Abstract
Cellular mechanisms of CO2 chemoreception are discussed and debated in terms of the stimuli produced during hypercapnic acidosis and their molecular targets: protons generated by the hydration of CO2 and dissociation of carbonic acid, which target membrane-bound proteins and lipids in brain stem neurons. The CO2 hydration reaction, however, is not the only reaction that CO2 undergoes that generates molecules capable of modifying proteins and lipids. Molecular CO2 also reacts with peroxynitrite (ONOO−), a reactive nitrogen species (RNS), which is produced from nitric oxide (•NO) and superoxide (•O2−). The CO2/ONOO− reaction, in turn, produces additional nitrosative and oxidative reactive intermediates. Furthermore, protons facilitate additional redox reactions that generate other reactive oxygen species (ROS). ROS/RNS generated by these redox reactions may act as additional stimuli of CO2 chemoreceptors since neurons in chemosensitive areas produce both •NO and •O2− and, therefore, ONOO−. Perturbing •NO, •O2−, and ONOO− activities in chemosensitive areas modulates cardiorespiration. Moreover, neurons in at least one chemosensitive area, the solitary complex, are stimulated by cellular oxidation. Together, these data raise the following two questions: 1) do pH and ROS/RNS work in tandem to stimulate CO2 chemoreceptors during hypercapnic acidosis; and 2) does nitrosative stress and oxidative stress contribute to CO2 chemoreceptor dysfunction? To begin considering these two issues and their implications for central chemoreception, this minireview has the following three goals: 1) summarize the nitrosative and oxidative reactions that occur during hypercapnic acidosis and isocapnic acidosis; 2) review the evidence that redox signaling occurs in chemosensitive areas; and 3) review the evidence that neurons in the solitary complex are stimulated by cellular oxidation.
Keywords: reactive oxygen species, reactive nitrogen species, carbon dioxide, central chemoreception, hypercapnia
MOLECULAR O2 AND MOLECULAR CO2 GENERATE REACTIVE OXYGEN SPECIES/REACTIVE NITROGEN SPECIES
molecular oxygen is a strong oxidant that is reduced sequentially to yield oxygen free radicals and their highly reactive nonradical derivatives. Known as “reactive oxygen species” (ROS), these strong oxidants include superoxide anion (•O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) among others (22). Molecular oxygen also reacts with l-arginine in the presence of reduced nicotinamide-adenine dinucleotide phosphate (NADPH) and nitric oxide synthase (NOS) to yield the free radical nitric oxide (•NO) and citrulline (45, 52). Nitric oxide is an important signaling molecule in the central nervous system (CNS) and a “reactive nitrogen species” (RNS). Nitric oxide reacts with •O2− to produce peroxynitrite (ONOO−), which is yet another type of RNS. These various oxidation-reduction (i.e., redox) reactions occur under normal physiological conditions and contribute to normal mechanisms of cellular signaling (22, 26). Under adverse conditions, redox reactions can also produce excessive levels of ROS/RNS that exceed the body's endogenous antioxidant defenses resulting in “oxidative and nitrosative stress” and cellular dysfunction (22, 80). Not surprisingly, ROS and RNS production have been implicated in the brain stem's sensitivity to hypoxia (13, 36, 40, 47, 62) and hyperoxia (16–19, 57).
In this minireview, I propose that molecular CO2 and H+ are two additional sources of ROS/RNS that modulate brain stem neurons, including those in CO2-chemosensitive areas. Molecular CO2 also reacts with ONOO− to generate the RNS called nitrosoperoxocarboxylate (ONO2CO2−)1 under physiological conditions (21, 27, 78, 89, 91). Nitrosoperoxocarboxylate, in turn, produces reactive intermediates capable of oxidation as well as nitration and nitrosylation reactions, i.e., nitro-oxidative reactions (27, 31, 80, 86, 91). In addition, protons, independently of molecular CO2, catalyze other redox reactions, most notably the Fenton reaction that produces the powerful oxidant, •OH, which nondiscriminately targets all components of the cell membrane (22, 70, 80). Consequently, the potential exists for increased CO2 and/or H+ during hypercapnic acidosis to facilitate various nitro-oxidative reactions in neurons that function in cardiorespiratory control (19).
The pervasiveness of CO2/H+-facilitated nitro-oxidative reactions in the bicarbonate (HCO3−)-buffered biological milieu raises two questions of potential importance for central CO2 chemoreception. First, if hypercapnic acidosis (respiratory acidosis) and isocapnic acidosis (metabolic acidosis) enhance redox reactions under physiological conditions, then does redox signaling participate in the cellular mechanism of CO2/H+ chemoreception? Putnam et al. (72) have proposed a “multiple factors and multiple targets” theory of CO2 chemoreception whereby neuronal chemosensitivity is explained by the combined effects of extracellular pH (pHo), intracellular pH (pHi), and molecular CO2/HCO3− on multiple populations of K+ and Ca2+ channels. For purposes of discussion here, I have proposed that CO2-/H+-dependent redox reactions and nitrosative reactions are additional “factors” that work in tandem with pH reactions to modulate the proteins and lipids forming the plasma membrane [the molecular “targets” (11)] of CO2 chemoreceptors during hypercapnic acidosis and isocapnic acidosis. I also expect that CO2-/H+-dependent redox reactions will influence other populations of cardiorespiratory neurons in the brain stem; however, as presented below, the initial evidence indicates that putative central chemoreceptors have a lower threshold for redox stimulation than nonchemosensitive neurons (19, 57). If this hypothesis proves to be correct, then the clinically relevant second question is how does oxidative stress and nitrosative stress influence central CO2 chemoreception and what role does it play in disorders of respiratory control that involve central chemoreceptors? Oxidative stress and nitrosative stress have been implicated in a number of neurological disorders and diseases (80) and may, likewise, contribute to onset of disorders of cardiorespiratory control and, in addition, result from O2/CO2/pH perturbations caused by disordered breathing patterns (43, 46, 51, 75, 79, 93).
I begin to address these issues and their possible implications for central chemoreception by 1) discussing nitro-oxidative reactions that are stimulated during hypercapnic acidosis and isocapnic acidosis in a HCO3−-buffered system under physiological conditions; 2) reviewing the evidence that neurons in chemosensitive areas of the brain stem express the requisite oxidative enzymes and reactants that would enable these specific CO2/H+-driven redox and nitrosative reactions; and 3) summarizing the evidence that neuronal activity in the solitary complex (SC), including putative CO2-chemoreceptor neurons, is stimulated by cellular oxidation that causes changes in mechanisms of excitability and pHi regulation.
REDOX AND NITROSATIVE REACTIONS ACTIVATED DURING HYPERCAPNIC ACIDOSIS AND ISOCAPNIC ACIDOSIS
The reaction between carbon dioxide/bicarbonate and peroxynitrite is important for understanding mechanisms of oxidant-mediated toxicity. First, normal plasma bicarbonate and carbon dioxide concentrations are 25 and 1.3 mM, respectively, but higher levels could be achieved during tissue pathological events such as respiratory distress or ischemia-reperfusion. Second, excess production of peroxynitrite has been documented to occur during these and other instances of accelerated tissue superoxide and nitric oxide production, enhancing the potential for coordinated reaction mechanisms to occur between CO2/HCO3−, peroxynitrite, and tissue target molecules.
—From Denicola et al. (Ref. 21)
CO2/HCO3− and peroxynitrite.
