Abstract
The dominant mode of intracellular Ca2+ release in adult mammalian heart is gated by ryanodine receptors (RyRs), but it is less clear whether inositol 1,4,5-trisphosphate (IP3)-gated Ca2+ release channels (IP3Rs), which are important during embryogenesis, play a significant role during early postnatal development. To address this question, we measured confocal two-dimensional Ca2+ dependent fluorescence images in acutely isolated neonatal (days 1 to 2) and juvenile (days 8–10) rat cardiomyocytes, either voltage-clamped or permeabilized, where rapid exchange of solution could be used to selectively activate the two types of Ca2+ release channel. Targeting RyRs with caffeine produced large and rapid Ca2+ signals throughout the cells. Application of ATP and endothelin-1 to voltage-clamped, or IP3 to permeabilized, cells produced smaller and slower Ca2+ signals that were most prominent in subsarcolemmal regions and were suppressed by either the IP3R-blocker 2-aminoethoxydiphenylborate or replacement of the biologically active form of IP3 with its L-stereoisomer. Such IP3R-gated Ca2+ releases were amplified by Ca2+-induced Ca2+ release (CICR) via RyRs since they were also reduced by compounds that block the RyRs (tetracaine) or deplete the Ca2+ pools they gate (caffeine, ryanodine). Spatial analysis revealed both subsarcolemmal and perinuclear origins for the IP3-mediated Ca2+ release events RyR- and IP3R-gated Ca2+ signals had larger magnitudes in juvenile than in neonatal cardiomyocytes. Ca2+ signaling was generally quite similar in atrial and ventricular cardiomyocytes but showed divergent development of IP3-mediated regulation in juveniles. Our data suggest that an intermediate stage of Ca2+ signaling may be present in developing cardiomyocytes, where, in addition to RyR-gated Ca2+ pools, IP3-gated Ca2+ release is sufficiently large in magnitude and duration to trigger or contribute to activation of CICR and cardiac contraction.
Keywords: IP3-gated Ca2+ release channels, ryanodine receptors, development, cardiac electrophysiology
cardiac excitation-contraction coupling in adult mammalian cardiomyocytes involves Ca2+ entry via L-type Ca2+ channels, Ca2+-induced Ca2+ release (CICR) from ryanodine receptors (RyR) on sarcoplasmic reticulum (SR), and Ca2+-mediated cellular contraction. In ventricular cells, Ca2+ signaling responses travel rapidly throughout the cell, as the action potential invades the network of t-tubules (3). However, it is not known in detail how the adult mode of Ca2+ signaling is established during embryonic and postnatal development, where the mammalian heart undergoes rapid changes in morphology and gene expression, including major modifications of the organelles and proteins that are associated with Ca2+ signaling. For instance, embryonic and neonatal cardiomyocytes lack the t-tubular network, whereas juvenile cardiomyocytes have an immature t-tubular system (15, 45). Similarly, SR in the fetal heart is sparse and has decreased capability to load Ca2+ compared with adult SR (33). Inositol 1,4,5-trisphosphate (IP3)- and RyRs are also differentially expressed with development and appear at the same time period when Ca2+ oscillations are first observed (12, 42). Interestingly, in embryonic cardiomyocytes, Ca2+ oscillations precede the expression of the ionic channels responsible for spontaneous depolarization (35, 42), and transgenic mice deficient in pacemaking channels demonstrate early embryonic spontaneous beating (41, 44, 50). Consistent with this idea, ryanodine (Ry) has been shown to diminish, but not eliminate, embryonic and neonatal cell contraction (36, 45, 51). Although functional RyRs are clearly present in postnatal cardiomyocytes (18), observations that inhibitors of SR function exert a more pronounced suppressive effect on cardiac action potential-mediated Ca2+ transients in juvenile (88%) vs. neonatal (15%) cardiomyocytes indicate an increased reliance on and upregulation of RyR as a function of age (10, 11). Antisense knockdown of IP3R1 in embryonic stem cells markedly reduces cardiac cellular oscillations (35). These data suggest that multiple mechanisms may be involved in cardiac pacing, including both IP3R- and RyR-gated Ca2+ signaling.
There is a clear contrast between cardiac Ca2+ signaling of embryonic and adult cardiomyocytes, but it remains unclear how the embryonic Ca2+ signaling phenotype transitions into the adult form. Here we studied the characteristics and roles of RyR- and IP3R-gated Ca2+ signaling in developing cardiomyocytes. Since culturing cardiomyocytes changes the physiology and gene expression of the cells, we used only acutely isolated developing cardiomyocytes. To focus on the intracellular Ca2+ release mechanisms, we eliminated the effects of membrane depolarization either by voltage-clamping the cells or permeabilizing the cell membrane. In the first case, we dialyzed the cells with high concentrations of fast fluorescent and slow nonfluorescent Ca2+ chelators [1 mM Fluo-4 + 2 mM EGTA; intracellular Ca2+ concentration ([Ca2+]i) ≅ 100 nM] to accentuate the rate of Ca2+ release while suppressing large local rises in [Ca2+]i (1, 49) that may support propagated waves of CICR. To allow reliable Ca2+ measurements in permeabilized cells, they were superfused with a simulated internal solution, which was also designed to accentuate the Ca2+ release, but contained lower concentrations of the Ca2+ buffers (0.04 mM Fluo-4 + 0.4 mM EGTA; [Ca2+]i ≅ 100 nM).
We tested the hypothesis that maturation of cardiomyocytes is accompanied by changes in the relative contribution of functional RyR- and IP3R-gated Ca2+ stores and that developing cardiomyocytes have specific Ca2+ signaling mechanisms, such as IP3-mediated Ca2+ release, that are capable of triggering Ca2+ transients, in part, by local CICR. We found that developing cardiomyocytes have functional RyR- and IP3R-gated Ca2+ releases with unique pharmacology and spatial profiles. Maturation from neonatal to juvenile cardiac phenotype involved an upregulation of both types of Ca2+ release channel. The IP3-mediated Ca2+ signaling was capable of generating both subsarcolemmal and perinuclear Ca2+ releases in developing cardiomyocytes, the former of which can directly contribute to CICR transients in the subsarcolemmal regions.
MATERIALS AND METHODS
Experiments were carried out in accordance with institutional and federal guidelines. Protocols were approved and supervised by Georgetown University Animal Care and Use Committee.
Cell isolation.
For preparation of cardiomyocytes from neonatal and juvenile rats, with the juveniles first being deeply anesthetized, the animals were decapitated, the chest cavities were opened, and the hearts were removed. The excised hearts were retrogradely perfused at 37°C through the aorta, first for 2 min with Ca2+ free Tyrode's solution composed of (in mM) 137 NaCl, 5.4 K-glutamate, 1 MgCl2, 10 glucose, and 10 HEPES titrated to pH 7.4 with NaOH and then with Ca2+ free Tyrode's solution supplemented with collagenase (0.8 mg/ml) and protease (0.1 mg/ml) for 4 min. The atrial and ventricular tissues were dissected and gently agitated to dissociate single cells. The freshly dissociated cells were plated onto glass coverslips, stored at room temperature in Tyrode's solution containing 0.2 mM CaCl2, and used within 8 h in Ca2+ imaging experiments where the membrane potential was either voltage-clamped or abolished by membrane permealibization. Cells that maintained their native elongated shape following the isolation procedure were selected for detailed examination over those that that assumed a more globular appearance.
Drugs were dissolved in the external experimental solutions and applied rapidly using a concentration-clamp device (6). Rapid application of 100 μM ATP (Sigma, St. Louis, MO), 10 mM caffeine (Sigma), 100 nM endothelin-1 (Sigma), and 1 or 20 μM IP3 (D-myo-IP3; Calbiochem, Gibbstown, NJ) was used to probe Ca2+ stores. All experiments were carried out at room temperature (22–24°C).
Whole-cell voltage-clamp procedures.
