Abstract
We tested the hypothesis that food deprivation alters body temperature (Tb) responses to bacterial LPS by enhancing inflammatory signaling that decreases Tb (cryogenic signaling) rather than by suppressing inflammatory signaling that increases Tb (febrigenic signaling). Free-feeding or food-deprived (24 h) rats received LPS at doses (500 and 2,500 μg/kg iv) that are high enough to activate both febrigenic and cryogenic signaling. At these doses, LPS caused fever in rats at an ambient temperature of 30°C, but produced hypothermia at an ambient temperature of 22°C. Whereas food deprivation had little effect on LPS fever, it enhanced LPS hypothermia, an effect that was particularly pronounced in rats injected with the higher LPS dose. Enhancement of hypothermia was not due to thermogenic incapacity, since food-deprived rats were fully capable of raising Tb in response to the thermogenic drug CL316,243 (1 mg/kg iv). Neither was enhancement of hypothermia associated with altered plasma levels of cytokines (TNF-α, IL-1β, and IL-6) or with reduced levels of an anti-inflammatory hormone (corticosterone). The levels of PGD2 and PGE2 during LPS hypothermia were augmented by food deprivation, although the ratio between them remained unchanged. Food deprivation, however, selectively enhanced the responsiveness of rats to the cryogenic action of PGD2 (100 ng icv) without altering the responsiveness to febrigenic PGE2 (100 ng icv). These findings support our hypothesis and indicate that cryogenic signaling via PGD2 underlies enhancement of LPS hypothermia by food deprivation.
Keywords: energy balance, fasting, inflammation, LPS, prostaglandins, temperature, fever, hypothermia
the ability of an organism to adjust its deep body temperature (Tb) in response to infection is an integral component of the host defense process. Humans (3, 11) and experimental animals (56, 65) respond to live bacteria or bacterial products with either fever (increased Tb) or hypothermia (decreased Tb). Fever prevails over hypothermia when systemic inflammation is less severe, whereas hypothermia prevails when systemic inflammation is more severe (1). Both fever and hypothermia seem to be regulated thermoregulatory responses brought about by brain-driven changes in thermoeffector activity (1, 2, 57). Fever is an energy-consuming strategy that enhances the bactericidal power of the immune system (4, 30). Hypothermia, an energy-conservation strategy (64), can have an adaptive value when inflammation is severe enough to compromise tissue perfusion (58) or when energy reserves would be threatened by the energetic cost of fever (69). Since the biological values of fever and hypothermia appear to be related to their different impacts on energy balance, it is important to understand how the state of energy balance plays a role in determining whether a host commits to fever or hypothermia.
Studies conducted in rabbits (28, 43, 72), guinea pigs (62), hamsters (6), and rats (23, 61) have shown that food deprivation attenuates the fever induced by bacterial LPS, and it can even turn fever into hypothermia. This switch from fever to hypothermia was thought to result from impairment in the ability of food-deprived animals to produce heat (62), but this idea has been challenged by recent studies (23, 61) reporting that food-deprived rats are fully capable of raising Tb in response to PGE2, an essential mediator of fever (15, 34, 79). Interestingly, food-deprived rats can produce as much PGE2 as free-feeding rats in response to LPS (23). The question then arises: how can food deprivation attenuate fever even when febrigenic signaling (at least via PGE2) is not attenuated? We hypothesized that food deprivation alters thermoregulatory responses to LPS by enhancing inflammatory signaling that decreases Tb [so-called cryogenic signaling (24)] rather than by suppressing inflammatory signaling that increases Tb [febrigenic signaling (24)]. We recognize that the terms cryogenic and antipyretic are sometimes used interchangeably to refer to substances that attenuate fever regardless of the mechanism involved (29, 32, 73), but we prefer to use the term cryogenic to specifically define a substance that exerts a Tb-lowering effect, which can result either in attenuation of fever or in hypothermia (67, 69), and we reserve the term antipyretic to define a substance that does not exert a thermoregulatory effect of its own but can attenuate fever by interrupting febrigenic signaling (12, 36, 46).
The hypothesis raised above was tested in the present study. To distinguish febrigenic from cryogenic inflammatory signaling, we took advantage of the fact that LPS affects Tb differently depending on the ambient temperature (Ta). At neutral or supraneutral Ta (warm environment), rats challenged with LPS primarily develop fever, even when the dose of LPS is high (for a review, see Ref. 53). At subneutral Ta (cool environment), low doses of LPS cause fever, and moderate-to-high doses cause hypothermia followed by fever, with the magnitude of the hypothermia increasing along with the LPS dose (for a review, see Ref. 53). These observations suggest that the Tb of rats at neutral Ta reflects mostly the action of febrigenic signals, whereas Tb of rats at subneutral Ta reflects the combined actions of febrigenic and cryogenic signals. A plausible explanation for this Ta-dependence is that febrigenic signals alter heat loss effectors that govern Tb at neutral Tas, whereas both febrigenic and cryogenic signals alter heat production effectors that govern Tb at subneutral Tas (52). Investigation of thermoregulatory responses to LPS at neutral Ta and subneutral Ta is emerging as a powerful strategy to determine the extent of febrigenic vs. cryogenic signaling (66, 67). In the present study, this strategy was employed in conjunction with measurement of the levels of inflammatory mediators in free-feeding and food-deprived rats. We measured typical febrigenic mediators, IL-1β, IL-6, and PGE2 (7, 35, 53), as well as mediators that have the potential to be cryogenic: TNF-α (5, 31, 37, 75) and PGD2 (26, 78). We also measured corticosterone, a hormone that has anti-inflammatory actions capable of attenuating fever (antipyretic action) or hypothermia in systemic inflammation (12, 33, 49). Furthermore, we evaluated the responsiveness of free-feeding and food-deprived rats to the thermoregulatory actions of PGE2 and PGD2.
MATERIALS AND METHODS
Animals.
This study was conducted in adult male Wistar rats originated from Charles River Laboratories (Raleigh, NC). The colony room was maintained at a temperature of 22–24°C and a 12:12-h light-dark cycle (lights on at 07:00 AM). Rats were initially caged in pairs; after surgery, they were caged individually. Each rat was handled 5 min per day for 8 days; during a handling session, a rat was habituated to being dressed with an infusion harness that was used later in the experiments. The rats weighed 310 ± 18 g on the day prior to the experiments, i.e., just before the start of the 24-h food manipulation period. At the end of the food manipulation period, food-deprived rats had lost 6 ± 2 g (∼1.9% of body mass), whereas free-feeding rats had gained 2 ± 2 g (∼0.6% of body mass), which resulted in a difference of 8 g (∼2.5%) in the mass of free-feeding and food-deprived rats. A considerable portion of this difference is known to reflect differences in the mass of food and feces in the gastrointestinal tract (38). All protocols were approved by the Animal Care and Use Committee of the Stratton Veterans Affairs Medical Center.
Surgical preparation.
