Abstract
Chronic systemic platelet cyclooxygenase (COX) inhibition with low-dose aspirin [acetylsalicylic acid (ASA)] significantly attenuates reflex cutaneous vasodilation in middle-aged humans, whereas acute, localized, nonisoform-specific inhibition of vascular COX with intradermal administration of ketorolac does not alter skin blood flow during hyperthermia. Taken together, these data suggest that platelets may be involved in reflex cutaneous vasodilation, and this response is inhibited with systemic pharmacological platelet inhibition. We hypothesized that, similar to ASA, specific platelet ADP receptor inhibition with clopidogrel would attenuate reflex vasodilation in middle-aged skin. In a double-blind crossover design, 10 subjects (53 ± 2 yr) were instrumented with four microdialysis fibers for localized drug administration and heated to increase body core temperature [oral temperature (Tor)] 1°C during no systemic drug (ND), and after 7 days of systemic ASA (81 mg) and clopidogrel (75 mg) treatment. Skin blood flow (SkBF) was measured using laser-Doppler flowmetry over each site assigned as 1) control, 2) nitric oxide synthase inhibited (NOS-I; 10 mM NG-nitro-l-arginine methyl ester), 3) COX inhibited (COX-I; 10 mM ketorolac), and 4) NOS-I + COX-I. Data were normalized and presented as a percentage of maximal cutaneous vascular conductance (%CVCmax; 28 mM sodium nitroprusside + local heating to 43°C). During ND conditions, SkBF with change (Δ) in Tor = 1.0°C was 56 ± 3% CVCmax. Systemic low-dose ASA and clopidogrel both attenuated reflex vasodilation (ASA: 43 ± 3; clopidogrel: 32 ± 3% CVCmax; both P < 0.001). In all trials, localized COX-I did not alter SkBF during significant hyperthermia (ND: 56 ± 7; ASA: 43 ± 5; clopidogrel: 35 ± 5% CVCmax; all P > 0.05). NOS-I attenuated vasodilation in ND and ASA (ND: 28 ± 6; ASA: 25 ± 4% CVCmax; both P < 0.001), but not with clopidogrel (27 ± 4% CVCmax; P > 0.05). NOS-I + COX-I was not different compared with NOS-I alone in either systemic treatment condition. Both systemic ASA and clopidogrel reduced the time required to increase Tor 1°C (ND: 58 ± 3 vs. ASA: 45 ± 2; clopidogrel: 39 ± 2 min; both P < 0.001). ASA-induced COX and specific platelet ADP receptor inhibition attenuate reflex vasodilation, suggesting platelet involvement in reflex vasodilation through the release of vasodilating factors.
Keywords: aspirin, plavix, thermoregulation
with rising body core temperature, skin blood flow is first increased by withdrawal of adrenergic vasoconstrictor tone, and then, on reaching a specific core temperature threshold, active cutaneous vasodilation occurs (25). Reflex vasodilation is mediated by cholinergic cotransmission (14), where several putative vasodilator mechanisms are involved, including the cotransmitter vasoactive intestinal peptide (1), histamine receptor activation (31), and neurokinin 1 receptor activation (29). Furthermore, full expression of reflex cutaneous vasodilation in young healthy human skin is dependent on nitric oxide (NO) synthase (NOS) (13, 27) and cyclooxygenase (COX) second-messenger mechanisms (18).
With healthy human aging, there is a significant attenuation in reflex cutaneous vasodilation due to a reduction in both NO- and cotransmitter-dependent vasodilation (8, 15). Our laboratory has recently demonstrated that, in otherwise healthy middle-aged humans, chronic low-dose aspirin [acetylsalicylic acid (ASA)] therapy (81 mg) taken for primary atherothrombotic disease prevention (23), consistently and significantly attenuates reflex cutaneous vasodilation (11). In a subsequent study examining the contribution of vascular COX-derived vasodilators in middle-aged skin, our laboratory demonstrated that acute, localized, nonisoform-specific COX inhibition with ketorolac did not attenuate reflex vasodilator responses during significant hyperthermia (10). Cumulatively, these data suggest that 1) local vascular COX-derived vasodilators do not significantly contribute to reflex vasodilation in this age group; and 2) it is unlikely that chronic low-dose, ASA-induced inhibition of vascular endothelial COX is a potential mechanism underlying attenuated reflex vasodilation observed in humans on chronic low-dose ASA therapy (11).
The primary mechanisms of low-dose ASA for atherothrombotic disease prevention is through the acetylation of platelet COX-1 in the presystemic (portal) circulation (24), thereby inhibiting COX for the life of the platelet (∼10 days), while preserving the ability of vascular endothelial cells to produce COX-dependent vasodilators. There are several putative mechanisms involving platelets and their potential role in reflex vasodilation that may be altered in subjects taking low-dose ASA. Importantly, activated platelets release known vasodilators, including NO, ATP, ADP, and 5-HT (5, 12, 20), all of which have the potential to directly stimulate cutaneous vasodilator pathways implicated in reflex vasodilation.
