Abstract
In the developing fetus, cerebral artery (CA) contractility demonstrates significant functional differences from that of the adult. This may be a consequence of differential activities of α1-adrenergic receptor (α1-AR) subtypes. Thus we tested the hypothesis that maturational differences in adrenergic-mediated CA contractility are, in part, a consequence of differential expression and/or activities of α1-AR subtypes. In CA from fetal (∼140 days) and nonpregnant adult sheep, we used wire myography and imaging, with simultaneous measurement of tension and intracellular Ca2+ concentration ([Ca2+]i), radioimmunoassay, and Western immunoblots to examine phenylephrine (Phe)-induced contractile responses. The α1A-AR antagonists (5-MU and WB-4101) completely inhibited Phe-induced contraction in adult but not fetal CA; however, [Ca2+]i increase was reduced significantly in both age groups. The α1D-AR antagonist (BMY-7378) blocked both Phe-induced contractions and Ca2+ responses to a significantly greater extent in adult compared with fetal CA. In both age groups, inhibition of α1A-AR and α1B-AR, but not α1D-AR, significantly reduced inositol 1,4,5-trisphosphate responses to Phe. Western immunoblots demonstrated that the α1-AR subtype expression was only ∼20% in fetal CA compared with the adult. Moreover, in fetal CA, the α1D-AR was expressed significantly greater than the other two subtypes. Also, in fetal but not adult CA, Phe induced a significant increase in activated ERK1/2; this increase in phosphorylated ERK was blocked by α1B-AR (CEC) and α1D-AR (BMY-7378) inhibitors, but not by α1A-AR inhibitors (5-MU or WB-4101). In conclusion, in the fetal CA, α1B-AR and α1D-AR subtypes play a key role in contractile response as well as in ERK activation. We speculate that in fetal CA α1B-AR and α1D-AR subtypes may be a critical factor associated with cerebrovascular growth and function.
Keywords: vascular smooth muscle, development, maturation
the adrenergic pathway is one of the main regulators of cerebrovascular tone and cerebral blood flow (CBF) (33), mediating the majority of its effects through α-adrenergic receptors (α-AR). In previous studies in fetal and adult ovine cerebral artery (CA), we have shown that α1-AR play a key role in contractile response (27, 28). In addition, α2-AR appear to be chiefly prejunctional, with the fetal CA having a significant component of postjunctional α2-AR (1). However, in the cerebral vasculature of most species studied several reports indicate that α1-AR are also abundant (6, 21, 41, 48), which suggests that cerebral vascular tone, and thus CBF, is regulated, in part, by activation/inhibition of these receptors. α1-AR belong to the G protein-coupled receptor (GPCR) superfamily that bind specifically to norepinephrine (NE) and epinephrine, and thus mediate sympathetic nervous system responses in vascular smooth muscle cells (SMCs). α1-AR stimulation leads to increase in inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], which in turn increases intracellular Ca2+ concentration ([Ca2+]i) and acts as a major signaling pathway (Ca2+-dependent mechanism) mediating vascular contraction (5, 27). Other signaling pathways coupled to α1-AR include activation of protein kinase C (PKC) and extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Ca2+ sensitization/independent mechanism) mediating vascular contraction (4, 22). Our previous studies (51, 52) have shown that α1-AR-mediated contraction of CA occurs by both Ca2+-dependent and Ca2+-independent mechanisms. We also have shown that in ovine CA adrenergic-mediated vasoconstrictor responses differ significantly in fetus and adult (27, 28, 51, 52). Recently, we have shown (8) significant changes in the Ca2+ sensitization pathway (PKC and ERK1/2) with developmental maturation from fetus to adult.
Radioligand binding and molecular cloning in several species have demonstrated that the α1-AR family has three structurally distinct subtypes (α1A, α1B, α1D), which are widely expressed in tissues and have different pharmacological properties and amino acid sequences (36, 53). Although the three α1-adrenoceptor subtypes have been reported in various cell types, little is known about their expression, physiological functions, or downstream pathways in CA (20, 22, 53). Of note, in fetal and adult CA we have demonstrated differing roles of NE (α1- and α2-AR agonist) and phenylephrine (Phe; α1-AR agonist) in contractility and downstream activation of PKC and ERKs (8, 29, 51, 52). Thus the question arises as to the role of the specific α1-AR subtypes and the role of downstream effectors in α-adrenergic-mediated contraction in CA, and their changes with maturation. In the present study, we tested the hypothesis that in CA differential expression and activation of α1-AR subtypes (α1A-, α1B-, and α1D-AR), in fetus compared with adult differentially activate downstream signaling pathway responsible for contractile response and/or growth and development. The present study provides a deeper understanding of CBF regulation during fetal and adult life and provides more specific targets for treatment of conditions such as dysregulation of CBF.
METHODS
Experimental animals and tissues.
All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the “Guiding Principles in the Care and Use of Animals” approved by the Council of the American Physiological Society and were approved by the Animal Care and Use Committee of Loma Linda University. For these studies, we used CA from near-term fetal (∼140 days) and nonpregnant adult (18–24 mo) sheep obtained from Nebeker Ranch (Lancaster, CA). For each experiment five or more animals were used; in case of fetal twins n was counted as 1. Pregnant and nonpregnant ewes were anesthetized with thiopental sodium (10 mg/kg iv), and anesthesia was maintained with inhalation of 1% isoflurane in oxygen throughout surgery. After the fetus was delivered by hysterotomy, the fetuses and ewes were killed with an overdose of the proprietary euthanasia solution Euthasol (pentobarbital sodium 100 mg/kg and phenytoin sodium 10 mg/kg; Virbac, Fort Worth, TX). Studies were performed in isolated cerebral vessels cleaned of adipose and connective tissue. To avoid the complications of endothelium-mediated effects, we removed the endothelium by carefully inserting a small wire three times, as previously described (26, 28). The vessels were used immediately for experiments.
Simultaneous measurement of [Ca2+]i and tension.