Peroxynitrite (ONOO−) is formed by the reaction of •O2− and •NO (20, 73, 86, 91) [Fig. 1, reaction (Rxn) 3]. The •O2−/•NO reaction (Fig. 1, Rxn 3) has been called the “radical switch” that diverts •NO away from its normal regulatory signaling actions toward potentially cytotoxic reactions that result in nitrosative and oxidative damage (27, 86). The •NO/•O2− reaction is diffusion limited and occurs more rapidly than the catalyzed dismutation reaction of •O2− (superoxide dismutase, SOD) to form H2O2 (4) (Fig. 1, Rxn 4). The •NO/•O2− reaction that forms ONOO− occurs in both intracellular and extracellular compartments. Peroxynitrite readily crosses the plasma membrane in its protonated form (peroxynitrous acid, ONOOH) to react with intracellular substrates. Peroxynitrite anion also crosses the cell membrane via a 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS)-sensitive anion exchanger that normally functions in Cl−/HCO3− exchange (78); this latter mechanism predominates at physiological pH (89). Once formed, ONOO− undergoes several reactions to yield molecular products of varying reactivity (91): oxidation of sulfhydryl (thiol) groups to disulfides; nitration of aromatic compounds; S-nitrosylation of sulfur atoms in amino acid residues resulting in posttranslational modification of proteins (i.e., S-nitrosylated proteins, SNO); protonation to produce peroxynitrous acid that decomposes to form nitrate; and reaction with CO2 to generate nitrosoperoxocarboxylate (ONO2CO2−) (21, 26, 27, 31, 70, 71, 73, 78, 89–91). It is the last reaction between CO2 and ONOO− that occurs ubiquitously in a CO2/HCO3−-buffered system (Fig. 1, Rxn 5) and, moreover, that is postulated here to be involved in nitrosative and oxidative modulation of brain stem neurons, including chemosensitive neurons, during hypercapnic acidosis and oxidative stress (Fig. 1, Rxn 6).
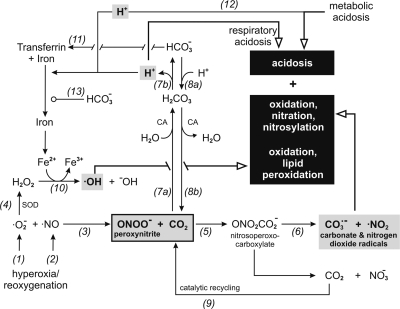
Fig. 1.
Schematic of the relationships between hypercapnic acidosis [respiratory acidosis; reactions (Rxns) 7a and 7b] and isocapnic acidosis (metabolic acidosis; Rxn 12) and production of nitrosative and oxidative intermediates via peroxynitrite (ONOO−; Rxns 5 and 6) and production of oxidative intermediates via the Fenton reaction (Rxn 10). Molecular oxygen produces both superoxide anion (•O2−) and nitric oxide (•NO) as described in the text (Rxns 1 and 2). These 2 ubiquitous radicals react to generate ONOO− (Rxn 3), which in a CO2/HCO3−-buffered system (Rxn 8) immediately reacts with CO2 to form nitrosoperoxocarboxylate (ONO2CO2−; Rxn 5) that is followed by several additional reactions summarized in the text; see also Vesela and Wilhelm (91). The most important reaction, in the context of the present discussion, is formation of carbonate radicals (CO3−•) and nitrogen dioxide radicals (•NO2) (Rxn 6). In the process, CO2 is regenerated and recycled for production of more ONO2CO2− (Rxn 9). In addition, •O2− (Rxn 1) is converted to hydrogen peroxide (H2O2) in a reaction catalyzed by superoxide dismutase (SOD; Rxn 4) and reacts with iron liberated from transferrin. Decreased pH increases dissociation of iron from transferrin (Rxns 7b and 12) and accelerates the Fenton reaction rightward, producing hydroxyl radicals (•OH; Rxn 10). Metabolic acidosis (i.e., ↑H+, ↓HCO3−, and no change Pco2) is a more effective stimulus than respiratory acidosis (i.e., ↑H+, ↑HCO3−, and ↑Pco2) for liberating free iron since HCO3− promotes binding of iron to transferrin (Rxn 11). CA, carbonic anhydrase; NO3−, nitrate anion; OH−, hydroxyl anion; solid black arrowhead, acceleration of reaction; open circle, inhibition of reaction; open arrowhead with thick line, cellular consequence of reaction, include acidosis, nitro-oxidation, and lipid peroxidation.
Peroxynitrite is unstable in CO2/HCO3−-buffered medium and is quickly consumed by its reaction with CO2 to yield ONO2CO2− (21, 70, 71, 89) (Fig. 1, Rxn 5). Addition of the enzyme carbonic anhydrase (CA), which catalyzes the hydration/dehydration reactions of CO2 and HCO3− (Fig. 1, Rxn 7/Rxn 8), also increases the rate of conversion of ONOO− to ONO2CO2− (89) (Fig. 1, Rxn 5). In the presence of CO2/HCO3−, a limited number of molecular targets react directly with ONOO− (27, 70). Instead, biomolecules react with the various RNS intermediates that are produced from the CO2/ONOO− reaction (3, 27, 70, 86, 91). The CO2/ONOO− reaction is one of the fastest reactions that ONOO− undergoes (reaction rate, k = 5.8 × 104 M−1·s−1 at 37°C), and it predominates in the extracellular space where the concentration of sulfhydryl groups is low and CO2/HCO3− concentration is high; that is, formation of ONO2CO2− is favored in the extracellular compartment over formation of disulfides (91). Radi (73) has proposed that over 90% of ONOO− initially reacts with CO2 in the extracellular space and from 30–40% of ONOO− initially reacts with CO2 in the intracellular space. As stated above, however, ONOO− has no problem crossing the cell membrane and gaining access to intracellular targets (78).
The CO2/ONOO− reaction occurs over a broad range of pH, including physiologically relevant pH that stimulates breathing in vivo and CO2-chemosensitive neurons under in vitro conditions, for example, arterial partial pressure of carbon dioxide (Pco2) and pH ranges, respectively, from ∼35–45 Torr and ∼7.35–7.45. An increase in arterial Pco2 of only a few Torr significantly increases minute ventilation (34, 59, 81). In rats, switching from air to 6% CO2 in air decreases cerebrospinal fluid pH (pHCSF) from 7.396 to 7.294 and neural pHi from 7.044 to 6.982 and stimulates minute ventilation. Breathing 11% CO2 in air decreases pHCSF further to 7.190 and brain pHi to 6.910 and stimulates breathing (83). In rat brain stem tissue slices, acute exposures to moderate levels of hypercapnia decreases pHi by ≤0.2 pH units from a baseline pHi of ∼7.24 (28, 76, 77), decreases membrane conductance, and stimulates firing rate of neurons in CO2-chemosensitive areas (14, 28, 42). Based on these studies, the physiologically relevant range of pHCSF/pHi for stimulation of chemoreceptors encompasses ∼7.4 down to 6.91. This range of pH overlaps with the range of pH (6.5–7.4) that the CO2/ONOO− reaction occurs (21, 71, 89). At pH 7.4, the predominance of the CO2/ONOO− reaction is demonstrated by the decrease in peroxynitrite-mediated oxidation products when CO2/HCO3− was added to the reaction vessel; that is, addition of CO2/HCO3− accelerated consumption of ONOO− to yield ONO2CO2− before ONOO− could react with other substrates and oxidize molecular targets such as glutathione and oxyhemoglobin (21). Increasing CO2 further accelerates consumption of ONOO− (21, 71, 86, 89, 91).