Some cells were voltage-clamped in the whole cell configuration (16) to 1) hold the membrane potential steady at −70 mV during recording of drug-induced Ca2+ transients, 2) measure membrane currents [e.g., Na+-Ca2+ exchanger current (INaCa) and Cl− current] that were activated during these interventions, and 3) subject the cells to repeated depolarizing pulses from −90 to 0 mV to maintain and equilibrate intracellular Ca2+ stores. The voltage-clamped cells were dialyzed with a Ca2+-buffered pipette solution containing (in mM) 110 CsOH, 110 aspartic acid, 5 NaCl, 20 TeaCl, 5 Mg-ATP, 0.2 cAMP, 2 EGTA, 1 K5Fluo-4, 1.2 CaCl2, and 20 HEPES (titrated to pH 7.2 with CsOH) (56). With the consideration of the binding constants of Fluo-4 (Kd ≅ 400 nM) and EGTA (Kd ≅ 100 nM), the addition of 1.2 mM CaCl2 was calculated to buffer [Ca2+]i of the dialyzing solution at ∼100 nM. Membrane currents were measured with a Dagan voltage-clamp amplifier using pClamp software. The conditioning pulses were initiated 3 min after rupture of the membrane, and fluorescence measurements were started 3 to 4 min later. The extracellular solution used during experiments contained (in mM) 137 NaCl, 5.4 K-glutamate, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (titrated to pH 7.4 with NaOH).
Permeabilization.
After being prestained with 20 μM Fluo-4 acetoxymethyl ester (Fluo-4 AM), the cells were permeabilized with saponin (9, 63). First, the cells were suspended in a solution containing (in mM) 100 K-aspartate, 20 KCl, 0.5 EGTA, 0.75 MgCl2, and 10 HEPES 10 (titrated to pH 7.2 with KOH). The cell surface membrane was permeabilized by adding 0.005% (wt/vol) saponin for 30 s. After 30 s, the bath solution was exchanged for a saponin-free simulated internal solution composed of 100 mM K-aspartate, 15 mM KCl, 5 mM KH2PO4, 5 mM MgATP, 0.4 mM EGTA, 0.04 mM K5Fluo-4, 0.2 mM CaCl2 ([Ca2+]i ≅ 100 nM), 0.75 mM MgCl2, 10 mM phosphocreatine, 5 U/ml creatine phosphokinase, 8% dextran, and 10 mM HEPES (titrated to pH 7.2 with KOH).
Two-dimensional confocal imaging.
Intracellular Ca2+ signals were measured with the fluorescent Ca2+-indicator dye Fluo-4, which was used in conjunction with the nonfluorescent Ca2+-chelator EGTA, as mentioned above. The cells were imaged using a Noran Odyssey XL rapid two-dimensional laser scanning confocal microscopy system (Noran Instruments, Madison, WI) attached to a Zeiss Axiovert TV135 inverted microscope fitted with an × 63 water-immersion objective lens. The excitation wavelength of the argon ion laser was set to 488 nm, and fluorescence emission was measured at wavelengths >515 nm. Cells were imaged confocally at 4–240 frames/s depending on the experiment.
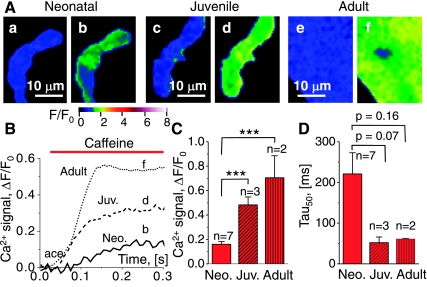
The average resting fluorescence intensity (F0) was calculated from several frames measured immediately before drug application. Images were filtered by 3 pixel × 3 pixel averaging. The amplitudes of the Ca2+-dependent cellular fluorescence signals were quantified as ΔF/F0 = F/F0 − 1, where F is the peak value and, like Fo, was measured by integration over the entire area of each cell. Tau50 quantifies the rate of rise of the Ca2+ signals and was calculated as 1.25× the time required for the transient to develop from 10% to 50% at the peak value (Fig. 1). Generally, the fluorescence images used in illustrations were normalized by dividing the images during activation [F(x,y)] by the average image before activation [Fo(x,y); Figs. 1–5, 7, 8]. The division was performed only provided Fo(x,y) exceeded a threshold that was chosen to generate a sharp transition from the color distributions of the cells to their surroundings, which are shown in black. The green-blue speckle seen in single frames before activation indicates the level of noise in the recordings, and thereby the type of low level signals that should be discarded as random fluctuations. In contrast, the signals of physiological interest were characterized by their larger amplitudes (yellow, orange, and red distributions) and systematic progression from frame to frame.
Fig. 1.
Caffeine-induced changes in Ca2+-dependent fluorescence in voltage-clamped neonatal (Neo), juvenile (Juv), and adult cardiomyocytes. A: normalized fluorescence images in neonatal (a and b), juvenile (c and d), and adult (e and f) cardiomyocytes show the noise in a single frame as blue mottle before activation (a, c, and e; F/Fo ≅ 1, see color scale) and local increases in intracellular Ca2+ as brighter color distributions (green-yellow-orange) following application of 10 mM caffeine (b, d, and f). The timing of the images (a–f) is indicated in B, which shows the time course of the caffeine-induced cellular Ca2+ transients (ΔF/F0) for representative neonatal, juvenile, and adult cardiomyocytes. The histograms show pooled data for cellular Ca2+ transients in terms of their magnitude (C) and the time to development of 50% of the full response (D). The level of significance (***P < 0.001, unpaired t-test) and the number of cells examined (n) are indicated in the histograms. The confocal fluorescence images were recorded at a rate of 120 frames/s, and the cells were voltage-clamped at −70 mV and dialyzed with Ca2+-buffered pipette solution.
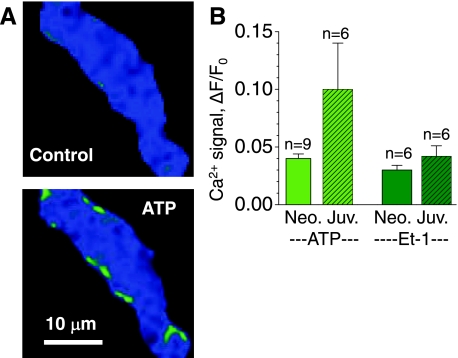
Fig. 2.
ATP-induced increases in intracellular Ca2+ concentration ([Ca2+]i) in Ca2+-buffered developing cardiomyocytes. A: normalized fluorescence images from a neonatal cardiomyocyte show low noise before activation (control; F/Fo ≅ 1) and subsarcolemmal increases in [Ca2+]i following application of 100 μM ATP. B: average cellular Ca2+ transients (ΔF/Fo) in neonatal (Neo; solid bars; 1 to 2 days old) and juvenile (Juv; hatched bars; 8–10 days old) cardiomyocytes in response to 100 μM ATP (left) or 100 nM endothelin-1 (ET-1; right). Voltage-clamped cells are same color scale as Fig. 1.
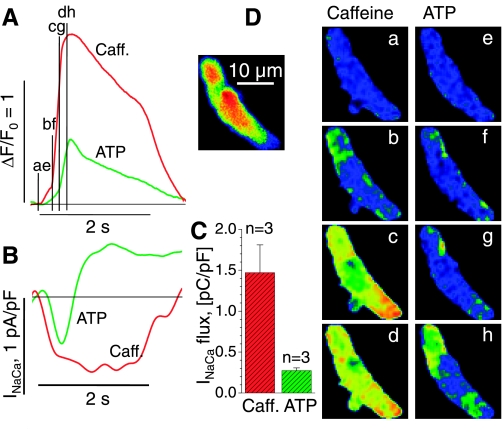
Fig. 3.
