Abstract
The slow-twitch soleus, but not fast-twitch muscle, of old vs. adult rats has previously been demonstrated to become insulin resistant for in vivo glucose uptake. We probed cellular mechanisms for the age effect by assessing whether insulin resistance for glucose uptake was an intrinsic characteristic of the muscle ex vivo and by analyzing key insulin signaling steps. We hypothesized that isolated soleus and epitrochlearis (fast-twitch) muscles from old (25 mo) vs. adult (9 mo) male Fisher-344 × Brown Norway rats would have insulin resistance for Akt2 Thr308 phosphorylation (pAkt2Thr308), AS160 phosphorylation Thr642 (pAS160Thr642), and atypical PKC (aPKCζ/λ) activity corresponding in magnitude to the extent of insulin resistance for [3H]-2-deoxyglucose (2-DG) uptake. Epitrochlearis insulin-stimulated 2-DG uptake above basal values was unaltered by age, and epitrochlearis pAkt2Thr308, pAS160Thr642, and aPKCζ/λ activity were not significantly different in adult vs. old rats. Conversely, insulin-stimulated 2-DG uptake by the soleus of old vs. adult rats was reduced with 1.2 nM (42%) and 30 nM (28%) insulin concomitant with an age-related decline in pAkt2Thr308 of the insulin-stimulated soleus. There were no age effects on pAS160Thr642 or aPKCζ/λ activity or abundance of Akt2, AS160, GLUT4 or Appl1 protein in either muscle. The results suggest the possibility that an age-related decline in pAkt2Thr308, acting by a mechanism other than reduced pAS160Thr642, may play a role in the insulin resistance in the soleus of old rats. Skeletal muscle insulin resistance in old age is distinctive compared with other insulin-resistant rodent models that are not selective for greater insulin resistance in the soleus vs. the epitrochlearis.
Keywords: glucose transport, GLUT4, AS160, Appl1, atypical PKC
several studies using the euglycemic-hyperinsulinemic clamp procedure at insulin doses expected to suppress hepatic glucose production have revealed an age-related decrement of ∼10–20% for 20- to 24-mo-old rats compared with 6- to 10-mo-old rats, implicating age-related insulin resistance in peripheral tissues, i.e., especially in skeletal muscle (13, 24, 51). By injecting radiolabeled 2-deoxy-d-glucose (2-DG) during the clamp, Escriva and colleagues directly assessed glucose uptake rates by several individual skeletal muscles (24). They found that the insulin-stimulated glucose uptake above basal values for the soleus, a predominantly slow-twitch muscle, was substantially lower for 24- vs. 8-mo-old rats (∼30–60% lower, depending on the insulin dose). In striking contrast, there was no evidence for an age-related decline in glucose uptake by the predominantly fast-twitch quadriceps muscles from the same animals. This result with aging differs remarkably from other commonly studied rat models of insulin resistance. For example, the relative decrements in glucose uptake in either the obese Zucker rat (64) or in high-fat diet fed rats (45) were quite similar for the soleus compared with predominantly fast-twitch muscles. In other words, age-related insulin resistance in skeletal muscle appeared to be fundamentally different from the two most widely studied models of insulin resistance in rats. In this study, we aimed to better understand the mechanism for this unusual effect of age on insulin resistance in skeletal muscle, a process that has major implications for health.
Skeletal muscle-specific differences in the magnitude of insulin resistance during a euglycemic-hyperinsulinemic clamp could reflect differences related to blood flow, concentrations of humoral factors (e.g., hormones, adipokines, lipids, etc.), neural recruitment, and/or differences intrinsic to the muscle itself. By studying isolated skeletal muscle in vitro, it is possible to separate the intrinsic characteristics of the muscle from short-lived and direct systemic effects. In this context, it is notable that, consistent with the lack of age-related insulin resistance in fast-twitch muscle in vivo, a number of previous studies using isolated preparations of the predominantly fast-twitch epitrochlearis muscle have indicated either very modest or no age-related insulin resistance for rats at 8–13 vs. 20–31 mo (8, 10, 11, 33). Accordingly, in this study, we evaluated the glucose uptake by both isolated soleus and epitrochlearis muscles from adult (9 mo) and old (25 mo) rats using a range of insulin concentrations.
Insulin-stimulated glucose transport in skeletal muscle is mediated via the insulin-regulated GLUT4 glucose transporter protein (30, 43, 65). Previous research has indicated no age-related decrease in GLUT4 protein abundance in rat skeletal muscle between 9 and 31 mo (8). We determined GLUT4 abundance and also assessed several key insulin signaling steps that could potentially account for insulin resistance in the absence of altered GLUT4 levels. Multiple isoforms of Akt are expressed in skeletal muscle, but only Akt2 appears to be important for insulin-stimulated glucose uptake (4, 14, 32, 48, 57), and aging effects on skeletal muscle Akt2, regardless of fiber type, have not previously been reported. We measured Akt1 and Akt2 abundance and insulin-mediated activation of total Akt, Akt1, and Akt2, as well as the abundance of Appl1, a protein that can regulate Akt activation (60). Phosphorylation of Akt substrate of 160 kDa (AS160) was recently identified as a missing link between insulin's activation of Akt2 and the increased GLUT4 translocation leading to elevated glucose transport rate (7, 12, 38, 58, 59). Insulin resistance for AS160 phosphorylation has been reported in a number of conditions of skeletal muscle insulin resistance (5, 39). Thus we also determined the abundance and insulin-stimulated phosphorylation of AS160. Finally, although Akt2 is important for insulin-induced glucose transport, Akt-independent mechanisms are also required for the full insulin-effect on glucose uptake. A great deal of evidence points to atypical PKC (aPKC) for this Akt-independent portion of insulin-mediated glucose transport in skeletal muscle (26–28). Because the influence of aging on aPKC is unknown, we also assessed the influence of age on aPKCζ/λ activity in soleus and epitrochlearis muscles. We hypothesized that muscle from older rats will have insulin resistance for key signaling steps (Akt2 phosphorylation, aPKCζ/λ activity, and AS160 phosphorylation) that correspond to the magnitude of insulin resistance for glucose uptake (i.e., little or no decrease in the epitrochlearis and a large decrease for the soleus).
