Abstract
On the basis of recent reports, the genioglossus (GG) negative-pressure reflex consists initially of excitation followed by a secondary state-dependent suppression phase. The mechanistic origin and functional role of GG suppression is unknown but has been hypothesized to arise from transient inhibition of respiratory active neurons as a protective reflex to prevent aspiration, as observed in other respiratory muscles (e.g., diaphragm) during airway occlusion. Unlike GG, tensor palatini (TP) is a tonic muscle with minimal respiratory phasic activation during relaxed breathing, although both muscles are important in preserving pharyngeal patency. This study aimed to compare GG vs. TP reflex responses to the same negative-pressure stimulus. We hypothesized that reflex suppression would be present in GG, but not TP. Intramuscular GG and TP EMGs were recorded in 12 awake, healthy subjects (6 female). Reflex responses were generated via 250-ms pulses of negative upper airway pressure (approximately −16 cmH2O mask pressure) delivered in early inspiration. GG and TP demonstrated reflex activation in response to negative pressure (peak latency 31 ± 4 vs. 31 ± 6 ms and peak amplitude 318 ± 55 vs. 314 ± 26% baseline, respectively). A secondary suppression phase was present in 8 of 12 subjects for GG (nadir latency 54 ± 7 ms, nadir amplitude 64 ± 6% baseline), but not in any subject for TP. These data provide further support for the presence of excitatory and inhibitory components of GG (phasic muscle) in response to brief upper airway negative-pressure pulses. Conversely, no reflex suppression below baseline was present in TP (tonic muscle) in response to the same stimuli. These differential responses support the hypothesis that GG reflex suppression may be mediated via inhibition of respiratory-related premotor input.
Keywords: respiratory reflexes, sleep-disordered breathing, suppression, apnea, lung
the ability of the upper airway dilator muscles to respond to subatmospheric (collapsing) pressure is crucial in maintaining a patent upper airway. The genioglossus (GG) is the largest pharyngeal dilator muscle and is reflexively activated to oppose upper airway collapse in response to negative upper airway pressure (17, 26, 27). Similarly, the palatal upper airway dilator muscle, tensor palatini (TP), also displays reflex activation in animals and humans in response to rapid application of negative upper airway pressure (3, 24, 39). However, the responsiveness of these excitatory reflexes may be attenuated during sleep (16, 38, 39) and, hence, may contribute to sleep-disordered breathing in individuals with an anatomically vulnerable upper airway. Thus, understanding the underlying mechanisms and behavior of upper airway dilator muscle reflexes provides important insight into the pathogenesis of sleep-disordered breathing.
While TP and GG have been shown to respond reflexively to rapid changes in upper airway pressure of relatively large magnitude (<10 cmH2O) in a similar manner (24, 39) during relaxed breathing, GG displays a phasic pattern of activation, whereas TP exhibits only tonic activation with no apparent respiratory modulation (36). Thus, GG activity increases during inspiration to dilate or stiffen the upper airway and oppose upper airway collapse, and GG is less active during expiration, when pharyngeal pressure is positive (20, 34). The phasic activation is believed to be generated via the combination of input from respiratory active neurons in the medulla and reflex feedback from negative pharyngeal pressure (1, 18, 20, 23). Conversely, the tonic level of activity displayed by TP during relaxed breathing is highly dependent on state [its activity decreases markedly from wakefulness to sleep (43)], but input from respiratory pattern generator neurons or the negative upper airway pressure that is generated during relaxed breathing (i.e., ∼1–2 cmH2O) is minimal (20, 37).
