Abstract
Murine reperfusion injury follows binding of specific IgM natural antibodies to neo-antigens exposed in ischemic tissue. Peptides that mimic the site of antibody binding in the injury prevent IgM binding when administered intravenously before reperfusion. To determine whether this pathogenic sequence is restricted to mice, we have tested the ability of the peptide to prevent reperfusion injury in a dissimilar species, the rat. Sprague-Dawley rats were subjected to 40 min of mesenteric ischemia followed by 180 min of reperfusion. The peptide mimic was administered intravenously prior to reperfusion. Gut injury was quantified using a scoring system based on the hematoxylin-and-eosin section. 125I-labeled albumin was used to assess local (gut) and remote (lung) injury. The macroscopic appearance of bowel from peptide-treated animals was less edematous and hemorrhagic. Microscopic analysis showed a significantly reduced injury score in peptide-treated animals. Permeability data indicated a significant reduction in local and remote injury in peptide-treated animals. The data demonstrate attenuation of rat gut microvillus injury, of gut edema, and of remote injury following mesenteric ischemia-reperfusion due to administration of an intravenous peptide mimic of a murine ischemia neo-antigen, indicating a second species uses a similar ischemia neo-antigen and corresponding natural antibody specificity to amplify reperfusion injury to the point of necrosis. This mechanism of inflammation is potentially applicable to higher species.
Keywords: ischemia/reperfusion injury
end-organ damage subsequent to an ischemic insult becomes evident after reperfusion and is the sum of permanent anoxic damage and the inflammatory response to damaged tissue (5). The syndrome of gastrointestinal ischemia and reperfusion injury (I/R), in particular, is associated with high mortality (70–90%) (10) and produces inflammation so severe that it becomes systemic with distinct pulmonary secondary injury. Serum complement activation is a significant component of the injury process, as treatment of rats prior to or at reperfusion with a complement antagonist resulted in attenuation of both local and remote injury (8), identical to effects seen in myocardial infarction models (16). This observation was extended to mice, a species in which complement pathways can be dissected using knockouts. C3 knockout mice showed reduced injury as did C4 knockouts (17), MBL knockouts (7, 19), and antibody-knockouts (RAG-/-) (17). Reconstitution of antibody knockouts using murine antibody fractions showed that only IgM from normal animals restored injury, presumably “natural” IgM (15, 18). B1 peritoneal lymphocytes are a known source of these IgM antibodies, and accordingly, mice with deficiencies of these cells but normal IgM levels were also protected from reperfusion injury (6, 12). Cloning of these cells led to identification of a very limited number of antibody specificities that initiated injury (3, 20), and then to identification of the antigens to which the IgM species bind as a result of injury (4, 21).
A highly conserved region of nonmuscle myosin heavy chain type II A and C is one of the antigens to which the pathogenic natural IgM binds and has led to the creation of 12-mer peptide mimics representing only the conserved region of the molecule. When given to mice intravenously at or just before reperfusion, this peptide mimeotope of the natural IgM binding site has been shown to reduce injury in murine models of skeletal (4), cardiac (M. C. Carroll, personal communication), and intestinal I/R (21).
The uncovering of this level of molecular specificity in the response to ischemia and reperfusion suggests that this mechanism of inflammatory triggering may not be confined to the mouse. In addition, the binding site of the pathogenic IgM in the hinge region of nonmuscle myosin heavy chain has a sequence that is highly conserved between microbes and mammals. We hypothesize that the mechanism is exactly conserved in additional species. Our direct testing in rats is presented below and appears to confirm our hypothesis.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (300–350 g) were purchased (Charles River Laboratories, Wilmington, MA). The protocol for this study was approved by the Harvard Medical School Institutional Animal Care and Use Committee. Animals in this study were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and those prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council [Department of Health, Education and Human Services, publication no. 85-23 (National Institutes of Health), revised 1985].
