Abstract
Mice with homozygous genetic disruption of the melanocortin-4 receptor gene (MC4R−/−) are known to be hyperphagic and become obese, while those with disruption of the melanocortin-3 receptor gene (MC3R−/−) do not become markedly obese. The contribution of MC3R signaling in energy homeostasis remains little studied. In the present work, we compare MC3R−/− mice with wild-type (WT), MC4R−/−, and mice bearing disruption of both genes (double knockout, DKO) on select feeding and neuroanatomical dimensions. DKO mice were significantly more obese than MC4R−/−, whereas MC3R−/− weighed the same as WT. In a food demand protocol, DKO and MC4R−/− were hyperphagic at low unit costs for food, due primarily to increased meal size. However, at higher costs, their intake dropped below that of WT and MC3R−/−, indicating increased elasticity of food demand. To determine whether this higher elasticity was due to either the genotype or to the obese phenotype, the same food demand protocol was conducted in dietary obese C57BL6 mice. They showed similar elasticity to lean mice, suggesting that the effect is of genotypic origin. To assess whether the increased meal size in MC4R−/− and DKO might be due to reduced CCK signaling, we examined the acute anorectic effect of peripherally administered CCK and subsequently the induction of c-Fos immunoreactivity in select brain regions. The anorectic effect of CCK was comparable in MC4R−/− , DKO, and WT, but it was unexpectedly absent in MC3R−/−. CCK-induced c-Fos was lower in the paraventricular nucleus in MC3R−/− than the other genotypes. These data are discussed in terms of demand functions for food intake, MC receptors involved in feeding, and their relation to actions of gut hormones, such as CCK, and to obesity.
Keywords: double knockout mice, cholecystokinin, c-Fos, diet-induced obesity, fixed unit price
obesity is one of the biggest threats to contemporary human health. Analysis of genetic determinants of body mass index has shown that polymorphisms in the melanocortin-4 receptor (MC4R) are implicated in 1–6% of early onset or severe adult obesity cases (1, 12).
Studies in mice have elucidated distinct roles for melanocortin-3 receptor (MC3R) and MC4R in food intake and body weight (7, 10). Homozygous deletion (−/−) of the MC4R results in an obese phenotype, including hyperphagia, indicating that this receptor has a role in limiting food intake and stimulation of energy expenditure (7, 9, 10). Homozygous deletion of the MC3R does not result in increased body weight, but it may be associated with higher body fat content compared with wild-type (WT) controls (7). Chen et al. (7) included a description of mice with deletions of both type 3 and 4 receptors (double knockout, DKO) but to our knowledge, further characterization has not been published. One purpose of this paper is to report feeding and physiology in DKO mice.
Food intake is organized in meals, and increased food intake could be realized by increased meal size, meal frequency, or both. One of the important determinants of intake is the cost or effort associated with obtaining food (3, 18). A demand function is the mathematical relationship between food intake and its cost; in fixed unit price (FUP) schedules, each unit of food costs a fixed number of behavioral responses. While many such protocols have used short sessions or open economies, the extension to continuous access and a closed economy, in which all of the food is earned, allows parameters of meal size and frequency to be determined. Previous work from our laboratory examined motivation to obtain food in MC4R−/− mice in a closed economy (19). We found that, compared with WT, MC4R−/− mice were hyperphagic and took larger meals under specific low-cost schedules. However, full demand functions were not determined in that study. The first part of this paper will determine full demand functions in WT, MC3R−/−, MC4R−/−, and DKO mice. In the second part, the same type of analysis will be presented with diet-induced obese (DIO) mice to try to dissociate phenotypic (obesity) effects from the results in genetically obese mice.
