Abstract
The significant decline in motor neuron number after ∼60 yr of age is accompanied by a remodeling of the neuromuscular system so that average motor unit force increases and the ability of old adults to produce an intended force declines. One possible explanation for the loss of movement precision is that the remodeling increases the difference in recruitment forces between successively recruited motor units in old adults and this augments force variability at motor unit recruitment. The purpose of the study was to compare the forces and discharge characteristics of motor units in a hand muscle of young and old adults at motor unit recruitment and derecruitment. The difference in recruitment force between pairs of motor units did not differ between young (n = 54) and old adults (n = 56; P = 0.702). However, old adults had a greater proportion of contractions in which motor units discharged action potentials transiently before discharging continuously during the ramp increase in force (young: 0.32; old: 0.41; P = 0.045). Force variability at motor unit recruitment was greater for old adults compared with young adults (P ≤ 0.010), but discharge rate and discharge variability did not differ between age groups (P ≥ 0.729). These results suggest that the difference in force between the recruitment of successive motor units does not differ between age groups, but that motor unit recruitment may be more transient and could contribute to the greater variability in force observed in old adults during graded ramp contractions.
Keywords: first dorsal interosseus, steadiness
due to a significant decline in the number of spinal motor neurons that begins around the sixth decade of life (5, 20, 38), muscle fibers that were once innervated by these motor neurons either disintegrate and disappear or are reinnervated by surviving motor neurons (5). The result of these changes is fewer but larger motor units in the muscles of old adults compared with young adults (5, 10, 11). It has been suggested that this remodeling contributes to the decline in force control observed in old adults (1, 17, 24, 39).
Those features of the remodeled neuromuscular system that are responsible for age-related impairments in force control, however, remain to be determined. One mechanism that has received some attention is the variability in the discharge times of action potentials discharged by motor units, which has a significant influence on the capacity of an individual to perform a steady contraction (1, 28). However, these studies have not found significant differences in the discharge characteristics between young and old adults that might influence force control (1, 28). Another possibility is that the increase in input conductance and decrease in rheobase that reduces motor neuron excitability (12, 27, 33) in old adults may alter the distribution of recruitment threshold forces within the motor unit pool and, thereby, influence the summation of motor unit forces. Furthermore, an increase in the difference in recruitment threshold force between successively recruited motor units would likely cause greater changes in force during a graded contraction that would impair the ability of old adults to produce an intended force.
The purpose of the study was to compare the forces and discharge characteristics of motor units in a hand muscle of young and old adults at motor unit recruitment and derecruitment. The approach was to record the difference in the recruitment and derecruitment threshold forces for pairs of motor units in the first dorsal interosseus muscle during a ramp increase and then decrease in muscle force. The discharge characteristics at recruitment and derecruitment of motor units were also recorded. The hypothesis was that the distribution of differences in recruitment and derecruitment threshold forces between pairs of motor units would differ for young and old adults and that the coefficient of variation (CV) for force at motor unit recruitment and derecruitment would be greater for old adults despite similar discharge characteristics for the two age groups.
METHODS
Pairs of single motor units were recorded from the first dorsal interosseus muscle of young (24.6 ± 4.87 yr) and old adults (76.3 ± 6.42 yr) during isometric contractions. All subjects reported having no known neurological impairments. The recruitment threshold forces, fluctuations in force at motor unit recruitment, and the discharge characteristics at recruitment were compared between young and old adults. Additionally, the derecruitment forces, fluctuations in force at derecruitment, and discharge characteristics at derecruitment were quantified for those motor units that could be tracked during the task. The Human Research Committee of the University of Colorado at Boulder approved the procedures, and informed consent was obtained from all subjects before participation in the study.
Experimental set-up.
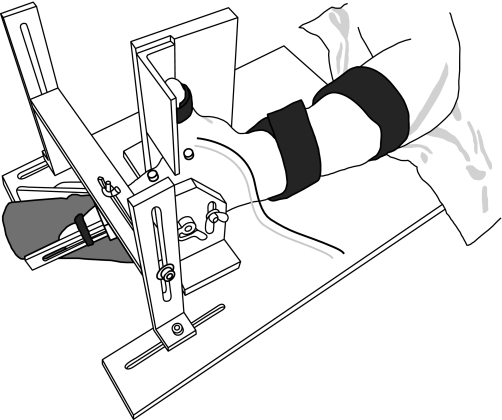
Subjects were seated in a chair with the left arm abducted ∼45° and the forearm restrained in the neutral position on an adjustable platform. The left hand was placed in a custom-made device that held the palm vertical and flexed the third through fifth digits by ∼45° at the metacarpophalangeal joints. The thumb was extended and restrained by a separate brace that maintained the thumb at a constant, comfortable angle relative to the index finger. The index finger was strapped to a hinged splint with the axis of rotation aligned with the metacarpophalangeal joint. The splint kept the interphalangeal joints extended and limited movement of the index finger to the abduction-adduction plane (Fig. 1).
Fig. 1.
The experimental setup consisted of a custom-made device that limited index finger movement to the abduction-adduction plane. A force transducer was aligned with the radial border of the proximal interphalangeal joint to record index finger abduction force. Surface EMG from the first dorsal interosseus muscle was obtained with 4-mm-diameter electrodes. Two fine-wire electrodes (three 50-μm-diameter insulated stainless steel wires) were inserted into the first dorsal interosseus muscle to record the discharge of action potentials by single motor units.
