Abstract
Obesity is thought to lead to sympathetic overactivity as a compensatory adjustment to weight gain. However, most of the experimental support for the hypothesis has been derived from white cohorts. Our previous study in blacks indicated that sympathetic nerve activity (SNA) is closely correlated with body mass index only in women, whereas, in black men, SNA is elevated and dissociated from adiposity (Abate et al., Hypertension 38: 379–383, 2001). To further determine whether total and regional adiposity are determinants of SNA in blacks, we performed a prospective weight loss study in 12 normotensive obese black men and 9 obese black women. SNA, body mass index, and abdominal fat mass were measured before and 16 wk after hypocaloric diet. The major new findings are that, in obese black men, the dietary-induced weight loss of 11.3 ± 0.8 kg resulted in reduction in plasma leptin, insulin, and visceral abdominal fat but had no effect on SNA (from baseline of 26 ± 4 to 28 ± 3 bursts/min, P = not significant). In contrast, in black women, weight loss of 8.0 ± 0.9 kg caused similar reductions in plasma leptin, insulin, and visceral abdominal fat and led to a reduction in SNA by 40% (from baseline of 22 ± 2 to 13 ± 3 bursts/min, P < 0.05). In conclusion, these new data from this prospective study provide strong support for a major adiposity-independent sympathetic activity in black men and adiposity-related sympathetic activity in black women.
Keywords: obesity, insulin, sympathetic nervous system, baroreceptor, blacks
obesity is a harbinger for a variety of cardiovascular diseases, including hypertension, left ventricular hypertrophy, and coronary artery disease. The fundamental mechanisms predisposing overweight subjects to hypertension and cardiovascular complications are still not completely understood, but a large body of clinical and animal investigations have implicated an important role of the sympathetic nervous system. Landsberg (14) hypothesized that obesity is accompanied by sympathetic overactivity as a compensatory mechanism to increase energy expenditure and minimize weight gain but at the cost of excessive sympathetic-mediated vasoconstriction and elevated blood pressure (BP). Previous studies in normotensive subjects have demonstrated that increasing adiposity is accompanied by increased sympathetic discharge, which is normalized after weight reduction (8, 10, 11, 23, 26). The mechanisms underlying such sympathetic activation are still unknown, but increased hormonal signals, such as leptin, insulin, or other metabolic signals from visceral fat depot (3, 8, 12, 14), are thought to play important roles.
Despite numerous evidence for adiposity-dependent sympathetic overactivity in humans, most of the data were derived from the white cohorts (8, 10, 11, 23, 26). Studies in other ethnic minorities, on the other hand, have not provided consistent findings. In Pima Indian men, levels of sympathetic nerve activity (SNA) were found to be lower than levels in white men and unrelated to percent body fat (29). In normotensive black adults, our previous study indicated that the relationship between SNA and obesity is dependent on sex. Although SNA in black women was highly correlated with body mass index (BMI) and multiple indices of obesity, SNA in black men was overall higher than that in white men and black women but dissociated from adiposity (1). Because our previous study was cross-sectional in design, we cannot exclude other confounding factors that may influence SNA and its relationship to obesity. Furthermore, measurement of visceral abdominal fat, which was suggested to have a larger influence on SNA than BMI at least in white subjects (3), was not performed.
Therefore, we conducted a prospective study to test the hypothesis that weight loss reduces sympathetic activity in black women but not in black men by assessing SNA responses to dietary weight reduction in obese black men and women. To further determine the role of regional fat distribution on SNA in this ethnic group, measurements of subcutaneous and visceral abdominal fat were also performed before and after weight loss.
METHODS
The protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all the subjects gave their written informed consent to participate. Twenty-one obese normotensive African Americans(12 men and 9 women) with BMI >30 kg/m2 and age <40 years were studied. All subjects were free of any history of cardiovascular disease or substance abuse. All subjects had fasting blood glucose concentrations <110 mg/dl at the time of study and had average 24-h ambulatory BP values of <130/80 mmHg. Baseline characteristics of all subjects are shown in Table 1.
Table 1.
