Abstract
The purpose of this study was to determine the effects of exercise on coronary blood flow and macrovascular atherosclerosis in response to stent deployment. Male Yucatan swine were placed on a control diet (C); on a high-fat/cholesterol diet (hypercholesterolemic; H); or on a high-fat/cholesterol diet and aerobically exercise trained (HX) starting after 36 wk on the diet. All pigs underwent coronary angiography and intravascular ultrasound (IVUS) guided placement of a bare metal stent in the circumflex coronary artery after 40 wk on diets and 3 wk later pigs underwent repeat angiography and IVUS and coronary blood flow (CBF) measurement. Average peak velocity (APV) was measured under basal conditions and in response to intracoronary application of the endothelium-independent vasodilator adenosine and the endothelium-dependent vasodilator bradykinin. There was a similar ∼8-fold increase in total cholesterol in H and HX compared with control. Baseline CBF was increased above control and H in HX (P < 0.05). At all doses adenosine-induced CBF was impaired in H, but preserved in HX. Similarly, bradykinin-induced CBF was impaired in H vs. control, yet was potentiated in HX. Microvessel density was decreased in H and preserved in HX vs. control. Native atheroma in HX was lower relative to H and control, while in-stent stenosis in HX was not different from H. Hyperlipidemia-induced microvascular dysfunction after stent deployment may be a result of reduction in microvessel density. This is the first report that short-term exercise training near the time of stenting prevents stent-induced microvascular dysfunction and attenuates native atheroma independent of changes in plasma cholesterol in this porcine model.
Keywords: microvessel, restenosis, exercise, hyperlipidemia, Yucatan swine
deployment of a stent in a coronary artery in the setting of flow-limiting stable coronary artery disease (CAD) results in initial improvement in functional capacity and quality of life. However, almost half of patients undergoing coronary angioplasty/stenting eventually experience recurrent angina, resulting in a decline in functional capacity, and require further medical attention (16). It has been shown that coronary stenting mechanically damages vascular cells in the target conduit artery segment, endothelium in peri-stent segments (30), and induces downstream microvascular dysfunction that persists for weeks and has been implicated in causing exertional ischemia (5, 28, 42, 59, 61). Camici and Crea (5) termed this phenomenon poststent “iatrogenic coronary microvascular dysfunction,” which may be due to enhanced alpha-adrenergic constriction (19), impaired autoregulation (28, 59), or microemboli that may cause microinfarcts (50) and perhaps more severe rarefaction (microvessel dropout). Despite enormous efforts to reduce in-stent stenosis and microvascular dysfunction, there remains significant room for improvement in addressing both of these post-stent procedure complications. This is especially important because drug-eluting stents, which are the main treatment for flow-limiting lesions, cause even greater microvascular dysfunction than bare metal stents (29, 39).
Exercise training of patients with coronary artery disease elicits beneficial effects, including improvement in exercise tolerance, left ventricular function, and reduction in the ischemic response to submaximal workload after exercise training, and increased dilation of conduit and microvessel coronary arteries (2, 7, 15, 21, 22, 38). Most of these studies have shown concomitant changes in plasma cholesterol: specifically, decreased total and low-density lipoprotein (LDL) and increased high-density lipoprotein (HDL) cholesterol (3, 26, 46). Hambrecht et al. (22) removed the confounding variable of hypercholesterolemia by excluding those patients from their study, thus implying that the beneficial effects of exercise may be due to more direct actions of exercise on the vasculature.
The benefits of exercise training may also extend to the population undergoing percutaneous coronary revascularization (3, 21, 26, 46). Belardinelli et al. (3) found that progression of macrovascular CAD in arterial segments proximal and distal to the angioplasty/stented segment, i.e., peri-stent CAD, was attenuated by exercise training and accompanied by an improved thallium perfusion stress test that is indicative of increased metabolic vasodilation of the coronary microvasculature. Further, exercise training may be more beneficial and cost effective than percutaneous coronary intervention in the setting of chronic stable CAD (21). Fleenor and Bowles (17) showed that long-term exercise training of pigs with normal plasma cholesterol attenuated conduit artery neointimal proliferation elicited by overexpansion injury from balloon angioplasty; however, microvascular effects were not determined. Further, the effects of plasma cholesterol and exercise training on in-stent stenosis, peri-stent CAD, and microvascular dysfunction after coronary stenting have not been studied. We have chosen to use the hyperlipidemic swine model, which is similar to human patients undergoing coronary stenting to test several hypotheses.
The purpose of this study was first to test the hypothesis that stent deployment in coronary arteries of hypercholesterolemic swine would elicit microvascular dysfunction and whether short-term exercise training would prevent the microvascular dysfunction and progression of macrovascular CAD after stenting. A second hypothesis tested whether exercise elicits beneficial effects by acting directly on the vascular wall, rather than indirectly via decreasing plasma risk factors (i.e., cholesterol). The final hypothesis tested whether a decrease in microvessel density, i.e., rarefaction, occurs after stenting in hypercholesterolemia, and whether exercise training would prevent this.
METHODS
All protocols involving animals were approved by an Institutional Animal Care and Use Committee and complied fully with standards (1, 43). Nineteen male Yucatan swine, 52–70 kg, were randomized to 43 wk of control (C, n = 8), high fat/cholesterol (H, n = 8), or H with short-term exercise training (HX, n = 3). The lower number of pigs in the HX group vs. C and H groups was used in strict compliance with the principle of reducing the number of animals exposed to surgical and exercise stress to the minimum number required for statistical power (1, 6, 44). H and HX were fed a diet consisting of 46% kcal from fat (41). The feed base consisted of mini pig chow with supplemental fat kilocalories from coconut oil (<1% trans fatty acids; Research Diets, New Brunswick, NJ) and cholesterol (Research Diets). Controls were fed a diet consisting of mini pig chow base with 8% kcal from fat.
Exercise training.
Animals randomized to the exercise group began treadmill training 4 wk before initial cardiac catheterization. During the first week of training, the exercise pigs ran on the treadmill at 3 mph (endurance) with 0% grade for 20–30 min and at 4 mph (sprint) for 15 min. The speed and duration of running were increased progressively over the course of the following 4 wk. During the 4th through the 7th week of training, a typical 70-min training session consisted of the following: 1) 5-min warm-up at 2.5 mph, 2) 15-min sprint at speeds of 5.5–6 mph, 3) 40- to 45-min endurance run at 3.5 mph, and 4) 5-min cool down at 2 mph. Ranges of running speeds are presented because the exercise training advanced during the course of the week. Following coronary stenting, each animal resumed symptom-limited exercise training. This regimen was maintained for 3 wk after stenting when the animals returned for repeat cardiac catheterization. Exercise protocols were compliant with guidelines from the American Physiological Society (6).