Seven to five days before an experiment, rats were implanted, as necessary, with an intravenous catheter (for administration of LPS or CL316,243, a thermogenic drug), an intracerebroventricular cannula (for administration of PGE2 or PGD2), and/or an intra-abdominal temperature datalogger (for recording of Tb). All procedures were performed under anesthesia with ketamine-xylazine-acepromazine (80:5:1 mg/kg ip) and antibiotic protection with enrofloxacin (2 mg/kg sc). During surgery, rats were maintained over a Delta phase isothermal pad (Braintree Scientific, Braintree, MA) warmed to 37°C.
For intravenous catheterization, a small longitudinal incision was made on the right ventral surface of the neck. The right jugular vein was exposed, freed from its surrounding connective tissue, and ligated. A 3-French polyurethane catheter (Instech Laboratories, Plymouth Meeting, PA) filled with heparinized (50 U/ml) saline was passed into the superior vena cava through the jugular vein and secured in place with ligatures. The distal end of the catheter was closed with a stainless steel plug, tunneled under the skin, and exteriorized at the nape. The intravenous catheters were flushed with heparinized saline every other day.
For intracerebroventricular cannulation, a rat was fixed to a stereotaxic apparatus (David Kopf, Tujunga, CA) with the incisor bar set at −3.3 mm. The skin was incised over the sagittal suture, the periosteum was excised, supporting microscrews were driven into the skull, and a steel guide cannula (Plastics One, Roanoke, VA) was implanted. The tip of the cannula was placed 0.5 mm dorsal to the right lateral ventricle by using the following stereotaxic coordinates: −0.5 mm from bregma, −1.5 mm from the midline, and 3.5 mm from the skull surface. The implanted cannula was attached to the supporting microscrews with acrylic cement.
For datalogger implantation, a midline laparotomy was performed and a spacer was used to keep the abdominal muscles separated. The rat was then positioned in lateral decubitus, and a SubCue miniature datalogger (Calgary, Alberta, Canada) was attached to the internal side of the dorsolateral abdominal wall with 4-0 silk sutures.
Food deprivation.
Food-deprivation was initiated at 11 am on the day before an experiment, and continued until the end of the experiment on the following day. During the food-deprivation period, a rat was placed in a cage equipped with an elevated metal grid floor to prevent coprophagy. Control rats had free access to food during the entire experiment, but were also placed on an elevated grid floor to ensure exposure to identical environmental conditions. Water was available ad libitum.
Experimental setup.
At 7 am on the day of the experiment, a rat in its cage was transferred to an environmental chamber (model NQ1; Environmental Growth Chambers, Chagrin Falls, OH), which contained fluorescent lamps that operated according to the light-dark cycle. In the chamber, Ta was maintained at 30.0 ± 0.1°C or 22.0 ± 0.1 °C; relative air humidity was always kept at 50 ± 5%. Whereas 30°C is a neutral Ta for rats in the chamber environment [as verified by the presence and variability of tail skin vasodilation (55)], 22°C is a subneutral Ta for rats in the same environment [as verified by the absence of tail skin vasodilation (55)]; see Supplemental Figure 1 (supplemental figures are available online at the American Journal of Physiology–Regulatory, Integrative and Comparative Physiology website). In a rat designated for intravenous drug administration, the preimplanted venous catheter was extended with a length of polyethylene-50 (PE-50) tubing. The extension was passed sequentially through a Covance infusion harness, which was worn by the rat, and a metal spring (Instech Solomon, Plymouth Meeting, PA), which protected the catheter extension from bites and scratches. The spring and extension, in turn, were connected to a swivel (Instech Solomon) mounted above the cage; the swivel prevented the extension from twisting as the rat moved about its cage. Another PE-50 extension, leaving the swivel, was passed to the exterior of the chamber through a wall port and connected to a 1-ml syringe. In a rat designated for intracerebroventricular drug administration, an injector needle (Plastics One) was fitted into the preimplanted guide cannula; the needle protruded 1 mm beyond the guide cannula to reach the lateral ventricle. The injector needle was coupled via a connector assembly (Plastics One) to a swivel (Instech) from which a PE-50 extension was passed to the exterior of the chamber and connected to a 10-μl Hamilton syringe. Both the intravenous and intracerebroventricular extensions were filled with the drugs of interest or their vehicles. This setup permits drug administration to be performed in a freely-moving, undisturbed rat.
With the infusion lines in place (usually around 8 AM), rats were allowed to become accustomed to the experimental setup for 3 h. Drug or its vehicle was administered at ∼11 am, i.e., 24 h after the initiation of the food manipulation period. If a rat was designated for measurement of Tb, it was left undisturbed for 360 min postinjection, then euthanized with pentobarbital sodium (100 mg/kg ip) for removal of the abdominal datalogger from which data were downloaded to a computer. If a rat was designated for blood collection, it was left undisturbed until the time point of interest (for details, see Results), at which the rat was anesthesized with ketamine-xylazine (8:0.5 mg/kg) via the extension of the venous catheter, and blood was collected by cardiac puncture.
Drug administration.
Systemic inflammation was induced by E. coli 0111:B4 LPS purchased from Sigma-Aldrich (St. Louis, MO). LPS was diluted to a final concentration of either 500 or 2,500 μg/ml in saline, and the resulting solution was bolus injected (1 ml/kg iv). The LPS doses administered were high enough to activate both febrigenic and cryogenic signaling (66, 67). Dosing of LPS to each rat was based on the body mass measured immediately before initiation of the 24-h food manipulation period, because a considerable portion of the mass lost by a rat over 24 h of food deprivation is simply a consequence of gastrointestinal emptying (38). Even if some actual body mass was lost during the 24-h food-deprivation period, it could not have exceeded the 2.5% difference in the mass of free-feeding and food-deprived rats (see Animals). Thus, any dosing difference between food-deprived and free-feeding rats is likely to be negligible in the present study.
CL316,243 (Sigma-Aldrich) was administered to compare the ability of food-deprived and free-feeding rats to increase their Tb in response to this thermogenic β3-receptor agonist (13, 21). A solution of CL316,243 (1 mg/ml) in saline was bolus injected (1 ml/kg iv). As explained above, dosing was based on the body mass of the rats immediately before initiation of the food manipulation period.
PGD2 and PGE2 (Cayman, Ann Arbor, MI) were administered to compare the responsiveness of food-deprived and free-feeding rats to a cryogenic substance and a febrigenic substance, respectively. Each drug was dissolved to a final concentration of 20 ng/μl in saline containing 1% of ethanol. The solution was infused intracerebroventricularly at a rate of 1 μl/min for 5 min. Correct placement of the intracerebroventricular injector needle was confirmed by postmortem verification using Chicago Sky Blue (0.1%, 5 μl icv).
Assays.
