Abstract
Walter B. Cannon's research on the sympathetic nervous system and neurochemical transmission was pioneering. Wisdom has endowed our body with a powerful autonomic neural regulation of the circulation that provides optimal perfusion of every organ in accordance to its metabolic needs. Exquisite sensors tuned to an optimal internal environment trigger central and peripheral sympathetic and parasympathetic motor neurons and allow desirable and beneficial adjustments to physiologic needs as well as to acute cardiovascular stresses. This short review, presented as The Walter B. Cannon Memorial Award Lecture for 2009, addresses the mechanisms that disrupt sensory signaling and result in a chronic maladjustment of the autonomic neural output that in many cardiovascular diseases results in excessive increases in the risks of dying. The hopes for any reduction of those risks resides in an understanding of the molecular determinants of neuronal signaling.
Keywords: sensory transduction, baroreceptors, chemoreceptors, ion channels
a letter from Dr. Martin Frank, the Executive Director of American Physiological Society, informed me that I was selected by the President of the American Physiological Society, Dr. Irving H. Zucker, to receive the Walter B. Cannon Memorial Award Lectureship. The letter indicated that the Lectureship is designed to honor Cannon's concept of The Wisdom of the Body and is the greatest honor that the Society could bestow on any one of its members. The presentation was scheduled by tradition on the first day of the Experimental Biology meeting on Saturday, April 18, 2009 in the Ernest N. Morial Convention Center, New Orleans, LA. This was an exceptional honor. In the following essay, I try to capture the event first by quoting some personal reflections from my opening remarks, then offering some comments on Dr. Cannon's wisdom, followed by an overview of the scientific presentation. Here is how I started.
“My goodness, thank you, Irv. Thank you all for being here.”
“I really can't believe it. I started coming to this meeting 50 years ago. Now those of you trying to figure out my age–forget it. You know I come from the old country and there we start real early. I married Doris when I was 10!! (chuckles).”
“Those of you sitting out there for your first meeting, you know you can get to stand here where I am–yes you can–you just have to be patient and lucky, lucky enough to meet two very nice guys: Irv Zucker and Martin Frank. You also need to have a lot of fun along the way.”
“Seriously, I am enormously honored and enormously grateful. Perhaps I am most grateful for having been accepted in the United States 54 years ago, one of the most powerful countries on Earth. I speak not of military power, but of the character of its people, who honor, not just individual fulfillment, but the struggle to realize the very best that is in us and in those around us as we pursue our God-given talents. I also realize that the award transcends its recipients. It allows us to set our goals high. It reminds us of what is good in our society and of the rich legacy that our past leaders have left us and on whose shoulders we now stand.”
One of those giants is Walter Bradford Cannon (supplemental Fig. S1; supplemental figures for this article are available online at the American Journal of Physiology–Regulatory, Integrative and Comparative Physiology website). Cannon was born in 1871 in Prairie du Chien, Wisconsin. That same year, Harvey Pickering Bowditch (who would become Cannon's mentor and teacher) was declared the “first full-time physiologist in the United States.” Twenty-five years later, in 1896, Cannon, as a first-year medical student at Harvard, began to work in the Bowditch laboratory. The American Physiological Society was then 10 years old. Advance the clock only 10 years. It is now 1906, and Cannon, a 35-year-old physician-scientist, assistant professor, succeeds Bowditch as the chairman of the physiology department at Harvard. He was the sixth APS president (1914–1916) and remained chairman of physiology at Harvard for 36 years until 1942. He died 3 years later in 1945. His early research as a medical student used the innovative, sophisticated technology of the newly discovered Rontgen rays. His work was certainly translational. He studied, with X-rays, the mechanism of swallowing and the motility of the stomach in the goose. He presented his work at the American Physiological Society meeting in December 1896 and published his research in the first volume of the American Journal of Physiology in the May and July issues of 1898.
Cannon's later research on the sympathetic nervous system and neurochemical transmission was pioneering. With insightful vision he enunciated the physiological concept of homeostasis, which he expanded in his book on The Wisdom of the Body (5a). I enjoyed enormously reading his book entitled The Way of an Investigator (5b). It is essentially an autobiography, which I encourage you all to read.
In his advice on some working principles, Cannon wrote in his Way of an Investigator on p. 108, “…what happens in our bodies is directed towards a useful end. And in the search for utility new discoveries may result.” He disagreed with those who thought “…that the province of science is strictly description…” and that turning away from “how” to “why” is entering “the realm of speculation.” He quotes the German physiologist, E. Von Bruecke who remarked, “Teleology is a lady without whom no biologist can live. Yet he is ashamed to show himself with her in public.” (5b).
In this essay entitled “In search of autonomic balance: the good, the bad, and the ugly,” we show no shame in joining lady Teleology (supplemental Fig. S2). This overview will focus on three topics: 1) sympathovagal imbalance and reciprocal dysautonomia, 2) molecular components of sensory signals [acid-sensing ion channels (ASICs) as mechano- and chemosensors], and 3) reactive oxygen species (ROS) as the insidious instigator of sympathoexcitation. We will conclude that parasympathetic activity is good, sympathetic overactivity is bad, and ROS is the ugly instigator of sympathetic excitation.
SYMPATHOVAGAL IMBALANCE AND RECIPROCAL DYSAUTONOMIA
A powerful autonomic neural control of our cardiovascular system determines the optimal cardiac output and its distribution to our organs to meet the metabolic demands. The sympathetic innervation of the heart and blood vessels being excitatory, causes vasoconstriction and increases heart rate and cardiac contraction. In contrast, the parasympathetic vagal innervation is inhibitory and decreases heart rate and cardiac contraction (8, 9, 36, 39). Increases in sympathetic activity are necessary during the flight-or-fight response, whereas increases in parasympathetic activity are necessary to calm the heart during emotionally driven increases in blood pressure. When an imbalance between the two systems occurs so that sympathoexcitation is excessive and parasympathetic activation is simultaneously suppressed and unable to restrain the cardiac and vasomotor sympathetic drive, as often happens in several cardiovascular disease states, the risk of morbidity and mortality increases substantially (38, 40, 54, 63).
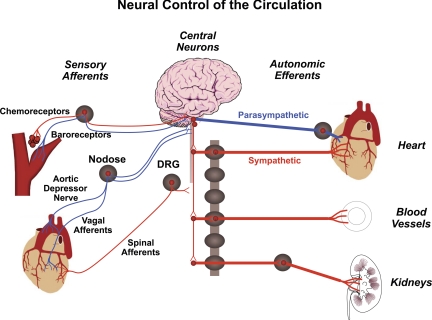
Two sets of powerful monitors, one of arterial blood pressure and the second of the oxygen content and pH of arterial blood are poised at the vascular entrance into the brain as if to ascertain an optimal perfusion and oxygen saturation of cerebral blood flow. In 1938, Corneille Heymans received the Nobel Prize in Physiology and Medicine for his discovery of the reflexogenic areas of the cardiovascular system (32, 33), where the carotid sinus nerves and aortic depressor nerves originate and exert their control on blood pressure, heart rate, and ventilation. These tiny nerves that innervate the very restricted areas of the vasculature (carotid sinuses, aortic arch, and carotid bodies) are exquisite mechanosensors of blood pressure fluctuations (baroreceptors) or chemosensors of hypoxia and acidosis (chemoreceptors) (Fig. 1). Action potentials generated at their terminals travel through the nodose and petrosal ganglia to the nucleus tractus solitarius, a medullary central station for autonomic control. The baroreceptor reflexes activated during a rise in pressure and the chemoreceptor reflexes activated with hypoxia and acidosis are powerful triggers of essential circulatory and ventilatory adjustments. Their effects on the sympathetic nerve activity is opposite. The baroreceptor reflex is inhibitory, whereas the chemoreceptor reflex is excitatory (1, 18, 27, 52, 72, 75).
Fig. 1.
Neural control of the circulation. Two major sensory signals are: 1) baroreceptors and mechanosensory sympathoinhibition, and 2) chemoreceptors and chemosensory sympathoexcitation. DRG, dorsal root ganglion.
Clinical Significance of Autonomic Adjustment
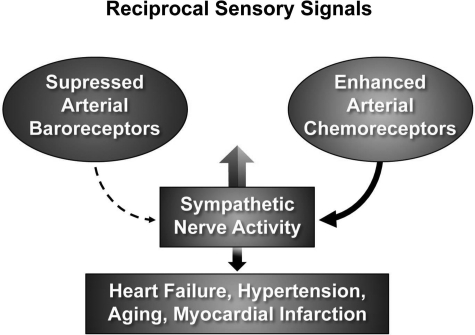
Teleologically the activation of baroreceptors, which suppresses sympathetic vasoconstriction and enhances parasympathetic bradycardia is a powerful beneficial adjustment during surges of arterial hypertension but a frightening maladjustment when it causes sudden loss of consciousness in neurogenic syncope (2). Conversely, the suppression of baroreceptors and activation of chemoreceptors are both excitatory (27, 59, 67, 72) (Fig. 2). The reciprocal modulation of these two sensory inputs results in a powerful additive sympathoexcitation that is associated with increased ventilation.
Fig. 2.