Earlier studies in healthy individuals that utilized moving-time-averaging smoothing techniques suggested that the reflex response of GG to rapid upper airway negative pressure was solely excitatory (17) and largely attenuated during sleep (16, 35, 38). However, more recent data demonstrate maintenance of GG reflex activity in response to negative-pressure pulse stimuli in the supine posture during non-rapid eye movement sleep (13, 14, 24) and the presence of a state-dependent longer-latency reflex suppression (14). The mechanistic origin and functional significance of GG reflex suppression are unknown but have been hypothesized to arise from transient inhibition of respiratory active neurons, as occurs in other respiratory phasic muscles (i.e., scalene, parasternal intercostals, and diaphragm) (7, 8, 14, 19). Reflex suppression of respiratory phasic muscles in response to brief airway occlusion or sudden upper airway obstruction/negative pressure has been postulated to be protective: via reducing intraluminal negative pressure to prevent aspiration of foreign objects or further upper airway collapse in situations of chronic airflow obstruction (e.g., snoring and asthma) (7, 8, 14, 19). Indeed, during wakefulness, reflex suppression of respiratory phasic muscles (i.e., scalene, parasternal intercostals, and diaphragm) in response to sudden respiratory loading during inspiration is more pronounced in obstructive sleep apnea patients, and there is a positive correlation between reflex suppression and the respiratory disturbance index (19). While such responses are likely beneficial for most phasic respiratory muscles, reduced output to the largest upper airway dilator muscle in the presence of rapid upper airway negative pressure is likely deleterious for patients with sleep-disordered breathing. In addition, the recently reported state dependence of GG reflex suppression (more pronounced reflex suppression during sleep, particularly rapid eye movement sleep) (14) further suggests that this phenomenon may be important in the pathophysiology of sleep-disordered breathing.
In this study, we utilized the known differential inputs to these two representative upper airway dilator muscles [GG (phasic with strong respiratory input) vs. TP (tonic with minimal respiratory input)] to gain insight into the potential contribution of respiratory-related premotor input to GG reflex suppression. Specifically, we aimed to compare the reflex responses of GG vs. TP to the same negative-pressure stimulus during a constant state (wakefulness) in healthy individuals. We hypothesized that reflex suppression would be present in the respiratory phasic GG, but not the tonic TP.
MATERIALS AND METHODS
Subject Selection
Thirteen healthy, nonsmoking men and women with no history of respiratory disease, sleep-disordered breathing, or regular medication use gave informed written consent to participate in the study. Female subjects were postmenopausal (2 subjects) or were studied in the follicular phase of the menstrual cycle (4 subjects). The study was approved by the Institutional Review Board of the Brigham and Women's Hospital.
Measurements and Equipment
Upper airway reflexes.
Both nostrils were decongested (0.05% oxymetazoline HCl), and the more patent nostril was anesthetized (4% lidocaine HCl) for insertion of two pressure-tipped catheters (model MCP-500, Millar, Houston, TX), which were used to monitor upper airway stimulus magnitude during negative-pressure pulses. One catheter was advanced 1–2 cm below the base of the tongue under direct visualization for measurement of pressure at the level of the epiglottis (Pepi) and the other to the level of the choanae (Pcho). Two Teflon-coated stainless steel fine-wire intramuscular electrodes (71 μm OD; catalog no. AS 765-36, Cooner Wire, Chatsworth, CA), with 2 mm removed from the tip, were inserted, via a 25-gauge needle, 3–4 mm on either side of the frenulum to a depth of ∼1.5 cm after surface anesthesia (4% lidocaine HCl) to create a bipolar EMG recording of GG activity (EMGGG). Similarly, two Teflon-coated fine-wire intramuscular electrodes were inserted at a 45° angle along the lateral surface of the medial pterygoid plate, to a depth of ∼10–15 mm into the palate, to create a bipolar EMG recording of TP activity (EMGTP). Each subject was fitted with a nasal mask (Gel Mask, Respironics, Murrysville, PA) equipped with a pneumotachograph (model 3700A, Hans Rudolf, Kansas City, MO) and differential pressure transducer (Validyne, Northridge, CA) for measurement of airflow. An additional pressure transducer was fitted to the mask [mask pressure (Pmask)]. End-tidal Pco2 (PetCO2) was also monitored at the nares. Upper airway negative-pressure pulses (nadir Pmask approximately −16 cmH2O, 250-ms duration) were delivered during early inspiration via a computer-controlled rapid-actuating solenoid-valve system, where one arm of the circuit was connected to room air and the other to a negative-pressure reservoir. The breathing circuit and the procedures utilized to deliver negative-pressure pulses were similar to those previously described (14). Analog signals were acquired on a 1401 Plus interface and Spike 2 software (Cambridge Electronic Design, Cambridge, UK). EMG and pressure signals were sampled at 2 kHz and filtered at 30–1,000 Hz.
