Abstract
The effect of hyperventilation-induced hypocapnic alkalosis (Hypo) on the adjustment of pulmonary O2 uptake (V̇o2p) and leg femoral conduit artery (“bulk”) blood flow (LBF) during moderate-intensity exercise (Mod) was examined in eight young male adults. Subjects completed four to six repetitions of alternate-leg knee-extension exercise during normal breathing [Con; end-tidal partial pressure of CO2 (PetCO2) ∼40 mmHg] and sustained hyperventilation (Hypo; PetCO2 ∼20 mmHg). Increases in work rate were made instantaneously from baseline (3 W) to Mod (80% estimated lactate threshold). V̇o2p was measured breath by breath by mass spectrometry and volume turbine, and LBF (calculated from mean femoral artery blood velocity and femoral artery diameter) was measured simultaneously by Doppler ultrasound. Concentration changes of deoxy (Δ[HHb])-, oxy (Δ[O2Hb])-, and total hemoglobin-myoglobin (Δ[HbTot]) of the vastus lateralis muscle were measured continuously by near-infrared spectroscopy (NIRS). The kinetics of V̇o2p, LBF, and Δ[HHb] were modeled using a monoexponential equation by nonlinear regression. The time constants for the phase 2 V̇o2p (Hypo, 49 ± 26 s; Con, 28 ± 8 s) and LBF (Hypo, 46 ± 16 s; Con, 23 ± 6 s) were greater (P < 0.05) in Hypo compared with Con. However, the mean response time for the overall Δ[HHb] response was not different between conditions (Hypo, 23 ± 5 s; Con, 24 ± 3 s), whereas the Δ[HHb] amplitude was greater (P < 0.05) in Hypo (8.05 ± 7.47 a.u.) compared with Con (6.69 ± 6.31 a.u.). Combined, these results suggest that hyperventilation-induced hypocapnic alkalosis is associated with slower convective (i.e., slowed femoral artery and microvascular blood flow) and diffusive (i.e., greater fractional O2 extraction for a given ΔV̇o2p) O2 delivery, which may contribute to the hyperventilation-induced slowing of V̇o2p (and muscle O2 utilization) kinetics.
Keywords: oxygen uptake kinetics, respiratory alkalosis, Doppler ultrasound, near-infrared spectroscopy
acute voluntary hyperventilation increases CO2 output, thereby reducing the end-tidal (PetCO2) and arterial partial pressure of CO2 (PaCO2) and lowering plasma and muscle [H+] (13, 26). As shown previously, hyperventilation-induced hypocapnic alkalosis results in a slower adjustment of pulmonary O2 uptake (V̇o2p) (13, 14, 33) and slower and greater breakdown of muscle phosphocreatine (PCr) (26) (both reflecting kinetics of muscle O2 utilization) compared with a normal breathing condition during moderate-intensity exercise. The increase in the fundamental (phase 2) component of V̇o2p during the transition to moderate-intensity exercise is not instantaneous but increases exponentially toward a new steady-state level. The adjustment of V̇o2p (and muscle O2 utilization) is believed to be limited by convective and diffusive delivery of O2 (i.e., blood flow) to the active muscles, to activation of rate-limiting enzymes that provide oxidative substrate for mitochondrial oxidative phosphorylation, or to a combination of these factors (29, 54, 68). Whether the slower V̇o2p adjustment during hyperventilation-induced hypocapnic alkalosis is related to either or both of these factors remains to be resolved.
Using near-infrared spectroscopy (NIRS), Chin et al. (13, 14) reported that the time course of muscle deoxygenation (Δ[HHb]; reflecting local muscle O2 utilization-to-microvascular blood flow ratio) at exercise onset was similar to that seen in the control condition, despite slower V̇o2p kinetics and similar V̇o2p amplitude, suggesting that the greater fractional O2 extraction and lower muscle O2 utilization early in the exercise transition were the consequences of an attenuated microvascular blood flow response in hyperventilation-induced hypocapnic alkalosis. Greater O2 extraction would be expected to lower microvascular Po2 and the capillary-to-muscle Po2 gradient and to impair O2 diffusion to the mitochondrial cytochrome c oxidase (assuming no change in muscle O2 diffusional conductance). An attenuated convective and diffusive O2 delivery could constrain the activation of mitochondrial oxidative phosphorylation and muscle O2 utilization, as evidenced by the slower V̇o2p kinetics.
To our knowledge, the effects of hyperventilation-induced hypocapnic alkalosis on blood flow and blood flow kinetics during exercise have not been examined in humans, although effects on resting blood flow have yielded conflicted findings. In animals mechanically ventilated to induce alkalosis, hindlimb blood flow was unchanged (41) or reduced (11, 31, 38, 40). In humans, forearm blood flow was shown to decrease in response to 6 min of voluntary hyperventilation (42), whereas increases in blood flow were measured in human forearm (12, 16, 57, 58) and calf (17) and inferred by decreased arteriovenous O2 difference across the leg muscle (46) during bouts of hyperventilation lasting between 1 and 15 min.
Therefore, with confounding information regarding the response of blood flow during acute hypocapnic alkalosis at rest and limited knowledge available during exercise in humans, the purpose of this study was to examine the adjustments of leg femoral conduit artery (“bulk”) blood flow (by Doppler ultrasound) and microvascular perfusion (by NIRS) in relation to V̇o2p during transitions to moderate-intensity exercise with normal breathing (control) and hyperventilation-induced hypocapnic alkalosis. It was hypothesized that in hyperventilation-induced hypocapnic alkalosis, compared with control, a slower adjustment of leg femoral conduit artery (and microvascular) blood flow would accompany the slower V̇o2p kinetics and greater and faster rate of muscle deoxygenation (and O2 extraction).
METHODS
Ethical Approval
This protocol and all procedures were approved by The University of Western Ontario Ethics Committee for Research on Human Subjects, in accordance with the Declaration of Helsinki. Subjects were given thorough verbal and written explanation of the experimental protocol, including possible risks and discomforts associated with the testing procedure. Subjects provided written consent before volunteering to participate in this study.
Subjects
Eight young male subjects (age, 26 ± 5 yr; mean ± SD) participated in this study and were all nonsmokers and free of known respiratory, cardiovascular, and metabolic disease.
Preexperimental Protocol
All tests were performed on a custom-built alternate-leg knee-extension ergometer as previously described (49). Briefly, subjects were in a seated upright position with their legs strapped to a padded “arm” that was attached to a Monark cycle ergometer (model 814E). Passive movement of the subject's legs was accomplished by having an assistant pedal the cycle ergometer, whereas active exercise involved having the subject alternately extend their legs over a 2-s duty cycle (i.e., 1-s contraction, 1-s relaxation), which resulted in a frequency of 30 extensions per minute (epm) per leg.