Submaximal stress test.
At weeks 1, 4, and 7 of exercise training the endurance-trained animals underwent a submaximal stress test consisting of running on a treadmill at 3.1 mph, 0% grade for 15 min at which point heart rate data were collected.
Stent procedure (12, 37, 55).
Each animal received antiplatelet therapy with aspirin 325 mg daily starting the day before the initial catheterization procedure and continuing through the completion of the study. Each pig was fasted overnight, and all animals arrived at the same time of day for procedure. Animals received preanesthesia with intramuscular injections of atropine (0.05 mg/kg), xylazine (2.2 mg/kg), and telazol (6.6 mg/kg). Following intubation, isoflurane (2–4%, with oxygen) was administered to maintain stable systemic hemodynamics and a stable level of anesthesia. Heart rate, blood pressure (tail cuff and aortic), respiratory rate, and electrocardiographic data were continuously monitored throughout the procedure. Under sterile conditions, the right femoral artery was exposed with surgical cut-down technique, and an 8-F vascular introducer sheath was inserted into the femoral artery followed by administration of heparin 200 U/kg. An 8-F Amplatz L (sizes 0.75–2.0) guiding catheter (Cordis, Miami, FL) was inserted through the sheath and advanced to engage the left main coronary ostium (Fig. 1A). Blood pressure measurements were recorded directly from the coronary ostium via a pressure transducer connected to the manifold assembly. A 0.014-in., flex-tip Doppler flow wire (FloWire; Cardiometrics, Rancho Cordova, CA) was advanced through the guiding catheter and positioned in the distal left circumflex artery with the aid of angiographic images. A 3.2-F, 30-MHz intravascular ultrasound (IVUS) catheter (Boston Scientific, Sunnyvale, CA) was advanced over the flow wire and positioned in the distal left circumflex coronary artery. IVUS interrogation of the left circumflex artery was performed using an automated pullback technique at a rate of 0.5 mm/s. Images were recorded on videotapes using Sonos Intravascular Imaging System (Hewlett Packard) for subsequent off-line analysis. Strict comparison of IVUS and angiographic images was taken to ensure accurate placement of the coronary stent. Following IVUS pullback, the IVUS catheter was removed and the coronary stent (3.0- to 4.0-mm diameter by 8- to 20-mm length, Multi-link Penta Coronary Stent System, Guidant, Temecula, CA) catheter was positioned in the left circumflex artery. Coronary stent size was chosen using angiogram and IVUS information to match the recipient artery diameter with stent: artery ratio at 1.0 and stent deployment at optimal inflation pressure (typically 6–10 atm). The coronary stent was deployed in the circumflex artery per routine deployment protocol. Repeat angiography was performed and apposition was confirmed by repeat IVUS interrogation. The IVUS catheter, guiding catheter, and introducer sheath were then removed and the right femoral artery was ligated. The skin was closed in two layers and the animal was allowed to recover from anesthesia.
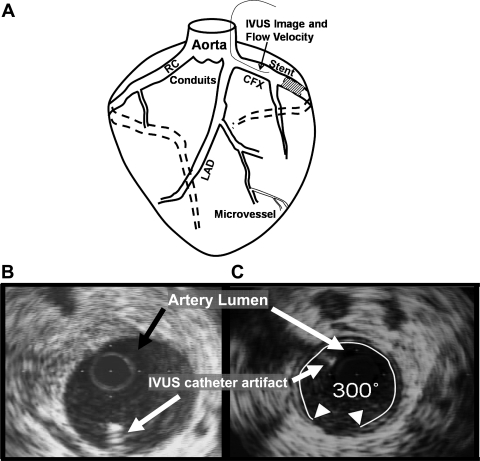
Fig. 1.
Schematic representation showing placement of coronary stent, positioning of intravascular ultrasound (IVUS) and flow wire in the circumflex artery, and IVUS images. A: for coronary blood flow (CBF) determinations, IVUS and flow wire (thin line in CFX) information were collected from a position in the circumflex (CFX) artery proximal to the coronary stent. The full length of the circumflex artery was interrogated with IVUS at stent placement and 3 wk later. The nonstented left anterior descending (LAD) artery was interrogated with IVUS only at the time the animal was euthanized, and the right coronary (RC) was not interrogated. B: representative IVUS image of coronary artery with minimal atherosclerosis. Arrows indicate location of lumen and artifact caused by IVUS catheter. C: image indicating percent degree atheroma calculation. This artery shows 300 degrees of atheroma (wall coverage) of a thin (<0.3 mm) layer of intimal thickening. Degrees of atheroma for an interrogated artery were summed and divided by (360°/mm × length of vessel in mm). This was multiplied by 100 to yield % degrees atheroma to normalize for different lengths of arteries (10).
Followup procedure.
Followup cardiac catheterization for coronary blood flow (CBF) analysis of in vivo microvascular function was performed 3 wk after initial stent placement. Preanesthesia and vascular access via the left femoral artery was similar to that described above. The left coronary ostium was engaged with the guiding catheter and a flow wire was positioned in the distal circumflex with angiographic guidance (41, 55). IVUS catheter was then advanced over the flow wire and positioned in the distal circumflex for IVUS interrogation via pullback as previously described in the peri-stent and in-stent regions. Following IVUS pullback, the IVUS catheter was removed and the flow wire was repositioned in the left circumflex artery proximal to the coronary stent. Strict comparison of IVUS and angiographic images were taken to ensure accurate placement of the flow wire for determination of CBF responses.
Average peak velocity (APV) measured by intracoronary flow wire was recorded continuously throughout the procedure using FloMap (Cardiometrics, Rancho Cordova, CA) for off-line analysis at a later time. The CBF velocity was allowed to reach a steady state (allowing any effects of preanesthesia to resolve) and baseline measurement of APV was obtained. Hyperemia was then induced with bolus doses of the endothelium-independent vasodilator adenosine (3, 1, 0.33, 0.167 μg/kg) given via the guiding catheter into the coronary artery. Peak APV, heart rate, and blood pressure were recorded for each adenosine administration. Subsequent doses of adenosine were administered only after APV had returned to baseline and stabilized, at which time the baseline parameters were again documented followed by the administration of the next adenosine dose. The effect of adenosine typically lasted 15–30 s. After completion of the graded adenosine doses we investigated the response to the endothelium-dependent vasodilator bradykinin. The APV was allowed to return to baseline and bradykinin (4, 2, 1 ng/kg) was administered. APV, heart rate, and blood pressure were continuously recorded as described above. Because of the prolonged response to bradykinin, hemodynamic and blood flow parameters were tracked for 2 min following each bradykinin to ensure return and stabilization of APV to its previous baseline. Subsequent doses of bradykinin were administered only after APV, heart rate, and blood pressure returned to baseline and stabilized.