Intestinal I/R
The animals were anesthetized with pentobarbital sodium (50 mg/kg ip), and body temperature was maintained with warming pads and the closure of the abdomen between manipulations. A midline laparotomy was performed, and a microvascular clip (Roboz, Gaithersburg, MD) was applied to the origin of the superior mesenteric artery, taking care to exclude the superior mesenteric vein. Thirty-five minutes after occlusion, animals received either peptide (300 μg/300 μl saline/animal) or normal saline (300 μl/animal) via tail vein injection. This dose was a weight-based multiple of the effective dose in mice. At 40 min, the microvascular clip was removed, and reperfusion was confirmed by the presence of pulsations observed in the mesenteric vessels and a return of color to the bowel from its ischemic pallor. All animals received 1.0 ml intravenous normal saline fluid resuscitation. Three hours after clip removal, the animals were euthanized with an overdose of pentobarbital sodium (90 mg/kg), and blood was obtained by cardiac puncture. Ischemic segments of distal small bowel were harvested from all animals and irrigated with intraluminal normal saline solution. Sham animals underwent an identical procedure without microvascular clip application.
Peptides
N2.
12-amino acid synthetic peptide (New England Peptide, Gardner, MA) with the amino acid sequence of the hinge region of nonmuscle myosin heavy chain II (LMKNMDPLNDNV).
SkMM.
12-amino acid synthetic peptide (New England Peptide, Gardner, MA) with the amino acid sequence of the corresponding site from skeletal muscle myosin heavy chain (LDKNKDPLNETV).
Permeability Index
Gut.
Animals for permeability measurements received 1 μCi of 125I-labeled albumin (ICN, Irvine, Ca) in 0.3 ml of normal saline by tail vein injection 10 min before reperfusion. The permeability index (PI) was calculated from counting and weighing intestine and blood: (cpm/g of jejunal loop)/(cpm/g of blood). The intestinal segments were dried in a convection oven (Precision Scientific Group, Chicago, IL) at 90°C for 72 h and then reweighed to correct for blood cpm in the intestine.
Lung.
The lungs were lavaged at death with 10 ml of normal saline for a total bronchoalveolar lavage (BAL) return of 6 ml. This was weighed and counted. The PI was calculated as (cpm/g of BAL)/(cpm/g of blood).
Western Blot Analysis
Gut samples from each of the three groups were homogenized in equal volumes of 2% SDS buffer with enzyme inhibitors, chilled, and then centrifuged at 16,000 g for 10 min to collect the supernatant as tissue extract. Equal amounts of protein were then reduced and separated on 4–20% graduated polyacrylamide minigels and transferred to nitrocellulose membranes. The membranes were blocked in buffer containing 5% nonfat milk and 0.1% Tween-20 overnight and then incubated in HRP-conjugated goat anti-Rat IgM (Southern Biotech, Birmingham, AL), at a dilution of 1:10,000. Membranes were developed with an enhanced chemiluminescent Western blot analysis kit (Amersham Biosciences, Piscataway, NJ).
Histopathology
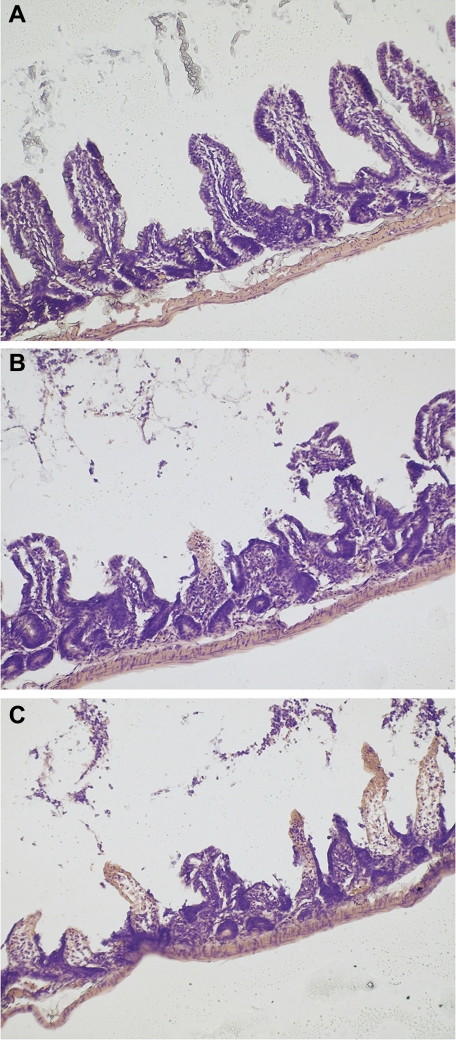
Paraffin sections of formaldehyde-fixed intestinal tissue were stained with hematoxylin and eosin and examined by light microscopy for injury. A modified injury scoring system was used on the basis of that of Williams et al. (17). Scores of 0, 1, or 2 were given to villi that were uninjured, partially injured, or severely injured, respectively. Partial injury was defined as any areas of denuded epithelium or partial shortening (<25%) of the villus. Severe injury was defined as more extensive areas of denuded epithelium, extreme shortening (>25%), or evidence of villus sloughing. Scores of 0, 1, or 2 were, therefore, assigned to each of 50 villi viewed under a high-power field. Six random high-power fields were viewed per animal, and the average was taken. A score of 0–100 was, therefore, obtained for each animal, ranging from completely normal intact villi (0) to maximally injured villi (100). (Fig. 1).