Each subject completed an initial incremental exercise test to volitional fatigue to determine their estimated lactate threshold (θ̂L), peak O2 uptake (V̇o2peak), and moderate-intensity work rate (WR). Testing began with 2 min of passive movement of the subject's legs, followed by an increase in work rate of 6 W (load, 200 g) every min, starting at 18 W (load, 600 g). The test ended when subjects were unable to sustain the kicking frequency of 30 epm despite verbal encouragement.
The θ̂L was determined by visual inspection and defined as the V̇o2p at which the following were observed to occur simultaneously: 1) CO2 output (V̇co2) increased out of proportion to the increase of V̇o2p, 2) ventilation (V̇e) increased out of proportion to the increase of V̇o2p, 3) the ventilatory equivalent for V̇o2p (V̇e/V̇o2p) began to rise with no systematic increase in the ventilatory equivalent for V̇co2 (V̇e/V̇co2), and 4) the end-tidal Po2 (PetO2) began to rise with a plateau in the end-tidal Pco2 (PetCO2). The V̇o2peak was calculated from the average of the final 20-s V̇o2p values of the exercise test. The WR that elicited ∼80% of the θ̂L (moderate-intensity exercise; Mod) also was determined from the results of the incremental exercise test.
Before data collection began, subjects familiarized themselves with the hyperventilation maneuver by establishing the degree of hyperventilation needed to attain the target PetCO2 of ∼20 mmHg from a normal PetCO2 of ∼40 mmHg (13, 45). Breath-by-breath PetCO2 data were collected and displayed (PowerLab Chart version 4.2; ADInstruments, Colorado Springs, CO) on a computer screen to allow subjects to monitor and adjust their breathing frequency and/or tidal volume to maintain PetCO2 relatively constant, even during exercise, at ∼20 mmHg.
Exercise Protocol
The protocol began with 5 min of normal breathing (preaccommodation), followed by an “accommodation period” of 20 min, when subjects either continued to breathe normally (Con; PetCO2 ∼40 mmHg) or were instructed to voluntarily hyperventilate to induce a hypocapnic alkalosis (Hypo; PetCO2 ∼20 mmHg). During Hypo trials, the duration of the accommodation period was sufficient to reduce and equilibrate body CO2 stores to the new lower level (10), and continued hyperventilation throughout the remainder of the exercise protocol maintained PetCO2 at the required level (i.e., PetCO2 was kept at ∼20 mmHg until the protocol was terminated).
The exercise component of the protocol began with 2 min of passive alternate-leg knee-extension (KE) exercise, where an assistant pedaled the cycle ergometer to move the subject's legs passively at 30 epm, followed by 6 min of active baseline KE exercise at 3 W (100 g), where the subject actively contracted at 30 epm. The inclusion of passive leg movement in this study minimized the mechanical effect at initiation of active exercise by the subject (49) and ensured a consistent pace was maintained between subjects at the onset of the exercise protocol (56). Step transitions in WR occurred from the active baseline exercise to a WR corresponding to ∼80% θ̂L (Mod). Each step lasted 6 min, and transitions were made instantaneously and without warning the subjects. The protocol was repeated four to six times per condition to improve the signal-to-noise ratio and, therefore, the confidence of the measured responses. Trials for each condition were randomized, and only a single trial was performed per visit, with each visit separated by at least 24 h.
Data Collection
Gas exchange.
Inspired and expired airflow and volumes were measured throughout the exercise protocol by a low dead space (90 ml) bidirectional volume turbine (VMM-110; Alpha Technologies, Laguna Hills, CA) that was calibrated before each test using a syringe of known volume (3.0 liters; Hans Rudolph, Kansas City, MO). Respired gases were sampled continuously (1 ml/s) at the mouth and analyzed for the fractional concentrations of O2, CO2, and N2 by mass spectrometry (AMIS 2000; Innovision, Lindvedvej, Denmark) following calibration with precision-analyzed gas mixtures. Inspired and expired volumes were time-aligned with changes in gas concentrations by measuring the time delay for a bolus of gas to travel through a capillary line from the turbine transducer and be detected by the mass spectrometer. The algorithms of Beaver et al. (8) were used to calculate breath-by-breath alveolar gas exchange.
Leg blood flow and heart rate.
Measures of femoral artery mean blood velocity (MBV) were made from the right leg using pulsed-wave Doppler ultrasound (Vingmed System FiVe; GE Medical Systems, Horten, Norway). Data were collected continuously using a 7.5-MHz probe with an insonation angle of 45° positioned at ∼2–3 cm distal to the inguinal ligament and proximal to the femoral artery bifurcation. This location was selected to avoid blood flow interference from the inguinal and surrounding regions, as well as to minimize the turbulence from the femoral bifurcation (49, 56). MBV was obtained from each trial by integrating the total area under the MBV profile and averaging over the 2-s duty cycle. Trials in the same condition for a given subject were averaged together to yield a single MBV profile for each subject in Con and Hypo trials. Although femoral artery (FA) diameters were not expected to change from rest and at any point during moderate-intensity exercise in Con [as demonstrated previously by Koga et al. (39), MacDonald et al. (47), MacPhee et al. (49), Radegran (55), and Radegran and Saltin (56)], hypocapnic alkalosis has been shown to reduce the diameter of peripheral microvessels in rabbits (40). As such, trials were recorded and stored on VHS tapes for further determination of FA diameters throughout the experimental protocol. Measures of diastolic FA diameters were made in triplicate using on-screen calipers included with the Doppler ultrasound and were averaged together to produce a single diameter value at that given time point. During Con trials, diameter measures were taken before the accommodation period, at the end of the accommodation period, during passive exercise, and during steady-state baseline and Mod. During the Hypo trials, diameter measures were obtained as follows: at least twice during the preaccommodation period; every 2 min during the accommodation period, passive exercise, and baseline; every 10 s during the first 2 min of the Mod transition; and every 30 s during the last 4 min of Mod. The FA diameter data were analyzed in a manner similar to MacDonald et al. (48), where averages for each subject by condition were fit with either a linear (for Con) or an exponential regression (for Hypo) to yield a single averaged response across time. With the averaged MBV data and the FA diameter data obtained from the regression equation, calculation of LBF was made as follows: LBF (l/min) = MBV (cm/s) × πr2 (mm2) × 0.0006, where r is the radius of the FA.
Beat-by-beat heart rate (HR) was monitored and recorded continuously using an electrocardiogram (LifePulse; HME, South Mimms, UK) with a three-lead arrangement and stored on a separate computer for further analysis (PowerLab Chart version 4.2).
Near-infrared spectroscopy.
