Abstract
Nesfatin-1 is an 82-amino acid protein encoded by the nucleobindin2 gene. When injected intracerebroventricularly, nesfatin-1, via a melanocortin ¾ receptor-dependent mechanism, potently decreased both food and water intakes and elevated mean arterial pressure in a dose-related manner. Because nesfatin-1 colocalized with oxytocin in hypothalamus and because nesfatin-1 had direct depolarizing effects on oxytocin-producing neurons in hypothalamic slice preparations, we hypothesized that the actions of nesfatin-1 required the presence of functional oxytocin receptors. We, therefore, pretreated conscious, unrestrained male rats with the oxytocin receptor antagonist, ornithine vasotocin (OVT), before treatment with nesfatin-1. We found that pretreatment with OVT reversed the effects of nesfatin-1 on both food and water intake and on mean arterial pressure, indicating that the central oxytocin system is a downstream mediator of these actions of nesfatin-1. Additionally, we found that OVT reversed the anorexigenic effect of α-melanocyte-stimulating hormone (α-MSH), suggesting that the central oxytocin system is downstream of the central melanocortin system. Taken together, these data suggest that nesfatin-1 acts through a serial neuronal circuit, in which nesfatin-1 activates the central melanocortin system, which, in turn, acts through the central oxytocin system, leading to an inhibition of food and water intake and an increase in mean arterial pressure.
Keywords: blood pressure, food intake
nesfatin-1 is a recently discovered, 82-amino acid protein derived from the nucleobindin2 precursor (21). Nesfatin-1 is produced in several hypothalamic nuclei, such as the paraventricular nucleus (PVN), supraoptic nucleus, arcuate nucleus (ARC), and lateral hypothalamic area (21), and in extra-hypothalamic areas as well, including the raphe pallidus, the Edinger-Westphal nucleus, and the nucleus of the solitary tract (NTS) (12). Although nesfatin-1 has been shown to colocalize with several well-described peptides, including cocaine and amphetamine-regulated transcript, CRF, oxytocin, and vasopressin, nesfatin-1 has not been visualized in axon terminals (12). This has led several groups (12, 30, 32) to speculate that nesfatin-1 does not signal via a classical axonal mechanism, but rather is released dendritically to act locally in a paracrine or autocrine fashion.
Nesfatin-1 originally was shown to be a potent inhibitor of both food and water intake via a leptin-independent, melanocortin receptor-dependent mechanism (21, 32). Nesfatin-1 is likely a physiologically relevant regulator of food intake, as chronic central administration of a morpholino antisense oligonucleotide led to exaggerated food intake and weight gain over missense-injected controls (21). Additionally, plasma levels of nesfatin-1 were significantly reduced by fasting, and this effect was reversed by refeeding (30). We recently have shown that nesfatin-1 increases mean arterial pressure (MAP) when injected into the lateral cerebroventricle (32). This effect, like the effect of nesfatin-1 on food intake, was blocked by pretreatment with the melanocortin ¾ receptor antagonist, SHU9119 (32). The increase in MAP induced by nesfatin-1 also was blocked by pretreatment with the nonspecific α-adrenergic antagonist, phentolamine, suggesting that nesfatin-1 acts through the central melanocortin system to increase sympathetic nervous system activity, leading to an elevation in MAP (32). In addition to the central melanocortin system, it appears that a forebrain site of action involving the recruitment of CRF neurons underlies the anorexigenic action of nesfatin-1, when administered into the lateral, but not the fourth, cerebroventricle (30). It is unknown, however, what other neuronal populations may be involved in the expression of the anorexigenic and hypertensive effects of nesfatin-1.