Baseline characteristics of all subjects
| Variable | Obese Black Women (n = 9) | Obese Black Men (n = 12) | Lean Black Men (n = 8) |
|---|---|---|---|
| Age, years | 32.6 ± 1.7 | 33.2 ± 1.5 | 39.3 ± 3.2 |
| Body weight, kg | 87.9 ± 5.9* | 103.8 ± 4.0 | 82.3 ± 4.9* |
| Body mass index, kg/m2 | 32.9 ± 1.7 | 32.6 ± 1.1 | 24.3 ± 0.4† |
| Waist circumference, cm | 100 ± 6 | 104 ± 3 | 87 ± 4† |
| Waist-to-hip ratio | 0.8 ± 0.03 | 0.9 ± 0.02 | 0.8 ± 0.02 |
| Fasting plasma glucose, mg/dl | 93 ± 2 | 99 ± 5 | 84 ± 5 |
| Total cholesterol, mg/dl | 170 ± 8 | 181 ± 12 | 184 ± 28 |
| Triglyceride, mg/dl | 70 ± 10 | 106 ± 18 | 118 ± 27 |
Values are means ± SE.
P < 0.05 vs. obese black men.
P < 0.01 vs. obese black men and women.
Experimental Protocol
Subjects were provided with hypocaloric diet prepared by metabolic kitchen of the General Clinical Research Center at the Parkland Memorial Hospital. The diet contained 57% carbohydrates, 20% fats, and 23% proteins with Na+ content of 110 mmol/day. Because of lower baseline body weight in women than in men, all women were given a 1,100-kcal diet per day and men were given a 1,700-kcal per day. Subjects consumed all meals off site. During the study, the subjects' body weight and compliance to dietary regimen were assessed on a biweekly basis at the outpatient unit of the General Clinical Research Center. To avoid potential confounding influence of exercise on SNA, each subject was instructed to maintain the same level of physical activity throughout the study. To avoid the confounding influence of salt restriction on SNA, each subject was instructed to add salt ad libitum to the diet.
Each subject underwent measurement of SNA, baroreflex sensitivity, 24-h ambulatory BP, 24-urinary sodium excretion, and visceral and total abdominal fat mass before and after 16 wk of hypocaloric diet. Fasting plasma glucose, insulin, and leptin levels were also measured in all subjects before and after dietary weight loss.
Twenty-four-hour ambulatory BP monitoring.
Ambulatory/nocturnal BP was monitored continuously, according to standard methods (6), using a Space Labs model 90207 monitor for 24 h. The BP monitor is programmed to measure BP every 20 min.
Measurement of SNA by microneurography.
All experiments were performed with the subjects in the supine position after an overnight fast. BP was measured by the oscillometric technique with the Vitalsigns monitor (CE00050, Welch Allyn, Tycos Instruments, Arden, NC). Heart rate (HR) was monitored continuously by a cardiotachometer triggered by R wave of an ECG lead. Postganglionic efferent sympathetic nerve discharge, HR, and respiratory rate were recorded continuously with the use of a multi-channel digital data recorder (MacLab/8S ML780, AD Instruments, Mountainview, CA).
Multiunit recordings of postganglionic SNA were obtained with unipolar tungsten microelectrodes inserted selectively into muscle nerve fascicles of the peroneal nerves using the microneurographic technique of Vallbo et al. (27). The nerve signals are amplified, filtered (bandwidth 700–2000 Hz), rectified, and integrated to obtain a mean voltage display of SNA. A recording of muscle sympathetic-nerve discharge was considered acceptable when the neurograms revealed spontaneous, pulse synchronous bursts of neural discharge, with the largest bursts showing a minimal signal-to-noise ratio of 3:1. SNA was analyzed by a single investigator (ZW), who was blinded to subject's sex and study condition (baseline and after weight loss). Nerve traffic was expressed as both the number of bursts per minute and the number of bursts per 100 heart beats, the latter as an HR-independent measure of sympathetic discharge. The interobserver and intraobserver variabilities in identifying bursts are <10% and <5%, respectively.
Arterial baroreflex testing.