Changes in local muscle oxy (Δ[O2Hb])-, deoxy (Δ[HHb])-, and total hemoglobin-myoglobin concentrations (Δ[HbTot]) were continuously measured by NIRS (NIRO 300; Hamamatsu Photonics, Hamamatsu, Japan). Optodes were housed in an optically dense rubber holder to ensure the specific distance between the optodes was fixed at 5 cm and then placed on the skin above the vastus lateralis muscle midway between the lateral epicondyle and greater trochanter of the femur of the right leg. A black vinyl sheet was taped to the skin surface to cover the optode assembly and to minimize the loss of near-infrared transmitted light from the region of interrogation and reduce the intrusion of extraneous light. To further secure the position of the optodes during the exercise protocol, an elastic bandage was wrapped around the leg to prevent any movement of the optode assembly while still permitting freedom of movement. The NIRS signals were monitored until a steady baseline was established, at which time the signals were set to zero.
A detailed explanation of the principle and theory of NIRS as used in the present study is described by Elwell (23). Briefly, four laser diodes produce different wavelengths (775, 810, 850, and 910 nm) that are pulsed in rapid succession and transmitted through fiber optic bundles to the tissue of interest. The transmitted light returns through a separate fiber optic bundle to a photomultiplier tube, where the light intensities are coupled with the relevant specific extinction coefficient and optical path length to give rise to changes (Δ) in [O2Hb], [HHb] and [HbTot] relative to steady-state resting values. Given the uncertainty of the optical path length in the vastus lateralis at rest and during exercise, NIRS data are presented as arbitrary units (a.u.). Changes in light intensities were monitored and recorded continuously at 2 Hz, and the raw attenuation signals were transferred and stored on a computer for later analysis.
Analysis of Data
V̇o2p, MBV, and HR data for each individual trial were initially filtered for erroneous data points that lay outside four standard deviations of the local mean, because they do not conform to a Gaussian distribution as described by Lamarra et al. (44). Data were then interpolated on a second-by-second basis and time-aligned to correspond to the onset of the Mod transition (time 0). The data for each repetition within a condition were further ensemble-averaged for each subject and time-averaged into 10-s bins to yield a single response profile. Second-by-second LBF (calculated from the averaged interpolated MBV and femoral artery radius) were averaged into 10-s time bins.
The on-transient responses for V̇o2p, MBV, LBF, and HR were modeled using a monoexponential of the form
| (1) |
where Y(t) represents V̇o2p, MBV, LBF, or HR as a function of time (t) throughout the exercise transient; Y(BSL) is the baseline of Y during steady-state baseline exercise before the step increase in WR; Amp is the amplitude of the increase in Y above the baseline value; τ is the time constant (i.e., time taken to reach 63% of the steady-state response); and TD is the time delay.
The fit for V̇o2p began at the phase 1-phase 2 transition [as previously described by Rossiter et al. (59) and Gurd et al. (30)], to the end of the exercise transition (i.e., 360 s). Fitting for MBV, LBF, and HR began at the first data point after the start of Mod, through to the end of the Mod bout.
The NIRS-derived Δ[O2Hb], Δ[HHb], and Δ[HbTot] data were time-aligned to the onset of Mod (time 0) and ensemble-averaged into 5-s bins to yield a single response for each subject for both Con and Hypo trials. The time delay for the Δ[HHb] response (i.e., TD-Δ[HHb]) at the onset of Mod was determined using second-by-second data for each transition and defined as the first increasing point that consistently remained above the nadir of the signal. This was performed on every trial for all subjects and averaged to yield one TD-Δ[HHb] value for Con and Hypo trials for each subject. To determine the time course for muscle deoxygenation, the Δ[HHb] data were modeled using an exponential function as in Eq. 1 from the TD-Δ[HHb] to 90 s of the WR step; extending the HHb fitting window beyond 90 s to 360 s did not affect the parameter estimations for Δ[HHb] kinetics. Visual inspection of the NIRS-derived Δ[HHb] profile and minimal variation of residuals around the Y-axis (Y = 0) suggest this fitting procedure provides a reasonable estimate of the time course of muscle deoxygenation during the period corresponding to the phase 2 V̇o2p response. The overall time course for muscle deoxygenation is shown as the mean response time (MRT = TD-Δ[HHb] + τΔ[HHb]). Analysis of the Δ[O2Hb] and Δ[HbTot] signals were restricted to steady-state baseline and end-exercise values, since these signals do not exhibit an exponential-like response.
Statistical Analysis
The kinetic parameter estimates for V̇o2p, MBV, LBF, HR, and Δ[HHb] were analyzed using one-way analysis of variance (ANOVA) for repeated measures. Comparisons for all variables across time were analyzed by two-way ANOVA for repeated measures with the main effects of condition and time. A significant F-ratio was analyzed using Tukey's post hoc analysis with statistical significance accepted at P < 0.05. All values are means ± SD.
RESULTS
“Steady-State” Responses Across Time During Hypo and Con Trials
Group-averaged profiles across time during Hypo and Con are shown for respiratory measures (Fig. 1), blood flow-related measures and heart rate (Fig. 2), and NIRS data (Fig. 3). Group means (±SD) are included for specific time points representing the end points for each of the step transitions of the protocol.
Fig. 1.
Averaged profiles across time of respiratory measures during normal breathing (control, Con; ○) and hyperventilation-induced hypocapnic alkalosis (Hypo; ●). Dotted lines indicate the transitions between the resting preaccommodation period (Pre-Acc), resting accommodation period (Acc), passive exercise (Pass), baseline knee-extension (KE) exercise (Bsl), and moderate-intensity KE exercise (Mod). Specific points represent group means ± SD. *P < 0.05, different from Con. †P < 0.05, different from Pre-Acc. ‡P < 0.05, different from Acc. #P < 0.05, different from Pass. $P < 0.05, different from Bsl. V̇o2, O2 uptake; V̇co2, CO2 output; PetCO2, end-tidal partial pressure of CO2. Frequency is breathing frequency given in breaths/min.
Fig. 2.
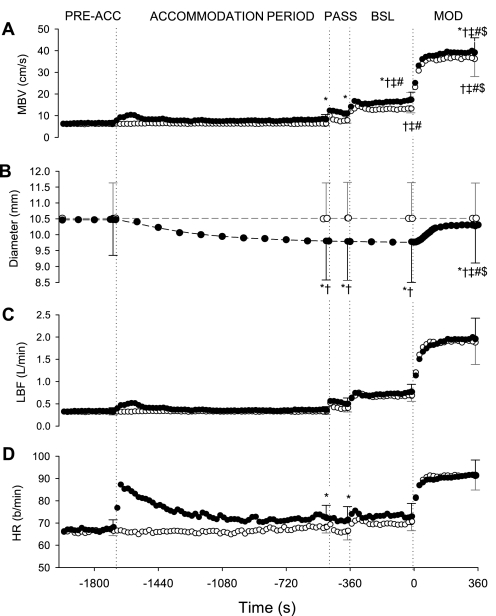
Averaged profiles across time of blood flow-related measures and heart rate (HR; beats/min) during Con (○) and Hypo (●). Dotted lines indicate the transitions between Pre-Acc, Acc, Pass, Bsl, and Mod. Specific points represent group means ± SD. *P < 0.05, different from Con. †P < 0.05, different from Pre-Acc. ‡P < 0.05, different from Acc. #P < 0.05, different from Pass. $P < 0.05, different from Bsl. MBV, mean blood velocity; LBF, leg blood flow.
