Abstract
Considerable progress has been made elucidating the cellular signals and ion channel targets involved in the response to increased CO2/H+ of brain stem neurons from chemosensitive regions. Intracellular pH (pHi) does not exhibit recovery from an acid load when extracellular pH (pHo) is also acid. This lack of pHi recovery is an essential but not unique feature of all chemosensitive neurons. These neurons have pH-regulating transporters, especially Na+/H+ exchangers, but some may also contain HCO3−-dependent transporters as well. Studies in locus ceruleus (LC) neurons have shown that firing rate will increase in response to decreased pHi or pHo but not in response to increased CO2 alone. A number of K+ channels, as well as other channels, have been suggested to be targets of these pH changes with a fall of pH inhibiting these channels. In neurons from some regions it appears that multiple signals and multiple channels are involved in their chemosensitive response while in neurons from other regions a single signal and/or channel may be involved. Despite the progress, a number of key issues remain to be studied. A detailed study of chemosensitive signaling needs to be done in neurons from more brain stem regions. Fully describing the chemosensitive signaling pathways in brain stem neurons will offer new targets for therapies to alter the strength of central chemosensitivity and will yield new insights into the reason why there are multiple central chemoreceptive sites.
Keywords: sodium/hydrogen exchanger, sodium-bicarbonate cotransporter, brain stem, control of breathing, acid base, potassium channel
a major tenet of central cardiorespiratory control for nearly a century has been that central chemosensitive neurons respond to respiratory stimuli through their responses to changes of pH (19, 34, 47, 67). Over the past 15 years, fluorescence imaging techniques to measure intracellular pH (pHi) in neurons from chemosensitive regions of the brain stem (5, 24, 53, 56, 64, 65) have enabled great strides to be made in understanding the role of pH changes in chemosensitive signaling. This minireview will provide a brief overview of that progress, highlighting our current understanding of the role played by changes of pH in central chemosensitive signaling and the implications of that understanding. The challenges that lie ahead will also be emphasized.
pH-REGULATING TRANSPORTERS IN CENTRAL CHEMOSENSITIVE NEURONS
By necessity, the vast majority of the studies of pH regulation in neurons from chemosensitive regions has been done in reduced preparations, usually brain slices (43, 53, 55), isolated ganglia (24), organotypic cultures (63, 64, 65) or in cultured neurons (5), superfused with artificial cerebral spinal fluid (aCSF). One of the earliest findings regarding the regulation of pH in these neurons is that they exhibit a maintained acidification in response to hypercapnic acidosis (HA: aCSF equilibrated with elevated CO2 such that fluid pH, pHo, falls) with no apparent tendency for pHi to recover back toward its initial value (43, 53, 64, 65). This was shown not to be due to the lack of pH recovery transporters on the membrane surface since these neurons exhibited brisk pHi recovery from acidification when pHo was held constant using isohydric hypercapnic (IH) solutions (aCSF containing elevated HCO3− and equilibrated with elevated CO2 such that pHo remained constant) (24, 53). The explanation for these findings is that neurons from chemosensitive regions clearly have pH-regulating transporters in their surface membranes but the activity of these transporters is suppressed during periods of acid challenge involving a fall of pHo (53).
Early work showed that the amiloride-sensitive Na+/H+ exchanger (NHE) played the major role in recovery from an acid load in these neurons. Whether neurons from chemosensitive regions were acidified using IH solutions or using an NH4Cl prepulse (4), the recovery from that acidification was largely inhibited by amiloride (an inhibitor of NHE) and largely unaffected by DIDS (an inhibitor of HCO3−-dependent membrane transporters). This was shown for rat neurons from the nucleus tractus solitarius (NTS) (53), the ventrolateral medulla (VLM) (53, 64, 65), and the retrotrapezoid nucleus (RTN) (43), and from snail neurons from the subesophageal ganglion (24).
There are at least nine isoforms of NHE, with only NHE1–5 thought to be present in the brain of rats (1, 9, 35). NHE1 is believed to be ubiquitous and is sensitive to inhibition by amiloride (46). Low levels of expression of mRNA for NHE2 have been reported for the brain stem (35), but so far no functional NHE2 has been described in neurons from this region. Very low levels of expression for NHE3 in brain stem have also been reported (35), but physiological evidence suggests that this isoform may play an important role in central chemoreception (see below). NHE4 activity has only been reported for hippocampal neurons (3), and its expression level is very low in the brain stem (35). Finally, NHE5 has been shown to be highly expressed in rat brain (1) although studies of its significance have been hindered by the lack of specific inhibitors for this isoform.