Blood samples were placed into EDTA-coated Eppendorf tubes, the tubes were centrifuged (6,000 g, 10 min, 4°C), and the resulting plasma was stored at −80°C. The plasma levels of TNF-α, IL-1β, and IL-6 were determined by sandwich ELISA using kits from Thermo Scientific (manufactured by Pierce Biotechnology, Rockford, IL) and a SpectroMax Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Plasma samples were assayed at a dilution of 1:200 in the TNF-α assay, 1:100 in the IL-6 assay, and 1:2 in the IL-1β assay. Assay ranges were 25.6–2,500 pg/ml for TNF-α and IL-1β, and 31–2,000 pg/ml for IL-6. All samples were run simultaneously, in duplicate.
Because cytokine production is modulated by the hypothamalo-pituitary-adrenal axis (77), we determined the plasma concentration of corticosterone, the final hormone of this axis. Corticosterone was assayed by competitive ELISA using a kit from Cayman Chemical (Ann Arbor, MI) and a SpectroMax Plus microplate spectrophotometer (Molecular Devices). Samples were assayed at a dilution of 1:100; the assay range was 41–8,000 pg/ml. All samples were run simultaneously, in duplicate.
Currently available antibody-based methods for prostaglandin analysis appear to be less accurate than chromatographic methods (22). Hence, we measured the plasma concentration of PGD2 and PGE2 by liquid chromatography-mass spectrometry according to the method of Masoodi and Nicolaou (40), with slight modifications. Each plasma sample (900 μl) was acidified to pH 3 with HCl (1 M) and subjected to solid-phase extraction as follows: after the sample was applied to a C18 Sep-Pak column (Waters, Milford, MA), the column was washed sequentially with water (2 ml) and 10% methanol in water (2 ml), then PGs were eluted from the column with methyl formate (2 ml). The methyl formate fraction was dried under a stream of nitrogen, and the resulting residue was reconstituted in 60 μl of methanol prior to liquid chromatography-mass spectrometry analysis. The analysis was performed using a Waters 600 liquid chromatography pump and injector coupled to a Waters Acquity tandem-quadrupole mass spectrometer. Prostaglandin separation was carried out in a 2.0 × 250 mm C18 Gemini column (Phenomenex, Torrance, CA) eluted isocratically with water-acetonitrile-formic acid (58:42:0.01) at a rate of 250 μl/min and temperature of 25°C; the injection volume was 20 μl. Detection was done by negative ion electrospray ionization tandem mass spectrometry using the following parameters: source temperature of 150°C, electrospray voltage of −3.6 kV, declustering potential of −21 V, and nitrogen gas collision at −16 eV. The method of detection was multiple reaction monitoring of the transition from the deprotonated molecules of m/z 351 to the most abundant fragment ion of m/z 271, which were present in the spectra of both PGD2 and PGE2; these PGs are geometrical isomers.
Calibration curves were made using standard methanolic solutions of PGD2 and PGE2 (both from Cayman). The retention time of PGD2 was 8.4 min, whereas the retention time of PGE2 was 7.3 min. For both compounds, the linear (r2 = 0.999) quantification range extended from 0.5 ng/ml to at least 50 ng/ml. Recoveries from the extraction procedure were 89% and 90% for PGD2 and PGE2, respectively.
Data analysis.
The initial (basal) value of Tb of each rat was calculated by averaging Tb values recorded during the 30 min that preceded drug administration. Thermoregulatory responses to drugs were evaluated based on Tb changes in relation to initial Tb. Changes in TNF-α, IL-1β, IL-6, corticosterone, PGD2, and PGE2 were evaluated based on the absolute concentrations of these substances in plasma. Statistical comparisons were performed using Statistica Advanced 8.0 (StatSoft, Tulsa, OK). Repeated-measures ANOVA was employed to evaluate the effects of feeding status and treatment on consecutive measures of Tb, whereas factorial ANOVA was employed to evaluate the effects of feeding status and treatment on initial Tb or on plasma levels of inflammatory mediators. Post hoc analysis was conducted using the Fisher least significant difference test. The level of significance was set at P < 0.05. Data are reported as means ± SE.
RESULTS
Effects of food deprivation on initial Tb and thermogenic capacity.
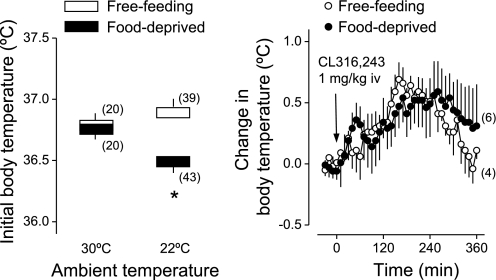
At neutral Ta (30°C), there was no difference between the initial (basal) Tb values of food-deprived (24 h) and free-feeding rats (Fig. 1, left). However, at subneutral Ta (22°C), the initial Tb of food-deprived rats was ∼0.5°C lower than the initial Tb of free-feeding rats (Fig. 1, left); this difference was statistically significant (P < 0.0001). To investigate whether the lower initial Tb of food-deprived rats could reflect thermogenic incapacity, we assessed the ability of rats to elevate Tb in response to CL316,243 at a dose (1 mg/kg iv) known to activate thermogenesis (21). Both free-feeding and food-deprived rats exposed to subneutral Ta responded to CL316,243 with a transient rise in Tb (Fig. 1, right): Tb was statistically higher than initial Tb at 150–250 min postinjection (P < 0.04), and there was no statistical difference between the free-feeding and food-deprived groups. This result indicates that thermogenic capacity was not diminished by the food-deprivation regimen used in the present study.
Fig. 1.
Influences of food deprivation (24 h) on initial (basal) body temperature and thermogenic capacity. Left: summary of initial body temperatures of free-feeding and food-deprived rats exposed to an ambient temperature of 30°C (warm environment) or 22°C (cool environment); this summary includes values obtained in all thermophysiological experiments. Right: effect of a thermogenic β3-receptor agonist (CL316,243; 1 mg/kg iv) on the body temperature of rats exposed to an ambient temperature of 22°C; within this experiment, initial body temperatures were 37.3 ± 0.1°C for free-feeding rats and 36.6 ± 0.3°C for food-deprived rats. The number of animals in each group is shown in parentheses. *Statistical differences between food-deprived and free-feeding rats; see text for more details about statistical results.
Effects of food deprivation on LPS-induced thermoregulatory responses.