Fig. 3.
Averaged near-infrared spectrometry (NIRS) profile across time during Con (shaded curves) and Hypo (solid curves). Dotted lines indicate the transitions between Pre-Acc, Acc, Pass, Bsl, and Mod. Specific points (Con, ○; Hypo, ●) represent group means ± SD. *P < 0.05, different from Con. Δ[HbTot], Δ[O2Hb], Δ[HHb], concentration changes of total, oxygenated, and deoxygenated hemoglobin-myoglobin; a.u., arbitrary unit.
Respiratory measures.
During the resting accommodation and through to the end of the exercise protocol, subjects were able to decrease (P < 0.05) and maintain PetCO2 at ∼20 mmHg in Hypo by doubling (P < 0.05) their ventilation (V̇e) compared with Con (Fig. 1). At end-exercise, tidal volume was higher (P < 0.05) in Hypo (1.9 ± 0.8 l/breath) than in Con (1.3 ± 0.2 l/breath), whereas breathing frequency was higher (P < 0.05) in Hypo (32 ± 15 breaths/min) compared with Con (21 ± 3 breaths/min). No difference was observed in V̇o2p between Hypo and Con conditions throughout the protocol, whereas overall V̇co2 was higher (P < 0.05) in Hypo compared with Con.
Blood flow-related and HR measures.
After the onset of hyperventilation, measured MBV was higher (P < 0.05) during Hypo than Con (Fig. 2). The FA diameter remained unchanged throughout the entire protocol during Con (∼10.5 mm). However, during Hypo, the FA diameter (which was not different from that during Con at the preaccommodation period) began to decrease during the accommodation period such that the FA diameter at each time point measured was smaller (P < 0.05) than preaccommodation values and those during Con (postaccommodation: Hypo, 9.8 ± 1.2 mm; Con, 10.5 ± 1.1 mm). During the transition to Mod, the FA diameter began to increase in an exponential-like manner (MRT = 99 ± 17 s) toward that observed in Con; the FA diameter at the end of exercise (10.3 ± 1.2 mm) was smaller (P < 0.05) than in Con (10.5 ± 1.1 mm) but greater than that measured during the postaccommodation, passive, and baseline periods (∼9.8 mm) in Hypo. A main effect of condition for LBF was observed, with an overall higher (P < 0.05) LBF in Hypo compared with Con. HR values were higher (P < 0.05) in Hypo compared with Con at the end of the accommodation period and during passive exercise.
NIRS measures.
There was a main effect of condition for the NIRS-derived Δ[HbTot], with an overall lower (P < 0.05) value in Hypo than in Con (Fig. 3). The Δ[O2Hb] in Hypo was lower (P < 0.05) at baseline and end-exercise compared with Con, whereas the Δ[HHb] tended to be similar between conditions.
Kinetic Responses During Moderate-Intensity Exercise
The average moderate WR intensity in the present study was 47 (±12) W, representing 78 (±7)% of θ̂L. Figure 4 presents the normalized group mean response profiles and model fits at the exercise transition for V̇o2p, MBV, and LBF and individual values for the respective time constants (τ) with group means (±SD). The parameter estimates for the kinetics of V̇o2p, MBV, LBF, and HR are presented in Table 1, whereas parameter estimates for Δ[HHb] are presented in Table 2.
Fig. 4.
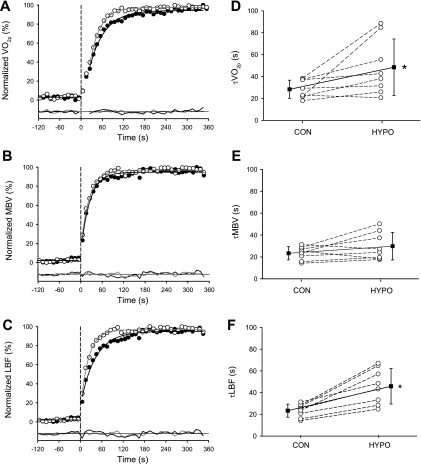
Averaged, normalized responses (A–C) and individual time constants (τ) with group means (D–F) of V̇o2p, MBV, and LBF for the transition to Mod exercise during Con (○) and Hypo (●). A–C: responses normalized to baseline and steady-state responses. Dashed lines indicate the onset of Mod. Model fit and associated residuals are shown for Con (shaded lines) and Hypo (solid lines). D–F: time constants for each subject (○) and group means ± SD (■). *P < 0.05, different from Con.
Table 1.
Kinetic parameter estimates of V̇o2p, MBV, LBF, and HR during Con and Hypo conditions
| Protocol | Baseline | Amplitude | τ, s | C95, s |
|---|---|---|---|---|
| V̇o2p, l/min | ||||
| Con | 0.50 ± 0.03 | 0.55 ± 0.17 | 28 ± 8 | 3 ± 1 |
| Hypo | 0.48 ± 0.05 | 0.55 ± 0.18 | 49 ± 26* | 4 ± 1 |
| MBV, cm/s | ||||
| Con | 13 ± 2 | 23 ± 7 | 23 ± 6 | 4 ± 1 |
| Hypo | 17 ± 3* | 22 ± 5 | 30 ± 13 | 4 ± 2 |
| LBF, l/min | ||||
| Con | 0.68 ± 0.14 | 1.22 ± 0.41 | 23 ± 6 | 4 ± 1 |
| Hypo | 0.74 ± 0.14* | 1.21 ± 0.35 | 46 ± 16* | 5 ± 2 |
| HR, beats/min | ||||
| Con | 70 ± 4 | 22 ± 6 | 21 ± 15 | 3 ± 1 |
| Hypo | 73 ± 6 | 19 ± 5* | 35 ± 19* | 5 ± 2 |
Values are means ± SD for parameter estimates of pulmonary O2 uptake (V̇o2p), mean blood velocity (MBV), leg blood flow (LBF), and heart rate (HR) during normal breathing (control; Con) and hyperventilation-induced hypocapnic alkalosis (Hypo). Baseline values are the average of the last 120 s of 3-W knee-extension exercise. τ, Time constant; C95, 95% confidence interval.
P < 0.05, different from Con.
Table 2.
Kinetic parameter estimates of Δ[HHb] (model fit to 90 s) during Con and Hypo
| Con | Hypo | |
|---|---|---|
| Baseline, a.u. | −1.49 ± 0.79 | −2.24 ± 2.51 |
| Amplitude, a.u. | 6.69 ± 6.31 | 8.05 ± 7.47* |
| MRT, s | 24 ± 3 | 23 ± 5 |
| TD, s | 15 ± 2 | 13 ± 2 |
| τ, s | 10 ± 4 | 10 ± 4 |
| C95, s | 2 ± 1 | 2 ± 1 |
Values are means ± SD for parameter estimates of muscle deoxyhemoglobin-myoglobin concentration changes (Δ[HHb]) during Con and Hypo. Baseline values are the average of the last 120 s of 3-W KE exercise. MRT, mean response time (TD + τ); TD, time delay.