Polysomnography.
Electroencephalograms, left and right electrooculograms, and surface submentalis EMG were applied to monitor wakefulness during reflex data collection and for sleep staging and arousal scoring during the overnight polysomnogram (PSG). In addition, after reflex data collection, a finger pulse oximeter was used to monitor blood arterial O2 saturation, surface tibialis EMG to measure leg movements, chest and abdominal belts to measure breathing movements, and a thermistor plus a nasal pressure probe to monitor airflow. PSG data were acquired using a commercially available system (Nihon Kohden, Tokyo, Japan).
Protocol
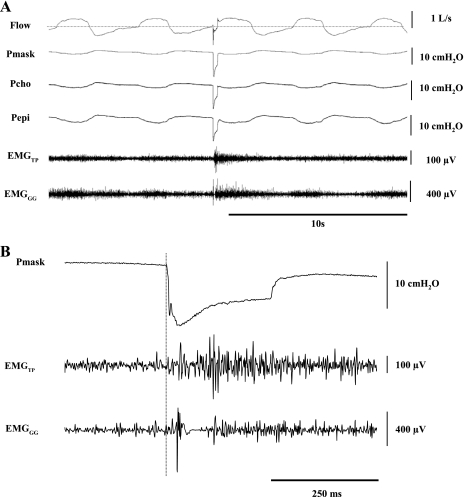
Subjects arrived at the laboratory at ∼6 PM having abstained from alcohol and caffeine for ≥12 h. After the subjects gave informed consent, the sensors and equipment were fitted, and several negative-pressure pulses were delivered for familiarization/habituation purposes. Subjects were studied in the supine posture. For elicitation of EMG reflex responses, negative-pressure pulses were delivered at random during stable relaxed breathing every 2–10 breaths during a 45-min wakefulness period (Fig. 1).
Fig. 1.
A: traces of airflow, mask pressure (Pmask), choanal pressure (Pcho), epiglottic pressure (Pepi), and raw tensor palatini (TP) and genioglossus (GG) EMGs (EMGTP and EMGGG) during a negative-pressure pulse delivered in early inspiration (3rd breath) and breaths immediately before and after pulse application in 1 individual subject (35-yr-old man). Note phasic activation of GG during inspiration and the tonic level of activation of TP. B: expanded view of Pmask and raw EMGTP and EMGGG responses during a negative-pressure pulse. Even during a single trial, reflex suppression is clearly present immediately following activation in GG, but not TP. Vertical dashed line represents stimulus onset.
At the completion of the reflex data collection period, the nasal mask and upper airway catheters were removed, and the subjects were outfitted with the remaining PSG equipment. Participants were then left undisturbed for 8 h, during which an overnight PSG was recorded to quantify the apnea-hypopnea index to rule out the presence of sleep-disordered breathing (defined as <10 events/h sleep) in these healthy individuals.
