Abstract
Objective
Surgical treatment of extreme obesity may be appropriate for some adolescents. We hypothesized that surgical weight loss outcomes may differ by preoperative level of extreme obesity (body mass index [BMI] ≥99th percentile).
Study design
A longitudinal assessment of clinical characteristics from 61 adolescents who underwent laparoscopic Roux-en-Y gastric bypass at a single pediatric center from 2002 until 2007 was performed. Patients were categorized into 1 of 3 preoperative BMI groups: group 1, BMI = 40.0 to 54.9 (n = 23); group 2, BMI = 55.0 to 64.9 (n = 21); group 3, BMI = 65.0 to 95.0 (n = 17). Changes in BMI and cardiovascular risk factors between baseline and year 1 were evaluated using repeated-measures mixed linear modeling.
Results
BMI in the overall cohort at baseline (60.2 ± 11 kg/m2) decreased by 37.4% at 1 year after surgery (P < .001). Percent BMI change varied little by preoperative BMI groups (−37.2%, −36.8%, and −37.7% for groups 1, 2, and 3 respectively; P = .8762). The rate of change in absolute BMI units significantly varied by preoperative BMI class (group × time interaction, P < .0001), with 1-year nadir BMI values for groups 1, 2, and 3 falling to 31 ± 4 kg/m2, 38 ± 5 kg/m2, and 47 ± 9 kg/m2, respectively. One year after surgery, only 17% of patients achieved a nonobese BMI (<30 kg/m2). Significant improvements in systolic and diastolic blood pressure (P < .0001), fasting insulin (P < .0001), total cholesterol (P = .0007), and triglyceride levels (P < .0001) were seen after surgery irrespective of baseline BMI class. Mean albumin levels remained normal despite significant caloric restriction and weight loss.
Conclusions
Laparoscopic gastric bypass resulted in improvement or reversal of cardiovascular risk factors and resulted in a decrease in BMI of approximately 37% in all patients, regardless of starting BMI, 1 year after surgery. The timing of surgery for adolescent extreme obesity is an important consideration, because “late” referral for bariatric surgery at the highest of BMI values may preclude reversal of obesity.
Pediatric obesity is recognized as one of the most significant public health issues in the United States.1,2 From 1999 to 2004, the proportion of overweight (body mass index [BMI] for age ≥95th percentile) adolescents (ages 12 to 19) in the United States increased from 14.8% to 17.4%.1 Morbid obesity in adolescents can lead to morbid conditions such as type 2 diabetes, cardiopulmonary disease, and the metabolic syndrome. In addition, numerous orthopedic, neurological, and gastroenterological conditions threaten the health of adolescents affected by excess weight.3–8 Increasingly, weight loss surgery is being performed in extremely obese adolescents to effectively treat these comorbidities.9 Early results from a retrospective study of outcomes in adolescents undergoing bariatric surgery suggest that health and weight loss outcomes will be similar if not better than results seen in adults.10
The BMI spectrum for adolescents seeking surgery is broad, with values in the literature ranging from 35 to 95 kg/m2, with average BMI values much higher than those seen in most adult surgical practices. However, to date, no analysis has been done that examines adolescent surgical outcomes stratified by baseline BMI. Even though many experts consider that the most important end points after bariatric surgery are the improvements in comorbidities and risk factors for future cardiovascular disease, it is also appropriate to place some emphasis on degree of weight loss as well, given the extremes of BMI encountered. Patients who plateau after surgery at a BMI of 40 or 50 kg/m2 probably remain at elevated risk of obesity-related health problems compared with patients who reach a postoperative plateau BMI that is no longer obese (BMI <30 kg/m2).
To begin to address predictors of success after surgical treatment of adolescent obesity, we assessed both cardiovascular risk factors and weight nadir in a cohort of adolescent patients 1 year after gastric bypass surgery, stratified by preoperative degree of extreme obesity. The central hypothesis tested in this analysis was that baseline BMI predicts weight loss after Roux-en-Y gastric bypass (RYGB). We further hypothesized that cardiovascular risk factors improve in all patients despite differences in preoperative weight.