The nature of the NHE transporters present in neurons from chemosensitive regions has been studied with pharmacological agents (31, 63, 64). In a recent paper, Kersh et al. (31) used isoform-specific inhibitors to study the presence of NHE isoforms in neurons from three different chemosensitive regions. NHE1 was the predominant isoform present in RTN neurons, accounting for about half of the pHi recovery from acidification, while NHE3 appeared to play a small role (5–10% of recovery). The remainder of the recovery may be due to NHE5 (31). A similar pattern was seen in NTS neurons, where ∼66% of recovery from acidification is mediated by NHE1 and ∼25% is mediated by NHE3 (31). Surprisingly, studies of pH recovery in neurons from another chemosensitive region, the locus ceruleus (LC), revealed no amiloride sensitivity to the pH recovery from acidification, suggesting that NHE isoforms play at most a small role in pHi recovery from acidification in LC neurons (31).
Our findings of at most a small contribution to acid extrusion by NHE3 in brain stem neurons is in agreement with the findings of Ma and Haddad (35) of a low level of expression of this isoform in brain stem. However, they are at odds with physiological data suggesting a role for NHE3 in CO2 sensing by the brain stem. In organotypic cultures of VLM neurons, an inhibitor of NHE3 resulted in neuronal acidification and an increase in firing rate (64), and this increase in firing rate was higher if both mild hypercapnia and the inhibitor were used together (63). These studies have been extended to show that exposure of rabbits to a blood-brain barrier-permeable form of an NHE3 inhibitor results in increased ventilation (32), and infusion of an NHE3 inhibitor increases respiratory frequency in anesthetized rats (50). Although these findings appear to conflict with our data showing little NHE3 activity in brain stem neurons, we believe that the two sets of data are consistent. The NHE3 inhibitors used in the animal studies may well have effects on other components of the respiratory network such as the pre-Bötzinger complex (50), which helps to set respiratory rhythm (21) and astrocytes, which affect central chemosensitivity (20, 27). Further, the NHE3 inhibitors appear to increase ventilation less than inspired hypercapnia (32). These findings suggest that NHE3 plays a role in increasing firing rate in only a limited number of central chemosensitive neurons while neurons from other central chemosensitive regions do not contain NHE3. This interpretation is consistent with the distributed nature of the central chemosensitive network (40).
The other major group of pH-regulating transporters is the HCO3−-dependent transporter family (9, 46, 57). There are many members of this class of transporters, including alkalinizing Na+-HCO3− cotransport (NBC: both electrogenic, NBCe, and electroneutral, NBCn) and Na-driven Cl−/HCO3− exchange (NDCBE) as well as acidifying Na-independent Cl−/HCO3− exchange (AE, anion exchange). While NHE appears to play a major role in pHi recovery in acid loads from brain stem neurons, there is evidence for the presence of functional HCO3−-dependent transporters as well. The best evidence is for the presence of AE in brain stem neurons (52). In response to an alkaline load at constant pHo, neurons from the VLM, but interestingly not the NTS, exhibit DIDS-inhibitable acidification. Further, removal of external Cl− results in DIDS-inhibitable alkalinization (most likely due to reversal of AE in the absence of external Cl−) in VLM but not NTS neurons (52). These data indicate that in neonatal rats, an acidifying AE is present in VLM but not NTS neurons. This is consistent with the fact that NTS neurons had a more alkaline resting pHi than VLM neurons (53), suggesting that the lack of acidifying AE in NTS neurons results in an elevated resting pHi. We have recently found that in adult rats the resting pHi of NTS neurons is lower than in neonates (41) and that these neurons appear to have AE activity (Martino PF and Putnam, unpublished observations). Interestingly, the pHi response of chemosensitive neurons to alkalinization (such as seen with hypocapnia) has not received much attention.
The presence of alkalinizing HCO3−-dependent transporters in response to acidification in neurons from chemosensitive regions is less clear. As mentioned above, LC neurons did not exhibit significant amiloride-inhibitable pHi recovery from acidification. However, the recovery from acidification was enhanced in the presence of HCO3−, compared with its absence, suggesting the presence of an alkalinizing HCO3−-dependent transporter in LC neurons (31). Since DIDS did not reduce recovery (31) it appears that LC neurons contain an alkalinizing DIDS-insensitive HCO3−-dependent transporter. Although this transporter has not been identified in LC neurons, one such transporter is an electroneutral variant of NBC, NBCn1, which has been shown to be largely unresponsive to inhibition by DIDS (10). Given the supposed importance of NDCBE for neuron pH regulation (8) it is likely that at least some neurons from some other chemosensitive regions will ultimately be shown to contain functional NDCBE and perhaps other HCO3−-dependent transporters.
