthop Scand 59: 447–451, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Dahl J, Li J, Bring DK, Renstrom P, Ackermann PW. Intermittent pneumatic compression enhances neurovascular ingrowth and tissue proliferation during connective tissue healing: a study in the rat. J Orthop Res 25: 1185–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dai G, Gertler JP, Kamm RD. The effects of external compression on venous blood flow and tissue deformation in the lower leg. J Biomech Eng 121: 557–564, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Delis KT. The case for intermittent pneumatic compression of the lower extremity as a novel treatment in arterial claudication. Perspect Vasc Surg Endovasc Ther 17: 29–42, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg 19: 261–269, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 241: 431–441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmunds NJ, Marshall JM. Vasodilatation, oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: roles of nitric oxide. J Physiol 532: 251–259, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egginton S. Invited review: activity-induced angiogenesis. Pflügers Arch 457: 963–977, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hepple RT, Hagen JL, Krause DJ. Oxidative capacity interacts with oxygen delivery to determine maximal O2 uptake in rat skeletal muscles in situ. J Physiol 541: 1003–1012, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105: 1405–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation 5: 7–23, 1998 [PubMed] [Google Scholar]
- 20.Ispanovic E, Haas TL. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol Cell Physiol 291: C579–C588, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 80: 829–837, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Kavros SJ, Delis KT, Turner NS, Voll AE, Liedl DA, Gloviczki P, Rooke TW. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg 47: 543–549, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labropoulos N, Wierks C, Suffoletto B. Intermittent pneumatic compression for the treatment of lower extremity arterial disease: a systematic review. Vasc Med 7: 141–148, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Chen LE, Seaber AV, Johnson GW, Urbaniak JR. Intermittent pneumatic compression of legs increases microcirculation in distant skeletal muscle. J Orthop Res 17: 88–95, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol 288: H759–H768, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol 284: H1668–H1678, 2003 [DOI] [PubMed] [Google Scholar]
- 29.McLaren AT, Marsden PA, Mazer CD, Baker AJ, Stewart DJ, Tsui AK, Li X, Yucel Y, Robb M, Boyd SR, Liu E, Yu J, Hare GM. Increased expression of HIF-1α, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol 292: R403–R414, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol 583: 753–766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1α and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol Heart Circ Physiol 289: H1315–H1320, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Milkiewicz M, Mohammadzadeh F, Ispanovic E, Gee E, Haas TL. Static strain stimulates expression of matrix metalloproteinase-2 and VEGF in microvascular endothelium via JNK- and ERK-dependent pathways. J Cell Biochem 100: 750–761, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol 227: 531–535, 1974 [DOI] [PubMed] [Google Scholar]
- 34.Morris RJ. Intermittent pneumatic compression − systems and applications. J Med Eng Technol 32: 179–188, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Morris RJ, Woodcock JP. Effects of supine intermittent compression on arterial inflow to the lower limb. Arch Surg 137: 1269–1273, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755–1767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, Roberts JP, Niemann CU. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 143: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol 97: 1119–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol 102: 2346–2351, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sadamoto T, Bonde-Petersen F, Suzuki Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur J Appl Physiol Occup Physiol 51: 395–408, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96: 34–40, 2000 [PubMed] [Google Scholar]
- 43.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA 91: 4678–4682, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan X, Qi WN, Gu X, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. J Biomech 39: 2430–2437, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63–69, 2004 [DOI] [PubMed] [Google Scholar]
- 46.van Bemmelen PS, Choudry RG, Salvatore MD, Goldenberg M, Goldman BI, Blebea J. Long-term intermittent compression increases arteriographic collaterals in a rabbit model of femoral artery occlusion. Eur J Vasc Endovasc Surg 34: 340–346, 2007 [DOI] [PubMed] [Google Scholar]
- 47.van Bemmelen PS, Gitlitz DB, Faruqi RM, Weiss-Olmanni J, Brunetti VA, Giron F, Ricotta JJ. Limb salvage using high-pressure intermittent compression arterial assist device in cases unsuitable for surgical revascularization. Arch Surg 136: 1280–1285, 2001 [DOI] [PubMed] [Google Scholar]
- 48.van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner DS. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 19: 1052–1058, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Wagner PD. Skeletal muscle angiogenesis. A possible role for hypoxia. Adv Exp Med Biol 502: 21–38, 2001 [PubMed] [Google Scholar]
- 50.Wang DL, Wung BS, Shyy YJ, Lin CF, Chao YJ, Usami S, Chien S. Mechanical strain induces monocyte chemotactic protein-1 gene expression in endothelial cells. Effects of mechanical strain on monocyte adhesion to endothelial cells. Circ Res 77: 294–302, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol 19: 2085–2093, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Zheng W, Christensen LP, Tomanek RJ. Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H2739–H2745, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-β. Am J Physiol Heart Circ Physiol 280: H909–H917, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]