The APVs, heart rates, blood pressures, and vasodilator-induced peak APVs, heart rates, and blood pressures were determined from off-line analysis of FloMap recordings. Coronary blood flow (ml/min) was calculated as APV (cm/s) × 0.5 × CSA (cm2) /60 (s/min) (41). Coronary vascular resistance (CVR, mmHg·ml−1·min−1) was calculated as mean arterial pressure (MAP) divided by CBF. The rate-pressure product (RPP, beats/min × mmHg) was calculated as heart rate (HR, beats/min) multiplied by systolic blood pressure (SBP, mmHg). Vessel cross-sectional area was calculated offline from recorded IVUS images using the commercially available Sonos Intravascular Imaging software package. End-systolic and end-diastolic cross-sectional areas were determined for three consecutive cardiac cycles. The average of these six measurements was taken as the CSA to be used in subsequent calculations and analysis.
Lipid analysis.
Blood was taken via the anterior vena cava and collected into vacutainers containing 0.117 ml of 15% EDTA after 18 h of fasting, centrifuged in the cold at 4°C for 20 min at 2,000 rpm in a Marathon 21,000 R Centrifuge (Fisher Scientific, Pittsburgh, PA), and frozen at −80°C. Plasma was analyzed for triglycerides, total cholesterol, and high-density lipoprotein (HDL) while low density-lipoprotein (LDL) was calculated from the Friedewald equation: LDL = total cholesterol − HDL − (triglyceride/5) (10).
For each plasma analysis, all samples for a given subject were analyzed in a single run. Plasma triglyceride and total cholesterol were assayed directly by standard enzymatic kit (Thermo Trace, Melbourne, Australia). HDL was measured by precipitating apolipoprotein B-containing lipoproteins with heparin-MnCl2 (8, 10, 11). The supernatant was used to assess HDL using the aforementioned total cholesterol kit and method.
Quantification of atherosclerosis and neointima formation.
To determine extent of atherosclerosis in the coronary arteries, we performed IVUS catheterization on the circumflex and left anterior descending arteries. The left anterior descending was not previously interrogated at the time of stent placement; thus the atheroma detected in this “nonstented” artery (Fig. 1A) was considered native, and uninfluenced by potential catheter-related nonspecific trauma of the stent procedure. The IVUS data were recorded on videotapes for analysis offline. Degrees of atheroma (percent wall coverage) were measured from IVUS images at 1-mm intervals. Degrees of atheroma at each interval of the given vessel were summed and divided by (360°/mm × length of vessel in mm). This was multiplied by 100 to yield percent degrees atheroma to normalize for different lengths of arteries (12, 37) (see Fig. 1B and Table 3).
Table 3.
Short-term exercise training prevents overall atheroma burden
| Percent Degree Atheroma | C | H | HX | Significance |
|---|---|---|---|---|
| CFX | 35.2 ± 5.5 | 59.5 ± 12.5 | 23.0 ± 1.1 | C < H; HX < C, H |
| LAD | 0 | 17.9 ± 2.4 | 3.9 ± 0.3 | C < H, HX; HX < H |
Values are means ± SE. CFX, circumflex artery; LAD, left anterior descending coronary artery. C, H, HX as denoted in Table 1. Statistical significance at P < 0.05.
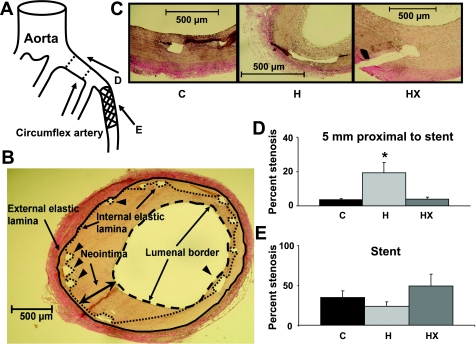
Verhoeff-van Gieson (Fig. 3C) and trichrome staining were performed on sections of stented arteries. Although technically very challenging, we obtained several sections for histology per animal per stain. In animals where more than one section was obtained, values were averaged. Neointima formation (Fig. 3B) was determined by obtaining area measurements bounded by the external elastic lamina and internal elastic lamina (tunica media), internal elastic lamina and lumen (neointima) using commercially available software (ImagePro 3.0). The percent stenosis was calculated by dividing the area of the neointima by the area demarcated by the internal elastic lamina × 100. Collagen content in the sections of the stented arteries was determined by colorimetric analysis (9). The adventitia, which is composed predominantly of collagen, was used as the reference color template against which the rest of the section was compared.
Fig. 3.
Percent stenosis in areas 5 and 10 mm proximal to stented section was significantly greater in H animals compared with control and HX. A: represents a diagram of the circumflex artery and relative location of the stented section and the sections proximal to the stent. B: representative image of the method used to calculate intima and media areas in histological section. Arrowheads indicate location of some stent struts and arrows indicate artery luminal border. Internal and external elastic laminas and neointima are identified. C: representative images of each histological section. Stented sections of the circumflex artery were stained with Verhoeff-van-Gieson stain to accentuate the internal and external elastic lamina. Areas of neointima and lumen were quantified to calculate percent stenosis values. D: summary data for the % stenosis determined at the location 5 mm proximal to the stent (peri-stent CAD). *P < 0.05, H vs. C and HX. E: summary data for the % stenosis obtained from histological analysis within the stent. There were no significant differences across groups within the stent.
Immunohistochemistry for coronary microvessels.
A small piece (1 × 1 cm2) of left ventricular myocardium was obtained at the time the animal was euthanized, and this piece was preserved in 10% formalin solution. Each piece was embedded in paraffin and sectioned (4 μm) for immunohistochemistry for smooth muscle α-actin. Immediately before treatment with antibodies, each section was deparaffinized and treated with an antigen retrieval process. A monoclonal primary antibody to smooth muscle α-actin was used as for identifying coronary vessel (1:1,000, Sigma Chemical, St. Louis, MO), incubated for 1 h. A biotinylated secondary antibody (Vectastain Elite, Vector Laboratories, Burlingame, CA) was used for the detection process. Stained samples were viewed under a light microscope, and images were captured with Coolpix digital camera. Only coronary arteries with internal diameter of 70–200 μm were used; sections of myocardium contained a wide range of microvessels and thus were normalized to cross-sectional area. Optical density (OD) of microvessel cross sections was normalized to the average of the control values and expressed as a percentage of control as indicated in the following: (OD of each individual vessel)/(average OD of control vessels) × 100% = OD as a percentage of control. Finally, microvessel density was measured by counting the number of microvessels in randomly selected 6.5-mm2 areas.