Arterial baroreflex sensitivity was quantified as the reflex decreases in SNA and HR during progressive increases in mean arterial pressure (MAP) of up to 15 mmHg above baseline during intravenous infusion of phenylephrine (0.5–2.0 μg/min) and as the reflex increases in SNA and HR during decreases in MAP of up to 15 mmHg below baseline during infusion of sodium nitroprusside (0.5–4.0 μg/min). Changes in HR and sympathetic bursts/min and percent changes in the total integrated activity (the product of average bursts/min multiplied by mean burst amplitude detected in 1 min) associated with changes in MAP at each dose of phenylephrine and sodium nitroprusside were calculated. The baroreflex gain was calculated as the slope of the curve relating increases or decreases in BP to SNA or HR.
Measurement of abdominal fat mass by magnetic resonance imaging.
Abdominal fat mass was determined using a 1.5-Tesla Gyroscan INTERA whole body system (Philips Medical Systems). During data acquisition, patients stayed in magnet in a prone position to suppress abdominal motion with body weight. Subcutaneous and visceral abdominal fat volumes were determined from high-resolution abdominal axial images. Average abdominal fat area was determined from abdominal axial images obtained in contiguous slices between L2 to L5 as previously described (30). Image analysis was performed by an observer blinded to patient clinical history using a commercially available workstation (MASS, Philips Medical Systems) and involved mapping of subcutaneous and intra-abdominal adipose tissue compartments on computer screen using a track ball.
Measurement of plasma glucose, leptin, and insulin.
Serum leptin levels (ng/ml) were measured by radioimmunoassay, using commercial kits (Linco Research, St Charles, MO). The lowest detection limit was 0.5 ng/ml. The inter- and intra-assay variability was <10%. Serum insulin levels were measured by a sensitive radioimmunoassay kit (Diagnostic Products, Los Angeles, CA). The sensitivity for this assay is 7 pmol/l (1.2 μU/ml). The inter- and intra-assay variabilities were <5%.
Statistical Methods
Statistical analyses were performed with SAS software (version 9.1, SAS Institute, Cary, NC), using two-factor repeated-measures ANOVA, with sex as the grouping factor and visit as the repeated factor. Analyses were used to compare levels of SNA, abdominal fat, BMI, plasma leptin, plasma insulin, 24-h ambulatory BP, 24-h ambulatory HR, baroreflex sensitivity, and 24-h urinary excretion at baseline with results after 16 wk of hypocaloric diet in black men and women. Pairwise comparisons were made with least-square means contrasts from the ANOVA models. Because distribution of subcutaneous and visceral fat mass was skewed, the data were log transformed before analysis. A P value of 0.05 or less was considered to indicate significance.
RESULTS
Baseline characteristics of our subjects are shown in Table 1. SNA in lean black men was similar to SNA in obese black men and obese black women (26 ± 4 vs. 26 ± 4 vs. 22 ± 2 bursts/min, respectively, P > 0.05). Hypocaloric diet caused a significant reduction in body weight by ∼11.3 ± 0.8 kg (from BMI of 32.6 ± 1.0 to 28.9 ± 0.9 kg/m2, P < 0.01; Table 2) in obese black men and 8.0 ± 0.9 kg (from BMI of 32.9 ± 2.8 to 30.0 ± 1.8 kg/m2, P < 0.01; Table 2) in obese black women. Baseline visceral abdominal fat mass was smaller in black women than in black men (Table 2, P < 0.01). However, visceral abdominal fat reduced significantly in both black men and women after weight loss (from 124 ± 18 to 84 ± 14 cm2 and from 62 ± 10 to 53 ± 9 cm2, respectively, P < 0.05; Table 2). Plasma leptin and insulin also reduced significantly in both black men and women after weight loss (Table 2). Average 24-h ambulatory diastolic BP reduced significantly after weight loss in black men but not in black women (Table 2). Average 24-h HR reduced significantly after weight loss in both men and women (from 76 ± 2 to 69 ± 3 and from 83 ± 2 to 77 ± 3 beats/min, respectively, P < 0.01; Table 2). There were no significant changes in baroreflex control of HR or SNA (Table 3) or 24-h urinary sodium excretion after weight loss in both men and women (Table 2).
Table 2.