The abduction force exerted at the proximal interphalangeal joint of the index finger was measured with two force transducers: a low-sensitivity transducer (0.02 V/N) measured forces ≥ 8.83 N and a high-sensitivity transducer (0.43 V/N) measured forces < 8.83 N. Force was digitized with a Power 1401 (CED, Cambridge, UK) at 200 Hz during the experimental task and stored on a computer for offline analysis. Visual feedback of the force exerted by the index finger was provided on a 43.2-cm computer monitor (resolution: 800 × 600 pixels) placed at eye level 1.2 m in front of the subject.
Single motor unit action potentials were recorded from the first dorsal interosseus muscle with one or two electrodes that were each comprised of three 50-μm stainless steel wires insulated with Formvar (California Fine Wire, Grover Beach, CA) and glued together at the tip with Krazy Glue (Elmer's Products). Each electrode was inserted into the muscle belly via a 27-gauge, 1.13-cm hypodermic needle. Electrodes were inserted into the muscle in random locations but were usually separated by a distance of 2–3 cm, and care was taken to avoid puncturing any visible blood vessels. Three wires were used in each electrode to allow for three bipolar configurations to increase the probability of identifying single motor unit action potentials. The insulation was removed from the recording tip and the opposite end of each wire. The quality of the single motor unit recording was optimized by using the different combinations of bipolar configurations and by slightly moving the position of the electrodes within the muscle. The electromyogram (EMG) recording was amplified 10,000×, band-pass filtered between 300 Hz and 8.5 kHz (S-series, Coulbourn Instruments, Allentown, PA), sampled at 20 kHz with the Power 1401 (CED) system, and stored on a computer. A reference surface electrode (silver-silver chloride, 4-mm diameter) was used for each intramuscular electrode and was placed over a bony prominence as close to the insertion site of the wire electrode as possible.
Surface EMG from the first dorsal interosseus was also measured with a pair of electrodes (silver-silver chloride, 4-mm diameter) placed over the muscle belly and the distal tendon. A ground electrode was attached over a bony prominence as close to the muscle as possible. Surface EMG was amplified 1,000×, band-pass filtered between 13 Hz and 1 kHz (V-series, Coulbourn Instruments), sampled at 1 kHz with a Power 1401 (CED), and stored on a computer.
Experimental protocol.
Subjects were able to participate in up to three experimental sessions in which at least one motor unit pair was tracked. Each subject began the first experimental session by performing a series of maximal grip contractions with the right and left hands. A hydraulic hand dynamometer (Fabrication Enterprises, Elmsford, NY) was used for the grip contractions. Grip force was measured alternately from the right and left hand three times for each hand, and the maximal grip force for each hand was recorded. Approximately 60 s of rest was given between maximal handgrip contractions.
Subjects then performed a series of maximal voluntary contractions (MVC) with the first dorsal interosseus muscle before surface or intramuscular EMG electrodes were attached. Strong verbal encouragement was provided to the subject as the abduction force exerted by the index finger during the isometric contraction was increased from rest to maximal over ∼3 s and maintained for ∼3 s. Visual feedback of the force was provided for the subjects during the task, and one investigator observed the subject's hand to ensure the task was done correctly. The peak force from up to five trials was used as the MVC force as long as it was within 5% of a previous trial.
Surface EMG from the first dorsal interosseus was recorded during two ramp contractions before the start of the experiment. The root mean square EMG amplitude was recorded to provide a baseline measure of EMG for estimating muscle fatigue at various times throughout the experiment. Subjects performed the ramp contractions by increasing the index finger force from rest to 20% of MVC force in 5 s and back to rest in 5 s. Visual feedback of the target force and the subject's force were provided on the computer monitor as horizontal red lines on a black background. The goal for the subject was to keep the finger force line in the middle of the screen over the target force line. When this contraction was repeated during the experiment and the EMG amplitude was greater than 15% of the baseline EMG amplitude, the muscle was considered to be experiencing a level of fatigue that would likely influence the experimental measures and the subject was provided with additional rest until the EMG amplitude during the contraction was less than 10% above the baseline value.
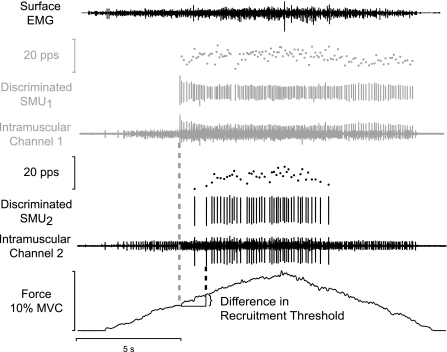
The goal of the experiment was to identify the motor unit action potentials from at least two motor units in the same ramp contraction. To ensure random sampling that included higher threshold motor units, subjects were instructed to abduct the index finger to a target of 65% of MVC force. Subjects repeated the contraction until a pair of candidate motor units was identified. Once a pair of motor units was identified, the target force was set to 120% of the recruitment threshold force of the higher threshold motor unit. At least three contractions were then performed to this target force to determine an average motor unit recruitment force. Subjects were provided with visual feedback of the index finger force and the target force, which was represented as a horizontal line on the computer monitor. Subjects were instructed to increase force from rest to the target force in ∼6 s and to return to rest in ∼6 s; these rates of change in force were observed in pilot sessions and in another study not to influence the recruitment threshold force of motor units (9). Consecutive contractions were separated by a minimum of 30 s with longer rests periods for stronger contractions. These procedures were repeated several times during an experimental session to increase the number of motor unit pairs recorded within a session. An example recording from one trial by a young adult is shown in Fig. 2. Experimental sessions were terminated after the subject had been in the laboratory for 2.5 h.