Oxytocin was originally described on the basis of its effects on uterine contractility and mammary tissue (8, 10). The central oxytocin system, which comprises both magnocellular (projecting to posterior pituitary and locally acting via dendritic release) and parvocellular (projecting to brain stem and other brain sites) oxytocin-producing neurons, is important for the expression of maternal, sexual, and feeding behaviors, and the control of cardiovascular function as well (19, 26). Because nesfatin-1 colocalized with oxytocin in neurons in the PVN (12), and because nesfatin-1 had direct depolarizing effects on oxytocin neurons in hypothalamic slice preparations (22), we hypothesized that the central oxytocin system was a downstream mediator of the anorexigenic, antidipsogenic, and hypertensive activities of nesfatin-1. Indeed, a recent report (16) demonstrated that the inhibitory effect of nesfatin-1 on food intake was dependent on central oxytocin receptors. Here, we confirm the findings of Maejima et al. (16) on food intake and add that the effects of nesfatin-1 on water intake and on MAP could be blocked by pretreatment with the oxytocin receptor antagonist, ornithine vasotocin (OVT). We also add that the anorexigenic action of α-melanocyte-stimulating hormone (α-MSH) was reversed by pretreatment with OVT, suggesting that nesfatin-1 acts through a serial neuronal circuit, in which nesfatin-1 activates the central melanocortin system, which, in turn, acts through the central oxytocin system to increase MAP and inhibit food and water intake.
MATERIALS AND METHODS
All procedures and protocols have been approved by the Saint Louis University Animal Use and Care Committee (protocol number 2041). Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed under controlled conditions (23–25°C, lights on 0600–1800) with free access to food and water. Rats (225–250 g; ∼7–8 wk of age) were anesthetized with a mixture of ketamine (60 mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8 mg/ml; TranquiVed, VedCo, Saint Joseph, MO) at a dose of 0.1 ml/100 g body wt, injected intraperitoneally, as previously described (32). Buprenorphine (0.05 mg/kg body wt, injected subcutaneously) was administered postanesthesia for analgesia. Fluid replacement included a subcutaneous injection of sterile saline (0.9% NaCl) to balance anticipated fluid loss (3:1). A stainless-steel cannula (23 mm, 17 gauge) was implanted into the right lateral cerebroventricle using a stereotaxic device (coordinates relative to the interaural line: A: +6.2, H: +7.4, L: −0.9) and immobilized using dental cement. Rats were then housed singly and observed for at least 4 days following surgery to ensure recovery of presurgery weight. Placement and patency of the intracerebroventricular cannula were confirmed by the dipsogenic effect of ANG II (50 pmol icv) (28).
For feeding studies, rats bearing an intracerebroventricular cannula were habituated to metabolic cages (Nalgene) for 3 days. Food and water intakes and body weights were monitored daily to ensure health. On the day of the experiment, food and water were removed from the cages at 1650, and rats were pretreated intracerebroventriclarly with 2 μl saline vehicle or 10 μg [d-(CH2)5,Tyr(me)2,Orn8]-vasotocin (OVT) (1). One to ten minutes later, rats were then injected with saline, 60 pmol nesfatin-1, an anorexigenic dose of oxytocin (0.5 μg) (20), or an anorexigenic dose of α-MSH (1.0 nmol) (24). Food and water bottles were replaced 10 min later, and food and water intakes were monitored at 30-min intervals for 1 h during the light phase (1700–1800) and 3 h during the dark phase (1800–2100), and again 24 h later. Experiments are conducted during this time frame so as to coincide with the natural light-entrained feeding cycle of our rat colony.
For cardiovascular experiments, an additional polyethylene cannula (PE-50) was implanted into the left carotid artery of rats minimally 5 days after implantation of the intracerebroventricular cannulas, and exteriorized between the shoulder blades as previously described (32). The cannula was filled with heparinized saline (200 U/ml in 0.9% NaCl). The next day (during lights on, between 0600 and 1800), rats were placed in a quiet room, and after minimally 2 h habituation, the carotid cannula was connected to a pressure transducer (DigiMed Blood Pressure Analyzer, Micro-Med, Louisville, KY). Baseline MAP and heart rate (HR) then were recorded at 1-min intervals for at least 30 min. Rats were pretreated with either 2 μl saline vehicle or vehicle containing 10 μg icv OVT. Ten minutes later, rats were treated with either saline vehicle or 180 pmol icv nesfatin-1, and MAP and HR were recorded for at least 15 min at 1-min intervals. Data are represented as change from preinjection baseline, which was determined by averaging the MAP or HR values for 5 min before injection of nesfatin-1 or saline vehicle.