Caffeine- and ATP-activated Ca2+ transients and inward currents in juvenile cardiomyocytes. A: superimposed cellular Ca2+ transients (ΔF/F0) upon extracellular application of 10 mM caffeine (Caff; red) and 100 μM ATP (green) for the voltage-clamped cell shown in the inset and in D. B: membrane current recorded at −70 mV during 2-s exposures to caffeine (red) and ATP (green). The duration of the solution exchange is indicated by the 2-s time bars in A and B. C: average values for Na+-Ca2+ current (INaCa)-flux during exposure to caffeine and ATP are based on the integral of the measured membrane current during its negative excursion (B). D: normalized images of Ca2+-dependent fluorescence before activation (a and e) and during exposure to caffeine (b–d) or ATP (f–h) at the times indicated in B. Voltage-clamped juvenile cells are same color scale as Fig. 1.
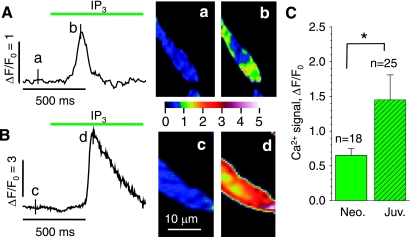
Fig. 4.
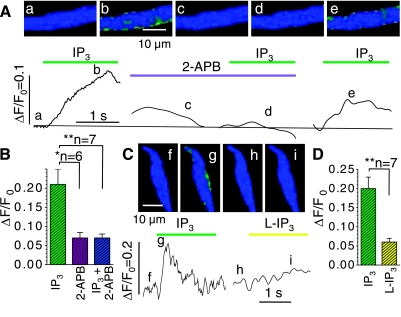
Inositol (1,4,5)-trisphosphate (IP3)-triggered Ca2+ release in permeabilized neonatal (A) and juvenile (B) cardiomyocytes. A and B: cellular Ca2+ transients (FΔ/F0) upon extracellular application of 20 μM IP3 and normalized fluorescence images before (a and c) and after (b and d) application of 20 μM IP3 at the times indicated next to the traces. The histogram in C compares the average responses in neonatal and juvenile cardiomyocytes (confocal imaging at 120 frames/s; the nonvoltage clamped cells were stained in Fluo-4AM and then permeabilized and finally maintained in simulated internal solution; nonpaired t-test; same color scale as Fig. 1). *P ≤ 0.05.
Fig. 5.
IP3-gated Ca2+ signals in juvenile, permeabilized cardiomyocytes are reversibly suppressed by the IP3-gated Ca2+ release channels (IP3R) antagonist 2-aminoethoxydiphenylborate (2-APB; A and B) and are triggered only weakly by L-IP3 (AB). A and C: normalized fluorescence images that were recorded in representative cardiomyocytes at the times indicated next to the tracings of cellular Ca2+ transients, i.e., before interventions (a, f, and h), during activation with 20 μM IP3 alone (b and g), and when IP3 was applied after 3 min incubation with 2 μM 2-APB (c), after 3 min of washout (e), or as the biologically inactive stereoisomer L-IP3 (i). B: average amplitude of fluorescence signals (ΔF/F0) evoked by IP3 (green) in cells that were then incubated with 2-APB (violet) and again challenged with IP3 (turquoise). D compares the average amplitudes of the cellular Ca2+ transients in another set of cells that were exposed sequentially to IP3 (green) and L-IP3 (yellow; permeabilized cells, paired t-test). *P ≤ 0.05; **P ≤ 0.01.
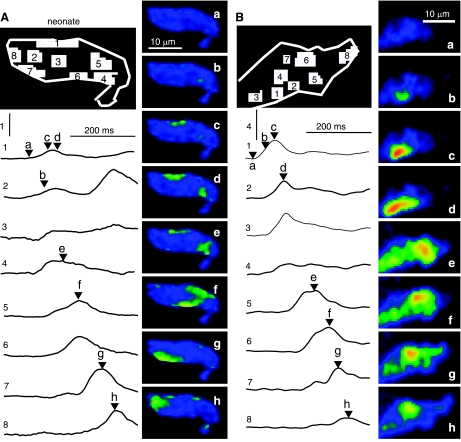
Fig. 7.
Spatial analysis of IP3-mediated Ca2+ signaling events in permeabilized neonatal (A) and juvenile (B) cardiomyocytes. Each panel shows a map that identifies 8 regions of interest, 8 traces (1–8) showing the time course of the fluorescence changes in these regions, and 8 normalized fluorescence images (a–h) that were measured at the times indicated by letters and arrow heads next to the traces. The vertical scale bars indicate ΔF/F0 values of 1 (A) or 4 (B; 20 μM IP3; permeabilized cells; same color scale as Fig. 1).
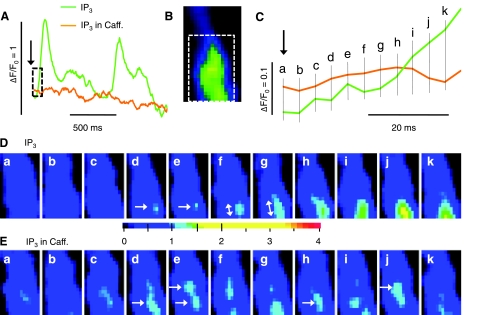
Fig. 8.
Time course of local IP3-activated Ca2 signal before and after depletion of the RyR-gated Ca2+ stores. A: cellular Ca2+ changes (ΔF/F0) upon extracellular application (arrow) of 20 μM IP3 before (green) and after (orange) 3-min incubation with 10 mM caffeine. The boxed regions of the traces and of the sample frame (B) were expanded to show details of the onset of the IP3-induced responses on an expanded time scale (C) and as rapidly changing normalized fluorescence images recorded at 240 frames/s (D and E) at the times indicated in C (permeabilized juvenile cardiomyocyte; expanded color scale).
To reduce photobleaching, the laser beam that was used for excitation of Fluo-4 was electronically shuttered and triggered to open by the command of the pClamp program only during acquisition of data.
Statistical analyses.
All data are presented as means ± SE, n, where n is the number of cells examined. The unpaired Student's t-test was used to evaluate differences between measurements from different populations of cells (e.g., neonatal vs. juvenile cardiomyocytes), whereas the paired t-test was used to compare different pharmacological interventions in the same population of cells (Figs. 5, B and D, and 6, C and D). Differences were considered statistically significant when P < 0.05. Higher degrees of significance are indicated with two (P < 0.01) or three (P < 0.001) asterisks.
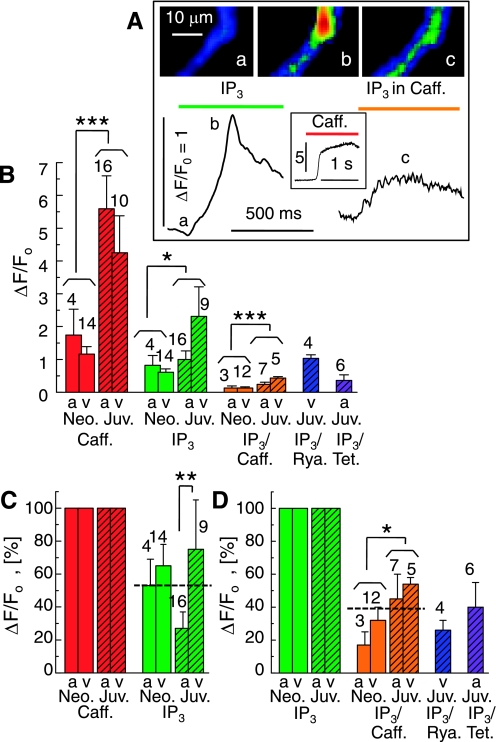
Fig. 6.