The purpose of this study was to examine the effect of specific platelet inhibition on reflex vasodilator mechanisms in middle-aged skin. We performed a randomized double-blinded crossover design study after 7 days of systemic low-dose ASA (81 mg) to inhibit platelet COX-1 and 7 days of clopidogrel (75 mg) to specifically inhibit platelet ADP receptors (purinoreceptor P2Y12). We hypothesized that, similar to low-dose ASA, specific platelet ADP receptor inhibition would attenuate reflex cutaneous vasodilation.
METHODS
Subjects.
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects before participation. Drug intervention studies were performed on 10 healthy subjects (5 men and 5 women). Three additional subjects served as time controls. Data from the baseline (no drug) experimental period and the first experiment for the time control subjects were pooled and previously reported (10), illustrating the effect of localized COX inhibition on reflex vasodilation.
All subjects underwent a complete medical screening, including a physician-supervised graded exercise test, to evaluate the existence of underlying cardiovascular disease, blood chemistry, coagulation study (prothrombin time and partial thromoplastin time), lipid profile evaluation (Quest Diagnostics Nichol Institute, Chantilly, VA), resting electrocardiogram, and physical examination. All subjects were screened for the presence of cardiovascular, dermatological, and neurological disease. No subjects were previously taking low-dose ASA, nor did any have a family history (first-degree relative) of atherothrombotic disease. Subjects were normally active, nondiabetic, nonsmokers, who were currently not taking medications, including vitamins, hormone replacement therapy, or oral contraceptives.
Systemic drug treatments.
Subjects were tested at enrollment into the study while not taking any systemic drugs. Subjects were instructed not to take anti-inflammatory medications for at least 2 wk before the baseline (no drug) experimental day. After initial testing, nonidentifiable capsules, compounded by a registered pharmacist (Boalsburg Pharmacy), were given to subjects over 7 days. Randomized doubled-blinded drug treatments consisted of a daily oral dose of either 81 mg of ASA (Bayer) or 75 mg of clopidogrel bisulphate (Plavix Bristol-Myers Squibb). Full platelet aggregation inhibition specific to arachidonic acid and ADP has been shown to occur within 4 days of initiating treatment (22, 26). Subjects took the compounded drugs each morning, with their last pill being ingested at 7:00 AM the day of the experiments. Experimental trials in the same subjects were separated by a minimum interval of 3 wk. This washout time period has been shown to be efficacious for full platelet recovery (22). Because the drug intervention group included a no-drug trial instead of a placebo control, three additional subjects served as time controls. These subjects underwent the same experimental procedures (whole body heating and intradermal microdialysis for localized drug administration) as the drug intervention groups; however, no systemic drug intervention was used. Experimental trials for the time control subjects were separated by a minimum of 3 wk. The data for the time control subjects were analyzed using a mixed-models repeated-measures analysis of variance (see Statistical analysis section).
Instrumentation and measurements.
Protocols were performed in a thermoneutral laboratory with the subject in the semisupine position, with the experimental arm at heart level. On arrival at the laboratory, subjects were instrumented with four intradermal microdialysis fibers (MD2000, Bioanalytical Systems) (10 mm, 20-kDa cutoff membrane) in the skin on the left ventral forearm. Microdialysis sites were at least 4.0 cm apart to ensure no cross-reactivity of pharmacological agents being delivered to the skin. Microdialysis fibers were placed at each site by first inserting a 25-gauge needle through unanesthetized skin using sterile technique. The entry and exit points were ∼2.5 cm apart. The microdialysis fibers were then threaded through the needle, and the needle was withdrawn, leaving the fibers in place. The microdialysis fibers were taped in place and initially perfused with lactated Ringer solution to ensure the integrity of the fiber and during the insertion trauma resolution period. Following this period, microdialysis sites were perfused with 1) 10.0 mM NG-nitro-l-arginine methyl ester (l-NAME) to inhibit NO production by NOS, 2) 10.0 mM ketorolac to nonspecifically inhibit local vascular COX isoforms, 3) a combination of 10.0 mM ketorolac and 10.0 mM l-NAME, and 4) lactated Ringer solution to serve as a control. All microdialysis drugs were perfused at a rate of 2.0 μl/min (Bee Hive controller and Baby Bee microinfusion pumps, Bioanalytical Systems) continuously throughout the protocol.