It was hoped that a lack of pHi recovery from hypercapnia-induced acidification would be characteristic of chemosensitive neurons, but several lines of evidence suggest that this is not the case. In regions like the VLM or NTS, where only about 30–50% of the neurons are CO2 sensitive (i.e., increase their firing rate in response to hypercapnia) (12, 14, 17, 41, 62), virtually all neurons from these regions exhibit a lack of pHi recovery from hypercapnic acidosis (53). Further, although neurons from nonchemosensitive regions, such as the inferior olive and the hypoglossal, of young neonates exhibit pHi recovery in response to hypercapnia-induced acidification, neurons from these regions in older neonates [older than postnatal day 11 (>P11)] no longer show recovery from hypercapnia-induced acidification (43). In other words, the pHi response to hypercapnia was similar in neurons from both chemosensitive and nonchemosensitive regions. Finally, in a direct comparison of cultured neurons from the chemosensitive raphé region vs. neurons from the nonchemosensitive hippocampal region, it was found that all neurons from all regions (both chemosensitive and nonchemosensitive) did not exhibit pHi recovery from hypercapnic acidosis (5). Thus it is clear that a lack of pHi recovery in response to hypercapnia-induced acidification is not a unique characteristic of chemosensitive neurons.
ROLE OF pH CHANGES IN NEURONAL CHEMOSENSITIVE RESPONSES
Hypercapnic acidosis could stimulate chemosensitive neurons through several potential signals, including increased CO2 per se, increased intracellular HCO3−, or decreased pHo or pHi (47). Most of the focus of research in chemosensitive signaling has been on the changes of pH. The main thrust of these studies is whether it is pHo or pHi that is the major factor in chemosensitive signaling. A typical approach is to study the firing rate response of chemosensitive neurons to various acid solutions, some with elevated CO2 and others not, and some with decreased pHo and others not. Such studies in LC (22) and VLM (63) neurons found that the factor that best correlates with the increased firing rate is the decrease of pHi and not pHo. A similar conclusion was made for raphé neurons (60) although pHi was not measured in that study.
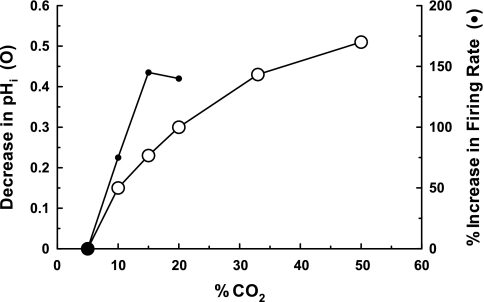
Despite the strong correlation between increased firing rate and decreased pHi, a simple relationship between these two parameters is problematic. One issue is that the increased firing rate appears to saturate, reaching a maximum in LC neurons with stimulation by solutions equilibrated with 15% CO2 (54), despite the fact that pHi continues to become more acid when solutions are equilibrated with values of CO2 higher than 15% (16) (Fig. 1). This saturation of the CO2 effect is also seen with increased phrenic nerve activity in response to increased inspired CO2 (11). It might be expected that the chemosensors would saturate at such pathologically high levels of CO2, but the mechanistic basis of this saturation of the chemosensitive response to very high levels of CO2 (and thus lower levels of pHo and presumably pHi) has not been addressed. A lack of a straightforward relationship between the changes of pHi and firing rate is also suggested, in LC neurons, by the fact that the decrease in pHi is larger in response to hypercapnic acidotic solutions compared with isohydric hypercapnic solutions, but the increase in firing rate is similar in response to these two acidic stimuli (22). The same result was reported for RTN neurons (56).
Fig. 1.
A graph showing the effect of increased CO2 [equilibrated with artificial cerebral spinal fluid (CSF) on intracellular pH (pHi) (○; left axis)] and on the increased firing rate of locus ceruleus (LC) neurons (●; right axis). The values for pHi were taken from Dean et al. (16) and represent the decrease in pHi from the initial value of pHi (at 5% CO2) of ∼7.25. The %increase in firing rate values were taken from Ritucci et al. (54) and represent the %increase in firing rate compared with control firing rate of ∼1 Hz (at 5% CO2). Note that the firing rate response reaches a plateau at 15–20% CO2 while pHi continues to decline up to 50% CO2.