Data analysis.
Statistical analysis was performed using commercially available software (SPSS version 10, SigmaStat 5.0). Coronary blood flow responses and hemodynamic parameters were compared between groups using repeated-measures ANOVA. Vessel cross-sectional area and percent degrees atheroma were compared between groups using single-factor ANOVA. The Dunnett's T3 multiple comparison test was used for data that were not normally distributed (microvessel density). In all cases, P < 0.05 was considered significant.
RESULTS
High-fat/cholesterol diet resulted in ∼8-fold increase in plasma total cholesterol, greater than 20-fold increase in LDL cholesterol, and a slight but significant increase in HDL cholesterol in H and HX relative to control (Table 1). These differences resulted in a 16-fold increase in LDL/HDL ratio in H and HX vs. control. Despite the significant cholesterol response to diet, the experimental design ensured that the body weight was not different between groups. Heart rate response was taken during a submaximal treadmill stress test with the pig running at 3.1 mph at 0% grade for 15 min. In HX, heart rate response to submaximal stress testing was ∼25% lower after 7 wk of training compared with performance on submaximal stress testing completed at the beginning of week 1 (Table 1), thus confirming the efficacy of the exercise training regimen. Heart weight, mean arterial pressure, and rate pressure products were not different between groups as shown in Table 1. IVUS interrogation revealed no flow-limiting stenosis in the studied vessel. Baseline conduit cross-sectional area was not different between groups and did not change with administration of vasodilators.
Table 1.
Phenotypic characteristics of control, H, and HX and adaptations to exercise
| Parameter | C | H | HX | Significance |
|---|---|---|---|---|
| Weight, kg | 59 ± 2 | 59 ± 4 | 58 ± 3 | None |
| Total cholesterol, mg/dl | 52 ± 4 | 466 ± 78 | 393 ± 112 | C < H, HX |
| LDL, mg/dl | 14 ± 3 | 408 ± 72 | 332 ± 115 | C < H, HX |
| HDL, mg/dl | 30 ± 3 | 51 ± 4 | 50 ± 8 | C < H, HX |
| LDL/HDL | 0.53 ± 0.1 | 7.97 ± 0.8 | 7.75 ± 3.4 | C < H, HX |
| TG, mg/dl | 34 ± 5 | 41 ± 9 | 61 ± 34 | None |
| Systolic blood pressure, mmHg | 77 ± 4 | 76 ± 6 | 86 ± 6 | None |
| Diastolic blood pressure, mmHg | 50 ± 4 | 52 ± 6 | 57 ± 6 | None |
| Heart rate, beats/min | 88 ± 6 | 77 ± 9 | 105 ± 9 | None |
| MAP, mmHg | 59 ± 4 | 60 ± 6 | 67 ± 6 | None |
| RPP, beats/min·mmHg | 6,730 ± 580 | 5,800 ± 820 | 9,000 ± 820 | None |
| CSA, mm2 | 12 ± 3 | 12 ± 2 | 12 ± 3 | None |
| Heart wt, g | 235 ± 12 | 265 ± 18 | 236 ± 7 | None |
| Submaximal stress test exercise heart rate, beats/min | ||||
| Start exercise training | 200 ± 30 | |||
| Mid exercise training | 160 ± 40 | |||
| End exercise training | 160 ± 10 | End < Start training |
Values are means ± SE. Male Yucatan swine were on a control diet (C); on a high-fat/cholesterol diet (H); or on a high-fat/cholesterol diet and aerobically exercise trained (HX). LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; MAP, mean arterial pressure; RPP, rate-pressure product; CSA, conduit cross-sectional area. Statistical significance at P < 0.05.
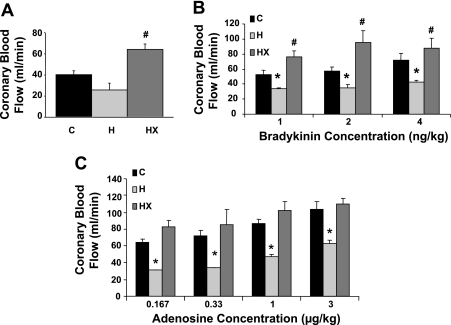
As shown in Fig. 2A, baseline CBF was increased ∼50% in HX relative to control and ∼100% relative to H (P < 0.05). The baseline CBF in H was lower vs. control but did not reach statistical significance (P = 0.08). Interestingly, bradykinin-induced CBF was ∼40% lower in H relative to control at all doses (Fig. 2B, P < 0.05). Short-term exercise training potentiated the CBF responses to bradykinin relative to control. Figure 2C demonstrated adenosine-induced CBF was reduced 50% in H vs. control at all doses (P < 0.05). Similarly, exercise training prevented the impaired CBF response to adenosine.
Fig. 2.
Baseline CBF and response to vasodilators. A: summary data illustrating baseline coronary blood flow was greater in swine placed on a high-fat/cholesterol diet and aerobically exercise trained (HX) vs. those on a high-fat/cholesterol diet (H) and those on a control diet (C). B: summary data demonstrating bradykinin-induced CBF was impaired in H and potentiated in HX relative to C at all doses. C: summary data demonstrating adenosine-induced CBF impairment is prevented with exercise training. *P < 0.05 relative to C; #P < 0.05 relative to both C and H.
Coronary vascular resistance is shown in Table 2. Baseline CVR was elevated in H relative to control (2.22 ± 0.23 vs. 1.46 ± 0.14 mmHg·ml−1·min−1), whereas short-term exercise training significant reduced baseline CVR (0.96 ± 0.05 mmHg·ml−1·min−1). Adenosine-induced CVR was approximately two times higher in H vs. control at all doses (i.e., 1.60 ± 0.15 vs. 0.88 ± 0.10 mmHg·ml−1·min−1 for adenosine 0.33 μg/kg). Short-term exercise training preserved the CVR response to adenosine (0.86 ± 0.16 mmHg·ml−1·min−1 at 0.33 μg/kg). Similarly, CVR in response to bradykinin administration was approximately 50–60% higher in H than control at all doses (i.e., 1.34 ± 0.05 vs. 0.82 ± 0.06 at bradykinin 4 ng/kg). Similar to adenosine, the impaired response to bradykinin was prevented with exercise training (0.80 ± 0.13 at bradykinin 4 ng/kg).