It has been found that large meal sizes characterize many genetic animal models of overeating and obesity. This suggests that some aspect of satiation or satiety may be impaired. CCK is released in the gut during meals and is believed to restrain meal size by stimulating glutamatergic vagal afferent fibers that project to the nucleus of the tractus solitarius (NTS), and/or by direct action in the area postrema (AP) (2, 4, 5, 8, 14). Release of α-MSH in the NTS from terminals of proopiomelanocortin (POMC) neurons is thought to modulate controls of meal size, including CCK action, in the NTS. There is now strong evidence that MC4Rs modulate release of glutamate from vagal afferents in the NTS by a presynaptic mechanism (20); in most NTS cells, the number of spontaneous excitatory postsynaptic events was enhanced by MC4R stimulation. One functional aspect of α-MSH action may be to augment CCK and/or other vagally mediated satiety signals. It follows that in the absence of MC4R, the satiety effect of CCK should be diminished, a prediction that has been confirmed by some investigators (6, 8). On the other hand, previous work in our laboratory has not supported this prediction (17); MC4R−/− mice showed slightly greater inhibition of liquid food intake after CCK injection compared with wild-type mice. The present work extends this analysis to a solid food and examines the effects of CCK in mice with deletion of MC3R and/or MC4R.
Additionally, since c-Fos is a marker of cellular activation and is induced in the NTS and other brain regions by peripheral administration of CCK (8), we compare the induction of c-Fos by CCK in the brain of these various mice with deletion of MC3R and/or MC4R.
METHODS
Subjects and housing.
MC3R−/− and WT mice on a mixed C57B6/129 background were generated by breeding heterozygous pairs from a colony originating at Merck Pharmaceutical. MC4R−/− and WT mice, also on a 129/B6 background, were generated by breeding heterozygous pairs from a colony originating at Millennium Pharmaceuticals. Double knockouts were generated by crossing MC4RKO−/− and MC3RKO−/− and genotyping the offspring. Resultant double MC4R/MC3R heterozygotes were then crossed. The double (+/+) mice from this cross were used to establish a WT colony. The MC3R−/− and MC4R+/− from this cross were bred to produce the double knockouts. All mice were genotyped from a tail snip taken at weaning, as described elsewhere (10). These colonies are maintained in the Cancer Genetics vivarium at the University of Florida. At 3–6 mo of age, mice were moved to the Psychology vivarium for the test procedures. Both facilities are managed by a centralized and accredited animal care program. For the diet-induced obesity protocol, 16 male C57BL/6 (B6) mice were purchased at 3 mo of age (Jackson Laboratories; Bar Harbor, ME) and housed in the Psychology Department vivarium at the University of Florida. B6 mice were chosen for this protocol because this strain readily develops metabolic syndrome and obesity when fed with a high-fat diet but remains lean and physically normal when fed a low-fat diet (16).
The Psychology vivarium was maintained on a 12:12-h light cycle (0700–1900) at an ambient temperature of 23 ± 2°C and relative humidity 50–70%. For at least a week before, and throughout behavioral testing, the mice were housed singly in standard polycarbonate cages with Sani Chips (Harlan, Madison WI) bedding and a red polycarbonate Igloo (BioServ) as enrichment (15). Food (5001 pellets; PMI International) and tap water were available at all times, except as noted. Animal use was in accordance with principles in the NRC Guide for Care and Use of Laboratory Animals, and the protocol was approved by the University of Florida Institutional Animal Care and Use Committee.
Operant behavior chambers and general procedure.
Twenty-four behavior test chambers (Med Associates; 13 × 13 × 12 cm), with Plexiglas and alloy walls and stainless-steel grill floor, were used for testing. Each was contained within a ventilated, noise-attenuating cabinet with the same 12:12-h light cycle as the vivarium via a 7-W bulb in a night light fixture run from a 24-h timer. All chambers were equipped with a nose poke device with a small cue light above. The nose poke device was located ∼2 cm above the floor and adjacent to a food receptacle. Water was supplied freely from sipper tubes mounted on the wall opposite the food receptacle. A record of total pellets obtained and number of responses (nose pokes) was acquired by MED-PC IV computer software (Med Associates). These data files allowed an analysis of the number of meals and the amount eaten at each meal. Data were accumulated in 15-min time bins throughout each 23-h period.