Effects of hypocaloric diet on anthropometric, hemodynamic, and metabolic and 24-hour urinary electrolyte excretion in black women and black men
| Black Women (n = 9) |
Black Men (n = 12) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline | 16 wk | P Value, Baseline vs. 16 wk in Women* | Baseline | 16 wk | P Value, Baseline vs. 16 wk in Men* | ANOVA P‡ |
| Body mass index, kg/m2 | 32.9 ± 1.7 | 30.0 ± 1.8 | <0.0001 | 32.6 ± 1.0 | 28.9 ± 0.9 | <0.0001 | 0.16 |
| Weight, kg | 87.9 ± 5.9 | 79.9 ± 5.9 | <0.0001 | 103.8 ± 4.0 | 92.5 ± 3.7 | <0.0001 | 0.02 |
| 24-h systolic BP, mmHg | 119 ± 3 | 119 ± 3 | 0.86 | 124 ± 2 | 119 ± 3 | 0.03 | 0.11 |
| 24-h diastolic BP, mmHg | 71 ± 2 | 73 ± 2 | 0.23 | 74 ± 2 | 71 ± 2 | 0.01 | 0.01 |
| 24-h heart rate, beats/min | 83 ± 2 | 77 ± 3 | 0.005 | 76 ± 2 | 69 ± 3 | 0.0006 | 0.81 |
| SNA bursts/min | 22 ± 2 | 13 ± 3 | 0.006 | 26 ± 4 | 28 ± 3 | 0.57 | 0.01 |
| SNA bursts/100 heart beats | 31 ± 4 | 20 ± 4 | 0.02 | 42 ± 6 | 45 ± 5 | 0.31 | 0.01 |
| SNA total activity, AU | 121 ± 14 | 79 ± 18 | 0.009 | 126 ± 19 | 136 ± 17 | 0.94 | 0.04 |
| 24-h Urine Na, mmol | 150 ± 29 | 118 ± 22 | 0.31 | 154 ± 12 | 99 ± 21 | 0.21 | 0.82 |
| Subcutaneous fat, cm2 | 428 ± 66 | 378 ± 63 | 0.06 | 302 ± 42 | 252 ± 34 | 0.009 | 0.58 |
| Visceral abdominal fat, cm2 | 62 ± 10 | 53 ± 9 | 0.02 | 124 ±18† | 84 ± 14 | <0.0001 | 0.02 |
| Fasting insulin, mU/ml | 7.9 ± 1.7 | 5.3 ± 1.2 | 0.003 | 5.0 ± 0.7 | 3.7 ± 0.5 | 0.004 | 0.58 |
| Plasma leptin, ng/ml | 23.5 ± 2.4 | 14.5 ± 2.2 | 0.008 | 8.2 ± 1.2 | 5.3 ± 0.9 | 0.0007 | 0.7 |
Values are means ± SE. AU, arbitrary unit; BP, blood pressure; SNA, sympathetic nerve activity.
Difference between the 2 visits (baseline vs. 16 wk) within each gender group.
P < 0.01 vs. black women at baseline.
Interaction between gender and visit from repeated-measures ANOVA models and represents difference in response between black women and black men.
Table 3.
Effects of dietary weight loss on baroreflex control of SNA and heart rate in black men and women
| Black Women (n = 9) |
Black Men (n = 12) |
|||
|---|---|---|---|---|
| Baseline | 16 wk | Baseline | 16 wk | |
| Phenylephrine | ||||
| ΔHR/ΔMAP | ||||
| beats·min−1·mmHg−1 | −1.2 ± 0.2 | −1.3 ± 0.4 | −1.0 ± 0.3 | 0.7 ± 0.2 |
| ΔMSNA/ΔMAP | ||||
| bursts·min−1·mmHg−1 | −1.5 ± 0.3 | −1.2 ± 0.4 | −1.2 ± 0.2 | −1.3 ± 0.4 |
| %integrated activity/mmHg | −7.2 ± 1.6 | −7.1 ± 1.0 | −5.6 ± 0.5 | −5.9 ± 1.2 |
| Nitroprusside | ||||
| ΔHR/ΔMAP | ||||
| beats·min−1·mmHg−1 | −1.9 ± 0.2 | −2.1 ± 0.3 | −2.0 ± 0.3 | −2.3 ± 0.4 |
| ΔMSNA/ΔMAP | ||||
| bursts·min−1·mmHg−1 | −3.1 ± 0.4 | −3.7 ± 0.5 | −3.3 ± 0.5 | −2.8 ± 0.4 |
| %integrated activity/mmHg | −22.6 ± 4.9 | −34.6 ± 5.8 | −28.4 ± 6.3 | −16.4 ± 1.9 |
Values are means ± SE. HR, heart rate; MAP, mean arterial pressure.