The CO2/ONOO− reaction redirects the primary reactivity of ONOO− in both intracellular and extracellular compartments to nitrosative and oxidative reactions through breakdown of ONO2CO2− into carbonate radicals (CO3−•) and nitrogen dioxide radicals (•NO2) and other species (70, 71, 91) (Fig. 1, Rxn 6). In the course of producing these derived nitro-oxidants, CO2 is regenerated and recycled for further production of ONO2CO2− (71) (Fig. 1, Rxn 9). Thus molecular CO2 acts as a catalyst for ONOO−/ONO2CO2− reaction (Fig. 1, Rxn 5). The formation of CO3−• and •NO2 is favored in the nonpolar environment of the plasma membrane (91). Carbonate radicals are more stable than •OH and can diffuse from their site of synthesis to induce oxidative and nitrosative stress with more species and molecular targets that are further removed. When CO3−• and •NO2 react in concert, they constitute an efficient nitrating system that is pivotal to biological nitration of protein tyrosine residues (70). The majority of CO3−• reactions are protein oxidations via hydrogen atom abstraction and electron transfer (3). Important targets for CO3−• include tyrosine, tryptophan, guanine, cysteine, sulfhydryl groups, and nucleic acids (27, 70, 86, 88, 91). Nitrogen dioxide is a RNS that can react with other radical species or oxidize biomolecules via electron transfer and hydrogen atom abstraction (3). Important targets include proteins, fatty acids, phenols, thiols, tyrosine, tryptophan, and cysteine residues (3, 70).
As stated above, ONOO− undergoes several other reactions to varying extent in the presence of CO2/HCO3− (26, 91). The lack of comment in the literature on the effects of pH on these other reactions suggests that they are either unremarkable in their pH sensitivity or that their pH sensitivity is unstudied to date. Regardless, I mention one of these ONOO− reactions here—the S-nitrosylation (SNO) reactions—for the following two reasons: 1) S-nitrosylation reactions have been implicated in cardiorespiratory control mechanisms in the nucleus tractus solitarius, which is the dorsal nucleus of the SC (see below) (47, 65); and 2) S-nitrosylation reactions redirect ONOO− away from reacting with CO2 and therefore influence formation of CO2-dependent RNS (21, 26, 71, 86, 89, 91). Peroxynitrite induces SNO reactions (26, 90) that cause reversible nitrations at cysteine and methionine residues that are thought to be involved in normal signaling mechanisms (31, 87), including cardiorespiratory control (47, 65). Conversely, irreversible SNO reactions occur principally from nitration of tyrosine residues forming nitrosocarbonate and are believed to be involved in pathological processes (31, 35, 88).
H+ and the Fenton reaction.
Trace amounts of metal ions, such as iron, are important activators of redox reactions (86). The Fenton reaction (Fig. 1, Rxn 10) describes the iron-catalyzed reaction in which H2O2 is converted to •OH. Ferrous iron (Fe2+) is oxidized by H2O2 to ferric iron (Fe3+) plus hydroxyl radical and hydroxyl anion: Fe2+ + H2O2 → Fe3+ + •OH + −OH. Hydroxyl radicals are more reactive and thus damaging than CO3−• and •NO2. They react with essentially all biomolecules that they collide with, which is why the effects of •OH are more random and widespread compared to the targeted reactions initiated by CO3−• and •NO2 (70). Under normal conditions, oxidative stress from excessive production of •OH is kept in check, in part, by the high-affinity iron-binding protein, transferrin (Tf), which is present in the CSF, plasma, extracellular fluid, and lymph (30). Binding of iron to Tf is strongly pH dependent (85), and decreasing pHo increases dissociation of iron from Tf (2, 82) (Fig. 1, Rxn 7b and Rxn 12). At pHo= 7.4, free Fe3+ binds to Tf and the transferrin receptor (TfR1) located on the plasma membrane. The Fe-Tf-TfR1 complex is internalized by endocytosis to form a vesicle (endosome) whose internal pH is maintained acidic (pH ∼6.0) via proton pumps. Vesicular acidification causes dissociation of Fe3+ from Tf-TfR1, which is reduced to Fe2+ by a ferrireductase (63). Ferrous iron then goes to the mitochondrion, or, alternatively, it exits the endosome into the cytosol via the divalent metal transporter, DMT1 (30). Presumably, during acidosis, decreasing pHo further acidifies vesicular pH causing enhanced release of Fe3+ from Tf-TfR1 and reduction to Fe2+, which increases the pool of free iron catalyzing the Fenton reaction within the cell (30). Binding of iron to Tf, however, is also dependent on HCO3−. Transferrin binds one ferric ion and one anion, which is usually HCO3− (23) (Fig. 1, Rxn 11). Cellular acidosis caused by metabolic acidosis (Fig. 1, Rxn 12), therefore, would favor dissociation of iron from Tf due to concurrent decreases in both pH and HCO3− (isocapnic acidosis), thereby providing Fe2+ for catalyzing the Fenton reaction and increasing production of •OH (74, 91).
On the other hand, hypercapnic acidosis would stabilize the Fe-Tf-TfR1 complex in part by producing equimolar amounts of HCO3− and H+ (Fig. 1, Rxn 7b), each species having opposing effects on the Fe-Tf-TfR1 complex (74, 91) (Fig. 1, Rxn 13). This is why hypercapnic acidosis is thought to provide neuroprotection during ischemia and hypercapnic hypoxia: increased levels of CO2/HCO3− stabilize the Fe-Tf-TfR1 complex and decrease free iron availability for catalyzing redox reactions (74, 91). In addition, as discussed above, rapid conversion of ONOO− to ONO2CO2− during hypercapnic acidosis effectively decreases the oxidative damage caused by ONOO−. At the same time, however, recall that the CO2/ONOO− reaction has its own potentially adverse consequences on cellular function through production of reactive intermediates that cause cellular oxidation and nitrosative processes (3, 5, 27, 33, 70) (Fig. 1, Rxn 5 and Rxn 6).
Acid-activated Fenton chemistry is an important mechanism in modulating neuronal function during pathological conditions such as ischemia, metabolic disorders, and chronically unstable breathing (22, 80, 93). Under more physiological conditions, the importance of the Fenton reaction in CO2 chemoreception during acidosis remains to be determined since cells, in addition to the iron binding proteins (10, 30), have H2O2 disposing systems (catalase, peroxidase) that keep their levels of H2O2 low under normal conditions (22, 38). Having said that, redox signaling can occur in restricted cellular domains that do not reflect the overall redox state of the cell (68). It is conceivable that the Fenton reaction could likewise be restricted to a particular subcellular compartment that enables it to act as a physiological modulator during acidosis of chemosensitive neurons and other cardiorespiratory neurons. For example, possible restricted subcellular domains for targeted redox signaling include the plasma membrane (lipids, proteins, and/or cytoskeleton), cytosol, mitochondrial matrix, or mitochondrial intermembrane space (11, 37, 68). Compartmentalization within a particular nanostructural domain of the cell is thought to be a critical factor in determining if a given redox reaction functions in physiological regulation or as an agent of nitrosative and oxidative stress (68).
Other redox reactions influenced by H+ and CO2/HCO3−.