We have described this technique in several reports (8, 24–26, 29). Briefly, fetal or adult middle CA rings of 2-mm length were incubated at 25°C for 40 min with the acetoxymethyl ester of fura-2 (fura-2 AM; Molecular Probes, Eugene, OR), a fluorescent Ca2+ indicator (24, 25, 26). After loading the dye, we mounted the arterial segments in a Jasco CAF-110, an intracellular Ca2+ analyzer (Jasco, Easton, MD). We then stabilized CA rings at 38°C for 40 min in an oxygenated standard Krebs solution. The bath chambers were continuously bubbled with 95% O2-5% CO2, and all experiments were conducted at 38°C (core temperature of sheep). After stabilization, on the basis of our previous studies, the optimum resting tension was 0.6 g for fetal and 0.7 g for adult CA, because at these tensions the contractility response to 125 mM KCl was maximum (28, 39). We then stimulated the isolated CA rings from fetal and adult sheep with 125 mM KCl, and after the contractile force plateaued 100 μM acetylcholine (ACh) was applied. The arteries that relaxed in the presence of ACh were discarded from the study, allowing for the evaluation of the relative quantity of contractile smooth muscle (34) and to determine the status of endothelium disruption (10). The contractile force due to 125 mM KCl was measured as grams (g) of tension, before stimulation of the test compound for each arterial segment, and was used to normalize the arterial contraction with other agonists and antagonists for variation in the smooth muscle mass (26). For all vessels we evaluated the contractile response for tension and fluorescence ratio by measuring the maximum peak height and expressing it in both absolute terms and as percent Kmax (a measure of “efficacy”), and calculated pD2 (negative logarithm of EC50, or half-maximal concentration, for NE or Phe and an index of tissue “sensitivity” or “potency”) (12, 26). As we have previously reported, although in absolute terms the maximal values of fetal CA K+- and NE-induced tension are 20–30% less than those of the adult (25, 26), expressing these in terms of Kmax helps to normalize the data and does not alter the interpretation of the results. In arteries used for response to a given agonist, i.e., NE (a nonselective agonist for both α1- and α2-AR) or Phe (a selective agonist for α1-AR) and/or selective α1-AR (subtype) antagonists, we added the antagonist for 20 min before administration of NE or Phe. Unless otherwise noted, all chemicals were obtained from Sigma Chemical (St. Louis, MO).
Role of α1-AR subtype blockers.
Three pharmacologically distinct α1-AR subtypes can be distinguished with competitive antagonists and alkylating agents. The α1A-AR is shown to be inhibited by 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride (WB-4101) (38), phentolamine (14), and 5-methyl-6[[3-[4-(2-methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil (5-MU). Moreover, WB-4101 is a noncompetitive antagonist, whereas 5-MU is a competitive antagonist and is more selective than other pharmacological agents for α1A-AR (9, 11, 17, 18). Furthermore, studies have demonstrated that the α1B-AR subtype is selectively inhibited by chlorethylclonidine (CEC) (14, 15, 35, 37). Other studies have established that 8-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-8-azaspiro(4,5)decane-7,9-dione (BMY-7378) selectively inhibits α1D-AR subtype (7, 23, 40, 42). All of these antagonists are well characterized, with known IC50 for vascular adrenergic receptors. Therefore, on the basis of these studies, we chose 5-MU and WB-4101 for α1A-AR, CEC for α1B-AR, and BMY-7378 for α1D-AR inhibition in the present study. First, using the fixed agonist, we performed dose-response curves with specific α1-AR subtype antagonists to determine the pIC50 in sheep CA (Table 1). Subsequently, to determine the role of the several subtypes in Phe-induced tension and [Ca2+]i, and the extent to which the selective α1-AR blockers inhibited the responses to Phe, we first measured control vascular tension and [Ca2+]i responses to 10−9–10−2 M Phe. Then after washout and 40 min reequilibration, we repeated the Phe dose-response curve in the presence of an appropriate concentration one of the α1-AR subtype blockers. For each agent we determined the dissociation constant KB.
Table 1.
pIC50 values of α1-adrenergic receptor subtype inhibitors
| IC50 Values |
|||
|---|---|---|---|
| Inhibitor | Parameter Measured | Adult | Fetus |
| 5-MU | Contractility | 7.4 ± 0.1 | 7.7 ± 0.1 |
| Ca2+ signaling | 7.3 ± 0.1 | 7.7 ± 0.1 | |
| WB-4101 | Contractility | 6.9 ± 0.1 | 7.2 ± 0.1 |
| Ca2+ signaling | 8.1 ± 0.1 | 6.7 ± 0.1 | |
| CEC | Contractility | 7.3 ± 0.1 | 6.4 ± 0.1 |
| Ca2+ signaling | 8.1 ± 0.1 | 6.4 ± 0.1 | |
| BMY-7378 | Contractility | 6.5 ± 0.1 | 6.8 ± 0.1 |
| Ca2+ signaling | 6.6 ± 0.1 | 7.1 ± 0.1 | |
Values are means ± SE. 5-MU, 5-methyl-6[[3-[4-(2-methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil; WB-4101, 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride; CEC, chlorethylclonidine; BMY-7378, 8-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-8-azaspiro(4,5)decane-7,9-dione; pIC50, negative logarithm of 50% inhibition concentration values. (n = 4 to 5.) See methods for details.
Ins(1,4,5)P3 quantification.
From each fetal and adult animal, cleaned CA was cut into segments weighing ∼20 mg (wet wt), placed in Krebs buffer, and bubbled with 95% O2-5% CO2 for 0.5 h. We exposed the arteries to various treatments outlined above (Phe stimulation with selective α1-AR subtype inhibition) at 38°C according to the protocol. Reactions were terminated by freezing in liquid N2, the frozen samples were homogenized in 2 ml of iced 16% trichloroacetic acid with a glass grinder and centrifuged for 30 min at 1,500 g, the supernatant was transferred, and the pellet was resuspended in 1 M NaOH for protein measurements (2). The supernatant was washed for 30 s with H2O-saturated ether (5× volume, 2 times). After ether evaporation, we determined Ins(1,4,5)P3 mass by competitive ligand binding assay in which a radioactive ligand competes with a nonradioactive ligand for a fixed number of receptor binding sites, as we have described previously (27). [3H]Ins(1,4,5)P3 assay kits were obtained from DuPont (Boston, MA); intra- and interassay coefficients of variation were 7% and 9%, respectively.
Immunoblot of α1-AR subtypes.
Isolated CA from fetal and adult sheep were cleaned of adventitia, and endothelium was denuded. These arteries were homogenized with a tissue grinder in ice-cold cell lysis buffer (Cell Signaling Technology, Danvers, MA) as we have described previously (29, 51). Protein concentrations were measured with a protein assay kit (Bio-Rad Laboratories, Hercules, CA), and bovine serum albumin (BSA) was used as a reference protein (2). The Mini Trans-Blot Electrophoretic Transfer Cell System (Bio-Rad Laboratories) was used to transfer proteins from the gel to a nitrocellulose membrane at 100 V for 3 h. We then performed an overnight incubation of subtype-specific primary antibodies (1:500 dilution) for α1A-, α1B-, and α1D-AR (Santa Cruz Biotechnology, Santa Cruz, CA). We used α-actin as an internal control for equal protein loading as well as the blocking peptide for each subtype-specific antibody as a negative control (Santa Cruz Biotechnology). The membrane was then incubated in chemiluminescence luminol reagent (Pierce, Rockford, IL) for 1 min, and the protein band was detected with the Alpha Innotech Chemiluminescent imaging system (San Leandro, CA). After protein detection, the membrane was striped with strip buffer (Pierce) and immunoblotted by anti-α-actin antibody.