METHODS
Materials.
Unless otherwise noted, all chemicals were purchased from Sigma Chemical (St. Louis, MO) or Fisher Scientific (Hanover Park, IL). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Anti-Akt (no. 9272), anti-phospho AktSer473 (pAktSer473; no. 9271), anti-phospho AktThr308 (pAktThr308; no. 9275), anti-phospho-(Ser/Thr) Akt substrate (anti-PAS; no. 9611), anti-GLUT4 (no. 2299), and anti-rabbit IgG horseradish peroxidase (no. 7074) were from Cell Signaling Technology (Danvers, MA). Anti-Akt1 (no. sc-7126) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt2 (no. AF23151) was from R&D Biosystems (Minneapolis, MN). Anti-phospho-AS160Thr642 (no. 07-802) and anti-AS160 (no. 07-741) were from Millipore (Billerica, MA). Anti-Appl1 (no. ab59592) was from Abcam (Cambridge, MA). 2-Deoxy-d-[3H]glucose ([3H]2-DG) and [14C]mannitol were from Perkin Elmer (Boston, MA).
Animal care.
The animal protocol for this study was approved by the University of Michigan Committee on Use and Care of Animals. Male Fisher-344 × Brown-Norway, F1 generation rats were obtained at 8 or 24 mo from Harlan (Indianapolis, IN) and housed for 1 mo before experimentation. Animals were individually housed in specific pathogen-free conditions in micro-isolation filter top cages and maintained on a 12:12-h light-dark cycle (lights out at 1700) and provided with standard rat chow (Lab Diet no. 5001; PMI Nutritional International, Brentwood, MO) and water ad libitum. Muscle experiments were performed on rats that were 9 (adult; n = 14) or 25 (old; n = 14) mo old.
Muscle dissection and incubation.
Food was removed from the cages of all rats on the morning of the experimental day between 0700 and 0800. Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) between 1030 and 1330. On loss of pedal reflexes, soleus and epitrochlearis muscles were removed and rapidly rinsed in warm (35°C) Krebs-Henseleit buffer (KHB). Muscles were longitudinally split into strips of similar size for each muscle (two strips for each epitrochlearis, and four strips for each soleus). Muscle strips were subsequently placed in vials containing the appropriate media and shaken and continuously gassed (95% O2/5% CO2) in a heated (35°C) water bath. In the first incubation step, all muscles were incubated in vials containing 2 ml of KHB supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, 6 mM mannitol, and either 0 nM (basal), 1.2 nM (physiological), or 30 nM (supraphysiological) insulin for 30 min. All muscles were then transferred to a second vial containing 2 ml of KHB/BSA solution, the same insulin concentration as the previous step, 1 mM 2-DG (including a final specific activity of 2.25 mCi/mmol [3H]-2-DG), and 9 mM mannitol (including a final specific activity of 0.022 mCi/mmol [14C]mannitol) for 20 min. Following the second incubation step, muscles were rapidly blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80°C for later processing and analysis.
Muscle lysate preparation.
Frozen muscles were weighed, transferred to prechilled glass tissue grinder tubes (Kontes, Vineland, NJ), and homogenized in ice-cold lysis buffer (1 ml/muscle) using a glass pestle attached to a motorized homogenizer (Caframo, Wiarton, ON). The lysis buffer contained Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL; no. 78510) supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate (Na3VO4), 1 mM β-glycerophosphate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Homogenates were transferred to microfuge tubes, rotated for 1 h at 4°C, and then centrifuged (15,000 g) for 15 min (4°C) to remove insoluble material. Protein concentration was measured using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL; no. 23225).
Immunoprecipitation.