Changes in pH modulate other redox reactions besides the Fenton reaction (70, 91). For instance, the protonated form of superoxide, HO2•, which is favored by acidification, has greater reactivity than •O2− and is thought to initiate membrane lipid peroxidation. Protonated superoxide is uncharged and more readily crosses the plasma membrane compared to the charged, impermeable superoxide anion (38). In addition, unconsumed H2O2 reacts with SOD, which is the catalytic enzyme that converts •O2− to H2O2 (Fig. 1, Rxn 4). As a result, a reactive intermediate of SOD is produced that behaves as a hydroxyl radical (SOD-Cu2+-•OH) and, in the process, inactivates SOD (91). In the presence of CO2/HCO3−, however, HCO3− reacts with the SOD-Cu2+-•OH to produce active SOD and CO3−• (91).
To summarize the first section, in a CO2/HCO3−-buffered system, increasing the level of CO2 results in formation of carbonic acid and nitrosoperoxocarboxylate. Both reactions are accelerated by carbonic anhydrase and occur in the extracellular and intracellular compartments. Carbonic acid immediately dissociates into H+ and HCO3− causing cellular acidosis. At the same time, ONO2CO2− reacts to generate RNS intermediates that result in cellular oxidation, nitration, and nitrosylation. Likewise, acidosis accelerates other redox reactions, producing additional ROS, particularly during metabolic acidosis. The available pool of stimuli during hypercapnic acidosis and metabolic acidosis, therefore, includes decreased pH and various reactive intermediates that can potentially modulate membrane-bound proteins and lipids (11) through various nitrosative and oxidative reactions. The pool of reactive species produced during respiratory acidosis vs. metabolic acidosis is predicted to be different depending on the respective contributions from Fenton chemistry (proton activated) vs. peroxynitrite chemistry (CO2/HCO3− activated). It is tempting to speculate that such differences in redox states could underlie differences in the brain stem chemoreceptor and ventilatory response to respiratory acidosis vs. metabolic acidosis (24).
EVIDENCE OF REDOX AND NITROSATIVE SIGNALING IN BRAIN STEM CHEMOSENSITIVE AREAS
What is the evidence, therefore, that neurons in chemosensitive areas produce the requisite enzymes (NOS, NADPH oxidase, and SOD) and reactants (•O2−, H2O2, •NO, and ONOO−) to drive the CO2/ONOO− reaction, Fenton reaction, and other pH-dependent redox reactions under physiological and pathological conditions of acidosis? There are multiple areas of CO2-chemosensitivity dispersed throughout the brain stem (59), including the dorsal medulla [solitary complex, SC (15)] and pons [locus ceruleus (LC)], midline medullary raphe, pre-Bötzinger complex (PBC), and ventrolateral medulla (VLM), including the retrotrapezoid nucleus (RTN) and rostroventral medulla (RVLM). Of these, only the SC has been investigated to date in the context of how oxidative stress affects putative CO2 chemoreceptors (19, 57, 58). The nucleus tractus solitarius (NTS) is recognized as a site of CO2 chemosensitivity in the dorsocaudal medulla oblongata (59, 81). For reasons discussed elsewhere, I have proposed (15) that the boundaries of the dorsocaudal chemosensitive area in the medulla encompass the caudal NTS and dorsal motor nucleus of the vagus nerve (DMV). Hence, evidence for redox signaling in NTS and DMV, which together comprise the SC, is considered below. While few labs have studied redox signaling as it pertains to CO2 chemoreception, several studies have investigated the mechanisms of ROS/RNS production in chemosensitive areas but from the perspective of understanding redox signaling mechanisms underlying cardiovascular control and dysfunction (8, 32, 41, 61) and the hypoxic ventilatory response (13, 39, 40, 47, 62).
Nitric oxide.
Nitric oxide, which is required for ONOO− production, is produced with l-citrulline from l-arginine in a NOS-catalyzed reaction that uses molecular O2 and NADPH (45, 52) (Fig. 1, Rxn 2). NOS immunoreactivity has been localized in the caudal NTS (40, 64, 67), DMV (94), PBC (48), RVLM (7, 13, 44, 64, 95), and dorsal raphe (66, 84). Microinjection of •NO donor unilaterally into the NTS in awake and unrestrained rats stimulated ventilation in both normoxia and hypoxia, whereas microinjection of NOS inhibitor into the NTS blunted the hypoxic ventilatory response (62). Bilateral injection of NOS inhibitor into the caudal NTS of awake rats increased arterial blood pressure and decreased the ventilatory response to hypoxic activation of the peripheral chemoreceptors (36, 39). In the PBC, 53% of the neurons expressing neurokinin-1 receptor immunoreactivity—the marker for PBC neurons—were colocalized with NOS (48). Other investigations have focused on the larger structure, the RVLM. Sympathoexcitatory neurons in RVLM also express NOS (44, 95); these neurons are thought to be chemosensitive or to receive input from chemoreceptors in the RTN (53, 54). Nitric oxide has been implicated in RVLM control of the hypoxic ventilatory response (13), pressor response (9, 32, 41, 61), and heart failure (8). Serotonergic neurons in the dorsal raphe also express NOS (66, 84).
In addition, S-nitrosothiols are NO donors that produce •NO, nitrosonium cations (NO+) and nitroxyl anions (NO−) through enzymatic cleavage reactions (31, 90). S-nitrosothiols target neurons in the NTS in a stereoselective fashion for reflex control of blood pressure and the hypoxic ventilatory response. Microinjection of S-nitroso-l-cysteine into the NTS caused a dose-dependent increase in heart rate and arterial blood pressure (65) and mimicked the stimulatory effects of hypoxia on minute ventilation (47). Likewise, microinjection of S-nitrosocysteinyl glycine into the NTS also mimicked the hypoxic ventilatory response (47).
Superoxide anion.
Superoxide anion, which is required for production of ONOO−, is generated from enzymatically catalyzed reactions involving NADPH oxidase and xanthine oxidase (22) (Fig. 1, Rxn 1). NADPH oxidase was localized in caudal NTS neurons (32, 92), and NADPH oxidase-dependent •O2− production was measured in NTS neurons (61). In addition, •O2− is produced nonenzymatically by reduction of molecular O2 at semiubiquinone at complexes I and III of the electron transport chain in mitochondria (22, 37) (Fig. 1, Rxn 1). Experiments in our laboratory indicate that •O2− is produced in caudal NTS and DMV (i.e., SC) neurons during normobaric hyperoxia and, paradoxically, hypoxia (16, 17). The source of •O2− during hypoxia is complex III in the mitochondria (17, 37). The source of •O2− during hyperoxia is not complex III, however, and has not yet been identified (17). Increased production of ROS in RVLM has been implicated in the control of blood pressure, maintenance of hypertension, chronic heart failure, and cardiorespiratory responses to intermittent hypoxia (9, 29, 41). Specifically, •O2− is produced in RVLM neurons from both NADPH oxidase (29) and mitochondrial electron transport (9, 60) and contributes to the pressor response. NADPH oxidase subunits (p40phox, p47phox and gp91phox) are expressed in RVLM neurons and their expression increases during chronic heart failure. Tempol, a •O2− scavenger, inhibits development of experimental chronic heart failure and decreases blood pressure in control and hypertensive rats (29).
Peroxynitrite.