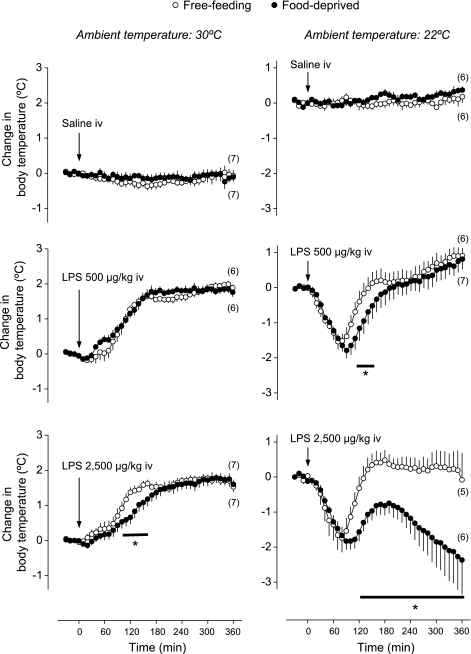
We subsequently studied how food deprivation affects thermoregulatory responses to LPS in rats kept at different Tas. The results of this experiment are depicted in Fig. 2. Intravenous administration of saline (the LPS vehicle) caused no change in Tb, regardless of Ta (30°C or 22°C) or feeding status (free-feeding or food-deprived). On the other hand, intravenous LPS evoked thermoregulatory responses that were dependent on Ta and feeding status. At neutral Ta, both moderate (500 μg/kg) and high (2,500 μg/kg) doses of LPS produced fever. Statistical difference between febrile Tb and initial Tb was observed at 70–360 min following injection of the moderate LPS dose (P < 0.04) and at 40–360 min following injection of the high dose (P < 0.04). At the peak of fever, Tb was 2.0°C higher than initial Tb regardless of dose. The febrile responses to LPS were similar in free-feeding and food-deprived rats, with the exception of a mild attenuation in the fever of food-deprived rats at 100–160 min following the high LPS dose (P < 0.04).
Fig. 2.
Effects of intravenous LPS (doses indicated) or its vehicle (saline) on the body temperature of free-feeding or food-deprived (24 h) rats exposed to an ambient temperature of 30°C or 22°C. Within this experiment, initial body temperatures were 36.8 ± 0.1°C for free-feeding rats at 30°C; 36.7 ± 0.1°C for food-deprived rats at 30°C; 37.0 ± 0.1°C for free-feeding rats at 22°C; and 36.6 ± 0.1°C for food-deprived rats at 22°C. The number of animals in each group is shown in parentheses. *Statistical differences between food-deprived and free-feeding rats.
At subneutral Ta, the same doses of LPS elicited typical hypothermic responses. In free-feeding rats, hypothermic Tb was statistically different from initial Tb at 30–110 min following injection of the moderate LPS dose (P < 0.009) and at 50–100 min following injection of the high dose (P < 0.01); within these time windows, Tb fell by 1.5°C and 1.8°C, respectively. Compared with free-feeding rats, food-deprived rats responded to LPS with enhanced hypothermic responses. Enhancement of hypothermia was mild, although statistically significant (P < 0.04; 110–150 min), in food-deprived rats injected with the moderate LPS dose (500 μg/kg). However, it was very prominent in food-deprived rats injected with the high dose (2,500 μg/kg), in which hypothermia was significantly enhanced from 120 to 360 min postinjection (P < 0.01). By the end of this period, Tb had fallen by 2.4°C (Fig. 2). Of note, the hypothermic response of food-deprived rats to the high LPS dose (2,500 μg/kg) was significantly (P < 0.03) enhanced even compared with the response of free-feeding rats to a twofold higher dose (5,000 μg/kg); see Supplemental Figure 2. This finding rules out any possibility that small differences in LPS dosing due to a 2.5% difference in the mass of free-feeding and food-deprived rats might have accounted for the observed differences in LPS hypothermia.
Effects of food deprivation on the levels of inflammatory mediators.
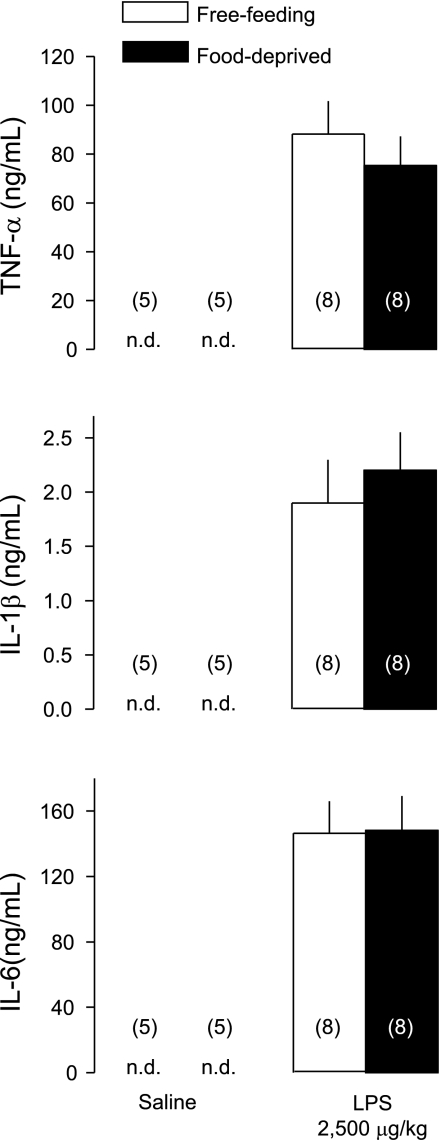
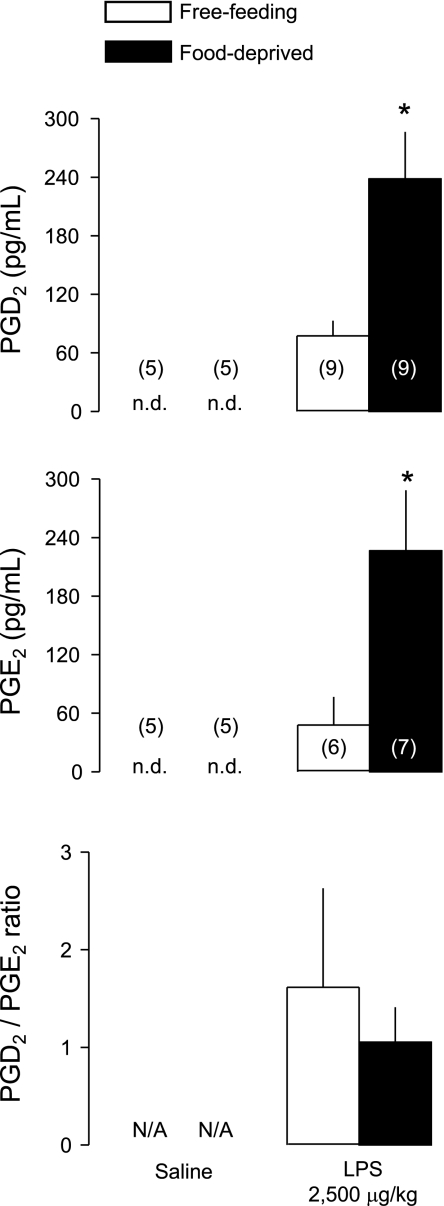
To investigate whether enhancement of LPS hypothermia by food deprivation was associated with changes in the levels of inflammatory mediators, we studied the plasma levels of cytokines (TNF-α, IL-1β, IL-6) and PGD2 and PGE2 known to be involved in the thermoregulatory responses to LPS. Plasma samples were collected 100 min after administration of LPS (2,500 μg/kg iv) or saline (iv) in free-feeding or food-deprived rats exposed to a subneutral Ta of 22°C. This time point corresponds to the maximal rate of separation between the hypothermic responses to LPS in free-feeding and food-deprived rats (see Fig. 2). None of the inflammatory mediators was detectable in the plasma of saline-treated rats, whereas all of them surged in the plasma of LPS-treated rats (Figs. 3 and 4). The LPS-induced surges in plasma TNF-α, IL-1β, and IL-6 did not differ between free-feeding and food-deprived rats (Fig. 3). However, the LPS-induced surges in PGD2 and PGE2 were more than threefold higher in food-deprived rats than in free-feeding rats (Fig. 4; P < 0.004 for PGD2; P < 0.02 for PGE2). It is interesting though that food deprivation augmented the surges in PGD2 and PGE2 to a similar extent so that the PGD2-to-PGE2 ratio was not altered (Fig. 4).
