Abstract
Indirect whole room calorimetry is commonly used in studies of human metabolism. These calorimeters can be configured as either push or pull systems. A major obstacle to accurately calculating gas exchange rates in a pull system is that the excurrent flow rate is increased above the incurrent flow rate, because the organism produces water vapor, which also dilutes the concentrations of respiratory gasses in the excurrent sample. A common approach to this problem is to dry the excurrent gasses prior to measurement, but if drying is incomplete, large errors in the calculated oxygen consumption will result. The other major potential source of error is fluctuations in the concentration of O2 and CO2 in the incurrent airstream. We describe a novel approach to measuring gas exchange using a pull-type whole room indirect calorimeter. Relative humidity and temperature of the incurrent and excurrent airstreams are measured continuously using high-precision, relative humidity and temperature sensors, permitting accurate measurement of water vapor pressure. The excurrent flow rates are then adjusted to eliminate the flow contribution from water vapor, and respiratory gas concentrations are adjusted to eliminate the effect of water vapor dilution. In addition, a novel switching approach is used that permits constant, uninterrupted measurement of the excurrent airstream while allowing frequent measurements of the incurrent airstream. To demonstrate the accuracy of this approach, we present the results of validation trials compared with our existing system and metabolic carts, as well as the results of standard propane combustion tests.
Keywords: indirect calorimetry, oxygen consumption, human energy expenditure, substrate oxidation
indirect calorimetry is the measurement of respiratory gas exchange [e.g., oxygen consumption (V̇o2) and carbon dioxide production (V̇co2)] to determine the metabolic rate of an organism. V̇o2 and V̇co2 are used to calculate the respiratory quotient (RQ = V̇co2/V̇o2), which provides a relative measurement of fat and carbohydrate oxidation. Indirect calorimeters typically use flow-through respirometry to measure the amount of a given gas produced or consumed by the organism. Flow-through respirometry is based on the principle that the amount of gas produced or consumed by an organism can be calculated from the concentrations of the gases in the incurrent and excurrent airstreams and the associated flow rates. For example, the equation to calculate V̇o2 using flow-through respirometry is V̇o2 = FRi(FiO2) − FRe(FeO2), where FRi and FRe are the flow rates of the incurrent and excurrent airstreams, and FiO2 and FeO2 are the fractional concentrations of oxygen in the incurrent and excurrent airstreams. FRi does not necessarily equal FRe, and understanding this inequality has important implications for understanding the potential sources of error in flow-through respirometry calculations.
The earliest human flow-through calorimeters (respiration chambers) were developed by Pettenkoffer (14) and Atwater and colleagues (1, 2). Since then, many other systems have been developed (6, 7, 9, 12, 13, 15–17, 20) and have been used to study human physiology. Most systems in use today can be broadly classified as either push (e.g., air is pushed through the respirometry chamber at a known flow rate) or pull (air is pulled through the respirometry chamber at a known flow rate) systems. Most room calorimetry systems in operation today, particularly in the United States, are pull-type systems (9, 13, 20), although a notable exception is the calorimeter located at Baylor College of Medicine (12), which is a hybrid push-pull closed circuit system, i.e., a pull system with a controlled, minimal, inward pressure gradient. A major obstacle to the accurate calculation of gas exchange rates in a pull system is that excurrent flow rates are increased above the incurrent flow rate (FiO2), because the organism produces water vapor and CO2; the added partial pressures of CO2 and water vapor increase the total pressure, and therefore flow rate of the excurrent airstream. At the same time, CO2 produced by the organism reduces the partial pressure of O2 (i.e., because CO2 is added to the excurrent air, the fractional concentration of O2 in the excurrent air will be reduced). All of these factors must be accounted for in the gas calculations. Although accurately measuring CO2 is not difficult, obtaining an accurate measurement of water vapor pressure so that its dilution effect can be corrected can be challenging. To circumvent this problem, most room calorimetry systems use a gas drying system to reduce water vapor in the excurrent air sample prior to analysis (12, 13, 20). However, if the sample is not completely dry, the measured oxygen concentration will be artificially low because of the dilution effect of water vapor pressure. Importantly, because most systems do not monitor whether water vapor has been completely removed from the airstream, errors can go undetected.
Aside from the problems associated with the dilution effect of water vapor pressure, room calorimeters are prone to two other major sources of error. The first, and generally larger, source of error, particularly with oxygen analyzers, is analyzer drift. Differential gas analyzers reduce, but do not eliminate, drift. Correcting for drift requires periodic recalibration of the analyzer to a standard gas (i.e., baselining) or the incurrent air (8). The latter can be used to monitor drift of the oxygen analyzer because O2 concentrations in the atmosphere are quite stable after adjusting for the variable gas components (water vapor and CO2). Thus, it is usually reasonable to assume that the fractional concentration of dry, CO2-free incurrent air is 0.2094 (11, 21), and analyzer drift can be corrected on this basis. In the case of CO2, analyzer drift is less of a problem, but accuracy is affected by the second source of error, which is variability in the CO2 concentration of the incurrent air (e.g., due to pollution, traffic levels, day/night cycles, and other environmental influences). Ideally, the gas concentrations in the incurrent airstreams should be periodically measured. Correcting either of the above sources of error requires switching the analyzer chains between the incurrent and excurrent airstreams. This introduces discontinuities in the metabolic data, which may explain why this approach is not commonly used. Most calorimeters are operated under the assumption that the concentrations of O2 and CO2 in the incurrent airstream are constant and do not adjust for analyzer drift or variability in FiCO2.
We operated a pull-type room calorimeter at the University of Colorado Denver from 1993 to 2006 that was designed after the calorimeters located at the National Institutes of Health Facility in Phoenix, AZ and Vanderbilt University in Nashville, TN (20). The source of incurrent air was recirculated building air, which proved to be very problematic because of variability in the incurrent CO2 concentration. In 2007, we moved to a new location, and a new pull-type calorimeter was constructed. In this new setup, the problem associated with recycled building air was circumvented by drawing incurrent air directly from the environment. However, this introduced an unanticipated problem related to the unique environmental conditions in Denver (elevation ∼5,280 ft), which include consistently low barometric pressures coupled with exceptionally dry ambient air, particularly in the winter months, when dew points are often below −10°C.