Data Analysis and Statistical Procedures
A trained sleep technician blinded to the reflex data defined the presence of arousals and performed sleep staging and scored respiratory events according to standard criteria (2, 31). PetCO2 immediately prior to and during the negative-pressure pulse protocol was analyzed on a breath-by-breath basis with use of custom-designed semiautomated software. The Pmask at which the rate of change in pressure was most negative during pulse presentation was identified to align each individual pulse to an accurately identifiable and highly reproducible reference point for EMG event-related analyses, as described previously (13, 14). This point was used to time-align all replicate pulses for ensemble averaging. Stimulus onset (time 0) was defined in the conventional manner as the last point preceding the sudden decrement in the ensemble-averaged Pmask following solenoid activation (Fig. 1B). Negative-pressure pulse stimulus magnitude was calculated for each subject as the nadir pressure observed in the ensemble-averaged waveform in each of the pressure channels (Pmask, Pcho, and Pepi). Negative-pressure pulses were excluded from analysis if 1) they were delivered during, or in the breath following, a sigh or a swallow, 2) they were delivered in a 30-s epoch immediately following any intrusion of sleep (as determined by EEG), 3) poor signal integrity (e.g., movement artifact or mucus accumulation on pressure catheters yielding artifacts). All remaining raw EMG recordings were full-wave rectified and ensemble-averaged to derive the EMGGG and EMGTP responses for each subject. Using custom-designed semiautomated software, each individual subject's ensemble-averaged, rectified EMG reflex responses were visually inspected to measure the presence, timing, and amplitude of each positive and negative component of the EMG response, as described previously (13, 14). Briefly, EMG reflex amplitude data were expressed as a percentage of the baseline average EMG activity for the 100 ms preceding pulse onset. This approach is similar to that described in previous studies, in which reflex amplitude data were expressed as the percent change from the preceding 100-ms period (9, 19). Excitation onset was defined as the point at which the rectified EMG signal crossed baseline prior to the first sustained (>10-ms) positive EMG peak. Where present, suppression onset was defined as the first point at which the rectified EMG recording crossed the baseline level for a sustained (>10-ms) period following the peak of the excitation response. Suppression cessation was defined as the first point at which the rectified EMG returned to baseline levels following the suppression nadir. Examples of representative EMGGG and EMGTP reflex responses and the criteria used to define the various reflex characteristics are displayed in Fig. 2.
Fig. 2.
Characterization criteria used to define GG (EMGGG) and TP (EMGTP) reflex components and timing properties for each subject. Traces represent ensemble average of rectified EMG (n = 85 replicate trials) and corresponding Pmask profile in a representative subject (54-yr-old woman).
GG and TP reflex latency and amplitude responses were compared using Student's paired t-tests. Statistical significance was inferred when P < 0.05. All group data are reported as means ± SE.
RESULTS
Anthropometric and Sleep Characteristics
One subject experienced sensations of claustrophobia when the nasal mask was applied; thus no reflex data were obtained from this subject. The mean age and body mass index for the 12 subjects (6 female) were 41 ± 3 (range 27–56) yr and 25 ± 1 (range 21–29) kg/m2, respectively. Absence of sleep-disordered breathing was confirmed in subjects with mean apnea-hypopnea index of 4 ± 1 (range 0–9) events/h sleep.
Upper Airway Muscle Reflex Responses to Brief Pulses of Negative Pressure
PetCO2 did not differ during the baseline period immediately before vs. during the negative-pressure pulse protocol (42.9 ± 1.1 vs. 43.3 ± 1.3 Torr, P = 0.831). Phasic activity of the raw EMGGG was observed in all subjects during relaxed breathing, whereas EMGTP showed a tonic level of activation without phasic changes with inspiration (Fig. 1). EMG peak reflex amplitudes, latency characteristics, the number of reflex suppression instances, stimulus properties, and the number of artifact-free negative-pressure pulse stimuli used to derive ensemble-average responses are summarized in Table 1. Negative-pressure pulse stimuli resulted in a short-latency peak in GG and TP (Figs. 1 and 2, Table 1). The onset of this initial peak occurred earlier for TP than GG (P = 0.02), but reflex amplitude and peak latency were not different (Table 1). A secondary reflex suppression phase of the rectified EMG below baseline was present in two-thirds of the subjects [4 female (2 premenopausal) and 4 male] for GG but was not present in any subject for TP (Fig. 2, Table 1).
Table 1.