Experiment 1: food demand analyses in melanocortin receptor knockout mice.
A total of 40 mice of MC4R−/− (n= 12; 6 females, 6 males), MC3R−/− (n = 8; 3 females, 5 males), DKO (n = 11; 7 females, 4 males) and WT (n = 9; 3 females, 6 males) littermates were used. Mice were between 4 and 9 mo of age during these tests. The WT group contained WT from both MC3R+/− and MC4R+/− breeding colonies; no differences were observed between these two types of WT mice in the present or any previous studies, so their data have been combined.
Prior to the main protocol, to habituate mice to the chambers and the novel pellets, a 4-day training period was applied, in which pellets were delivered contingent upon FUP2 (each pellet delivery is contingent on the completion of two nose poke responses) with the same nose poke operant. Mice were deemed to have learned the contingency when they earned enough pellets over 23 h to maintain their body weight, typically within 2 days (6). No food deprivation was imposed at any time during the experiment. During the main protocols, mice lived in test chambers for 23 h per day and obtained a 20-mg pellet (Purina Test Diet 5TUM, a grain-based tablet with 10.4% kcal from fat and 24.1% from protein) when they completed a unit cost (n, nose poke responses) as specified by a programmed fixed unit prices (FUPn) schedule of reinforcement. After training, mice were tested using an increasing series of unit costs (FUP2, 5, 10, 25, 50) with 4 days at each unit price.
At the end of the study in the second batch of mice (n = 23), and after about 1 wk of free feeding to regain any lost weight, mice were anesthetized (Sleepaway), and the subcutaneous, visceral, and pericardial (thoracic) white adipose tissue depots were dissected and weighed.
Experiment 2: food demand in diet-induced obese mice.
Sixteen B6 mice were randomly divided in two dietary groups of 8: high-fat (HF) and low-fat (LF). The diets were purchased from Research Diets; HF #D12492: 34.9% fat and 60% energy; LF # D01060501, 4.3% fat wt/wt, and 10% energy. For the next 9 wk, mice received the designated diet in their home cages. Throughout this period, HF fed mice gradually gained more weight than LF fed mice and displayed significantly higher body weight and a distinct diet-induced obesity. After this phase, all mice were run in the operant test chambers, where they worked for 5TUM (low fat) pellets for the same training period and main protocol as in experiment 1.
Experiment 3: effects of CCK.
Three CCK studies were performed. In the first, WT, MC4R−/−, and DKO were included. In the second, MC4R−/−, MC3R−/−, and corresponding WT from two different age ranges were tested. In the third, after at least 1 wk of free chow intake, mice from the first study were used to study induction of c-Fos immunoreactivity.
Study A: CCK anorexia in dessert protocol.
Mice were 4–9 mo of age at the time of testing. Food intake was measured in 30-min test sessions using a “dessert” protocol, in which the mice were adapted to receive a highly preferred food treat at the same time each day. The dietary treats were Fruit Crunchies (Bio-Serv; No. F05798; 52% carbohydrate, 20% protein, 6% fat by weight, with carbohydrate fraction ∼75% monosaccharides and disaccharides), 190-mg spherical pellets about the same macronutrient composition as chow and available in grape, apple, and orange flavors (15). During training, the mice received 10 pellets of mixed flavors in a 10-ml glass beaker, hung inside the cage from a metal stirrup. On the first day, the beaker was left in place for as long as needed for robust intake to occur. Thereafter, the time per day was tapered rapidly to 30 min. We found that most (∼90%) WT mice readily consumed Crunchies (15), whereas ∼25% of MC4R−/− and DKO mice did not eat this novel food, even after many days of presentation. Thus, sufficient mice were initially trained so that at least 6 reliable eaters were obtained for each genotype.