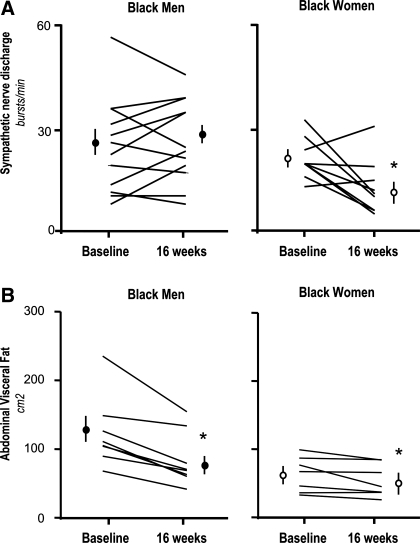
Weight reduction in black women led to significant reduction in SNA (from baseline of 22 ± 2 to 13 ± 3 bursts/min, P = 0.03; Fig. 1, Fig. 2, and Table 2). In contrast, despite significant reduction in body weight, visceral abdominal fat, plasma leptin, and insulin, SNA was unaffected by weight loss in black men (from baseline of 26 ± 4 to 28 ± 3 bursts/min, P = not significant; Figs. 1–2 and Table 2). In a subset of male subjects (n = 6), who maintained the same level 24-h urinary sodium excretion after weight loss (from 143 ± 21 to 152 ± 24 mmol/day), SNA and 24-h mean ambulatory BP were also unaffected by weight loss (from baseline of 27 ± 7 to 29 ± 5 bursts/min and from 94 ± 4 to 94 ± 6 mmHg, respectively, P = not significant). In the remaining six subjects who reduced sodium intake after weight loss (from 165 ± 11 to 45 ± 17 mmol/day), 24-h mean ambulatory BP decreased from 91 ± 3 to 84 ± 2 mmHg, but this magnitude of reduction, compared with subjects who did not reduce sodium intake, was not statistically significant (P = 0.11 by repeated-measures ANOVA). No correlation was found between changes in 24-h urine sodium and changes in 24-h MAP from baseline in black men (r2 = 0.02, P = 0.65). There was also no correlation between changes in SNA and changes in body weight, plasma leptin, insulin, or abdominal fat mass after weight loss in both black men and women (data not shown).
Fig. 1.
Recordings of muscle sympathetic nerve activity (SNA) in a 32-year-old black woman (top) and a 24-year-old black man (bottom) studied at baseline and 16 wk after dietary weight loss. On these mean-voltage displays of muscle sympathetic-nerve activity to the muscle, each peak represents a spontaneous burst of sympathetic nerve discharge. In the 32-year-old black woman, SNA reduced by 50% after 16 wk of hypocaloric diet, whereas SNA remained unchanged in the 24-year-old black man despite a similar amount of weight loss.
Fig. 2.
A: summary data of all subjects showing muscle SNA in bursts/min and bursts/100 heart beats (bursts/110RR). B: total and visceral abdominal fat measured by MRI in black women (n = 9) and black men (n = 12) at baseline and 16 wk after dietary weight loss. *P < 0.05 vs. baseline. Data are expressed as means ± SE.
DISCUSSION
The major findings from our present study are twofold. First, in black women, weight reduction was accompanied by reduction in sympathetic activity, consistent with Landsberg's hypothesis. Second, in black men, levels of SNA remained unchanged despite substantial reduction in body weight and many variables thought to stimulate central sympathetic outflow, such as leptin, insulin, and visceral abdominal fat, an unexpected exception to Landsberg's hypothesis.