To determine plasma oxytocin levels, rats that were habituated to a quiet room were injected intracerebroventricularly with either saline vehicle or 180 pmol nesfatin-1. Ten minutes later, rats were killed by rapid decapitation, and trunk blood was collected. Plasma oxytocin levels were determined by radioimmunoassay, as previously described (27). The lower limit of sensitivity was defined as minimally 95% of total binding (2 pg/ml plasma).
Feeding data were analyzed using ANOVA with Scheffé's multiple comparison test. Cardiovascular data were analyzed using a nonparametric test (Mann-Whitney U), as MAP/HR data were transformed to represent change from preinjection baseline because of the natural variation of resting MAP/HR between animals. Radioimmunoassay data were analyzed using a t-test. All peptides were purchased from Phoenix Pharmaceuticals (Burlingame, CA). Doses of 60 pmol and 180 pmol nesfatin-1 were used for feeding experiments and cardiovascular experiments, respectively, because these were the most effective doses in each experimental paradigm (32). The dose of OVT used in this study was determined based on the dose previously reported in the literature (1).
RESULTS
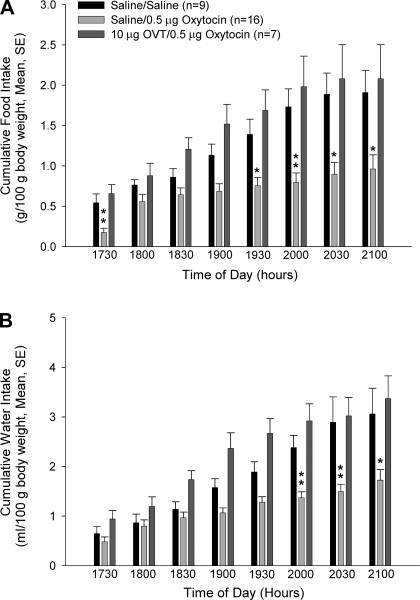
Oxytocin inhibited both food and water intake when injected into the lateral cerebroventricle (Fig. 1, A and B). Although food intake was decreased at all time points compared with saline-injected controls, this effect was most clear during later time points, when the effect attained significance. The effect of oxytocin on water intake was less robust than the effect on food intake but did attain significance during the last three time intervals. Pretreatment with the oxytocin receptor antagonist, OVT, reversed oxytocin-induced anorexia and adipsia.
Fig. 1.
The anorexigenic and antidipsogenic effects of oxytocin are blocked by pretreatment with ornithine vasotocin (OVT). Rats were pretreated with either saline vehicle or vehicle containing 10 μg OVT icv, and then they were administered either saline or 0.5 μg icv oxytocin. While oxytocin significantly inhibited both food (A) and water (B) intake, these effects were reversed by pretreatment with OVT. Data were analyzed using ANOVA with Scheffé's multiple comparison test (*P < 0.05, **P < 0.01, vs. saline-injected controls).