Fig. 2.
Sample data from a young subject. Motor unit recruitment was defined as the force at which a motor unit began discharging action potentials repetitively. Recruitment threshold was determined by moving a 0.5-s window forward in time in 1-ms increments through the data until the coefficient of variation (CV) for interspike interval within the window was < 50%. The force corresponding to the first discharge within this window was taken as the recruitment threshold force. Derecruitment threshold force was obtained with the same algorithm, except the last discharge within the window was taken as the derecruitment threshold force. SMU, single motor unit; MVC, maximal voluntary contraction; pps, pulses per second.
Data analysis.
Motor unit action potentials were discriminated offline both visually and with the template matching features of Spike2 software (version 5.02, CED). The recruitment threshold force of each motor unit was identified with an algorithm that used a 0.5-s duration sliding window. The window was advanced in 1-ms increments through the discriminated trains of action potentials until the CV for interspike interval (ISI) was <50%. The force corresponding to the first discharge in this window was taken as the recruitment threshold force for the motor unit. The same algorithm was used to determine the derecruitment force of motor units; however, the force corresponding to the last discharge within the sliding window was taken as the derecruitment force. The difference in recruitment and derecruitment forces between pairs of motor units activated in the same ramp contraction, as well as the recruitment and derecruitment forces of individual motor units, were compared between young and old adults (Fig. 2). As motor units discharged action potentials before and after the defined recruitment and derecruitment thresholds, respectively, the proportion of ramps that had an action potential 0.25 s before the recruitment threshold and within 2 s of the ramp onset were quantified for both age groups. The inverse algorithm was used to compare the proportion of ramps with action potentials after derecruitment between young and old adults.
The variability in force in absolute (SD of force; SD) and relative (CV for force) terms was determined over a 0.25-s period commencing at recruitment and 0.25 s before derecruitment of the higher threshold motor unit within each ramp contraction. Force variability was only quantified about the recruitment and derecruitment of the higher threshold motor unit as lower threshold motor units were often activated before the force transducer detected the index finger force. It was not possible to quantify the derecruitment forces and discharge characteristics of every motor unit as some could not be tracked throughout the entire contraction; this occurred for 14 of the 71 motor units recorded in young adults and 22 of the 77 motor units recorded in old adults. Measures of force variability at derecruitment were also not quantified when a motor unit was derecruited after the force had returned to baseline.
The CV for ISI and discharge rate of each motor unit was determined over a 0.5-s window commencing at recruitment and 0.5 s before derecruitment. The CV for ISI was compared between age groups, between recruitment and derecruitment, and between the low- and high-threshold motor units within a ramp contraction. These comparisons were necessary as the CV for ISI of the higher threshold units had the potential to be quantified over a relatively smaller increase in force compared with the lower threshold motor units. The CV for ISI could, therefore, differ between the low- and high-threshold motor units as previous studies have shown that the CV for ISI decreases rapidly as force is increased just after recruitment (1, 28).
To estimate the involvement of persistent inward currents in the observed discharge characteristics, differences in discharge rate of the lower threshold motor units at the recruitment and derecruitment of the higher threshold motor units were quantified. The difference in discharge rate is used as an index of the strength of persistent inward currents, which is attributed to the activation of metabotropic receptors that can result in the self-sustained discharge of action potentials by motor units (18).
Statistical analysis.
A two-factor ANOVA was used to compare the MVC force, maximum handgrip strength, and handedness between young and old adults (age group) and men and women. Independent samples Mann-Whitney U-tests were used to compare recruitment and derecruitment thresholds of motor units between young and old adults, as well as between recruitment and derecruitment within each age group. Chi-square tests were used to compare the proportion of ramps in which a motor unit discharged an action potential before and after recruitment and derecruitment, respectively. A two-factor ANOVA was used to assess differences in mean force between young and old adults and between force at recruitment and derecruitment (ramp-phase group). A two-factor analysis of covariance (ANCOVA) with mean recruitment force as the covariate was used to assess differences in force variability (SD and CV) of the higher threshold motor units between age groups and between recruitment and derecruitment. A three-factor ANOVA was used to compare the discharge characteristics between age groups, between low- and high-threshold motor units, and between recruitment and derecruitment. Independent samples t-tests, with Bonferroni corrections, were used to evaluate differences between groups when significant differences were found.
Linear regression analysis was used to determine the association between the recruitment force and derecruitment force, the SD of force, and the discharge characteristics of motor units at recruitment. Linear regression analysis was also used to determine the associations between the derecruitment force and the SD of force and the discharge characteristics of motor units at derecruitment. All statistics were performed in SPSS version 16.0 (SPSS, Chicago, IL). An α level of 0.05 was considered significant, and values are reported as means ± SD.