Fig. 1.
Photomicrographs of hematoxylin-and-eoesin (H&E) stained rat terminal ileum, at ×40 magnification, as examples of quantitative intestinal injury scoring system for intestinal reperfusion injury. 0, no injury (A); 1, moderate injury with villus shortening (B); 2, severe injury with villus disruption and completely absent villi due to sloughing (C).
Immunohistochemistry Analysis
IgM and C3c staining was performed on paraformaldehyde-fixed serial sections of distal jejunum. All slides were baked at 60°C for 1 h before deparaffinization and rehydration. For IgM staining (1:500) HRP-labeled goat anti-rat IgM (Southern Biotech, Birmingham, AL) was used. For C3, sections were stained with (1:100) Rabbit anti-human C3c (Dako, Carpinteria, CA) followed by (1:500) biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) then (1:1,000) avidin-HRP (eBioscience, San Diego, CA). The slides were developed with 3,3′-diaminobenzidine (Sigma, St. Louis, MO), according to the standard protocol.
Statistical Analysis
Data are presented as means ± SE. Comparisons between groups were performed by one-way ANOVA, and when significant difference was found, Student's t-test with the Bonferroni correction for multiple comparisons was applied.
RESULTS
The macroscopic appearance of the bowel provided a striking example of the protective effect of peptide administration (Fig. 2). Similar regions of terminal small bowel were used for macroscopic and microscopic injury evaluation. Gut from animals that did not receive N2 was edematous, with regions of patchy discoloration and evidence of intraluminal hemorrhage. In contrast, administration of intravenous N2 just prior to reperfusion revealed a more normal looking appearance to the bowel, generally more comparable to the sham animal.
Fig. 2.
Representative gross images of rat terminal ileum from sham (A), ischemia and reperfusion injury (I/R) injured (B), and a 12-amino acid synthetic peptide (N2)-treated I/R injured animals (C) following 40 min of ischemia and 3 h of reperfusion. N2-treated gut from I/R injured animals is slightly discolored compared with sham injured animals. Gut from I/R injured but untreated animals shows gross hemorrhagic discoloration.
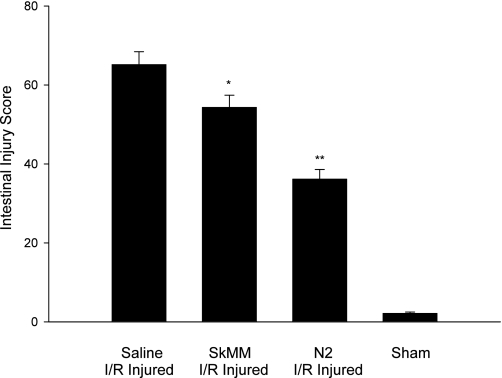
On microscopy, using the gut injury scoring system to grade six random fields, we observed that there was significant protection against mucosal injury in the N2 peptide-treated animal group, compared with I/R injured, untreated animals (Fig. 3). Rat gut injury score was 65.2 ± 11.3 (n = 6) in I/R injured animals. For N2-treated animals, the injury score was 36.2 ± 8.4 (n = 6) (P < 0.01 compared with injured, untreated animals). Animals with sham injury had an injury score 2.1 ± 1.1 (n = 6). The animals that received control peptide (SkMM) scored 54.3 ± 10.6 (n = 6) (P < 0.05), a decrease in injury with respect to the saline group, but not as significant as in the N2 treatment group. SkMM was less protective than N2 (P < 0.01).
