Abstract
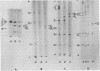
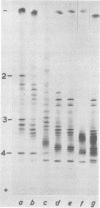
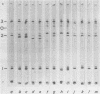
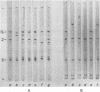
The structural polypeptides of foot-and-mouth disease virus were analyzed by electrofocusing in a polyacrylamide gel containing 9 M urea. Three versions of the technique were used to accomodate the widely differing isoelectric points of the four polypeptides. VP2 was identified by comparing mature virus with procapsids. The selective actions of proteases on virions of two serotypes and on their 12S particles were examined. From this emerged a simple test for distinguishing the similarly sized polypeptides: VP1, VP2, and VP3. The effects of carbamylation and succinylation on the charge of the polypeptides were investigated. From the properties of polypeptides modified either chemically or by mutation, it was concluded that all amino acid substitutions that might be expected to cause a charge change would be detected except for neutral-to-histidine substitutions in the most basic polypeptide, VP1. In a sample of 73 temperature-sensitive mutants, 11 classes of variant polypeptides were distinguished on the basis of charge. Their molecular weights were unchanged. Alterations were found in all structural polypeptides except VP4. Mutations affecting VP2 caused similar shifts in the precursor, VP0.
Full text
PDF

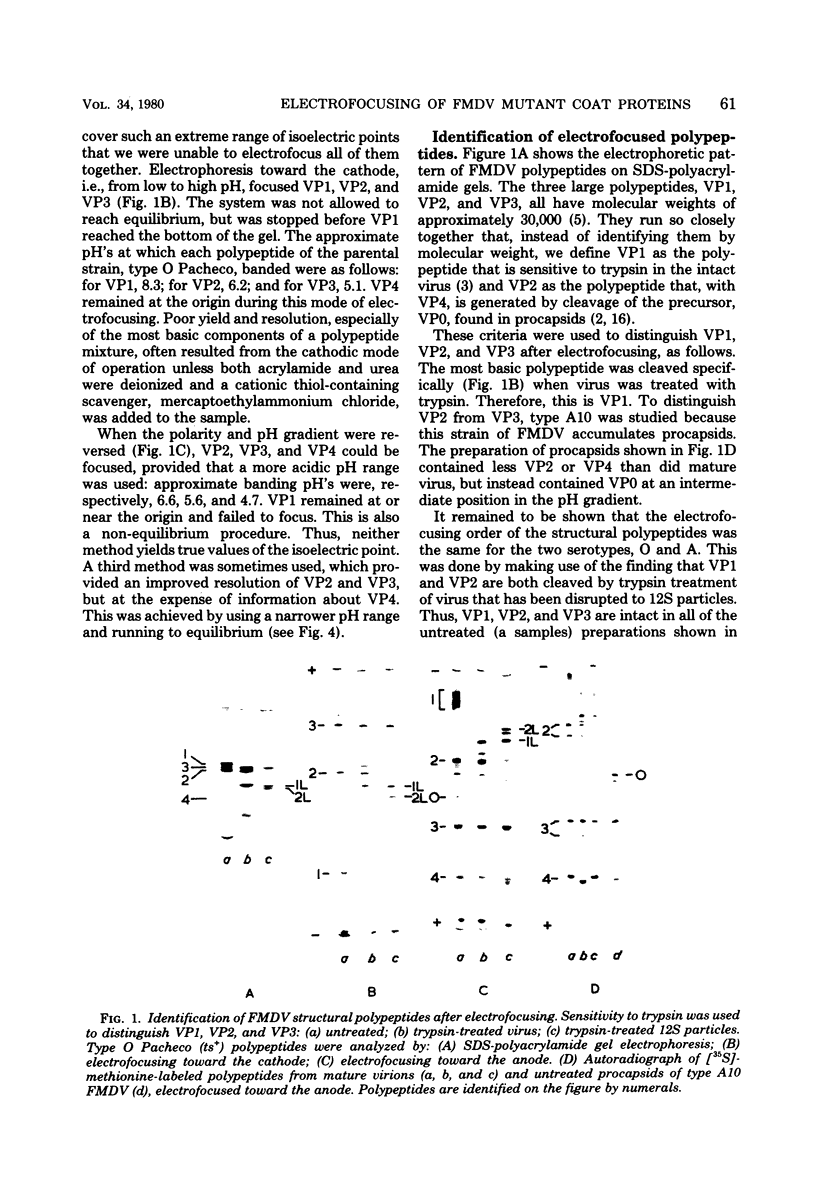
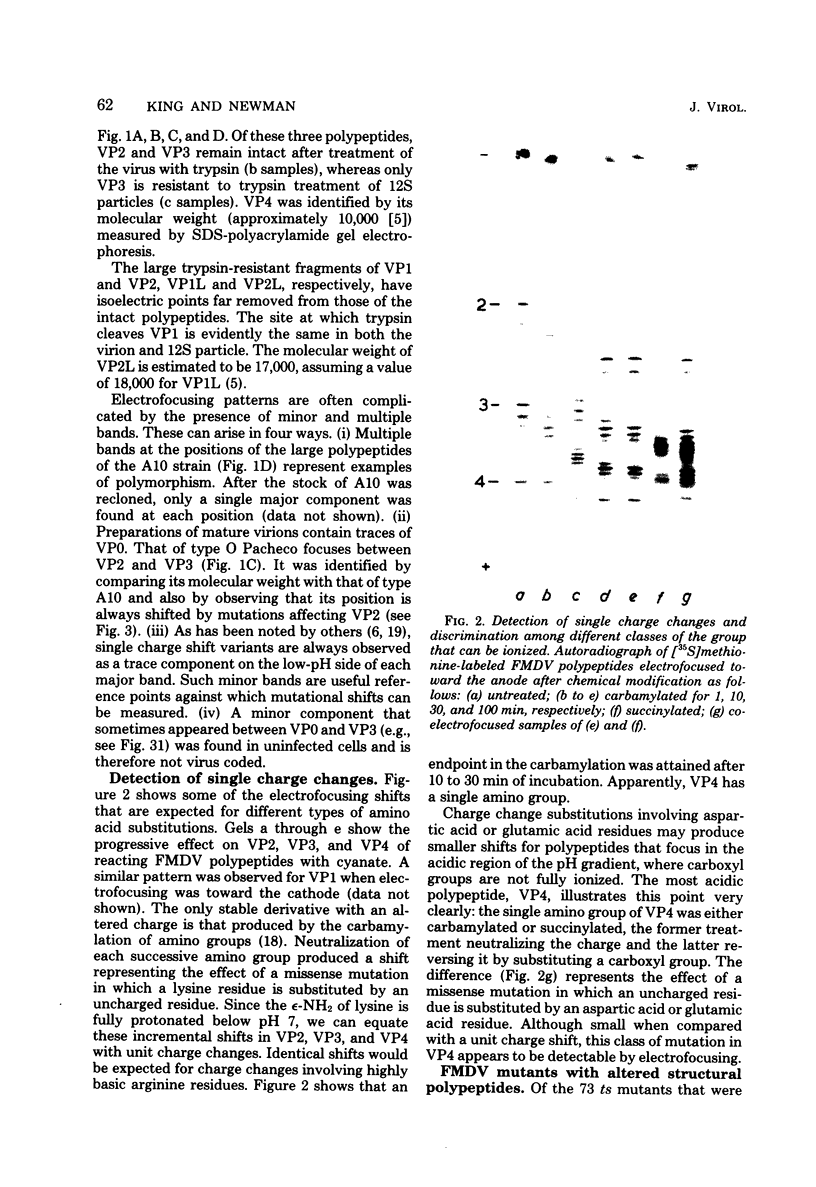




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRACH H. L., TRAUTMAN R., BREESE S. S., Jr CHEMICAL PHYSICAL PROPERTIES OF VIRTUALLY PURE FOOT-AND-MOUTH DISEASE VIRUS. Am J Vet Res. 1964 Mar;25:333–342. [PubMed] [Google Scholar]
- Black D. N. Proteins induced in BHK cells by infection with foot-and-mouth desease virus. J Gen Virol. 1975 Jan;26(1):109–119. doi: 10.1099/0022-1317-26-1-109. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Rowlands D. J., Sangar D. V., Talbot P., Brown F. Further evidence for multiple proteins in the foot-and-mouth disease virus particle. J Gen Virol. 1971 Oct;13(1):73–84. doi: 10.1099/0022-1317-13-1-73. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Sangar D. V., Rowlands D. J., Brown F. Immunogenic and cell attachment sites of FMDV: further evidence for their location in a single capsid polypeptide. J Gen Virol. 1977 Apr;35(1):149–158. doi: 10.1099/0022-1317-35-1-149. [DOI] [PubMed] [Google Scholar]