An index of skin blood flow was obtained by measuring cutaneous red blood cell flux with an integrated laser-Doppler flowmeter probe placed in a local heater maintained at 33°C (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK) on the skin directly above each microdialysis membrane. All laser-Doppler probes were calibrated using Brownian standard solution. Cutaneous vascular conductance (CVC) was calculated as flux divided by mean arterial pressure (MAP).
Mean skin temperature was controlled by water-perfused suit that covered the entire body, except head, hands, and experimental arm. Subjects also wore a water-impermeable outer garment over the water-perfused suit to minimize evaporative heat loss. The subject's electrocardiogram was monitored throughout the protocol, and blood pressure was measured via brachial auscultation with every 0.1°C rise in oral temperature (Tor). Tor was continuously monitored during baseline and throughout whole body heating as an index of body core temperature with a thermistor placed in the sublingual sulcus. The thermistor was secured in the same location in the sublingual sulcas, and the subject's mouth was taped shut. The subjects were instructed to keep the thermistor in the same location in the sublingual sulcus and not to open their mouths or speak during the protocol. Mean skin temperature was calculated as the unweighted average from six copper-constantan thermocouples placed on the chest, middle back, abdomen, upper arm, thigh, and calf. During the period of insertion trauma resolution and baseline measurement periods, thermoneutral water (34°C) was perfused through the suit to clamp mean skin temperature. During whole body heating, 50°C water was perfused through the suit, which increased mean skin temperature to 40°C. 50°C water was continually pumped through the suit to raise subject's Tor by 1.0°C. Local skin temperature over each microdialysis site was maintained at 33°C (Moor Instruments SHO2, Devon, UK).
Experimental protocol.
Red cell flux over each microdialysis site was monitored as insertion trauma resolved over a 75- to 90-min period. Four microdialysis sites were randomly assigned to their specific pharmacological treatment. All drugs were mixed just before each experiment, dissolved in lactated Ringer solution, and sterilized using syringe microfilters (Acrodisc, Pall, Ann Arbor, MI).
Microdialysis sites were perfused continuously for at least 75 min before the start of the baseline and during the baseline and heating periods with assigned pharmacological agents at a rate of 2.0 μl/min. Baseline data were collected for 20 min before the start of whole body heating, after which whole body heating was initiated. After Tor had increased by 1°C and clamped for 10 min, mean skin temperature was returned to baseline, and 28.0 mM sodium nitroprusside (SNP; Nitropress, Abbot Laboratories, Chicago, IL) was perfused through all sites at a rate of 4 μl/min to achieve maximal CVC (CVCmax). Additionally, local heating of the skin to 43°C, in combination with 28 mM SNP, was conducted to ensure CVCmax had been achieved.
Data acquisition and analysis.
Data were acquired using Windaq software and Dataq data-acquisition systems (Akron, OH). The data were collected at 40 Hz, digitized, recorded, and stored on a personal computer for further analysis. CVC data were averaged over 3-min periods for baseline and every 0.1°C rise in Tor and are presented as a percentage of CVCmax (%CVCmax). Absolute CVCmax in each microdialysis site was calculated as the average of the stable plateau (10 min) in laser-Doppler flux during 28 mM SNP infusion and local heating to 43°C, divided by MAP.
Statistical analyses.
Using previously published skin blood flow data with these systemic drug interventions (26), a power calculation was performed, where it was determined that a sample size of nine subjects (power = 0.80, α = 0.05) would be sufficient to determine a meaningful physiological difference of 12% CVCmax between microdialysis treatment sites and systemic drug intervention trials. A three-way mixed-models ANOVA with repeated measures was conducted to determine 1) differences between systemic drug treatments (no drug, ASA, and clopidogrel) across the rise in Tor; and 2) differences between localized microdialysis drug treatment across the rise in Tor. A two-way ANOVA with repeated measures was conducted to determine differences in absolute CVCmax (flux/MAP) between systemic drug treatment and localized microdialysis drug treatment. A one-way ANOVA with repeated measures was conducted to determine differences in the time required to increase body core temperature 1.0°C. The level of significance was set at α = 0.05. Specific planned comparisons with Bonferroni corrections were performed when appropriate. Values are presented as means ± SE.
RESULTS
Subject characteristics are presented in Table 1. There were no differences between the subjects who participated in the systemic drug intervention studies and the time control subjects. Furthermore, no differences were observed between the sexes for localized microdialysis treatment or systemic drug intervention; therefore, the data from both sexes in each group were combined.
Table 1.