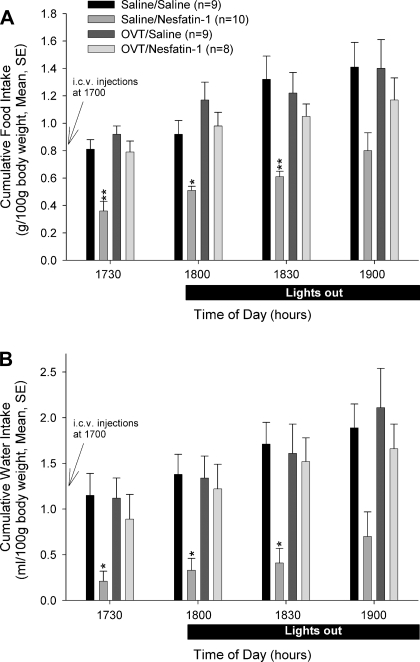
Central administration of 60 pmol nesfatin-1 significantly inhibited cumulative food intake as previously reported (21, 30, 32). This decrease attained significance at the first three sampling periods compared with cumulative food intakes in animals administered saline vehicle alone or the oxytocin antagonist and saline (nesfatin-1 induced an ∼55% decrease in food intake compared with controls at 1730) (Fig. 2A). Pretreatment with OVT reversed the inhibitory action of nesfatin-1, but did not affect food or water intake when injected alone. This reversal attained significance at the 30- and 60-min sampling intervals. Cumulative food intakes in animals administered nesfatin-1 after OVT at no time point differed significantly from intakes observed in saline vehicle controls or animals administered OVT and saline. When the data were analyzed by ANOVA, no significant differences in cumulative food intakes among treatment groups were observed at any subsequent (following 1900) sampling intervals (data not shown).
Fig. 2.
The anorexigenic and antidipsogenic effects of nesfatin-1 are blocked by pretreatment with OVT. Rats were pretreated intracerebroventricularly with either saline vehicle or vehicle containing 10 μg OVT, then treated with either saline vehicle or 60 pmol nesfatin-1. While nesfatin-1 significantly reduced both food (A) and water (B) intake, these effects were reversed by pretreatment with OVT. Data were analyzed using ANOVA with Scheffé's multiple comparisons (*P < 0.05, **P < 0.01, vs. saline-injected controls).
Central administration of 60 pmol nesfatin-1 significantly inhibited cumulative water intake as previously reported (32). This decrease attained significance at the first three sampling periods compared with cumulative water intakes in animals administered saline vehicle alone or the oxytocin antagonist and saline (nesfatin-1 induced an ∼82% decrease in water intake compared with controls at 1730) (Fig. 2B) and in the fourth interval (1900) compared with water consumed in OVT and saline-treated animals. Cumulative water intakes in animals administered nesfatin-1 after OVT at no time point differed significantly from intakes observed in saline vehicle controls or animals administered OVT and saline. However, a significant reversal of the inhibitory effect of nesfatin-1 on water drinking was observed following OVT pretreatment at 90 min. While more water was consumed by animals treated with OVT and nesfatin-1 compared with those treated with saline and nesfatin-1 at all sampling times, this attained significance only at the 90-min sampling interval. Increased water consumption was observed in the nesfatin-1-treated animals following the fourth sampling interval (after 1900), such that no significant differences in cumulative water consumption were observed compared with controls thereafter (data not shown).
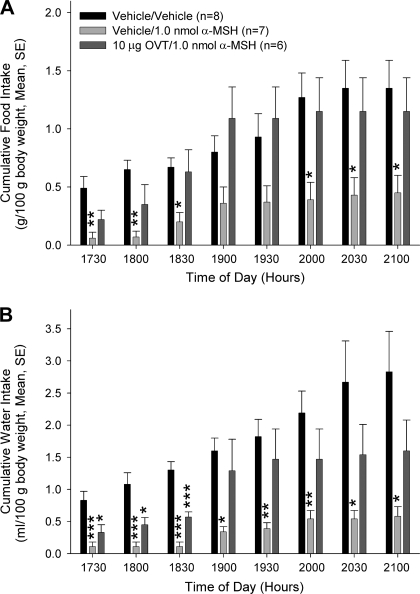
Because the anorexigenic and antidipsogenic effects of nesfatin-1 were completely reversed by pretreatment with a melanocortin ¾ receptor antagonist (21, 32) and an oxytocin receptor antagonist (Fig. 2, A and B; 16), we hypothesized that nesfatin-1 acts through a serial neuronal circuit to exert its activities. Since melanocortin agonists previously were shown to increase oxytocin release (25), we reasoned that the central oxytocin system acts as a downstream mediator of the central melanocortin system. To test this hypothesis, we pretreated rats with either saline or OVT intracerebroventricularly prior to central administration of saline or an anorexigenic dose of α-MSH (24). Animals that received saline and α-MSH consumed significantly less food and water than animals that were injected with saline alone (Fig. 3, A and B). However, the effect of α-MSH on food intake was reversed by pretreatment with OVT. OVT also reversed the effect of α-MSH on water intake, although this effect did not attain significance until 1900.