The only chemosensitive region in which ONOO− activity has been measured is the RVLM; no other chemosensitive area has been investigated to my knowledge. In the RVLM, ONOO− has been implicated in fatal cardiovascular depression (7, 8) and endotoxin-induced apoptosis (25). That said, it is plausible that ONOO− is synthesized in other chemosensitive areas given reports that NOS/•NO and NADPH oxidase/•O2− and SOD are localized in a number of chemosensitive areas (see above). For example, formation of ONOO− is thought to be regulated by SOD, which can lower the concentration of •O2− (Fig. 1, Rxn 4) available for producing ONOO− (86). Superoxide dismutase has also been identified in the SC (61, 94). Likewise, •NO and •O2− activity have been identified in SC (16, 17, 32, 61, 92).
Fenton reaction.
Cellular acidosis accelerates the Fenton reaction (Fig. 1, Rxn 10) by increasing dissociation of iron from Tf (Fig. 1, Rxn 11), making it available for reducing H2O2 to •OH. Transferrin is located throughout the medulla and pons (1, 55), with high concentrations noted in the LC (55), dorsal raphe (56), and SC (49, 55). Hydrogen peroxide, which reacts with iron in the Fenton reaction, is produced from •O2− via the dismutase reaction catalyzed by SOD (22) (Fig. 1, Rxn 4). The presence of SOD, therefore, indicates ongoing production of H2O2 from •O2− [and also possible control of ONOO− production, as discussed above (86)]. To date, •O2− production has been demonstrated in the SC (16, 17, 32, 61, 92) and RVLM (9, 32, 60). Immunoreactivity for manganese-superoxide dismutase (Mn-SOD) and cooper/zinc-superoxide dismutase (Cu/Zn-SOD) was detected in SC neurons (61, 94). Transfection of adenovirus vectors encoding the Mn-SOD gene or Cu/Zn-SOD into the NTS reduced blood pressure in spontaneously hypertensive rats (41). Injection of H2O2 into the NTS induced a dose-dependent, transient bradycardia and hypotension in anesthetized rats. Likewise, injection of catalase inhibitor, which delays conversion of H2O2 to H2O, thereby allowing accumulation of endogenously produced H2O2, caused a similar bradycardia and hypotension (6). The ANG II-induced pressor response elicited from RVLM neurons involves Ca2+-dependent increase in mitochondrial •O2− production. Overexpression of Mn-SOD, which accelerates conversion of •O2− to •OH, decreases the magnitude of ANG II-induced ROS production and the pressor response (60).
To summarize the second section, the data, which come mostly from studies of the SC and RVLM, indicate that neurons in chemosensitive areas express NOS, NADPH oxidase, and SOD under baseline conditions and employ signaling mechanisms that use •O2−, •NO, ONOO−, and SNO. Perturbing these redox signaling mechanisms through their activation or inhibition produces alterations in cardiorespiratory output. The relative contribution of these redox reactions to CO2 chemoreception, however, is untested to date. It will be important to determine how perturbation of these redox reactions affects neuronal chemosensitivity in vitro as well as the ventilatory response to hypercapnia in intact and chemodenervated animals. For example, one could study mechanisms of whole animal and cellular CO2 chemosensitivity against a background of acute and chronic oxidative and nitrosative conditioning to determine the role of redox signaling in CO2 chemoreception (19). For example, the ventilatory response or cellular response to hypercapnic acidosis and metabolic acidosis could be studied by employing sustained hyperoxia to induce ROS/RNS production. These studies, of course, will need to redefine the control O2 conditions, which are usually hyperoxic (see below)2. Alternatively, dietary manipulations that upregulate or downregulate endogenous antioxidant defenses can be employed, or animal models lacking the requisite oxidative enzymes for producing ROS and RNS (e.g., SOD, NOS knockout mice, etc.).
OXIDATION AND ACIDOSIS STIMULATE SC NEURONS
If neurons in CO2-chemosensitive areas of the brain stem employ redox signaling mechanisms, then oxidative stress is anticipated to perturb neuronal excitability, pHi regulation, and alveolar ventilation. The evidence to date indicates that putative CO2-chemoreceptor neurons in the SC are stimulated and acidified by oxidative stress (57, 58). Other chemosensitive areas have not yet been studied. In addition, hyperoxia, particularly isocapnic hyperoxia, is a powerful oxidant and stimulus of breathing, even following carotid body denervation, which indicates that hyperoxic stimuli—ROS/RNS—are acting centrally. The significance of this so called “hyperoxic hyperventilation” as evidence of redox modulation of respiratory networks was reviewed previously in Journal of Applied Physiology (19). To date, three sources of oxidative stress have been tested on neurons in the SC in rat brain stem slices: 1) hyperoxia, which increases •O2−, •NO, and ONOO−; 2) oxidizing agents that target the amino acids cysteine and methionine to form disulfides; and 3) chemical sources of ROS (•O2− and H2O2) at constant hyperoxia (95%O2).
Hyperoxia and chemical oxidants.
Molecular oxygen has a positive redox potential (E = +0.82 V) making it an excellent oxidant that readily accepts electrons. Accordingly, hyperoxia is a reliable test stimulus for activating redox signaling mechanisms, especially those involving •O2−, •NO, and ONOO− (16–18, 20). Essentially all studies of neurons in reduced tissue preparations—such as rat brain stem slices—are done under hyperoxic control conditions (95%O2 in CO2/HCO3− medium). The only way to increase neural tissue partial pressure of oxygen (Po2) further is to increase barometric pressure and use of hyperbaric oxygen (HBO2), which is the experimental approach we have used to investigate the effects of oxidative stress on CO2-sensitive neurons in SC (18, 19, 57). Hyperbaric O2 decreased membrane conductance and stimulated integrated firing rate (IFR) in 38% (n = 43/113) of the SC neurons tested. Of this group, 60 neurons, which included HBO2-sensitive and -insensitive neurons, were also exposed to hypercapnic acidosis and/or chemical oxidants. Of these neurons, 48% (n = 29/60) were stimulated by HBO2 and/or chemical oxidants; and of these, 90% (n = 26/29) were CO2 excited. The excitatory effects of HBO2 were blocked by the antioxidant, Trolox C, a membrane-permeable vitamin E analog. The excitatory effects of HBO2 were mimicked by application of a chemical oxidant in 95% O2 (57). Two chemical oxidants were tested, chloramine-T (CT) and N-chlorosuccinimide (NCS), neither of which produces ROS/RNS, but both are fairly specific oxidizers of cysteine and methionine. These chemical oxidants stimulated both CO2-excited neurons and CO2-insensitive neurons; altogether, 63% of the SC neurons tested were stimulated by CT/NCS. Whenever high CO2 and HBO2 were coadministered, the combined stimulation was greater than either stimulus alone. In half the neurons tested, the IFR response to hyperoxia and hypercapnia was additive, whereas in the other half of neurons tested the combined IFR response to hyperoxia and hypercapnia was greater than the sum of the individual responses.
The unspoken caveat in brain slice studies is that equilibrating nutrient medium with 95% O2 produces tissue hyperoxia throughout a 300- to 400-μm slice, including the core, that is equivalent to a rat breathing >2 atm of HBO2 (18). Hyperoxic control medium induces ROS production in brain slices and increases cell death (12, 16, 17). Given that HBO2 stimulates certain SC neurons maintained in 95% O2 at room pressure (57), and that •O2− production increases significantly in brain slices maintained in 95% O2 for 4 h (12, 16, 17), we have initiated experiments using a lower control level of O2 (20–40%). Under these conditions, SC neurons in 300- to 400-μm-thick transverse slices, harvested from neonates and weaned rats, remained viable for many hours based on electrophysiology criteria (50) and capacity for cells to produce ROS during O2 manipulation (16, 17). Under these new control conditions, acute exposure to normobaric hyperoxia (95% O2) also stimulated IFR neurons in the SC, including CO2-excited and CO2-inhibited neurons (50).