The augmented sympathetic nerve activity (SNA) in hypertension, heart failure, myocardial infarction, diabetes, and aging may result in large part from impaired baroreceptor activity (dashed-lined arrow) and enhanced chemoreceptor activity (solid-line arrow). Both increase SNA.
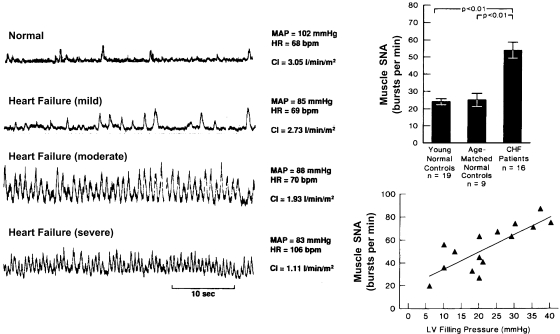
Also, teleologically, this reciprocal sensory modulation may be beneficial in states of circulatory collapse and shock when severe hypotension and hypoxia coexist and mutually enhance sympathetic drive and ventilation to overcome the acute crisis. However, when this reciprocal dysautonomia occurs in chronic states as it does in heart failure, hypertension, myocardial infarction, diabetes, and aging (1, 18, 21, 27, 43, 52, 59, 67, 72, 75), it represents a serious maladjustment. The exaggerated and sustained sympathetic activity and reduced parasympathetic activity correlate strongly with increased mortality (10). Figure 3 portrays dramatic increases in sympathetic nerve activity in three patients with mild, moderate, and severe heart failure compared with a normal subject. The magnitude of increase in nerve activity correlated well with severity of heart failure reflected in the progressive increase in left ventricular filling pressure.
Fig. 3.
Direct recordings of muscle SNA in patients with congestive heart failure (CHF) show dramatic increases in severe failure. The increases in SNA correlate with increases in left ventricular (LV) filling pressures and are significantly greater than in young and age-matched normal controls (bar graph). MAP, mean arterial pressure; HR, heart rate. [From Ferguson et al. (21) and Leimbach et al. (43).]
In an important New England Journal of Medicine article, Cohn et al. (10) reported <10% 2-year survival of patients in heart failure if their circulating plasma norepinephrine levels were >800 pg/ml, reflecting an excessive degree of sympathetic nerve activity. In contrast, nearly 60% 5-year survival was reported if norepinephrine levels were <400 pg/ml (supplemental Fig. S3).
Similarly, La Rovere et al. (38) reported that cardiac mortality after myocardial infarction in patients with a left ventricular ejection fraction of <35 was more than twice as high (18%) when their baroreceptor sensitivity was <3 ms/mmHg compared with 8% in those with baroreceptor sensitivity >3 ms per mmHg. The low baroreceptor sensitivity would contribute to excessive sympathetic nerve activity.
Reciprocal Interaction Between Baro- and Chemoreceptors: Pathological Implications
Chronic, excessive sympathetic nerve activity in cardiovascular disease is pathological. Three of the mechanisms that contribute to it will be considered here. One is the reduced baroreceptor activity, the other is the increased chemoreceptor activity, and a third is a facilitatory interaction between baroreceptors and chemoreceptors, whereby the chemoreceptor response is enhanced by the reduced baroreceptor activity. In earlier studies, we defined this interaction in dogs by showing that elevation of carotid sinus pressure not only reduces vascular resistance in the perfused gracilis muscle, but it also inhibits the vasoconstrictor response to chemoreceptor activation by carotid artery perfusion with hypoxemic blood (30). Conversely, when the baroreceptor activity is reduced by lowering carotid sinus pressure, baseline vascular resistance increases and the vasoconstrictor response to carotid hypoxemia is enhanced (supplemental Fig. S4).
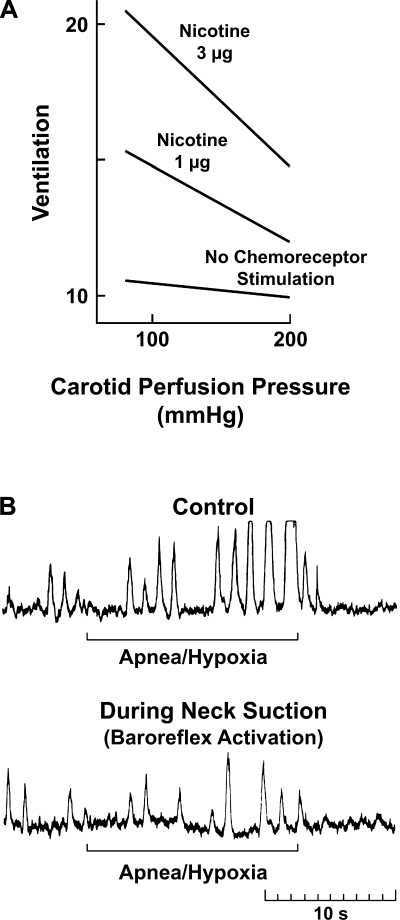
Similar results were obtained when the ventilatory response to chemoreceptor stimulation was tested with intracarotid nicotine injections in dogs (31). The response was minimized during activation of baroreceptors by increasing the carotid perfusion pressure and enhanced at low carotid sinus pressure (Fig. 4A). We observed a similar interaction in humans (74). The pronounced increase in sympathetic nerve activity in a hypertensive subject during apnea on 10% oxygen was dramatically suppressed by activation of carotid baroreceptors with a neck-suction device. Neck suction distends the carotid sinus region and increases the transmural arterial pressure simulating hypertension (73) (Fig. 4B).
Fig. 4.
Reciprocal dysautonomia. Baroreceptor activation inhibits the chemoreceptor reflex and vice-versa. A: chemoreceptor stimulation. Ventilatory responses to intracarotid nicotine were reduced significantly at high carotid perfusion pressures [from Heistad et al. (31)]. B: SNA in a hypertensive subject is markedly increased with apnea and hypoxia. The increase is abrogated by neck suction [from Somers et al. (73)] (see also Ref. 74).
These results support our notion of the morbid consequences of reciprocal dysautonomia, i.e., decreased baroreceptor reflex and enhanced chemoreceptor reflex. These reciprocal changes in baro- and chemosensory activities promote sympathovagal imbalance in cardiovascular diseases and increase mortality.
Beneficial Consequences of the Restoration of Sympathovagal Balance
As mentioned above, the push-pull cardiovascular influence of the sympathetic and parasympathetic systems is essential for physiologic adjustments to stresses. The push of sympathetic activation may be balanced by the pull of parasympathetic activation. It is when the sympathetic activation is increased, while parasympathetic activation is decreased, in chronic states that cardiovascular damage and risks are enhanced. I wish to mention three experiments that demonstrate beneficial effects of restoring the autonomic balance.
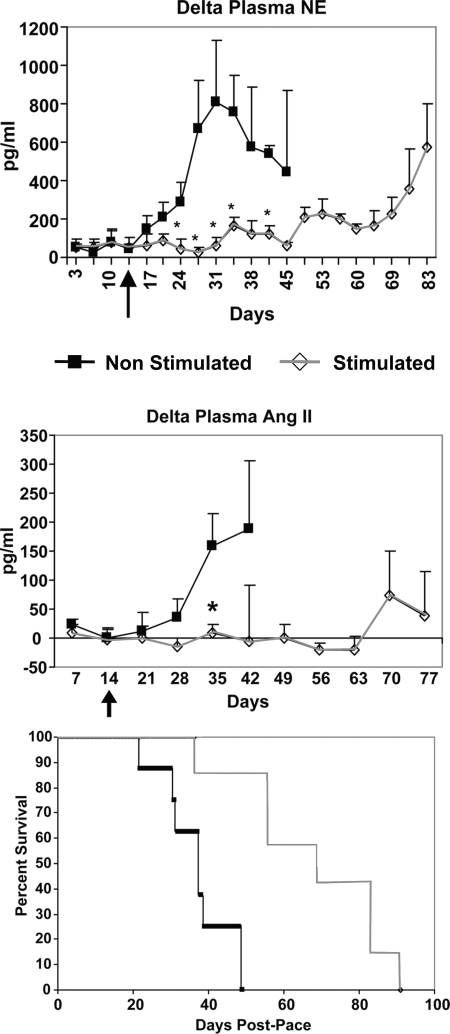
First, there is evidence that chronic electrical activation of carotid sinus baroreceptors enhances survival in dogs in heart failure induced by cardiac pacing. Figure 5 shows a significant prolongation of survival associated with the reduction of neurohumoral indicators of sympathetic nerve activity, namely circulating norepinephrine and angiotensin levels (85). Thus, chronic suppression of sympathetic activity is protective in heart failure. These results also support the human clinical trials of electrical stimulation of carotid baroreceptors in refractory hypertension (22, 82).
Fig. 5.
Chronic baroreceptor activation enhances survival in dogs in heart failure (pacing-induced) and suppresses the increases in plasma norepinephrine (NE) and angiotensin (ANG II). Arrow, time carotid sinus stimulation was initiated. *Significant differences between the 2 groups at specific time points (P < 0.05). [From Zucker et al. (85).]