For evaluation of AS160 phosphorylation (PAS-AS160), 200 μg of protein from each sample was combined with a 1:1,000 titer of PAS antibody and rotated overnight (4°C). Protein G-agarose beads (Upstate, Lake Placid, NY; no. 16-266) were washed three times with lysis buffer and resupended in lysis buffer to yield a 50% slurry. After initial antibody incubation, 100 μl of 50% slurry mix of protein G-agarose beads were added to the lysate/antibody mix and rotated 2 h at 4°C. Protein G-agarose beads were isolated by centrifugation (4,000 g at 4°C for 1 min) and washed three times in lysis buffer. Antigens were eluted from the beads with 45 μl of 2× SDS loading buffer and were boiled for 5 min before SDS-PAGE, as described below. For evaluation of Akt1 and Akt2 phosphorylation, ExactaCruz C beads (Santa Cruz Biotechnology; sc-45040) were prepared by washing three times with phosphate-buffered saline (PBS) and resuspended in 500 μl of PBS. Goat anti-Akt1 or anti-Akt2 antibody (3 μg/sample) was incubated with ExactaCruz C beads (50 μl/sample) and rotated 3 h at 4°C. The antibody-bead complex was then washed three times with PBS and resuspended to yield a 50% slurry. An aliquot 50 μl of the bead-antibody complex was then added to each sample of 200 μg of muscle lysate in 1 ml of PBS and slowly rotated overnight at 4°C. The immunoprecipitation matrix (bead-antibody-antigen) for each sample was washed three times with PBS, aspirating buffer completely after final wash, and then 50 μl of 2× Laemmli buffer was added. Samples were boiled for 5 min and centrifuged, and supernatants were subjected to 10% SDS-PAGE. Resolved proteins were transferred to PVDF membranes that were subsequently incubated in blocking solution [5% nonfat milk protein and Tris-buffered saline (TBS) plus 0.1% Tween-20 (TBST), pH 7.5] for 1 h at room temperature. Blots were then washed (3 × 5 min) in TBST and subsequently incubated in a 5% BSA TBST solution with anti-pAktThr308 (1:1,000) overnight at 4°C. Blots were subjected to secondary antibody incubation and visualized as described below.
Immunoblotting.
An equal amount of protein of each sample was mixed with 6× Laemmli buffer boiled for 5 min and separated using SDS-PAGE (7% resolving gel) before being transferred to nitrocellulose membranes. Membranes were blocked in 5% milk in TBST for 1 h at room temperature and transferred to 5% BSA-TBST with the appropriate primary antibody overnight at 4°C. Membranes were washed three times for 5 min in TBST and incubated with secondary antibody (1:20,000) for 1 h at room temperature. Blots were washed three times for 5 min in TBST, then washed two times for 5 min in TBS (pH 7.5), and then incubated with West Dura Extended Duration Substrate (Pierce; no. 34075) to visualize protein bands. Immunoreactive proteins were quantified by densitometry (AlphaEase FC; Alpha Innotech, San Leandro, CA). Values are expressed relative to the normalized average of the 30 nM insulin samples on each blot.
Atypical PKCζ/λ activity.
Briefly, aPKCζ/λ were immunoprecipitated from lysates (500 μg) with a rabbit polyclonal antibody (Santa Cruz Biotechnologies; sc-216) that recognizes COOH terminus of both PKC-ζ and PKC-λ. Sepharose-AG beads (Santa Cruz Biotechnologies) were added and incubated for 8 min at 30°C in 100 μl of buffer containing 50 mM Tris·HCl (pH 7.5), 100 μM Na3VO4, 100 μM Na4P2O7, 1 mM NaF, 100 μM PMSF, 4 μg of phosphatidylserine, 50 μM [γ-32P]ATP (NEN Life Science Products, Waltham, MA), 5 mM MgCl2, and, as substrate, 40 μM serine analog of the PKC-ε (BioSource, Carlsbad, CA). After incubation, 32P-labeled substrate was trapped on P-81 filter papers, which were placed in scintillation vials with scintillation cocktail and quantified by liquid scintillation counting.
2-Deoxy-d-glucose uptake.
Aliquots (200 μl) of the supernatants were combined in a vial with 10 ml of scintillation cocktail (Research Products International, Mount Prospect, IL), and a scintillation counter (Perkin Elmer, Waltman, MA) was used to determine 3H and 14C disintegrations per minute. These values were used to determine [3H]-2-DG uptake as previously described (8, 34). Additionally, to assess insulin's effects above baseline, Δ values for 2-DG uptake were determined by subtracting the 0 nM (basal) insulin value from the corresponding insulin-stimulated values (1.2 or 30 nM) from the same rat.
Statistical analysis.
Two-way ANOVA was used to determine significant main effects (age and insulin concentration) and interactions. Bonferroni t-tests were used for post hoc analysis to identify the source of significant variance (SigmaStat; SPSS, Chicago, IL). For masses (body, epididymal fat pad, or muscle strip) and total abundance of each protein, a Student's t-test was used to compare adult and old groups. Data are presented as means ± SE. A P value of ≤0.05 was considered statistically significant.
RESULTS
Body mass, epididymal fat pad mass, and muscle strip mass.
Body mass was 27% greater (P < 0.05) in old (559.4 ± 7.5 g) vs. adult (441.0 ± 8.5 g) rats. Epididymal fat pad mass was 63% greater (P < 0.05) in old (13.0 ± 0.6 g) vs. adult (8.0 ± 0.4 g) rats. The epididymal fat pad-to-body mass ratio was 28% greater (P < 0.05) in old (0.023 ± 0.001 g/g) vs. adult (0.018 ± 0.001 g/g) rats. The muscle strip mass of the epitrochlearis was 13% greater (P < 0.05) in old (78.6 ± 0.2 mg) vs. adult (69.5 ± 0.2 mg) rats. The mass of soleus strips was not significantly different between old (47.0 ± 0.2 mg) and adult (44.0 ± 0.2 mg) rats.
Total protein abundance.
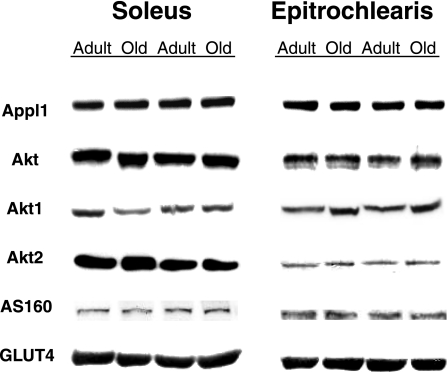
There were no age-related differences in total protein abundance for Appl1, total Akt, Akt1, Akt2, AS160, or GLUT4 in either the epitrochlearis or soleus muscles (Fig. 1).