Fig. 3.
The anorexigenic and antidipsogenic effects of α-melanocyte stimulating hormone (α-MSH) are blocked by pretreatment with OVT. Rats were pretreated with either saline vehicle or vehicle containing 10 μg icv OVT, then administered either saline or an anorexigenic dose (1.0 nmol icv) of α-MSH. While α-MSH significantly inhibited both food (A) and water (B) intake, these effects were reversed by pretreatment with OVT. Data were analyzed using ANOVA with Scheffé's multiple comparisons (*P < 0.05, **P < 0.01, ***P < 0.001, vs. saline-injected controls).
Because nesfatin-1 acts through the central melanocortin system to affect both food and water intake and MAP (21, 32), we sought to determine whether the central oxytocin system was a point of convergence or divergence for the different effects of the protein (i.e., appetitive vs. autonomic). We, therefore, tested the ability of OVT to block the hypertensive action of nesfatin-1.
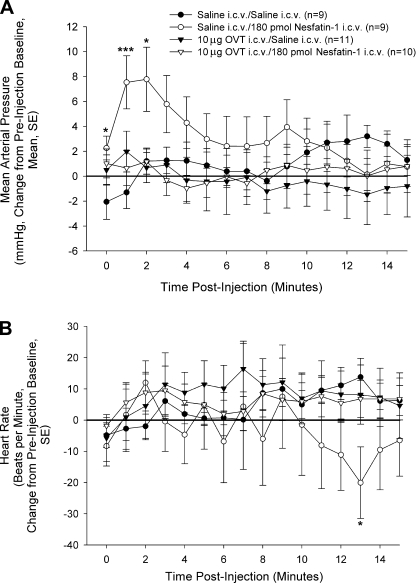
In conscious, freely moving rats, 180 pmol nesfatin-1 led to significant increases in MAP (Fig. 4A), as previously reported (32). Pretreatment with 10 μg OVT did not affect resting MAP (Table 1), nor did OVT/saline-treated rats exhibit any alterations from preinjection baseline. However, pretreatment with OVT completely abolished the stimulatory effect of nesfatin-1 on MAP. No significant increases in HR were observed in any of the treatment groups (Fig. 4B). HR was only significantly decreased in saline/nesfatin-1-treated rats at one time point, 13 min postinjection (preinjection HR baselines: saline/saline = 388 ± 10; saline/nesfatin-1 = 387 ± 11; OVT/saline = 408 ± 14; OVT/nesfatin-1 = 388 ± 19).
Fig. 4.
Nesfatin-1-induced elevation in mean arterial pressure (MAP) is blocked by pretreatment with OVT. Rats bearing intracerebroventricular and carotid cannulas were pretreated with 10 μg icv OVT or saline vehicle. Ten minutes later, rats were treated with either saline vehicle or 180 pmol nesfatin-1 (time 0). While nesfatin-1 led to significant increases in MAP (A), this effect was blocked by pretreatment with OVT. None of the treatment groups exhibited any significant increases in heart rate (HR; B). Data are represented as change from preinjection baseline (average 5 min before intracerebroventricular injection). Data were analyzed using a Mann-Whitney U test (*P < 0.05, ***P < 0.001, vs. saline-injected controls).
Table 1.