Fig. 3.
Plasma levels of the cytokines TNF-α, IL-1β, and IL-6 at 100 min after administration of LPS (2,500 μg/kg iv) or saline (iv) in free-feeding or food-deprived (24 h) rats exposed to an ambient temperature of 22°C. The time of sample collection (100 min) corresponds to the maximal rate of separation between the hypothermic responses to LPS in free-feeding and food-deprived rats (see Fig. 2, bottom, right). The number of animals in each group is shown in parentheses. n.d., Nondetectable levels. There was no statistical difference between the levels found in food-deprived and free-feeding rats.
Fig. 4.
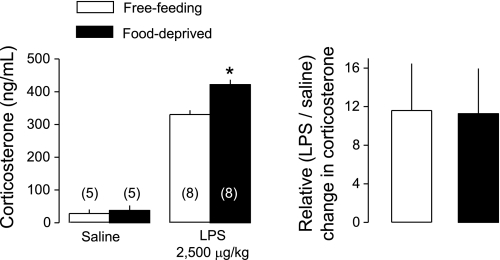
Plasma levels of PGD2 and PGE2, as well as the ratio between them, at 100 min after administration of LPS (2,500 μg/kg iv) or saline (iv) in free-feeding or food-deprived (24 h) rats exposed to an ambient temperature of 22°C. The number of animals in each group is shown in parentheses. N/A, not applicable. *Statistical differences between food-deprived and free-feeding rats.
In addition, plasma samples were assayed for corticosterone, a glucocorticoid hormone that is modulated by both food deprivation and LPS (16, 19) and can modulate thermoregulatory responses to LPS (12, 33, 49). In the absence of inflammation (saline treatment), plasma corticosterone tended to be higher in food-deprived rats (37 ± 15 ng/ml) than in free-feeding rats (28 ± 11 ng/ml), but this difference did not reach statistical significance. Compared with saline, LPS significantly (P < 0.0001) increased plasma corticosterone in both free-feeding and food-deprived rats (Fig. 5, left). Absolute corticosterone levels were higher in those LPS-treated rats that were food-deprived than in those that were free-feeding (P < 0.0001). However, the relative increase in corticosterone level (i.e., the ratio between levels in LPS- and saline-treated rats) did not differ between free-feeding and food-deprived rats (Fig. 5, right).
Fig. 5.
Plasma levels of corticosterone at 100 min after administration of LPS (2,500 μg/kg iv) or saline (iv) in free-feeding or food-deprived (24 h) rats exposed to an ambient temperature of 22°C. Relative changes in corticosterone are shown as the ratios between corticosterone levels in LPS-treated rats and in the corresponding saline-treated controls. The number of animals in each group is shown in parentheses. *Statistical differences between food-deprived and free-feeding rats.
Effects of food deprivation on the thermoregulatory responses to PGD2 and PGE2.
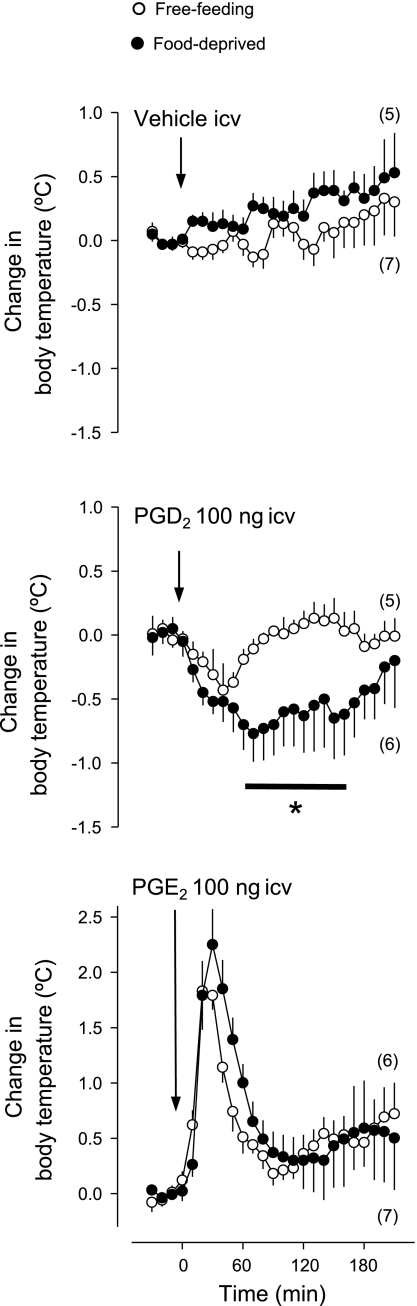
In addition to understanding how food deprivation alters the levels of inflammatory mediators, it is important to understand how it affects the responsiveness of the body to inflammatory mediators. Hence, we investigated whether food deprivation alters the ability of PGD2 and PGE2 to produce hypothermia and fever, respectively. Each substance or its vehicle was administered into the lateral cerebral ventricle at a dose (100 ng) known to affect Tb in rats (71, 78). The experiment was conducted in rats exposed to subneutral Ta (22°C), which permits the development of fever or hypothermia (53). The results of this experiment are shown in Fig. 6. Vehicle administration did not significantly alter the Tb of free-feeding or food-deprived rats. PGD2 caused a modest (0.4°C) fall in the Tb of free-feeding rats, which was significantly different (P < 0.03) from initial Tb at 40–50 min postinjection. The hypothermic response to PGD2 was largely exaggerated in food-deprived rats: not only was the response of the food-deprived rats longer-lasting than the response of free-feeding rats (P < 0.04; 60–170 min), but it was also more pronounced (0.8°C) in magnitude (P < 0.04). In contrast to PGD2, PGE2 induced fever. The fever induced by PGE2 was virtually identical in free-feeding and food-deprived rats. The Tb of PGE2-treated rats peaked 1.8°C above initial Tb, and it was statistically different from initial Tb at 20–50 min postinjection (P < 0.02).
Fig. 6.