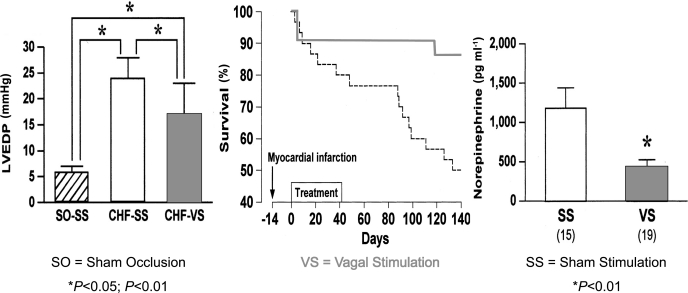
Second is the evidence that vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats subjected to coronary occlusion (44). Figure 6 shows that the dramatic survival is accompanied by significant reductions in circulating norepinephrine levels and reduction in left ventricular end-diastolic pressure.
Fig. 6.
Vagal nerve stimulation (VS) markedly improves rat long-term survival after CHF and significantly reduces left ventricular end-diastolic pressure (LVEDP) and plasma norepinephrine levels [from Li et al. (44)].
Third is the demonstration of a protective influence of vagal nerve stimulation from the inflammatory responses that occur with injection of LPS in rats (supplemental Fig. S5). The vagal outflow innervates the reticuloendothelial system in the liver and intestines and may inhibit the release of TNF and 1L-1 by activating cholinergic receptors on macrophages. Vagotomy worsens, and vagal nerve stimulation attenuates both the LPS-induced release of cytokines and the endotoxin shock and hypotension (4).
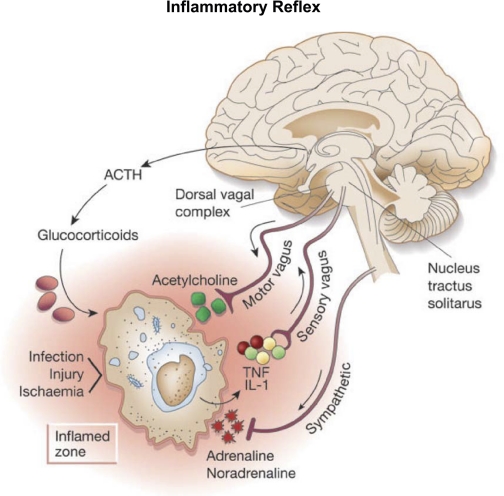
Work by Tracey (79) emphasizes the concept of an inflammatory reflex (Fig. 7). The afferent arm of the reflex consists of the vagal sensory nerve endings in the reticuloendothelial system that are activated by release of cytokines (TNF and IL-1) at the site of an inflammatory process. The activated vagal afferents project into the medullary nucleus tractus solitarus as do baroreceptor and chemoreceptor afferents. The signals are processed through medullary neurons reaching the dorsal motor nucleus of the vagus and activate vagal efferents, which innervate the macrophages exerting a negative feedback action on cytokine synthesis and release through a cholinergic anti-inflammatory process.
Fig. 7.
Inflammatory reflex. Inflammatory products of tissue injury (TNF, IL-1) activate sensory vagal afferent signals that are relayed to nucleus tractus solitarus and reflexly activate vagus efferent activity to inhibit cytokine synthesis through the cholinergic anti-inflammatory pathway [from Tracey (79)].
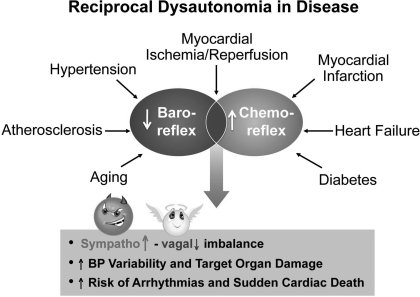
These and other experiments that address structural, metabolic, and cellular effects of adrenergic and cholinergic autonomic neurotransmitters, in addition to the hemodynamic effects, allow us to conclude that sustained excessive sympathetic activation is damaging and chronic parasympathetic activation is protective (12, 53). In terms of our declaration of the three adjectives: good, bad, and ugly, it would be logical to conclude that under those circumstances sympathetic nerve activity is the “bad” actor and parasympathetic nerve activity is the “good” actor. The lethal consequences of the pronounced sympathovagal imbalance result in large part from the high blood pressure variability and target organ damage and high risk of arrhythmias and sudden cardiac death (38, 40, 54, 63) (Fig. 8).
Fig. 8.
Sympathovagal imbalance resulting from reciprocal dysautonomia has catastrophic consequences in cardiovascular diseases.
MOLECULAR COMPONENTS OF SENSORY SIGNALS
An understanding of the mechanisms of sensory dysfunction requires a determination of the molecular components of mechanotransduction in the baroreceptor neurons and chemoreceptor transduction in the glomus cells of the carotid bodies.
Mechanosensory Transduction in Baroreceptors
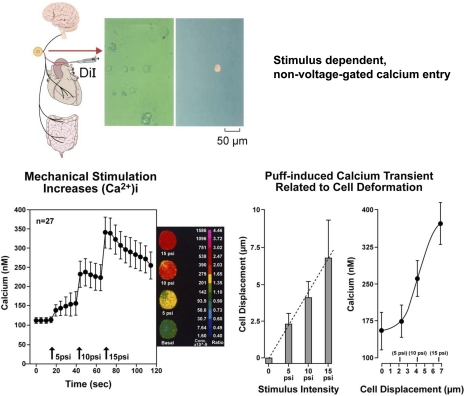
Our attempts to characterize the molecular determinants of mechanotransduction began in 1994 when we showed that gadolinium (Gd3+) blocked carotid baroreceptor nerve activity (28). We then isolated aortic baroreceptor nodose neurons from rats and demonstrated significant increases in intracellular calcium during their deformation by mechanical probing and by puffing buffer solutions through an ejecting pipette using a pneumatic Pico Pump (68, 76) (Fig. 9). The graded ejection pressures caused progressive deformations of the neurons and induced parallel increases in Ca2+ transients that coincided with the linear increases in neuronal deformation (76) (Fig. 9). The calcium transients depended on extracellular Ca2+, and the influx was through non-voltage-gated Ca2+ channels that were primarily sensitive to Gd3+, but much less sensitive to lanthanum (La3+). Although both Gd3+ and La3+ are trivalent lanthenides equally effective in blocking voltage-gated Ca2+ channels, we found that Gd3+ blocked preferentially the mechanically gated channels (76) as had been reported earlier (83). We also identified mechanically induced inward currents and the opening of single ion channels in aortic baroreceptor neurons that were blocked by Gd3+ (39, 40, 42).
Fig. 9.
Isolation of baroreceptor neurons [1,1′-dioleyl-3,3,3,′3′-tetramethylindocarbo-cyanine methanesulfonate (DiI) labeled] and mechanically-induced Ca2+ transients. Ca2+ transients in isolated aortic baroreceptor neurons are proportionate to the intensity of mechanical stimulation (bar graph) and the magnitude of cell deformation [from Sharma et al. (68) and Sullivan et al. (76)].
Degenerin/epithelial sodium channel superfamily.
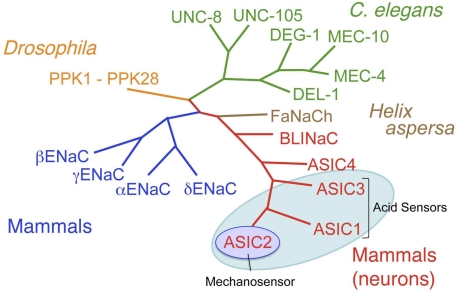
The molecular characterization of any vertebrate mechanosensor including the auditory hair cell (26, 50, 80) has remained elusive. In invertebrates, however, and specifically in Caenorhabditis elegans, random chemical mutations that caused a loss of touch sensitivity allowed the identification of several genes of which two appeared essential for touch sensitivity: Mec-4 and Mec-10 (6, 7, 16, 56). Sequence homology helped define numerous members of what became known as the degenerin/epithelial sodium channel (DEG/ENaC) superfamily, which had preferential selectivity for sodium in a variety of invertebrates (Fig. 10).
Fig. 10.
DEG/ENaC ion channel family members (PPK, UNC-8, UNC-105, MEC, DEL-1). Evolutionary conservation of mechanical signaling. ASIC, acid-sensing ion channels; ENaC, epithelial sodium channel; DEG, degenerin; FaNaCh, FMRF amide-gated Na+ channel; BLINaC: brain-liver-intestine Na+ channel.
In mammals, the α-, β-, γ-subunits of the ENaC, which are critical for sodium retention and homeostasis, were first identified as members of the superfamily. We promptly hypothesized that they may be part of the mechanosensitive complex in arterial baroreceptors. We were delighted with the discovery that β- and γ-ENaC are expressed in rat aortic and carotid baroreceptor neurons and in their terminals (17). We also confirmed that the pharmacological blockers of ENaC, amiloride and benzamil, blocked the mechanically induced Ca2+ transients and depolarizations (17, 70) in isolated aortic baroreceptor neurons as well as the carotid sinus nerve activity and the carotid sinus baroreflex in anesthetized rabbits (17). Unfortunately, the deletion of ENaC subunits in mice was postnatally lethal, and we could not test its influence on the baroreflex in the whole animal.
Acid-sensing ion channels as a DEG/ENaC neuronal subfamily.