The problem with using acid solutions of various compositions to study chemosensitive signaling is that while acid challenges can be studied at constant CO2 or constant pHo, all acid challenges involve a decrease of pHi. To test whether a decrease of pHi is necessary for chemosensitive signaling, we developed a technique to clamp pHi at normal control values (measured in 5% CO2) during exposure to HA solutions (25). When LC neurons were exposed to HA solutions with pHi clamped to control values, firing rate still increased, clearly indicating that a change of pHi is not required for chemosensitive signaling in LC neurons. Interestingly, firing rate does not increase in response to IH solutions (which have the same pHo as control pHo) when pHi is also clamped to control values (Hartzler LK, Dean JB, and Putnam RW, unpublished observation). These findings clearly indicate that a decrease of pHi, a change of pHo, or both are sufficient to increase LC firing rate, but that an increase of CO2 alone is not sufficient to increase firing rate in LC neurons. These findings have led us to propose a multiple-factors model for chemosensitive signaling in LC neurons (47). It is not known at this point whether a similar signaling pattern occurs in neurons from other chemosensitive regions or whether the components of chemosensitive signaling are dependent on the neurons being studied.
Another issue in cellular chemosensitive signaling is where on the neuron acid stimuli are sensed. In neurons, the critical changes of pH and the ion channel targets of those changes could reside on the soma, on the dendrites, or throughout the cell. As we study more fully the cellular basis for chemosensitivity, this question takes on greater importance since the pHi responses of dendrites to acidic stimuli can differ in magnitude and kinetics from the responses to the same stimuli in the soma (58, 66) and the distribution of channels may differ between soma and dendrites (33, 49). Based on neuronal morphology, the argument has been made that chemosensitive signaling occurs mainly on specialized regions of the dendrites. Neurons from the VLM seem to have at least one dendritic process that projects toward the ventral medullary surface (30, 44), suggesting that this process may be “sampling” fluid chemistry at the medullary surface. A similar morphology has also been observed in RTN neurons, which send dendritic projections to the ventral medullar surface that run considerable distances along the surface limiting layer, thus exposing a large dendritic surface area to the surface solution (37). A variation on this theme is the observation that chemosensitive serotonergic neurons send dendritic projections that make close contact with large medullary arteries, suggesting that these projections are sensing the chemistry of the arterial blood (6). Based on these morphological studies, it appears that the dendrites of at least some chemosensitive neurons play a critical role in chemosensitive sensing.
The role of the soma vs. the dendrites has only been directly addressed experimentally in LC neurons (54). In these studies, an LC neuron within a brain stem slice was patched and loaded with fluorescent dye so that both the soma and dendrites could be visualized. A second, larger pipette containing aCSF equilibrated with 100% CO2 and containing a fluorescent dye for visualization was brought close to the surface of the patched neuron. Acidic solution was continuously puffed on only a large dendrite (as determined by the area of spread of the fluorescent dye in the pipette) or only on the soma (and the initial segments of the primary dendrites), and the firing rate response was measured. Surprisingly, only when acidic solution was placed on the soma did the LC neuron firing rate increase (54). There was no increase in firing rate if normal aCSF (equilibrated with 5% CO2) was puffed on the neuron. Further, the increased firing rate of LC neurons in response to a regional acidification only on the soma was as large as the maximal firing rate response to a generalized acidification due to exposing the whole slice to aCSF equilibrated with 15% CO2 (54). These findings clearly indicate that the chemosensitive machinery in LC neurons resides within or very close to the cell soma. It may well be that LC neurons are sampling the local extracellular space and their cellular localization of the chemosensory machinery differs from that in neurons from other chemosensitive regions. Whether LC neurons are unique in having somatic chemosensitive signaling or whether this is a generalized finding in all chemosensitive neurons must await further experiments, of the type described here, in neurons from other chemosensitive regions such as the NTS, RTN, and VLM.
ION CHANNEL TARGETS OF CHEMOSENSITIVE SIGNALING
It is widely assumed that the targets for hypercapnia-induced acidification are pH-sensitive ion channels, especially K+ channels (29, 47). The assumption is that acid pH, either pHo or pHi, results in inhibition of K+ channels, neuronal depolarization, and increased firing rate. There are many candidate pH-sensitive channels, most of which are K+ channels (29, 47). Possible K+ channels involved in chemosensitive signaling include inwardly rectifying K+ channels (Kir) (45), especially the heteromeric Kir4.1–5.1 channel with a pK of ∼7.4 (13, 69), TASK channels (2), the rapidly activating and inactivating A current (15, 18, 48), and a delayed-rectifying K+ channel (18, 48).
Three main approaches have been adopted to study possible channels involved in the response of chemosensitive neurons: the use of channel inhibitors; direct measurements of the effects of pH on channel function using voltage clamp; and studies of the response to CO2/H+ in neurons from knockout mice. Based on the evidence to date, it appears that the number and types of channels involved in chemosensitive signaling differ in neurons from different chemosensitive regions (Fig. 2).