- Hamann A., Drzeniek R. Isoelectric focusing of viral polypeptides in urea. A methodological study on poliovirus. J Chromatogr. 1978 Jan 11;147:243–262. doi: 10.1016/s0021-9673(00)85136-5. [DOI] [PubMed] [Google Scholar]
- King A. M., Slade W. R., Newman J. W., McCahon D. Temperature-sensitive mutants of foot-and-mouth disease virus with altered structural polypeptides. II. Comparison of recombination and biochemical maps. J Virol. 1980 Apr;34(1):67–72. doi: 10.1128/jvi.34.1.67-72.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Priston A. J., Slade W. R. A genetic recombination map of foot-and-mouth disease virus. J Gen Virol. 1975 Jun;27(3):355–367. doi: 10.1099/0022-1317-27-3-355. [DOI] [PubMed] [Google Scholar]
- Lake J., Mackenzie J. S. Improved technique for the isolation of temperature-sensitive mutants of foot-and-mouth disease virus. J Virol. 1973 Sep;12(3):665–668. doi: 10.1128/jvi.12.3.665-668.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahon D., Slade W. R., Priston R. A., Lake J. R. An extended genetic recombination map for foot-and-mouth diseases virus. J Gen Virol. 1977 Jun;35(3):555–565. doi: 10.1099/0022-1317-35-3-555. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Slade W. R., Elworthy P., O'Sullivan M. Properties of temperature-sensitive mutants of the KENYA 3/57 strain of foot-and-mouth disease virus. J Gen Virol. 1970 Feb;6(2):213–220. doi: 10.1099/0022-1317-6-2-213. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Crowther J. R., King A. M., Brown F. Comparative biochemical and serological analysis of five isolates of a single serotype of foot-and-mouth disease virus. J Gen Virol. 1979 Dec;45(3):579–590. doi: 10.1099/0022-1317-45-3-579. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Brown F. Biochemical mapping of the foot-and-mouth disease virus genome. J Gen Virol. 1977 May;35(2):281–297. doi: 10.1099/0022-1317-35-2-281. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Morrissett H., Gold L. An electrofocusing system for the analysis of proteins and their genetic variants. Anal Biochem. 1978 Mar;85(1):224–229. doi: 10.1016/0003-2697(78)90293-2. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Talbot P., Brown F. A model for foot-and-mouth disease virus. J Gen Virol. 1972 May;15(2):163–169. doi: 10.1099/0022-1317-15-2-163. [DOI] [PubMed] [Google Scholar]