Fig. 3.
Microscopic intestinal injury score in I/R injured vs. N2-treated, I/R injured vs. sham injured animals. Six animals in each group, score is a mean of 6 random high-power fields. A significant reduction in intestinal injury was produced by N2 pretreatment (**P < 0.01), and to a lesser degree by the related peptide for skeletal muscle myosin (SkMM) (*P < 0.05).
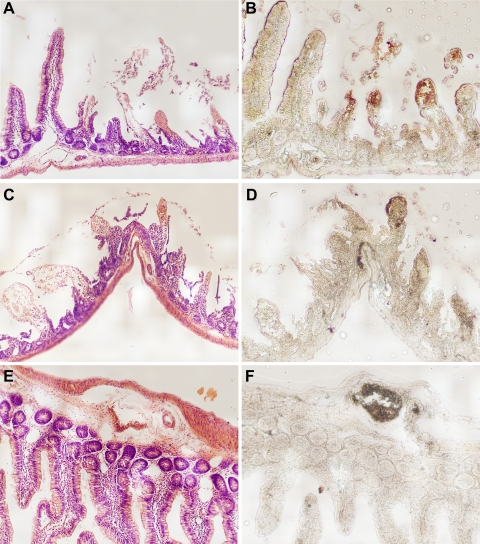
Immunohistochemistry was used to demonstrate the contribution of complement and the classical pathway to the pathogenesis of rat I/R injury. Representative slides identify IgM and C3c as deposited in the injured villi (Fig. 4). C3c is a stable cleavage product of C3b, covalently bound to target surfaces as a result of complement activation, only. Notably, villi that were extremely injured or sloughed off were so disintegrated that the locus of IgM or C3c staining could not be seen, so areas of lesser injury were selected, instead of the random sections used for the intestinal scoring system above. Both IgM- and C3-dependent staining were seen at the tips of injured villi. The control slide used sham-injured tissue, and IgM staining was only seen within the serum of the normal vasculature. C3c cannot be demonstrated, as this protein is generated only by complement activation. Use of this technique to compare the degree of deposition of C3c and IgM between I/R injured animals vs. N2-treated I/R injured animals proved impossible as injured villi in injured, untreated animals were not visible for quantitation. Instead, whole tissue homogenates were made for Western blot analysis to compare relative amounts of tissue IgM content. This demonstrated a marked increase in intestinal IgM content in I/R injured animals compared with N2-treated I/R injured animals or sham injured animals. (Fig. 5).
Fig. 4.
Intestinal I/R injury as analyzed by immunohistochemistry for C3c and IgM deposition. A, C, and E: H&E stains. B, D, and F: immunoperoxidase stains of adjacent sections. A and B: adjacent sections of I/R-injured intestine stained with H&E and for IgM deposition, respectively. C and D: adjacent sections of I/R-injured intestine stained with H&E and for C3c deposition, respectively. E and F: adjacent sections of sham-injured intestine stained with H&E and for IgM deposition, respectively. A–D: moderately injured intestine containing intact villi (grade 0) with no C3c or IgM, grade 1 villi with both C3c and IgM deposition, and grade 2 villi that are completely absent and unable to be assessed. E and F: uninjured intestine that has grade 0 villi and IgM staining only within the serum of normal blood vessels. C3c staining is not seen, as no complement activation has taken place.
Fig. 5.
Western blot of SDS-PAGE (reduced) of whole intestinal homogenates, stained for peroxidase activity after reaction with HRP-conjugated goat anti-rat IgM. The 90-kDa band of the IgM heavy chain is prominent in the homogenate from I/R injured animals, which is reduced in N2-treated I/R injured animals, and absent in sham-treated animals. The 25-kDa band corresponding to the IgM light chain is poorly visualized with this antibody. This antibody also recognizes murine albumin (65 kDa), showing that there is more material loaded in the N2 treatment lane than the other lanes.