The purpose of this paper is to describe a new flow-through respirometry system (Sable Systems International, Las Vegas, NV) for indirect room calorimetry that minimizes errors related to the incomplete removal of water vapor, analyzer drift, and variability in gas concentrations of incurrent air. To evaluate the accuracy of the Sable Systems flow-through respirometry system (Sable system), we conducted extensive validation trials using a variety of approaches. First, we performed simultaneous, parallel measurements with the new Sable system and the existing system during propane combustion tests when the dew point in the incurrent air was both above and below 0°C. Second, we measured V̇o2, V̇co2, energy expenditure (EE), and RQ during several different activities performed in the calorimeter and compared the measurements obtained using both the Sable and the existing systems to those obtained using a metabolic cart. Third, we performed simultaneous, parallel 24-h measurements using the Sable and existing systems. Finally, we tested the dynamic response of the 24-h RQ measurements by studying human subjects under conditions known to induce dramatic changes in RQ (i.e., manipulation of macronutrient intake).
METHODS
Institutional Approval
The human study protocols included in this analysis were approved by the Colorado Multiple Institutional Review Board and the Scientific Advisory Board of the Clinical Translation Research Center (CTRC) at the University of Colorado Denver, Denver CO. All subjects provided informed written consent.
Description of the Room Calorimeter
The calorimeter room measures ∼3.6 × 3.3 × 2.4 m (length × width × height), with a total volume of ∼28.5 m3. The room is located in the CTRC inpatient unit on the 12th (top) floor of the University of Colorado Hospital. Incurrent air is drawn from the external environment above the building into a storage buffer above the calorimeter. The buffer was intended to ensure stability of the O2 and CO2 concentration of the incurrent air, and is kept at a slight positive pressure relative to the surrounding environment. The volume of the buffer is ∼20 m3 (∼1.1 × 3.2 × 5.8 m). Temperature of the air in the buffer is controlled at 23.9°C, but is not otherwise conditioned. Temperature (22.0 ± 1.0°C) and relative humidity (∼30–50%) inside the calorimeter room are controlled using a ceiling-mounted fan coil unit (model TSC-08; McQuay International, Minneapolis, MN) (Fig. 1). The fan coil unit is also used to perform mixing of the air within the room; airflow within the room is recirculated at a rate of ∼20 m3/min. At this rate, the volume of air inside the calorimeter is mixed approximately every 2 min, which ensures fast response time for the measurement of V̇o2 and V̇co2. Temperature, barometric pressure, and relative humidity inside the room are measured continuously using a dew point hygrometer (General Eastern Optica Dewpoint Meter; GE Infrastructure Sensing, Billerica, MA). Technical specifications and descriptions of the Sable and existing systems are contained in the appendix.
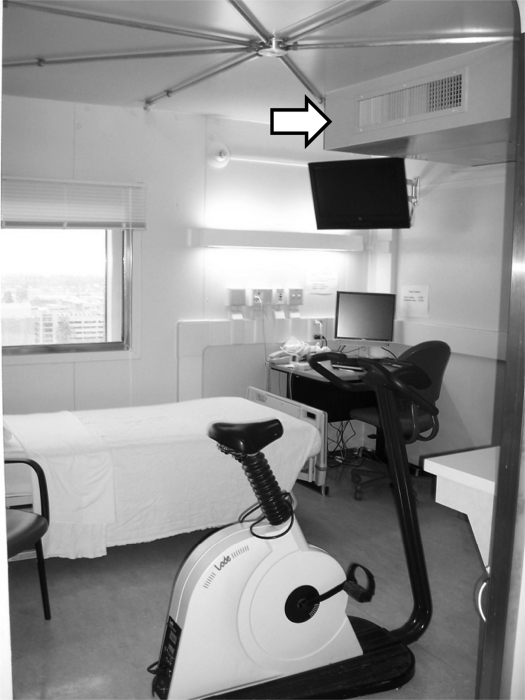
Fig. 1.
The room calorimeter located at the University of Colorado Denver. The fan coil unit is indicated by the solid arrow. The pipes in the spider configuration on the ceiling are the sampling ports.
Installation of the Sable system was performed such that the existing system and Sable system could be run in parallel. The existing system continued to use its mass flow meter to measure the excurrent airflow pulled into the FlowKit-500 mass flow generator (80 l/min), while the Sable system used the flow reading obtained from its own mass flow generator. Samples of excurrent air for both systems were drawn downstream from the Sable system's mass flow generator. In this manner, it was possible to obtain simultaneous, noninterfering measurements from both systems.
Calculations
In the case of the Sable system, the rates of V̇o2 and V̇co2 were calculated using equations for gaseous exchange in open-circuit indirect calorimetry derived by Brown et al. (4): V̇o2 = FRe [(FiO2 − FeO2) − FiO2 * (FeCO2 − FiCO2)]/ (1 − FiO2) and V̇co2 = FRe [(FeCO2 − FiCO2) + FiCO2 * (FiO2 − FeO2)]/(1 + FiCO2), where FRe is the excurrent flow rate, corrected for the dilution effect of water vapor pressure, Fi is the fractional concentration of a gas in the incurrent airstream, and Fe is the fractional concentration of a gas in the excurrent airstream. The equations for the existing system have been previously described (13).
Validation Approach
To demonstrate the accuracy of this new approach, we performed several different tests.
Propane tests.
Verification of the accuracy in measuring V̇o2, V̇co2, EE, and RQ is routinely performed based on the recovery of O2 and CO2 while propane is burned. We expect all variables to be >98% of anticipated values. We performed simultaneous propane tests with both the Sable and the existing systems when the dew point was both above and below 1°C to demonstrate the effect of ineffective drying and the dilution effect of water vapor pressure.
Validation against metabolic cart.