Immunoblotting of ERK1/2.
After tension measurement, fetal and adult sheep CA were frozen rapidly in liquid N2. Frozen samples were homogenized in the 1× cell lysis buffer (Cell Signaling Technology) containing 1× phosphatase inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride. Nuclei and debris were pelleted by centrifugation at 1,000 g for 10 min. The supernatant was collected and stored at −80°C. SDS-gel and Western immunoblots were performed, as mentioned above for α1-AR immunoblot by using phospho- and total-ERK1/2 antibody (Cell Signaling Technology), as we have described previously (8).
Statistical analysis.
All values were calculated as means ± SE. In all cases, n values refer to the number of vessels (which corresponds to the number of animals and is included in Figs. 1–6) for a particular study. Because of the nature of these studies, we used several statistical tests to examine for significant differences. For testing differences between two groups, we used a simple unpaired Student's t-test. For multiple comparisons, we used one-way and two-way analysis of variance (ANOVA; vessel, age) coupled with Duncan's multiple range test. Where appropriate, we used ANOVA with repeated measures (Prism, GraphPad Software, La Jolla, CA). A P value of <0.05 was considered significant.
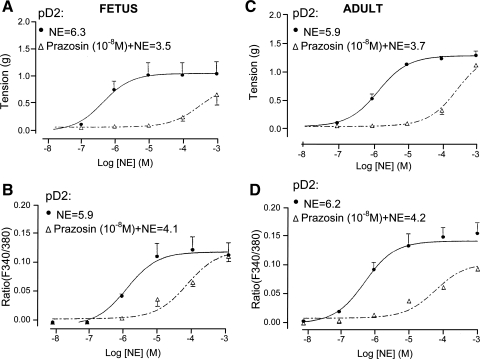
Fig. 1.
Norepinephrine (NE) dose-response relations for fetal and adult cerebral arteries (CA) in the presence or absence of the α1-adrenergic receptor (α1-AR) antagonist prazosin. A and C: vascular tension (g) for fetal CA (A) and adult CA (C) in response to NE under control conditions and after administration of 10−8 M prazosin (n = 4). B and D: fluorescence ratio (F340/380), i.e., intracellular Ca2+ concentration ([Ca2+]i), for fetal CA (B) and adult CA (D) in response to NE under control conditions and after addition of 10−8 M prazosin. pD2, negative logarithm of EC50. (n = 5 each.)
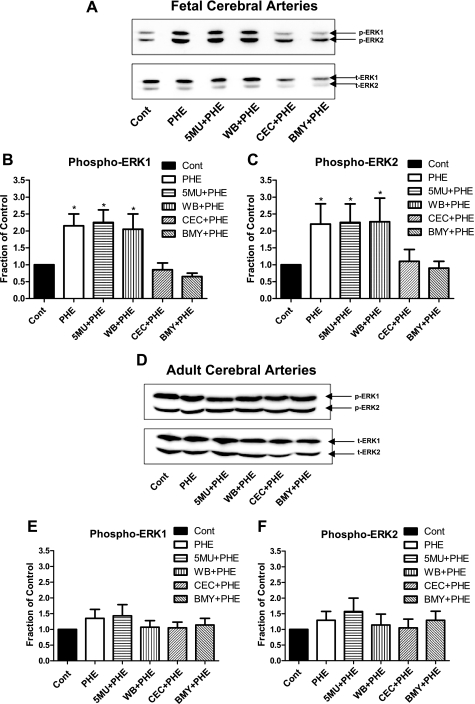
Fig. 6.
Western immunoblot and densitometric analysis of total (t) and phosphorylated (p) p44/p42 extracellular signal-regulated kinases (ERK1/2) in near-term fetal and adult CA. Intact CA were stimulated by exposure to 10−5 M Phe for 5 min in the absence or presence of the α1A-AR antagonists 5-MU (10−8 M) or WB-4104 (10−7 M), the α1B-AR antagonist CEC (10−5 M), or the α1D-AR antagonist BMY-7378 (10−7 M). A and D: bands for phosphorylated ERK1/2, total ERK1/2, and α-actin in fetal (A) and adult (D) CA. B and E: densitometric analysis of phosphorylated ERK1, p44, normalized to the α-actin band (n = 4; *P < 0.05, compared with control) in fetal (B) and adult (E) CA. C and F: densitometric analysis of phosphorylated ERK2, p42, normalized to the α-actin band (n = 4; *P < 0.05, compared with control) in fetal (C) and adult (F) CA.
RESULTS
Norepinephrine-induced CA contraction through α1-AR.
To establish the extent to which NE mediates its effects through α1-AR in both fetal and adult CA, we measured NE-induced contractile and [Ca2+]i responses in the presence and absence of the α1-AR antagonist prazosin (selective α1-AR blocker). As seen in Fig. 1, in the presence of prazosin (10−8 M), the pD2 values for NE responses of tension and fluorescence ratio for both adult and fetal vessels were significantly decreased by >1 log unit. Also, as shown in Table 2, in both fetal and adult CA, the dissociation constants (KB values) for prazosin-mediated inhibition of NE-induced vascular contraction and [Ca2+]i responses were similar, suggesting that both events were mediated by the α1-AR.
Table 2.
Dissociation constants (KB values) of antagonist inhibition
| Receptor | Drug | Response | Adult, M | Fetus, M |
|---|---|---|---|---|
| α1 | Prazosin (10−8 M) | Contraction | 6.4 × 10−10 | 1.1 × 10−10 |
| [Ca2+]i | 1.1 × 10−10 | 1.6 × 10−10 | ||
| α1A | 5-MU (10−8 M) | Contraction | 1.1 × 10−9 | 0.2 × 10−9 |
| [Ca2+]i | 1.1 × 10−9 | 0.7 × 10−9 | ||
| α1B | CEC (10−5 M) | Contraction | 5.3 × 10−10 | No significant inhibition |
| [Ca2+]i | 0.8 × 10−10 | |||
| α1D | BMY-7378 (10−7 M) | Contraction | 6.7 × 10−9 | No significant inhibition |
| [Ca2+]i | 25 × 10−9 |
[Ca2+]i, intracellular Ca2+ concentration. See methods for details.