RESULTS
Fifteen young (11 men) and 7 old (4 women) adults participated in the experimental protocol. There were no age- or sex-related differences in handedness (P ≥ 0.583), and the average laterality quotient (Edinburgh Handedness Inventory; 29) for all subjects was 0.57 ± 0.53, indicating that they were moderately right-handed. Handgrip strength of the right hand was weaker for old adults compared with young adults, but the difference was not statistically significant (30.3 ± 8.6 and 43.3 ± 13.0 kg, respectively; P = 0.082). Handgrip strength of the left hand for old adults (24.7 ± 5.19 kg) was significantly weaker than that of the young adults (40.3 ± 11.6 kg; P = 0.007). Handgrip strength for both hands was significantly weaker for the women (right: 26.0 ± 4.93 kg; left: 24.8 ± 4.53 compared with the men (right: 46.6 ± 9.92 kg; left: 45.3 ± 9.00 kg; P ≤ 0.005). Index finger abduction strength (MVC force) did not differ between young (33.7 ± 8.96 N) and old adults (30.8 ± 13.1 N; P = 0.966), but women (25.7 ± 6.6 N) were weaker than men (36.8 ± 9.9 N; P = 0.01).
Motor unit recruitment and derecruitment threshold.
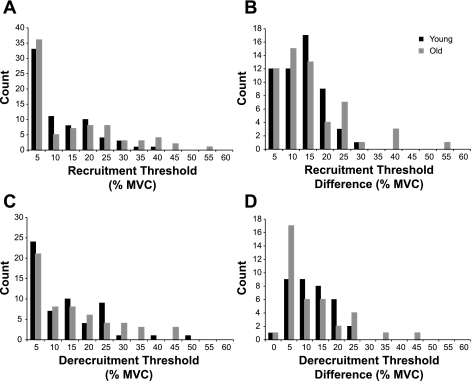
Similar numbers of motor units were recorded in young (n = 71) and old (n = 77) adults. Motor unit recruitment forces ranged from 0 to 37.7% MVC force for young adults and from 0 to 53.6% MVC force for old adults (Fig. 3A). Average recruitment threshold force of motor units did not differ significantly between young (8.94 ± 9.25% MVC force) and old adults (12.3 ± 13.2% MVC force; P = 0.276) (Fig. 3A). The recordings included 54 pairs of motor units in young adults and 56 pairs in old adults. The difference in recruitment threshold force between motor units in each pair ranged from 0.46 to 29.0% MVC force for young adults and from 0.70 to 53.0% MVC force for old adults (Fig. 3B). The mean difference in recruitment threshold force for these pairs was 10.8 ± 6.49% MVC force for young adults and 12.8 ± 10.2% MVC force for old adults (P = 0.702) (Fig. 3B).
Fig. 3.
Histograms of the distributions for motor unit recruitment and derecruitment threshold forces for young and old adults. A: recruitment threshold forces for motor units in young (n = 71) and old (n = 77) adults. Motor unit recruitment threshold forces did not differ significantly between age groups (P = 0.276). B: difference in recruitment threshold forces between pairs of motor units for young (n = 54) and old (n = 56) adults. The difference in recruitment threshold forces for pairs of motor unit did not differ between age groups (P = 0.702). C: derecruitment threshold forces for motor units in each age group (n = 57). Derecruitment threshold forces did not differ between age groups (P = 0.644). D: difference in derecruitment threshold forces for pairs of motor units in young (n = 35) and old (n = 38) adults did not differ between age groups (P = 0.453).
Only 57 motor units could be tracked at derecruitment in each age group. Derecruitment thresholds ranged from 0 to 46.8% MVC force for young adults and from 0.03 to 44.0% MVC force for old adults (Fig. 3C). The average derecruitment force of these motor units did not differ significantly between young (10.8 ± 9.74% MVC force) and old adults (12.8 ± 12.1% MVC force; P = 0.644) (Fig. 3C). Approximately half of the pairs of motor units identified at recruitment could also be discriminated at derecruitment for young (n = 35) and old (n = 38) adults. The difference in derecruitment force between the high- and low-threshold motor units ranged from −0.47 to 21.1% MVC force for young adults and from 0.45 to 42.7% MVC force for old adults (Fig. 3D). The mean derecruitment threshold difference between motor unit pairs was 9.54 ± 5.91% MVC force for young adults and 10.1 ± 9.65% MVC force for old adults (P = 0.453) (Fig. 3D).
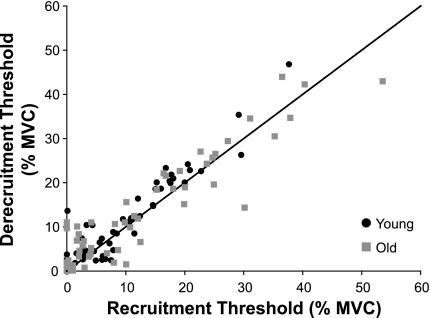
There were no differences between the recruitment and derecruitment forces for the motor units that were tracked at both recruitment and derecruitment in either age group (P ≥ 0.127). Linear regression analysis revealed that the motor unit derecruitment forces were positively associated with the recruitment forces for both young (r2 = 0.89) and old adults (r2 = 0.87; P ≤ 0.001) (Fig. 4). However, the majority of data points were above the line of identity for both young and old adults, which indicated that derecruitment forces tended to be greater than recruitment forces. As such the difference between motor unit recruitment and derecruitment threshold forces for young and old adults were −1.50 ± 3.26 and −0.75 ± 4.73% MVC force, respectively.