Although obesity is proposed to lead to sympathetic overactivity as a compensatory autonomic adjustment to weight gain, the experimental support for this hypothesis is derived mainly from white cohorts. In our study, SNA responses to weight loss in black women appear to be similar to those reported previously in white men and women (8, 9). In these studies, increased baroreflex restraint of SNA was thought to be the mechanism mediating reduction in SNA in obese white men and women after weight loss (8, 9). However, reduction in SNA in black women after weight loss was not accompanied by changes in baroreflex gain in our study. On the other hand, circulating plasma insulin, plasma leptin, or visceral adiposity may contribute to elevated SNA in obese black women because these three variables reduced significantly after weight loss. In normotensive white subjects, insulin infusion has been shown to acutely increase sympathetic outflow (5). However, chronic elevation of endogenous insulin levels has not been shown to have any effect on SNA or BP in patients with insulinoma (22). In anesthetized rats, continuous administration of intravenous leptin increased BP and sympathetic activity to the kidney and adipose tissue (12, 24). However, effects of leptin administration on SNA have not been determined in humans. Visceral abdominal fat was also shown to be a stronger determinant of SNA than total body fat or subcutaneous abdominal fat (2, 3), but studies have not confirmed that selective reduction in visceral fat mass leads to reduction in SNA in humans. Because changes in SNA in black women after weight loss were not correlated with changes in visceral fat mass or circulating levels of leptin and insulin, we cannot exclude that other factors contribute to weight loss-induced sympathoinhibition in black women.
The current cross-sectional data in black men confirm findings from our previous study that SNA results were similar between obese and lean subjects, suggesting that obesity is not associated with sympathetic activation in this ethnic-sex group. Our prospective study further extends the evidence that diet-induced reductions in circulating leptin and insulin levels and visceral abdominal fat are not sufficient to lower SNA in obese black men. The negative finding is not due to inadequate weight reduction because even smaller amounts of weight loss resulting in higher BMI in black women caused a significant reduction in SNA. Furthermore, this amount of weight reduction in black men is sufficient to cause significant reduction in 24-h ambulatory HR. Sodium restriction is not likely to be responsible for sustained sympathetic activation because, overall as a group, there was no significant reduction in 24-h urinary sodium excretion after weight loss. In contrast, sodium restriction may contribute to reduction in BP in a subset of black men after weight loss. However, our subgroup analysis is limited by small sample size. Other potential explanations for SNA to remain unchanged after weight loss include occult sleep apnea, which is known to be associated with sympathetic overactivity via chemoreflex sensitization, independent of obesity (20). However, this is less likely because none of our subjects reported history of insomnia or loud snoring. Alternatively, elevation in SNA in black men could be the primary event that promotes subsequent development of obesity via downregulation of β-adrenergic receptor in the adipose tissue, leading to reduced thermogenesis and resting energy expenditure. One recent study provides support for this hypothesis, demonstrating that withdrawal of sympathetic neural drive with trimethaphan caused a much smaller reduction resting energy expenditure in the obese subjects, despite a larger reduction in BP, than in the lean subjects (25). Thus we speculate that black men who maintain normal β-adrenergic receptor function will be protected from obesity despite elevated SNA and BP, whereas those with downregulation of β-adrenergic receptor are more prone to develop both obesity and hypertension from elevated SNA.
Several aspects of our experiments limit our ability to draw inferences about the mechanisms of obesity-induced hypertension in African Americans in general. First, because we measured SNA targeted mainly to the skeletal muscle vasculature, the results cannot be extrapolated to sympathetic outflow to other organs such as splanchnic vascular bed, which could contribute to BP reduction observed in black men. In our study, weight reduction led to significant reduction in 24-h ambulatory HR in both black men and women, which could signify reduction in sympathetic outflow to the heart. However, this is unlikely because a previous study has indicated that obese subjects already have lower levels of cardiac sympathetic activity, as evidenced by cardiac norepinephrine spillover, despite higher levels of renal and hepatomesenteric norepinephrine spillover than lean individuals (28). In this regard, increased cardiac vagal activity after weight loss, which is suggested by a previous study in whites, is more likely to be the explanation (13). Second, because weight loss in our study was achieved by hypocaloric diet, we cannot exclude the possibility that negative energy balance from dietary restriction alone is responsible for reduction in SNA in black women (16), independent of reduction in fat depot. Third, given the small sample size of 12, we have only 80% power to detect reduction in SNA by ∼25% or 6.6 bursts/min in black men. However, average SNA in black men in our study did not show even a tendency to decrease after weight loss but rather increased by 1.4 bursts/min. Thus we do not believe that inadequate sample size explains failure of SNA to reduce in black men in our study.