Role of α1-AR subtypes in cerebrovascular contraction and [Ca2+]i responses.
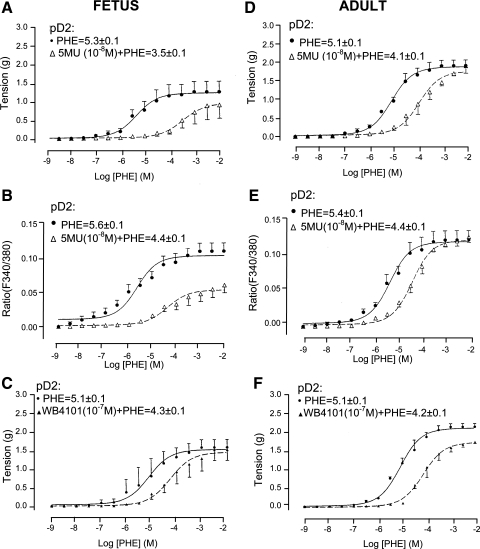
We measured the vascular tension and [Ca2+]i responses in the presence of selective inhibitors for each of these subtypes. We first conducted α1-AR selective antagonist dose-response curves with 5-MU, WB-4101, CEC, and BMY-7378 in the presence of 10−2 M Phe. Table 1 presents the pIC50 values of the several α1-AR subtype antagonists. We then quantified α1-AR selective agonist Phe (10−9–10−2 M)-induced contraction and [Ca2+]i responses in the presence and absence of α1A-AR antagonist 5-MU (10−8 M). As shown in Fig. 2, in the presence of 5-MU both fetal and adult CA showed a significant right shift of the Phe dose-response curve for contraction (Fig. 2, A and D), as well as increases in [Ca2+]i (Fig. 2, B and E; see Table 2 for KB values and Fig. 2 for pD2 values). This demonstrates that in both fetal and adult CA α1A-AR play a significant role in Phe-induced contractile response, acting through a Ca2+-dependent pathway. Moreover, 5-MU had a greater effect in fetal CA compared with that in adult CA. To confirm this possibility further, we used a different α1A-AR antagonist, WB-4101 (10−7 M), to test Phe-induced tension and [Ca2+]i responses in another series of CA. As shown in Fig. 2F for adult CA, the tension responses were similar to those in the presence of 5-MU (see Table 2 for KB values). For fetal CA (Fig. 2C), the KB value for the effect of WB-4101 on tension was similar to that of adult CA (Table 2).
Fig. 2.
Fetal and adult CA tension and [Ca2+]i responses to phenylephrine (Phe) under control conditions and in the presence of α1A-AR inhibition by 5-methyl-6[[3-[4-(2-methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil (5-MU, 10−8 M). A and D: vascular tensions (g) for fetal CA (A) and adult CA (D) in response to Phe alone and in the presence of 10−8 M 5-MU (n = 4). B and E: fluorescence ratio (F340/380), i.e., [Ca2+]i for fetal CA (B) and adult CA (E) in response to Phe alone or after 5-MU. C and F: fetal (C) and adult (F) CA tension responses to Phe under control conditions and in the presence of α1A-AR inhibition by 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride (WB-4101). (n = 4 to 5 each.)
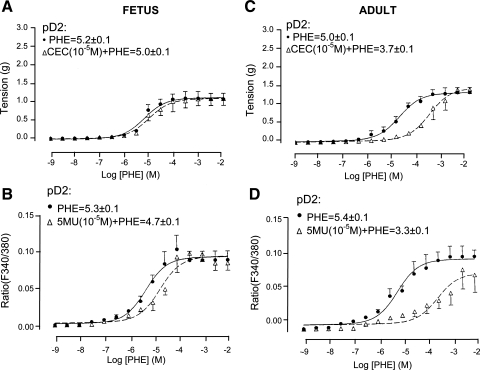
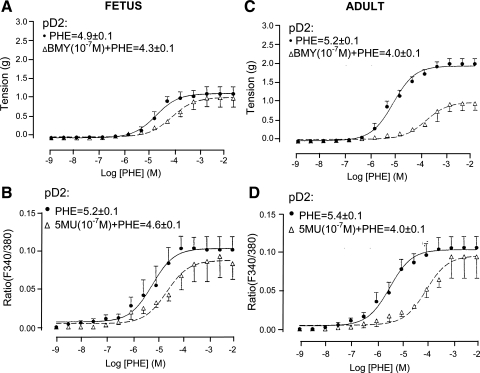
To examine the effect of α1B-AR inhibition on Phe concentration-response curves, we performed a Phe dose-response curve in the presence and absence of the α1B-AR antagonist CEC (10−5 M). In adult CA, in the presence of CEC (10−5 M) both tension (Fig. 3C) and [Ca2+]i (Fig. 3D) were significantly right-shifted. In contrast, in fetal CA 10−5 M CEC had no significant effect on the Phe-induced vascular tension (Fig. 3A) and only modestly right-shifted the Phe dose response for [Ca2+]i (Fig. 3B); see Table 2 for CEC KB values. Thus in fetal CA, α1B-AR has significantly less role in Phe-induced contractile response compared with adult CA.
Fig. 3.
Adult and fetal CA tension and [Ca2+]i responses to Phe under control conditions and in the presence of α1B-AR inhibition by chlorethylclonidine (CEC). A and C: vascular tensions (g) for fetal (A) and adult (C) CA in response to Phe alone and in the presence of 10−5 M CEC (n = 4 each). B and D: fluorescence ratio (F340/380) for fetal (B) and adult (D) in response to Phe alone or after 10−5 M CEC.
To determine the extent to which α1D-AR inhibition altered Phe-induced tension and [Ca2+]i responses, we measured these variables in response to increasing doses of Phe (10−9–10−2 M) in the absence or presence of the α1D-AR antagonist BMY-7378 (10−7 M). As shown in Fig. 4C, in response to increasing Phe alone, adult CA showed a typical increase in vascular tension. After α1D-AR inhibition, however, the maximal Phe-induced contractile response and [Ca2+]i was attenuated compared with control (P < 0.01 for each; Fig. 4, C and D). As shown in Fig. 4A, in response to increasing Phe alone, fetal CA showed a typical contraction. In the presence of α1D-AR antagonist BMY-7378, the Phe-induced contractile and [Ca2+]i responses were decreased, but significantly less so than those in adult (see Table 2 for KB values for BMY-7378).
Fig. 4.