V̇o2, V̇co2, EE, and RQ were measured during three different activities (resting, bench stepping, stationary cycling) using a metabolic cart (TrueOne 2400; ParvoMedics, Sandy, UT). Measurements were obtained on six individuals (2 male, 4 females). Subjects arrived in the CTRC at ∼ 7:00 AM following an overnight fast and having abstained from any strenuous exercise for at least 24 h. Subjects rested quietly for 30 min, after which time the resting metabolic rate (RMR) was measured for 15 min using the ventilated hood technique. The final 10 min of the measurement were averaged to determine the RMR. Subjects then performed 20 min of bench stepping at a cadence of 72 steps/min. Cadence was controlled using an auditory metronome. Respiratory gas exchange was measured during minutes 1–10 and 16–20, and minutes 5–10 and 16–20 were averaged to determine steady-state V̇o2, V̇co2, EE, and RQ. Finally, subjects performed 30 min of stationary cycling at a workload corresponding to ∼ 55% of age-predicted maximal heart rate. Respiratory gas exchange was measured during minutes 1–10, 16–20, and 26–30, and minutes 5–10, 16–20, and 26–30 were averaged to determine steady-state V̇o2, V̇co2, EE, and RQ. During the stepping and cycling activities, respiratory gas exchange was measured using a mouthpiece with a nonrebreathing apparatus. On a separate day (within 1–2 days), these same activities were performed in the room calorimeter with simultaneous measurements obtained using the existing system and the new Sable system. Subjects arrived in the CTRC at ∼ 7:30 AM following an overnight fast and having abstained from strenuous exercise for at least 24 h. Subjects entered the calorimeter at ∼ 8:00 AM and remained at rest, but awake, for the first 60 min while lying supine. Because minute-to-minute variability is greater with the room calorimeter compared with a metabolic cart, particularly during resting activities, the last 30 min of this period was used to represent RMR. Subjects then sat quietly, either watching TV or using the internet. After 30 min, subjects performed 20 min of bench stepping at the same cadence as before. After another 30-min quiet period, subjects performed 30 min of stationary cycling, followed by another 60-min rest period before exiting the calorimeter. EE, V̇o2, V̇co2, and RQ measured by the two room calorimeter systems and metabolic cart were compared during the separate activities. Steady-state values for bench stepping and cycling were the averages of minutes 5–20 and 5–30, respectively. EE, V̇o2, V̇co2, and RQ measured by the two room calorimeter systems over the entire 4-h measurement period were also compared.
Simultaneous 24-h measurements were obtained August through October 2009 during on-going study visits in our room calorimeter. We obtained 24 simultaneous measurements during this period from several different research study protocols.
We studied two individuals, both consuming a low-fat (20% fat, 65% carbohydrate, 15% protein) or high-fat (50% fat, 35% carbohydrate, 15% protein) diet. Energy content of the diets was identical, only the macronutrient composition of the diet varied. This manipulation in macronutrient intake would be expected to produce pronounced differences in 24-h RQ (18); the measured RQ would be expected to be close to the food quotient of the low-fat and high-fat diets (0.914 and 0.8270, respectively). Subjects consumed the diets for 3 days prior to the calorimeter study day and were studied in the room calorimeter on the fourth day, while still consuming the same diet.
Statistical Analysis
Differences in all outcomes between systems were determined using repeated-measures ANOVA. Significance for all tests was set at P = 0.05. Data are presented as means ± SE unless otherwise specified.
RESULTS
Propane Tests
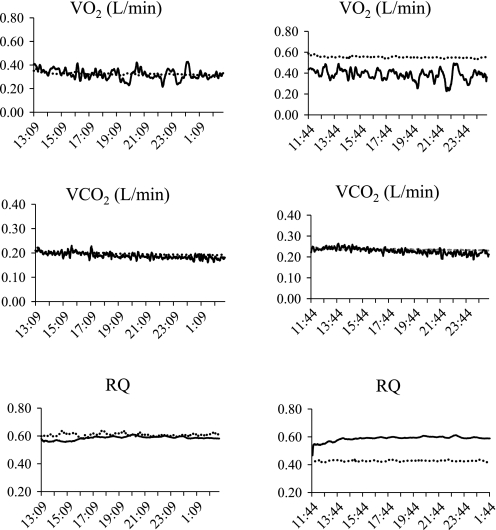
Results of two separate propane tests are listed in Table 1. The first test was conducted when the dew point of the incurrent air was >1°C throughout the entire test. Both systems provided >98% of expected recovery of V̇o2 and V̇co2, calculated EE and RQ were both within 2% of expected, and there was close agreement between systems throughout the test (Fig. 2). The second test was conducted on a day when the dew point of the incurrent airstream was below 1°C. V̇co2 was still >98% of expected values with both systems. However, V̇o2 was ∼100% of expected values with the Sable system, but 141% of expected values with the existing system. As a result, the EE calculated with the existing system was 135%, but RQ was only 71%, of the expected value. V̇co2 tracked very closely over the entire measurement period with both systems, but there was an obvious divergence in measured V̇o2 and RQ.
Table 1.
Results of propane test with positive and negative ambient dew points
| Positive Dew Point |
Negative Dew Point |
|||||
|---|---|---|---|---|---|---|
| Expected | Existing System | Sable System | Expected | Existing System | Sable System | |
| V̇o2, liters | 374.4 | 372.1 (99.4) | 370.4 (98.9) | 322.2 | 456.7 (141.7) | 322.7 (100.1) |
| V̇co2, liters | 224.6 | 228.0 (101.4) | 218.0 (97.0) | 193.4 | 195.1 (100.9) | 191.3 (98.9) |
| EE, kcal | 1,724.0 | 1,718.5 (99.7) | 1,700.8 (98.7) | 1,483.4 | 2,105.7 (135.9) | 1,483.4 (99.9) |
| RQ | 0.6000 | 0.6130 (102.2) | 0.5859 (97.7) | 0.6000 | 0.4272 (71.2) | 0.5879 (97.9) |
During the positive dew point test, 147 g of propane was combusted over 1,168 min (∼0.13 g/min), and during the negative dew point test, 126 g was combusted over 827 min (∼0.15 g/min). Numbers in parentheses indicate %expected value. V̇o2, oxygen consumption; V̇co2, carbon dioxide production; EE, energy expenditure; RQ, respiratory quotient.
Fig. 2.
Concurrent oxygen consumption (V̇o2, l/min; top), carbon dioxide production (V̇co2, l/min; middle), and respiratory quotient (RQ; bottom) measured during two separate propane tests with the existing system (dashed line) and Sable system (solid line). Graphs were obtained on a day with a positive (left) and a negative (right) dew point in the incurrent airstream.