Subject characteristics
| Drug Treatment Group | Time Control | |
|---|---|---|
| Sex (M, F) | 5, 5 | 2, 1 |
| Age, yr | 57 ± 3 | 57 ± 5 |
| BMI, kg/m2 | 26 ± 1 | 24 ± 1 |
| Total cholesterol, mg/dl | 159 ± 8 | 156 ± 20 |
| HDL, mg/dl | 56 ± 3 | 60 ± 9 |
| LDL, mg/dl | 103 ± 7 | 99 ± 15 |
| Fasting blood glucose, mg/dl | 94 ± 3 | 92 ± 5 |
| MAP, mmHg | 85 ± 3 | 78 ± 7 |
| Baseline Tor, °C | 36.2 ± 0.1 | 36.4 ± 0.2 |
Values are means ± SE. M, male; F, female; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MAP mean arterial pressure; Tor, oral temperature.
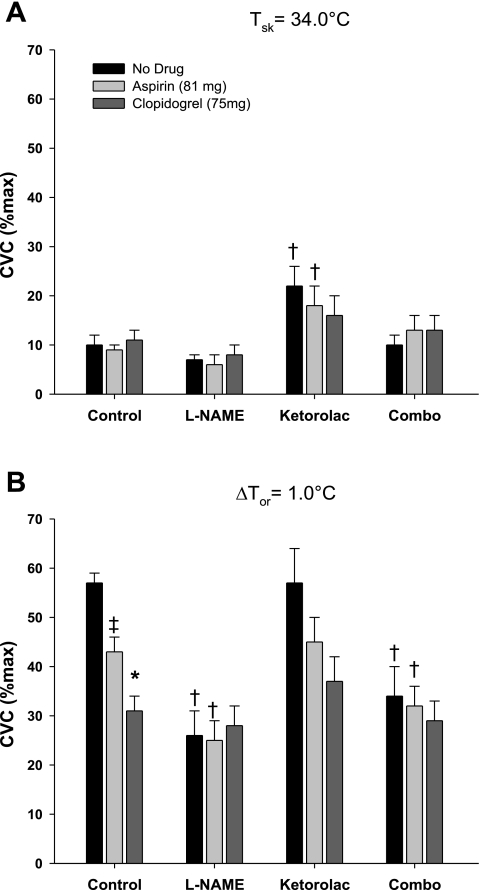
%CVCmax at thermoneutral baseline and with a 1.0°C rise in body core temperature is illustrated in Fig. 1 for all microdialysis treatment sites. Similar to previously reported studies, localized COX inhibition augmented baseline %CVCmax in no systemic drug and systemic low-dose ASA trials (P < 0.001) and did not affect %CVCmax during significant hyperthermia [change (Δ) in Tor = 1.0°C].
Fig. 1.
Group mean ± SE maximal cutaneous vascular conductance (%CVCmax) at thermoneutral baseline [change in oral temperature (ΔTor) = 0.0°C; A] and during hyperthermia (ΔTor = 1°C; B), measured as a percentage of maximum (28 mM sodium nitroprusside) for control, nitric oxide synthase-inhibited (NOS-I) [NG-nitro-l-arginine methyl ester (l-NAME)], cyclooxygenase-inhibited (COX-I) (ketorolac), and the combination of l-NAME and ketorolac (combo) sites during no drug, after 7 days of low-dose aspirin (81 mg), and after 7 days of clopidogrel (75 mg). Local treatment with ketorolac increased %CVCmax baseline with no systemic drug and low-dose aspirin treatment, which was attenuated with concurrent NOS inhibition. At plateau, there was no difference between ketorolac-treated and control sites. Systemic low-dose aspirin and clopidogrel both attenuated reflex cutaneous vasodilation compared with the control. †P < 0.05 between control and drug-treated sites within trial. ‡P < 0.05 between no-drug and low-dose aspirin trials. *P < 0.05 between low-dose aspirin and clopidogrel trials. Tsk, skin temperature.