Group GG and TP reflex and stimulus characteristics to negative-pressure pulse stimuli
| GG | TP | |
|---|---|---|
| Reflex characteristics | ||
| Excitation phase | ||
| Onset latency, ms | 19 ± 2 | 9 ± 2* |
| Peak amplitude, %baseline | 318 ± 56 | 314 ± 26 |
| Peak latency, ms | 31 ± 4 | 31 ± 6 |
| Duration, ms | 26 ± 3 | N/A† |
| Suppression phase | ||
| No. of subjs in which suppression occurred | 8/12 | 0/12 |
| Onset latency, ms | 42 ± 4 | N/A |
| Nadir amplitude, %baseline | 64 ± 6 | N/A |
| Nadir latency, ms | 54 ± 7 | N/A |
| Duration, ms | 25 ± 4 | N/A |
| Stimulus properties | ||
| Nadir Pmask, cmH2O | −15.7 ± 0.8 | |
| Nadir Pcho, cmH2O | −14.5 ± 1.1 | |
| Nadir Pepi, cmH2O | −9.1 ± 0.6 | |
| No. of artifact-free pulse presentations per subj | 77 ± 5 | |
Values are means ± SE. GG, genioglossus; TP, tensor palatini; Pmask, mask pressure; Pcho, pressure at chonae; Pepi, pressure at epiglottis.
Significantly different from GG.
Because excitation duration was defined as time from excitation onset to suppression onset, excitation duration could not be quantified for TP.
DISCUSSION
The main finding of this study was that, after an initial excitatory phase, a secondary reflex suppression component was present in the respiratory phasic GG, but not the tonic TP, in response to the same negative-pressure pulse stimuli. These data provide further evidence for the presence of excitatory and inhibitory components to GG and support the hypothesis that GG reflex suppression may originate from transient inhibition of respiratory-related premotor neurons, which normally provide phasic respiratory excitation of hypoglossal motoneurons.
Until recently (13, 14), the presence of inhibitory components of the GG negative-pressure reflex was overlooked because of the use of moving-time-averaging smoothing techniques. More recent studies, in which GG reflex suppression was identified, were conducted solely in healthy, young male subjects (13, 14). The data from the present study extend these observations by demonstrating GG reflex suppression in male and female subjects and in a middle-aged group. In the original studies (13, 14), GG reflex suppression was present in all subjects; in the present study, the secondary suppression phase was present in two-thirds of the subjects. While the reasons for this apparent discrepancy remain uncertain, the larger negative-pressure pulse stimulus employed in the present study (nadir Pmask approximately −16 vs. approximately −10 cmH2O) may have led to a more pronounced initial reflex excitation phase, which then overwhelmed the suppression component in some instances. In support of this hypothesis, the initial GG reflex excitation phase was larger in the present study than in the previous studies (∼320 vs. ∼230% of baseline). Nonetheless, GG reflex suppression was observed in the majority of cases, providing supportive evidence for the prior report.
All the previous studies investigating the reflex response of TP to negative-pressure pulse stimuli employed a smoothing moving time average (50–100 ms) of the rectified raw EMG signal (24, 39). As recently highlighted (13, 14), this technique is useful for characterizing generalized patterns of activity during the relatively slow time frame of tidal breathing. However, it inevitably distorts the details of the EMG response, such as the relationship between excitatory and inhibitory responses and, particularly, their latencies. Thus, elucidation of transient short-latency excitatory and/or inhibitory components is likely to be beyond the resolution of the previously employed techniques. Thus, by analyzing the rectified raw EMGTP without using a sliding-window smoothed average in the present study, we have revealed a short-latency excitatory reflex response with no evidence of reflex suppression. While the onset occurred more rapidly for TP than GG, the timing and amplitude of the excitatory peaks were quantitatively similar.
Mechanisms for GG Reflex Suppression
The GG negative-pressure reflex arc is believed to be mediated via pressure-sensitive mechanoreceptors in the pharyngeal airway that relay sensory information via the superior laryngeal nerve to the nucleus tractus solitarius within the brain stem. Recent data from rat experiments suggest that an adjacent group of neurons, termed the “periobex,” may also play a role in regulating the reflex response to negative pressure (10). The motor output to GG originates at the nearby hypoglossal motor nucleus and is relayed via the medial branch of the hypoglossal nerve (15, 20, 25, 32, 40). Although fewer data are available, the excitatory phase of the TP negative-pressure reflex is believed to be mediated in a manner similar to that of the GG negative-pressure reflex; however, it is innervated via the mandibular branch of the trigeminal nerve and trigeminal motor nucleus, rather than the hypoglossal motoneurons (6, 20, 39). Consistent with this notion, the initial reflex excitation component was remarkably similar in GG and TP, in terms of peak reflex latency and amplitude to the same negative-pressure stimulus.