Baseline MAP values before and after OVT pretreatment for saline, saline and nefatin, OVT and saline, and OVT and nesfatin-1 in rats
| Treatment | n | Baseline Before OVT Pretreatment | Baseline After OVT Pretreatment |
|---|---|---|---|
| Saline/saline | 9 | 124.3 ± 4.3 | 123.1 ± 4.2 |
| Saline/180 pmol nesfatin-1 | 9 | 116.6 ± 2.8 | 118.7 ± 2.5 |
| 10 μg OVT/saline | 11 | 123.7 ± 4.2 | 127.7 ± 3.6 |
| 10 μg OVT/180 pmol nesfatin-1 | 10 | 136.0 ± 3.6 | 133.6 ± 3.8 |
Data are presented as average MAP ± SE for 5 min before or after pretreatment with either 10 μg icv OVT or saline vehicle. Neither ornithine vasotocin (OVT) nor saline pretreatment altered baseline mean arterial pressure (MAP).
The effects of nesfatin-1 were shown previously to be dependent on the central melanocortin system (21, 32). Because melanocortin agonists were shown to stimulate the release of oxytocin from dendrites but inhibit axonal release of oxytocin (25), we sought to determine whether central injection of nesfatin-1 would alter plasma oxytocin levels. Rats were, therefore, treated with either saline or 180 pmol icv nesfatin-1. Ten minutes later, trunk bloods were collected and plasma oxytocin levels were determined by radioimmunoassay. Nesfatin-1 did not significantly alter plasma oxytocin levels [saline = 10.4 ± 0.6 (n = 14); nesfatin-1 = 9.8 ± 1.0 (n = 14)].
DISCUSSION
Oxytocin has been shown to act in brain to inhibit food intake (20) and alter cardiovascular function (19, 31a) and is a potential downstream mediator of the cardiovascular effects of substance P (17) and neuropeptide FF (13). Oxytocin colocalizes with nesfatin-1 in the PVN (12, 14), and nesfatin-1 has direct depolarizing effects on oxytocin neurons in hypothalamic slice preparations (22). These findings suggest that nesfatin-1 could interact with central oxytocin receptors to exert its anorexigenic and hypertensive effects.
In these studies, we found that the actions of nesfatin-1 on food and water intake and MAP could be blocked by pretreatment with the oxytocin receptor antagonist, OVT, suggesting that functional oxytocin receptors are required to mediate the actions of nesfatin-1. Although it has been reported previously (11) that OVT alone led to an exaggeration of water intake, this effect was not observed in our experiments. This is likely due to strain differences, as the studies by Fitts et al. (11) used Long-Evans rats, while all of our experiments were conducted using Sprague-Dawley rats. In our experiments, OVT did not alter food intake. This is in accordance with a previous report (3), which demonstrated that OVT does not lead to exaggerated food intake unless injected into the fourth ventricle (our injections are made into the lateral ventricle). We previously have reported that the activities of nesfatin-1 also require the presence of functional melanocortin receptors (32). While it is possible that the central melanocortin system and the central oxytocin system operate in parallel to simultaneously exert the effects of nesfatin-1, these two neuronal circuits may also act in series. Melanocortin agonists and oxytocin exerted similar actions on a variety of physiological functions when injected into the brain, including the initiation of the yawning-stretching reflex and sexual behaviors (26). Central administrations of oxytocin (20, 23, 31a) and melanocortin agonists (7) led to a potent inhibition of food intake and altered cardiovascular function. These similarities suggest a circuit in series.