Table 2.
Short-term exercise training prevents CVR derangements
| CVR (mmHg/ml/min) | C | H | HX | Significance |
|---|---|---|---|---|
| Baseline | 1.46 ± 0.14 | 2.22 ± 0.23 | 0.96 ± 0.05 | C < H; HX < C, H |
| Adenosine, μg/kg | ||||
| 0.167 | 0.96 ± 0.08 | 1.80 ± 0.20 | 0.83 ± 0.03 | C, HX < H |
| 0.33 | 0.88 ± 0.10 | 1.60 ± 0.15 | 0.86 ± 0.16 | C, HX < H |
| 1 | 0.71 ± 0.05 | 1.18 ± 0.07 | 0.67 ± 0.03 | C, HX < H |
| 3 | 0.58 ± 0.05 | 0.91 ± 0.13 | 0.60 ± 0.03 | C, HX < H |
| Bradykinin, ng/kg | ||||
| 1 | 1.14 ± 0.08 | 1.72 ± 0.14 | 0.86 ± 0.11 | C < H; HX < C, H |
| 2 | 1.01 ± 0.06 | 1.71 ± 0.38 | 0.73 ± 0.15 | C < H; HX < C, H |
| 4 | 0.82 ± 0.06 | 1.34 ± 0.05 | 0.80 ± 0.13 | C, HX < H |
Values are means ± SE. CVR, coronary vascular resistance. C, H, HX as denoted in Table 1. Statistical significance at P < 0.05.
In addition to assessment of microvascular function, we investigated the effect of exercise on the macrovascular atheroma along the entire length of the artery that was interrogated by IVUS. Results of IVUS analysis are shown in Table 3. The overall macrovascular atheroma in H was almost twofold greater than control as shown as circumflex percent degrees atheroma. Remarkably, this response was completely prevented with exercise training. At death the left anterior descending (LAD), which had not been previously accessed, was also interrogated with IVUS to determine the effects of short-term exercise on native atheroma not related to coronary stenting. As shown in Table 3 there was significantly higher percent degrees atheroma in LAD of H vs. control, which was completely absent of atheroma. Importantly, exercise reduced LAD atheroma. Note that overall atheroma burden was evident in the CFX in the control group only because of the in-stent stenosis in controls contributed substantially to the overall atheroma burden. Finally, to address the effect of hyperlipidemia and exercise on the in-stent atherosclerotic processes, analyses for neointima formation (percent stenosis) and collagen content were performed on the stented sections of the circumflex artery (Fig. 3A). We report no statistical difference across groups with respect to percent stenosis (Fig. 3E). This is in stark contrast to the percent stenosis that was determined 5 mm (Fig. 3, A and D) and 10 mm (data not shown) proximal to the stent, as H pigs had significantly greater percent stenosis in both locations compared with the control and HX groups. In addition, we found significantly higher percent collagen in the stented sections of the H animals (21.5 ± 6.8%) compared with the control (0.9 ± 0.3%) or HX (2.1 ± 0.8%). Taken together, these data suggest that the higher amount of atheroma in the circumflex artery of the H animals was predominantly in the nonstented sections of the interrogated artery, which is strongly supported by the summary comparison data of histology in Fig. 3, D and E.
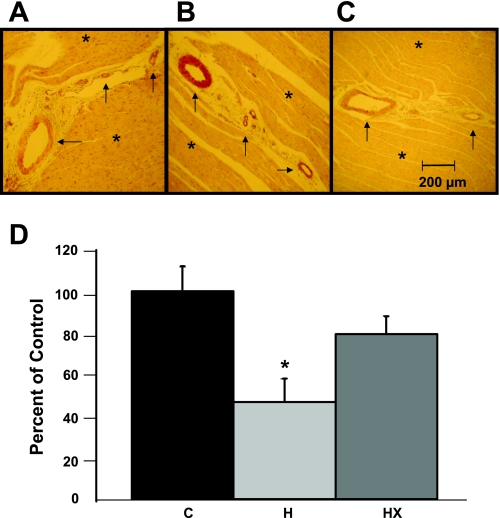
Finally, Fig. 4, A–D, represents microvessel density in the left ventricular sections obtained from all pigs. Microvessel density was significantly decreased in H compared with control (47% of C). Microvessel density in the HX group was higher than in the H group (80% of C), suggesting that exercise training partially prevented this reduction in microvessel density.
Fig. 4.
Coronary microvessel density after stenting was significantly less in the H animals vs. control (P < 0.05). Left ventricular sections obtained from each animal were preserved in formalin and stained with an antibody for α-smooth muscle actin to identify arteriolar microvessels. A–C: representative section of left ventricular tissue from a C, H, and HX animal. Asterisk (*) indicates areas of cardiac muscle; arrow indicates coronary microvessels. D: summary data for the microvessel density obtained from immunohistochemical analysis in the myocardium. *P < 0.05 vs. C and HX.
DISCUSSION
To our knowledge, this is the first study examining the effects of exercise training on microvascular function and macrovascular atheroma after coronary stenting in a model of hypercholesterolemia and CAD. Additionally, we provide evidence that microvascular rarefaction may underlie the blunted hyperlipidemia-induced response and normalized exercise training-induced blood flow responses to adenosine and bradykinin in the coronary microcirculation. Coronary stenting has been shown to mechanically damage vascular cells in the target conduit-artery segment, endothelium in peri-stent segments (30), and induces downstream microvascular dysfunction that persists for weeks and has been implicated in causing exertional ischemia (5, 28, 42, 59, 61). A major finding of this study is bare metal stent deployment in our hyperlipidemic swine model results in microvascular dysfunction, as assessed by the decreased CBF response to intracoronary bradykinin and adenosine administration and a reduction in baseline CBF (Fig. 2, A–C). Second, short-term exercise training prevented the microvascular dysfunction in response to intracoronary bradykinin and/or adenosine (Fig. 2, B and C). A third major finding was that hyperlipidemia decreased microvessel density (Fig. 4), thus providing a structural mechanism for the in vivo responses to adenosine and bradykinin reported herein. Interestingly, exercise training prevented the reduction in microvessel density (Fig. 4D). These data demonstrate the efficacy of short-term exercise training on preventing early pathophysiological responses to coronary stent deployment in hyperlipidemic swine. Equally remarkable is that the beneficial effects of exercise training were independent of changes in plasma lipids. Taken together, we conclude that, in addition to the well-known effect of exercise training to improve plasma cholesterol profile, exercise may act directly on the vascular wall or other plasma risk factors not measured here.