Methods
The Follow-up of Adolescent Bariatric Surgery (FABS) study is a single-center, longitudinal outcome study of adolescents and young adults (age ≤21 years) seeking obesity treatment at Cincinnati Children’s Hospital Medical Center (CCHMC). Patients enrolled in FABS who underwent laparoscopic RYGB surgery between August 2002 and January 2007 at CCHMC were included in this analysis. The operation was performed in a similar fashion in all patients with a side-to-side jejunojejunostomy and an end-to-side gastro-jejunostomy. The gastric pouch was limited to 30 mL in volume and the jejunum was divided 15 to 20 cm from the Ligament of Treitz. In addition to baseline demographic data (date of birth, sex, race), anthropometric and other clinical measures were evaluated at 6 months and then annually with data abstracted from clinical records for those subjects enrolled in the FABS study. This research was approved by the CCHMC Institutional Review Board.
Anthropometric measurements were performed during routine clinical visits by clinic staff. Measures recorded at baseline (initial preoperative contact) and postoperative visits (6 [range ±3 months] and 12 [range, 9 to 18 months]) were included in the analysis. Adolescents were weighed and measured in light clothing and without shoes. Body weight was recorded using a digital scale (Model 5002 Stand-On Scale; Scale Tronix, Inc., White Plains, New York), and height was measured using a calibrated wall-mounted stadiometer (Ayrton Stadiometer Model S100; Ayrton Corporation, Prior Lake, Minnesota). BMI was calculated as body weight in kilograms divided by height in meters squared. To evaluate the impact of preoperative BMI on bariatric surgical outcomes, patients were categorized into 3 groups, based on arbitrary cutoffs of baseline BMI (group 1, BMI = 40.0 to 54.9 kg/m2; group 2, BMI = 55.0 to 64.9 kg/m2; group 3, BMI = 65.0 to 95.0 kg/m2). Systolic and diastolic blood pressures were measured by trained clinic personnel using an appropriately sized cuff with the patient in a seated position. Fasting serum lipids (total cholesterol, HDL, LDL, triglycerides), insulin, and albumin were measured at a CLIA-certified clinical laboratory at baseline and each follow-up visit.
Statistical Analyses
Comparisons of categorical variables by preoperative BMI group were performed using χ2 or Fisher exact tests. Similar comparisons for continuous variables were conducted using equal and unequal variance analysis of variance. Pearson correlation and simple linear regression analyses were performed to relate baseline BMI values with those gathered 1 year after surgery. To longitudinally assess the impact of preoperative BMI on postoperative BMI (absolute BMI units and percent BMI change from baseline), blood pressure, fasting serum lipids (total cholesterol, HDL, LDL, triglycerides), insulin, and albumin, separate repeated-measures mixed linear models were fit using the MIXED procedure in SAS v9.1 (SAS System. Version 9.1, Cary, North Carolina). To account for the correlation of within-patient repeated observations, a variance-covariance matrix was selected for each model, based on the Akaike Information Criterion. The following covariates were considered for potential inclusion into each model: age at surgery, sex, race, time between baseline consultation and surgery, and Roux length. The interaction between preoperative BMI category and time was also assessed. The Tukey-Kramer adjustment method for multiple comparisons was used for all pairwise least-squares means comparisons. A P value of <.05 was considered statistically significant for all analyses.
Results
Over a 5-year period (2002 to 2007), 61 consecutive adolescents underwent laparoscopic RYGB surgery. The mean BMI at baseline for these patients was 60.2 kg/m2, ranging from 41.4 to 95.5 kg/m2. Nearly 70% of this cohort was female, with 83.6% self-identified as white race (Table I). The mean age at surgery was 17.2 years (range, 13 to 23 years). The average time from initial baseline consultation to surgery was 5.0 months. The mean Roux length was 119.2 cm. Preoperative BMI groups were similar in terms of age at surgery (P = .4294), sex (P = .1014), race (P = .1158), time between baseline and surgery (P = .3581), and Roux length (P = .2753).