Test intakes were recorded as the number of Crunchies eaten; bedding was searched for half pellets, but these or smaller crumbs were minimal: most eating of this size of food item seems to be largely all-or-none. After about 1 wk adaptation to the dessert regimen, as well as to handling, a baseline was established by recording intake for 3 consecutive days. On test days, mice were injected subcutaneously at about 1300 with the same volume of either the vehicle (saline, 0.02 ml/10 g body wt) or CCK (6 μg/kg body wt in a volume of 0.02 ml/10 g body wt). These doses were chosen on the basis of our previous work (18). The procedure was repeated 5–7 days later using a cross-over design. There was no order effect, so data were combined for analysis. Intakes after vehicle or peptide were expressed as % of the baseline for each individual, and data were analyzed using one-way ANOVA and post hoc Tukey tests (P < 0.05). CCK (as the sulfated octapeptide) was purchased from Sigma Chemical. Intake after drug was also analyzed as percent of the intake by each individual after vehicle injection. No food deprivation was imposed in this protocol.
Study B: CCK anorexia in deprivation protocol.
In the second study, MC3R−/− and MC4R−/− mice of two age ranges were used, young adults (3–4 mo), and middle-aged (7–8 mo). Littermate MC3R+/+ and MC4R+/+ were tested as controls, but no middle-aged MC4R+/+ were available. To address, in part, body weight differences between groups, two doses of CCK (3 and 6 μg/kg) were tested. To be consistent with studies from other laboratories (6, 8), we used an overnight (18 h) food deprivation protocol with a standard chow pellet presented as the test food. As before, injections were made 5 min before food was presented, and intake was measured after 30 min. Three tests were conducted at 1-wk intervals, with each mouse receiving vehicle and 3 or 6 μg/kg CCK, in random order. Data will be presented as absolute intakes and expressed as a percentage of the individual intakes on the vehicle test day. These data were subjected to ANOVA and post hoc Tukey tests, with a significance criterion of P < 0.05.
Study C: c-Fos-immunoreactivity.
Using the mice that had been in the first study, but now 1–2 mo older, we examined induction of c-Fos immunoreactivity by CCK. This was performed in several batches, with all genotypes represented in each batch; no differences were found as a result of batch, so data were combined, as planned. On each test day, chow pellets were removed from the home cages 1–2 h beforehand to prevent recent spontaneous meals.
Mice were injected with CCK (6 μg/kg) or saline vehicle and 1 h later were anesthetized (Sleepaway; 1 ml/kg), and perfused transcardially with heparinized saline followed by paraformaldehyde. Brains were removed, stored in paraformaldehyde overnight, and then sliced by vibratome into coronal 75-μm sections at the levels of the paraventricular nucleus (PVN) of the hypothalamus and the AP and adjacent NTS. Sections were then incubated with primary (c-Fos 4 polyclonal; Santa Cruz Biotechnology) and secondary (biotinylated goat anti-rabbit IgG, Zymed) antibodies, and the reaction product visualized using ABC (Vector Laboratories), as we have described in detail elsewhere (11). Sections were mounted on slides, and c-Fos-positive cells in regions of interest were examined under a microscope and counted manually by two observers; to ensure a blind procedure, animal identifiers were obscured during this phase. The counts from the two observers showed <5% variation and were averaged.
Data analysis.
In the operant behavior study, as we have described before (3), the raw data showed the number of responses emitted each 23-h period (1380 min) and the number of pellets earned, with a temporal resolution of 15 min. Nonresponding (noneating) episodes were defined as time bins with zero pellets received. The data stream was separated into meals using a 15-min (at least one zero separating nonzero bins) minimum criterion. Note that on average, an animal will have stopped in the middle of the interval preceding a zero entry and will have started eating in the middle of the interval following a zero entry; thus, this 15-min meal criterion, in fact, defines an average minimum intermeal interval of 30 min, but with a range from 15–45 min. After the numbers of meals per day were determined for each mouse, the mean meal size was derived by dividing the number of total pellets by the number of meals for each day. No statistical difference was noted across the 4 days of each schedule for either total intake or meal parameters. Thus, for each mouse, the mean number of meals, total pellets earned, and meal sizes were averaged across the 4 days of each reinforcement schedule. These data were then treated using a repeated-measures ANOVA (SPSS), with genotype as a between-subject variable and the increasing schedules as a within-subject variable. Follow-up Tukey tests were employed to assess specific differences; in all cases, P < 0.05 was considered significant.