Similar impairment of endothelium-dependent microvascular function and CBF have been reported in the setting of hypercholesterolemia alone (4, 18, 47, 48, 51). Possible mechanisms for the hypercholesterolemia-induced endothelium-dependent dysfunction include an increase in endothelin-1 (ET-1) (4), a reduction in nitric oxide (NO) synthase (56), or responsiveness to NO (60). We did not measure protein levels of ET-1 or NO synthase or responsiveness to NO in the microvessel preparations in the present study. Furthermore, coronary interventions can damage vascular cells and induce endothelial dysfunction in arterial segments distal to the angioplasty/stent site (30), with one study suggesting that endothelial dysfunction following coronary interventions is related to inducible ischemia (42). Importantly, the impaired microvascular function and CBF could also be secondary to direct endothelial cell damage. The present findings of impaired responses to adenosine (largely endothelium-independent vasodilator) suggest that the microvascular dysfunction was not limited solely to a dysfunctional endothelium, but suggests smooth muscle function was also adversely affected. We acknowledge that a component of adenosine-induced relaxation is endothelium dependent in rat vasculature (24); however, the effects of adenosine are mediated through a primarily endothelium-independent pathway, which is the predominant vasodilatory pathway in porcine coronary microvessel.
Previous reports have shown beneficial effects of exercise training including improved myocardial perfusion and improved endothelial function (20, 36, 57). However, these studies did not involve revascularized patients. More recent studies involving patients undergoing coronary interventions report improvements in functional capacity and quality of life but report conflicting results regarding recurrent events and rehospitalizations, and these studies did not directly assess microvascular function (3, 25, 31, 40, 46). Hosokawa et al. (26) directly assessed endothelial function in vivo and reported regular exercise training improved endothelial function in non-infarct-related coronary arteries following coronary interventions for myocardial infarction. Importantly, that study did not evaluate function of the culprit artery. Finally, it is important to note that these studies involved long-term exercise training, which contrasts sharply to the short-term (7 wk) regimen in the present study.
Short-term exercise training resulted in significantly lower native atheroma burden as determined by IVUS interrogation of the left anterior descending (nonintervened vessel) (Table 3). These data are consistent with other investigators who have shown that long-term exercise training has beneficial cardioprotective effects with some evidence suggesting that exercise training may delay the progression or even induce regression of CAD (45, 52, 53). Additionally, IVUS interrogation of the circumflex artery revealed that short-term exercise training significantly decreased the macrovascular atheroma response following stenting. This result is noteworthy in light of recent human studies involving long-term exercise training following coronary interventions that did not result in significant differences in restenosis rates (3, 25, 31, 46). Interestingly, the formation of neointima within the stent was not different across groups; thus the differences in atheroma burden across groups stem from difference in the nonstented regions. This is noteworthy as little is known regarding the progression of CAD in a stented vessel proximal and distal to the stent. Because of the increase in collagen content in the H animals in the tunica media, the mechanisms responsible for the alteration in cellular composition in each of these regions warrant additional investigation. Further punctuating these present findings is the fact that coronary stenting is clearly the most widely accepted treatment option for patients with CAD, yet restenosis still affects 15–25% of patients and often requires repeated interventions even with recent advances in therapy (35, 49). The exact mechanisms involved in the beneficial effects of short-term exercise training are still not clearly elucidated but could involve neurohumoral, mechanical, direct, or indirect effects on the vascular wall.
Revascularization of an occluded artery with percutaneous angioplasty has been shown to impair conduit and microvascular dilation (5, 19, 28, 30, 42, 50, 61). We investigated one potential underlying mechanism related to microvascular dysfunction in the present study—the reduction in microvascular density in the hypercholesterolemic swine (Fig. 4D). Since the hyperemic response to adenosine and bradykinin is a result of microvessel vasodilation, it stands to reason that one potential common explanation for a blunted response to both adenosine and bradykinin could be a reduction in number of microvessels per tissue area. As for the relative importance of different mechanisms for CBF changes, we have no definitive conclusions yet based on our current studies. Since short-term exercise training potentiated bradykinin-induced CBF in HX relative to both H and C, the endothelium-dependent factors may be more exercise sensitive in terms of CBF changes.
The smaller pig number in the HX group could be considered a limitation of our study; however, this design was strictly compliant with the principle of reducing the number of animals exposed to surgical and exercise stress to the minimum number required for statistical power (1, 6, 44). Further, evidence for the validity of the results from the small pig number in the HX group was shown by similar results in our report that exercise training significantly decreased atheroma burden in Ossabaw miniature swine with metabolic syndrome that were treated with coronary stents (13). Another limitation of our study is the animals did not receive a typical medical regimen that would be considered standard of care for human patients undergoing coronary interventions. Pigs in our study were given daily aspirin and periprocedural heparin but did not receive clopidogrel. Further, the pigs did not receive typical lipid-lowering agents, by design, which have also been shown to have an ameliorating effect on endothelial dysfunction and even atheroma burden as well as outcomes in patients with CAD (14, 27, 33, 34, 54, 58) and our model (9, 23, 32). Our rationale for translation of these results to human patients is that those patients who experience side effects of statins (e.g., liver dysfunction, rhabdomyolysis), clopidogrel (e.g., excess bleeding, gastrointestinal irritation), angiotensin converting-enzyme inhibitors and beta blockers (e.g., cough, orthostatic hypotension) might be able to decrease their dosage to avoid side effects because of the profound effects of exercise training. Additionally, by design, our study did not address how long the beneficial exercise effect was maintained, nor did it address whether the effect was preserved following cessation of exercise. Clearly, future studies should include exercise superimposed on all standard care medications and the effects of cessation of exercise.
The results of this study demonstrate that short-term exercise training preserves endothelium-independent coronary microvascular responses, potentiates endothelium-dependent coronary microvascular function, and significantly diminishes the overall macrovascular atheroma burden after coronary stenting in a porcine model of hyperlipidemia and CAD. Our results add direct evidence for microvessel dropout (rarefaction), which may explain the parallel CBF responses to endothelium-dependent (bradykinin) and endothelium-independent (adenosine) dilators. Combined with the exercise-induced decrease in macrovascular atheroma burden, these data provide a compelling basis for exercise training to ameliorate complications associated with coronary stenting in hyperlipidemic swine. To our knowledge this is the first study to report such effects associated with peri-procedural short-term exercise training independent of favorable changes in plasma cholesterol. These phenomenal effects of exercise training, if translatable to human clinical medicine, could offer an outstanding adjunct to drug-eluting stent and pharmacological treatment for flow-limiting lesions if exercise training can ameliorate the even greater microvascular dysfunction in drug-eluting compared with bare metal stents (29, 39).