Fetal and adult CA tension and [Ca2+]i responses to Phe under control conditions and in the presence of α1D-AR inhibition by 8-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl)-8-azaspiro(4,5)decane-7,9-dione (BMY-7378; BMY). A and C: vascular tension (g) for fetal (A) and adult (C) in response to Phe alone and in the presence of 10−7 M BMY-7378 (n = 4 each). B and D: fluorescence ratio (F340/380) for fetal (B) and adult (D) CA in response to Phe alone or after 10−7 M BMY-7378 (n = 4 each).
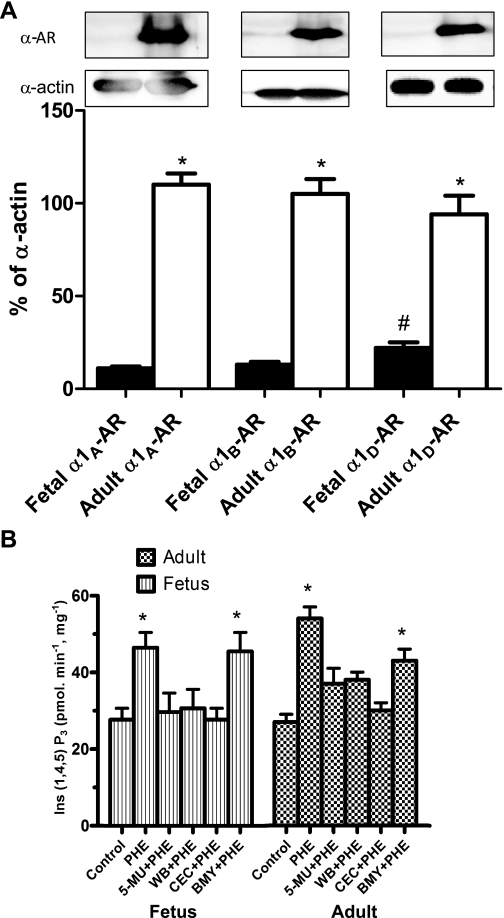
Protein abundance of α1-AR subtypes.
Figure 5A illustrates the α1-AR profiles from whole cell extracts that were subjected to Western immunoblot analysis using enhanced chemiluminescence substrate, as described in methods. As shown, adult ovine CA express relatively abundant amounts of each of the subtype α1A-, α1B-, and α1D-AR. In contrast, near-term fetal CA demonstrated low levels of the three α1-AR subtypes. Immunoblots of α-actin are shown as an internal control. Of interest, in fetal CA the α1D-AR subtype is expressed to a relatively greater degree than the other two subtypes (Fig. 5A).
Fig. 5.
A: α1-AR subtype profiles in fetal and adult CA. Note the relatively strong expression of the α1A-, α1B-, and α1D-AR proteins in adult CA. In contrast, in fetal CA note the very low levels of these proteins compared with the adult. Histograms show the densitometric analysis of the Western immunoblots. Data are means ± SE for 4 separate experiments. *P < 0.01 for fetus compared with adult. #P < 0.01 for fetal α1D-AR compared with α1A- and α1B-AR. B: inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] values in fetal and adult CA. Values are means ± SE; n = 3 for each group and parameter tested. *P < 0.01. WB, WB-4101.
α1-AR subtypes and Ins(1,4,5)P3 responses.
To examine the downstream signaling of the several α1-AR subtypes, we measured Phe-induced Ins(1,4,5)P3 levels (pmol·min−1·mg protein−1) in fetal and adult CA. As shown in Fig. 5B, in adult CA 3 × 10−5 M Phe stimulation resulted in a significant increase in Ins(1,4,5)P3 levels, being 54 ± 3 from a basal value of 27 ± 2 (P < 0.01). Similarly in fetal CA, Phe induced a significant increase in Ins(1,4,5)P3 levels, being 47 ± 4 from 28 ± 3 (P <0.01). In the presence of the α1A-AR antagonist 5-MU (10−8 M), Phe did not increase Ins(1,4,5)P3 levels significantly in either fetal or adult CA, which were 30 ± 5 and 37 ± 4, respectively. Similarly, in the presence of another α1A-AR antagonist, WB-4101 (10−5 M), Phe did not induce significant Ins(1,4,5)P3 responses, levels being 38 ± 2 in the adult and 31 ± 5 in the fetus. These Ins(1,4,5)P3 levels were statistically similar to those of control (unstimulated) arteries. Additionally, in the presence of the specific α1B-AR antagonist CEC (10−5 M), the Phe-induced Ins(1,4,5)P3 values in adult and fetal CA did not differ significantly from control (30 ± 2 and 28 ± 3, respectively). Of interest, in both adult and fetal CA the selective α1D-AR antagonist BMY-7338 (10−5 M) caused significantly less inhibition of the Phe-induced Ins(1,4,5)P3 responses. In adult CA, Ins(1,4,5)P3 responses induced by Phe + BMY-7338 were significantly less than those induced by Phe alone, whereas in fetal CA Ins(1,4,5)P3 levels showed no significant difference from control (Fig. 5B).
α1-AR subtypes and ERK1/2 interaction.
Figure 6, A–C, show the levels of activated (phosphorylated) ERK1/2 in fetal CA in the absence or presence of the α1-AR subtype antagonists. In control experiments, the antagonists for α1A-AR (5-MU and WB-4104), for α1B-AR (CEC), and for α1D-AR (BMY-7378) alone had no significant effect on basal total or activated ERK1 and 2 levels (data not shown). In the fetal arteries, Phe stimulation (10−5 M) showed a significant (100%) increase in both phosphorylated ERK1 and 2 (Fig. 6A). Also, Phe in the presence of α1A-AR antagonists 5-MU (10−8 M) or WB-4104 (10−7 M) showed similar increases in phosphorylated ERK1/2 levels. In contrast, in the presence of CEC (10−5 M; α1B-AR antagonist) or BMY-7378 (10−7 M; α1D-AR antagonist), there was no significant Phe-induced phosphorylation of ERKs (Fig. 6). Figure 6, B and C, show the densitometric analysis of phosphorylated-p44 (ERK1) and phosphorylated-p42 (ERK2) in fetal CA normalized to the α-actin band (n = 4), which confirms these observations. By way of contrast, in adult CA (Fig. 6, D–F), Phe stimulation (10−5 M) showed no significant increase in activated ERKs. Also under basal conditions in the adult arteries, the antagonists 5-MU or WB-4101, CEC, and BMY-7378 showed no significant inhibition of activated ERK1/2 levels. In addition, in the adult vessels, none of the α1-AR subtype inhibitors significantly affected the activated ERK levels after Phe (10−5 M) stimulation (Fig. 6, D–F).