Table I.
Baseline bariatric patient characteristics by preoperative body mass index classification
| %(N)/x̄(SD) | Overall | Group 1 BMI 40–54 | Group 2 BMI 55–64 | Group 3 BMI ≥65 | P value |
|---|---|---|---|---|---|
| n | 61 | 23 | 21 | 17 | |
| Age at surgery (y) | 17.2 (1.88) | 17.6 (2.39) | 16.8 (1.68) | 17.1 (1.20) | .4294 |
| Sex (female) | 67.2% (n = 41) | 78.2% (n = 18) | 71.4% (n = 15) | 47.1% (n = 8) | .1014 |
| Race (white) | 83.6% (n = 51) | 86.4% (n = 19) | 95.2% (n = 20) | 70.6% (n = 12) | .1158 |
| Time to surgery (mo) | 5.0 (4.16) | 5.2 (5.01) | 4.0 (2.54) | 5.9 (4.46) | .3581 |
| Roux length (cm) | 119.2 (25.00) | 113.1 (24.52) | 120.0 (24.49) | 126.7 (25.82) | .2753 |
The average length of stay was 3.6 (±1.7) days, with no deaths, no transfusions, and no conversions to open operations. There were 18 complications that occurred in 16 of 61 subjects within 30 days of surgery. The nature of the complications was as follows: dehydration requiring readmission, peristomal ulcer, bowel obstruction, intestinal leakage, wound infection, and anastomotic stricture.
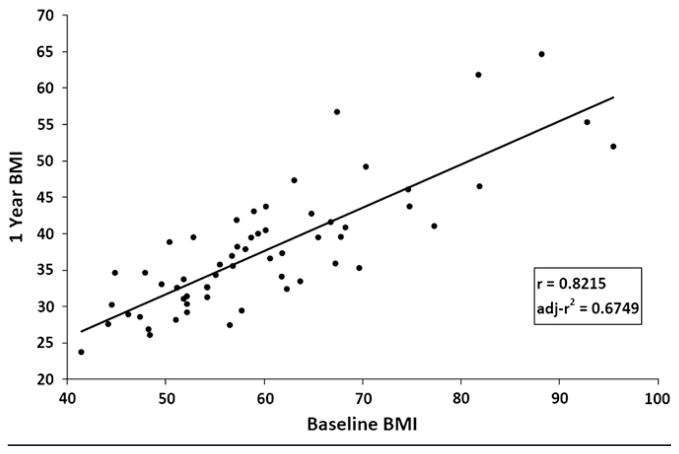
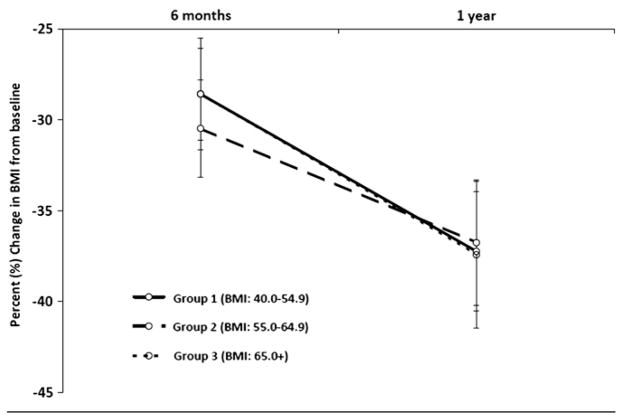
Baseline BMI was found to be highly positively correlated with BMI at 1 year (r = .8215), accounting for 67% of the variance in BMI 1 year after surgery (adjusted r2 = .6692; Figure 1). Table II (available at www.jpeds.com) shows baseline and 1-year outcome measures by preoperative BMI classification. One year after surgery, body weight declined by 37.4%, but height did not significantly change (P = .4787; data not shown). In the overall cohort, the mean BMI declined by 37.4% 1 year after surgery (P < .0001). The percent BMI loss did not differ by preoperative BMI class (P = .8762). Further, the rate of change in percent BMI lost over time was also found to be similar for each group (group × time interaction, P = .2330; Figure 2). However, the slope of decline in BMI units through 1 year after surgery differed by preoperative BMI class (group × time interaction, P < .0001), with the initial group differences in BMI diminishing over time (Figure 3). At each time point, group 3 (BMI >65 kg/m2) maintained a significantly higher BMI than groups 1 (BMI, 40 to 54.9 kg/m2) and 2 (BMI, 55 to 65 kg/m2), as did group 2 compared with group 1. One year after RYGB, 10 subjects (17.0%) were able to achieve a nonobese BMI (<30 kg/m2), the majority of whom (n = 8) came from group 1.