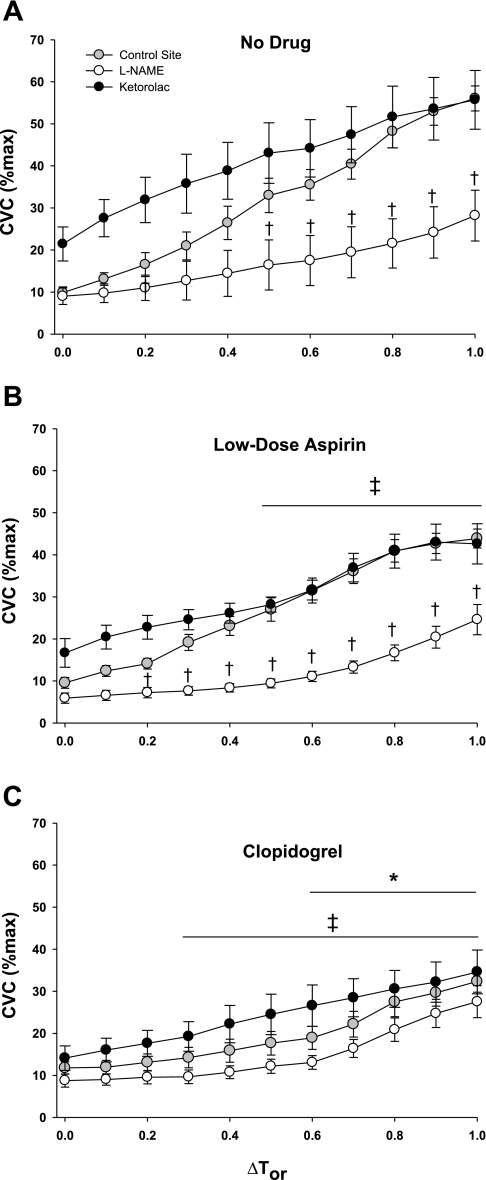
Figure 2 illustrates the control, NOS-inhibited (NOS-I), and COX-inhibited (COX-I) microdialysis sites across the rise in body core temperature. During no systemic drug trials, NOS inhibition attenuated reflex vasodilation with ΔTor ≥ 0.5°C (P < 0.001). Furthermore, there was no difference between control sites and COX-I sites with ΔTor ≥ 0.5°C. Compared with no systemic drug trials with systemic ASA treatment, reflex vasodilation was attenuated with ΔTor ≥ 0.5°C at the control site. NOS inhibition attenuated reflex vasodilation with ΔTor ≥ 0.2°C, and there was no difference between COX-I sites and the control sites with ΔTor ≥ 0.5°C. Systemic clopidogrel treatment significantly attenuated reflex vasodilation compared with no drug and systemic ASA treatment at ΔTor ≥ 0.3°C and ΔTor ≥ 0.5°C, respectively (both P < 0.001). In contrast to no systemic drug and systemic ASA treatment, there was no difference between the control, NOS-I, or COX-I sites with systemic clopidogrel treatment. In all trials (no drug, ASA, and clopidogrel), there was no difference between the NOS-I sites and the NOS + COX-I sites (omitted for clarity in Fig. 2).
Fig. 2.
Group mean ± SE %CVCmax during passive whole body heating. The control site, cyclooxygenase-inhibited (ketorolac) site, and NOS-I site are shown across the change in core body temperature (ΔTor). The combination l-NAME + ketorolac site has been omitted for clarity; however, there was no difference between this site and the l-NAME site. A: no-drug trial: local treatment with ketorolac augmented %CVCmax compared with control from baseline, but not with substantial hyperthermia. l-NAME significantly attenuated reflex cutaneous vasodilation. B: low-dose aspirin: %CVCmax was attenuated compared with the no-drug trial with ΔTor ≥ 0.5°C. C: clopidogrel: %CVCmax was attenuated compared with the no-drug trial with ΔTor ≥ 0.3°C and compared with the low-dose aspirin trial with ΔTor ≥ 0.6°C. †P < 0.05 between control and drug-treated sites within trial. ‡P < 0.05 between no-drug and low-dose aspirin trials. *P < 0.05 between low-dose aspirin and clopidogrel trials.
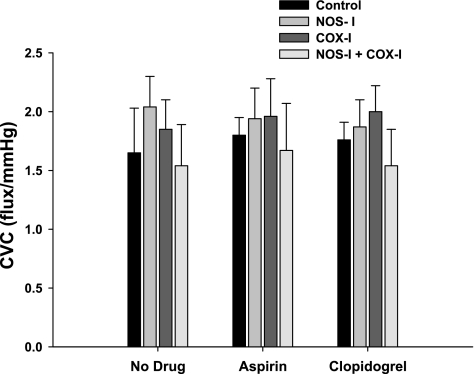
Figure 3 shows the absolute CVCmax (flux/MAP) for all localized microdialysis drug treatments and across all trials. There were no differences in absolute CVC due to either local microdialysis treatment (P = 0.89) or systemic drug intervention (P = 0.86).
Fig. 3.
Group mean ± SE absolute CVC at maximal vasodilation (sodium nitroprusside + 43.0°C). There was no difference in CVCmax due to localized microdialysis drug treatment or systemic drug treatment.
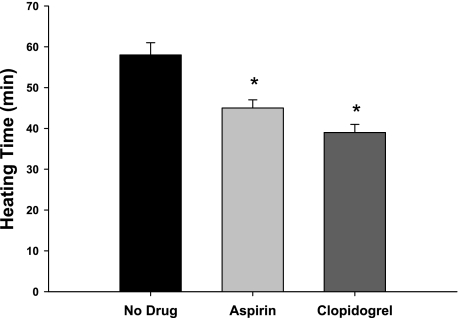
The time required to increase body core temperature by 1.0°C is shown in Fig. 4. Both systemic ASA and clopidogrel reduced the time required to increase body core temperature by 1.0°C compared with the no-drug trial (both P > 0.001); however, there were no differences between the ASA and clopidogrel trials.