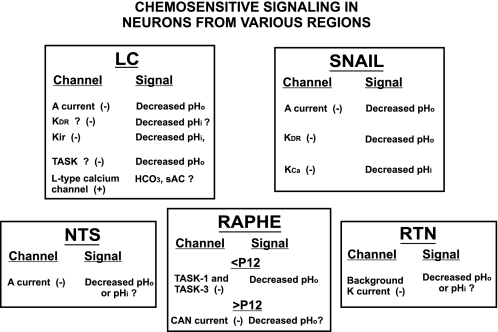
Fig. 2.
A summary figure of the ion channels and cellular signals involved in the chemosensitive response of neurons from 4 different brain stem regions or from the snail Helix aspersa. − indicates that the channel is inhibited while + indicates that it is activated by the stated signal. ? indicates the identity of the channel or of the signal is uncertain. Note that some neurons, like those from the LC or from snail, seem to involve multiple signals and multiple ion channels in chemosensitive signaling, while neurons from other regions such as the nucleus tractus solitarius (NTS) and retrotrapezoid nucleus (RTN), appear to involve one ion channel and one chemosensitive signal. In neurons from the raphé region, the ion channel responsible for chemosensitive signaling appears to be developmentally dependent, with TASK channels predominating in rodents younger than postnatal day 12 (<P12) while a calcium activated nonselective cation current (CAN current) mediates signaling in animals older than P12 (>P12). Kir, inwardly rectifying K+ channel; KCa, Ca2+-activated K+ channel; Kdr, delayed rectifying K+ channel; sAC, soluble adenylate cyclase.
In LC neurons, evidence suggests that many different channels are involved in the firing rate response to acidic stimuli. Inhibitor studies have revealed that a tetraethylammonium (TEA)-sensitive channel is active at resting pH and is inhibited by pH, and that 4-aminopyridine (4-AP) and nifedipine reduce but do not eliminate CO2/H+-induced increased firing rate (23, 36, 48). These findings suggest that the chemosensitive response in LC neurons involves L-type Ca2+ channels and TEA- and 4-AP-sensitive K+ channels. Voltage-clamp studies have confirmed that LC neurons contain CO2/H+-inhibitable transient (4-AP-sensitive current, which is likely an A current) and sustained (TEA-sensitive current, which may be due to a delayed-rectifying K+ channel) currents (48). The A current in LC neurons is largely inhibited by decreased pHo (Li K-Y and Putnam, unpublished observation), and we speculate that the TEA-sensitive sustained current is inhibited by decreased pHi. In addition, LC neurons have been shown to have CO2/H+-inhibitable Kir channels (45). These channels are inhibited by a decrease in pHi, which may change the charge state on intracellular polyamines thereby decreasing the activity of Kir channels (45). In LC neurons, pHo-sensitive TASK channels are strongly expressed and may play a role in chemosensitive signaling as well. Interestingly, it appears that L-type Ca2+ channels are activated by increased CO2/H+ in LC neurons (23), although the basis for this increase is not known. As shown in peripheral chemoreceptive cells (59), it may be that increased CO2 results in an increase in intracellular HCO3−, which activates soluble adenylate cyclase, cAMP production, and protein kinase A activity. This leads to phosphorylation and activation of L-type Ca2+ channels. Thus it appears likely that the response to CO2/H+ in LC neurons involves multiple ion channels, perhaps as many as five different types.
A similar finding has previously been made in chemosensitive neurons from the snail Helix aspersa (18). These snails were found to contain three pH-sensitive K+ currents: a transient current (likely an A current), a delayed rectifying K+ current, and a calcium-activated K+ current. Thus, like LC neurons, chemosensitive neurons from the snail appear to involve increased CO2/H+ modulating multiple ion channels.
A different picture is indicated for chemosensitive NTS neurons. In these neurons, the increased firing rate response is entirely eliminated by 4-AP (15, 36). Although voltage clamp has not been performed on these chemosensitive neurons, it appears, based on the inhibitor studies, that an A current may be solely responsible for the firing rate response of chemosensitive NTS neurons to CO2/H+. It is not known whether the A current in NTS neurons is largely inhibited by pHo or pHi. Thus, unlike chemosensitive LC and snail neurons, NTS neurons may involve a single chemosensitive signal acting on a single ion channel.