Figure 1.
Plot of baseline and one year post-operative BMI values.
Table II.
Baseline and 1-year outcome measures by preoperative body mass index classification
| x̄±SD(N) | Overall (n = 61) | Group 1 BMI 40–54 (n = 23) | Group 2 BMI 55–64 (n = 21) | Group 3 BMI ≥65 (n = 17) |
|---|---|---|---|---|
| BMI | ||||
| Baseline | 60.2 ± 11.76 (61) | 49.7 ± 3.63 (23) | 59.3 ± 2.78 (21) | 75.3 ± 9.55 (17) |
| 1 y | 37.7 ± 8.58 (59) | 31.2 ± 3.83 (22) | 37.5 ± 4.85 (21) | 46.9 ± 8.99 (16) |
| % Change | −37.4%† | −37.2%† | −36.8%† | −37.7%† |
| SBP | ||||
| Baseline | 121.0 ± 13.20 (57) | 120.4 ± 10.42 (20) | 120.4 ± 13.31 (21) | 122.6 ± 16.52 (16) |
| 1 y | 110.3 ± 9.88 (58) | 110.4 ± 8.69 (22) | 107.6 ± 8.69 (20) | 113.4 ± 12.20 (16) |
| % change | −8.8%† | −8.3%‡ | −10.6%† | −7.5%‡ |
| DBP | ||||
| Baseline | 75.6 ± 9.05 (57) | 76.8 ± 9.46 (20) | 76.4 ± 8.64 (21) | 73.2 ± 9.14 (16) |
| 1 y | 65.4 ± 7.46 (58) | 64.9 ± 7.62 (22) | 64.0 ± 7.14 (20) | 67.9 ± 7.50 (16) |
| % Change | −13.5%† | −15.5%† | −16.2%† | −7.2%‡ |
| Cholesterol | ||||
| Baseline | 178.1 ± 44.80 (45) | 178.2 ± 60.74 (17) | 182.0 ± 26.72 (15) | 173.5 ± 39.58 (13) |
| 1 y | 148.2 ± 39.81 (37) | 135.9 ± 37.29 (14) | 164.3 ± 45.70 (13) | 144.5 ± 30.34 (10) |
| % Change | −16.8%† | −23.7%* | −9.7%‡ | −16.7%‡ |
| HDL | ||||
| Baseline | 42.8 ± 23.77 (52) | 45.0 ± 23.39 (20) | 37.5 ± 9.43 (17) | 33.1 ± 3.37 (15) |
| 1 y | 44.5 ± 10.09 (47) | 47.1 ± 12.04 (16) | 45.8 ± 8.89 (19) | 38.9 ± 7.23 (12) |
| % Change | +4.0%‡ | +4.7% | +22.1%‡ | +17.5%‡ |
| LDL | ||||
| Baseline | 113.0 ± 42.27 (51) | 127.3 ± 57.77 (19) | 110.0 ± 21.27 (17) | 99.5 ± 33.01 (15) |
| 1 y | 93.3 ± 29.08 (47) | 83.4 ± 20.31 (16) | 100.9 ± 35.56 (19) | 94.3 ± 25.69 (12) |