Several lines of evidence support the hypothesis that oxytocin is a downstream mediator of the central melanocortin system. Intracerebroventricular administration of the melanocortin ¾ receptor agonist, α-MSH led to c-Fos accumulation in oxytocin-producing neurons (6). Pretreatment with OVT reversed the anorexigenic effect of leptin (3), a peptide that is dependent on the central melanocortin system to exert its activities (9). Here, we also show that the anorexigenic effect of α-MSH is reversed by pretreatment with OVT (see Fig. 3). Additionally, α-MSH was shown to increase the dendritic release of oxytocin in hypothalamus, but to inhibit the release of oxytocin from axon terminals in the posterior pituitary gland leading to a decrease in plasma oxytocin (OT) levels (25). Nesfatin-1 may indeed increase dendritic release of oxytocin, as a recent report (16) indicated that nesfatin-1 led to an increase in oxytocin secretion within the PVN, and evidence from our laboratory indicates that nesfatin-1 does not affect basal plasma levels of oxytocin in conscious male rats. In our experiments, we did not observe a decrease in plasma OT levels, unlike the decrease in oxytocin reported by Sabatier et al. (25) after treatment with melanocortin agonists. The difference is likely due to differences in model systems. Sabatier et al. (25) conducted their studies using female rats that were injected with hypertonic saline to increase basal oxytocin levels, while all of our experiments were performed using male rats, in which basal oxytocin levels were extremely low. Nesfatin-1 may also increase axonal release of oxytocin from parvocellular neurons, as nesfatin-1 led to an increase in c-Fos expression in parvocellular PVN neurons (16). A recent report (31) also provided evidence that conditional Sim1 knockout mice are hyperphagic and obese, and they exhibit a marked reduction in melanocortin 4 receptor and oxytocin expression in the hypothalamus. Their data support our hypothesis of an action of nesfatin-1 on proopiomelanocortin (POMC) neurons in ARC, resulting in the activation of OT neurons in PVN by α-MSH derived from those ARC POMC neurons.
Stengel et al. (30) have reported that nesfatin-1 injected into the lateral cerebroventricle reduced food intake, and this effect was reversed with the CRF2 receptor antagonist, astressin2-B (30), suggesting an action of nesfatin-1 on CRF neurons in the forebrain. CRF is another potential downstream mediator of the central melanocortin system, as the anorexigenic effect of the melanocortin agonist MTII was abolished by a CRF receptor antagonist (15), and Briscoe et al. (5) demonstrated that, like nesfatin-1 (32) and oxytocin (2, 19, 23, 31), central administration of CRF resulted in an increase in MAP. Thus, interactions with not only the melanocortin and oxytocin pathways, but also with forebrain CRF neurons may underlie the anorexigenic and the sympathostimulatory actions of nesfatin-1.
After the completion of our studies described here, while preparing this manuscript for publication, Maejima et al. (16) reported that the effect of nesfatin-1 on food intake was blocked by pretreatment with the oxytocin receptor antagonist, H4928. Those authors also provided evidence that pretreatment with the melanocortin ¾ receptor antagonist, SHU9119, reversed the effect of oxytocin on food intake, indicating that nesfatin-1 may act through the central oxytocin system to activate the central melanocortin system, leading to an inhibition of food intake. However, data from other groups (3, 6, 25), as mentioned above, and data from the studies described here suggest that the order of activation is reversed. Our data are in agreement with those of Maejima et al. (16), supporting a role for OT neurons in the anorexigenic action of nesfatin-1. Here, we add that an oxytocin receptor antagonist, OVT, also abrogated the antidipsogenic and hypertensive effects of nesfatin-1. These two additional, novel observations further support the hypothesis that the central nervous system's (CNS's) actions of nesfatin-1 are dependent upon not only the activation of the central melanocortin system (21, 32), but also on the activation of OT neurons that project to CNS sites known to be important in the control of appetite and autonomic regulation.
Perspectives and Significance
Since nesfatin-1 has not been visualized in axon terminals (4, 12) and because the protein was localized to secretory vesicles in perikarya (16), it is tempting to hypothesize that nesfatin-1 is a locally acting, modulatory protein. Target sites of nesfatin-1 action may include areas where the protein is produced, such as the hypothalamus and the NTS. Nesfatin-1 likely exerts the same activities in both of these (and in other) brain sites, since nesfatin-1 inhibited food intake when injected into the lateral ventricle, the third ventricle, the fourth ventricle, or cisterna magna (21, 30, 32). Interestingly, Skibicka and Grill (29) recently have shown that melanocortin agonists increased heart rate and thermogenesis and inhibited food intake regardless of the injection site (two forebrain sites and three hindbrain sites). Which neuronal system(s) is (are) the primary target of nesfatin-1 remains unknown. Nesfatin-1 colocalized with both oxytocin and POMC in brain (12, 14), and the actions of nesfatin-1 were blocked by pretreatment with both SHU9119 (21, 32) and oxytocin receptor antagonists (16, this article). Further studies are required to determine whether nesfatin-1 is acting through these two systems simultaneously or whether there exists a hierarchy of neural networks organized to mediate the effects of nesfatin-1 on both appetite and autonomic function that has as an anatomic basis a shared series element, requiring activation initially of POMC and then OT neurons, or vice-versa, both potentially recruiting CRF neurons in the hypothalamus (30). Behavioral actions of nesfatin-1 (18, 32) may require activation of similar circuitry as well.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
G. L. C. Yosten is supported by a National Institutes of Health (NIH) predoctoral fellowship (5T32GM008306). W. K. Samson is supported by NIH Grant HL66023.