Fig. 4.
Derecruitment threshold force of motor units plotted as a function of recruitment threshold force for young and old adults. Clustering of the data points around the line of identity indicated that derecruitment thresholds were similar to recruitment thresholds for both young (P = 0.127) and old adults (P = 0.334). Derecruitment threshold was linearly associated with the recruitment threshold for both young (r2 = 0.89; y = 1.07x + 0.896) and old adults (r2 = 0.87; y = 0.871x + 2.29; P ≤ 0.001).
Although there was no difference in motor unit recruitment or derecruitment forces between young and old adults, the proportion of ramps with at least one action potential before recruitment was greater for old (0.410) compared with young adults (0.325; P = 0.045). Conversely, the proportion of ramps with at least one action potential after derecruitment did not differ between young (0.319) and old adults (0.325; P = 0.895).
Steadiness at motor unit recruitment and derecruitment.
The mean force and absolute (SD) and relative (CV) force fluctuations were quantified over a 0.25-s time period commencing at recruitment and 0.25 s before derecruitment of the higher threshold motor unit within a ramp contraction. The mean force of these units did not differ between recruitment (n = 66; 18.6 ± 11.8% MVC force) and derecruitment (n = 57; 17.3 ± 10.8% MVC force) when averaged across young and old adults (P = 0.564). Mean force averaged across recruitment and derecruitment was greater for old adults (20.5 ± 13.0% MVC force) than for young adults (15.7 ± 8.86% MVC force; P = 0.020). The absence of an interaction between age and ramp phase (P = 0.821) indicated that the mean force at both recruitment and derecruitment was greater for the old adults compared with the young adults.
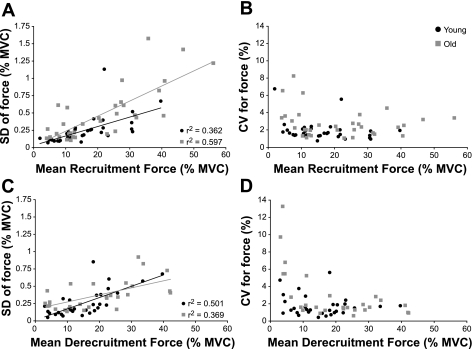
The SD of force increased linearly with mean recruitment and derecruitment force for both young adults (recruitment: r2 = 0.37; derecruitment: r2 = 0.50) and old adults (recruitment: r2 = 0.60; derecruitment: r2 = 0.37; P ≤ 0.001). The SD of force did not differ between recruitment (0.37 ± 0.33% MVC force) and derecruitment (0.30 ± 0.21% MVC force) when averaged across young and old adults (P = 0.228). However, the SD of force averaged across recruitment and derecruitment was greater for old adults (recruitment: 0.48 ± 0.38% MVC force; derecruitment 0.36 ± 0.21% MVC force) compared with the young adults (recruitment: 0.25 ± 0.21% MVC force; derecruitment: 0.25 ± 0.20% MVC force; P = 0.010). The lack of an interaction between age and ramp phase (P = 0.142) indicated that the SD of force was greater for the old adults compared with the young adults at both recruitment and derecruitment (Fig. 5).
Fig. 5.
Absolute (SD) and relative (CV) measures of force variability at recruitment and derecruitment of the higher threshold motor unit in each pair. A: the SD of force increased linearly with mean recruitment force for young [y = 1.36(10−2)x + 3.19(10−2)] and old adults [y = 2.18(10−2)x + 1.89(10−2); P < 0.001] and was greater for old adults (P = 0.010). B: the CV for force decreased with mean recruitment force for both age groups, but was greater for old adults (P < 0.001). C: the SD of force increased linearly with mean derecruitment force for young [y = 1.65(10−2)x + 5.14(10−3)] and old adults [y = 1.03(10−2)x + 0.158; P ≤ 0.001] and was greater for old adults (P = 0.010). D: the CV for force decreased with mean derecruitment force for both age groups but was greater for old adults (P < 0.001).
In contrast to the SD of force, the CV for force decreased with an increase in mean force at recruitment and derecruitment for young and old adults. As with the SD of force, however, the CV for force did not differ (P = 0.674) between recruitment (2.17 ± 1.52%) and derecruitment (2.34 ± 2.29%) forces when averaged across age groups. Furthermore, the CV for force averaged across recruitment and derecruitment was greater for old adults (recruitment: 2.59 ± 1.68%; derecruitment: 2.97 ± 2.97%) compared with young adults (recruitment 1.74 + 1.22%; derecruitment: 1.76 ± 1.21%; P ≤ 0.001). The lack of an interaction between age group and ramp phase (P = 0.625) also indicated that the CV for force was greater for old adults compared with young adults at recruitment and derecruitment (Fig. 5).
Motor unit discharge characteristics at recruitment and derecruitment.