Despite these limitations, our observation may have important clinical implications. The sympathetic nervous system has been shown to contribute to pathogenesis of hypertension and adverse cardiovascular outcomes even in populations with or without cardiovascular diseases (7, 17–19, 31). Failure of SNA to decrease despite substantial reductions in body weight and visceral fat depot in obese normotensive black men may explain greater susceptibility for hypertension and the associated cardiovascular complications in black men (4) independent of BMI (21) and abdominal obesity (15) than in men of other ethnic groups. On the other hand, the adiposity-dependent sympathetic activity in black women, the population with the highest prevalence of obesity (21), may predispose them to hypertension and hypertensive-related target organ damage vs. women of other ethnic groups in the United States. Elucidating the precise mechanisms of adiposity-independent sympathetic activity in black men could lead to the identification of new drug targets to prevent hypertension and improve cardiovascular prognosis.
GRANTS
W. Vongpatanasin was funded by grants from the Donald W. Reynolds Cardiovascular Clinical Research Center and the O'Brien Kidney Center and National Center for Research Resources Grant M01 RR-00633; B. Adams-Huet was funded by grants from the Clinical and Translational Sciences Award (National Center for Research Resources Grant UL1 RR-024982); and R. Victor was funded by grants from Donald W. Reynolds Cardiovascular Clinical Research Center and National Heart, Lung, and Blood Institute Grant 5P50 HL-55988.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Present address of R. Victor and L. Szczepaniak: The Heart Institute, Cedars Sinai Medical Center, 8700 Beverly Blvd., Los Angeles, CA 90048.
REFERENCES
- 1. Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in Black Americans. Hypertension 38: 379–383, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol 287: H414–H418, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002 [DOI] [PubMed] [Google Scholar]
- 4. American Heart Association Heart and Stroke Statistics—2009 Update. Dallas, TX: American Heart Association, 2009 [Google Scholar]
- 5. Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appel LJ, Stason WB. Ambulatory blood pressure monitoring and blood pressure self-measurement in the diagnosis and management of hypertension. Ann Intern Med 118: 867–882, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell'Oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 49: 535–541, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Grassi G, Seravelle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Gudbjornsdottir S, Lonnroth P, Sverrisdottir YB, Wallin BG, Elam M. Sympathetic nerve activity and insulin in obese normotensive and hypertensive men. Hypertension 27: 276–280, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol 83: 1242–1247, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Landsberg L. Hyperinsulinemia: possible role in obesity-induced hypertension. Hypertension 19, Suppl I: I-61, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 15: 216–224, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Mark AL. Weight reduction for treatment of obesity-associated hypertension: nuances and challenges. Curr Hypertens Rep 9: 368–372, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Masuo K, Mikami H, Ogihara T, Tuck ML. Familial hypertension, insulin, sympathetic activity, and blood pressure elevation. Hypertension 32: 96–100, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Masuo K, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42: 474–480, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Masuo K, Mikami H, Ogihara T, Tuck ML. Sympathetic nerve hyperactivity precedes hyperinsulinemia and blood pressure elevation in a young, nonobese Japanese population. Am J Hypertens 10: 77–83, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98: 772–776, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Scherrer U, Owlya R, Trueb L. Sympathetic-nerve activity before and after resection of an insulinoma. N Engl J Med 335: 1240–1242, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 89: 2634–2640, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409–414, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 49: 27–33, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension 17: 669–677, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Vallbo AB, Hagbarth KE, Toreebjork HE, Wallin BG. Somatosensory proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 28. Vaz M, Jennings G, Turner A, Cox H, Lambert G ME. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension 36: 531–537, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Zib I, Jacob AN, Lingvay I, Salinas K, McGavock JM, Raskin P, Szczepaniak LS. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 55: 230–236, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cateliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105: 1354–1359, 2002 [DOI] [PubMed] [Google Scholar]