When another mammalian subfamily of DEG/ENaC was identified in peripheral nerves and human brain (25, 61, 62, 81), we explored its possible contribution to mechanosensing in baroreceptor neurons. It was designated as the ASICs subfamily. However, not all of its members were equally sensitive to acid (3). The ASIC subtypes ASIC1 and ASIC3 were exquisitely acid sensitive and implicated in the transduction of acid-evoked nociception in spinal sensory afferents and, as we will show later, in pH sensitivity of carotid body glomus cells. ASIC2 was the least pH-sensitive molecule, and Price et al. (61) had reported that its deletion reduced mechanosensing in some of the rapidly adapting cutaneous fibers; hence our hypothesis that it contributes to the mechanosensitivity of arterial baroreceptor nerves (Fig. 10).
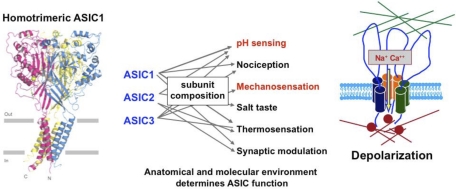
The topography of the ASIC channels and their properties are widely shared by family members. They are nonselective, cationic, non-voltage-gated channels with predominant Na+ conductances that are amiloride sensitive. They have a large extracellular domain that may be tethered to extracellular matrix proteins, two transmembrane domains, and short intracellular COOH and amino terminals linked to cytoskeletal actin and other proteins that form a mechanically gated complex (Fig. 11).
Fig. 11.
Left: crystal structure of ASIC1 [from Jasti et al. (35)]. Middle: subunit composition determines function [from Hattori et al. (29)]. Right: ASIC channel and associated proteins form a mechanosensitive complex [from Drummond et al. (17)].
Hetero- or homomultimers of the various subunits (probably as trimers) make up the channels. The subunit composition defines the intensity of the acid sensitivity, and, depending on the type of sensory terminal where they are expressed, the ASICs mediate different functions including nocicepton, mechanosensation, thermosensation, salt tasting, pH sensing, and synaptic modulation (Fig. 11). The crystal structure of ASIC1 was recently shown to conform with the predicted topography (35).
ASIC2 as a mechanosensory ion channel in baroreceptors.
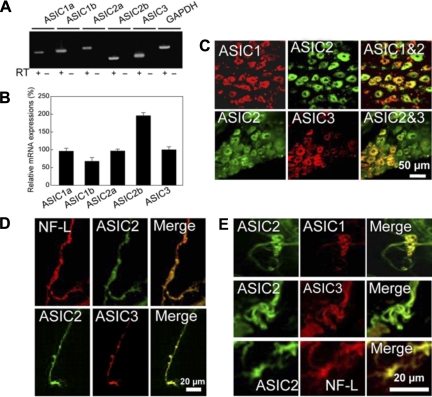
Here we describe our results published recently in Neuron (48). We first identified by RT-PCR that ASICs were expressed in mouse nodose ganglia. All five isomers ASIC1a, -1b, -2a, -2b, and -3 were expressed with prominence of ASIC2b. We then colocalized by immunofluorescence ASIC1, -2, and -3 in various nodose neurons, with varying intensities, and most importantly, in the baroreceptor nerve fibers and their terminals in the aortic arch (Fig. 12).
Fig. 12.
A and B: expression of ASICs in mouse nodose ganglia by RT-PCR. C: colocalization of ASICs proteins in mouse nodose ganglion. NF-L, neurofilament light polypeptide. Expression of ASICs in baroreceptor nerve fibers (D) and nerve endings (E) [from Lu et al. (48)].
FUNCTIONAL PHENOTYPE OF ASIC2 NULL MICE.
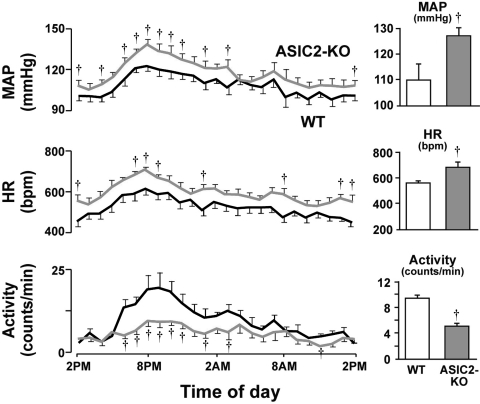
The results obtained from the radiotelemetry recordings of blood pressure and heart rate provided all the hemodynamic manifestations of impairment of the baroreflex in the ASIC2 null mice. They were hypertensive with average levels of blood pressure and heart rate that were significantly higher over a 24-h period compared with wild-type (WT) mice. Diurnal fluctuations in blood pressure and heart rate were also greater despite a reduced level of activity compared with WT mice (Fig. 13). Spontaneous baroreflex sequences of reciprocal changes in blood pressure and heart rate reflected a significant reduction of baroreceptor sensitivity (48).
Fig. 13.
Cardiovascular phenotype of ASIC2 deletion in mice. Values were obtained from continuous recordings of MAP and HR, which increased in ASIC2 knockout (KO) mice. Motor activity was reduced (gray lines and columns) when compared with wild-type (WT; black lines, white columns). †Significant differences at specific time points throughout the diurnal cycle, as well as in the average 24-h measurements represented in the bar graphs for MAP, HR, and locomotor activity (P < 0.05 by 2-way ANOVA). [From Lu et al. (48).]
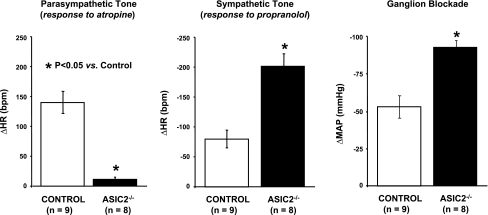
Moreover, there was clear evidence for the sympathovagal imbalance that results from a loss of baroreflex control over autonomic activity in the ASIC2 null mouse (Fig. 14). The enhanced heart rate response to propranolol and suppressed response to atropine, as well as the pronounced drop in arterial blood pressure with ganglion blockade, represented the significantly enhanced sympathetic drive of heart rate and vasomotor tone, as well as the reduced parasympathetic control of heart rate.
Fig. 14.
Sympathovagal imbalance in conscious ASIC2 KO mice reveals a high level of sympathetic control of heart rate (HR) and vasomotor tone (MAP) and suppressed parasympathetic control of HR [from Lu et al. (48)].
EVIDENCE OF A BARORECEPTOR DEFECT IN ASIC2 NULL MICE.
Our initial hypothesis was that ASIC2 contributes to the mechanosensitivity of the baroreceptors in the afferent arm of the reflex arc. The foregoing studies showing an impaired baroreflex in the conscious ASIC2 null mice would be supportive of the hypothesis only if the central and peripheral motor components of the reflex were not impaired. This was proven when the electrical stimulation of the central end of the aortic depressor nerves caused equivalent hypotension and bradycardia in WT and ASIC2 null mice (supplemental Fig. S6) (48).
ISOLATED NEURONS.
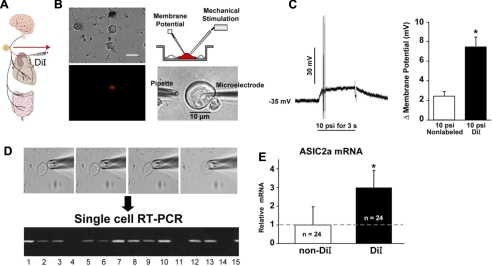
We then proceeded to test directly whether ASIC2 is a mechanosensor in isolated nodose neurons. The neurons were impaled with sharp microelectrodes (Fig. 15) and were deformed by puffing buffer solutions as described above in Fig. 9.
Fig. 15.
DiI-labeled aortic baroreceptor neurons patch-clamped and mechanically stimulated (A and B) showed greater depolarization than non-DiI neurons (C) and expressed more ASIC2a mRNA by single-cell RT-PCR (D and E). *Significant differences between DiI and non-DiI values (C and E; P < 0.05). Arrow points to RT-PCR of 15 single neurons isolated as shown (D). [From Lu et al. (48).]
We found that 1,1′-dioleyl-3,3,3,′3′-tetramethylindocarbo-cyanine methanesulfonate (DiI)-labeled aortic baroreceptor neurons depolarized much more than nonlabeled nodose neurons during mechanical stimulation. Correspondingly, single-cell RT-PCR revealed significantly higher ASIC2a mRNA levels in DiI vs. non-DiI neurons (Fig. 15). Moreover, when mechanical responses of ASIC2 null and WT nodose neurons were compared, a significantly smaller number of neurons depolarized and the depolarizations were less in ASIC2 null mice compared with WT mice. On the other hand, the neurons from transgenic mice overexpressing ASIC2 showed significantly greater and more sustained depolarizations than WT neurons (supplemental Fig. S7) (48).
These correlations between mechanically induced depolarization and ASIC2 expression provide support for a mechanosensing property of ASIC2.
IN VIVO STUDIES.