Validation with the Metabolic Cart
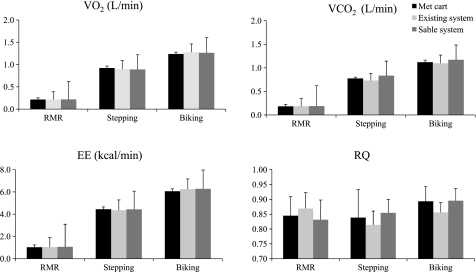
At rest and during stepping and cycling, V̇o2, V̇co2, EE, and RQ measured by the metabolic cart did not differ with measurements obtained by either of the room calorimeter systems (Fig. 3). It is interesting to note the RQ response. RQ is lowest during resting activities (reflecting a higher use of fat as a substrate) but increases during even light activities, such as stepping, and will further increase with increasing exercise intensity. Increases in RQ from rest to stepping and then cycling were only detected with the Sable system. Over the entire 4-h measurement period, V̇o2 measured by the existing calorimeter was significantly higher than that measured by the Sable system (Table 2). However, the magnitude of this difference was small (∼5 liters), and the measurements were significantly correlated. Likewise, total EE measured by the existing system was significantly higher than with the Sable system (average difference ∼17 kcal), but the measurements were highly correlated. In contrast, V̇co2 measured by the existing system was significantly lower than that measured by the Sable system, but again the magnitude of difference was small (∼2 liters) and the measurements were highly significantly correlated (r = 0.99). Finally, average RQ measured by the existing system (0.84 ± 0.05) was lower (P = 0.05) than that measured by the Sable system (0.87 ± 0.04). The correlation between the existing and Sable systems for RQ was r = 0.89.
Fig. 3.
Average V̇o2 (l/min), V̇co2 (l/min), energy expenditure (EE; kcal/min), and RQ measured in 6 individuals at rest [resting metabolic rate (RMR)] and during bench stepping and cycling activities. Values are means ± SD.
Table 2.
Calorimeter results at 4 h
| Existing System | Sable System | P | r | |
|---|---|---|---|---|
| V̇o2, liters | 110.6 ± 22.6 | 105.4 ± 20.4 | <0.01 | 0.99 |
| V̇co2, liters | 92.6 ± 18.0 | 95.5 ± 18.6 | 0.47 | 0.99 |
| EE, kcal | 538.0 ± 109.4 | 520.9 ± 100.6 | 0.05 | 0.99 |
| RQ | 0.84 ± 0.05 | 0.87 ± 0.04 | 0.01 | 0.89 |
Values are means ± SD.
24-h Comparisons
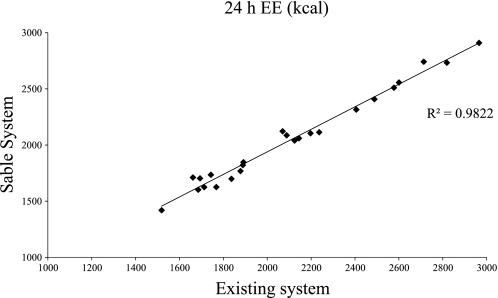
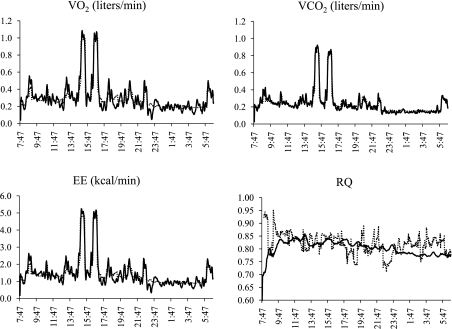
Similar to the 4-h tests, 24-h V̇o2 and EE measured by the existing system was significantly higher than that measured by the Sable system (Table 3). However, the magnitude of the differences were small (∼15 liters, 60 kcal), and the measurements were highly correlated (Fig. 4). There were no differences in total V̇co2. As in the 4-h tests, 24-h RQ was ∼ 0.03 higher (P < 0.01) with the Sable system. The correlation for RQ between the two systems was r = 0.54. A sample 24-h measurement in a single subject (Fig. 5) demonstrates the level of agreement between the two systems.
Table 3.
Calorimeter results at 24 h
| Existing System | Sable System | P | r | |
|---|---|---|---|---|
| V̇o2, liters | 434.5 ± 83.7 | 418.8 ± 85.7 | <0.01 | 0.99 |
| V̇co2, liters | 362.1 ± 73.2 | 363.6 ± 71.3 | 0.47 | 0.99 |
| EE, kcal | 2,112.9 ± 409.4 | 2,052.7 ± 414.5.4 | <0.01 | 0.99 |
| RQ | 0.83 ± 0.04 | 0.86 ± 0.05 | <0.01 | 0.69 |
Values are means ± SD.
Fig. 4.
Correlation between 24-h EE measured with the existing system and Sable system (n = 24).
Fig. 5.
Sample concurrent measurement of 24-h V̇o2 (l/min), V̇co2 (l/min), EE (kcal/min), and RQ with the existing system (dashed line) and Sable system (solid line). Increases in EE at ∼14:00 and 16:00 are periods of activity (30 min of bench stepping at 72 steps/min).
Response of 24-h RQ
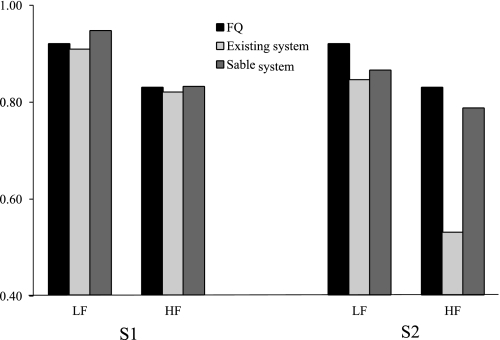
When fed the low-fat diet, 24-h RQ for the first subject (S1) was close to the food quotient of the diet, as measured by each system (Fig. 6). When fed the high-fat diet, 24-h RQ decreased close to the food quotient of the diet, and similar values were obtained for each system. The 24-h EE measured by the existing system was 2,145 and 2,197 kcal/day for the low-fat and high-fat diets, respectively, and 2,060 and 2,105, respectively, when measured by the Sable system. For the second subject (S2), the 24-h RQ measured by each system was below the food quotient of the low-fat diet, but similar between systems. When fed the high-fat diet, 24-h RQ decreased as expected. Unexpectedly, the dew point was negative on this day. As a result, V̇o2 measured by the existing system (732 liters) was substantially higher than that measured by the Sable system (495 liters), although there was close agreement in the measured V̇co2 (395 and 389 liters, respectively). Thus, once again, we demonstrate that when the incurrent airstream is not completely dried and the dilution effect of water vapor pressure on the partial pressure of O2 is not accounted for, the resulting calculated V̇o2 will be too high (and RQ will be too low).