The discharge rate of motor units at recruitment ranged from 6.28 to 24.2 pulses per second (pps) for young adults and from 6.19 to 21.2 pps for old adults and was positively and linearly associated with the recruitment force for both age groups (young: r2 = 0.23; old: r2 = 0.29; P ≤ 0.001). The discharge rate at derecruitment ranged from 6.54 to 17.6 pps for young adults and from 4.86 to 15.8 pps for old adults and was also positively and linearly associated with the force of derecruitment for both age groups (young: r2 = 0.24; old: r2 = 0.33; P ≤ 0.001). Motor unit discharge rate differed between recruitment (9.91 ± 3.18 pps) and derecruitment (9.21 ± 2.11 pps) when averaged across age groups (P ≤ 0.001). Moreover, the discharge rate of motor units averaged across recruitment and derecruitment did not differ significantly between young (9.41 ± 2.81 pps) and old adults (9.66 ± 2.87 pps; P = 0.729). The lack of an interaction between age group and ramp phase (P = 0.622) indicated that discharge rate was greater at recruitment compared with derecruitment for both young and old adults.
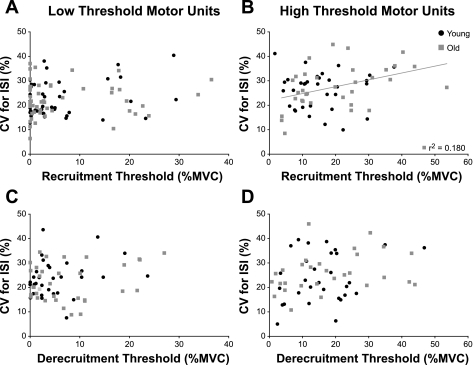
The CV for ISI did not differ between age groups, between recruitment and derecruitment, or between the low- and high-threshold motor units (P ≥ 0.247). However, the CV for ISI at recruitment tended to be less for the low-threshold motor units (22.5 ± 6.8%) compared with the high-threshold motor units (26.9 ± 8.05%; P ≥ 0.247 ) (Fig. 6, A and B). Linear regression analysis revealed that the CV for ISI for the low-threshold motor units from young (22.6 ± 6.81%) and old (22.4 ± 6.88%) adults was not significantly associated with the recruitment force (P ≥ 0.085) (Fig. 6A). The CV for ISI of the higher threshold motor units was also not associated with recruitment force for young adults (26.2 ± 7.59%; P = 0.963) but was significantly associated with the recruitment force for old adults (27.6 ± 8.55%; r2 = 0.18; P = 0.014) (Fig. 6B).
Fig. 6.
The CV for interspike interval (ISI) of the low-threshold (A and C) and high-threshold (B and D) motor units at recruitment and derecruitment. A: the CV for ISI of the low-threshold motor units was not associated with the mean recruitment force for either age group (P ≥ 0.085). B: the CV for ISI of the high-threshold motor units was not associated with the mean recruitment force for young adults (P = 0.963) but was for old adults (y = 0.280x + 22.0; P = 0.014). C: the CV for ISI of the low-threshold motor units was not associated with the mean derecruitment force for either age group (P ≥ 0.194). D: the CV for ISI of the high-threshold motor units was not associated with the mean derecruitment force for either age group (P ≥ 0.107).
Similar to the findings for recruitment, the CV for ISI at derecruitment tended to be less for the low-threshold motor units (22.9 ± 7.6%) compared with the high-threshold motor units (25.1 ± 9.02%; P ≥ 0.247). Linear regression analysis indicated that the CV for ISI was not associated with the derecruitment force for either the high- or low-threshold motor units or for either age group (P ≥ 0.107) (Fig. 6, C and D).
Indexes of persistent inward currents.
The strength of any possible persistent inward current was quantified as the difference between the discharge rate of the low-threshold motor unit at the recruitment and derecruitment of the high-threshold motor unit (ΔD). The ΔD was only quantifiable for 10 and 13 ramps in young and old adults, respectively. ΔD was 2.94 ± 2.72 pps for young and 2.66 ± 3.12 pps old adults and did not differ statistically (P = 0.483).
DISCUSSION
Contrary to expectations, the difference in recruitment and derecruitment threshold forces for pairs of motor units did not differ between age groups. However, motor unit recruitment was more transient for old adults and the fluctuations in force at motor unit recruitment were also greater for old adults. Similar to previous reports on the first dorsal interosseus muscle, the mean discharge rate and discharge variability at recruitment and derecruitment did not differ significantly between young and old adults. Furthermore, an indirect estimate of persistent inward currents in motor neurons did not differ between young and old adults during the graded contractions.
Motor unit recruitment and derecruitment threshold.
Contrary to expectations and previous findings (17, 22), the average recruitment threshold force for the sample of motor units in the present study did not differ between young and old adults. Furthermore, the differences in recruitment force and derecruitment force for pairs of motor units activated in the same ramp contraction did not differ between young and old adults. Differences in the task and algorithm used to define motor unit recruitment between the present study and those that found age-related differences in motor unit recruitment forces likely contributed to the divergent findings.