Contribution of ryanodine receptors (RyR)-gated Ca2+ stores to the IP3-triggered Ca2+ releases. A: cellular Ca2+ transients (ΔF/F0) in a permeabilized juvenile cardiomyocyte upon extracellular application of 20 μM IP3 (left), 10 mM caffeine (inset), and IP3 after 3 min incubation in caffeine (right). Selected frames (a-c) show normalized fluorescence images at the times indicated. B: comparison of the average Ca2+ transients (ΔF/F0) in atrial (a) and ventricular (v) cardiomyocytes from neonatal (Neo; open bars) and juvenile (Juv; hatched bars) rats. The Ca2+ transients were evoked by 10 mM caffeine (red) or 20 μM IP3, either by itself (green) or after 3 to 4 min incubation with 10 mM caffeine (orange), 40 μM ryanodine (blue), or 1 mM tetracaine (purple). C: IP3-activated Ca2+ transients normalized relative to the caffeine-induced transients measured in the same cells. D: suppression of IP3-activated Ca2+ signals by pretreatment with caffeine, ryanodine, and tetracaine. Permeabilized cells are same color scale as Fig. 1; n values are indicated at each bar. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
RESULTS
RyR Ca2+ signals in dialyzed cardiomyocytes.
Excitation-contraction coupling in neonatal cardiomyocytes appears to be regulated by Ca2+ influx through the sarcolemma, with the L-type Ca2+ channel being the main source for trans-sarcolemmal Ca2+ flux (2, 7, 14, 34, 55). However, although RyRs are functional in neonatal cardiomyocytes, Ry only reduces Ca2+ transients by ∼15% in the day 1 neonatal rat heart versus 88% in juvenile cardiomyocytes, suggesting an increased reliance on RyR as a function of age (10, 11). Thus, to test the hypothesis that RyRs are upregulated as a function of maturation, we compared RyR-gated Ca2+ release in acutely isolated neonatal (days 1 to 2) and juvenile (days 8 to 10) cardiomyocytes, using the results from the widely studied adult cardiomyocytes as a benchmark for full maturation of RyR-mediated Ca2+ signaling.
Freshly isolated cardiomyocytes were voltage-clamped and dialyzed with a Ca2+ buffered internal solution, and conditioning pulses from −90 to −10 mV were applied at 5-s intervals to maintain the Ca2+ load of the SR throughout the experiments. In between conditioning pulses and with the cardiomyocytes being voltage-clamped at −70 mV to prevent activation of voltage-gated ion channels, RyR-mediated Ca2+ release was activated using rapid application of 10 mM caffeine, a RyR agonist, and changes in intracellular Ca2+ were detected using confocal Ca2+ imaging.
Figure 1A shows a comparison of the caffeine-activated Ca2+ transients in neonatal (b), juvenile (d), and adult (f) cardiomyocytes, with the normalized images recorded before activation being shown as well (a, c, and e, respectively), to give an indication of the spatial distribution of the caffeine-induced Ca2+ signal. The fluorescent signal Ca2+ transients of the neonatal (Neo), juvenile (Juv), and adult cardiomyocytes imaged at 120 Hz are shown in Fig. 1B. Comparisons of both the images (Fig. 1A) and the Ca2+ transients (Fig. 1B) indicate that maturation from neonate to adult is accompanied by larger and more uniformly distributed caffeine-activated Ca2+ transients. Indeed, analysis of the amplitude of the caffeine-induced Ca2+ transients (Fig. 1C) reveals that the magnitude of the caffeine Ca2+ transient increased with the age of the cardiomyocytes, with the neonates having the smallest amplitude (ΔF/F0 = 0.160 ± 0.022; n = 7) and significantly larger amplitudes being observed in both juveniles (Juv: ΔF/F0 = 0.483 ± 0.065; n = 3; P = 0.0003) and adults (ΔF/F0 = 0.705 ± 0.18; n = 2; P = 0.0005). Figure 1D shows that the time required for development of 50% of the full amplitude of the caffeine-induced Ca2+ transient was generally shorter in the juvenile (Tau50 = 52 ± 14 ms; n = 3) and adult (Tau50 = 61 ± 1 ms; n = 2) compared with the neonatal (Tau50 = 220 ± 50 ms; n = 7) cardiomyocytes. The data demonstrate an increase in the velocity and magnitude of RyR-gated Ca2+ release as a function of development and support our hypothesis that RyRs are upregulated during postnatal development, with neonatal and juveniles representing two stages of progressive maturation.
ATP-activated IP3R Ca2+ signals in dialyzed developing cardiomyocytes.
Although IP3-mediated Ca2+ signaling plays a large role in excitation-contraction coupling (ECC) of embryonic cardiomyocytes (43, 53), the role of IP3-activated Ca2+ signaling in acutely isolated developing cardiomyocytes has never been studied before. The documented differences in the maturity of RyR-gated Ca2+ stores in neonatal and juvenile cardiomyocytes make these stages of development ideal to test whether parallel changes take place in IP3-activated Ca2+ signaling, with IP3-activated Ca2+ signaling perhaps having a reduced role as the role of RyR-gated Ca2+ signaling increases. To test this hypothesis, we compared the Ca2+ signals of acutely isolated neonatal and juvenile cardiomyocytes in response to activation of IP3 by rapid application of 100 μM ATP, which binds to extracellular purinergic receptors to activate the breakdown of PIP2 into IP3 (52). Similarly, 100 nM endothelin-1 (ET-1), which binds to specific cell surface receptors to generate IP3 (54) was tested as an alternative means to activate the IP3-signaling pathway.
Ca2+-buffered, voltage-clamped rat cardiomyocytes produced variable responses to ATP and ET-1, ranging from rapid whole cell Ca2+ transients to an increase in the diastolic [Ca2+]i. Figure 2A shows representative normalized images of a voltage-clamped neonatal cardiomyocyte before (control) and after exposure to ATP, revealing that ATP activated an increase in Ca2+ fluorescence mostly confined to the subsarcolemmal area of the cell. The spatial profiles for ET-1 Ca2+ responses were virtually identical (not shown). As summarized in Fig. 2B, application of ATP generated an increase in Ca2+-dependent fluorescence in both age groups with the neonatal cardiomyocytes producing a ΔF/F0 = 0.040 ± 0.004 (n = 9) and the juveniles a ΔF/F0 = 0.10 ± 0.04 (n = 6). Exposure of the same cells to ET-1 produced similar results, revealing an increase in the basal Ca2+ level of ΔF/F0 = 0.030 ± 0.004 (n = 6) in the neonates and ΔF/F0 = 0.040 ± 0.009 (n = 6) in the juveniles. Interestingly, maturation from the neonatal to the juvenile state appeared to be accompanied not only by an increase in RyR-gated Ca2+ stores but also by maintenance, or slight upregulation, of the IP3R-gated Ca2+ stores. Although the differences in IP3-mediated Ca2+ signaling only represent a trend, it was a surprising observation that we subjected to more extensive testing in permeabilized cells.
Figure 3 shows a representative example selected from a number of voltage-clamped Ca2+-buffered juvenile cardiomyocytes where we compared the magnitude and time course of the ATP- and caffeine-activated Ca2+ transients. As shown in Fig. 3A, superimposing the whole cell Ca2+ fluorescence traces for the two interventions on the same graph reveals different kinetics and magnitudes to the caffeine and ATP responses. Tau50 of the caffeine-induced Ca2+ transient was shorter than that of ATP (26 ms vs. 240 ms). In addition, the amplitude of the caffeine-induced Ca2+ transient was larger than that of ATP (ΔF/F0 = 0.70 vs. 0.25). The cellular images shown in Fig. 3D, taken at the time points indicated in the corresponding traces in Fig. 3A, show that caffeine-induced rise in Ca2+ occurred simultaneously in both the peripheral and central regions of the cell (Fig. 3D, b-d). The appearance of the caffeine-activated Ca2+ signal at the top the cell (Fig. 3D, b and c) corresponds to the area of the cell exposed to caffeine first. These data indicate that RyRs are distributed throughout the developing cardiomyocytes and agree with the findings of other researchers (15).
The ATP Ca2+ signal appears to develop first in the membrane-associated peripheral region (Fig. 3D, f and g), with delayed and incomplete invasion of the center of the cell (Fig. 3D, h). The subsarcolemmal origin of the response correlates with the data from neonatal cardiomyocyte shown in Fig. 2. The following, much larger magnitude of the ATP-activated Ca2+ transient, as well as the spread of the signal to some parts of the cell, suggests activation of the RyRs secondary to the primary IP3-activated Ca2-release.