Fig. 6.
24-h RQ in 2 individuals studied after consuming a low-fat (LF) or high-fat (HF) diet. S1, subject 1; S2, subject 2. Black bars indicate the food quotient (FQ) of the respective diets.
DISCUSSION
In this report, we describe the accuracy of a new approach to performing whole room indirect calorimetry. This new approach incorporates two novel aspects of operation: 1) constant measurement of water vapor pressure in the incurrent and excurrent airstreams, permitting the accurate adjustment of the measured V̇o2 for the dilution effect of water vapor pressure, which eliminates the need of drying the gasses; and 2) implementation of a new switching approach that permits constant, uninterrupted measurement of the excurrent airstream, while allowing frequent measurements of the incurrent airstream, and that allows for continuous and dynamic adjustments to the incurrent gas concentrations due to both changes in gas concentrations in the incurrent air and analyzer drift. To determine the accuracy of this new approach, we performed several different validation studies. Using propane combustion, the current standard for validating the performance of room calorimeters, we demonstrated that recoveries of O2 and CO2 were within 2% of expected values. We also demonstrated that under conditions where water vapor pressure is not removed from the incurrent airstream (i.e., the dew point is <1°C), recoveries of O2 and CO2 were still >98%. In fact, the Sable system consistently yields recoveries for both O2 and CO2 that are greater than ∼98% of expected values (Table 1). Next, we compared V̇o2, V̇co2, RQ, and EE measured at rest and during physical activity against that measured using a metabolic cart. Results of this study showed good agreement between the metabolic cart and the Sable system. We also compared 4-h continuous measurements obtained with our existing system and the Sable system. Although there were significant differences in V̇o2 and EE, the magnitude of these differences was relatively small. We then performed simultaneous 24-h measures with both systems. As in the 4-h studies, there were significant differences in V̇o2 and EE, but again the magnitude of these differences was relatively small. Importantly, the magnitude of the differences over 24 h (2–3% difference between systems) was not greater than that observed during the 4-h studies (3–4%). We also demonstrated that the dynamic response of 24-h RQ was appropriate when there was a change in dietary fat intake. The results of this study indicate that this new approach provides accurate readings of V̇o2 and V̇co2. Importantly, this new approach represents the first major advance in room calorimeter measurements approaches in several years.
As described in the introduction, the environmental conditions in Denver (dry air coupled with constantly low barometric pressure) create a unique set of challenges for implementing a pull-type indirect calorimetry. Although our choice to use environmental air as a source of reference gas for the differential analyzers eliminated one source of error (i.e., potentially large fluctuations in concentrations of O2 and CO2 in the incurrent air), it introduced a new, unanticipated source of error (i.e., low dew point in the incurrent air, particularly during the winter months). Because we employed a chilled water cooler, which can only chill the sample gasses to 1°C, to dry both the incurrent and excurrent airstreams, this introduces an error into the measurement of V̇o2. Although the dew point of the incurrent airstream is periodically < 1°C, the dew point of the incurrent airstream will always be positive (because the subject is respiring, water vapor is added to the excurrent air). Under this situation, the chilled water cooler will dry the gas in the excurrent air, but not the incurrent air, thus inducing an error in the calculated V̇o2 because the dilution effect of water vapor pressure on O2 in the incurrent air was not considered in the calculations. The Sable system circumvents this problem, and the need for drying, by constantly measuring and adjusting for the dilution effect of water vapor pressure on O2 and CO2. Importantly, even small fluctuations in the difference in water vapor pressure in the incurrent and excurrent air can introduce substantial errors in the measured O2 concentrations. Thus, if unequal drying occurred in the two samples (incurrent and excurrent), a potentially large and undetected error could occur. Thus, the continued use of this type of drying system in room calorimeters is questioned.
Prior to installing the Sable system, we explored several alternative approaches for drying the gasses. One possibility was to use a desiccant column; a commonly used column is calcium sulfate (gypsum). However, a characteristic of gypsum is that it absorbs CO2, and would therefore introduce another source of error in the metabolic calculations. We also considered the use of a counterflow approach, as used in the calorimeters in Department of Human Biology, Maastricht University, Maastricht, The Netherlands (15). This approach uses a specialized drying tube that has a center tube surrounded by a membrane that permits exchange of water vapor. The center tube is surrounded by an outer tube, and a dry gas (e.g., 100% oxygen or nitrogen) flows through the outer tube in a direction counter to the flow of the sample gas. However, supplying a source of continuous gas is expensive; it requires a source of dry gas be delivered via central lines in the building to the calorimeter, or tanks of gas that are replaced on a regular basis. Prior to installing the Sable system, we circumvented the problem induced when the dew point of the incurrent air was <0°C by installing water baths upstream from the chilled water cooler. The samples from both the incurrent and excurrent airstreams were passed through small flasks that were filled to ∼75% with water (the gasses passed through the airspace above the water). In this manner, the samples would be moistened and would therefore raise the dew point of both samples above 0°C. Although we did not measure the dew point to confirm this, we conducted numerous propane tests when the dew point was <0°C; the result was that recovery of O2 was restored to >98% (data not shown). However, we were aware that this was not an ideal setup; because CO2 is highly soluble in water, this probably induced a lag in CO2 response time. Although 24-h recoveries of CO2 during the propane tests were also >98%, we were skeptical of CO2 measurements over short periods of time. Thus, we deemed this approach unacceptable, which ultimately led us to consider the Sable system.