DISCUSSION
In the present study, we have demonstrated several important findings regarding the role of α1-AR subtypes in mediating contractile responses in fetal and adult CA. 1) Each of the three known α1-AR subtypes is present in fetal and adult CA. Moreover, in fetal CA all three subtypes are present in significantly lower amounts than in the adult. Nonetheless, in fetal CA, α1D-AR are present in significantly greater amounts than the other two subtypes. 2) In adult CA, each of the three subtypes mediates a Phe-induced contractile response. In fetal CA, in contrast, α1A-AR is the major subtype involved in Phe-induced contractility. 3) In both fetal and adult CA, Phe stimulates increased Ins(1,4,5)P3 responses with increased [Ca2+]i. Moreover, in both fetal and adult CA, α1A-AR and α1B-AR inhibition completely blocked these Ins(1,4,5)P3 responses. In contrast, in adult CA α1D-AR inhibition decreased the Ins(1,4,5)P3 response to a much lesser extent compared with those produced by α1A-AR and α1B-AR inhibition (P = 0.01). In fetal CA no inhibition of Ins(1,4,5)P3 responses was observed in the presence of α1D-AR inhibitor. 4) In fetal, but not adult, CA, Phe stimulates phosphorylation of ERK1/2, and this Phe-mediated ERK phosphorylation was inhibited by α1B-AR and α1D-AR blockers. These novel and important findings provide a deeper understanding of the role of adrenergic-mediated cerebral contractility, which may be important in the regulation of CBF. The findings of differential roles of the α1-AR subtypes may lead to development of specific therapeutic agents with fewer adverse effects during fetal life.
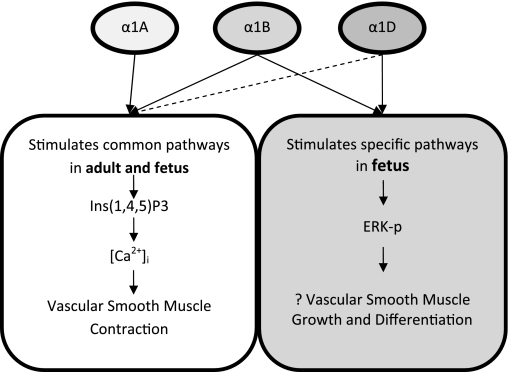
For a mechanistic overview, Fig. 7 presents a schema, based on both our present findings and other data (8), for the role of specific α1-AR subtype-mediated mechanisms in fetal and adult CA. In the fetus and the adult (Fig. 7, left), the present study supports the canonical pathway of Ca2+-dependent contractility, with the α1A- and α1B-AR subtypes stimulating Ins(1,4,5)P3 production to increase [Ca2+]i and activate myosin light chain (MLC) kinase (MLCK) and MLC20, thus stimulating vascular contraction by thick and thin filament interaction. Of importance, α1B-AR and α1D-AR function quite differently in the fetus (Fig. 7, right). In fetal CA, Phe stimulates the α1B-AR and α1D-AR subtypes, which in turn activate ERK1/2 by a mechanism as yet unclear. By activation of ERK directly (with or without involvement of other kinases), α1B-AR and α1D-AR in the fetus may play a critical role in vascular development by stimulating maturation and growth of SMCs. In addition, the α1B-AR subtype-mediated production of diacylglycerol (DAG) activates PKC, which leads to phosphorylation in MAPK and ERK1/2. Thus the several α1-AR subtypes function in a very different manner in the fetus compared with the adult. A grasp of these interactions, along with subtype-specific information, is essential for understanding the regulation of cerebrovascular tone and CBF. This is important, as the unique features of these subtypes may play a role in dysregulation of CBF in the fetus and premature newborn, as well as in the adult. Quite obviously, we yet have much to learn in regard to α1-AR subtypes and their changing role with maturation. A caveat of these studies concerns the relative selectivity of the purported pharmacological antagonists for the several α1-AR subtypes (53), because subtype-selective agents may not be overly selective and some interact with other adrenergic and nonadrenergic receptors. Both α1A-AR antagonists, 5-MU (11, 31) and WB-4101 (38), are relatively selective, although they differ in action. In turn, the alkylating agent CEC (16) and BMY-7378 (7) have been demonstrated to be quite selective antagonists for α1B-AR and α1D-AR, respectively.
Fig. 7.
Proposed signal transduction pathways for specific α1-AR subtype-mediated Ca2+-dependent and Ca2+-independent smooth muscle cell (SMC) contraction in adult and fetal CA. Left: α1A-AR and α1B-AR subtypes mediate production of Ins(1,4,5)P3, with the result of increasing intracellular Ca2+ release, and effect contraction in the Ca2+-dependent pathway in both fetus and adult. In addition, α1D-AR with dashed line indicates activation of Ins(1,4,5)P3 pathway only in adult. Right: fetus, in contrast to adult, α1B- and α1D-AR activate PKC, which in turn activates the extracellular regulated kinases ERK1/2 to stimulate gene expression to result in SMC differentiation and vascular growth.
α1-AR subtype expression and vascular reactivity.
The International Union of Pharmacology has designated α1-AR subtypes as α1A/a, α1B/b, and α1D/d, with the uppercase letters representing the pharmacologically identified subtypes and the lowercase letters representing the subtype molecular clones (19). Important mediators of sympathetic nervous response, the α1-AR are widely distributed throughout many cell types, and in SMCs regulate vascular tone and blood flow (53). In SMCs, the α1-AR subtypes and their levels vary considerably in their distribution in various circulatory beds, in different-size vessels of a given vascular bed, and in different species (16, 36, 41, 48). The present findings of a paucity of expression of the several α1-AR subtypes in the fetal CA agrees with the concept of an increase in α1-AR density with development (13, 32). For instance, in rats with aging from 1 mo to 24 mo, both α1B-AR mRNA and protein levels of α1A-AR increased, while α1D-AR levels remained constant (13). Moreover, in another report, after 10 wk of age α1D-AR have been reported to decrease, so that by 12 mo of age vasoconstrictor response to the α1A-AR subtype was predominant (32). Also, in comparing 7-day-old and adult rats, each of the three subtypes increased in brain tissue, α1B-AR increased in heart but decreased in liver, and none of the subtypes changed significantly in kidney (43).
α1-AR subtypes and intracellular signaling.