| % Change | −17.7%† | −34.5%† | −8.3%‡ | −5.2%‡ |
| Triglycerides | ||||
| Baseline | 140.3 ± 70.82 (51) | 136.6 ± 70.35 (19) | 147.9 ± 85.82 (17) | 136.5 ± 55.03 (15) |
| 1 y | 87.9 ± 35.51 (47) | 88.2 ± 34.90 (16) | 91.7 ± 35.88 (19) | 81.6 ± 37.91 (12) |
| % Change | −37.3%† | −35.4%* | −38.0%* | −40.2%* |
| Insulin | ||||
| Baseline | 40.0 ± 21.04 (43) | 40.4 ± 28.76 (14) | 40.8 ± 16.30 (14) | 38.9 ± 17.63 (15) |
| 1 y | 10.5 ± 5.58 (44) | 10.0 ± 5.31 (14) | 10.7 ± 5.70 (17) | 10.8 ± 6.11 (13) |
| % Change | −73.8%† | −75.2%† | −73.8%† | −72.2%† |
| Albumin | ||||
| Baseline | 4.1 ± 0.36 (42) | 4.2 ± 0.40 (15) | 4.0 ± 0.24 (15) | 4.2 ± 0.43 (12) |
| 1 y | 4.0 ± 0.32 (42) | 4.1 ± 0.31 (16) | 4.1 ± 0.38 (14) | 3.9 ± 0.37 (12) |
| % Change | −2.4%‡ | −2.4%‡ | +2.5%‡ | −7.1%‡ |
P < .05.
P < .01.
P > .05 (not significant).
Figure 2.
Percent Change in BMI from Baseline by Pre-operative BMI Classification and Follow-up Visit.
Figure 3.
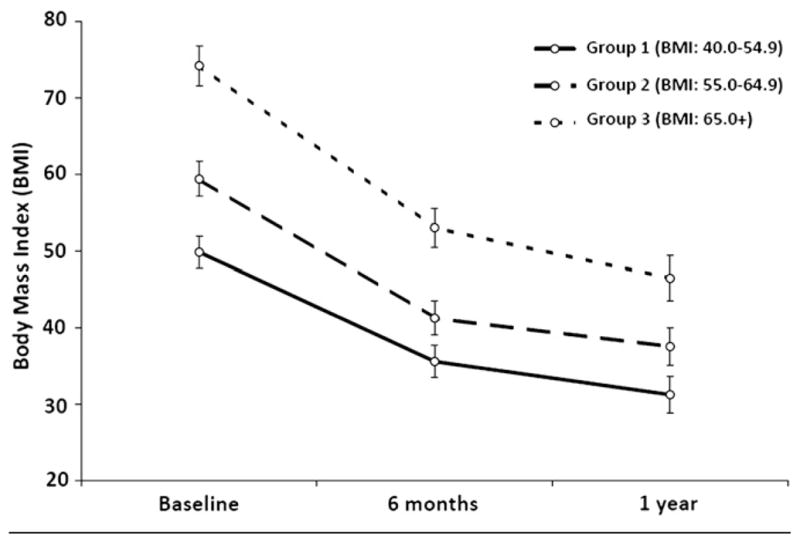
BMI by Pre-operative BMI Classification and Follow-up Visit.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) each significantly decreased 1 year after surgery by 8.8% and 13.5%, respectively (each P < .0001). However, these measures did not differ by preoperative BMI group (SBP, P = .2633; DBP, P = .9759), nor did the rate of decrease over time differ for either by BMI group (group × time interaction: SBP, P = .4171; DBP, P = .2036). Total cholesterol level decreased by 16.8% after 1 year (P = .0007), although no significant changes occurred after 6 months (P = .2671). Overall, cholesterol was similar for all preoperative BMI groups (P = .2415), with the rate of decline through 1 year similar as well (group × time interaction, P = .6940).