Fig. 1.
Total protein abundance for Appl1, total Akt, Akt1, Akt2, AS160, and GLUT4. There were no statistically significant differences between the age groups. There was n = 8 muscles per group.
2-Deoxy-d-glucose uptake.
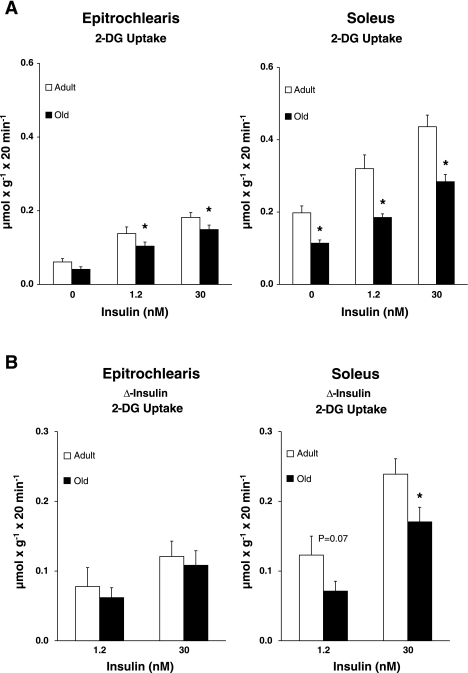
Epitrochlearis 2-DG uptake was not statistically different between adult and old rats with 0 nM insulin (Fig. 2A). Epitrochlearis 2-DG uptake was significantly decreased (P < 0.05) for old vs. adult rats with 1.2 nM (25% decrease) and 30 nM (18% decrease) insulin. To assess insulin's effects above baseline (Δ-insulin), insulin stimulation of 2-DG uptake above basal values was determined by subtracting the value of the 0 nM group from the values of 1.2 and 30 nM insulin for each rat (Fig. 2B). Δ-Insulin 2-DG uptake values for the epitrochlearis were not significantly different between the adult and old groups with either 1.2 or 30 nM insulin. Soleus 2-DG uptake was significantly decreased (P < 0.05) in old vs. adult rats for each of the three (0 nM, 1.2 nM, and 30 nM) insulin concentrations (decreases of 42, 42, and 35%, respectively; Fig. 2A). There was a trend (P = 0.07) for Δ-insulin 2-DG uptake values in the soleus to be decreased in old vs. adult rats with 1.2 nM (42% decrease) insulin. There was a significant age-related decrease (P < 0.05) for Δ-insulin 2-DG uptake values in the soleus with 30 nM (28% decrease) insulin (Fig. 2B).
Fig. 2.
A: 2-deoxy-d-glucose (2-DG) uptake in epitrochlearis and soleus muscles with 0, 1.2, or 30 nM insulin. B: the insulin-stimulated increase (Δ-insulin) for 2-DG uptake was determined by subtracting the 0 nM insulin value from each muscle in the insulin-stimulated groups (1.2 or 30 nM) within the same rat. Data are means ± SE (n = 10–14 muscles per age and insulin concentration). *P < 0.05 vs. adult in the same insulin treatment group.
Akt phosphorylation.
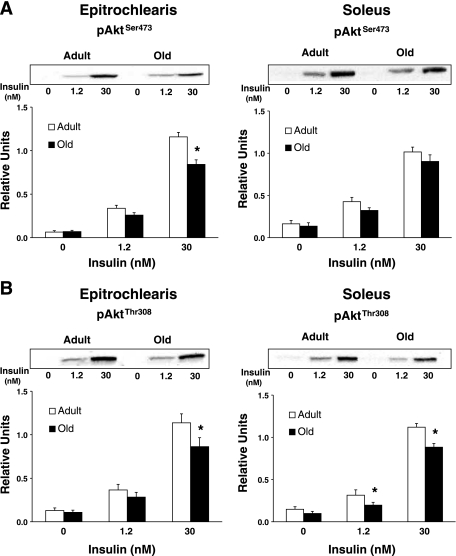
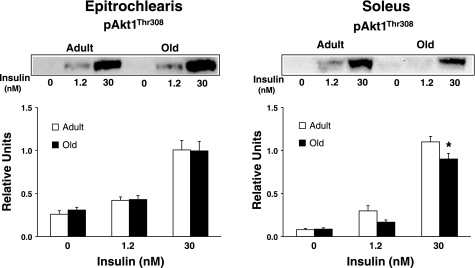
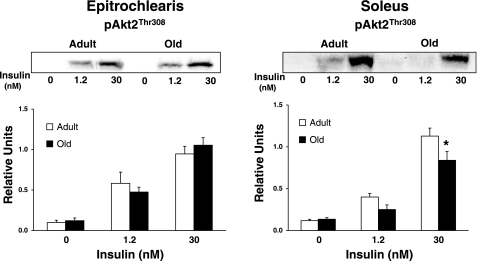
In the epitrochlearis, there was also no age-related difference in phosphorylation of Akt on either the Ser473 residue (pAktSer473; Fig. 3A) or Thr308 residue (pAktThr308; Fig. 3B) with 0 or 1.2 nM insulin, but there was a significant age-related decrease (P < 0.05) in both pAktSer473 and pAktThr308 with 30 nM insulin. Muscle lysates were immunoprecipitated with either Akt1 or Akt2 antibody and immunoblotted with pAktThr308 to determine phosphorylation of Akt1 (pAkt1Thr308; Fig. 4) or Akt2 (pAkt2Thr308; Fig. 5). For the epitrochlearis, there was no age-related difference for pAkt1Thr308 or pAkt2Thr308, regardless of insulin concentration.