Fig. 4.
The time required (minutes) to increase body core temperature by 1.0°C using the water-perfused suit in the no-drug, low-dose aspirin, and clopidogrel trials. Both systemic low-dose aspirin and clopidogrel decreased the time required to increase body core temperature by 1.0°C compared with the no-drug trials. *P < 0.05 vs. no-drug trial.
The mean %CVCmax values for each microdialysis treatment sites (ΔTor = 1.0°C) and for the time required to increase body core temperature by 1.0°C for the time control subjects are presented in Table 2. There was no difference in %CVCmax for each microdialysis site or heating time between the experimental trials for the time control group.
Table 2.
Time control data
| Experiment Day 1 | Experiment Day 2 | |
|---|---|---|
| Control, %CVCmax | 64 ± 4 | 63 ± 5 |
| l-NAME, %CVCmax | 35 ± 6 | 33 ± 3 |
| Ketorolac, %CVCmax | 61 ± 7 | 61 ± 8 |
| l-NAME + ketorolac, %CVCmax | 37 ± 8 | 33 ± 8 |
| Heating time, min | 46 ± 5 | 48 ± 6 |
Values are means ± SE. The mean percentage of maximal cutaneous vascular conductance (%CVCmax) for each microdialysis treatment sites (ΔTor = 1.0°C) and for the time required to increase body core temperature by 1.0°C for the time control subjects are shown. l-NAME, NG-nitro-l-arginine methyl ester.
DISCUSSION
The principal findings of this study were 1) specific platelet ADP receptor inhibition with clopidogrel significantly attenuated reflex vasodilation to a greater extent than platelet COX-1 inhibition with systemic low-dose ASA; and 2) 1 wk of systemic low-dose ASA therapy in healthy middle-aged humans moderately attenuated reflex cutaneous vasodilation during hyperthermia. Similar to previously reported findings, localized vascular COX inhibition augmented baseline %CVCmax during thermoneutral conditions, but did not affect %CVCmax during significant hyperthermia across all systemic drug interventions (9). These data suggest that platelets may be involved in reflex cutaneous vasodilation through either 1) the release of vasodilating factors, and/or 2) by altering blood viscoelastic properties, thus decreasing the shear stimulus on the cutaneous microvascular endothelium during hyperthermia. Finally, both ASA and clopidogrel treatments significantly reduced the time required to increase body core temperature by 1.0°C using the water-perfused suit to induce passive whole body heating, suggesting that systemic platelet inhibition may have functional thermoregulatory consequences through decreased dry heat loss mechanisms.
Recently, we observed that reflex cutaneous vasodilation was consistently and significantly attenuated in otherwise healthy middle-aged human subjects voluntarily engaging in long-term, chronic, low-dose ASA therapy (>1 yr) (11). Initially, we hypothesized that low-dose ASA may be inhibiting cutaneous vascular COX, an enzyme known to synthesize vasodilating factors that contribute to reflex vasodilation in young healthy humans. Subsequently, we explored the role of vascular COX in reflex vasodilation in healthy middle-aged subjects. We found that local vascular COX inhibition with ketorolac (intradermal microdialysis) 1) augmented thermoneutral skin blood flow (skin temperature = 34°C); and 2) did not affect %CVCmax during significant hyperthermia. Collectively, these data suggested that inhibition of vascular COX was not the underlying mechanisms explaining the significant attenuation in reflex vasodilation with chronic low-dose ASA therapy (10). Instead, based on the pharmacological mechanisms of action of low-dose ASA, these data pointed to a potential role for platelet COX-1 in reflex vasodilation.
The present study was designed to examine the effect of platelet inhibition on reflex vasodilator mechanisms. Both low-dose ASA and clopidogrel were utilized because of their distinct pharmacological mechanisms of action. Low-dose ASA acetylates COX-1 in the portal circulation inhibiting platelet COX-mediated PGH2 and thromboxane synthesis for the life of the platelet (∼10 days). Full systemic inhibition of platelet COX-1 with low-dose ASA is observed within 3 days (21, 22). In contrast, clopidogrel is metabolized in the liver, where its active metabolite irreversibly inhibits platelet ADP P2Y12 surface receptors and the potentiation of platelet ADP release. There is no direct evidence for interaction between these platelet activation pathways and is the clinical reason why low-dose ASA and clopidogrel are administered in combination for maximum platelet inhibition (21–23).