HDL cholesterol exhibited a nonsignificant 4.0% increase 1 year after RYGB (P = .9559). Similarly, this overall measure (P = .7039) and the rate of change over time (group × time interaction, P = .6746) did not differ by preoperative BMI group. In contrast, LDL cholesterol significantly decreased by 17.7% from baseline (P = .0098). Corresponding with total cholesterol, no significant change in LDL was observed after 6 months (P = .5674). Overall, there was no difference in LDL by preoperative BMI group (P = .8168), but the rate of decrease 1 year after surgery differed across these groups (group × time interaction, P = .0588). Specifically, LDL levels for BMI groups 2 and 3 remained uniform over each time point, and group 1 had a significant decrease at 6 months (P = .0019), with no significant change noted thereafter (P = .9945).
Overall, serum triglyceride levels fell markedly (−37.3%) 1 year after RYGB (P < .0001), although no significant change was detected after 6 months (P = .0982). No differences were noted by preoperative BMI group for overall triglyceride level (P = .3930) or by the rate of decline through 1 year (group × time interaction, P = .9247). Insulin level decreased dramatically (−75.8%) 6 months after RYGB (P < .0001), with no significant change observed thereafter (P = .7811). Each pre-operative BMI group had a similar pattern of decline in insulin over time (group × time interaction, P = .7733) while also maintaining comparable insulin levels overall (P = .7458). Despite the significant weight loss, no preoperative or postoperative differences in albumin were identified.
In general, the use of medications for cardiovascular risk factors was low in this population. Most of the medications were discontinued after operation, but the limited size of the population using medication limits the usefulness of the change data. For instance, 30% of the subjects in this analysis had dyslipidemia at baseline, but only 2 were receiving pharmacotherapy at baseline. In both of these 2, the medications for dyslipidemia were discontinued in the year after operation. Similarly, 9 subjects in this series were diabetic at baseline, and all were receiving medications at baseline. However, only 1 was still taking medication for diabetes after surgery. Finally, 20% in this series presented with hypertension at baseline, but only one subject was treated with medication, and this treatment was discontinued soon after surgery.
Discussion
Extreme obesity is now recognized as major pediatric health problem. Recent estimates indicate that 4% of children ages 5 to 17 years in the United States are extremely obese.11 Nearly two thirds of such children have 2 or more cardiovascular risk factors, and a substantial proportion have significant metabolic comorbidities typically seen in adults, including obstructive sleep apnea, diabetes mellitus, nonalcoholic steatohepatitis, and significant hypertension and left ventricular hypertrophy.12–15 Freedman et al11 found that without intervention, most extremely obese children will remain extremely obese as adults—88% had an adult BMI of ≥35 kg/m2 and 65% had an adult BMI ≥40 kg/m2. At present, diet and lifestyle interventions for pediatric patients with extreme obesity have not been successful in achieving and maintaining meaningful improvements in weight and associated risk factors.16–18 Bariatric surgery has been the only treatment shown to achieve meaningful and sustained weight loss in extremely obese adolescents.19
One of the most commonly used bariatric procedures in the United States today is laparoscopic RYGB.20 Recent data have demonstrated significant improvements in type 2 diabetes and insulin resistance,21 dyslipidemia,17,21 sleep efficiency,22 obstructive sleep apnea,12 hypertension and cardiac hypertrophy,23 proteinuria,24 depressive symptoms,25 quality of life,25 and body composition26 in extremely obese adolescents after weight loss surgery. However, the range of BMI in adolescents undergoing bariatric surgery is large, typically 35 to >90 kg/m2, and results are conventionally presented as average improvements for the entire cohort. In this investigation, we found that most adolescents within the highest ranges of baseline BMI (BMI ≥65 kg/m2) remained extremely obese, with BMIs ≥40 kg/m2 1 year after surgery, despite BMI reductions averaging nearly 40%.