In cells where the caffeine- and ATP-induced Ca2+ transients were relatively large (Fig. 3A), we found that they were accompanied by significant transient inward currents (Fig. 3B; INaCa) of the type that in previous studies has been associated with activation of the Na+-Ca2+ exchanger by elevation of [Ca2+]i (4). Note that the initial Ca2+ fluorescence time course (Fig. 3A) is similar to that of the transient inward current (Fig. 3B), with both sets of traces revealing a bigger delay for the ATP-induced response. Despite the great disparity in the amplitudes of the cellular Ca2+ transients produced by the two interventions, the amplitudes of the associated inward currents were comparable (n = 3), suggesting that the Na+-Ca2+ exchanger is quite sensitive to the localized subsarcolemmal Ca2+ transients evoked by ATP. The ATP-activated inward current was often followed by an outward maintained current that reversed at ECl and could be blocked by DIDS (unpublished data, Tufan and Morad). As summarized for three cells in Fig. 3C, the integral INaCa flux was larger for the caffeine- than for the ATP-activated Ca2+ release (1.47 ± 0.34 pC/pF vs. 0.27 ± 0.03 pC/pF), mirroring the larger magnitude of caffeine-induced Ca2+ fluorescence shown in Fig. 3A (Figs. 1 and 2). These results indicate that IP3-induced Ca2+ release even contributes to cellular Ca2+ transients in highly Ca2+-buffered cells exclusive of Na+ or L-type Ca2+ channel activation.
IP3R-gated Ca2+ signals in permeabilized cardiomyocytes.
Although the voltage-clamped, Ca2+-buffered cardiomyocytes provided insight into the magnitude of RyR- and IP3R-gated Ca2+ signals, one of the limitations in the previous set of experiments is the possibility that variability in the expression of extracellular receptors may limit the size and frequency of ATP- and ET-1-activated Ca2+ signals. Therefore, direct application of IP3 in permeabilized cells, although not representing a physiological response, was used to evoke Ca2+ signals and to compare the size of IP3R-gated Ca2+ stores in neonatal and juvenile cardiomyocytes, as well as the ability of IP3-activated Ca2+ release to directly activate CICR.
As shown in Fig. 4, application of 20 μM IP3 activated Ca2+ transients in both acutely isolated neonatal (Fig. 4A) and juvenile (Fig. 4B) cardiomyocytes. The insets show normalized fluorescence images before (Fig. 4B, a and c) and after (Fig. 4B, b and d) application of IP3. As summarized in Fig. 4C, IP3 evoked significantly larger Ca2+ transients in juvenile than in neonatal cardiomyocytes (ΔF/F0 = 0.65 ± 0.10, n = 18 vs. ΔF/F0 = 1.45 ± 0.36, n = 25, P = 0.07). The use of 1 μM instead of 20 μM IP3 produced fluorescence signals with similar amplitudes, but with delayed kinetics (data not shown).
To ensure that the observed effects of IP3 were due to IP3-dependent Ca2+ release, myocytes were exposed to IP3 before and after incubation in solution containing the IP3R antagonist 2-aminoethoxydiphenylborate (2-APB). The traces and the corresponding images in Fig. 5A show that IP3 given before (Fig. 5A, a and b) and after washout of 2 μM 2-APB (Fig. 5A, e) produced an increase in the cytosolic [Ca2+] that was absent when the cells were treated with 2-APB alone (Fig. 5A, c) and with IP3 in the presence of 2-APB (Fig. 5A, d). Average values from six cells (Fig. 5B) demonstrate that the Ca2+ signal produced by IP3 alone (ΔF/F0 = 0.20 ± 0.04) was much larger than the Ca2+ signals that were measured after incubation with 2-APB when the cells were either exposed to IP3 (ΔF/F0 = 0.07 ± 0.01; P = 0.02) or a recording was performed without intervention to ascertain the decay of the preceding Ca2+ signal and evaluate noise, bleaching, and the stability of the cells (ΔF/F0 = 0.07 ± 0.01; P = 0.01).
Similarly, Fig. 5C shows sample records from a series of experiments where individual cells were found to respond more strongly to the naturally occurring D-IP3 (Fig. 5C, f and g) than to its stereoisomer L-IP3 (Fig. 5C, h and i). As illustrated in the Fig. 5D, the average Ca2+ signals produced by 20 μM D-IP3 were greatly reduced when the cells were exposed to the same concentration of the L-isomer (ΔF/F0: 0.20 ± 0.03 vs. 0.06 ± 0.01; n = 7; P = 0.0011). These control experiments show that the observed IP3-activated Ca2+ transients are specific for IP3Rs and IP3.
Contribution of RyR-gated Ca2+ release to IP3-mediated Ca2+ signal.
To evaluate the contribution of RyR-gated Ca2+ release to the IP3-activated Ca2+ signals, we performed experiments where Ca2+ release via RyRs was blocked by different interventions. Figure 6A shows changes in the IP3-activated Ca2+ signal in a representative juvenile cardiomyocyte before (Fig. 6A, a and b, left) and after (Fig. 6A, c, right) incubation of the cardiomyocyte with 10 mM caffeine to deplete RyR-gated Ca2+ secondary to the initial large Ca2+ release as seen in the inset. Fig. 6B compares the accumulated results from atrial (Fig. 6B, a) and ventricular (v) cardiomyocytes from neonates (Neo) and juveniles (Juv). The Ca2+ signals in response to caffeine (red) were almost fourfold (3.9 ± 0.9) larger in juvenile than in neonatal cells, and similar increases were observed in response to IP3 in the absence (green) or presence (orange; Fig. 6B) of caffeine. These observations are based on pooled data from atrial and ventricular cardiomyocytes (Fig. 4C) since the permeabilization studies generally showed no significant differences between these cell types when examined at the same age. The only departure from this pattern was found in the response to IP3 alone, where the upregulation in juvenile cardiomyocytes cells surprisingly appeared to be limited to the ventricular cells. When normalized relative to the caffeine-induced Ca2+ signals, those evoked by IP3 amounted to about half (53 ± 9%; n = 43; dashed line) and, overall, showed no significant increase in juveniles although in this age group the difference between atrial and ventricular cardiomyocytes was noticeable (Fig. 6C). Pretreatment with caffeine reduced the IP3-induced Ca2+ signals to 39 ± 5% (n = 27), and similar reductions were found when the RyR-gated Ca2+ stores were depleted with 40 μM ryanodine or 1 mM tetracaine was added to block the gating of the RyRs (Fig. 6D).
These data, which are consistent with the findings from Ca2+-buffered voltage-clamped cardiomyocytes (Fig. 2), suggest that, although the maturation of juvenile cardiomyocytes is accompanied by an increase in both caffeine-sensitive RyR-gated Ca2+ stores and IP3R-gated Ca2+ stores, a change in the relative contribution of the two signaling pathways is seen only as differential development of atrial versus ventricular cells.
Spatial analysis of IP3-mediated Ca2+ signaling events.
The above results suggest that the IP3-activated Ca2+ release directly activates the RyRs by CICR in both neonatal and juvenile cardiomyocytes, but it remains to be shown where in the cell this communication may occur and how it may correlate with the locations of the IP3- and RyRs. Figure 7 illustrates the spatial development of IP3-induced Ca2+ transients in representative neonatal (Fig. 7A) and juvenile (Fig. 7B) cardiomyocytes. Regions of interest in the cells were identified with numbers on the sketched cells, and the Ca2+ signals in these numbered regions were followed in the corresponding numbered traces (1–8), thereby mapping the time course of the IP3-generated local Ca2+ responses. Labeled time marks on the traces correspond to the normalized cellular images (Fig. 7B, a–h). In both age groups, the IP3-activated responses were first observed at subsarcolemmal locations (Figs. 7A, c, and B, b) and traveled along the periphery of the cell before variably moving into the center. This wave-like spread of the IP3-generated Ca2+ signals near the cell surface suggests subsarcolemmal colocalization of IP3Rs and RyRs and was characteristic of permeabilized cells, where failure of whole cell signal integration may be directly linked to the loss of membrane potential. The minimal involvement of the central portions of the cell (e.g., Figs. 7A region/trace 3, and B, region/trace 4) may be indicative of a spatial disconnect between IP3Rs and RyRs in the center.