Another novel aspect of the Sable system is the use of fuel cell oxygen sensors to measure the fractional concentrations of O2. Paramagnetic oxygen analyzers are highly accurate, with a very short response time and high resolution, and their use in room calorimetry is well established. Fuel cell oxygen analyzers have only recently attained the level of resolution of paramagnetic analyzers; however, they are continuing to improve. Because fuel cell oxygen analyzers are electrochemical in nature, they have the potential to yield signals with less noise than paramagnetic oxygen analyzers, which use various complex systems that transduce the forces exerted by oxygen concentration differences within inhomogeneous magnetic fields. For example, using the same dual-absolute fuel cell oxygen analyzer we used in this study, atmospheric scientists have attained a resolution of 0.3 ppm O2 against a background of atmospheric air (19). On the other hand, the response times of fuel cell oxygen analyzers are significantly slower than those of paramagnetic oxygen analyzers (∼ 7 s vs. 0.2 s). However, in the case of room calorimetry, or indeed in most forms of flow-through respirometry, response times are primarily determined by the time constant of the respirometry chamber (volume/flow rate), which is usually in the range of minutes to (in the case of room calorimetry) hours. Most time-response correction algorithms for room calorimeters employ the first derivative of the gas analyzer signals and are thus extremely sensitive to analyzer noise. Under these circumstances, analyzer noise is a more important determinant of overall system performance than analyzer response time.
In addition to solving the problem with inadequate drying of the sample gasses, we have observed several other advantages to operating the Sable system. First, eliminating the drying step eliminates a need for regular maintenance of the chilled water cooler; the peristaltic pumps that remove the condensed water needed to be replaced approximately every 12–18 mo. It is likely that other drying systems require periodic maintenance as well. Second, because the Sable system continuously baselines the gas analyzers, there is no need for daily calibration of the gas analyzers, which can be a time-consuming process (as well as another source of potential error). Finally, since the Sable system is an integrated system specifically designed for the purpose of measuring metabolism in flow-through respirometers, it is very user friendly. Our experience with our previous systems and knowledge of other room calorimeters is that these systems have been developed with “off the shelf” components and often employ user-written customized software.
Perspectives and Significance
We have demonstrated the validity of a new approach to measuring gas exchange in humans using whole room indirect calorimetry. The novel aspects of this approach are the continuous measurement of relative humidity and temperature, permitting the accurate and continuous calculation of water vapor pressure and frequent measurement of incurrent gas concentrations without interrupting the measurement of the excurrent airstream. These features permit continuous adjustment for the dilution effect of water vapor pressure and changes in the background concentrations of O2 and CO2, thus accounting for the two major sources of error in pull-type room calorimeters.
APPENDIX
Description of the Room Calorimeter
The calorimeter has an external window and is furnished with a hospital bed, desk, chair, flat-screen, wall-mounted television, computer with internet connection, telephone, and a sink and toilet. Two air-locking passages are mounted in the wall and are used to pass meals to the subject and collect biological specimens (e.g., urine samples) with minimal disturbance of the internal environment of the room. A blood sampling port is also mounted in the wall of the calorimeter; when the covers are removed, the subject can extend his/her arm through a rubber iris sleeve for blood sampling, again without disturbing the internal environment of the room. A video camera is mounted in the corner of the room with video monitors located in the calorimeter control room and at the nursing station. The calorimeter is also equipped with an intercom system, which facilitates communication with the research subjects during the study periods.
Description of the Existing Respirometry System
Air is pulled from the room at a constant rate of 80 l/min and replaced with fresh air from the buffer using an adjustable DC-powered fan (Rotron Minispiral Regenerative Blower; Ametek, Paoli, PA). Excurrent flow rate is measured using a mass flow meter (Teledyne Hastings HFM-200 LFE; Teledyne Hastings Instruments, Hampton, VA). The difference in gas concentrations in the incurrent and excurrent airstreams is measured using a differential paramagnetic oxygen analyzer (Siemens Oxymat 6E Oxygen Gas Analyzer; Siemens, Houston, TX) and a differential infrared carbon dioxide analyzer (ABB Advance Optima Uras 14 NDIR CO2 Analyzer; ABB, Zurich, Switzerland). Span ranges of the O2 and CO2 analyzers are 20–21% and 0–1%, respectively. Subsamples of both the incurrent and excurrent airstreams are passed through a chilled water gas cooler (ABB Model SCC-C Sample Gas Cooler). The chilled water cooler is placed upstream from the gas analyzers, and samples from both the incurrent and excurrent airstreams are chilled to 1°C. This condenses the moisture from the samples and the airstreams are dried to a water vapor pressure corresponding to 1°C, which is ∼0.66 kPa or 4.93 Torr. Flow through the cooler is controlled (∼1 l/min) by using an ABB model SCC-F Sample Gas Feed Unit that also provides control for the flow rate through the gas analysis chain. The temperature of the chilled water cooler is 1°C; as the gas passes through the cooler, moisture condenses and is removed using peristaltic pumps. The CO2 and O2 analyzers are aligned in series. After exiting the CO2 analyzer, the airstreams are warmed to 37°C by using a waterbath to ensure constant temperature of both the incurrent and excurrent airstreams entering the O2 analyzer. Analog data signals are converted to digital using a 16-bit A/D converter (model PCI-DAS1602/16; Measurement Computing). Data are collected at 60 Hz, for 8 s of each minute, and averaged to obtain average minute values. A custom-written program controls data collection, and data are processed using a fast-response algorithm that suppresses noise and identifies trends yielding improved transient response (13). Briefly, this algorithm determines the two connected exponential equations, using the least-squares method, that best fit the previous 30 min of data from the present minute, and calculates the smoothed gas concentration and time derivative from 15 min prior to the present minute. These values are then substituted into the respiration equations, and the process is repeated every minute. The system is calibrated prior to each study visit. Before the subjects enter the calorimeter, the O2 and CO2 analyzers are calibrated to a zero value and a span value. During the zero calibration, both channels of the analyzers are fed gas from a tank containing an ambient gas mixture of 20.94% O2 and 0.03% CO2; thus the recorded difference between the analyzer channels is 0.0%. During the span calibration, the sample line from the calorimeter is fed a gas mixture containing ∼20.1% O2 and 0.9% CO2, and the reference line is fed ambient tank gas; thus, the span value differences are ∼0.8% for both the O2 and CO2 analyzers. Periodically (approximately once a month), the precision of the system is tested using propane combustion tests. A bottle of propane is combusted at a rate of ∼ 0.15 g/min, as determined by serial data recorded from a balance scale (Denver Instrument model FX-1502; Fisher Scientific, Pittsburgh, PA) beneath the bottle, and the expected volume of O2 and CO2 is determined based on expected production of 2.55 and 1.53 l of O2 and CO2, respectively, per gram of propane burned (22). The total V̇o2 and V̇co2 measured by the system are expressed as a percentage of the expected values. Initially, these tests showed that the previous system was substantially in error. The manufacturer-supplied calibration gas concentrations were therefore adjusted by back calculation to yield appropriate propane burn results.