Fig. 3.
A: AktSer473 phosphorylation in epitrochlearis and soleus muscles. B: AktThr308 phosphorylation in epitrochlearis and soleus muscles. Data are means ± SE (n = 10–14 muscles per age and insulin concentration). *P < 0.05 vs. adult in the same insulin treatment group.
Fig. 4.
pAkt1Thr308 in epitrochlearis and soleus muscles. Muscle lysate was immunoprecipitated with the Akt1 antibody before immunoblotting with the phospho-AktThr308 antibody. Data are means ± SE (n = 10–14 muscles per age and insulin concentration). *P < 0.05 vs. adult in the same insulin treatment group.
Fig. 5.
pAkt2Thr308 in epitrochlearis and soleus muscles. Muscle lysate was immunoprecipitated with the Akt2 antibody before immunoblotting with the phospho-AktThr308 antibody. Data are means ± SE (n = 10–14 muscles per age and insulin concentration). *P < 0.05 vs. adult in the same insulin treatment group.
In the soleus, there was no age-related difference in pAktSer473 (Fig. 3A) regardless of insulin concentration. There was no age-related difference in pAktThr308 (Fig. 3B) with 0 nM but a significant decrease (P < 0.05) in old vs. adult rats for 1.2 and 30 nM insulin. For the soleus, there was also no age-related difference in pAkt1Thr308 (Fig. 4) or pAkt2Thr308 (Fig. 5) with 0 or 1.2 nM insulin, although it tended (P = 0.109) to be lower in old rats at 1.2 nM. There were significant decreases (P < 0.05) in pAkt1Thr308 and pAkt2Thr308 in the old compared with the adult rats with 30 nM insulin.
AS160 phosphorylation.
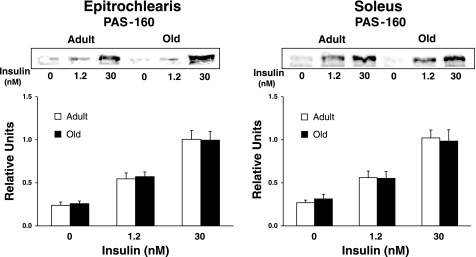
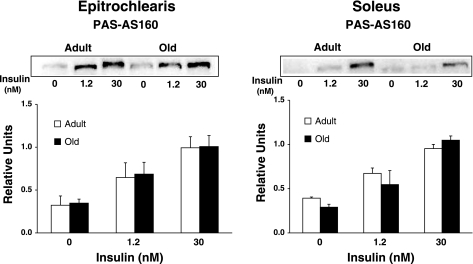
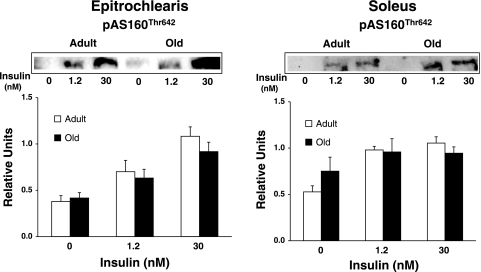
We evaluated AS160 phosphorylation using three approaches: 1) immunoblotting muscle lysates using the anti-PAS antibody and quantifying the 160-kDa band, which corresponds to the migration of AS160 for rat skeletal muscle (29) (Fig. 6); 2) using the anti-PAS to immunoprecipitate muscle lysate followed by immunoblotting with anti-AS160 (Fig. 7); and 3) using anti-pAS160Thr642 to probe blots of muscle lysates (Fig. 8). None of these analyses revealed any significant age-related differences for soleus or epitrochlearis muscles, regardless of insulin concentration.
Fig. 6.
Immunoreactivity of the 160-kDa band after immunoblotting with the phospho-Akt substrate (PAS) antibody in the epitrochlearis and soleus muscles. Data are means ± SE (n = 10–14 muscles per age and insulin concentration).
Fig. 7.
Phosphorylation of AS160 in epitrochlearis and soleus muscle lysates that were immunoprecipitated with the phospho-Akt substrate (PAS) antibody before immunoblotting with the AS160 antibody. Data are means ± SE (n = 6 muscles per age and insulin concentration).
Fig. 8.
AS160 phosphorylation at the Thr642 residue in epitrochlearis and soleus muscles. Data are means ± SE (n = 10–14 muscles per age and insulin concentration).
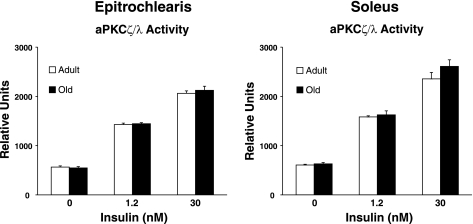
aPKCζ/λ activity.
There was no age-related difference in aPKCζ/λ activity in either the epitrochlearis or soleus muscles regardless of insulin concentration (Fig. 9).
Fig. 9.