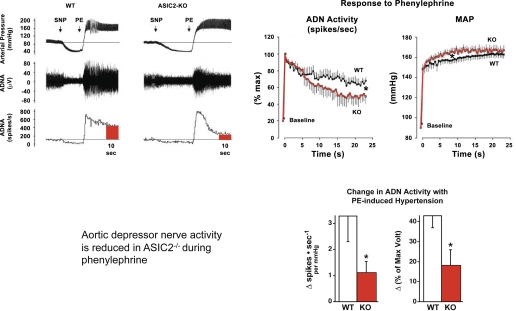
Additional support for a mechanosensitive ASIC2 was obtained from whole-animal experiments in anesthetized WT and knockout (KO) mice in which we contrasted the aortic depressor nerve activity (ADNA) during the pressor response to phenylephrine after sodium nitroprusside. Despite a significantly greater pressor response, the ADNA declined rapidly to a significantly lower level in the KO (Fig. 16). The increases in integrated voltage of the neurogram, as well as the spike frequency calculated per mmHg, increase in blood pressure were less than half to a third of the activity seen in WT.
Fig. 16.
Aortic depressor nerve activity (ADNA) declines to a significantly lower level in ASIC2 KO mice compared with WT during the sustained pressor response (MAP) to intravenous phenylephrine (PE). Tracings show a significantly lower ADN activity expressed as %maximum spikes/s in KO than in WT mice, despite a higher MAP in KO mice (*P < 0.001 by ANOVA). Bar graphs show a significantly lower Δ spikes/s−1 and Δ %maximum integrated voltage over a 10-s period in KO vs. WT mice (*P < 0.05, unpaired t-test). SNP, sodium nitroprusside. [From Lu et al. (48).]
Another in vivo approach was to measure the pressor response to bilateral carotid occlusion (BCO) in anesthetized mice. The occlusion mechanically unloads the carotid baroreceptors provoking a reflex rise in systemic arterial pressure. Thus the magnitude of this increase in pressure reflects the decrease in carotid baroreceptor nerve activity during the fall in carotid sinus pressure and the opposing increase in aortic baroreceptor nerve activity that occurs simultaneously as a result of the rise in systemic arterial pressure.
So, by sectioning the aortic depressor nerves, the pressor response to BCO was enhanced significantly in the WT mice but was unchanged in the KO mice (supplemental Fig. S8) (48). This indicates that the ADNA during the reflex rise in pressure with carotid occlusion must have been significantly higher in WT compared with KO mice, causing an effective buffering of arterial pressure that is removed by section of the ADNs. In contrast, the ADNA in the ASIC2 null mice must have been significantly blunted during the rise in pressure with BCO so that a section of the ADNs subsequently did not result in any significant augmentation of the response to a repeated BCO. Moreover, the significantly attenuated pressor response to BCO after ADN section in the KO, reflects, most likely, a blunted baseline carotid baroreceptor afferent activity (48).
Our conclusion from this set of experiments is that ASIC2 is an important determinant of baroreceptor sensitivity and likely a molecular pressure sensor. Its absence results in disruption of the baroreceptor reflex with a significant, sustained enhancement of sympathetic activity (the “bad”) and loss of parasympathetic activity (the “good”) that increases cardiovascular risk and mortality.
Chemoreceptor Transduction in Carotid Bodies
Bilateral carotid occlusion.
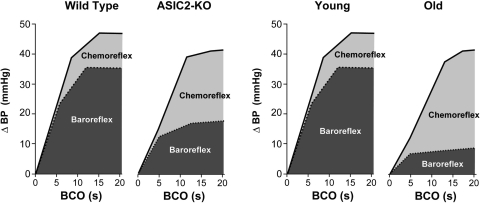
The results of the BCO experiments described above to test the contribution of ASIC2 to carotid baroreceptor activity were done on mice breathing 100% O2 to eliminate a possible effect of activation of the carotid chemoreceptor reflex by the ischemic hypoxia during the occlusion (34). Generally, that contribution of the chemoreceptor reflex to the pressor response to BCO is small compared with that of the baroreflex, but it becomes very significant with senescence and in ASIC2 KO compared with young and WT mice, respectively, as shown in Fig. 17.
Fig. 17.
Schematic representation of contributions of the baroreflex vs. chemoreceptor reflex to the pressor response to bilateral carotid occlusion (BCO) in young (∼3 mo) vs. old (∼18 mo) and 5–8 mo WT and ASIC2 KO mice. The relatively small contribution of the chemoreflex in WT and in young mice is increased significantly in ASIC2 KO mice and in old mice as the baroreflex components decline (see Refs. 34 and 64). BP, blood pressure.
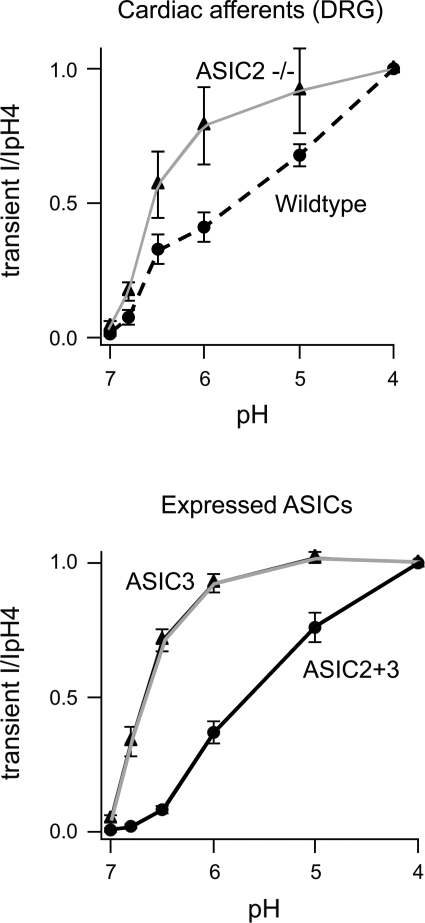
Our preliminary findings in ASIC2 null mice indicated an increased chemoreceptor component of the BCO pressor response, which was eliminated in mice with the additional deletions of ASIC1 and -3 (64). These early results raised the possibility that acid-sensitive ion channels ASIC1 and -3 may contribute to activation of chemoreceptors, and their sensitivity may be enhanced when ASIC2 is deleted (64).
Isolated glomus cells.
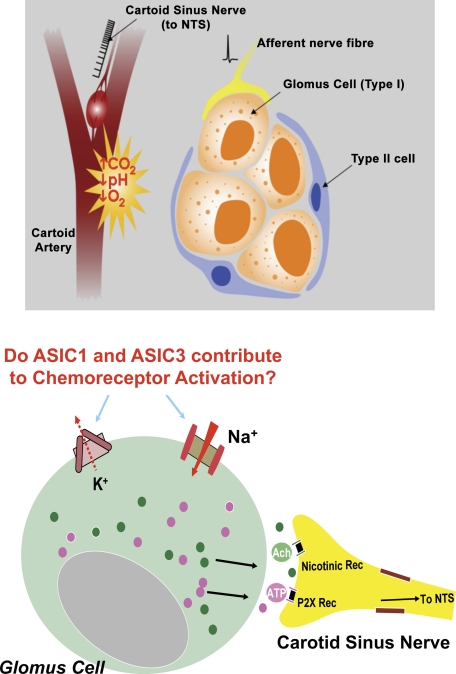
Glomus type I cells are generally accepted as the primary site of chemotransduction and activation of chemoreceptors in response to hypoxemia, hypercapnia, and acidosis (Fig. 18). The activation leads to the release of ATP, acetylcholine, and other transmitters that trigger action potentials in adjacent carotid nerve endings (19, 47, 55, 57, 60). The molecular identity of the sensors that result in depolarization of the glomus cells and the Ca2+ transients that evoke the release of the transmitters has been explored. Closure of K+ channels, including the large-conductance Ca2+-activated, has been supported as a mediator of the effect of hypoxemia (47, 57).
Fig. 18.
Chemosensory transduction in carotid bodies occurs in glomus cells (Type I). ASICs and K+ channels contribute to pH sensitivity (see Ref. 77). NTS, nucleus tractus solitarius; P2X Rec, P2X receptors ATP-activated channels.
Extracellular pH-sensitive conductances including Cl− currents and TASK-like currents have been considered as mediators of the depolarization of extracellular acidosis (5, 58). The question of whether ASICs play a role in the enhanced chemoreceptor sensitivity noted in ASIC2 null mice required first the determination of whether carotid bodies express ASICs.
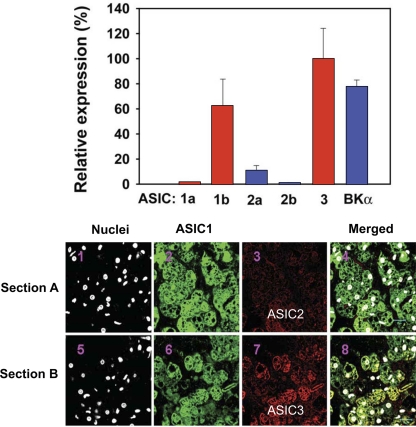
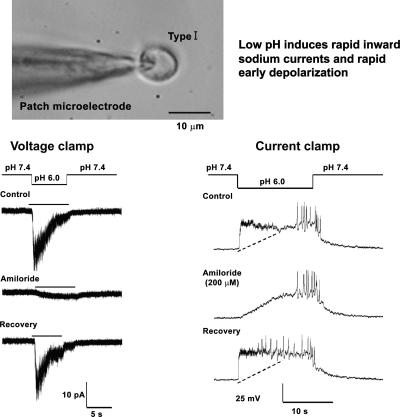
Our results with RT-PCR and immunofluorescence showed both a predominant expression of ASIC1 and -3 mRNA in carotid bodies, as well as the channel proteins of ASIC1, -2, and -3 coexpressed in clusters of glomus cells (77; Fig. 19). By patch-clamping glomus cells from rat carotid bodies we could ascribe the initial rapid inward current and early transient depolarization that occurred in response to low pH primarily to ASIC3, since they were blocked selectively by amiloride and not by Psalmotoxin venom, the blocker of ASIC1a. In addition to ASIC3, we noted the contribution of the pH-sensitive potassium leak current TASK, which closed at low pH and induced a sustained depolarization that extended the early depolarization caused by ASIC3 (Fig. 20) (77).