P < 0.05, different from Con.
V̇o2p.
The amplitude for V̇o2p was similar between conditions (Table 1), whereas the phase 2 time constant of V̇o2p (τV̇o2p) was greater (P < 0.05) during Hypo (49 ± 26 s) than during Con (28 ± 8 s) (Fig. 4), with differences between conditions being greater than the 95% confidence intervals for each condition (Table 1), reflecting a slower adjustment of V̇o2p during Hypo.
MBV, LBF, and HR.
The MBV amplitude and time constant were not different between Hypo and Con conditions (Table 1). The LBF amplitude (Hypo, 1.21 ± 0.35 l/min; Con, 1.22 ± 0.41 L/min) and ratio (ΔLBF × 2)/ΔV̇o2p (∼4.5 liters of blood per liter of O2 uptake for Hypo and Con) were similar between conditions; however, as a result of the gradual increase in FA diameter during Hypo, the adjustment of LBF was slower (P < 0.05) in Hypo (τLBF, 46 ± 16 s) than in Con (τLBF, 23 ± 6 s) (Fig. 4).
The HR amplitude was lower (P < 0.05) during Hypo (19 ± 5 b/min) than during Con (22 ± 6 beats/min), and the τHR was greater (P < 0.05) during Hypo (35 ± 19 s) than during Con (21 ± 15 s) (Table 1), showing slower HR kinetics during Hypo.
NIRS.
The greater (P < 0.05) Δ[HHb] amplitude (Hypo, 8.1 ± 7.5 a.u.; Con, 6.7 ± 6.3 a.u.) (Table 2) and ratio Δ[HHb]/ΔV̇o2p (Hypo, 14.5 ± 10.9 a.u.·l−1·min−1; Con, 12.2 ± 9.5 a.u.·l−1·min−1) and similar time constant, time delay, and mean response time of Δ[HHb] between the conditions (Table 2) reflect a faster adjustment and greater Δ[HHb] response relative to muscle O2 utilization during Hypo.
DISCUSSION
Slower V̇o2p kinetics during the transition to Mod exercise were shown previously to occur with hyperventilation-induced hypocapnic alkalosis, although the mechanism(s) related to this slower response was not established (13, 14, 33). In agreement with our previous findings, the present study reported slower V̇o2p kinetics in Hypo than in Con conditions, whereas Δ[HHb] kinetics were not different between conditions but the Δ[HHb] amplitude (and Δ[HHb]/ΔV̇o2p) was greater in Hypo. The new findings reported in the present study were that 1) absolute MBV was higher in Hypo than in Con, but MBV kinetics were similar between conditions; 2) FA diameter remained at preaccommodation levels throughout the protocol in Con (consistent with previous studies), but in Hypo, FA diameter decreased during the accommodation period and increased in an “exponential-like” manner back toward, but did not reach, control levels during the transition to Mod (the response time for vasodilation being ∼100 s); and 3) leg femoral conduit artery (bulk) blood flow kinetics during the exercise transition were slower in Hypo than in Con. These data suggest that as a consequence of the slower adjustment of femoral artery (bulk) blood flow (and microvascular blood flow) in Hypo, there was a greater reliance on O2 extraction in this condition, which would be expected to lower microvascular O2 content and Po2 and thus slow O2 diffusion to the mitochondrial cytochrome c oxidase. Therefore, with an attenuated O2 diffusion, a greater provision of one or more of the other oxidative substrates (i.e., reducing equivalents, ADP, Pi) would be required to achieve the required rate of oxidative ATP production and contribute to the slow adjustment of muscle O2 utilization, and V̇o2p, during Hypo conditions.
Hyperventilation-Induced Hypocapnic Alkalosis and LBF
At rest.
Previous studies that measured blood flow at rest during Hypo yielded inconsistent results, with studies reporting an increase (12, 16, 17, 46, 57, 58), a decrease (11, 31, 38, 40, 42), or an unchanged blood flow response (41). In the present study, measured LBF at the end of the resting accommodation period was similar to preaccommodation LBF, as a result of a higher (∼27%) MBV and a lower (∼7%) FA diameter that accompanied the fall in PetCO2 (and the decrease in arterial CO2 and [H+] ) (13).
The constriction of blood vessels is dependent on vascular smooth muscle (VSM) contraction in response to an increase in intracellular calcium concentration ([Ca2+]i) (4). This is in turn related to an increase in intracellular and extracellular pH (4, 73). Plasma pH influences the extracellular pH to which VSM are exposed (73), and although plasma pH was not measured in the present study, estimations can be made from PaCO2. Based on our previous study (13), in which blood was sampled during a hyperventilation protocol identical to that used in this study, postaccommodation and end-exercise arterial pH was 7.63 and 7.54, respectively (compared with 7.51 and 7.41, respectively, in Con). Thus, in the present study, a pH effect on VSM is likely. Support for a Hypo-induced FA vasoconstriction comes from studies that demonstrated increases in [Ca2+]i (2, 7, 63, 64, 70) and tension (2, 3) following alkalinization of VSMs (either by removal of CO2 from the superfusate or exposure to NH4Cl or NaOH).
There were studies, however, that found vasodilation with alkalinization of VSMs (36, 66), whereas others observed a biphasic response, where a transient vasodilation [lasting from 1 (50) to 10 min (20)] preceded sustained vasoconstriction. A biphasic response was observed by Kontos et al. (42) in humans during voluntary hyperventilation, where an induced vasorelaxation in the forearm was followed, after 4 min, by vasoconstriction and a reduction in forearm blood flow. In the present study, the reduction in FA diameter was not significant until ∼4 min after the start of hyperventilation. Therefore, the apparent time-dependent vasoconstrictor response with hyperventilation may explain the varied findings of previous studies at rest, where the duration of hyperventilation varied between 1 and 20 min (12, 16, 17, 40–42, 46, 57, 58) and 1–2 h (11, 31, 38).
During mod exercise.
To our knowledge, no studies have reported blood flow kinetics in humans during transitions to exercise during Hypo. In the present study, steady-state FA (i.e., bulk) LBF tended to be higher in Hypo than in Con during baseline and exercise, due to the elevated MBV. However, during the exercise transition, the adjustment of LBF was slower in Hypo than in Con, which was related more to the time course of the increase in FA diameter, since MBV, although tending to be slower, was not different between conditions (τMBV: Hypo, 30 s; Con, 23 s).