Yet a different pattern for hypercapnia-induced increased firing rate is indicated for chemosensitive neurons from the medullary raphé. In young neonatal mice [younger than postnatal day 12 (P12)] that have had TASK channel isoforms 1, 3, or both 1 and 3 knocked out, the increased firing rate in response to elevated CO2/H+ in serotonergic raphé neurons is eliminated (38). This suggests that chemosensitivity arises from TASK-1 and TASK-3 in raphé neurons from mice younger than P12. It may be that these two isoforms make a heteromeric channel that is inhibited by decreases in pHo (38). The magnitude of the chemosensitive response in raphé neurons from these young mice is very small (38), and in fact Hodges et al. (26) recently showed that a large chemosensitive response to hypercapnia is only seen in raphé neurons from mice older than P12. Further, the large hypercapnia-induced increase in firing rates of raphé neurons from older neonates appears to be due to an acid-sensitive calcium-activated nonselective cation (CAN) current (68). It has not been determined whether this channel is sensitive to changes of pHo or pHi. The chemosensitive response of raphé neurons, therefore, appears to be developmentally dependent, with a small chemosensitive response, perhaps dependent on TASK channels in young neonates, that changes to a larger response dependent on a CAN current after age P12 (Fig. 2).
Interestingly, in these same TASK-1 and -3 knockout mice, the chemosensitive response to increased CO2/H+ is unaffected in RTN neurons (38). This indicates that TASK-1 and -3 are not involved in the chemosensitive response of RTN neurons. Nevertheless, the acid-sensitive channel that is responsible for the chemosensitive response of RTN neurons has a current-voltage plot that indicates that it is a background K+ channel (38), either one that is not a TASK channel or an isoform of TASK channels that is neither TASK-1 nor TASK-3. It is unclear whether the chemosensitive channel(s) in RTN neurons is/are responsive to changes of pHo or pHi.
In summary, chemosensitive signaling in neurons from some regions (LC, snails) involves multiple channels and presumably multiple signals, while in neurons from other regions (NTS and RTN) a single channel may be responsible for chemosensitive signaling. Finally, in raphé neurons the channel responsible for chemosensitive signaling appears to change with development.
Future Studies
This brief review points to several significant unanswered questions that could serve as the focus for future studies. While we have learned a great deal about pHi regulation in chemosensitive neurons it will ultimately be important to know which isoforms of which transporters are present in the neurons from different chemosensitive regions. This is especially true given the evidence that NHE3 may play a role in ventilatory control. The exact localization of this NHE isoform, in neurons or glia, in which neurons, and somatic vs. dendritic distribution, should be a high priority since this information could yield important insights into the role played by neurons from chemosensitive regions that contain NHE3 in ventilatory control. It is also likely that HCO3−-dependent transporters play a greater role than currently appreciated in the response of chemosensitive neurons to acidic stimuli. Thus studies of the expression and activity of various HCO3−-dependent transporters in neurons from different chemosensitive regions should be a focus of future research. There is already evidence that the expression of pH-regulating transporters in brain stem neurons varies with development (43; Martino and Putnam, unpublished observation), and it will be important in the future to determine developmental changes of the expression of pH-regulating transporters in neurons from the various chemosensitive regions. Finally, detailed studies such as those performed in LC neurons need to be done in neurons from other chemosensitive regions. The most important experiments will be those done with pHi clamped during exposure to HA in an attempt to elucidate whether the neurons from various chemosensitive regions are responding to changes of pHi, changes of pHo, changes of CO2, or to a combination of these stimuli.
There is also considerable work left to do identifying which ion channels are involved in the response to acid stimuli of neurons from different chemosensitive regions (Fig. 2). A major part of this work will be to further identify the cellular localization of these putative chemosensitive channels, whether on the soma, dendrites, or throughout the neuron. Especially if we find that many chemosensitive neurons are responding to acid challenges based on processes that occur on their dendrites, studies of pHi regulation and of the proximate stimulus for these channels will need to be designed to examine pH changes and pHi clamping on the dendritic processes, a daunting task but most likely amenable using modern technological advances in imaging.
Whatever model emerges from these studies of the signals and ion channel targets in chemosensitive neurons will have to be able to explain the apparent saturation of the firing rate response of these neurons to stimuli of increasing strength (Fig. 1). This saturation phenomenon may arise from a diminishing ability of decreasing pH to inhibit K+ channels further or it may be due to activation of some process that limits further increases in firing rate with increasing acidic stimulation. Such a process may involve the Ca2+-activated K+ channel (KCa) since it has been shown in LC neurons that hypercapnic solutions activate L-type Ca2+ channels (23). At some point this process could result in sufficient intracellular Ca2+ accumulation to activate KCa, limiting further membrane depolarization and increased firing rate. The mechanism by which increased acidic stimuli saturate the firing rate or the ventilatory response is an important process to elucidate.