Definitions of successful weight loss outcomes after adolescent bariatric surgery have been difficult to characterize, as have predictors of success. Successful outcomes probably will be measured by examining a broad range of medical and psychosocial outcomes, as well as BMI change. In that regard, our results demonstrate that irrespective of initial BMI, the percent decline in BMI at 1 year is unexpectedly predictable (range, 36.8% to 37.7%). Therefore, patients who present at higher weights and BMI values lose more weight than those who present at lower weights but also plateau at a higher weight on average. The biological and potentially behavioral reasons for this are unclear. Because the operation performed was similar, it would seem that most of the variability in weight loss during the first year after gastric bypass is related to starting BMI. Whether starting BMI will differentially affect long-term weight maintenance or regain remains unknown. Of concern, long-term data (5 years) in adults after gastric bypass suggest that the higher extremes of baseline BMI (>50 kg/m2) were not only associated with lower nadir BMI but also with greater prevalence of weight regain.27
Our 1-year results parallel those reported in adults. In adults, higher preoperative BMI and body weight positively correlate with higher absolute maximum weight loss after gastric bypass.28,29 Adults with preoperative BMI values ≥60 kg lost less excess weight when compared with those with BMI values <60 kg (51% versus 64% excess weight loss, P < .001), but both groups lost an equivalent percent of original weight (36% versus 37%).30 In several multivariate analyses examining predictors of outcome, baseline weight was one of the strongest predictors of weight loss at 1 year.29,31,32
Despite the fact that most patients in our adolescent cohort remained obese at 1 year after surgery, those with the highest preoperative BMI demonstrated decreases in SBP and DBP and hyperinsulinemia that were similar to patients with lower baseline BMI values. Likewise, all 3 groups also demonstrated comparable and significant decreases in serum levels of total cholesterol and triglycerides after surgery. Metabolic improvements after gastric bypass occur independent of degree of weight loss or absolute BMI at 1 year after surgery. Even those who remain extremely obese at 1 year had significant psychosocial improvement.25 However, the effect of persistent though significantly ameliorated obesity and excess weight on arthropathy or quality of life in those who remain obese after bariatric surgery is a concern and deserves greater study.
Strengths of this investigation primarily relate to the data source used. Sixty-one consecutive patients undergoing laparoscopic RYGB were included in the analysis, thus minimizing the potential impact of selection bias in these results. Additionally, data for the primary measure of interest, BMI, were available for 59 of the 61 (97%) patients through 1 year after surgery. Therefore, bias from missing BMI values is likely to be negligible. However, this study was subject to several limitations. First, this investigation used data retrospectively collected from clinical records from a surgical weight management program; each outcome measure had some level of missing values, ranging from 3.3% to 41.0% missing at 1 year after surgery. However 97% of BMI values were available 1 year after surgery. Additionally, beyond preoperative BMI, there may be other important indicators of weight loss efficacy, such as adherence to postoperative guidelines for postoperative physical activity, caloric intake, and dietary composition. These variables were not systematically collected but must be included in future studies.
Finally, in the adult bariatric outcomes literature, medication use is commonly analyzed in conjunction with change in cardiovascular risk factors. However, in general, the use of medications for cardiovascular risk factors was low in this adolescent population limiting the usefulness of the change data.
In summary, we have demonstrated that preoperative BMI serves as an accurate indicator of nadir BMI at 1 year after surgery. This finding suggests that the timing of surgery for adolescent obesity is an important consideration, as “late” referral for bariatric surgery at higher BMI values may preclude reversal of obesity or extreme obesity within the first postoperative year and may increase the risk of weight regain over the long term.
A greater understanding of the specific surgical procedures and postoperative management approaches that optimize success is needed. The definition of success will take into account not only weight loss efficacy but also change in comorbidity, quality of life, psychosocial status, and minimization of surgical, medical, and nutritional complications. We are currently collecting a broad array of outcome data in the prospective multicenter Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study.33 We hope that this study will help define the merits and limitations of surgical interventions directed at weight loss in morbidly obese adolescents as compared with adults.
Acknowledgments
This study was funded in part by investigator-initiated grant support from Ethicon Endosurgical, Blue Ash, Ohio. The authors declare no conflicts of interest.