Effects of intracerebroventricular PGD2 (100 ng), PGE2 (100 ng), or their vehicle (1% ethanol in saline) on the body temperature of free-feeding or food-deprived (24 h) rats exposed to an ambient temperature of 22°C. Within this experiment, initial body temperatures were 36.7 ± 0.1°C for the free-feeding rats and 36.3 ± 0.1°C for the food-deprived rats. The number of animals in each group is shown in parentheses. *Statistical differences between food-deprived and free-feeding rats.
DISCUSSION
The objective of the present study was to investigate how food deprivation alters febrigenic vs. cryogenic inflammatory signaling during systemic inflammation induced by LPS. We chose to deprive rats of food for no more than 24 h (prior to drug administration) to avoid the thermogenic incapacity known to disrupt thermoregulation following longer periods of food deprivation (20, 63). Indeed, food-deprived rats in the present study were fully able to increase Tb in response to a drug (CL316,243) that directly activates β3-driven thermogenesis in brown fat. Interestingly, though, their basal Tb was slightly reduced at subneutral Ta (cool environment), but not at neutral Ta (warm environment). This observation may reflect the fact that food deprivation decreases the threshold for cold-induced thermogenesis even when it does not impair thermogenic capacity (60). The thermogenic threshold governs Tb at subneutral Ta, but it is of little importance for maintenance of Tb at neutral Ta (55). The Tb of rats at neutral Ta is governed by heat-loss effectors (55), which are unaffected by deprivation of food for as long as 3 days (60). To account for small (≤ 0.5°C) differences in basal Tb related to Ta and feeding status, we evaluated thermoregulatory responses to LPS based on the extent of Tb change in relation to initial (basal) Tb.
The present study was the first to evaluate whether food deprivation alters thermoregulatory responses to LPS in adult animals exposed to neutral Ta in which fever is the predominant thermoregulatory response and is virtually under the sole influence of febrigenic inflammatory signaling (68, 70). The results indicate that food deprivation has little or no effect on LPS fever at neutral Ta: the fever induced by the moderate LPS dose was not affected by food deprivation; the fever induced by the high dose was attenuated, but this attenuation was weak. Although these results do not rule out the possibility that food deprivation may weakly attenuate febrigenic signaling via a yet unidentified mechanism, they indicate that febrigenic signaling is not largely affected by food deprivation. To investigate whether food deprivation alters cryogenic signaling, we studied the thermoregulatory responses to LPS in rats exposed to subneutral Ta in which fever or hypothermia can occur as a reflection of the balance between febrigenic and cryogenic inflammatory signaling (67, 69, 73). Moderate and high doses of LPS caused hypothermia in rats at subneutral Ta, and the hypothermic response to either dose was enhanced by food deprivation. Enhancement of hypothermia by food deprivation was particularly pronounced in rats injected with the high LPS dose. Such an enhancement of hypothermia likely reflected an enhancement in cryogenic signaling, as our experiments at neutral Ta show that food deprivation does not exert a pronounced influence on febrigenic signaling.
The present study also provides insights into the mechanisms by which food deprivation enhances cryogenic signaling. Inflammatory mediators were assayed in samples collected at the time (100 min) corresponding to the maximal rate of separation between the hypothermic responses of free-feeding and food-deprived rats injected with LPS, when their cryogenic signaling is most likely to differ. The cytokines we assayed have different roles in thermoregulation. IL-1β and IL-6 appear to be exclusive mediators of fever (35), whereas TNF-α appears to be febrigenic at low doses but cryogenic at high doses (5). An increasing production of TNF-α, as systemic inflammation becomes more severe, is thought to promote a switch from fever to hypothermia (31, 37, 75). In the present study, the LPS-induced surges in TNF-α, IL-1β, and IL-6 were not altered by food deprivation, at least not at 100 min post-LPS. A study by Inoue et al. (23) agrees with the present results in that it demonstrated unaltered surges of IL-1β and IL-6 in the plasma of food-deprived rats injected with LPS, but it is not in line with the present results in that it reported a reduced surge of TNF-α in the plasma of the same animals. In yet another study, Faggioni et al. (16) demonstrated that food deprivation can augment the plasma TNF-α response to LPS. These discrepancies regarding TNF-α levels may be related to differences in the regimen of food deprivation and LPS dose. Compared with the present study, Inoue et al. (23) employed a longer (2-day) period of food deprivation and a lower dose of LPS, whereas Faggioni et al. (16) employed a longer (2-day) regimen of food deprivation in combination with a higher LPS dose. Regardless, the present results indicate that altered plasma levels of TNF-α are unlikely to be necessary for enhancement of cryogenic signaling by food deprivation, because plasma TNF-α was unaltered when the hypothermic response of food-deprived rats to LPS was enhanced at the highest rate. The present results, however, do not rule out possible alterations in other components of the TNF-α pathway, such as membrane-bound TNF-α, soluble TNF receptor antagonist, and cellular TNF receptors (14, 76).
Interestingly, plasma levels of PGE2 and PGD2 were augmented during the enhanced hypothermic response of food-deprived rats to LPS. This augmentation, at least in the case of PGE2, may be the result of an increased synthesis in peripheral tissues, as it has been reported that food deprivation does not augment the synthesis of PGE2 in the brain of LPS-injected rats (23). Regardless of its origin, circulating PGE2 is fully capable of activating brain thermoregulatory circuitries to produce fever (39, 51, 54, 68). Circulating PGD2 may also be able to activate brain thermoregulatory circuitries, as dense PGD2 binding occurs in the preoptic region (80), a brain site that plays key roles in thermoregulation (45) and is accessible to circulating substances via the organum vasculosum of the lamina terminalis (8, 42, 59). Alternatively, PGD2 receptors in leptomeninges may convey signals of circulating PGD2 to the brain (50). Ueno et al. (78) were the first to report that PGD2 causes hypothermia when microinjected intracerebroventricularly or into the preoptic area of conscious rats at subneutral Ta. Since then, the hypothermic effect of intracerebroventricular PGD2 in rats has been reproduced in a study by Kandasamy and Hunt (26) and in the present study. On the contrary, Gao et al. (17) reported that PGD2 administered into the cisterna magna does not cause hypothermia in rats and, in fact, causes a delayed fever. The basis for this conflicting observation is unknown, but could be related to an insufficient amount of intracisternal PGD2 reaching the preoptic area or to the fact that Gao et al. (17) briefly anesthetized rats at the time of PGD2 administration. Another consideration is that the spontaneous decomposition of PGD2 in aqueous solutions is faster than the decomposition of many other prostaglandins (10). Presumably for this reason, we observed that PGD2 solutions caused hypothermia only if stored no longer than 3 wk at −80°C.