Atypical protein kinase C (aPKCζ/λ) activity in epitrochlearis and soleus muscles. Data are means ± SE (n = 10–14 muscles per age and insulin concentration).
DISCUSSION
We evaluated the effect of age on glucose uptake, along with several key insulin signaling proteins that regulate glucose uptake, in the predominantly slow-twitch soleus (∼80–93% type I and 7–20% type II fibers) (2, 3, 9) and the predominantly fast-twitch epitrochlearis (∼8–15% type I and ∼85–92% type II fibers) (49, 63) muscles from adult (9 mo) and old (25 mo) rats. From a functional perspective, the most important new finding in this study was the age-related insulin resistance for glucose uptake by the isolated soleus muscles from 25- vs. 9-mo-old rats. In contrast, for the epitrochlearis, insulin's ability to increase glucose uptake above basal values was similar between the two age groups, confirming the results from many earlier studies that reported little or no insulin resistance in the epitrochlearis of rats aged 23–31 mo compared with 8–13 mo (8, 10, 11, 33). The present results indicated that age-related insulin resistance for glucose uptake by isolated skeletal muscles is not uniform for all muscles. From a mechanistic perspective, the most important new result was the identification of an age-associated reduction in Akt2 phosphorylation of the insulin-stimulated soleus, but not in the epitrochlearis. Thus the relationship between age and Akt2 phosphorylation in each muscle corresponded with the influence of age on insulin-mediated glucose uptake.
The substantial age-related insulin resistance for the soleus without a comparable level of insulin resistance in the epitrochlearis is notable because other commonly studied models of insulin resistance, including the obese Zucker rat or the high-fat diet-fed rat, are not characterized by exaggerated insulin resistance in the soleus compared with other muscles (45, 61, 64). Many previous studies have evaluated glucose uptake for both the soleus and the epitrochlearis from rats with insulin resistance induced by genetic defects, dietary manipulations, exposure to various hormones, or treatment with drugs (17, 18, 25, 35, 41, 42, 47, 52). None of these insulin resistant conditions of diverse origins were characterized by selectively greater insulin resistance for the soleus vs. the epitrochlearis.
Although the selective, age-related insulin resistance in the isolated soleus described in the present study appears to be unusual, the results are reminiscent of those for age effects on in vivo glucose uptake recently reported by Escriva and colleagues (24). They found a substantial, age-related (8 vs. 24 mo) decline in insulin-stimulated glucose uptake by the soleus without any age-related insulin resistance in the predominantly fast-twitch quadriceps muscle in Wistar rats. Thus, whether glucose uptake is measured in vitro or in vivo, substantial age-related insulin resistance was found in the soleus without comparable insulin resistance in predominantly fast-twitch muscle from the same rats. In vivo insulin resistance depends on both the muscle's intrinsic glucose uptake capacity and the modulation of this capacity by systemic influences, including the circulating concentrations of hormones, adipokines, and lipids, the muscle recruitment pattern, neural input, and the rate of muscle blood flow. The age-related insulin resistance in the isolated soleus reflects the muscle's intrinsic glucose capacity together with only relatively persistent consequences of the prior in vivo condition.
What instrinsic characteristics of the soleus might account for the age-related insulin resistance? There was no evidence for an age-related decline in GLUT4 protein abundance in either the soleus or the epitrochlearis, consistent with previously published results (8). We also previously reported that GLUT1 protein levels are also unchanged between 9 and 31 mo in epitrochlearis and soleus muscles of Fisher-344 × Brown Norway rats (8). In skeletal muscle, insulin increases glucose transport secondary to inducing GLUT4, but not GLUT1, to move from the cell's interior to the surface membranes (19). The absence of an age-related decrease in GLUT4 protein levels suggests a compromised ability to recruit GLUT4 to the cell surface membranes, perhaps because of defects in insulin signaling or the GLUT4 trafficking machinery. As a first step in assessing possible mechanisms, we focused on studying several key insulin signaling proteins that are important for GLUT4 translocation.
Akt2, but not Akt1 or Akt3, plays a central role in insulin-stimulated glucose transport, and the isoform-specificity of Akt2 for increasing GLUT4 translocation appears to be the consequence of the specific subcellular localization with insulin-stimulation (32). Knockdown of Akt2 by siRNA markedly lowered insulin-stimulated glucose uptake, whereas knockdown of Akt1 had very little effect (37, 40). Akt2-null mice have reduced muscle glucose uptake (14, 48), but Akt1- (15) and Akt3-null (21) mice have normal glucose regulation. Furthermore, the rapid activation of Akt2 is sufficient to increase GLUT4 in cells (50). In the epitrochlearis, there was a small but significant age-related reduction in the total pAktThr308 even though neither pAkt1Thr308 nor pAkt2Thr308 were lower for the old vs. adult group. Skeletal muscle has been reported to express all three Akt isoforms (Akt1, Akt2, and Akt3), and each isoform becomes phosphorylated in response to insulin (6, 16, 57). The observed age-related decrease in total pAktThr308 in the epitrochlearis with 30 nM insulin despite no decrease in pAkt1Thr308 or pAkt2Thr308 in this muscle suggests the possibility that there may have been an age-related decline in pAkt3Thr308. Regardless, alterations in Akt3 activation would not be expected to play an important role in altered insulin sensitivity or glucose metabolism (20, 21). The influence of aging on Akt2Ser473 in the soleus remains to be determined, as does its potential role in the observed insulin resistance for glucose transport. However, phosphorylation of AktThr308, independent of altered phosphorylation on AktSer473, is essential for the full activation of Akt activity and insulin-stimulated glucose transport (1, 44). Based on the available evidence, the reduction in Akt2Thr308 phosphorylation in the soleus of the old compared with adult rats is likely to play a role in the reduced insulin-stimulated glucose uptake.