Spatial analysis of IP3-activated Ca2+ release after depletion of RyR-gated Ca2+ stores.
Although the strong IP3-induced Ca2+ signals, which spread near the surface of neonatal and juvenile cells, are likely to reflect subsarcolemmal colocalization of IP3Rs and RyRs, as found in adult atrial cells (31), it is unclear whether these signals may swamp the less conspicuous Ca2+ release activity of functional IP3Rs that may be present at central locations but may operate without the support of CICR from nearby RyR-gated Ca2+ stores. Therefore, we measured the subcellular distribution of IP3-induced Ca2+ signals under conditions where the RyR-mediated component of the Ca2+ release was inhibited.
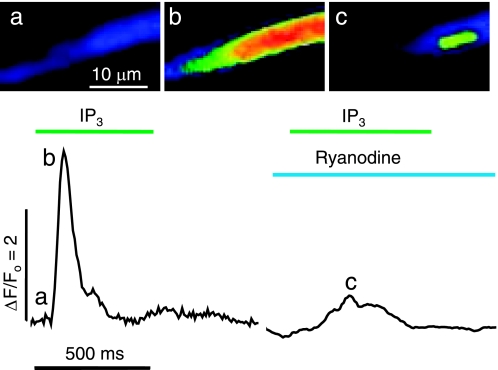
Figure 8 shows the spatial development of an IP3-induced Ca2+ transient in a representative juvenile cardiomyocyte before and after incubation in caffeine to deplete and prevent refilling of the RyR-gated Ca2+ stores. As shown in Fig. 8A, the whole cell Ca2+ fluorescence changes were followed before (green) and after (orange) depletion of the RyR-gated Ca2+ pools via long-term incubation (3 min) in 10 mM caffeine. Application of IP3 (arrow) under control conditions produced a strong response (green trace) that had a rapid upstroke (Tau50 = 12 ms) indicative of CICR and was regenerative with a period of ∼800 ms. The cellular response to IP3 was greatly suppressed in the presence of caffeine (orange trace). To focus on the genesis of these Ca2+ signals, we expanded the time scale of the first 40 ms of the recordings (Fig. 8C) and inspected associated consecutive frames recorded at 240 Hz (Fig. 8, D and E). Before the caffeine treatment, the IP3-activated Ca2+ transient was generally seen to originate at a single location near the cell surface (Fig. 8D, image d) and to spread steadily, primarily in the longitudinal direction of the cell (starting at Fig. 8D, frame f), eventually developing large cellular Ca2+ transients with distributions (not shown) similar those in Fig. 7. Although the large cellular Ca2+ transients disappeared after long-term incubation with caffeine (Fig. 8A), IP3 was now seen to generate brief localized Ca2+ signals throughout the cytoplasm (Fig. 8E). In some cases, these Ca2+ signals could be seen clearly in consecutive frame (Fig. 8E, d and e, lower arrow), but more often a clear Ca2+ release event in a single frame was accompanied only by faint signals at the same location in the previous or following frame. Some sites appeared to produce repeated Ca2+ releases at intervals of ∼20 ms (e.g., Fig. 8E, e → j, top arrow, and d and e → h, bottom arrow). These results suggest that functional IP3R-gated Ca2+ stores are present throughout the cardiomyocyte. Similar results were seen in the two neonatal and three juvenile cells analyzed.
To test for the presence of IP3R Ca2+ stores in the perinuclear area, as has been described in cultured neonatal cardiomyocytes and adult cardiac cells (25), we performed experiments where we carefully adjusted the confocal plane to focus on the nucleus. Figure 9 shows whole cell changes in Ca2+ fluorescence in a representative juvenile cardiomyocyte in response to IP3 under control conditions (Fig. 9b) and after incubation in 40 μM ryanodine to deplete RyR-gated Ca2+ stores (Fig. 9c). The IP3 response under control conditions revealed a whole cell Ca2+ transient with a short rise time, whereas the IP3 response after incubation with ryanodine was of a smaller magnitude (ΔF/F0 = 3.05 for IP3 vs. 0.76 for IP3 in Ry) and developed much more slowly, which is consistent with the loss of RyR-mediated CICR. Fluorescence images at the peak of the cellular Ca2+ transients show that the Ca2+ signal invaded the entire cell before treatment with ryanodine (Fig. 9, image b), but afterward was largely limited to the nuclear region (Fig. 9, image c). Subsequent exposure to caffeine produced no response (data not shown). Similar results were seen in five cardiomyocytes. These findings indicate that developing cardiomyocytes have substantial RyR-insensitive IP3R-gated Ca2+ stores in the perinuclear area.
Fig. 9.
IP3-mediated perinuclear Ca2+ release in a permeabilized juvenile cardiomyocyte. Representative cellular Ca2+ transients (ΔF/F0) and fluorescence images (a–c) evoked by extracellular application of 20 μM IP3 before (left) and after (right) 5-min incubation in 40 μM ryanodine. The normalized fluorescence images were recorded at 240 frames/s before activation (a) and at the peak of the IP3-activated responses, which normally invaded the entire cell (b) but were confined to the nuclear region after ryanodine treatment (c).
DISCUSSION
Here we have demonstrated that freshly dissociated cardiomyocytes from neonatal and juvenile rats have functional IP3R-gated Ca2+ stores with kinetics and magnitudes that differ significantly from those of the RyR-gated Ca2+ releases (Figs. 1–4). The IP3-mediated Ca2+ release is suppressed, not only by blocking the IP3R with 2-APB or substituting L- for D-IP3 (Fig. 5) but also by depleting the RyR-gated Ca2+ stores with caffeine or Ry or blocking the RyRs with tetracaine (Fig. 6). As discussed below, the kinetics, magnitudes, and subcellular distributions of the Ca2+ signals suggest that the primary subsarcolemmal IP3-mediated Ca2+ release in both age groups is capable of activating local RyRs to produce a secondary CICR, even in the presence of high concentrations of fluorescent (Fluo-4) and nonfluorescent (EGTA) Ca2+ buffers (Figs. 3, 4, and 7). Comparisons of the RyR- and IP3R-gated Ca2+ releases during development show that both release systems are upregulated from the neonatal (days 1 to 2) to the juvenile (days 8-10; Fig. 6B) stage but that the IP3-mediated response with age overall changes little when normalized relative to CICR (Fig. 7C). Spatial analysis revealed Ry-insensitive IP3-mediated Ca2+ signals that are present throughout the cells at central (Fig. 8) and perinuclear (Fig. 9) locations but are most prominent in subsarcolemmal regions (Figs. 1–5 and 7) where they evoke CICR (Figs. 3, 4, and 6) and significant inward INaCa (Fig. 3). Overall, our data indicate that developing cardiomyocytes have an intermediate Ca2+ signaling phenotype between the early postnatal and adult cardiomyocytes showing vestigial IP3R- gated Ca2+ signaling in both atrial and ventricular myocytes capable of directly triggering CICR.
RyR-gated Ca2+ release in postnatal developing cardiomyocytes.
Analysis of the caffeine-activated Ca2+ release pools in voltage-clamped, Ca2+ buffered cells suggests enhancement of the size and kinetics of RyR-gated Ca2+ stores during transition from neonatal to juvenile cardiomyocytes (Fig. 1). In permeabilized myocytes this enhancement was nearly fourfold (Fig. 6). The upregulation of the size of the RyR-gated Ca2+ stores is consistent with previous studies of developing cardiomyocytes (15, 45). The faster kinetics in juvenile versus neonatal cells reported here (Fig. 1D) may reflect an altered sensitivity or distribution of the RyRs (10).