During Con, the FA diameter during MOD remained unchanged from rest, as observed in previous studies (39, 47, 49, 55, 56). Indeed, under control conditions, the initial increase in blood flow during submaximal exercise is achieved by microcirculatory vasodilation via a muscle contraction-induced mechanical compression or distortion of resistance vessels and/or contraction-induced release of vasodilator substances (69), which negates the need to increase conduit artery diameters (56). During Hypo, however, the restoration of FA diameter from its constricted state occurred with an exponential-like time course (effective MRT, 99 ± 17 s) at the onset of Mod (Fig. 2). Although the hyperventilation protocol was maintained throughout Mod (thereby maintaining PetCO2 at ∼20 mmHg), microvessels in close proximity to recruited muscle fibers will be exposed to CO2 released by muscle fibers. Thus, as mitochondrial CO2 production increases during the transition to exercise, it is expected that CO2 diffusion out of active muscle fibers will contribute to local arteriolar vasodilation with a time course somewhat slower than the activation of mitochondrial oxidative phosphorylation. The vascular network (consisting of arterioles, capillaries, and venules) is arranged such that microvessels are in close proximity to each other (22, 53), thus allowing diffusion of CO2 to occur between neighboring microvessels. Furthermore, the vasodilatory signal originating from arterioles (62, 71, 72), capillaries (9, 18), or venules (19) is capable of being transmitted through endothelial and smooth muscle cells via gap junctions (6, 15, 24) and ascending up the vascular network to produce dilation of the feed artery (71). This could explain the increase in FA diameter that was seen at the start of Mod in the present study. As discussed above, CO2 has the ability to evoke changes in VSM cells through alterations in intra- and extracellular pH and subsequent changes in [Ca2+]i. In particular, VSM cells respond to lowered pH by reducing [Ca2+]i and promoting relaxation (2, 3, 50, 64).
Another possible contributor to the increase in FA diameter is lactate−, which is capable of causing vasodilation independently of acid-base changes (1, 34, 51, 52). Lactate− accumulation in arterial plasma was greater in Hypo (∼5 mM) than in Con (∼3 mM) during Mod cycling exercise (13, 45), a consequence of a slower activation of pyruvate dehydrogenase (PDH; requiring a greater substrate level phosphorylation) with Hypo (45) and enhanced transport out of the muscle via the sarcolemma monocarboxylate transporter (37). A lactate−-induced vasodilation has been shown to cause a decrease in systemic vascular resistance in pigs (1) and relaxation of isolated precontracted mesenteric arteries in rats (51) and coronary arteries in dogs and pigs (34, 52). Furthermore, vasodilation was observed with lactate− concentrations as low as 1 mM (34), with vascular relaxation occurring in a concentration-dependent manner (51, 52). Thus, because the present study shares an exercise protocol similar to that of the study by Chin et al. (13), it is reasonable to assume that an increase in arterial plasma lactate− concentration over the course of the Mod bout in Hypo conditions might contribute to the gradual increase in FA diameter observed in the present study.
Hyperventilation-Induced Hypocapnic Alkalosis and V̇o2p
The slower V̇o2p kinetics during exercise in Hypo observed in the present study are consistent with the findings from other published reports (13, 14, 33) and reflect slower muscle O2 utilization kinetics. In addition, Forbes et al. (26) reported that muscle PCr breakdown kinetics [a proxy measure of muscle O2 utilization (59)] was slowed and the fall in muscle PCr content was greater in Hypo during the transition to moderate-intensity plantar-flexion exercise, supporting the relationship between V̇o2p and muscle O2 utilization kinetics (5, 28, 43).
Hayashi et al. (33) proposed that slower V̇o2p kinetics observed during Mod cycling with prior hyperventilation of 2 min was the result of a leftward shift of the oxyhemoglobin dissociation curve, which attenuated the offloading of O2 from hemoglobin. Therefore, a lower microvascular Po2 would be expected for any level of O2 extraction in Hypo compared with Con. In addition, LeBlanc et al. (45), using a hyperventilation protocol similar to that used in the present study, reported that the active form of the mitochondrial PDH complex was lower during the first minute of cycling exercise at 55% V̇o2max compared with a normal control condition. Although V̇o2p was not measured in that study, the slower activation of PDH, which regulates the conversion of pyruvate to acetyl-CoA and provision of carbohydrate-derived substrates for the tricarboxylic acid cycle and reducing equivalents (i.e., NADH, FADH2) to the electron transport chain (65), suggests a metabolic explanation for slower V̇o2p kinetics observed during respiratory alkalosis. Hence, slower activation of PDH may contribute to slowed V̇o2p adjustment during hyperventilation and Mod; however, PDH was not measured in the present study.
Hyperventilation-Induced Hypocapnic Alkalosis and NIRS
Microvascular changes within a muscle can be inferred from NIRS measures. The kinetics of muscle deoxygenation (Δ[HHb]) reflect fractional muscle O2 extraction (21) and, when considered in combination with V̇o2p kinetics, provide information on the adjustment of muscle microvascular blood flow (21, 25, 32). In the present study, the time course of Δ[HHb] was similar between conditions despite slower V̇o2p kinetics (reflecting slower adjustment of muscle O2 utilization), which is consistent with our previous findings (13, 14, 26). In addition, the Δ[HHb] amplitude and Δ[HHb]-to-ΔV̇o2p ratio were greater during Hypo, reflecting a greater fractional O2 extraction with hyperventilation. These findings, along with the observation that conduit FA LBF kinetics were slowed in Hypo, are consistent with slowed adjustment of muscle blood flow at the microvascular level during Hypo, thereby requiring a greater fractional O2 extraction to meet the muscle O2 requirements. This in turn would potentiate the fall in microvascular Po2, thereby reducing O2 diffusive flux into the muscle.
Also, a lower Δ[HbTot] and Δ[O2Hb], as seen in this study during Hypo, would suggest a lower muscle blood (hemoglobin) volume and O2 availability in muscle (compared with Con) before and during Mod. Because red blood cell (RBC) spacing in capillaries contributes to the functional capillary surface area for O2 diffusion and thus is an important determinant of O2 diffusive conductance (Do2) (35), a lower Δ[HbTot] might reflect a lower Do2, which, combined with a lower microvascular Po2, would impair O2 flux between the RBC and muscle mitochondrial cytochrome c oxidase.
Microvascular blood volume in humans (13) and microcirculation in animals (31, 40) at rest were previously shown to be lower with Hypo. However, this is in contrast to bulk (conduit artery) blood flow measured at the FA in the present study, where steady-state blood flow was not attenuated during Hypo (as discussed above). Interestingly, however, a reduction of the diameter of microvessels within the ear chamber of rabbits recently was demonstrated with hypocapnia (PetCO2 ∼15–20 mmHg) (40), consistent with the FA constriction observed at rest during Hypo in the present study. Therefore, although conduit arteries and peripheral microvessels appear to respond in a similar manner to Hypo, the latter appear to be affected by Hypo to a greater extent.