Differences in α1-AR subtype expression levels with development would be anticipated to have important physiological significance. Moreover, coupling of different α1-AR with different signal transduction pathways increases such significance. Relatively few studies have examined these relations in the vascular smooth muscle, and none to our knowledge in CA, much less comparing the immature organism with the adult. In adult but not in fetal CA, it has been well documented that the three α1-AR subtypes, members of the Gq/11 protein-coupled receptors, activate PLC-β to hydrolyze phosphatidylinositol 4,5-bisphosphate to Ins(1,4,5)P3 and DAG, the latter of which, in turn, activates PKC. α1D-AR inhibition appears to cause modest decrease in Phe-induced Ins(1,4,5)P3 responses, however, the contractility and Ca2+ responses being inhibited to a significant extent. α1D-AR may be mediating these effects through other signal transduction pathways such as RhoA/Rho kinase and/or activation of L-type Ca2+ channels and so forth.
In fetal but not adult CA we also observed a pronounced increase in ERK phosphorylation with Phe, which was inhibited by α1B-AR and α1D-AR antagonists. Of interest, recently we have shown (8) that PKC also differentially activates ERK in fetal and adult CA. Other reports indicate that GPCR such as α1-AR acting through Gq/11 also can activate the MAPK pathway via PKC and/or the Ras/Raf pathway by adapter proteins (30, 54). In 1- to 2-day-old rat cardiac myocytes, MAPK phosphorylation appeared to be mediated by the α1B-AR (45). To our knowledge, no studies in cerebrovascular SMCs have examined the relation of α1-AR subtypes to activation of the MAPK cascade and downstream effects of cell growth and differentiation, and this requires further investigation. In cultured rat aortic SMCs, protein synthesis and hypertrophy appeared to be mediated by the prominent α1B-AR (3). Similarly in rabbit aortas, α1B-AR stimulated protein synthesis (44). Along this line, in cultured rat thoracic aorta SMCs stably transfected with full-length α1-AR cDNAs, the α1D-AR appeared to couple to MAPK-mediated protein synthesis and cell growth, and these responses could be inhibited by the MEK inhibitor PD-98059 (46).
Perspectives and significance.
The present study extends our understanding of the adrenergic receptor pathway in CA contractility. It is a logical extension of our previous findings and illustrates a small facet of this complexity, e.g., that having to do with developmental maturation. The study provides details regarding maturational shift in expression and functions of α1-AR subtypes, as well as coupling with downstream pathways. However, this study makes more apparent the complexity of intracellular signaling pathways and raises a number of new questions. For instance, what is the functional significance of α1B- and α1D-AR during fetal life? To what extent do the G protein coupling pathways differ in the maturing fetus from those of the adult? During fetal life, to what extent is ERK activation responsible for contractility and/or vascular growth? What are the pathways involved in α1D-AR-mediated contractile responses in adult CA? To what extent do α1-AR mediate angiogenesis in the adult? If not via MAPK, then what are the pathways involved? Insight into these pathways and physiological mechanisms may prove of great value in developing therapeutic interventions in preventing and/or ameliorating the sequelae of functional dysregulation of CBF, such as occur in the developing fetus and newborn infant.
GRANTS
This work was supported by National Institutes of Health Grant HD/HL-03807 to L. D. Longo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Wen Long and Yu Zhao for technical assistance and Brenda Kreutzer for preparing the manuscript.
REFERENCES
- 1.Bishai JM, Penninga L, Nijland R, Meulenaar R, Gheorghe CP, Zhao Y, Buchholz JN, Zhang L, Longo LD. Pre- and postjunctional alpha2-adrenergic receptors in fetal and adult ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 282: R1654–R1662, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Xin X, Eckhart AD, Yang N, Faber JE. Regulation of vascular smooth muscle growth by alpha1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem 270: 30980–30988, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Cobb MH, Robbins DJ, Boulton TG. ERKs, extracellular signal-regulated MAP-2 kinases. Curr Opin Cell Biol 3: 1025–1032, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol 36: 481–509, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Fagura MS, Lydford SJ, Dougall IG. Pharmacological classification of alpha1-adrenoceptors mediating contractions of rabbit isolated ear artery: comparison with rat isolated thoracic aorta. Br J Pharmacol 120: 247–258, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DL., Jr BMY 7378 is a selective antagonist of the D subtype of alpha1-adrenoceptors. Eur J Pharmacol 272: R5–R6, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 297: H2242–H2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graziadei I, Zernig G, Boer R, Glossman H. Stereoselective binding of niguldipine enantiomers to alpha1A-adrenoceptors labeled with [3H]5-methyl-urapidil. Eur J Pharmacol 172: 329–337, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Greenberg B, Kishiyama S. Endothelium-dependent and -independent responses to severe hypoxia in rat pulmonary artery. Am J Physiol Heart Circ Physiol 265: H1712–H1720, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Gross G, Hanft G, Rugevics C. 5-Methyl-urapidil discriminates between subtypes of the alpha1-adrenoceptor. Eur J Pharmacol 151: 333–335, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 13.Gurdal H, Tilakaratne N, Brown RD, Fonseca M, Friedman E, Johnson MD. The expression of alpha1 adrenoceptor subtypes changes with age in the rat aorta. J Pharmacol Exp Ther 275: 1656–1662, 1995 [PubMed] [Google Scholar]
- 14.Han C, Abel PW, Minneman KP. Alpha1-adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature 329: 333–335, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Han C, Abel PW, Minneman KP. Heterogeneity of alpha1-adrenergic receptors revealed by chlorethylclonidine. Mol Pharmacol 32: 505–510, 1987 [PubMed] [Google Scholar]
- 16.Han C, Li J, Minneman KP. Subtypes of alpha1-adrenoceptors in rat blood vessels. Eur J Pharmacol 190: 97–104, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Hanft G, Gross G. Subclassification of alpha 1-adrenoceptor recognition sites by urapidil derivatives and other selective antagonists. Br J Pharmacol 97: 691–700, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanft G, Gross G, Beckeringh JJ, Korstanje C. Alpha1-adrenoceptors: the ability of various agonists and antagonists to discriminate between two distinct [3H]prazosin binding sites. J Pharm Pharmacol 41: 714–716, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR., Jr International Union of Pharmacology. X. Recommendation for nomenclature of alpha1-adrenoceptors: consensus update. Pharmacol Rev 47: 267–270, 1995 [PubMed] [Google Scholar]