Preliminary single motor unit analyses support the notion that reflex inhibition, rather than motor unit synchrony, is the cause of the secondary GG reflex suppression phase (14). The absence of TP reflex suppression, despite similar initial reflex activation in response to the same negative-pressure pulse stimuli in the present study, provides further support for GG reflex inhibition, rather than motor unit synchrony. Given the strong input from respiratory pattern generator neurons to GG, but not TP, the observation that reflex suppression to the same negative-pressure pulse stimulus was present in GG, but not TP, is consistent with inhibition of respiratory active neurons to GG as the origin of the observed suppression. Likely sites within the GG negative-pressure reflex arc in which inhibition of respiratory active neurons may lead to reflex suppression are the hypoglossal motor nucleus and the nearby, recently emphasized, periobex region (10). Indeed, inhibition of the periobex region has been shown to block the GG negative-pressure reflex in rats (10). Thus these data raise the possibility that GG reflex excitation could be due to disinhibition and that reflex inhibition may be due to disfacilitation of an excitatory input. However, such hypotheses are difficult to test in humans.
The present study and the existing literature on GG respiratory reflex suppression have focused on inspiratory reflex suppression (13, 14). Recent reports utilizing single motor unit techniques within GG have identified various subclasses of motor unit firing patterns, including some motor units that fire predominantly during expiration (4, 33, 42). Thus, it would be interesting to utilize the combined techniques of improved multiunit temporal resolution (as employed in the present study) and single motor unit analyses, which provide improved spatial resolution, to gain further insight into the mechanisms of GG reflex suppression and its potential role in the commonly observed expiratory airway collapse (28).
Finally, the timing and characteristics of the reflex inhibition observed in other respiratory muscles in response to respiratory loading (e.g., diaphragm, parasternal intercostal, and scalene) (7–9, 11, 13, 19, 30) were very similar to the previous GG reflex suppression reports (13, 14) and the present observations. Thus, taken together, these data suggest that similar mechanisms may be involved in the genesis of these reflex components.
Methodological Considerations
We studied upper airway reflex responses during wakefulness in middle-aged healthy men and women, rather than patients with disease or during sleep, because of the many potential confounding factors associated with sleep-disordered breathing and sleep-related effects on upper airway muscle tone. While the present design allowed us to examine accurately the differential effects of GG vs. TP negative-pressure reflexes and gain insight into the likely origin of GG reflex suppression, reflex responses may differ in elderly subjects (22), in patients with obstructive sleep apnea (5, 29), between sexes, according to menstrual phase/menopausal status, or during sleep (16, 38, 39) [particularly in rapid eye movement sleep, when muscle tone is markedly reduced (12, 21, 41) and profound GG reflex inhibition is present (14, 35)].
In addition, the use of lidocaine for instrumentation in our experimental set-up may have altered afferent sensations and the measured EMG responses. However, while it remains possible that minor residual effects of this agent may have had a small effect on the reflex responses, we believe that a major effect is unlikely because of the small (<1-ml) doses of lidocaine and the short (<30-min) duration of its effects, and experimental manipulations were not performed until well after (>60 min) the anesthetic agent's effects had worn off.
Summary and Conclusions
Upper airway dilator muscle reflexes to negative pressure are important in maintaining airway patency. Thus, understanding the underlying mechanisms and behavior of upper airway dilator muscle reflexes provides important insight into the pathogenesis of sleep-disordered breathing. This study has demonstrated the presence of an initial excitatory reflex component in GG and TP of similar peak latency and amplitude in response to the same negative-pressure pulse stimuli in healthy middle-aged men and women during wakefulness. In addition, a secondary reflex suppression phase was present in GG, but not TP. These data provide further evidence for excitatory and inhibitory components of GG. Furthermore, given the known differential inputs to these two upper airway dilator muscles [GG (phasic with strong respiratory input) vs. TP (tonic with minimal respiratory input)], these data support the hypothesis that GG reflex suppression likely arises from transient inhibition of respiratory-related premotor neurons, which normally provide phasic respiratory excitation of hypoglossal motoneurons. The therapeutic implications of our findings are unclear.