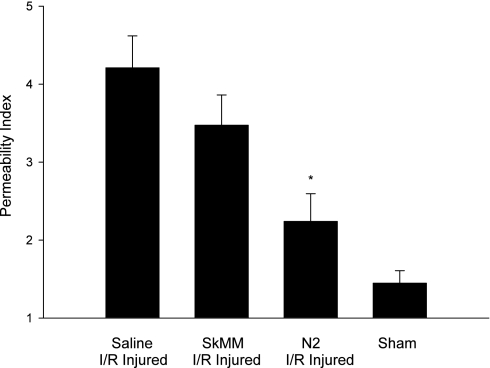
Another manifestation of the local effects of I/R injury is increased tissue edema. There was a statistically significant decrease in the gut PI of N2 peptide-treated animals from I/R injured saline-treated animals: N2 treated rat gut PI = 2.240 ± 0.355 (n = 6), compared with PI = 4.211 ± 0.408 (n = 6) for I/R injured saline-treated animals (P < 0.01). Treatment with control peptide SkMM resulted in PI = 3.474 ± 0.387 (n = 6), statistically different from the N2 group (P < 0.05) but not statistically significant when compared with the saline group. Sham-injured rat gut PI = 1.448 ± 0.159 (n = 6). (Fig. 6).
Fig. 6.
Intestinal permeability as determined by radio-labeled albumin extravasation. Intestinal permeability index in N2-treated, I/R injured animals is significantly less than in injured, untreated animals (*P < 0.01). There was no significant decrease seen with the control peptide, SkMM. Intestine from N2-treated animals still demonstrated more edema than that of sham-injured animals.
Gut I/R injury typically produces a pronounced secondary lung injury. Bronchoalveolar lavage was performed to assess lung extravasation of radio-labeled albumin, which is a reflection of pulmonary microvascular integrity. This technique showed a statistically significant decrease in pulmonary permeability index (PI) in the I/R injured, N2 peptide-treated group PI = 1.197 ± 0.194 (n = 6) compared with the I/R injured, untreated rat lung PI = 2.398 ± 0.286 (n = 6), P < 0.05. The control peptide SkMM was found to have a PI = 1.751 ± 0.136 (n = 6), significantly different from N2 (P < 0.05), but not statistically different from the saline group. The PI of sham-injured rats was 0.894 ± 0.11 (n = 6). (Fig. 7).
Fig. 7.
Pulmonary permeability index as determined by extravasation of radio-labeled albumin into bronchial-alveolar lavage. Pulmonary permeability index in N2-treated, I/R injured animals is significantly less than in injured, untreated animals (*P < 0.05). There was no significant decrease seen with the control peptide, SkMM. Permeability index in N2-treated animals was greater than in sham-injured animals.
DISCUSSION
In this model of rat gut I/R injury, we provide evidence for the attenuation of rat I/R injury following administration of N2, a 12-mer peptide that is the highly conserved binding site on the injury neo-antigen expressed during ischemia in mice and to which pathogenic IgM binds. This drug was given after ischemia and just prior to reperfusion, similar to clinically encountered circumstances.
When similar regions of rat ileum were compared following I/R, a dramatic macroscopic difference was noted (Fig. 2). Untreated I/R rat gut appeared edematous and necrotic with areas of intraluminal hemorrhage, an appearance shared only to a small degree by I/R-injured N2-treated intestine and not at all by sham injured intestine. These findings were an indication of 1) the gross effects of I/R injury with increased vascular permeability, vascular disruption, and necrosis and 2) the ability of N2 peptide to prevent this.
For N2 peptide to have this effect in the rat, there must be both pathogenic antibody specificity directed to N2, the same neo-antigen (nonmuscle myosin heavy chain) and the same expression of this antigen occurring after ischemia. We confirmed this gross finding in several ways. First, conventional histological quantification of injury at the level of the intestinal villi showed that significant protection from I/R-induced mucosal injury was conferred by N2 peptide treatment (Fig. 3). Second, we attempted to use immunohistochemistry and demonstrated deposition of both IgM and C3c on the injured villi (Fig. 4), implicating activation of the same antibody and serum complement components that had been seen in previous models of I/R injury (15, 18). The use of C3c in this analysis allows identification of products that can result only from complement activation and not as aggregations of necrotic tissue or blood. But, the most injured villi simply slough and are unavailable to make a comparison of treated to untreated animals. Therefore, Western blots of intestinal tissue homogenates were compared for the presence of IgM. IgM was dramatically increased in I/R injured tissue and, in turn, reduced in N2-treated I/R injured tissue, even with the N2 lane containing relatively more tissue homogenate (Fig. 5). This result indicates not only lessened inflammatory activation in the N2-treated animals but also that N2 peptide was acting by its anticipated mechanism: interference with natural IgM binding to ischemic tissue (4, 21).