In the present study, subjects increased force from rest to a target in ∼6 s and recruitment threshold was defined as the force corresponding to the first discharge within a sliding 0.5-s window in which the CV for ISI was < 50%. In the study by Galganski et al. (17), recruitment threshold force was identified as the minimum force that sustained the repetitive discharge of the motor unit, which may have reduced the possibility of recording low-threshold motor units from old adults who have difficulty performing contractions at low forces (1, 17, 24, 36, 39). Consequently, the rightward shift in the distribution of motor unit recruitment thresholds of old adults observed by Galganski et al. (17) may have been a result of the task used to identify recruitment threshold. In the study by Knight and Kamen (22), recruitment threshold forces were only quantified for motor units < 20% MVC force, which is approximately one-third of the recruitment range for motor units in the first dorsal interosseus muscle. Furthermore, Knight and Kamen (22) did not observe motor units with recruitment thresholds < 1.5% MVC force in old adults, which is contrary to the results of the present study. Thus the greater average motor unit recruitment force of old adults observed by Knight and Kamen (22) may have been due to the absence of low-threshold motor units in their sample from old adults.
In contrast to these studies, the distribution of recruitment thresholds in the present study was skewed to include more low-threshold motor units compared with high-threshold motor units for both young and old adults. The results of the present study also agree with the findings from some studies that did not find a difference in recruitment threshold forces between young and old adults (14, 37, 40). Furthermore, the average recruitment threshold force for motor units sampled from the first dorsal interosseus muscle can even be lower in old adults compared with young adults (1, 13, 28).
The average difference in motor unit recruitment threshold force between pairs of units did not differ between young and old adults. The experimental task, however, likely limited the sample of motor unit pairs activated below 5% MVC force as subjects often increased force rapidly to targets below 5% MVC force. As rapid rates of force development decrease recruitment threshold forces, (3, 9), these data were discarded in the present study. The fewer pairs of motor units below 5% MVC force likely biased the results and may account for the similar distribution of differences in recruitment threshold forces for pairs of motor units in young and old adults. However, it is noteworthy that only the old adults had recruitment threshold force differences between pairs of motor units that were greater than 35% MVC.
Increased coactivation by old adults during the ramp contractions may have also contributed to the lack of a difference in recruitment and derecruitment thresholds between age groups. Although the activity of the antagonist muscle, second palmar interosseus, was not measured in the present study, it is possible that older adults had greater amounts of coactivation (4), which can reduce the recruitment threshold forces of motor units. Previous studies with similar tasks have found that derecruitment forces can be either greater (8, 15, 26, 31) or similar (31, 37) to the recruitment threshold force of motor units and that this may differ for young and old adults (31). Hence, similar recruitment and derecruitment thresholds may be due to increased coactivation during the ramp-down phase of the contraction (4, 37).
Although there was no difference in recruitment or derecruitment forces between young and old adults, the older adults had more contractions with action potentials before the defined recruitment threshold of the motor unit. This finding indicates that motor unit recruitment may be a more transient process for old adults than for young adults, which may be a consequence of greater synaptic noise onto the motor neuron pool of old adults during the ramp increase in force. This finding differs from sustained contractions when it appears that synaptic noise is greater for young adults (30, 32). Thus the amount of active synaptic inputs onto the motor neuron pool may differ across tasks for young and old adults (2). A more transient recruitment process may also increase the force fluctuations around recruitment for old adults.
Steadiness at motor unit recruitment and derecruitment.
The SD of force increased with force as observed in other studies (1, 6, 17, 24, 28), but the SD of force during the ramp contractions was significantly greater for old adults in contrast to other findings (1, 17, 24, 28). The greater SD of force for old adults indicates that the recruitment and derecruitment of motor units with larger twitch forces (7, 11, 35) may increase the variability in force during ramp contractions. The CV for force was also greater for old adults compared with young adults at recruitment and derecruitment of the higher threshold motor units during a ramp contraction, which is consistent with the findings from other studies on the index finger (1, 17, 24, 36).
Motor unit discharge characteristics at recruitment and derecruitment.
Similar to the results of previous studies (1, 28), the rate at which motor units began to discharge action potentials was similar for young and old adults and was positively associated with the recruitment threshold force. The discharge rate at derecruitment was also similar for young and old adults and positively associated with the derecruitment force. The positive associations between discharge rate at recruitment and derecruitment likely indicate a matching between the discharge and contractile properties of motor units, such that motor units with briefer contraction and half-relaxation times will discharge at greater rates to increase twitch fusion (21).
The CV for ISI was similar for all motor units at recruitment and derecruitment and averaged 24.2%. The observed discharge variability is lower than previously reported (1, 22, 28); however, the CV for ISI was quantified over a 0.5-s period when force was increasing and decreasing, compared with measurements during a constant force contraction (1, 28) or during a force-modulation task (22). As the CV for ISI declines rapidly at forces just above recruitment, the CV for ISI in the present study was slightly lower than the values obtained during constant force contractions.
The discharge variability of motor units is an important factor contributing to the fluctuations in muscle force for both young and old adults (1, 28). Although the CV for ISI was similar for motor units of young and old adults, it is likely that discharge variability had a greater influence on the force fluctuations for old adults, especially at low forces. As old adults have fewer and larger motor units (5, 10, 11, 20, 38) and the influence of newly recruited motor units on muscle force is greatest at low forces (16, 25, 26, 32), similar motor unit discharge variability may result in larger force fluctuations for old adults than young adults. Support for this assertion comes from a training study that found old adults reduced the CV for acceleration of the index finger during shortening and lengthening contractions performed with the first dorsal interosseus by reducing the discharge variability of motor units (23).