Characterization of IP3R-gated Ca2+ release in postnatal developing cardiomyocytes.
Although other cellular studies of cardiac development have used cultured cardiomyocytes, to our knowledge this is the first study where IP3R-gated Ca2+ signals were studied in acutely isolated cells. We pursued this strategy because cultured cardiomyocytes have been known to form spontaneously beating interconnected monolayers and drift to heterogeneous populations with variable functional and electrophysiological characteristics (39), dedifferentiation of cardiac structure (27, 38), and altered expression of IP3Rs (58) and other Ca2+ signaling proteins (48), all of which tend to complicate the interpretation of results and compromise physiological relevance. Nevertheless, it should also be recognized that, even without subsequent culturing, the enzymatic dispersion of cells appears to cause marked changes in the phosphoinositol-signaling pathways (57, 58), and it may be asked whether permeabilized cells may be better suited than dialyzed voltage-clamped cells to evaluate IP3-signaling by excluding confounding factors resulting from heterogeneity or enzymatic degradation of sarcolemmal ATP- and ET-1 receptors.
Application of ATP and ET-1 in voltage-clamped, Ca2+-buffered cells (Figs. 2 and 3) and IP3 in permeabilized cells (Figs. 4–9) produced results, ranging from a rise in the basal [Ca2+]i level to Ca2+ waves and transients throughout the cells at subsarcolemmal, central (Fig. 8), and perinuclear (Fig. 9) locations. Both voltage-clamp (Fig. 3) and permeabilization (Fig. 7) experiments revealed IP3-induced Ca2+ transients that followed a subsarcolemmal activation pathway. Although the IP3R-gated Ca2+ signals were upregulated with age (Figs. 2 and 6), the magnitude of these responses was found to depend on prior exposure to compounds that targeted the RyR-gated Ca2+ stores (Figs. 6 and 8), indicating that the two types of Ca2+ signal are interdependent.
Interplay between RyR- and IP3R-gated Ca2+ stores.
The reduction in the IP3-mediated Ca2+ signals following prolonged exposure to caffeine (Fig. 6) suggests that Ca2+ release via IP3R may stimulate CICR from nearby, functionally distinct, RyR-gated Ca2+ stores. Alternatively, some subcellular Ca2+ storage compartments may be gated by both Ry- and IP3Rs. The first possibility is consistent with our observations that the IP3-mediated Ca2+ signals 1) generally displayed an initial slow rise that is followed by a fast caffeine-sensitive spike (Figs. 3A, 4, 6A, and 8); 2) propagated slowly under the membrane (Figs. 7 and 8); and 3) were suppressed equally by caffeine and Ry, both of which deplete the RyR-gated Ca2+ pools, and by tetracaine (Fig. 6), which is known to enhance the SR Ca2+ content by stabilizing RyRs in the closed state (13, 19, 37, 46). In addition, CICR is a promiscuous process that can be initiated by diverse Ca2+ triggers including Ca2+ channels within the same t-SR junctions, reverse mode Ca2+ transport by NCX (26), T-type Ca2+ current (47), or photolysis of caged Ca2+ (29), and, therefore, probably also to the IP3-induced Ca2+ signals. Our use of moderate and high concentrations of Ca2+ buffers was, in part, intended to reduce the free diffusion distance of Ca2+ (1) and thereby intercept the IP3R-gated Ca2+ signals before they could trigger CICR. In this regard, we may have been successful in limiting the spread of CICR to the interior of the cells, but it would appear that higher concentrations of fast Ca2+ buffers, in combination with ultrastructural studies, would be required to quantify distances between IP3- and RyRs.
On the other hand, the alternative possibility that some IP3-gated Ca2+ pools may be partially emptied by prior exposure to caffeine does not necessarily require a high degree of colocalization of the two receptor types, as seen in adult atrial cells (31), only equilibration of Ca2+ between the subcompartments they gate. Such transfer of Ca2+ has been observed between SR and perinuclear stores, but it notably only occurs on the time scale of minutes (59). Finally, the open probability of IP3Rs is steeply dependent on Ca2+ in the range from 10 to 100 nM (40), but our buffering of [Ca2+]i at 100 nM probably minimized such effects. Overall, present and published results favor the interpretation that the initial IP3-mediated Ca2+ release is boosted by local CICR via RyRs.
Spatial profile of IP3R-gated Ca2+ signaling.
In highly Ca2+-buffered cells, application of ATP or ET-1 (Figs. 2 and 3) generated increases in the cytosolic Ca2+ at the cell periphery that, in juvenile cells, infrequently triggered global Ca2+ transients. In permeabilized cells, IP3 produced RyR-sensitive global Ca2+ transients that originated in subsarcolemmal regions and propagated to different degrees into the cell interior (Figs. 7 and 8). These IP3-activated Ca2+ transients in neonatal and juvenile cardiomyocytes have spatial Ca2+ profiles similar to those of adult atrial cells (21, 28, 31, 32) where they may be explained by the existence of two regularly arranged populations of atrial RyRs, of which those near the cell surface colocalize with IP3R in the junctional SR (28, 31). Our caffeine-activated Ca2+ profiles (Figs. 1, 3, 6, and 9) suggest that functional RyRs are uniformly distributed throughout the cell in the developing cardiomyocytes. However, mapping of IP3-activated CICR pathways in either voltage-clamped (Fig. 3) or permeabilized (Fig. 7) cells suggests that a similar spatial disconnect exists between the sarcolemma and the interior SR, as that found in atrial cardiomyocytes (32). The presence of functional IP3Rs throughout the cell (Fig. 8E), in combination with the predominant subsarcolemmal localization of IP3R-gated Ca2+ signals (Figs. 2–5 and 7), suggests that those in the interior may be relatively few or spatially dissociated from the centrally located RyRs.
Thus, in developing cardiomyocytes, IP3Rs may have a closer spatial proximity to RyRs in the peripheral versus central and nuclear regions. The general absence of differences in RyR- and IP3R-gated Ca2+ signaling between atrial and ventricular cardiomyocytes in the two age groups (Fig. 6) may indicate that developing cardiomyocytes are physiologically similar to atrial cardiomyocytes, with the distinctive ventricular physiology becoming more apparent after postnatal day 10. The finding that IP3-triggered Ca2+ signals of juveniles are larger in ventricular than in atrial myocytes (Fig. 6B) is surprising considering the importance of IP3-mediated signaling in adult atrial cells (32).
Role of IP3R-gated Ca2+ signaling in postnatal cardiomyocytes.
There is evidence that IP3-dependent Ca2+ release can exert a positive inotropic effect in adult atrial and ventricular cells (8, 62), but it is generally found to be minor in nondiseased tissue, especially the ventricle. In our voltage-clamped and permeabilized cells, ATP- or IP3-activated Ca2+ transients suggest that IP3 is capable of triggering Ca2+ transients independent of depolarization of the membrane. In addition, the finding that IP3-triggered Ca2+ transients that were suppressed in the presence of caffeine, ryanodine, and tetracaine implicates a role for IP3 in CICR in developing postnatal cardiomyocytes, where the resulting inward NCX current (Fig. 3) may contribute to membrane depolarization, as seen in pacing embryonic heart cells (23, 43) and in the genesis of arrhythmias (25).
The observation of a Ry-insensitive perinuclear IP3-induced Ca2+ signal (Fig. 9) is consistent with previous studies (20, 22, 30, 60) and may implicate Ca2+-dependent nuclear transcription factors (25). Similarly, it has been suggested that the Ca2+ that is released in response to IP3 may be taken up preferentially by mitochondria that thereby influence the spread of the Ca2+ signals (32) and are stimulated to synthesize ATP (17, 22, 24). These observations suggest that neurohumoral regulation of IP3 production could affect the inotropic, chronotropic, and metabolic states of developing cardiomyocytes.