Chemical oxidants, ROS, and intracellular pH.
Acute exposure to chemical oxidants and generators of ROS in 95% O2 caused significant decreases in pHi in SC neurons. Chemical oxidants CT and NCS significantly inhibited Na+/H+ exchange leading to intracellular acidosis. Likewise, H2O2 and dihydroxyfumarate, which generates extracellular •O2−, acidified SC neurons (58). The effect of normobaric hyperoxia on pHi, by comparison, is more complicated than the other oxidative stimuli tested since O2 availability influences a broader range of redox reactions through simultaneous production of •O2− and •NO and activation of multiple downstream redox reactions. In addition, hyperoxia will affect cellular respiration and neuronal activity. All of these effects, in turn, will influence pHi. In SC neurons, pHi is more alkaline during normobaric hyperoxia and decreases with decreasing tissue Po2. Specifically, average pHi plateaus at 7.44–7.45 in superfusate equilibrated with 80–95% O2. Decreasing steady-state O2 to 60–40% decreases pHi to another plateau averaging 7.34–7.35 (69). These data suggest that the excitatory effect of hyperoxia on the IFR of SC neurons (57) is mediated primarily by cellular oxidation rather than hyperoxia-induced acidification. At constant O2, however, conditions that increase production of ROS and cause cellular oxidation would both acidify and oxidize SC neurons and IFR would be determined by the balance between oxidation and acidification (58), as illustrated next.
In the case of CT and NCS, one must consider the effects of both cellular oxidation and intracellular acidosis; that is, because chemical oxidants applied at constant O2 resulted in decreased pHi, were SC neurons stimulated by intracellular acidosis caused by oxidative stress or was the effect of cellular oxidation separate from that of cellular acidosis? To determine this, Mulkey et al. (58) developed a novel method for clamping pHi at control level during exposure to CT using CO2/HCO3− manipulations. These experiments showed that SC neurons increased IFR in the absence of intracellular acidosis indicating cellular oxidation alone stimulated SC neurons. Exposure to simultaneous cellular oxidation and acidification tended to cause a larger stimulation of IFR (58).
To summarize the final section, the foregoing discussion indicates that neurons in the dorsocaudal chemosensitive area of the medulla oblongata are stimulated by various sources of oxidative stimuli under in vitro conditions. The excitatory effects of hyperoxia are caused by decreased membrane conductance and are reversibly blocked by a membrane-permeable antioxidant. Putative CO2-chemoreceptor neurons, that is, neurons stimulated by hypercapnic acidosis, are likewise stimulated by HBO2 and chemical oxidants. Exposure to hypercapnic HBO2 caused a greater stimulation of IFR than exposure to either stimulus alone. Chemical oxidants and generators of ROS at constant O2 acidify SC neurons by inhibition of Na+/H+ exchange and stimulate IFR. The excitatory effects of oxidation and acidification occur independently of each other however. While the stimulatory effects of hyperoxia on IFR occur primarily in chemosensitive SC neurons, the effects of chemical oxidants (at constant O2) on IFR and pHi regulation indicate that redox stimulation influences the functions of a larger population of SC neurons. The functional significance of sensitivity to hyperoxia and chemical oxidants suggests that certain neurons in the SC, including CO2-excited neurons (i.e., putative CO2 chemoreceptors), use redox signaling mechanisms to carry out certain aspects of their normal functions (19). The excitability of SC neurons is determined, we propose, by the relative levels of pHo/pHi and ROS/RNS at any given time (58), both of which decrease membrane conductance. Presumably, different populations of pH-sensitive channels and redox-sensitive channels are involved, which include potassium channels (57, 58, 72). Redox- and nitrosative-sensitive components of the plasma membrane that are targeted by ROS and RNS during hyperoxia likewise are affected, we hypothesize, by ROS and RNS generated during hypercapnic acidosis (via CO2/ONOO− reaction) or isocapnic acidosis at constant O2 (via Fenton reaction) (Fig. 1). These same redox sensitive mechanisms would also render neurons in the SC vulnerable to oxidative and nitrosative stress during pathological conditions of ischemia, hyperoxia, and chronic respiratory acidosis and metabolic acidosis.
Perspectives
Carbon dioxide, like O2 and several other gas species (70), is able to effect redox signaling mechanisms through production of oxidative and nitrosative derivatives. These effects of hypercapnia and acidosis on redox signaling have rarely been considered in studies of central CO2 chemoreception (19, 57). During CO2 retention, therefore, the scope of their importance in defining brain stem activity and, ultimately, alveolar ventilation, is unknown and requires further research. The ventilatory response in the conscious animal to changes in CO2/pH is incredibly sensitive (34, 59, 81). I am postulating that these CO2/H+-sensitive redox reactions are poised, under physiological conditions, such that small perturbations in CO2/pH produce changes in redox state that, in tandem with changes in cellular pH, affect central chemoreceptors (and other cardiorespiratory neurons) and thus alveolar ventilation. Clearly, the data indicate that neurons in multiple chemosensitive areas express the biochemical machinery for producing significant quantities of ROS/RNS during hypercapnic acidosis and isocapnic acidosis. Moreover, neurons in at least one chemosensitive area (SC) are stimulated directly by CO2-induced acidification and oxidation, and indirectly through oxidation-induced acidification (57, 58). Thus chemoreceptor activity, at least in the SC, is going to be determined by the balance between the relative levels of pHi and redox state during hypercapnic acidosis (58). Furthermore, the influence of any CO2/H+-driven redox reaction will be amplified by conditions that enhance production of their reactants; that is, •O2− and •NO for the CO2/ONOO− reaction, and •O2−, iron, and H2O2 for the Fenton reaction. Hence, hypoxia, posthypoxic reoxygenation, and hyperoxia (17), when combined with CO2 retention, will result in increased ROS/RNS production through the combined redox and nitrosative chemistry of molecular O2 and CO2/H+.
GRANTS
Research and ideas discussed herein are based in part on work performed in the author's laboratory, often in collaboration with Dr. Robert Putnam, which was funded by the Office of Naval Research, Undersea Medicine Program (ONR Grant N000140710890 to J. B. Dean) and the National Heart, Lung, and Blood Institute (Grant R01-HL-56683 to R. W. Putnam and J. B. Dean).
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the author.
ACKNOWLEDGMENTS
J. B. Dean gratefully acknowledges discussions with Drs. Daniel K. Mulkey (Univ. of Connecticut), Robert W. Putnam (Wright State Univ.), and Dominic P. D'Agostino (Univ. of South Florida) that helped to solidify several key ideas presented in this minireview.
Footnotes
Nitrosoperoxocarboxylate (ONO2CO2−) has also been called nitrosoperoxycarbonate anion (78), nitrosocarbonate anion (91), and by its IUPAC name, 1-carboxylateo-2-nitrosodioxidane (78).