Third, tissue edema and vascular permeability were used as an independent method to assess the effect of N2 peptide. The gut lumen was flushed with saline to remove any radioalbumin that accumulated as a result of mucosal necrosis or bleeding. Additionally, the superior mesenteric vein was specifically excluded from clamping to remove vasodilatation as a cause of radioalbumin retention, a feature also not identified on H&E. The PI assessment in the gut itself confirmed that animals treated with N2 peptide were relatively protected from injury (Fig. 6). The lungs, which are a remote target for I/R injury, were found to be relatively protected from injury as well (Fig. 7).
Thus, treatment of rats with N2 peptide 5 min prior to reperfusion substantially attenuated the effects of ischemia and reperfusion injury, namely, gross alterations in gut appearance, mucosal villus sloughing, intestinal deposition of IgM, and both gut and pulmonary permeability edema. We do not know whether a higher dose or an altered time of dosing would have reduced injury parameters to nil. We suspect not, as this N2 dose is similar to that seen in mice (4, 21). This indicates to us that the sum total of measurable ischemia and reperfusion injury is both the intrinsic ischemic injury to the cell and the subsequent invoked inflammatory reaction, which N2 appears to largely prevent. A homologous peptide derived from the hinge region of skeletal muscle myosin and very similar to N2 was used to test the specificity of the rat pathogenic antibody. The minor activity of SkMM on one of the three quantitative measures of injury confirms the specificity of rat pathogenic IgM for N2.
N2 peptide is a 12-amino acid peptide that contains the exact sequence of the hinge region between the major globular subunits of nonmuscle myosin heavy chain IIC, a protein that appears to be the key ischemic injury antigen (4, 21). Mice have a preformed, naturally circulating IgM that recognizes this antigen when it is exposed in the course of ischemic or thermal (14) injury and produces complement and mast cell (1) activation with eventual excessive necrosis of involved tissue. The biology of expression of nonmuscle myosin heavy chain in response to injury is not understood at present. It is proposed that N2 peptide is taken up within the binding site of pathogenic natural IgM for nonmuscle myosin, thereby competitively depleting the amount of available pathogenic natural antibody to drive a reperfusion injury. Given the specific antibodies and antigens involved, it would not be surprising to discover that the same principle of an injury neo-antigen and corresponding pathogenic antibody might exist in another species, particularly given the absolute conservation of the primary structure of the hinge region of nonmuscle myosin heavy chain from yeast to humans. What is unexpected, though, is that the specificity of the binding site in pathogenic IgM has not changed, as antibodies are viewed as highly changeable since minor differences in primary structure have a profound effect on binding affinity. For N2 to have any effect in the rat, the rat has to have the identical injury antigen, the identical pathogenic IgM, and the same mechanism of amplifying a reperfusion injury. To argue that N2 peptide is active through a different mechanism ignores the three following features. First, a small peptide is instantaneously degraded in serum, unless complexed within a protective protein pocket, such as within the IgM that recognizes N2. Second, the spectrum of activity in rats is identical to what we have seen in mice. Third, we have provided evidence of reduced IgM binding after N2 administration. Our assessments of the concentration of pathogenic IgM in mice suggest that it is 1–10% of the circulating IgM. One final point concerns the structure of IgM, which is a pentamer with 10 antigen binding sites. For it to activate complement, the shape of the protein must be altered by the binding of the majority of its sites to the target. Thus, the ability of the N2 peptide to prevent complement activation by this type of antibody may be augmented, as only a few of the 10 binding sites would have to be occupied by N2 to disrupt the complement-activating conformation.