GRANTS
This work was supported by National Institutes of Health Grants RR-013223 and HL-062552 to M. Sturek, HL-052490 to M. H. Laughlin, and T32-HL-007094 and T32-AR-048523 to M. Sturek; a Translational Research Fellowship from the Indiana University School of Medicine to J. M. Edwards; and an American Heart Association Predoctoral Fellowship to X. Long.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Shawn G. Kaser and Eric A. Mokelke for excellent technical support and contributions to the manuscript.
REFERENCES
- 1. Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett BT, Pascoe P, Shull E, Cork LC, Francis-Floyd R, Amass KD, Johnson R, Schmidt RH, Underwood W, Thornton GW, Kohn B. 2000 Report of the AVMA panel on euthanasia. JAMA 218: 669–696, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 97: 553–561, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 37: 1891–1900, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Best PJM, McKenna CJ, Hasdai D, Holmes DR, Jr, Lerman A. Chronic endothelin receptor antagonism preserves coronary endothelial function in experimental hypercholesterolemia. Circulation 99: 1747–1752, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 356: 830–840, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Committee to Develop a Resource Book for Animal Exercise Protocols. Resource Book for the Design of Animal Exercise Protocols. Bethesda, MD: Am. Physiol. Soc., 2006 [Google Scholar]
- 7. Conn E, Williams RS, Wallace AG. Exercise responses before and after physical conditioning in patients with severely depressed left ventricular function. Am J Cardiol 49: 296–300, 1982 [DOI] [PubMed] [Google Scholar]
- 8. Demacker PN, Hessels M, Toenhake-Dijkstra H, Baadenhuijsen H. Precipitation methods for HDL-C measurement compared, and final evaluation under routine operating conditions of a method with a low sample-to-reagent ratio. Clin Chem 43: 663–668, 1997 [PubMed] [Google Scholar]
- 9. Dixon JL, Shen S, Vuchetich JP, Wysocka E, Sun G, Sturek M. Increased atherosclerosis in diabetic dyslipidemic swine: protection by atorvastatin involves decreased VLDL triglycerides but minimal effects on the lipoprotein profile. J Lipid Res 43: 1618–1629, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Dyson M, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006 [PubMed] [Google Scholar]
- 11. Dyson M, Mokelke EA, Vuchetich J, Sturek M. Use of computed tomography to evaluate intra-abdominal fat stores in a swine model of the metabolic syndrome (abstract). FASEB J 19: A191, 2005 [Google Scholar]
- 12. Edwards JM, Alloosh M, Long X, Dick GM, Lloyd PG, Mokelke EA, Sturek M. Adenosine A1 receptors in neointimal hyperplasia and in-stent stenosis in Ossabaw miniature swine. Coron Artery Dis 19: 27–31, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85: 631–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, Takeshita A. Reduction in serum cholesterol with pravastatin improves endothelium dependent coronary vasomotion in patients with hypercholesterolemia. Circulation 89: 2519–2514, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Ehsani AA, Heath GW, Hagberg JM, Sobel BE, Holloszy JO. Effects of 12 months of intense exercise training on ischemic ST-segment depression in patients with coronary artery disease. Circulation 64: 1116–1124, 1981 [DOI] [PubMed] [Google Scholar]
- 16. Feuerstein GZ. Restenosis: basic research and clinical perspective. In: Restenosis: From Genetics to Therapeutics, edited by Feuerstein GZ. New York: Dekker, 1997, p. 1–4 [Google Scholar]
- 17. Fleenor BS, Bowles DK. Exercise training decreases the size and alters the composition of the neointima in a porcine model of percutaneous transluminal coronary angioplasty (PTCA). J Appl Physiol 107: 937–945, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilligan DM, Guetta V, Panza JA, García CE, Quyyumi AA, Cannon RO. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation 90: 35–41, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Gregorini L, Marco J, Farah B, Bernies M, Palombo C, Kazakova M, Bossi IM, Cassagneau B, Fajadet J, Di Mario C, Albiero R, Cugno M, Grossi A, Heusch G. Effects of selective α1- and α2-adrenergic blockade on coronary flow reserve after coronary stenting. Circulation 106: 2901–2907, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu JT, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease. Circulation 109: 1371–1378, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca2+ ATPase uptake is impaired in coronary smooth muscle distal to chronic occlusion. Am J Physiol Heart Circ Physiol 281: H223–H231, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hinschen AK, Rose'meyer RB, Headrick JP. Age-related changes in adenosine-mediated relaxation of coronary and aortic smooth muscle. Am J Physiol Heart Circ Physiol 280: H2380–H2389, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Hofman-Bang C, Lisspers J, Nordlander R, Nygren A, Sundin O, Ohman A, Ryden L. Two-year results of a controlled study of residential rehabilitation for patients treated with percutaneous transluminal coronary angioplasty. A randomized study of a multifactorial programme. Eur Heart J 20: 1465–1474, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Hosokawa S, Hiasa Y, Takahashi T, Itoh S. Effect of regular exercise on coronary endothelial function in patients with recent myocardial infarction. Circulation 67: 221–224, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Huggins GS, Pasternak RC, Alpert NM, Fischman AJ, Gewirtz H. Effects of short-term treatment of hyperlipidemia on coronary vasodilator function and myocardial perfusion in regions having substantial impairment of baseline dilator reverse. Circulation 98: 1291–1296, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Kern MJ, Puri S, Bach RG, Donohue TJ, Dupouy P, Caracciolo EA, Craig WR, Aguirre F, Aptecar E, Wolford TL, Mechem CJ, Dubois-Rande JL. Abnormal coronary flow velocity reserve after coronary artery stenting in patients. Role of relative coronary reserve to assess potential mechanisms. Circulation 100: 2491–2498, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Kern MJ. Attenuated coronary collateral function after drug-eluting stent implantation: a new downside of drug-eluting stents? J Am Coll Cardiol 49: 21–22, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kim JW, Seo HS, Park JH, Na JO, Choi CU, Lim HE, Kim EJ, Rha SW, Park CG, Oh DJ. A prospective, randomized, 6-month comparison of the coronary vasomotor response associated with a Zotarolimus- versus a Sirolimus-eluting stent: differential recovery of coronary endothelial dysfunction. J Am Coll Cardiol 53: 1653–1659, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Kubo H, Yano K, Hirai H, Yabuki S, Machii K. Preventive effect of exercise training on