Fig. 19.
Expression of ASICs mRNA in rat carotid body by RT-PCR (bar graph) and by immunofluorescence. BKα, large-conductance Ca2+-activated K+ channel-α. Two sections of carotid body clusters were stained with ASIC1 and -2 (Section A) and with ASIC1 and -3 (Section B). [From Tan et al. (77).]
Fig. 20.
Whole-cell patch-clamping of an isolated glomus cell. Low pH induces rapid inward sodium currents and early depolarizations, which are blocked by amiloride [from Tan et al. (77)].
Molecular mechanisms of enhanced chemoreceptor sensitivity.
In the result described earlier in Fig. 4, we demonstrated a central occlusive interaction between the baroreceptors and chemoreceptors in both animals and humans (supplemental Fig. S4) (30, 31, 74). This interaction, whereby decreased baroreceptor activity enhances chemoreceptor responses, proved to be clinically very relevant. In several cardiovascular diseases, the impaired baroreflex is associated with enhanced chemoreceptor sensitivity resulting in excessive sympathoexcitation of dire pathological consequences (38, 40, 54, 63, 67).
Now, with a better understanding of the role of ASICs in baro- and chemosensory signaling, we wanted to determine the molecular basis of increased chemoreceptor sensitivity in ASIC2 null mice.
One possibility we considered was that the heteromultimeric composition of the channels, i.e., the relative expression of the various subunits of ASIC in glomus cells, may define their pH sensitivity. In support of that concept, the work of Hattori et al. (29) shows that the activation kinetics and pH sensitivity of ASIC3 heterologously expressed in COS-7 cells are much faster and greater, respectively, than those of ASIC2 expressed in COS-7 cells. Hattori et al. (29) showed further that coexpression of ASIC2 and ASIC3 results in a heteromultimeric channel of a lesser pH sensitivity that is enhanced once ASIC2 is deleted. This reciprocal interaction was confirmed in cardiac and other dorsal root ganglion neurons, which show a markedly enhanced pH sensitivity in ASIC2 null mice compared with WT mice (Fig. 21). A similar molecular alteration in channel composition and pH sensitivity of glomus cells may account for the enhanced chemoreceptor reflex we saw with BCO in ASIC2 null mice (64).
Fig. 21.
Absence of ASIC2 enhances acid sensitivity of ASIC3 heterologously expressed in COS-7 cells. Targeted deletion of ASIC2 in DRG neurons from ASIC2 null mice causes significant increases in their pH sensitivity compared with DRG neurons from WT mice. [From Hattori et al. (29).]
To recapitulate our results on the molecular determinants of mechano- and chemotransduction, we found that: 1) in nodose neurons, ASIC2 and associated tethering proteins contribute to mechanical depolarization during a rise in pressure and trigger sympathoinhibition; 2) in glomus cells, ASIC3 contributes to the pH sensitivity, causing sympathoexcitation; and 3) in the ASIC2 null mice the baroreflex is impaired primarily because of reduced sensitivity of the afferent arm of the reflex resulting in a hypertensive phenotype with enhanced sympathetic cardiac and vasomotor tone, and the chemoreceptor reflex is augmented when baroreceptor input is reduced. We had proposed in our earlier studies that there is a central occlusive interaction between these two inputs.
We now propose that, in addition, at the level of signal transduction, the pH sensitivity of ASIC3 in glomus cells may be enhanced in ASIC2 null mice as a result of a change in the composition of heterotrimeric channels made up of ASIC2 and -3 subunits that become more pH sensitive in the absence of ASIC2. Although we have not actually shown that glomus cells from ASIC2 null mice are more pH sensitive, the results of Hattori et al. (29) with cardiac dorsal root ganglion neurons from ASIC2 null mice support that premise.
Spontaneously Hypertensive Rats: Do ASICs Mediate Baro- and Chemotransduction?
Having shown that ASIC2 and -3 play a role in the reciprocal changes in baro- and chemoreceptor sensitivities, respectively, in the ASIC2 null hypertensive mouse model, we decided to test whether these molecular changes pertain to an established model of genetic hypertension, namely the spontaneously hypertensive rat (SHR). The SHR is known to have impaired baroreceptor and enhanced chemoreceptor sensitivities (supplemental Fig. S9) (20, 23). These reciprocal sensitivities occur also in human hypertension. We tested whether the decreased baroreceptor activity is related to a decline in ASIC2 expression in baroreceptor neurons and the increased chemoreceptor activity is associated with increased ASIC3 expression in carotid bodies.
In preliminary experiments (49, 69), we found a significant suppression of the depolarization with mechanical stimulation of nodose neurons of SHR associated with a reduction of ASIC2a expression compared with Sprague-Dawley and Wistar-Kyoto (WKY) rats.
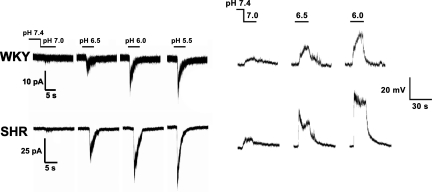
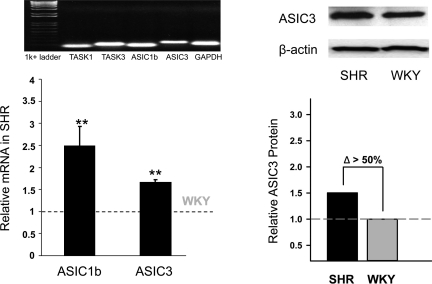
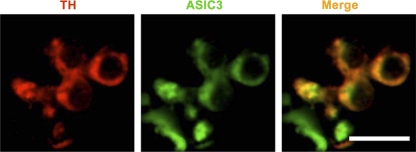
With respect to chemoreceptor responses, we found enhanced pH-dependent rapid inward currents and early depolarizations in glomus cells of young SHR compared with WKY (Fig. 22). Correspondingly, there was greater overexpression of ASIC1 and ASIC3 mRNA and ASIC3 protein in the carotid bodies of SHR compared with WKY rats (Fig. 23). Moreover, ASIC3 and TASK proteins were colocalized by immunofluorescence in clusters of carotid body cells along with tyrosine hydroxylase, the specific marker of glomus cells (Fig. 24) (78).
Fig. 22.
Acid sensitivity is significantly enhanced in spontaneously hypertensive rat (SHR) vs. Wistar-Kyoto (WKY) rat glomus cells. Left: tracings portray inward currents. Right: tracings portray depolarizations. [From Tan et al. (78).]
Fig. 23.
ASIC1 and ASIC3 expression are also increased in carotid bodies of SHR vs. WKY rats. **Significant increases in mRNA expression compared to WKY rats (P < 0.01). [From Tan et al. (78).]
Fig. 24.
Carotid body immunofluorescence showing colocalization of ASIC3 with tyrosine hydroxylase (TH). Bar = 10 μm. [From Tan et al. (78).]
The results obtained in SHR were of great interest because they seemed to replicate the phenotype of ASIC2 deletion. The ASIC2 null mice had decreased baroreceptor sensitivity, which we ascribed to the role of ASIC2 as mechanosensor at the baroreceptor nerve terminal (48). In the SHR, the suppressed mechanosensitivity in nodose neurons was also associated with significant reductions in ASIC2 expression (49).
The ASIC2 null mouse also had an enhanced chemoreceptor reflex, which we ascribed to the proton-sensitive ASIC3 identified in the glomus cells of the carotid body. In the SHR glomus cell, pH sensitivity was enhanced and was associated with overexpression of ASIC3 in the carotid bodies (78).
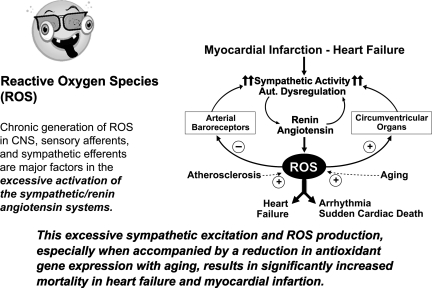
CONTRIBUTION OF ROS TO EXCESSIVE SYMPATHETIC NERVE ACTIVITY AND PATHOLOGIC STATES
An important insight was gleaned from preliminary experiments in which the ASIC-2 KO mice were treated with tempol, the pharmacologic mimic of superoxide dismutase (65). Tempol treatment for 1 wk caused a surprising restoration of baroreceptor sensitivity, reduction in blood pressure and sympathoexcitation, and restoration of parasympathetic tone. This led us to ask how pervasive and ubiquitous is the influence of ROS in contributing to the enhanced sympathoexcitation and its dire consequences.