Although food deprivation similarly enhanced the LPS-induced surges in PGD2 and PGE2, it selectively intensified the responsiveness of rats to the cryogenic action of PGD2 without interfering with the responsiveness to febrigenic PGE2. This is an important finding of the present study as it reveals that enhancement of cryogenic signaling via PGD2 is associated with enhancement of LPS hypothermia by food deprivation. Regarding PGE2, the augmented rise in its plasma level may have functional implications to prevent excessive falls in Tb. It should be noted, however, that any augmentation in PGE2 levels by food deprivation does not seem to have functional implication for LPS fever at neutral Ta, as fever was not enhanced by food deprivation. There are at least two possible interpretations to this intriguing observation. On one hand, it is possible that febrigenic signaling reaches a ceiling at PGE2 levels encountered in free-feeding rats so that it remains unchanged even when PGE2 levels are further increased in food-deprived rats. On the other hand, it is possible that food deprivation does not enhance the LPS-induced surge in PGE2 levels when rats are exposed to neutral Ta, even though it does so when rats are exposed to subneutral Ta. Future studies are necessary to investigate whether Ta has the ability to change the network of inflammatory mediators.
Another important finding of the present study is that enhancement of LPS hypothermia by food deprivation was not associated with impairment of the immune activation of corticosterone production. Such an impairment may occur following relatively long (at least 2 days) periods of food deprivation (16), and it would be expected to result in exaggeration of LPS hypothermia (33, 49), but it did not occur when rats in the present study were deprived of food for a single day. On the contrary, the regimen of food deprivation employed in the present study elevated the absolute concentration of corticosterone in the plasma of LPS-treated rats, even though the relative (LPS-induced) change in corticosterone was not altered. Nevertheless, the elevation in the absolute concentration of corticosterone was not strong enough to suppress LPS-induced surges in cytokines (which were unaltered by food deprivation), LPS-induced surges in prostaglandins (which were augmented by food deprivation), or LPS-induced hypothermia (which was enhanced by food deprivation).
In conclusion, the present study supports the hypothesis that food deprivation alters thermoregulatory responses to LPS mainly by enhancing cryogenic inflammatory signaling, and not by suppressing febrigenic signaling, at least not to a considerable extent. Enhancement of cryogenic signaling by food deprivation may involve PGD2, as food deprivation not only enhanced the level of PGD2 during the hypothermic response to LPS, but also increased the responsiveness of rats to the cryogenic (hypothermic) action of PGD2.
Perspectives and Significance
The present study paves a new path for researching the mechanisms linking energy balance to cryogenic inflammatory signaling via PGD2. Future studies will be necessary to determine which molecular alterations account for the enhanced responsiveness to cryogenic PGD2 in food-deprived animals. Such alterations may occur at the level of PGD2 receptor expression and signaling (9, 44), as well as at the level of PGD2 transport across the blood-brain barrier (25, 27). Future studies will also be necessary to determine how feeding status interferes with each step of the PGD2 biosynthetic pathway, which involves three sequential reactions catalyzed by phospholipase A2, cyclooxygenase (also known as PGH synthase), and PGD synthase (47). Of these enzymes, cyclooxygenase isoforms have been shown to play important roles in LPS-induced fever and hypothermia, with fever being mediated by PGE2 produced via cyclooxygenase-2 (15, 39, 41, 68) and hypothermia being mediated by a yet unidentified product of cyclooxygenase-1 (67). Such a product may be PGD2 because of its cryogenic action (present study and Refs. 26, 78), although it should be noted that the direct involvement of PGD2 in the mediation of LPS hypothermia has not yet been demonstrated. Inhibitors of PGD synthase and antagonists of PGD2 receptors are becoming available, but their ability to block LPS hypothermia has never been tested. However, it is surprising that EDJ300520, a drug that preferentially inhibits the synthesis of PGD2 over PGE2 in macrophages, has been reported to attenuate LPS fever in rats (18). As EDJ300520 awaits a more detailed pharmacological characterization, the possibility that it attenuates fever via a mechanism other than inhibition of PGD2 synthesis needs to be considered. Another possibility is that PGD2 might have a dual role in mediating both fever and hypothermia. For instance, while peripherally acting PGD2 may be febrigenic (as suggested in Ref. 18), centrally acting PGD2 seems to be cryogenic (present study and Refs. 26, 78). It is worth mentioning that the relevance of the present study may extend beyond thermoregulation, since PGD2 may be involved in other aspects of the pathophysiology of systemic inflammation, including sleep and pain (48, 74).
GRANTS
The study was supported by funds from the Albany College of Pharmacy and Health Sciences (to A. A. Steiner) and by a National Institute of Health Grant R15-AI072744 (to C. Feleder). X. Yao was the recipient of a scholarship from the China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Luciana B. Lopes for critical comments on the manuscript and Dr. HaiAn Zheng (both from Albany College of Pharmacy and Health Sciences, Albany, NY) for assistance with the liquid chromatography-mass spectrometry system.
REFERENCES
- 1.Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 23: 3359–3367, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE 1: e1, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med 27: 699–707, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Baracos VE, Whitmore WT, Gale R. The metabolic cost of fever. Can J Physiol Pharmacol 65: 1248–1254, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Bibby DC, Grimble RF. Temperature and metabolic changes in rats after various doses of tumour necrosis factor α. J Physiol 410: 367–380, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilbo SD, Nelson RJ. Melatonin regulates energy balance and attenuates fever in Siberian hamsters. Endocrinology 143: 2527–2533, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Blatteis CM. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther 111: 194–223, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Blatteis CM. Role of the OVLT in the febrile response to circulating pyrogens. Prog Brain Res 91: 409–412, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cao H, Xiao L, Park G, Wang X, Azim AC, Christman JW, van Breemen RB. An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Anal Biochem 372: 41–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemmer TP, Fisher CJ, Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. Crit Care Med 20: 1395–1401, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Coelho MM, Souza GE, Pela IR. Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol 263: R423–R427, 1992 [DOI] [PubMed] [Google Scholar]
- 13.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA. Proinflammatory cytokines. Chest 118: 503–508, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 6: 1137–1138, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol 156: 1781–1787, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W, Schmidtko A, Lu R, Brenneis C, Angioni C, Schmidt R, Geisslinger G. Prostaglandin D2 sustains the pyrogenic effect of prostaglandin E2. Eur J Pharmacol 608: 28–31, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Gao W, Schmidtko A, Wobst I, Lu R, Angioni C, Geisslinger G. Prostaglandin D2 produced by hematopoietic prostaglandin D synthase contributes to LPS-induced fever. J Physiol Pharmacol 60: 145–150, 2009 [PubMed] [Google Scholar]
- 19.Giovambattista A, Chisari AN, Corro L, Gaillard RC, Spinedi E. Metabolic, neuroendocrine and immune functions in basal conditions and during the acute-phase response to endotoxic shock in undernourished rats. Neuroimmunomodulation 7: 92–98, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Nagasaka T. Suppression of norepinephrine-induced thermogenesis in brown adipose tissue by fasting. Am J Physiol Endocrinol Metab 245: E582–E586, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic β3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Il'yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol 14: 793–797, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Inoue W, Somay G, Poole S, Luheshi GN. Immune-to-brain signaling and central prostaglandin E2 synthesis in fasted rats with altered lipopolysaccharide-induced fever. Am J Physiol Regul Integr Comp Physiol 295: R133–R143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IUPSThermal Commission Glossary of terms for thermal physiology. Jpn J Physiol 51: 245–280, 2001 [Google Scholar]