- 20.Hirasawa A, Awaji T, Xu Z, Shinoura H, Tsujimoto G. Regulation of subcellular localization of alpha1-adrenoceptor subtypes. Life Sci 68: 2259–2267, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Hussain MB, Marshall I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol 122: 849–858, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao X, Gonzalez-Cabrera PJ, Xiao L, Bradley ME, Abel PW, Jeffries WB. Tonic inhibitory role for cAMP in alpha1a-adrenergic receptor coupling to extracellular signal-regulated kinases 1/2. J Pharmacol Exp Ther 303: 247–256, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kenny BA, Chalmers DH, Philpott PC, Naylor AM. Characterization of an alpha1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol 115: 981–986, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long W, Zhang L, Longo LD. Cerebral artery KATP- and KCa-channel activity and contractility: changes with development. Am J Physiol Regul Integr Comp Physiol 279: R2004–R2014, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Long W, Zhang L, Longo LD. Cerebral artery sarcoplasmic reticulum Ca2+ stores and contractility: changes with development. Am J Physiol Regul Integr Comp Physiol 279: R860–R873, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Long W, Zhao Y, Zhang L, Longo LD. Role of Ca2+ channels in NE-induced increase in [Ca2+]i and tension in fetal and adult cerebral arteries. Am J Physiol Regul Integr Comp Physiol 277: R286–R294, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Longo LD, Ueno N, Zhao Y, Pearce WJ, Zhang L. Developmental changes in alpha1-adrenergic receptors, IP3 responses, and NE-induced contraction in cerebral arteries. Am J Physiol Heart Circ Physiol 271: H2313–H2319, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, alpha1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Heart Circ Physiol 270: H915–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Longo LD, Zhao Y, Long W, Miguel C, Windemuth RS, Cantwell AM, Nanyonga AT, Saito T, Zhang L. Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am J Physiol Regul Integr Comp Physiol 279: R1419–R1429, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science 275: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Low AM, Lu-Chao H, Wang YF, Brown RD, Kwan CY, Daniel EE. Pharmacological and immunocytochemical characterization of subtypes of alpha-1 adrenoceptors in dog aorta. J Pharmacol Exp Ther 285: 894–901, 1998 [PubMed] [Google Scholar]
- 32.Lu ZZ, Zhang YY, Han QD. Comparison of functional alpha1-adrenoceptor subtypes in aortae between 12 month- and 10 week-old rats. Sheng Li Xue Bao 49: 414–418, 1997 [PubMed] [Google Scholar]
- 33.McCalden TA. Sympathetic control of the cerebral circulation. J Auton Pharmacol 1: 421–431, 1981 [DOI] [PubMed] [Google Scholar]
- 34.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74: 365–507, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Minneman KP, Atkinson B. Interaction of subtype-selective antagonists with alpha1-adrenergic receptor-mediated second messenger responses in rat brain. Mol Pharmacol 40: 523–530, 1991 [PubMed] [Google Scholar]
- 36.Minneman KP, Esbenshade TA. Alpha1-adrenergic receptor subtypes. Annu Rev Pharmacol Toxicol 34: 117–133, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Minneman KP, Han C, Abel PW. Comparison of alpha1-adrenergic receptor subtypes distinguished by chlorethylclonidine and WB 4101. Mol Pharmacol 33: 509–514, 1988 [PubMed] [Google Scholar]
- 38.Morrow AL, Creese I. Characterization of alpha1-adrenergic receptor subtypes in rat brain: a reevaluation of [3H]WB4104 and [3H]prazosin binding. Mol Pharmacol 29: 321–330, 1986 [PubMed] [Google Scholar]
- 39.Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Piascik MT, Guarino RD, Smith MS, Soltis EE, Saussy DL, Jr, Perez DM. The specific contribution of the novel alpha-1D adrenoceptor to the contraction of vascular smooth muscle. J Pharmacol Exp Ther 275: 1583–1589, 1995 [PubMed] [Google Scholar]
- 41.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular alpha1-adrenergic receptors by vessel bed and age. Circulation 100: 2336–2343, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Saussy DL, Jr, Goetz AS, Queen KL, King HK, Lutz MW, Rimele TJ. Structure activity relationships of a series of buspirone analogs at alpha-1 adrenoceptors: further evidence that rat aorta alpha-1 adrenoceptors are of the alpha-1D-subtype. J Pharmacol Exp Ther 278: 136–144, 1996 [PubMed] [Google Scholar]
- 43.Shen H, Peri KG, Deng XF, Chemtob S, Varma DR. Distribution of alpha1-adrenoceptor subtype proteins in different tissues of neonatal and adult rats. Can J Physiol Pharmacol 78: 237–243, 2000 [PubMed] [Google Scholar]
- 44.Siwik DA, Brown RD. Regulation of protein synthesis by alpha1-adrenergic receptor subtypes in cultured rabbit aortic vascular smooth muscle cells. J Cardiovasc Pharmacol 27: 508–518, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Wenham D, Rahmatullah RJ, Rahmatullah M, Hansen CA, Robishaw JD. Differential coupling of alpha1-adrenoreceptor subtypes to phospholipase C and mitogen activated protein kinase in neonatal rat cardiac myocytes. Eur J Pharmacol 339: 77–86, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Xin X, Yang N, Eckhart AD, Faber JE. Alpha1D-adrenergic receptors and mitogen-activated protein kinase mediate increased protein synthesis by arterial smooth muscle. Mol Pharmacol 51: 764–775, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Koike K. Characterization of alpha1-adrenoceptor-mediated contraction in the mouse thoracic aorta. Eur J Pharmacol 424: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Yokoo H, Kobayashi H, Minami S, Shiraishi S, Yamamoto R, Yanagita T, Tsuchiya K, Mohri M, Wada A. Alpha1-adrenergic receptor subtypes in rat cerebral microvessels. Brain Res 878: 183–187, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Xiao D, Longo LD, Zhang L. Regulation of alpha1-adrenoceptor-mediated contractions of uterine arteries by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H2282–H2289, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Pearce WJ, Longo LD. Noradrenaline-mediated contractions of ovine uterine artery: role of inositol 1,4,5-trisphosphate. Eur J Pharmacol 289: 375–382, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Long W, Zhang L, Longo LD. Extracellular signal-regulated kinases and contractile responses in ovine adult and fetal cerebral arteries. J Physiol 551: 691–703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y, Zhang L, Longo LD. PKC-induced ERK1/2 interactions and downstream effectors in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 289: R164–R171, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol 375: 261–276, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Zhong H, Minneman KP. Differential activation of mitogen-activated protein kinase pathways in PC12 cells by closely related alpha1-adrenergic receptor subtypes. J Neurochem 72: 2388–2396, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Zhou L, Zhao Y, Nijland R, Zhang L, Longo LD. Ins(1,4,5)P3 receptors in cerebral arteries: changes with development and high-altitude hypoxia. Am J Physiol Regul Integr Comp Physiol 272: R1954–R1959, 1997 [DOI] [PubMed] [Google Scholar]