Akt2 is a Ser/Thr kinase, and AS160 was the first Akt substrate to be identified as a participant in insulin-stimulated GLUT4 translocation and glucose uptake (7, 22, 38, 46, 59). In muscles from Akt2-null mice, insulin-stimulated phosphorylation of AS160 (measured with anti-PAS or anti-pAS160Thr642) was nearly completely eliminated (46). Several conditions with insulin resistance for glucose disposal are characterized by attenuated insulin-stimulated AS160 phosphorylation (5, 39, 62). We expected to find age-related insulin resistance for Akt2 phosphorylation in the soleus to be accompanied by reduced AS160 phosphorylation, but there was no evidence for an age effect on either AS160 abundance or AS160 phosphorylation.
Although AS160 phosphorylation did not track closely with glucose uptake, it is possible that we failed to detect age-related changes in other regulatory aspects of AS160. For example, we measured AS160 phosphorylation with anti-pAS160Thr642 or anti-PAS (which appears to primarily detect pThr642 and possibly pSer588 to a lesser extent) (38). Although Thr642 appears to be an especially important phospho-site for AS160's role in controlling GLUT4 (59), AS160 can be phosphorylated on several other sites by both Akt and other kinases (31, 59). In addition to phosphorylation, AS160 is regulated by binding to 14-3-3 proteins, and in 3T3-L1 cells the ability of AS160 to bind 14-3-3 proteins appears to be critical for regulating GLUT4 redistribution (53). Finally, it is possible that age can influence the subcellular localization of AS160 (53), which is likely to also be important for its ability to regulate GLUT4. Thus it remains possible that there are age effects on AS160 function that are relevant for the age-related insulin resistance.
An alternative scenario is that the insulin resistance for glucose uptake in the soleus of Old rats was the result of an Akt2-dependent, but AS160-independent mechanism. Other Akt substrates that have been implicated as potential regulators of GLUT4 localization include TBC1D1 (54) and myosin Vb (36). Another possibility is that lesser Akt activation in the old soleus results in attenuated suppression of Akt's substrate GSK-3, which may in turn induce insulin resistance as the result of greater serine-phosphorylation of the insulin receptor and/or insulin receptor substrates (18, 23).
To probe possible mechanisms for the reduced Akt activation, we studied Appl1, a protein that can interact with and apparently regulate Akt. Reducing Appl1 expression using shRNA in 3T3-L1 cells resulted in reduced insulin-stimulated Akt phosphorylation, GLUT4 translocation, and glucose uptake (55). However, there was no evidence of an age effect on an abundance of Appl1 in either muscle. It remains possible that the older rats have altered association of Appl1 with Akt.
Finally, although the decrease in Akt2 activation is an attractive potential candidate for the age-related insulin resistance, the present results do not establish causality, and it remains possible that other mechanisms are essential for the insulin resistance in the soleus. A great deal of evidence indicates that aPKC is an insulin-regulated but Akt-independent signaling protein for increasing GLUT4 translocation and glucose uptake (26–28, 56). For example, muscle-specific (aPKCζ/λ-null mice) have reduced insulin-stimulated glucose uptake by skeletal muscle (28). However, there was no evidence in the present study for a difference between age groups for aPKCζ/λ activity in either muscle.
In conclusion, the present study demonstrates age-related insulin resistance for glucose uptake in the isolated soleus muscle from 9- vs. 25-mo-old rats without a comparable decrement of insulin's ability to increase glucose uptake in the epitrochlearis muscle from the same rats. Reduced activation of Akt2 is supported as a potential component of the mechanism for this selective age effect on the soleus. Our working hypothesis is that the diminished activation of Akt2 results in reduced phosphorylation of AS160 at a site other than Thr642 and/or another Akt2 substrate other than AS160. Future studies using selective pharmacological and/or genetic approaches to test the role of Akt2 in age-related insulin resistance is essential. The substantial insulin resistance in the soleus without comparable insulin resistance in other skeletal muscles of old vs. adult rats is strikingly different from other rodent models for insulin resistance. It would be important for future research to determine whether the age-related insulin resistance is unique to the soleus or is a common feature of aging in other predominantly slow-twitch muscles. If other slow-twitch muscles are characterized by insulin resistance similar to the soleus, it would be valuable to determine whether selective age-related insulin resistance is found in the slow-twitch fibers within predominantly fast-twitch muscles. The present results are a compelling example of the heterogeneity that aging can have on different tissues from the same organism.
GRANTS
This research was supported by the National Institute on Aging (AG-010026 to G. D. Cartee).
ACKNOWLEDGMENTS
The authors would like to thank Carlos Castorena for technical assistance.