For in vivo studies, it will be necessary to compare the ventilatory response to CO2 in intact animals versus carotid body denervated animals. The caveats of using 100% O2 to chemically denervate the peripheral CO2 chemoreceptors have been reviewed elsewhere (19).
REFERENCES
- 1. Aldred AR, Dickson PW, Marley PD, Schreiber G. Distribution of transferrin synthesis in brain and other tissues in the rat. J Biol Chem 262: 5293–5297, 1987 [PubMed] [Google Scholar]
- 2. Aruoma OI, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J 241: 273–278, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, DeMenezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med 32: 841–859, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bonini MG, Radi R, Ferrer-Sueta G, Ferreira AM, Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J Biol Chem 274: 10802–10806, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cardoso LM, Colmbari DSA, Menani JV, Toney GM, Chianca DA, Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 297: R462–R469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JYH, Wu CHY, Tsai CY, Chen HL, Dai KY, Chan SHH, Chang AYW. Transcriptional up-regulation of nitric oxide synthase II by nuclear factor-kB at rostral ventrolateral medulla in a rat mevinphos intoxication model of brain stem death. J Physiol 581: 1293–1307, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan SH, Wang LL, Ou CC, Chan JYH. Contribution of peroxynitrite to fatal cardiovascular depression induced by overproduction of nitric oxide in rostral ventrolateral medulla of the rat. Neuropharmacology 43: 889–898, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chan SH, Wu KL, Chang AY, Tai MH, Chan JY. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 53: 217–227, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem 91: 9–18, 2002 [DOI] [PubMed] [Google Scholar]
- 11. D'Agostino DP, Colomb DG, Dean JB. Effects of hyperbaric gases on membrane nanostructure and function in neurons. J Appl Physiol 106: 996–1003, 2009 [DOI] [PubMed] [Google Scholar]
- 12. D'Agostino DP, Putnam RW, Dean JB. Superoxide •O2− production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol 98: 1030–1041, 2007 [DOI] [PubMed] [Google Scholar]
- 13. De Paula PM, Branco LGS. Nitric oxide in the rostral ventrolateral medulla modulates hyperpnea but not anapyrexia induced by hypoxia. Brain Res 977: 231–238, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol 129: 83–100, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Dean JB, Landon CS, D'Agostino DP. Oxygen-induced superoxide production in solitary complex neurons in rat medullary slices. 2010 Experimental Biology Meeting Abstracts [on CD-ROM]. In press, 2010 [Google Scholar]
- 17. Dean JB, Landon CS, D'Agostino DP, Putnam RW. Hypoxia and hyperoxia both increase superoxide production in nucleus tractus solitarius (NTS) neurons in rat brain tissue slices. 2009 Experimental Biology Meeting Abstracts [on CD-ROM]. Abstract no. 1038.8, 2009 [Google Scholar]
- 18. Dean JB, Mulkey DK, Garcia AJ, 3rd, Putnam RW, Henderson RA., 3rd Neuronal sensitivity to hyperoxia, hypercapnia and inert gases at hyperbaric pressures. J Appl Physiol 95: 883–909, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Dean JB, Mulkey DK, Henderson RA, 3rd, Potter SJ, Putnam RW. Hyperoxia, reactive O2 species, and hyperventilation: O2 sensitivity of brain stem neurons. J Appl Physiol 96: 784–791, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Demchenko IT, Atochin DN, Boso AE, Astern J, Huang PL, Piantadosi CA. Oxygen seizure latency and peroxynitrite formation in mice lacking neuronal or endothelial nitric oxide synthases. Neurosci Lett 344: 53–56, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys 333: 49–58, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Edeker BL, Rasmussen GT, Britigan BE. Bicarbonate and phosphate ions protect transferrin from myeloperoxidase-mediated damage. Leukocyte Biol 58: 59–64, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidosis. J Physiol 358: 285–297, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faith CH, Chan JYH, Chan SHH, Chang AYW. In the rostral ventrolateral medulla, the 70-kDa heat shock protein (HSP70), but not HSP90, confers neuroprotection against fatal endotoxemia via augmentation of nitric-oxide synthase I (NOS 1)/protein kinase G signaling pathway and inhibition of NOS II/peroxynitrite cascade. Mol Pharmacol 68: 179–192, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ferdinandy P. Peroxynitrite: just an oxidative/nitrosative stressor or a physiological regulator as well? Br J Pharmacol 148: 1–3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. Am Chem Soc Chem Biol 4: 161–177, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Filosa J, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541: 493–509, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Garrick MD, Garrick LM. Cellular iron transport. Biochim Biophys Acta 1790: 309–325, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Gaston B. Nitric oxide and thiol groups. Biochim Biophys Acta 1411: 323–333, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Glass MJ, Chan J, Frys KA, Oselkin M, Tarsitano MJ, Iadecola C, Pickel VM. Changes in the subcellular distribution of NADPH oxidase subunit p47phox in dendrites of rat dorsomedial nucleus tractus solitarius neurons in the response to chronic administration of hypertensive agents. Exp Neurol 205: 383–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein S, Czapski G. Formation of peroxynitrite from the reaction of peroxynitrite with CO2: evidence for carbonate radical production. J Am Chem Soc 120: 3458–3468, 1998 [Google Scholar]
- 34. Gonzalez C, Dinger BF, Fidone editors SJ. Mechanisms of Carotid Body Chemoreception. New York: Dekker, 1995, p. 391–471 [Google Scholar]
- 35. Gow A, Duran D, Thom SR, Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch Biochem Biophys 333: 42–48, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Granjeiro EM, Machado BH. NO in the caudal NTS modulates the increase in respiratory frequency in response to chemoreflex activation in awake rats. Respir Physiol Neurobiol 166: 32–40, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Guzy RD, Schumaker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Halliwell B, Gutteridge JMC. Antioxidant defences: endogenous and diet derived. In: Free Radicals in Biology and Medicine, edited by Halliwell B, Gutteridge JMC. Oxford: Oxford Univ. Press, 2007, p. 79–186 [Google Scholar]
- 39. Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res 72: 511–516, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Haxhiu MA, Chang CH, Dreshaj IA, Erokwu B, Prabhakar NR, Cherniack NS. Nitric oxide and ventilatory response to hypoxia. Respir Physiol 101: 257–266, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Hirooka Y. Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci 3: 20–24, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Huang RQ, Erlichman JS, Dean JB. Cell-cell coupling between CO2-excited neurons in the dorsal medulla oblongata. Neuroscience 80: 41–57, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Ruther E, Kornhuber J, Bleich S. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm 113: 239–254, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H, Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventral medulla in stroke-prone spontaneously hypertensive rats. Hypertension 39: 264–268, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochemistry 298: 249–258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lavie L, Lavie P. Oxidative stress-the culprit of obstructive sleep apnea syndrome. Progr Respir Res 35: 97–104, 2006 [Google Scholar]
- 47. Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-Nitrosothiols signal the ventilatory response to hypoxia. Nature 413: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Liu YY, Wong-Riley MTT. Distribution and colocalization of neurotransmitters and receptors in the pre-Botzinger complex of rats. J Appl Physiol 91: 1387–1395, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat 37: 182–195, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Matott MP, Putnam RW, Dean JB. Effects of a lower level of control O2, hyperoxia and hypercapnia on solitary complex neurons in medullary tissue slices. 2009 Experimental Biology Meeting Abstracts [on CD-ROM]. Abstract no. 621.14, 2009 [Google Scholar]