Finally, the focus of this review has been solely on neurons that are activated by hypercapnia, but in several brain stem regions neurons have been described that are inhibited by hypercapnia (12, 17, 28, 41, 51, 61, 62). There has yet to be a cellular mechanism proposed by which acid stimuli result in a reversible decrease in neuronal firing rate. However, it has long been postulated that the normal response of neurons to acid environments is an inhibitory response (7), and the mechanism in neurons from chemosensitive regions may involve K+ channels that are activated by acidic stimuli. Neurons inhibited by hypercapnia are often present in low proportions, but recently a model system has been described in which acid-inhibited neurons constitute as much as 30% of NTS neurons (42). Such a system has a sufficient percentage of inhibited neurons to serve as an excellent model system for the study of the cellular mechanism(s) involved in the inhibition of neurons from chemosensitive regions by hypercapnia.
CONCLUSIONS
A detailed knowledge of the signals and targets that mediate the activation of chemosensitive neurons in response to hypercapnic acidosis would be extremely valuable. The signals being “sensed” will reveal a great deal about what is being regulated (e.g., ventilation or brain pH), and the ion channels that are affected by the signals will make attractive targets for therapies aimed at altering the strength of central chemosensitivity. Further, since central chemosensitivity appears to be due to a distributed network of chemosensitive neurons (40), knowledge of how changes of CO2/H+ are sensed in neurons from different regions may yield insights into the role played by each of those regions in the overall control of ventilation in the organism. Finally, since it appears likely that chemosensitive signaling differs in neurons from different brain stem regions (Fig. 2), the findings to date are consistent with a distributed central pattern of chemosensitivity that arose in a hierarchical fashion, with new chemosensory components added throughout evolution as organisms passed through key transitions such as those from water to air breathing, the maintenance of a high and constant body temperature, and the development of sleep (39).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-56683.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1. Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem 274: 4383–4388, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” channel expressed in brain stem respiratory neurons. Respir Physiol 129: 159–174, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bookstein C, Musch MW, DePaoli A, Xie Y, Rabenau K, Villereal M, Rao MC, Chang EB. Characterization of the rat Na+/H+ exchanger isoform NHE4 and localization in rat hippocampus. Am J Physiol Cell Physiol 271: C1629–C1638, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouyer PG, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurons cultured from rat medullary raphé or hippocampus. J Physiol 559: 85–101, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401–402, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Carpenter DO, Hubbard JH, Humphrey DR, Thompson HK, Marshall WH. Carbon dioxide effects on nerve cell function. In: Carbon Dioxide and Metabolic Regulation, edited by Nahas G, Schaefer KE. New York: Springer, 1974, p. 49–62 [Google Scholar]
- 8. Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF. Expression and localization of Na-driven Cl-HCO3 exchanger (SLC4A8) in rodent CNS. Neuroscience 153: 162–74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chesler M. The regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Coates EL, Li A, Nattie EE. Widespread sites of brainstem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol 166: 4–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui N, Giwa LR, Xu H, Rojas A, Abdulkadir L, Jiang C. Modulation of the heteromeric Kir4.1-Kir51 channels by Pco2 at physiological levels. J Cell Physiol 189: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Dean JB, Gallman EA, Millhorn DE. Electrophysiology and morphology of CO2-sensitive neurons in the dorsal vagal complex studied in vitro. Soc Neurosci Abstr 16: 1235, 1990 [Google Scholar]
- 16. Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol 129: 83–100, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res 76: 656–661, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Denton JS, McCann FV, Leiter JC. CO2 chemosensitivity in Helix aspersa: three potassium currents mediate pH-sensitive neuronal spike timing. Am J Physiol Cell Physiol 292: C292–C304, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidosis. J Physiol 358: 285–297, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol 85: 1599–1604, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Feldman JR, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Ann Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541.2: 493–509, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Goldstein JI, Mok JM, Simon CM, Leiter JC. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive regions of Helix aspersa. Am J Physiol Regul Integr Comp Physiol 279: R414–R423, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Biol Med 605: 333–337, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Hodges MR, Wu Y, Richerson GB. Chemosensitivity of mouse 5-HT neurons to hypercapnia acidosis is dependent upon age and temperature in brainstem slices in vitro. Soc Neurosci Abstr 89 24, 2009 [Google Scholar]