Using two pharmacologically distinct platelet inhibitors, we observed that 1 wk of systemic low-dose ASA or clopidogrel attenuated reflex cutaneous vasodilation in middle-aged humans. One potential explanation to explain this finding is that platelets may be activated either neurogenically through sensory nerves and/or through platelet vessel wall interactions, causing the release of vasodilating substances during hyperthermia. There is in vitro (3, 17) and in vivo (19, 26) direct evidence for 1) platelet COX-mediated vasodilation in models of neurogenic inflammation; and 2) platelet ADP-receptor-mediated, endothelium-dependent vasodilation. Specifically, platelet ADP-receptor stimulation causes the release of dinucleotides stored in platelet dense granule, which further regulates platelet aggregation and also mediates endothelium-dependent vasodilation (3). The precise mechanism of platelet vessel wall interactions is complex and incompletely understood; however, there is clear evidence for platelets releasing multiple vasodilating factors, some of which are known to contribute to cutaneous reflex vasodilation (2, 13, 18, 29, 31).
Another potential explanation for the attenuation in reflex vasodilation observed with platelet inhibition is through a reduction in shear-mediated vasodilation. Unlike conduit vasculature, there is significant controversy whether shear-mediated vasodilation occurs in the cutaneous vasculature. Part of this controversy is due to 1) a lack of an obligatory role for NO in cutaneous reactive hyperemia (30); and 2) methodological limitations in humans for directly measuring cutaneous microvessel diameter in addition to flow for a calculation of shear rate. Using an elegant model, Green and colleagues (7) have recently demonstrated that repeated heating of the skin can induce improvements in microvascular function, only if it is associated with hyperemia and increased shear stress. Furthermore, these authors suggest that the shear stress stimulus in the cutaneous microvasculature (through either passive heating or exercise training) is obligatory. Along these lines, whole blood viscosity may play an important role that might be capable of modulating the microvascular shear stress stimulus. Whole blood viscoelasticity is determined by plasma volume, total plasma protein concentration, red blood cell number, erythrocyte's internal viscosity, and erythrocyte and platelet aggregation tendencies (28). Both low-dose ASA and clopidogrel decrease whole blood viscoelastic (4, 21–23) properties. However, it is unclear how changes in whole blood viscoelastic properties induced by platelet inhibition may impact cutaneous vasodilation.
Regardless of the mechanism through which platelet inhibition attenuates reflex cutaneous vasodilation, there may be functional thermoregulatory consequences through decreased dry heat loss mechanisms. Although unexpected, we found that the time required to increase body core temperature by 1.0°C was significantly decreased with both systemic low-dose ASA and clopidogrel treatments. This seems counterintuitive from the perspective of convective heat transfer alone, which should be decreased with a lower skin blood flow. Yet the platelet-inhibited conditions consistently led to shorter heating times to reach a 1.0°C increase in Tor. We utilized the water-perfused suit passive heating model to induce hyperthermia in our study design, which reversed the core-to-skin gradient and clamped mean skin temperature at a constant 40°C across all systemic treatment conditions (no drug, ASA, and clopidogrel). Further research using more physiologically relevant models of heat stress (i.e., exercise in the heat) is needed to fully address the functional thermoregulatory and cardiovascular consequences of systemic platelet inhibition.
Clinical perspective.
Both low-dose ASA and clopidogrel are widely and successfully used for primary and secondary atherothrombotic disease prevention, respectively. Our data demonstrate that, in healthy middle-aged human subjects, these commonly used drugs alter skin blood flow responses during hyperthermia. While these data may provide some insight into the mechanisms mediating reflex vasodilation in healthy subjects, the potential thermoregulatory effects in cardiovascular disease populations are unknown. It may be that, in thrombotic-prone populations, treatment with these drugs helps prevent cardiovascular complications during heat stress and may even help normalize skin blood flow responses. However, given the results of the present study, the potential exists for anticoagulatory therapy to significantly reduce skin blood flow, imposing significant thermoregulatory and cardiovascular risk in these vulnerable populations (16).
Limitations.
First, given the multiple and redundant neurovascular pathways that contribute to reflex vasodilation, it is difficult to determine what potential signaling mediators may be underlying the attenuation in skin blood flow with platelet inhibition. In this study, the NOS-I microdialysis sites suggest that the NO pathway may be attenuated. This would be consistent with reduced intraluminal release of either ADP (3) and/or platelet-activating factor (17). Second, we did not specifically test our subjects' blood after platelet inhibition with either arachidonic acid or ADP, respectively. In healthy subjects, Rousseau et al. (26) found complete platelet inhibition after 4 days of treatment, with either low-dose ASA or clopidogrel (26). We treated our subjects for 7 consecutive days with 100% compliance and found that functional measures of skin blood flow were reduced with these systemic treatments. Third, it may be possible that P2Y12 receptors exist on the vascular endothelium and systemic treatment, with clopidogrel-inhibited important vascular mechanisms contributing to reflex vasodilation through P2Y12 vascular receptors. However, the literature to date only shows improvement in microvascular function with clopidogrel, independent of its effects on vascular P2Y12 receptors in a model of endothelial dysfunction (6). Finally, while 1 wk of systemic low-dose ASA attenuated reflex vasodilation based on our laboratory's previous cross-sectional study with chronic long-term ASA therapy (>1 yr), we anticipated a more significant attenuation in skin blood flow. These differences between our studies are likely due to the length of systemic ASA therapy (1 wk vs. >1 yr) and potential differences in our human subject populations.