- 51. Maurizi CP. Could exogenous melatonin prevent sudden infant death syndrome? Med Hypotheses 49: 4250427, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 97: 1676–1689, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morrell MJ, Heywood P, Moosavi SH, Stevens J, Guz A. Central chemosensitivity and breathing asleep in unilateral medullary lesion patients: comparisons to animal data. Respir Physiol 129: 269–277, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Morris CM, Candy JM, Bloxham CA, Edwardson JA. Distribution of transferrin receptors in relation to cytochrome oxidase activity in the human spinal cord, lower brainstem and cerebellum. J Neurol Sci 111: 158–172, 1992 [DOI] [PubMed] [Google Scholar]
- 56. Morris CM, Candy JM, Omar S, Bloxham CA, Edwardson JA. Transferrin receptors in the Parkinsonian midbrain. Neuropathol Appl Neurobiol 20: 468–472, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Mulkey DK, Henderson RA, 3rd, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol 95: 910–921, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Mulkey DK, Henderson RA, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases intracellular pH and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol Cell Physiol 286: C940–C951, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Nattie EE. CO2, brainstem chemoreceptors and breathing. Progr Neurobiol 59: 299–331, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, Ide T, Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens 26: 2176–2184, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Nozoe M, Hirooka Y, Koga Y, Sagara Y, Kishi T, Engelhardt JF, Sunagawa K. Inhibition of Rac 1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension 50: 62–68, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Ogawa H, Mizusawa A, Kikuchi Y, Hida W, Miki H, Shirato K. Nitric oxide as a retrograde messenger in the nucleus tractus solitarii of rats during hypoxia. J Physiol 486: 495–504, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nature Genet 37: 1264–1269, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohta A, Takagi T, Matsui T, Hamai Y, Lida S, Esumi H. Localization of nitric oxide synthase-immunoreactive neurons in the solitary nucleus and ventrolateral medulla oblongata of the rat: their relation to catecholaminergic neurons. Neurosci Lett 158: 33–35, 1993 [DOI] [PubMed] [Google Scholar]
- 65. Ohta H, Bates JN, Lewise SJ, Talman WT. Actions of S-nitrosocysteine in the nucleus tractus solitarii are unrelated to release of nitric oxide. Brain Res 746: 98–104, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Okere CO, Waterhouse BD. Acute restraint increases NADPH-diaphorase staining in distinct subregions of the rat dorsal raphe nucleus: implications for raphe serotonergic and nitrergic transmission. Brain Res 1119: 174–181, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Pajolla GP, Accorsi-Mendonca D, Lunardi CN, Bendhack LM, Machado BH, Llewellyn-Smith IJ. Immunoreactivity for neuronal NOS and fluorescent indication of NO formation in the NTS of juvenile rats submitted to chronic intermittent hypoxia. Auton Neurosci 148: 55–62, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Pani G, Bedogni B, Colavitti R, Anzevino R, Borrello S, Galeotti T. Cell compartmentalization in redox signaling. Life 52: 7–16, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Potter SJ. Effects of Oxygen on Intracellular pH and pH Regulation in Nucleus Tractus Solitarius and Hypoglossal Neurons in Brain Slices (Master's Thesis). Dayton, OH: Dept. of Neuroscience, Cell Biology, and Physiology, Wright State Univ., 2005, p. 89 [Google Scholar]
- 70. Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies JA. Free radical biology and medicine: it's a gas, man. Am J Physiol Regul Integr Comp Physiol 291: R491–R511, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Pryor WA, Lemercier JN, Zhang H, Uppu RM, Squadrito GL. The catalytic role of carbon dioxide in the decomposition of peroxynitrite. Free Radic Biol Med 23: 331–338, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol 11: 720–721, 1998 [DOI] [PubMed] [Google Scholar]
- 74. Rehncrona S, Hauge HN, Siesjo BK. Enhancement of iron-catalyzed free radical formation by acidosis in brain homogenates: difference in effect by lactic acid and CO2. J Cereb Blood Flow Metab 9: 65–70, 1989 [DOI] [PubMed] [Google Scholar]
- 75. Reid G, Tervit H. Sudden infant death syndrome: oxidative stress. Med Hypotheses 52: 577–580, 1999 [DOI] [PubMed] [Google Scholar]
- 76. Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and non-chemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 275: R1152–R1163, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 273: R433–R441, 1997 [DOI] [PubMed] [Google Scholar]
- 78. Romero N, Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite in the presence of carbon dioxide. Arch Biochem Biophys 368: 23–30, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Sanfilippo-Cohn B, Sai S, Zhan G, Fenik P, Practico D, Mazza E, Veasey SC. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep 29: 152–159, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Current Medicinal Chem 8: 721–738, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Scheid P, Putnam RW, Dean JB, Ballantyne D, Guest Editors. Special Issue: Central chemosensitivity. Respir Physiol 129: 1–278, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Siesjo BK, Bendek G, Koide T, Westerberg E, Wieloch T. Influence of acidosis on lipid peroxidation in brain tissues in vitro. J Cereb Blood Flow Metab 5: 253–258, 1985 [DOI] [PubMed] [Google Scholar]
- 83. Siesjo BK, Folbergrova J, MacMillan V. The effect of hypercapnia upon intracellular pH in the brain, evaluated by the bicarbonate-carbonic acid method and from the creatine phosphokinase equilibrium. J Neurochem 19: 2483–2495, 1972 [DOI] [PubMed] [Google Scholar]
- 84. Simpson KL, Waterhouse BD, Lin RCS. Differential expression of nitric oxide in serotonergic projection neurons: neurochemical identification of dorsal raphe inputs to rodent trigeminal somatosensory targets. J Comp Neurol 466: 495–512, 2003 [DOI] [PubMed] [Google Scholar]
- 85. Sipe DM, Murphy RF. Binding to cellular receptors results in increases iron release from transferrin at mildly acidic pH. J Biol Chem 266: 8002–8007, 1991 [PubMed] [Google Scholar]
- 86. Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med 25: 392–403, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Stademan ER, Levine RL. Protein oxidation. Ann NY Acad Sci 899: 191–208, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Tien M, Berlett BS, Levine RL, Chock PB, Stadtman ER. Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration: pH dependence of carbonyl formation, tyrosine nitration, and methionine oxidation. Proc Natl Acad Sci USA 96: 7809–7814, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Uppu RM, Squadrito GL, Pryor WA. Acceleration of peroxynitrite oxidations by carbon dioxide. Arch Biochem Biophys 327: 335–343, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Van der Vliet A, Chr't Hoen PA, Wong PS-Y, Bast A, Cross CE. Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J Biol Chem 273: 30255–30262, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Vesela A, Wilhelm J. The role of carbon dioxide in free radical reactions of the organism. Physiol Res 51: 335–339, 2002 [PubMed] [Google Scholar]
- 92. Wang G, Anrather J, Huang J, Speth RC, Pickel VM. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci 24: 5516–5524, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weinberg ED. Iron, infection and sudden infant death. Med Hypotheses 56: 731–734, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Yu WA. Spatial and temporal correlation of nitric oxide synthase expression with CuZn-superoxide dismutase reduction in motor neurons following axotomy. Ann NY Acad Sci 962: 111–121, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Zanzinger J. Mechanisms of action of nitric oxide in the brain stem: role of oxidative stress. Auton Neurosci 98: 24–27, 2002 [DOI] [PubMed] [Google Scholar]