- 27. Holleran J, Babbie M, Erlichman JS. Ventilatory effects of impaired glial function in a brain stem chemoreceptor region in the conscious rat. J Appl Physiol 90: 1539–1547, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Huang RQ, Erlichman JS, Dean JB. Cell-cell coupling between CO2-excited neurons in the dorsal medulla oblongata. Neuroscience 80: 41–57, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol 145: 115–126, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. J Physiol 492.1: 277–292, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kersh AE, Hartzler LK, Havlin K, Belcastro Hubbell B, Nanagas V, Kalra A, Chua J, Whitesell R, Ritucci NA, Dean JB, Putnam RW. pH regulating transporters in neurons from various chemosensitive brainstem regions in neonatal rats. Am J Physiol Regul Integr Comp Physiol 297: R1409–R1420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiwull-Schöne H, Wiemann M, Frede S, Bingmann D, Wirth KJ, Heinelt U, Lang HJ, Kiwull P. A novel inhibitor of the Na+/H+ exchanger type 3 activates the central respiratory CO2 response and lowers the apneic threshold. Am J Respir Crit Care Med 164: 1303–1311, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol 525: 621–639, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol 332: 1–24, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma E, Haddad GG. Expression and localization of Na+/H+ exchangers in rat central nervous system. Neuroscience 79: 591–603, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Martino PF, Putnam RW. The effect of 4 aminopyridine (4AP) on the hypercapnic response of locus coeruleus (LC) neurons. Soc Neurosci Abstr 297. 7, 2007 [Google Scholar]
- 37. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14049, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nattie EE. Central chemosensitivity, sleep, wakefulness. Respir Physiol 129: 257–268, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex (SC) neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296: R763–R773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nichols NL, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats. Respir Physiol Neurobiol 168: 272–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nottingham S, Leiter JC, Wages P, Buhay S, Erlichman JS. Developmental changes in intracellular pH regulation in medullary neurons of the rat. Am J Physiol Regul Integr Comp Physiol 281: R1940–R1951, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Pilowsky P, Llewellyn-Smith IJ, Arnolda L, Lipski J, Minson J, Chalmers J. Are the ventrally projecting dendrites of respiratory neurons a neuroanatomical basis for the chemosensitivity of the ventral medulla oblongata? Sleep 16, Suppl 8: S53–S55, 1993 [PubMed] [Google Scholar]
- 45. Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Putnam RW. Intracellular pH regulation of neurons in chemosensitive and nonchemosensitive areas of brain slices. Respir Physiol 129: 37–56, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Putnam RW, Li KY. Transient and sustained potassium currents are inhibited by hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr 89 16, 2009 [Google Scholar]
- 49. Riazanski V, Becker A, Chen J, Sochivko D, Lie A, Wiester OD, Elger CE, Beck H. Functional and molecular analysis of transient voltage-dependent K+ currents in rat hippocampal granule cells. J Physiol 537: 391–406, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribas-Salgueiro JL, Matarredona ER, Sarmiento M, Ribas J, Pásaro R. Respiratory response to systemic inhibition of the Na+/H+ exchanger type 3 in intact rats. Respir Physiol Neurobiol 165: 254–260, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 275: R1152–R1163, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 273: R433–R441, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol 289: C1094–C1104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ritucci NA, Erlichman JS, Dean JB, Putnam RW. A fluorescence technique to measure intracellular pH of single neurons in brainstem slices. J Neurosci Meth 68: 149–163, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol 538: 371–382, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Summers BA, Overholt JL, Prabhakar N. CO2 and pH independently modulate L-type Ca2+ current in rabbit carotid body glomus cells. J Neurophysiol 88: 604–612, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and Pco2. J Physiol 540: 951–970, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang W, Pizzonia JJ, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511.2: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wellner-Kienitz MC, Shams H. CO2-sensitive neurons in organotypic cultures of the fetal rat medulla. Respir Physiol 111: 137–151, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Wiemann M, Bingmann D. Ventrolateral neurons of medullar organotypic cultures: intracellular pH regulation and bioelectric activity. Respir Physiol 129: 57–70, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Wiemann M, Schwark JR, Bonnet U, Jansen HW, Grinstein S, Baker RE, Lang HJ, Wirth KK, Bingmann D. Selective inhibition of the Na+/H+ exchanger type 3 activates CO2/H+-sensitive medullary neurones. Pflügers Arch 438: 255–262, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Wiemann M, Baker RE, Bonnet U, Bingmann D. CO2-sensitive medullary neurons: activation by intracellular acidification. Neuroreport 9: 167–170, 1998 [DOI] [PubMed] [Google Scholar]
- 66. Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol 544: 487–499, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Winterstein H. Chemical control of pulmonary ventilation. III. The “reaction theory” of respiratory control. N Engl J Med 255: 331–337, 1956 [DOI] [PubMed] [Google Scholar]
- 68. Wu Y, Hodges MR, Wang W, Zaykin A, Wylie CJ, Deneris ES, Richerson GB. Hypercapnic acidosis inhibits a calcium-activated non-selective cation current in mature serotonergic neurons. Soc Neurosci Abstr 89 17, 2009 [Google Scholar]
- 69. Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. J Physiol 524: 725–735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]