It may not seem significant that a drug developed for mouse innate immunity interferes with the rat system. While earlier phylogenetic studies placed the mouse and rat in the same phylum, rodentia, their similarity was largely attributed to shared anatomical features (13). Their genetic similarity or disparity has been more recently elucidated in conjunction with genomic study. Primarily of interest are the sizes of the different genomes, human 2.9 gigabases (Gb), rat 2.75 Gb and mouse 2.6 Gb. (2, 11). Furthermore, only about 30% of rat euchromatin has been shown to align with the mouse, a considerable portion of which is defined as rodent-specific repeats. The majority of the remainder is rat specific, such as genes that are involved in immunity, chemosensitization, and proteolysis. By this more modern analysis, rats are as different from mice as they are from humans. Since there is conservation of the injured tissue binding site, the antibody binding site, and mechanism of injury between two different species, we hypothesize administration of peptide to a higher species might also result in attenuation of I/R injury, both local and systemic.
These rat studies extend our prior work in mice to show that this agent that prevents local reperfusion injury in the gut also prevents secondary injury in the lungs, an issue that is difficult to demonstrate in mice due to small tracheobronchial tree volume. The bigger issue is the transition of a novel biology up the phylogenetic tree to the point that it is reasonable to test its existence in humans, an undertaking associated with formidable expense. There have been innumerable cases in the past 20 years of novel and promising murine biologic agents that have translated ineffectively in humans, presumably because of more redundancy in human proinflammatory systems. Here, we are proposing to use rats as intermediate species based on genetic distance from mice. Encouraging rat data from mouse biology can then lead to large animal experiments, prior to human introduction, rather than the more common path to failure of using animals for toxicology. What is needed is an agreed-upon sequence of animal species to test, after which efficacy in humans can be assumed.
GRANTS
This article is supported by U.S. Public Health Service Grant P50 GM52585 and the Trauma Research Foundation.
DISCLOSURES
Dr. Moore is a cofounder of DecImmune, Inc., Boston, MA.
REFERENCES
- 1.Abonia JP, Friend DS, Austen WG, Jr, Moore FD, Jr, Carroll MC, Afnan J, Chan R, Humbles A, Gerard C, Knight P, Kanaoka Y, Yasuda S, Morokawa Austen KF N, Stevens RL, Gurish MF. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol 174: 7285–7291, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins RM, Gelke EL, Rowe D, Honeycutt RL. Molecular phylogeny and divergence time estimates for major rodent groups: evidence from multiple genes. Mol Biol Evol 18: 777–791, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Austen WG, Jr, Zhang M, Chan R, Friend DM, Hechtman HB, Carroll MC, Moore FD., Jr Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery 136: 401–406, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr Attenuation of skeletal muscle reperfusion injury with iv 12 amino acid peptides that bind to pathogenic IgM. Surgery 139: 236–243, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Cotran RS. Robbins Pathological Basis of Disease St. Louis, MO: Saunders, 1999, p. 7–12 [Google Scholar]
- 6.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol 169: 2126–2133, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol 174: 6373–6380, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor Type 1 ameliorates the local and remote organ injury following intestinal ischemia-reperfusion injury in the rat. J Immunol 149: 1723–1728, 1992 [PubMed] [Google Scholar]
- 9.Hill JH, Ward PA. The phylogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med 133: 885–900, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence JB. Mesenteric Vascular Disease. Current Diagnosis and Treatment in Gastroenterology, edited by Friedmanv SL. New York: McGraw-Hill, Section I.9, 2003 [Google Scholar]
- 11.Rat Genome Sequencing Project Consortium. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428: 493–521, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Reid R, Woodcock S, Shimabukuro-Vornhagen A, Austen WG, Jr, Kobzik L, Zhang M, Hechtman HB, Moore FD, Jr, Carroll MC. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol 169: 5433–5440, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proc Natl Acad Sci 100: 1056–1061, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suber F, Carroll MC, Moore FD., Jr Innate response to self-antigen significantly exacerbates burn wound depth. Proc Natl Acad Sci 104: 3973–3977, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 183: 2343–2348, 1996. 8642343 [Google Scholar]
- 16.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249: 146–151, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Williams J, Pechet TV, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol 86: 938–942, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Williams JP, Pechet TTV, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Phys 86: 938–942, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Takahashiu K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RAB, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol 177: 4727–4734, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia-reperfusion injury. Proc Natl Acad Sci USA 101: 3886–3891, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–52, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]