In summary, platelet inhibition with systemic low-dose ASA or clopidogrel independently and differentially attenuated reflex cutaneous vasodilation in healthy middle-aged humans. There are several potential mechanisms that may be mediating this attenuation, including: 1) inhibition of platelet-induced release of vasodilating factors causing endothelium-dependent vasodilation; and 2) a reduction in the shear-stress stimulus on the cutaneous microvasculature, resulting from a decrease in whole blood viscoelastic properties. The consistent and significant reduction in skin blood flow with systemic platelet inhibitors was associated with a reduction in the time required to increase body core temperature by 1.0°C, suggesting greater thermal strain. Further research is necessary to examine the mechanisms of platelet vessel wall interactions underlying this attenuated skin blood flow response, as well as the potential cardiovascular and thermoregulatory consequences.
GRANTS
This study was supported by National Institutes of Health Grants R01-AG-07004-19 and M01-RR-10732 (General Clinical Research Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are appreciative for the technical assistance of Jane Pierzga and for data collection assistance from Jess Dahmus and Jeremy Sorkin.
REFERENCES
- 1. Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud's phenomenon. Arthriris Res Ther 7: R1103–R1112, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Busse R, Ogilvie A, Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am J Physiol Heart Circ Physiol 254: H828–H832, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Ciuffetti G, Lombardini R, Pirro M, Lupattelli G, Mannarino E. Clopidogrel: hemorheological effects in subjects with subclinical atherosclerosis. Clin Hemorheol Microcirc 25: 31–39, 2001 [PubMed] [Google Scholar]
- 5. Forstermann U, Mugge A, Bode SM, Frolich JC. Response of human coronary arteries to aggregating platelets: importance of endothelium-derived relaxing factor and prostanoids. Circ Res 63: 306–312, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Giachini FR, Osmond DA, Zhang S, Carneiro FS, Lima VV, Inscho EW, Webb RC, Tostes RC. Clopidogrel, independent of vascular P2Y12 receptor, improves the arterial function in small mesenteric arteries from Ang II-hypertensive rats. Clin Sci 118: 463–471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green DJ, Carter HH, FitzSimons MG, Cable NT, Thijssen DHJ, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J Appl Physiol 107: 1121–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle aged human skin. J Appl Physiol 107: 1121–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holowatz LA, Kenney WL. Chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol 106: 500–505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaul S, Padgett RC, Waack BJ, Brooks RM, Heistad DD. Effect of atherosclerosis on responses of the perfused rabbit carotid artery to human platelets. Arterioscler Thromb 12: 1206–1213, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol 272: H1609–H1614, 1997. [DOI] [PubMed] [Google Scholar]
- 16. Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kikuchi M, Shirasaki H, Himi T. Platelet-activating factor (PAF) increases NO production in human endothelial cells-real-time monitoring by DAR-4M AM. Prostaglandins Leukot Essent Fatty Acids 78: 305–309, 2008 [DOI] [PubMed] [Google Scholar]
- 18. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Noguchi K, Matsuzaki T, Shiroma N, Ojiri Y, Sakanashi M. Involvement of nitric oxide and eicosanoids in platelet-activating factor-induced haemodynamic and haematological effects in dogs. Br J Pharmacol 118: 941–950, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oskarsson HJ, Hofmeyer TG. Platelets from patients with diabetes mellitus have impaired ability to mediate vasodilation. J Am Coll Cardiol 27: 1464–1470, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, Davi G, Forni L. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 72: 1177–1184, 1985 [DOI] [PubMed] [Google Scholar]
- 22. Patrono C, Coller B, Dalen JE, FitzGerald GA, Fuster V, Gent M, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 119: 39S–63S, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Patrono C, Rocca B. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb 28: s25–s32, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med 311: 1206–1211, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol 136: 489–497, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rousseau P, Tartas M, Fromy B, Godon A, Custaud MA, Saumet JL, Abraham P. Platelet inhibition by low-dose aspirin but not by clopidogrel reduces the axon-reflex current-induced vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 294: R1420–R1426, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Stoltz JF, Donner M, Larcan A. Introduction to hemorheology: theoretical aspects and hyperviscosity syndromes. Int Angiol 6: 119–132, 1987 [PubMed] [Google Scholar]
- 29. Wong BJ, Minson CT. Neurokinin-1 receptor desensitisation attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1054, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]