Hyperventilation-Induced Hypocapnic Alkalosis and HR
HR was not different between the conditions, although the adjustment of HR was slower in Hypo, suggesting a slower adjustment of cardiac output could have contributed to the slower LBF kinetics, and thus the slower V̇o2p kinetics, seen in Hypo in the present study. This finding is in contrast to that of Chin et al. (13, 14) and Hayashi et al. (33), where no differences in HR kinetics were reported between conditions. In the present study and those of others (13, 14, 33), the exercise intervention was in the Mod domain. However, the present study used KE exercise rather than leg cycling exercise (13, 14, 33), where end-exercise HR reached ∼90 beats/min compared with ∼ 115 beats/min for leg cycling. Importantly, autonomic control of HR increases may differ somewhat between these two exercise modalities. Parasympathetic (i.e., vagal) withdrawal contributes to the increase in HR up to ∼100 beats/min, whereas further increases in HR to above ∼100 beats/min rely on sympathetic activation (60). Vagal tone has been shown to be attenuated with hyperventilation (27), suggesting that vagal reactivity has been altered and that there is a diminished ability to increase HR by vagal withdrawal during Hypo. Indeed, older inactive adults who display reduced vagal tone (61) have shown slower increases in HR in response to exhaustive isometric hand-grip exercise (67) relative to younger controls. Since in the present study the increase in HR during moderate KE exercise relies mainly on parasympathetic withdrawal, an attenuated vagal reactivity associated with hyperventilation may contribute to slower HR kinetics during Hypo compared with Con. Although the slower HR kinetics (and cardiac output) may have contributed to slower LBF kinetics in the present study, we believe that the hyperventilation-induced reduction in FA diameter and subsequent slow rate of FA (and microvascular) dilation at exercise onset may play a more important role, since other studies using leg cycling exercise reported slowed V̇o2p kinetics with hyperventilation in the absence of slowed HR kinetics (13, 14, 33).
Conclusion
In the present study, a prolonged hyperventilation (Hypo) maneuver (>20 min) was associated with a slowing of V̇o2p and conduit artery (FA) LBF kinetics but not with the kinetics of muscle fractional O2 extraction (as assessed by NIRS-derived Δ[HHb]). Also, FA diameter was reduced at rest during accommodation to Hypo, and an exponential-like increase in FA diameter toward Con values during the transition to Mod contributed, in part, to the slower LBF kinetics in Hypo, since the adjustment of MBV was not different between conditions. In addition, slower microvascular blood flow kinetics (inferred from the faster and greater Δ[HHb]-to-V̇o2p) suggests a slowed microcirculatory adjustment during the transition to moderate-intensity exercise. These findings support the concept that the adjustment of muscle O2 utilization (as reflected by V̇o2p kinetics) may be affected by peripheral vascular adjustments that control muscle conduit artery (bulk) and microvascular blood flow and thus local convective and diffusive O2 delivery to contracting skeletal muscle.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council of Canada grants. Additional support was provided by Western's Graduate Thesis Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Brad Hansen for technical support, as well as all the subjects who participated in this study.
REFERENCES
- 1. Aduen JF, Burritt MF, Murray MJ. Blood lactate accumulation: Hemodynamics and acid base status. J Intensive Care Med 17: 180–185, 2002 [Google Scholar]
- 2. Austin C, Wray S. The effects of extracellular pH and calcium change on force and intracellular calcium in rat vascular smooth muscle. J Physiol 488: 281–291, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Austin C, Wray S. A quantitative study of the relation between intracellular pH and force in rat mesenteric vascular smooth muscle. Pflügers Arch 427: 270–276, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Austin C, Wray S. Interactions between Ca2+ and H+ and functional consequences in vascular smooth muscle. Circ Res 86: 355–363, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Barstow TJ, Lamarra N, Whipp BJ. Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise. J Appl Physiol 68: 979–989, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol 278: H604–H612, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Batlle DC, Peces R, LaPointe MS, Ye M, Daugirdas JT. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol Cell Physiol 264: C932–C943, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Beaver WL, Lamarra N, Wasserman K. Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51: 1662–1675, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol Heart Circ Physiol 272: H2693–H2700, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Brandi G, Clode M. CO2 washout during hyperventilation in man. Respir Physiol 7: 163–172, 1969 [DOI] [PubMed] [Google Scholar]
- 11. Brice AG, Welch HG. Effect of respiratory alkalosis on skeletal muscle metabolism in the dog. J Appl Physiol 58: 658–664, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Burnum JF, Hickam JB, McIntosh HD. The effect of hypocapnia on arterial blood pressure. Circulation 9: 89–95, 1954 [DOI] [PubMed] [Google Scholar]
- 13. Chin LM, Leigh RJ, Heigenhauser GJ, Rossiter HB, Paterson DH, Kowalchuk JM. Hyperventilation-induced hypocapnic alkalosis slows the adaptation of pulmonary O2 uptake during the transition to moderate-intensity exercise. J Physiol 583: 351–364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chin LMK, Heigenhauser GJF, Paterson DH, Kowalchuk JM. Effect of hyperventilation and prior heavy exercise on O2 uptake and muscle deoxygenation kinetics during transitions to moderate exercise. Eur J Appl Physiol 108: 913–925, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Christ GJ, Spray DC, El-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues: evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res 79: 631–646, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Clarke RSJ. The effect of voluntary overbreathing on the blood flow through the human forearm. J Physiol 118: 537–544, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coffman JD, Kelly P. Hyperventilation and human calf blood flow. Am J Physiol 211: 1255–1260, 1966 [DOI] [PubMed] [Google Scholar]
- 18. Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol 278: H1916–H1923, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Danthuluri NR, Deth RC. Effects of intracellular alkalinization on resting and agonist-induced vascular tone. Am J Physiol Heart Circ Physiol 256: H867–H875, 1989 [DOI] [PubMed] [Google Scholar]
- 21. DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Ellsworth ML, Ellis CG, Popel AS, Pittman RN. Role of microvessels in oxygen supply to tissue. News Physiol Sci 9: 119–123, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elwell CE. A Practical User's Guide to Near Infrared Spectroscopy. London: Hamamatsu Photonics KK, 1995 [Google Scholar]
- 24. Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol 98: 1820–1828, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Forbes SC, Kowalchuk JM, Thompson RT, Marsh GD. Effects of hyperventilation on phosphocreatine kinetics and muscle deoxygenation during moderate-intensity plantar flexion exercise. J Appl Physiol 102: 1565–1573, 2007 [DOI] [PubMed] [Google Scholar]