Oxidative Stress Enhances Sympathetic Drive
ROS may certainly enhance sympathetic activity at the ganglionic level (51). It can also mediate the central action of angiotensin at the level of the subfornical organs in the hypothalamus, resulting in excessive sympathetic drive with depressed myocardial contractility following myocardial infarction (84) and also at the level of the rostral ventrolateral medulla in heart failure (24). Oxidative stress contributes to increased sympathetic vasomotor tone and decreased baroreflex sensitivity in hypertensive and hypercholesterolemic mice (42). Short-duration antioxidant therapy can restore baroreflex sensitivity and cardiovagal tone in old mice (41).
Our earlier work revealed a marked inhibitory influence of ROS generated by xanthine and xanthine oxidase on carotid baroreceptor nerve activity in rabbits (46). Catalase, in addition to superoxide dismutase, was necessary to restore the nerve activity implicating hydrogen peroxide (H2O2) as the inhibitory ROS. In these experiments, we showed that oxygen-derived free radicals contributed significantly to baroreceptor dysfunction in atherosclerotic rabbits (46).
Isolated Nodose Neurons
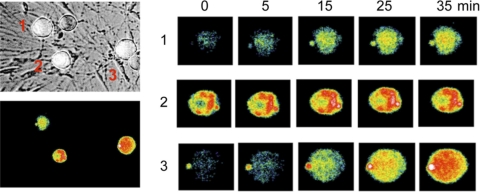
In more recent preliminary experiments, we noted a significant inhibition of excitability of nodose ganglion neurons for several minutes after a brief period of sustained electrical activation. This activity-dependent inhibition of excitability was associated with the appearance of ROS in the neurons detected by immunofluorescence. Both the inhibition of excitability and the ROS generation were eliminated by polyethylene glycol-catalase (71). A parallel in vivo response was noted with antidromic stimulation of the aortic depressor nerve, which resulted in reduction of baroreceptor activity, which was also reversed by catalase (66). Thus, an endogenous generation of ROS may be responsible for this activity-dependent resetting. By analogy, a similar process may also contribute to the known baroreceptor resetting in hypertension. In Fig. 25 we show a preliminary experiment that demonstrates autocrine increases in ROS immunofluorescence in three nodose neurons during activation of glutamate receptors with N-methyl-d-aspartate.
Fig. 25.
Reactive oxygen species (ROS) are generated in nodose neurons (1–3) in culture during stimulation of glutamate receptors with N-methyl-d-aspartate.
We believe that a sustained or enhanced inhibitory influence of ROS in baroreceptor neurons impairs the baroreceptor reflex and contributes significantly to the dysautonomia in ASIC2 null mice (65), as well as the dysautonomia in old mice (41), and possibly in pathological states of hypertension and hypercholesterolemia (42).
ROS and Chemoreceptor Activation
Others have shown that ROS generated in carotid bodies contributes to the excessive chemoreceptor activation seen in both hypertension and heart failure (15, 45, 67, 86). ROS overexpression at the level of sympathetic ganglia may also increase sympathetic activity. Moreover, the age-related acceleration of atherosclerosis correlates with failure to upregulate antioxidant genes (11).
These results indicate that ubiquitous neuronal generation of ROS at sensory, central, and peripheral autonomic neurons can sustain chronic sympathoexcitation and precipitate its consequential pathological processes. Thus, ROS deserve the label of the “ugly” actor in the saga of autonomic control (Fig. 26).
Fig. 26.
Positive feedback enhancement of ROS expression at various neuronal sites increases SNA, causing catastrophic increases in cardiovascular risks. CNS, central nervous system.
SUMMARY
First, in humans and in animal models of cardiovascular diseases, the autonomic imbalance of excessive sympathetic activity and reduced parasympathetic activity represents a major risk factor for cardiovascular mortality. The exaggerated sympathetic drive is a function not only of loss of the inhibitory influence of baro- and mechanosensory afferents but also a simultaneously increased activity of the excitatory chemoreceptors: a state we refer to as reciprocal dysautonomia.
Second, nonvoltage-gated ion channels of the ASIC subfamily of the DEG/ENaC superfamily are important components of the mechano- and chemotransduction mechanisms. ASIC2, the least acid-sensitive, is an important mechanosensing molecule in baroreceptors, and ASIC3 is important in the rapid transduction of acid sensitivity by chemoreceptors.
Third, deletion of ASIC2 results in a phenotype of hypertension with decreased baroreceptor sensitivity and vagal influence, increased chemoreceptor sensitivity, and sympathetic influence. The ASIC2 disruption replicates the phenotype of the genetically hypertensive rat (SHR) with exaggerated sympathetic nerve activity, decreased baroreceptor sensitivity, reduced ASIC2 expression in nodose ganglia, and enhanced chemoreceptor sensitivity and ASIC3 expression in carotid bodies.
Fourth, ubiquitous ROS generation in sensory, central, and peripheral autonomic neurons has been linked to excessive sympathetic drive in models of heart failure, and mice with induced deletion of ASIC2 and in SHR with constitutive reduction in ASIC2 expression. We found preliminary evidence that autocrine release of ROS during activation of baroreceptor neurons leads to their prolonged postexcitatory inhibition.
CONCLUSIONS AND CONCEPTS
Chronic sympathetic excitation is a major cardiovascular risk.
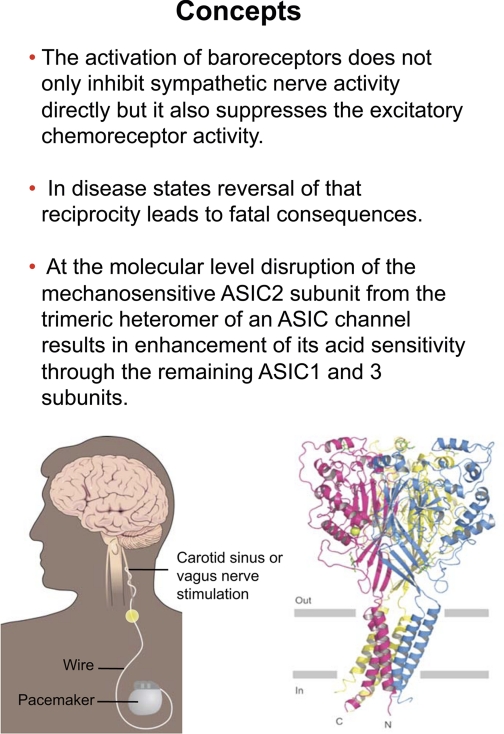
Reciprocal changes in sensory signaling (decreased baroreceptor activity and increased chemoreceptor activity to which we refer as reciprocal dysautonomia) occur frequently in cardiovascular diseases. They sustain a morbid excess in sympathoexcitation and prevent a putatively beneficial parasympathetic activation.
The prolonged activation of baroreceptors not only inhibits sympathetic nerve activity but it also suppresses the excitatory chemoreceptor activity and reduces arterial pressure in patients with drug-resistant hypertension (Fig. 27).
ASIC subfamily members are important components of mechanotransduction and chemoreceptor transduction.
At the molecular level, the reciprocal dysautonomia may be explained by the fact that disruption of the mechanosensitive ASIC2 subunit from the trimeric heteromer of an ASIC channel results in enhancement of its acid sensitivity through the remaining ASIC1 and/or ASIC3 subunits (Fig. 27).
ROS is an insidious ubiquitous promoter of sympathoexcitation that can accelerate or worsen cardiovascular disease processes and cardiovascular risks (Fig. 26).
Fig. 27.
Concepts. A dual role of baroreceptor impairment provides a strong mechanism for excessive sympathoexcitation and a reciprocal dysautonomia. At the molecular level, the heteromutimeric ASIC channel structure explains the enhanced acid sensitivity in ASIC2 KO mice.
GRANTS
The major research support for the work was from National Heart, Lung, and Blood Institute Grant HL-14388-38, a Veterans Affairs Merit Review Award from the Department of Veterans Affairs, and grants from the Howard Hughes Medical Institute and the American Heart Association.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
I particularly recognize the contribution of Dr. Mark Chapleau's laboratory to the work presented in this manuscript from the Cardiovascular Research Center at the University of Iowa. I also recognize the important collaborations received from the laboratories of Drs. Welsh, Benson, and Rahmouni. Several individuals made important contributions to various concepts and protocols. These include 1) baroreceptor activity: Mark Chapleau, Heather Drummond, Zhi Li, George Hajduczok, Xiuying Ma, Rasna Sabharwal, Donald Morgan; 2) cell culture: Carol A. Whiteis; 3) Ca2+ imaging: Heather Drummond, Margaret Sullivan, Ram Sharma, Elena Petroff; 4) patch-clamping: Klaus Bielefeldt, J. Thomas Cunningham, George Hajduczok, Vladislav Snitsarev, Zhiyong Tan; 5) molecular identity: Christopher Benson, Vivian Costa-Bartlett, Heather Drummond, Yongjun Lu, Maggie Price, Mike Welsh; and 6) human studies: Donald Heistad, Allyn Mark, and Virend Somers.
I thank Courtney Cable for assiduous work on preparation of the manuscript and Shawn Roach for artistic renditions of the figures.