The prominent role of the IP3-signaling pathways in cardiac diseases is generally characterized by activation of the renin-angiotensin and endothelin, enhanced synthesis of IP3, and upregulation of IP3Rs (25). In the present context, it may be relevant to emphasize the unexpected finding that IP3 in juvenile rat ventricular cardiomyocytes generates strong subsarcolemmal Ca2+ signals (Figs. 2, 3, 5, and 7), with the distribution typically seen in atrial cells (21, 28, 31, 32) where upregulation of IP3R is associated with the relatively common human condition of atrial fibrillation (5, 61).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute RO1-HL-16152 (to M. Morad).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol 108: 435–454, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artman M, Ichikawa H, Avkiran M, Coetzee WA. Na+/Ca2+ exchange current density in cardiac myocytes from rabbits and guinea pigs during postnatal development. Am J Physiol Heart Circ Physiol 268: H1714–H1722, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Callewaert G, Cleemann L, Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol Cell Physiol 257: C147–C152, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Cao K, Xia X, Shan Q, Chen Z, Chen X, Huang Y. Changes of sarcoplamic reticular Ca2+-ATPase and IP3-I receptor mRNA expression in patients with atrial fibrillation. Chin Med J (Engl) 115: 664–667, 2002 [PubMed] [Google Scholar]
- 6.Cleemann L, Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol 432: 283–312, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res 78: 15–25, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 294: H596–H604, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Endo M, Titazawa T. E-C coupling studies in skinned cardiac fibres. In: Biophysical Aspects of Cardiac Muscle, edited by Morad M. New York: Academic, 1978, p. 307–327 [Google Scholar]
- 10.Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Mejia-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol 286: H971–H978, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gomez JP, Potreau D, Raymond G. Intracellular calcium transients from newborn rat cardiomyocytes in primary culture. Cell Calcium 15: 265–275, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Gorza L, Vettore S, Tessaro A, Sorrentino V, Vitadello M. Regional and age-related differences in mRNA composition of intracellular Ca2+-release channels of rat cardiac myocytes. J Mol Cell Cardiol 29: 1023–1036, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Gyorke S, Lukyanenko V, Gyorke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol 500: 297–309, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddock PS, Artman M, Coetzee WA. Influence of postnatal changes in action potential duration on Na-Ca exchange in rabbit ventricular myocytes. Pflügers Arch 435: 789–795, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, Jafri MS, Artman M. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res 85: 415–427, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Hansford RG. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol 102: 1–72, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Hatem SN, Sweeten T, Vetter V, Morad M. Evidence for presence of Ca2+ channel-gated Ca2+ stores in neonatal human atrial myocytes. Am J Physiol Heart Circ Physiol 268: H1195–H1201, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol 48: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose M, Stuyvers B, Dun W, Ter Keurs H, Boyden PA. Wide long lasting perinuclear Ca2+ release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cells. J Mol Cell Cardiol 45: 176–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaconi M, Bony C, Richards SM, Terzic A, Arnaudeau S, Vassort G, Puceat M. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca2+ spiking. Mol Biol Cell 11: 1845–1858, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol 581: 1113–1127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsuragi T, Sato C, Usune S, Ueno S, Segawa M, Migita K. Caffeine-inducible ATP release is mediated by Ca2+-signal transducing system from the endoplasmic reticulum to mitochondria. Naunyn Schmiedebergs Arch Pharmacol 378: 93–101, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leblanc N, Hume JR. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science 248: 372–376, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Lipp P, Huser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol 497: 589–597, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol 10: 939–942, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Lipp P, Niggli E. Fundamental calcium release events revealed by two-photon excitation photolysis of caged calcium in Guinea-pig cardiac myocytes. J Physiol 508: 801–809, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo D, Yang D, Lan X, Li K, Li X, Chen J, Zhang Y, Xiao RP, Han Q, Cheng H. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium 43: 165–174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li WH, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol 541: 395–409, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117: 6327–6337, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Mahony L, Jones LR. Developmental changes in cardiac sarcoplasmic reticulum in sheep. J Biol Chem 261: 15257–15265, 1986 [PubMed] [Google Scholar]
- 34.Masuda H, Sumii K, Sperelakis N. Long openings of calcium channels in fetal rat ventricular cardiomyocytes. Pflügers Arch 429: 595–597, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Mery A, Aimond F, Menard C, Mikoshiba K, Michalak M, Puceat M. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Mol Biol Cell 16: 2414–2423, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi T, Seguchi M, Takao A. Development of the myocardial contractile system. Experientia 44: 936–944, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol 502: 471–479, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poindexter BJ, Smith JR, Buja LM, Bick RJ. Calcium signaling mechanisms in dedifferentiated cardiac myocytes: comparison with neonatal and adult cardiomyocytes. Cell Calcium 30: 373–382, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Pollack PS, Carson NL, Nuss HB, Marino TA, Houser SR. Mechanical properties of adult feline ventricular myocytes in culture. Am J Physiol Heart Circ Physiol 260: H234–H241, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J 75: 834–839, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter H, Henderson SA, Han T, Mottino GA, Frank JS, Ross RS, Goldhaber JI, Philipson KD. Cardiac excitation-contraction coupling in the absence of Na+-Ca2+ exchange. Cell Calcium 34: 19–26, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Rosemblit N, Moschella MC, Ondriasa E, Gutstein DE, Ondrias K, Marks AR. Intracellular calcium release channel expression during embryogenesis. Dev Biol 206: 163–177, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J Gen Physiol 130: 133–144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275: 39193–39199, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res 58: 535–548, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Sipido KR. Efficiency of L-type Ca2+ current compared to reverse mode Na/Ca exchange or T-type Ca2+ current as trigger for Ca2+ release from the sarcoplasmic reticulum. Ann N Y Acad Sci 853: 357–360, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Snopko RM, Aromolaran AS, Karko KL, Ramos-Franco J, Blatter LA, Mejia-Alvarez R. Cell culture modifies Ca2+ signaling during excitation-contraction coupling in neonate cardiac myocytes. Cell Calcium 41: 13–25, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ′Ca2+ spikes′ in rat cardiac myocytes. J Physiol 512: 677–691, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100: 15235–15240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka H, Shigenobu K. Effect of ryanodine on neonatal and adult rat heart: developmental increase in sarcoplasmic reticulum function. J Mol Cell Cardiol 21: 1305–1313, 1989 [DOI] [PubMed] [Google Scholar]
- 52.Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81: 767–806, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Viatchenko-Karpinski S, Fleischmann BK, Liu Q, Sauer H, Gryshchenko O, Ji GJ, Hescheler J. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci USA 96: 8259–8264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vigne P, Breittmayer JP, Marsault R, Frelin C. Endothelin mobilizes Ca2+ from a caffeine- and ryanodine-insensitive intracellular pool in rat atrial cells. J Biol Chem 265: 6782–6787, 1990 [PubMed] [Google Scholar]
- 55.Wibo M, Bravo G, Godfraind T. Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res 68: 662–673, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Woo SH, Cleemann L, Morad M. Diversity of atrial local Ca2+ signalling: evidence from 2-D confocal imaging in Ca2+-buffered rat atrial myocytes. J Physiol 567: 905–921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodcock EA, Lambert KA. Acute effects of cell isolation on InsP profiles in adult rat cardiomyocytes. J Mol Cell Cardiol 29: 3275–3283, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Woodcock EA, Tanner JK, Fullerton M, Kuraja IJ. Different pathways of inositol phosphate metabolism in intact neonatal rat hearts and isolated cardiomyocytes. Biochem J 281: 683–688, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99: 283–291, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada J, Ohkusa T, Nao T, Ueyama T, Yano M, Kobayashi S, Hamano K, Esato K, Matsuzaki M. [Up-regulation of inositol 1, 4, 5-trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation]. J Cardiol 39: 57–58, 2002 [PubMed] [Google Scholar]
- 62.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555: 607–615, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zima AV, Kockskamper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol 550: 765–783, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]