- BMI
Body mass index
- CCHMC
Cincinnati Children’s Hospital Medical Center
- DBP
Diastolic blood pressure
- FABS
Follow-up of Adolescent Bariatric Surgery
- RYGB
Roux-en-Y gastric bypass
- SBP
Systolic blood pressure
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–8. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128:608–15. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 4.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16:124–9. doi: 10.1002/ppul.1950160208. [DOI] [PubMed] [Google Scholar]
- 6.Loder RT, Aronson DD, Greenfield ML. The epidemiology of bilateral slipped capital femoral epiphysis: a study of children in Michigan. J Bone Joint Surg Am. 1993;75:1141–7. doi: 10.2106/00004623-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–74. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 8.Roberts EA. Nonalcoholic steatohepatitis in children. Curr Gastroenterol Rep. 2003;5:253–9. doi: 10.1007/s11894-003-0028-4. [DOI] [PubMed] [Google Scholar]
- 9.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161:217–21. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 10.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–8. doi: 10.1016/S1091-255X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–7. e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti B, et al. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–9. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 13.Van Putte-Katier N, Rooman RP, Haas L, Verhulst SL, Desager KN, Ramet J, et al. Early cardiac abnormalities in obese children: importance of obesity per se versus associated cardiovascular risk factors. Pediatr Res. 2008;64:205–9. doi: 10.1203/PDR.0b013e318176182b. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe JA, Naylor LH, Jones TW, Davis EA, O’Driscoll G, Ramsay JM, et al. Impact of obesity on diastolic function in subjects < or =16 years of age. Am J Cardiol. 2006;98:691–3. doi: 10.1016/j.amjcard.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226–32. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 16.Zeller M, Kirk S, Claytor R, Khoury P, Grieme J, Santangelo M, et al. Predictors of attrition from a pediatric weight management program. J Pediatr. 2004;144:466–70. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Lawson ML, Kirk S, Mitchell T, Chen MK, Loux TJ, Daniels SR, et al. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41:137–43. doi: 10.1016/j.jpedsurg.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30:318–28. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- 19.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248:763–76. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 20.Fernstrom MH, Courcoulas AP. Bariatric surgery for the severely obese adolescent. Aesthet Surg J. 2008;28:331–4. doi: 10.1016/j.asj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123:214–22. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 22.Kalra M, Mannaa M, Fitz K, Kumar S, Chakraborty R, Sheng X, et al. Effect of surgical weight loss on sleep architecture in adolescents with severe obesity. Obes Surg. 2008;18:675–9. doi: 10.1007/s11695-008-9472-4. [DOI] [PubMed] [Google Scholar]
- 23.Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51:1342–8. doi: 10.1016/j.jacc.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24:851–5. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 25.Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2008.644. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inge T, Wilson KA, Gamm K, Kirk S, Garcia VF, Daniels SR. Preferential loss of central (trunk) adiposity in adolescents and young adults after laparoscopic gastric bypass. Surg Obes Relat Dis. 2007;3:153–8. doi: 10.1016/j.soard.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Diniz MD, Passos VM, Barreto SM, Linares DB, de Almeida SR, Rocha AL, et al. Different criteria for assessment of Roux-en-Y gastric bypass success: does only weight matter? Obes Surg. 2008 doi: 10.1007/s11695-008-9669-6. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Czupryniak L, Pawlowski M, Kumor A, Szymanski D, Loba J, Strzelczyk J. Predicting maximum Roux-en-Y gastric bypass-induced weight reduction: preoperative plasma leptin or body weight? Obes Surg. 2007;17:162–7. doi: 10.1007/s11695-007-9042-1. [DOI] [PubMed] [Google Scholar]
- 29.Hatoum IJ, Stein HK, Merrifield BF, Kaplan LM. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2009;17:92–9. doi: 10.1038/oby.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farkas DT, Vemulapalli P, Haider A, Lopes JM, Gibbs KE, Teixeira JA. Laparoscopic Roux-en-Y gastric bypass is safe and effective in patients with a BMI > or = 60. Obes Surg. 2005;15:486–93. doi: 10.1381/0960892053723466. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, Mason R, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16:1227–31. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 32.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008;12:250–5. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 33.Inge TH, Zeller M, Harmon C, Helmrath M, Bean J, Modi A, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42:1969–71. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]