Description of Sable System
The Sable system is designed to minimize errors related to the incomplete removal of water vapor, analyzer drift, and variability in gas concentrations of incurrent air. The primary enhancements are 1) the use of a fuel cell-based oxygen analyzer; 2) high-precision continuous measurement of relative humidity and temperature, permitting the accurate and continuous calculation of water vapor pressure; and 3) frequent measurements of incurrent gas concentrations. The first two enhancements eliminated the need for drying the airstreams and, more importantly, eliminated errors introduced when the dew point of the incurrent air was below 0°C. The third enhancement was accomplished by using a novel switching approach that permits constant, uninterrupted measurement of the excurrent airstream, while allowing frequent measurements of the incurrent airstream.
New hardware and software were installed for the room calorimeter in June 2009 and operated in parallel with the existing system. The Sable system is completely contained (i.e., all analyzers and components manufactured by Sable Systems International). A variable rate (0–500 l/min) mass flow generator (Sable Systems FlowKit-500) pulls the air from the chamber. A subsample of the excurrent airstream (∼400 ml/min) is pulled through a flow-switching unit (Sable Systems Background Baselining Unit), using a variable subsampling pump (Sable Systems model SS-4), from which it passes through two complete gas analysis systems that comprise two water vapor-, two carbon dioxide-, and two oxygen-analyzers plus a barometric pressure sensor. For water vapor analysis, two Sable Systems RH-300 water vapor analyzers (with direct readout in kPa water vapor pressure, resolution 0.01 Pa) are used. For CO2 analysis, two Sable Systems CA-10 CO2 analyzers (resolution; 1 ppm CO2) are used, while for O2 analysis a dual-channel O2 analyzer (Sable Systems FC-2 Oxzilla; resolution 1 ppm O2) is used. The barometric pressure analyzer inside the FC-2 O2 analyzer resolves to 0.1 Pa (0.0001 kPa).
Sable System Data Acquisition and Processing
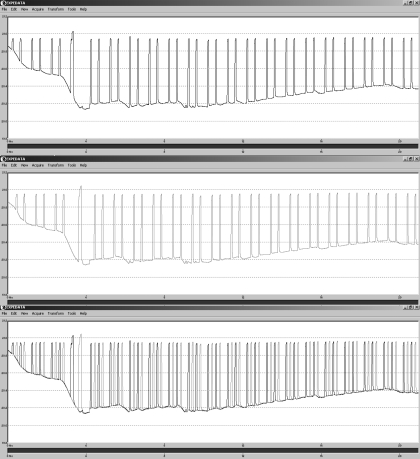
Data acquisition is performed using Sable Systems ExpeData software that employs a novel switching approach that permits constant, uninterrupted measurement of the excurrent airstream, while allowing frequent measurements of the incurrent airstream. In operation, the Sable system passes excurrent air from the chamber through both analyzer chains, but every ∼15 min, switches the input of one of the analyzer chains so that it measures incurrent air instead for 3.5 min (Fig. A1). The analyzer measuring incurrent air is then switched back to measuring excurrent air. Both analyzer chains then continue to measure excurrent air, after which the input to the other analyzer chain is switched so that it now measures incurrent air instead. After the measurement, the analyzer chain returns to measuring excurrent air. The cycle repeats, and although each analyzer chain is baselined (allowed to measure incurrent gas concentrations) multiple times per hour, at least one (and often both) of the analyzer chains is measuring excurrent gas concentrations at any given time. The switching control is also controlled during data acquisition using the Sable Systems Expedata software. All raw data are saved to a file for subsequent calculations.
Fig. A1.
Sample data collection for excurrent fraction of O2 (FeO2) by the Sable system. FeO2 is sampled by two separate channels (top and middle ). Alternately, each channel is baselined, i.e., switches to measure the incurrent fraction of O2 (FiO2). The two channels are stitched together (bottom), resulting in an uninterrupted excurrent measurement. Drift correction occurs during data processing, as described in the text.
During data analysis, the raw data are processed using Sable Systems ExpeData. The Sable system does not, at present, analyze data during acquisition. Every step in the process of data reduction is documented in an executable script that can be modified as desired. Briefly, the O2 and CO2 channels are corrected for water vapor dilution by multiplying them by BP/(BP − WVP), where BP is barometric pressure and WVP is water vapor pressure in equivalent units. The dried incurrent O2 intervals (baselines) in each O2 channel are then spanned to FiO2 = 0.2094 using a Catmull-Rom splining technique (5), essentially eliminating analyzer drift. Delta channels are then created of (FiO2 − FeO2) and (FeCO2 − FiCO2), also using the multiple incurrent readings on both gases in conjunction with Catmull-Rom splining. The concurrent measurements from the two excurrent channels are averaged, and the excurrent sections of the two delta O2 and two delta CO2 channels are then matched and stitched together, thus creating a single delta O2 and a single delta CO2 channel, both of them drift corrected and without any interruptions from incurrent measurements. Flow rate is multiplied by (BP − WVP)/BP to remove the water vapor added by the subject to the excurrent flow. Standard equations (see below) are then used to calculate V̇o2 and V̇co2. Sable Systems International has applied for a patent for the above-described background baselining technique.