REFERENCES
- 1.Abboud FM. The sympathetic system in hypertension: state-of-the-art review. Hypertension 4, Suppl II: 208–225, 1984 [PubMed] [Google Scholar]
- 2.Abboud FM. Ventricular syncope: is the heart a sensory organ? N Engl J Med 320: 390–392, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Buckler KJ, Williams BA, Honore E. An oxygen-, acid-, and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525: 135–142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Cannon WB. The Wisdom of the Body New York: Norton, 1932, 1967 [Google Scholar]
- 5b.Cannon WB. Way of an Investigator. A Scientist's Experience in Medical Research New York: Norton, 1945 [Google Scholar]
- 6.Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243: 1027–1033, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Chalfie M, Driscoll M, Huang M. Degenerin similarities. Nature 361: 504, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Chapleau MW, Abboud FM. The baroreceptor reflex: novel methods and mechanisms. In: Neural Mechanisms of Cardiovascular Regulation (edited by Dun NJ, Machado BH, Pilowsky PM.), Boston, MA: Kluwer Academic, 2004, p. 1–29 [Google Scholar]
- 9.Chapleau MW, Li A, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann NY Acad Sci 940: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 829–823, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Collins A, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104: e42–e54, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation 98: 1329–1334, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Cunningham JT, Wachtel RE, Abboud FM. Mechanosensitive currents in putative aortic baroreceptor neurons in vitro. J Neurophysiol 73: 2094–2098, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Cunningham JT, Wachtel RE, Abboud FM. Mechanical stimulation of neurites generates an inward current in putative aortic baroreceptor neurons in vitro. Brain Res 757: 149–154, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Li YL, Zimmerman MC, Davisson RL, Schultz HD. Role of CuZn superoxide dismutase on carotid body function in heart failure rabbits. Cardiovasc Res 81: 678–685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349: 588–593, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lampert G. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol 28: 986–989, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Eyzaguirre C, Fidone SJ. Transduction mechanisms in carotid body: glomus cells, putative neurotransmitters, and nerve endings. Am J Physiol Cell Physiol 239: C135–C152, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Fazan PV, Junior FR, Salgado CH, Barreira AA. Morphology of aortic depressor nerve myelinated fibers in normotensive Wistar-Kyoto and spontaneously hypertensive rats. J Auton Nerv Syst 77: 133–139, 1999 [PubMed] [Google Scholar]
- 21.Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16: 1125–1134, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Filippone JD, Bisognano JD. Baroreflex stimulation in the treatment of hypertension. Curr Opin Nephrol Hypertens 16: 403–408, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst 19: 1–11, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1α to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci 21: 2678–2686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352: 685–701, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hajduczok G, Chapleau MW, Ferlic RJ, Mao HZ, Abboud FM. Gadolinium inhibits mechano-electrical transduction in rabbit carotid baroreceptors: implication of stretch-activated channels. J Clin Invest 94: 2392–2396, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ Res 105: 279–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes: modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest 53: 1226–1236, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J Appl Physiol 39: 411–416, 1975 [DOI] [PubMed] [Google Scholar]
- 32.Heymans C, Neil E. Reflexogenic Areas of the Cardiovascular System Boston, MA: Little, Brown, 1958 [DOI] [PubMed] [Google Scholar]
- 33.Heymans C. Le Sinus Carotidien London: HK Lewis; 1929 [Google Scholar]
- 34.Iturriaga R, Alcayaga J, Zapata P. Contribution of carotid body chemoreceptors and carotid sinus baroreceptors to the ventilatory and circulatory reflexes produced by common carotid occlusion. Acta Physiol Pharmacol Latinoam 38: 27–48, 1988 [PubMed] [Google Scholar]
- 35.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev 56: 100–176, 1976 [DOI] [PubMed] [Google Scholar]
- 37.Kraske S, Cunningham JT, Hajduczok G, Chapleau MW, Abboud FM, Wachtel RE. Mechanosensitive ion channels in putative aortic baroreceptor neurons. Am J Physiol Heart Circ Physiol 275: H1497–H1501, 1998 [DOI] [PubMed] [Google Scholar]
- 38.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 351: 478–484, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Landgren S. On the excitation mechanism of the carotid baroceptors. Acta Physiol Scand 26: 1–34, 1952 [DOI] [PubMed] [Google Scholar]
- 40.Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden AC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet Med 14: 82–85, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Lazartigues E, Maheshwari N, Whiteis CA, Strauss HM, Davisson RL, Abboud FM, Chapleau MW. Restoration of baroreflex sensitivity and cardiovagal tone in old mice by short-duration antioxidant therapy (Abstract). Clin Auton Res 14: 339, 2004 [Google Scholar]
- 42.Lazartigues E, Whiteis CA, Maheshwari N, Abboud FM, Strauss HM, Davisson RL, Chapleau MW. Oxidative stress contributes to increased sympathetic vasomotor tone and decreased baroreflex sensitivity in hypertensive and hypercholesterolemic mice (Abstract). FASEB J 18: A299, 2004 [Google Scholar]
- 43.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof F, Mark A. Direct evidence from intraneural recording for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res 75: 546–554, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res 79: 802–811, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by Po2 in type I chemoreceptor cells. Science 241: 580–582, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64: 885–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, Whiteis CA, Chapleau MW, Abboud FM. Decreased mRNA expression of ASIC2a in nodose sensory ganglia is associated with development of hypertension in SHR (Abstract). FASEB J 21: A1405, 2007 [Google Scholar]
- 50.Lumpkin EA, Bautista DM. Feeling the pressure in mammalian somatosensation. Curr Opin Neurobiol 15: 382–388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X, Zhang HJ, Whiteis CA, Kregel KC, Abboud FM, Chapleau MW. Oxidative stress in sympathetic ganglia: a possible mechanism of increased sympathetic nerve activity and impaired baroreflex sensitivity in atherosclerosis (Abstract). Hypertension 44: 523, 2004 [Google Scholar]
- 52.Mancia G, Grassi G, Giannattasio C, Serravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 34: 724–728, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Mann DL, Kent RL, Parsons B, Cooper G., IV Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85: 790–804, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 96: 3450–3458, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 56.O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Peers C, Green FK. Inhibition of Ca2+-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol 437: 589–602, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petheo GL, Molnar Z, Roka A, Makara JK, Spat A. A pH-sensitive chloride current in the chemoreceptor cell of rat carotid body. J Physiol 535: 95–106, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol 92: 39–44, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol 96: 359–366, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem 271: 7879–7882, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke 34: 705–712, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Sabharwal R, Chapleau MW, Price MP, Welsh MJ, Abboud FM. Molecular mechanisms of baro- and chemoreceptor activation: evidence that ASIC1 and ASIC3 contribute to chemoreceptor activation (Abstract). Circulation 112: 156, 2005 [Google Scholar]
- 65.Sabharwal R, Stauss HM, Lazartigues E, Whiteis CA, Price MP, Welsh MJ, Davisson RL, Abboud FM, Chapleau MW. ASIC2−/− mice: a genetic model of impaired baroreceptor activation and oxidative stress-dependent neurogenic hypertension (Abstract). Hypertension 48: e31, 2006 [Google Scholar]
- 66.Santana-Filho VJ, Abboud FM, Chapleau MW. Hydrogen peroxide mediates post-excitatory depression of baroreceptor afferent activity in vivo (Abstract). FASEB J 23: 1008.15, 2009 [Google Scholar]
- 67.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension 50: 6–13, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Sharma RV, Chapleau MW, Hajduczok G, Wachtel RE, Waite LJ, Bhalla RC, Abboud FM. Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience 66: 433–441, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Snitsarev V, Iyer K, Whiteis CA, Chapleau MW, Abboud FM. Novel molecular defects in mechanosensitivity of aortic baroreceptor neurons from spontaneously hypertensive rats (Abstract). FASEB J 19: A607, 2005 [Google Scholar]
- 70.Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton Neurosci 98: 59–63, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Snitsarev V, Yermolaieva Y, Whiteis C, Abboud FM, Chapleau MW. Activity-dependent ”resetting“ of baroreceptor and vagal afferent neurons mediated by endogenous generation of hydrogen peroxide/hydroxyl radical (Abstract). Hypertension 43: 1349, 2004 [Google Scholar]
- 72.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somers VK, Dyken ME, Mark AL, Abboud FM. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnea in hypertension. Clin Auto Res 2: 171–176, 1992 [DOI] [PubMed] [Google Scholar]
- 74.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953–1957, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 11: 608–612, 1988 [DOI] [PubMed] [Google Scholar]
- 76.Sullivan MJ, Sharma RV, Wachtel RE, Chapleau MW, Waite LJ, Bhalla RC, Abboud FM. Non-voltage-gated Ca2+ influx through mechanosensitive ion channels in aortic baroreceptor neurons. Circ Res 80: 861–867, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res 101: 1009–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Tan ZY, Lu Y, Whiteis CA, Simms A, Paton JFR, Chapleau M, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res 106: 536–545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tracey K. The inflammatory reflex. Nature 420: 853–859, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 30: 339–365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 54: 530–536, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Yang X, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243: 1068–1071, 1989 [DOI] [PubMed] [Google Scholar]
- 84.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 50: 904–910, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84: 217–232, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.