- 27. George DT, Nutt DJ, Walker WV, Porges SW, Adinoff B, Linnoila M. Lactate and hyperventilation substantially attenuate vagal tone in normal volunteers: A possible mechanism of panic provocation? Arch Gen Psychiatry 46: 153–156, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80: 988–998, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Gurd BJ, Heigenhauser GJF, Paterson DH, Kowalchuk JM. An integrative approach to control of oxidative phosphorylation during exercise. Physiol News 69: 36–38, 2007 [Google Scholar]
- 30. Gurd BJ, Scheuermann BW, Paterson DH, Kowalchuk JM. Prior heavy-intensity exercise speeds V̇o2 kinetics during moderate-intensity exercise in young adults. J Appl Physiol 98: 1371–1378, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson U, Sjoberg F, Lewis DH, Thorborg P. The effect of hypocapnia on skeletal muscle microcirculatory blood flow, oxygenation and pH. Int J Microcirc Clin Exp 12: 131–141, 1993 [PubMed] [Google Scholar]
- 32. Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Human femoral artery and estimated muscle capillary blood flow kinetics following the onset of exercise. Exp Physiol 91: 661–671, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Hayashi N, Ishihara M, Tanaka A, Yoshida T. Impeding O2 unloading in muscle delays oxygen uptake response to exercise onset in humans. Am J Physiol Regul Integr Comp Physiol 277: R1274–R1281, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Hester RK, Weiss GB, Willerson JT. Basis of pH-independent inhibitory effects of lactate on 45Ca movements and responses to KCl and PGF2α in canine coronary arteries. Circ Res 46: 771–779, 1980 [DOI] [PubMed] [Google Scholar]
- 35. Honig CR, Connett RJ, Gayeski TE. O2 transport and its interaction with metabolism; a systems view of aerobic capacity. Med Sci Sports Exerc 24: 47–53, 1992 [PubMed] [Google Scholar]
- 36. Jensen PE, Hughes A, Boonen HC, Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res 73: 314–324, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev 77: 321–358, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Karlsson T, Stjernstrom EL, Stjernstrom H, Norlen K, Wiklund L. Central and regional blood flow during hyperventilation. An experimental study in the pig. Acta Anaesthesiol Scand 38: 180–186, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Koga S, Poole DC, Shiojiri T, Kondo N, Fukuba Y, Miura A, Barstow TJ. Comparison of oxygen uptake kinetics during knee extension and cycle exercise. Am J Physiol Regul Integr Comp Physiol 288: R212–R220, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Komori M, Takada K, Tomizawa Y, Nishiyama K, Kawamata M, Ozaki M. Permissive range of hypercapnia for improved peripheral microcirculation and cardiac output in rabbits. Crit Care Med 35: 2171–2175, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Kontos HA, Mauck HPJ, Richardson DW, Patterson JL., Jr Circulatory responses to hypocapnia in the anesthetized dog. Am J Physiol 208: 1201–1210, 1965 [DOI] [PubMed] [Google Scholar]
- 42. Kontos HA, Richardson DW, Raper AJ, Zubair-ul-Hassan, Patterson JL., Jr Mechanisms of action of hypocapnic alkalosis on limb blood vessels in man and dog. Am J Physiol 223: 1296–1307, 1972 [DOI] [PubMed] [Google Scholar]
- 43. Krustrup P, Jones AM, Wilkerson DP, Calbet JA, Bangsbo J. Muscular and pulmonary O2 uptake kinetics during moderate and high-intensity sub-maximal knee-extensor exercise in humans. J Physiol 587: 1843–1856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 62: 2003–2012, 1987 [DOI] [PubMed] [Google Scholar]
- 45. LeBlanc PJ, Parolin ML, Jones NL, Heigenhauser GJ. Effects of respiratory alkalosis on human skeletal muscle metabolism at the onset of submaximal exercise. J Physiol 544: 303–313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lennox WG, Gibbs EL. The blood flow in the brain and the leg of man, and the changes induced by alteration of blood gases. J Clin Invest 11: 1155–1177, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol 85: 1622–1628, 1998 [DOI] [PubMed] [Google Scholar]
- 48. MacDonald MJ, Tarnopolsky MA, Hughson RL. Effect of hyperoxia and hypoxia on leg blood flow and pulmonary and leg oxygen uptake at the onset of kicking exercise. Can J Physiol Pharmacol 78: 67–74, 2000 [DOI] [PubMed] [Google Scholar]
- 49. MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM. Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol 99: 1822–1834, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Matthews JG, Graves JE, Poston L. Relationships between pHi and tension in isolated rat mesenteric resistance arteries. J Vasc Res 29: 330–340, 1992 [DOI] [PubMed] [Google Scholar]
- 51. McKinnon W, Aaronson PI, Knock G, Graves J, Poston L. Mechanism of lactate-induced relaxation of isolated rat mesenteric resistance arteries. J Physiol 490: 783–792, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mori K, Nakaya Y, Sakamoto S, Hayabuchi Y, Matsuoka S, Kuroda Y. Lactate-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. J Mol Cell Cardiol 30: 349–356, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Pittman RN. Influence of microvascular architecture on oxygen exchange in skeletal muscle. Microcirculation 2: 1–18, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Poole DC, Barstow TJ, McDonough P, Jones AM. Control of oxygen uptake during exercise. Med Sci Sports Exerc 40: 462–474, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383–1388, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol 274: H314–H322, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Richardson DW, Wasserman AJ, Patterson JL., Jr General and regional circulatory responses to change in blood pH and carbon dioxide tension. J Clin Invest 40: 31–43, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roddie IC, Shepherd JT, Whelan RF. Humoral vasodilatation in the forearm during voluntary hyperventilation. J Physiol 137: 80–85, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 518: 921–932, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Seals DR, Monahan KD, Bell C, Tanaka H, Jones PP. The aging cardiovascular system: changes in autonomic function at rest and in response to exercise. Int J Sport Nutr Exerc Metab 11: 189–195, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol 256: H838–H845, 1989 [DOI] [PubMed] [Google Scholar]
- 63. Siskind MS, McCoy CE, Chobanian A, Schwartz JH. Regulation of intracellular calcium by cell pH in vascular smooth muscle cells. Am J Physiol Cell Physiol 256: C234–C240, 1989 [DOI] [PubMed] [Google Scholar]
- 64. Smirnov SV, Knock GA, Belevych AE, Aaronson PI. Mechanism of effect of extracellular pH on L-type Ca2+ channel currents in human mesenteric arterial cells. Am J Physiol Heart Circ Physiol 279: H76–H85, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Spriet LL, Heigenhauser GJ. Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev 30: 91–95, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Spurway NC, Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol 393: 57–71, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taylor JA, Hayano J, Seals DR. Lesser vagal withdrawal during isometric exercise with age. J Appl Physiol 79: 805–811, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol 86: 1101–1113, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Weirich J, Dumont L, Fleckenstein-Grün G. Contribution of store-operated Ca2+ entry to pHo-dependent changes in vascular tone of porcine coronary smooth muscle. Cell Calcium 35: 9–20, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol 273: H156–H163, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol 274: H178–H186, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Wray S, Smith RD. Mechanisms of action of pH-induced effects on vascular smooth muscle. Mol Cell Biochem 263: 163–172, 2004. [DOI] [PubMed] [Google Scholar]