Approximately every 2 wk, the Sable system CO2 analyzers are calibrated with nitrogen (CO2 zero) and CO2 span gas (∼1.0%), using the span gas manufacturer's published gas concentrations without correction. The O2 analyzer does not require spanning because this calibration is performed during data analysis, after correction for water vapor dilution, each time incurrent gas concentration is measured (see above). The water vapor pressure analyzers are calibrated during each run by switching a magnesium perchlorate desiccant column into the airstream while the incurrent airstream is selected. The dry air allows the water vapor pressure analyzer to be zeroed, and the increase in FiO2 resulting from airstream desiccation allows water vapor pressure to be calculated (10) and is used to span the water vapor pressure analyzer automatically under ExpeData control. The system is validated ∼ 1/mo using propane combustion, as described above.
The Sable system uses a conservative approach to response correction, modeled on first-order, gas-mixing kinetics as described elsewhere (3). It is applied after complete V̇o2 and V̇co2 traces have been calculated. In essence, the first derivative of the trace is calculated, multiplied by a constant derived from the volume of the room and the volumetric flow rate, and added to the original data. Because of the long time constant of the room (∼5 h), the multiplicative constant is very large. Consequently, any significant noise in the derivatized data will overwhelm the original trace. A number of algorithms and approaches to low-noise derivatization exist (as do other approaches to response correction). After extensive testing, the following approach was adopted for the V̇o2 and V̇co2 traces as the best compromise between low noise and speed of response. Briefly, starting at the beginning of the V̇o2 and V̇co2 traces, successive half-hour sections of each trace (sampled at 1 Hz) were fitted by least squares fifth-degree polynomial regression. After each half-hour section was fitted, the resulting equation was utilized to calculate the best-fit prediction of the data over that section. The predicted values were added to a storage buffer. The section to be fitted was then shifted forward by 7.5 min, and the process was repeated until the end of the trace was reached. The mean of the summed predictions in the storage buffer was calculated, derivatized, and processed as described above.
GRANTS
This study was supported by a University of Colorado Denver Clinical and Translational Science Award through a National Center of Research Resources Grant 1UL1-RR-025780.
DISCLOSURES
John Lighton is the President of Sable Systems International.
ACKNOWLEDGMENTS
The lead author would like to express his gratitude to his colleagues around the world who have shared their knowledge and expertise in designing and operating room calorimeters. We are also grateful to Dr. Robert Eckel, director of the Discovery Translational Pillar Program of the Colorado Clinical and Translational Science Institute, for his support of this project.
REFERENCES
- 1.Atwater WO, Benedict FG. Metabolism of Matter and Energy in the Human Body Washington DC: Department of Agriculture OoES, 1899 [Google Scholar]
- 2.Atwater WO, Woods CD, Benedict FG. Report of Preliminary Investigations on the Metabolism of Nitrogen and Carbon in the Human Organism with a Respiration Chamber of Special Construction Washington DC: Department of Agriculture OoES, 1897 [Google Scholar]
- 3.Bartholomew GA, Vleck D, Vleck CM. Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in Sphingid and Saturniid moths. J Exp Biol 90: 17–32, 1981 [Google Scholar]
- 4.Brown D, Cole TJ, Dauncey MJ, Marrs RW, Murgatroyd PR. Analysis of gaseous exchange in open-circuit indirect calorimetry. Med Biol Eng Comput 22: 333–338, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Catmull E, Rom R. A class of interpolating splines. In: Computer Aided Geometric Design, edited by Riesenfeld B. New York: Academic, 1974, p. 317–326 [Google Scholar]
- 6.Dauncey MJ, Murgatroyd PR. A direct and indirect calorimeter for studies on energy expenditure in man over 24 h periods. J Physiol 284: 7P-–8P., 1978 [PubMed] [Google Scholar]
- 7.Henning B, Lofgren R, Sjostrom L. Chamber for indirect calorimetry with improved transient response. Med Biol Eng Comput 34: 207–212, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Jensen DR, Gayles EC, Ammon S, Phillips R, Eckel RH. A self-correcting indirect calorimeter system for the measurement of energy balance in small animals. J Appl Physiol 90: 912–918, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr 38: 989–998, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Lighton JRB. Flow through respirometry: the equations. In: Measuring Metabolic Rates: a Manual for Scientists New York: Oxford University Press, 2008, p. 100–104 [Google Scholar]
- 11.Machta L, Hughes E. Atmospheric oxygen in 1967 to 1970. Science 168: 1582–1584, 1970 [DOI] [PubMed] [Google Scholar]
- 12.Moon JK, Vohra FA, Valerio Jimenez OS, Puyau MR, Butte NF. Closed-loop control of carbon dioxide concentration and pressure improves response of room respiration calorimeters. J Nutr 125: 220–228, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput 41: 572–578, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Pettenkoffer M. Uber die respiration. Annalen Der Chemie und Pharmacie 123: 1–52, 1862 [Google Scholar]
- 15.Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol 83: 2064–2072, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Seale JL, Rumpler WV, Moe PW. Description of a direct-indirect room-sized calorimeter. Am J Physiol Endocrinol Metab 260: E306–E320, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Shetty PS, Sheela ML, Murgatroyd PR, Kurpad AV. An open-circuit indirect whole body calorimeter for the continuous measurement of energy expenditure of man in the tropics. Indian J Med Res 85: 453–460, 1987 [PubMed] [Google Scholar]
- 18.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood JC, Windhauser MM, Bray GA. Fat and carbohydrate balances during adaptation to a high-fat diet. Am J Clin Nutr 71: 450–457, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Stephens BB, Bakwin T, Tans PP, Teclaw R, Baumann D. Application of a differential fuel-cell analyzer for measuring atmospheric oxygen variations. J Atmos Oceanic Technol 24: 82–94, 2007 [Google Scholar]
- 20.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol 76: 2686–2691, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Tohjima Y, Machida T, Tomonori W, Akam I, Amara T, Moriwak Y.Preparation of gravimetric standards for measurement of atmospheric oxygen and reevaluation of atmospheric oxygen concentration. J Geophys Res 110: D11302, 2005 [Google Scholar]
- 22.Withers PC. Design, calibration, and calculation for flow-through respirometry systems. Aus J Zool 49: 445–461, 2001 [Google Scholar]