REFERENCES
- 1.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides 10: 89–93, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Bernatova I, Rigatto KV, Key MP, Morris M. Stress-induced pressor and corticosterone responses in oxytocin-deficient mice. Exp Physiol 89: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148: 5088–5094, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Briscoe RJ, Cabrera CL, Baird TJ, Rice KC, Woods JH. Antalarmin blockade of corticotropin releasing hormone-induced hypertension in rats. Brain Res 881: 204–207, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Caquineau C, Leng G, Guan XMM, Jiang M, Van der Ploeg L, Douglas AJ. Effects of alpha-melanocyte-stimulating hormone on magnocellular oxytocin neurons and their activation at intromission in male rats. J Neuroendocrinol 18: 685–691, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cone R. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 8.den Hertog CE, de Groot AN, van Dongen PW. History and use of oxytocics. Eur J Obstet Gynecol Reprod Biol 94: 8–12, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res Bull 50: 215–221, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG. The synthesis of oxytocin. J Am Chem Soc 76: 3115–3121, 1954 [Google Scholar]
- 11.Fitts DA, Thornton SN, Ruhf AA, Zierath DK, Johnson AK, Thunhorst RL. Effects of central oxytocin receptor blockade on water and saline intake, mean arterial pressure, and c-Fos expression in rats. Am J Physiol Regul Integr Comp Physiol 285: R1331–R1339, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156: 563–579, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Jhamandas JH, MacTavish D. Central administration of neuropeptide FF causes activation of oxytocin paraventricular hypothalamic neurons that project to the brainstem. J Neuroendocrinol 15: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149: 1295–1301, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23: 7863–7872, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh-I S, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Maier T, Dai WJ, Skikos T, Jirikowski GF, Unger T, Culman J. Oxytocin pathways mediate the cardiovascular and behavioral responses to substance P in the rat brain. Hypertension 31: 480–486, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology 201: 115–123, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. Am J Physiol Heart Circ Physiol 284: H2269–H2276, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol 20: 245–250, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Richard P, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiol Rev 71: 331–370, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effects of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139: 4428–4431, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XMM, Jiang M, Van der Ploeg L, Leng G. α-Melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci 23: 10351–10358, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatier N, Caquineau C, Douglas AJ, Leng G. Oxytocin released from magnocellular dendrites: a potential modulator of alpha-melanocyte-stimulating hormone behavioral actions? Ann NY Acad Sci 994: 218–224, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Samson WK, Baker JR, Samson CK, Samson HW, Taylor MM. Central neuropeptide B administration activates stress hormone secretion and stimulates feeding in male rats. J Neuroendocrinol 16: 842–849, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1505–R1509, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150: 5351–5361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Lambrecht NW, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology 150: 4911–4919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci 30: 3803–3812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Wsol A, Cudnoch-Jedrezejewska A, Szczepanska-Sadowska E, Kolwalewski S, Puchalska L. Oxytocin in the cardiovascular responses to stress. J Physiol Pharmacol 59: 123–127, 2008 [PubMed] [Google Scholar]
- 32.Yosten GLC, Samson WK. Nesfatin-1 exerts cardiovascular effects in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol 297: R330–R336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]