REFERENCES
- 1. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 2. Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21: 51–55, 1973 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278: 49530–49536, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, Zierath JR. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55: 785–791, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brozinick JT, Jr, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes 52: 935–941, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Cartee GD, Bohn EE, Gibson BT, Farrar RP. Growth hormone supplementation increases skeletal muscle mass of old male Fischer 344/brown Norway rats. J Gerontol A Biol Sci Med Sci 51: 214–219, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol 75: 972–978, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol Regul Integr Comp Physiol 266: R1443–R1447, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Catalano KJ, Bergman RN, Ader M. Increased susceptibility to insulin resistance associated with abdominal obesity in aging rats. Obes Res 13: 11–20, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Cozzone D, Frojdo S, Disse E, Debard C, Laville M, Pirola L, Vidal H. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from Type 2 diabetic patients. Diabetologia 51: 512–521, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Doh KO, Park JO, Kim YW, Park SY, Jeong JH, Jeon JR, Lee SK, Kim JY. Effect of leptin on insulin resistance of muscle–direct or indirect? Physiol Res 55: 413–419, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab 288: E1188–E1194, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter.” Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem 265: 13427–13430, 1990 [PubMed] [Google Scholar]
- 20. Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26: 8042–8051, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 25: 1869–1878, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA 94: 9660–9664, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Escriva F, Gavete ML, Fermin Y, Perez C, Gallardo N, Alvarez C, Andres A, Ros M, Carrascosa JM. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol 194: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Etgen GJ, Jr, Wilson CM, Jensen J, Cushman SW, Ivy JL. Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat. Am J Physiol Endocrinol Metab 271: E294–E301, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab 283: E1–E11, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans 33: 350–353, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Funai K, Cartee GD. Contraction-stimulated glucose transport in rat skeletal muscle is sustained despite reversal of increased PAS-phosphorylation of AS160 and TBC1D1. J Appl Physiol 105: 1788–1795, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol 80: 569–578, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA 106: 7004–7009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gulve EA, Henriksen EJ, Rodnick KJ, Youn JH, Holloszy JO. Glucose transporters and glucose transport in skeletal muscles of 1- to 25-mo-old rats. Am J Physiol Endocrinol Metab 264: E319–E327, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76: 979–985, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Hunt DG, Ding Z, Ivy JL. Clenbuterol prevents epinephrine from antagonizing insulin-stimulated muscle glucose uptake. J Appl Physiol 92: 1285–1292, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol 295: C1016–C1025, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100: 7569–7574, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-Stimulated Phosphorylation of the Akt Substrate AS160 Is Impaired in Skeletal Muscle of Type 2 Diabetic Subjects. Diabetes 54: 1692–1697, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Katome T, Obata T, Matsushima R, Masuyama N, Cantley LC, Gotoh Y, Kishi K, Shiota H, Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J Biol Chem 278: 28312–28323, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Kim JY, Nolte LA, Hansen PA, Han DH, Kawanaka K, Holloszy JO. Insulin resistance of muscle glucose transport in male and female rats fed a high-sucrose diet. Am J Physiol Regul Integr Comp Physiol 276: R665–R672, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Kinnick TR, Youngblood EB, O'Keefe MP, Saengsirisuwan V, Teachey MK, Henriksen EJ. Modulation of insulin resistance and hypertension by voluntary exercise training in the TG(mREN2)27 rat. J Appl Physiol 93: 805–812; discussion 797, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Koistinen HA, Zierath JR. Regulation of glucose transport in human skeletal muscle. Ann Med 34: 410–418, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Kondapaka SB, Zarnowski M, Yver DR, Sausville EA, Cushman SW. 7-Hydroxystaurosporine (UCN-01) inhibition of Akt Thr308 but not Ser473 phosphorylation: a basis for decreased insulin-stimulated glucose transport. Clin Cancer Res 10: 7192–7198, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Kraegen EW, Storlien LH, Jenkins AB, James DE. Chronic exercise compensates for insulin resistance induced by a high-fat diet in rats. Am J Physiol Endocrinol Metab 256: E242–E249, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Leturque A, Hauguel S, Sutter Dub MT, Maulard P, Girard J. Effects of placental lactogen and progesterone on insulin stimulated glucose metabolism in rat muscles in vitro. Diabete Metab 15: 176–181, 1989 [PubMed] [Google Scholar]
- 48. McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Nesher R, Karl IE, Kaiser KE, Kipnis Epitrochlearis muscle DM. I. Mechanical performance, energetics, and fiber composition. Am J Physiol Endocrinol Metab 239: E454–E460, 1980 [DOI] [PubMed] [Google Scholar]
- 50. Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3–L1 adipocytes. Cell Metab 7: 348–356, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Nishimura H, Kuzuya H, Okamoto M, Yoshimasa Y, Yamada K, Ida T, Kakehi T, Imura H. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol Endocrinol Metab 254: E92–E98, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Nolte LA, Yarasheski KE, Kawanaka K, Fisher J, Le N, Holloszy JO. The HIV protease inhibitor indinavir decreases insulin- and contraction-stimulated glucose transport in skeletal muscle. Diabetes 50: 1397–1401, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Roach WG, Chavez JA, Mãrinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem 282: 32280–32287, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Sajan MP, Standaert ML, Miura A, Kahn CR, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol 18: 2513–2521, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277: 11910–11917, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133: 486–497, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Sherman WM, Katz AL, Cutler CL, Withers RT, Ivy JL. Glucose transport: locus of muscle insulin resistance in obese Zucker rats. Am J Physiol Endocrinol Metab 255: E374–E382, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol 292: C729–C739, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl 564: 1–80, 1987 [PubMed] [Google Scholar]
- 64. Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51: 3492–3498, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci 31: 215–222, 2006. [DOI] [PubMed] [Google Scholar]