- 25.Ivanov AI, Scheck AC, Romanovsky AA. Expression of genes controlling transport and catabolism of prostaglandin E2 in lipopolysaccharide fever. Am J Physiol Regul Integr Comp Physiol 284: R698–R706, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kandasamy SB, Hunt WA. Involvement of prostaglandins and histamine in radiation-induced temperature responses in rats. Radiat Res 121: 84–90, 1990 [PubMed] [Google Scholar]
- 27.Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, Busija DW. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol 100: 1392–1399, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kleitman N, Satinoff E. Behavioral responses to pyrogen in cold-stressed and starved newborn rabbits. Am J Physiol Regul Integr Comp Physiol 241: R167–R172, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 71: 93–127, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science 188: 166–168, 1975 [PubMed] [Google Scholar]
- 31.Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol Regul Integr Comp Physiol 269: R23–R29, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Kozak W, Kluger MJ, Tesfaigzi J, Kozak A, Mayfield KP, Wachulec M, Dokladny K. Molecular mechanisms of fever and endogenous antipyresis. Ann NY Acad Sci 917: 121–134, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Krakauer T, Buckley M. Dexamethasone attenuates staphylococcal enterotoxin B-induced hypothermic response and protects mice from superantigen-induced toxic shock. Antimicrob Agents Chemother 50: 391–395, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 10: 1131–1133, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Leon LR. Cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol 92: 2648–2655, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Leon LR, Kozak W, Rudolph K, Kluger MJ. An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol Regul Integr Comp Physiol 276: R81–R89, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol Regul Integr Comp Physiol 275: R269–R277, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Levine S, Saltzman A. Feeding sugar overnight maintains metabolic homeostasis in rats and is preferable to overnight starvation. Lab Anim 34: 301–306, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Perlik V, Feleder C, Tang Y, Blatteis CM. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol 290: R1262–R1270, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 20: 3023–3029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci 9: 2819–2826, 2004 [DOI] [PubMed] [Google Scholar]
- 42.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Molnar D, Milner RD. Metabolic and hormonal response to endotoxin fever in fed and starved one-week rabbits. Biol Neonate 44: 309–314, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 98: 1942–1948, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19: 67–74, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Mouihate A, Boisse L, Pittman QJ. A novel antipyretic action of 15-deoxy-Δ12,14-prostaglandin J2 in the rat brain. J Neurosci 24: 1312–1318, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res 43: 3–35, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci 8: d520–d550, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Ochalski SJ, Hartman DA, Belfast MT, Walter TL, Glaser KB, Carlson RP. Inhibition of endotoxin-induced hypothermia and serum TNF-α levels in CD-1 mice by various pharmacological agents. Agents Actions 39: C52–C54, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Oida H, Hirata M, Sugimoto Y, Ushikubi F, Ohishi H, Mizuno N, Ichikawa A, Narumiya S. Expression of messenger RNA for the prostaglandin D receptor in the leptomeninges of the mouse brain. FEBS Lett 417: 53–56, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol 294: R1294–R1303, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Romanovsky AA. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol 287: R992–R995, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10: 2193–2216, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Romanovsky AA, Ivanov AI, Karman EK. Blood-borne, albumin-bound prostaglandin E2 may be involved in fever. Am J Physiol Regul Integr Comp Physiol 276: R1840–R1844, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92: 2667–2679, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am J Physiol Regul Integr Comp Physiol 271: R244–R253, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol 270: R693–R703, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses 50: 219–226, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Roth J, Harre EM, Rummel C, Gerstberger R, Hubschle T. Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci 9: 290–300, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Sakurada S, Shido O, Sugimoto N, Hiratsuka Y, Yoda T, Kanosue K. Autonomic and behavioural thermoregulation in starved rats. J Physiol 526: 417–424, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shido O, Nagasaka T, Watanabe T. Blunted febrile response to intravenous endotoxin in starved rats. J Appl Physiol 67: 963–969, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Shojoony MJ. Effects of fasting on heat balance and nonshivering thermogenesis in febrile adult guinea pigs. J Therm Biol 10: 239–243, 1985 [Google Scholar]
- 63.Sivitz WI, Fink BD, Donohoue PA. Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology 140: 1511–1519, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Steiner AA, Branco LG. Hypoxia-induced anapyrexia: implications and putative mediators. Annu Rev Physiol 64: 263–288, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se–not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol 289: R348–R352, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Steiner AA, Dogan MD, Ivanov AI, Patel S, Rudaya AY, Jennings DH, Orchinik M, Pace TW, O'Connor KA, Watkins LR, Romanovsky AA. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J 18: 1949–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or -2–which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol 297: R485–R494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, Romanovsky AA. Cellular and molecular bases of the initiation of fever. PLoS Biol 4: e284, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steiner AA, Romanovsky AA. Leptin: at the crossroads of energy balance and systemic inflammation. Prog Lipid Res 46: 89–107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steiner AA, Rudaya AY, Robbins JR, Dragic AS, Langenbach R, Romanovsky AA. Expanding the febrigenic role of cyclooxygenase-2 to the previously overlooked responses. Am J Physiol Regul Integr Comp Physiol 289: R1253–R1257, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Sugimoto N, Simons CT, Romanovsky AA. Vagotomy does not affect thermal responsiveness to intrabrain prostaglandin E2 and cholecystokinin octapeptide. Brain Res 844: 157–163, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Szekely M. Nutritional state and endotoxin fever of newborn rabbits. Acta Physiol Acad Sci Hung 53: 279–283, 1979 [PubMed] [Google Scholar]
- 73.Szekely M, Romanovsky AA. Pyretic and antipyretic signals within and without fever: a possible interplay. Med Hypotheses 50: 213–218, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Telleria-Diaz A, Ebersberger A, Vasquez E, Schache F, Kahlenbach J, Schaible HG. Different effects of spinally applied prostaglandin D2 on responses of dorsal horn neurons with knee input in normal rats and in rats with acute knee inflammation. Neuroscience 156: 184–192, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Tollner B, Roth J, Storr B, Martin D, Voigt K, Zeisberger E. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflügers Arch 440: 925–932, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Toussirot E, Wendling D. The use of TNF-α blocking agents in rheumatoid arthritis: an overview. Expert Opin Pharmacother 5: 581–594, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79: 1–71, 1999 [DOI] [PubMed] [Google Scholar]
- 78.Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc Natl Acad Sci USA 79: 6093–6097, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395: 281–284, 1998 [DOI] [PubMed] [Google Scholar]
- 80.Yamashita A, Watanabe Y, Hayaishi O. Autoradiographic localization of a binding protein(s) specific for prostaglandin D2 in rat brain. Proc Natl Acad Sci USA 80: 6114–6118, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.