Indexes of persistent inward currents.
Persistent inward currents can enable a motor neuron to sustain the discharge of action potentials in the absence of synaptic input. The functional consequence of these depolarizing currents is that less excitatory synaptic input is needed to maintain the discharge of a motor unit, which might lead to lower derecruitment thresholds. Nonetheless, the derecruitment threshold force of a motor unit is generally greater than its recruitment threshold force (8, 15, 26, 31), particularly in the absence of coactivation (37). To examine the potential involvement of persistent inward currents in the present study, the technique of Gorrasini et al. (18) was used to estimate the strength of persistent inward currents (ΔD). The ΔD value was similar for both young and old adults and represented an average reduction of 21% in the synaptic input needed to activate vs. maintain the discharge of the higher threshold motor units, which is approximately half the reduction in synaptic input (∼40%) observed in the tibialis anterior muscle (19). These findings suggest that any involvement of persistent inward currents in maintaining the discharge of active motor units was similar for young and old adults.
In conclusion, despite larger and fewer motor units in the muscles of old adults, there was no difference in either the recruitment and derecruitment threshold forces of motor units or the difference in recruitment force between pairs of motor units for young and old adults. However, motor unit recruitment may be a more transient process for old adults, which suggests there are differences in synaptic input onto the motor neuron pools of young and old adults. Furthermore, the larger motor units of old adults likely augments force variability during tasks that involve the recruitment and derecruitment of motor units.
GRANTS
This study was funded by National Institute on Aging Grant R01-AG-09000.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the authors.
ACKNOWLEDGMENTS
We thank Jane Litsey for assistance with the experimental design and testing sessions.
REFERENCES
- 1. Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Büdingen HJ, Freund HJ. The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflügers Arch 362: 61–67, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol 89: 61–71, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christou E, Grossman M, Carlton L. Modeling variability of force during isometric contractions. J Mot Behav 34: 67–81, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999 [DOI] [PubMed] [Google Scholar]
- 8. De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264: 673–693, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doherty TJ, Brown WF. The estimated numbers and relative sizes of thenar motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve 16: 355–366, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol 82: 93–101, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Englehardt JK, Morales FR, Yamuy J, Chase MH. Cable properties of spinal motoneurons in adult and aged cats. J Neurophysiol 61: 194–201, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Erim Z, Beg FM, Burke DT, De Luca C. Effects of aging on motor-unit control properties. J Neurophysiol 82: 2081–2091, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fling BW, Knight CA, Kamen G. Relationship between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res 197: 125–133, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Freund HJ, Büdingen HJ, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol 38: 933–946, 1975 [DOI] [PubMed] [Google Scholar]
- 16. Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70: 2470–2488, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69: 2108–2115, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Gorassini M, Yang JF, Siu M, Bennet DJ. Intrinsic activation of human motoneurons: possible contribution of motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kawamura Y, Okazaki H, O'Brien PC, Dych PJ. Lumbar motoneurons of man: I) number and diameter histogram of alpha and gamma axons of ventral root. J Neuropathol Exp Neurol 36: 853–860, 1977 [DOI] [PubMed] [Google Scholar]
- 21. Kernell D. Organized variability in the neuromuscular system: a survey of task-related adaptations. Arch Ital Biol 130: 19–66, 1992 [PubMed] [Google Scholar]
- 22. Knight CA, Kamen G. Modulation of motor unit firing rates during a complex sinusoidal force task in young and older adults. J Appl Physiol 102: 122–129, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol 98: 2072–2080, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23: 600–612, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Milner-Brown H, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230: 359–370, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milner-Brown H, Stein RB, Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol 230: 371–390, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morales FR, Boxer PA, Fung SJ, Chase MH. Basic electrophysiological properties of spinal cord motoneurons during old age in cat. J Neurophysiol 58: 180–194, 1987 [DOI] [PubMed] [Google Scholar]
- 28. Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Oldfield RC. The assessment and analysis of handedness: the Endinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- 30. Pascoe MA, Holmes MR, Gaw ME, Enoka RM. Motor unit recruitment in the biceps brachii of older adults during a fatiguing contraction. Abstr Soc Neurosci S-104606, 2008 [Google Scholar]
- 31. Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol 83: 128–143, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Riley ZA, Maerz AH, Litsey JC, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586: 2183–2193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve 20: 679–690, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Selen LPJ, Beek PJ, van Dieën JH. Can co-activation reduce kinematic variability? A simulation study. Biol Cybern 93: 373–381, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol 84: 358–366, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Sosnoff JJ, Newell KM. Are age-related increases in force variability due to decrements in strength? Exp Brain Res 174: 86–94, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Spiegel KM, Stratton J, Burke JR, Glendinning DS, Enoka RM. The influence of age on the assessment of motor unit activation in a human hand muscle. Exp Physiol 81: 805–819, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977 [DOI] [PubMed] [Google Scholar]
- 39. Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging 24: 25–35, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Welsh SJ, Dinenno DV, Tracy BL. Variability of quadriceps femoris motor neuron discharge and muscle force in human aging. Exp Brain Res 179: 219–233, 2007. [DOI] [PubMed] [Google Scholar]