recurrent stenosis after percutaneous transluminal angioplasty (PTCA). Jpn Circ J 56: 413–421, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Lee DL, Wamhoff BR, Katwa LC, Reddy HK, Voelker DJ, Dixon JL, Sturek M. Increased endothelin-induced Ca2+ signaling, tyrosine phosphorylation, and coronary artery disease in diabetic dyslipidemic swine are prevented by atorvastatin. J Pharmacol Exp Ther 306: 132–140, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Leung W, Lau CP, Wong CK. Beneficial effect of cholesterol-lowering therapy on coronary endothelium-dependent relaxation in hypercholesterolemic patients. Lancet 341: 1496–1500, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Levine GN, Keaney JR, Jr, Vita JA. Cholesterol reduction in cardiovascular disease: clinical benefits and possible mechanisms. N Engl J Med 332: 512–521, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Lincoff AM, Califf RM, Topol EJ. Platelet glycoprotein IIb/IIIa receptor blockade in coronary artery disease. J Am Coll Cardiol 35: 1103–1115, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Linxue L, Nohara R, Makita S, Hosokawa R, Hata T, Okuda K, Hamazaki H, Fujita M, Sasayama S. Effects of long-term exercise training on regional myocardial perfusion changes in patients with coronary artery disease. Jpn Circ J 63: 73–78, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Lloyd PG, Sheehy AF, Edwards JM, Mokelke EA, Sturek M. Leukemia inhibitory factor is upregulated in coronary arteries of Ossabaw miniature swine after stent placement. Coron Artery Dis 19: 217–226, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Maddahi J, Garcia EV, Berman DS, Waxman A, Swan H, Forrester J. Improved noninvasive assessment of coronary artery disease by quantitative analysis of regional stress myocardial distribution and washout of thallium-201. Circulation 64: 924–935, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Meier P, Zbinden R, Togni M, Wenaweser P, Windecker S, Meier B, Seiler C. Coronary collateral function long after drug-eluting stent implantation. J Am Coll Cardiol 49: 15–20, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Mobius-Winkler S, Hollriegel R, Schuler G, Adams V. Endothelial progenitor cells: implications for cardiovascular disease. Cytometry A 75: 25–37, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Monnink S, Tio R, Veeger N, Amoroso G, Van Boven AJ, Van Gilst WH. Exercise-induced ischemia after successful percutaneous coronary intervention is related to distal coronary endothelial dysfunction. J Investig Med 51: 221–226, 2003 [DOI] [PubMed] [Google Scholar]
- 43. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996 [Google Scholar]
- 44. National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: National Academy Press, 2003 [PubMed] [Google Scholar]
- 45. O'Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, Jr, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 80: 234–244, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Pasquali S, Alexander K, Coombs L, Lytle B, Peterson E. Effect of cardiac rehabilitation on functional outcomes after coronary revascularization. Am Heart J 145: 445–451, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Pitkänen OP, Nuutila P, Raitakari OT, Porkka K, Lida H, Nuotio I, Rönnemaa T, Viikari J, Taskinen MR, Ehnholm C, Knuuti J. Coronary flow reserve in young men with familial combined hyperlipidemia. Circulation 99: 1678–1684, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Pitkänen OP, Raitakari OT, Niinikoski H, Nuutila P, Lida H, Voipio-Pulkki LM, Härkönen R, Wegelius U, Rönnemaa T, Viikari J, Knuuti J. Coronary flow reserve is impaired in young men with familial hypercholesterolemia. J Am Coll Cardiol 28: 17 05–1711, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Popma JJ, Califf RM, Topol EJ. Clinical trials of restenosis after coronary angioplasty. Circulation 84: 1426–1436, 1991 [DOI] [PubMed] [Google Scholar]
- 50. Prati F, Pawlowski T, Gil R, Labellarte A, Gziut A, Caradonna E, Manzoli A, Pappalardo A, Burzotta F, Boccanelli A. Stenting of culprit lesions in unstable angina leads to a marked reduction in plaque burden: A major role of plaque embolization?: A serial intravascular ultrasound study. Circulation 107: 2320–2325, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol 23: 833–843, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Schuler G, Hambrecht R, Schlierf G, Grunze M, Methfessel S, Hauer K, Kübler W. Myocardial perfusion and regression of coronary artery disease in patients on a regimen of intensive physical exercise and low fat diet. J Am Coll Cardiol 19: 34–42, 1992 [DOI] [PubMed] [Google Scholar]
- 53. Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M, Kübler W. Regular physical exercise and low-fat diet: Effects on progression of coronary artery disease. Circulation 86: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 54. Schwartz R, Pearson TA, Kalaria V, Mackin M, Williford DJ, Awasthi A, Shah A, Rains A, Guido J. Prospective serial evaluation of myocardial perfusion and lipids during first six months of prvastatin therapy: coronary artery disease regression single photon emission computed tomography monitoring trial. J Am Coll Cardiol 42: 600–610, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA, Brisbin IL., Jr Ossabaw island miniature swine: cardiometabolic syndrome assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques, edited by Swindle MM. Boca Raton, FL: CRC, 2007, p. 397–402 [Google Scholar]
- 56. Taner CB, Severson SR, Best PJM, Lerman A, Miller VM. Treatment with endothelin-receptor antagonists increases NOS activity in hypercholesterolemia. J Appl Physiol 90: 816–820, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Thompson PD. The benefits and risks of exercise training in patients with chronic coronary artery disease. JAMA 259: 1537–1540, 1988 [PubMed] [Google Scholar]
- 58. Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med 332: 481–487, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Van Liebergen RAM, Piek JJ, Koch KT, De Winter RJ, Lie KI. Immediate and long-term effect of balloon angioplasty or stent implantation on the absolute and relative coronary blood flow velocity reserve. Circulation 98: 2133–2140, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Weisbrod RM, Griswold MC, Du Y, Bolotina VM, Cohen RA. Reduced responsiveness of hypercholesterolemic rabbit aortic smooth muscle cells to nitric oxide. Arterioscler Thromb Vasc Biol 17: 394–402, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Werner GS, Emig U, Bahrmann P, Ferrari M, Figulla HR. Recovery of impaired microvascular function in collateral dependent myocardium after recanalisation of a chronic total coronary occlusion. Heart 90: 1303–1309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]