GRANTS
D. J. Eckert is supported by an Overseas Based Biomedical Fellowship from the National Health and Medical Research Council of Australia (510392). Other support includes National Heart, Lung, and Blood Institute Grants HL-73146, R01 HL-085188-01A2, R01 HL-090897-01A2, and K24 HL-093218-01A1 and the American Heart Association.
DISCLOSURES
D. J. Eckert and A. S. Jordan consult for Apnex Medical (<$20,000/year). A. Malhotra consults for several companies including Philips Respironics (<$20,000/year). D. P. White is Chief Medical Officer for Philips Respironics. While a number of the authors have some industry affiliations related to the treatment of apnea, we do not see these as relevant to this physiological investigation that is funded via peer review grant mechanisms.
ACKNOWLEDGMENTS
The authors thank Karen Stevenson, Lauren Hess, Eric Smales, and Scott Smith for technical support.
REFERENCES
- 1. Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol 531: 677–691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Sleep Disorders Association EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 173–184, 1992 [PubMed] [Google Scholar]
- 3. Amis TC, O'Neill N, Wheatley JR, van der Touw T, di Somma E, Brancatisano A. Soft palate muscle responses to negative upper airway pressure. J Appl Physiol 86: 523–530, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 94: 1875–1882, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci 28: 3535–3545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler JE, McKenzie DK, Crawford MR, Gandevia SC. Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. J Physiol 487: 273–281, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler JE, McKenzie DK, Gandevia SC. Impaired reflex responses to airway occlusion in the inspiratory muscles of asthmatic subjects. Thorax 51: 490–495, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butler JE, McKenzie DK, Glanville AR, Gandevia SC. Pulmonary afferents are not necessary for the reflex inhibition of human inspiratory muscles produced by airway occlusion. J Neurophysiol 78: 170–176, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JN, Sears TA. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol 209: 711–738, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 135: 957–964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Effects of hypoxia on genioglossus and scalene reflex responses to brief pulses of negative upper-airway pressure during wakefulness and sleep in healthy men. J Appl Physiol 104: 1426–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol 581: 1193–1205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436: 31–44, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol 476: 141–151, 1994 [PMC free article] [PubMed] [Google Scholar]
- 17. Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436: 15–29, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 55: 793–798, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Jeffery S, Butler JE, McKenzie DK, Wang L, Gandevia SC. Brief airway occlusion produces prolonged reflex inhibition of inspiratory muscles in obstructive sleep apnea. Sleep 29: 321–328, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol 160: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax 62: 798–804, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, Kikinis R, White DP. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 119: 72 e79–e14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 165: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, Kleverlaan D, White DP. Postural effects on pharyngeal protective reflex mechanisms. Sleep 27: 1105–1112, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathew OP. Upper airway negative-pressure effects on respiratory activity of upper airway muscles. J Appl Physiol 56: 500–505, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. J Appl Physiol 52: 445–450, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 52: 438–444, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 158: 1974–1981, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Mortimore IL, Douglas NJ. Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med 156: 867–873, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Murray NP, McKenzie DK, Gorman RB, Gandevia SC, Butler JE. Reproducibility of the short-latency reflex inhibition to loading of human inspiratory muscles. Respir Physiol Neurobiol 162: 216–222, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Rechtschaffen A, Kales A. (Editors). A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, UCLA, 1968 [Google Scholar]
- 32. Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol 537: 251–265, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Sauerland EK, Mitchell SP. Electromyographic activity of the human genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc 35: 69–73, 1970 [PubMed] [Google Scholar]
- 35. Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol 520: 897–908, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol 73: 1058–1066, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol 70: 2574–2581, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 148: 597–605, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Wheatley JR, Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol 75: 2117–2124, 1993 [DOI] [PubMed] [Google Scholar]
- 40. White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 172: 1363–1370, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol 71: 488–497, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep 31: 525–533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 85: 908–920, 1998. [DOI] [PubMed] [Google Scholar]