For acute CCK feeding tests and the fat pad analysis, one- and two-way ANOVA (SPSS) and post hoc Tukey tests (where appropriate) were used to examine the genotype effects and/or drug on the % suppression of food intake or the dissected body fat content. For the c-Fos studies, the numbers of c-Fos-stained cells in each region of treated mice were compared across genotypes using one-way ANOVAs and Tukey post hoc contrasts.
RESULTS
Experiment 1: food demand analyses in melanocortin receptor knockout mice.
No consistent differences in meal patterns or intakes were found between males and females, so these data were not analyzed for sex differences. The experiment was run in two replications to increase the sample size, with 17 and 23 mice, respectively, in each phase and with each genotype represented. The data from both studies were similar and were combined for analysis, as planned.
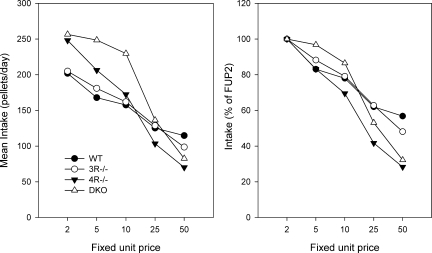
The demand for food as a function of unit price is shown in Fig. 1. There were differences in intake as a function of unit price (P < 0.001) with intake declining as unit price increased. There was a significant main effect of genotype (P = 0.006) and a genotype × unit price interaction (P < 0.001). As is evident from the Fig. 1, left, at the lower costs (FUP2, FUP5, FUP10) the MC4R−/− and the DKO mice ate more food per day than either MC3R−/− or WT mice (P < 0.001). However, as unit cost increased further, the intakes of the MC4R−/− and DKO declined more sharply than those of MC3R−/− or WT, so that at the highest price (FUP50), the former groups consumed the least (P < 0.05). Figure 1, right, expresses the intake of each group as a percentage of their intake at the lowest cost (FUP2); intakes at the highest cost (FUP50) had fallen by 40–50% in MC3R−/− and WT, while intakes of the MC4R−/− and DKO had fallen by ∼70%.
Fig. 1.
Daily (23 h) food intake, shown as number of 20-mg pellets eaten, in wild-type (WT), mice with disruption of the melanocortin-3 receptor gene (MC3R−/−), mice with disruption of the melanocortin-4 receptor gene (MC4R−/−), and double knockout (DKO) mice exposed to increasing fixed unit prices (nose pokes) per pellet. Each datum is the average of 4 days at each price step and 8–12 mice per genotype. Left: intake is shown in absolute terms. Right: intake is expressed relative to percentage of their intake at the lowest cost (FUP2).
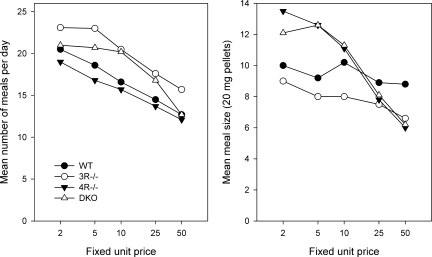
The meal parameters are shown in Fig. 2. The number of meals (left) taken per day did not differ as a function of genotype but declined as unit prices increased (P < 0.001 for all 4 genotypes). Mean meal size (right) was comparable in MC3R−/− and WT and did not decline significantly across the unit cost series in these genotypes. In contrast, the mean meal size of the MC4R−/− and DKO dropped rapidly as unit cost increased (P < 0.001). Thus, the decline in total food intake as unit prices increased (Fig. 1) was the result of fewer and smaller meals in MC4R−/− and DKO, whereas in WT and MC3−/−, it was a result of fewer meals only (Fig. 2).
Fig. 2.
Intake data from Fig. 1 analyzed for number of meals (left) and mean meal size (right).
Body weights and fat pads.
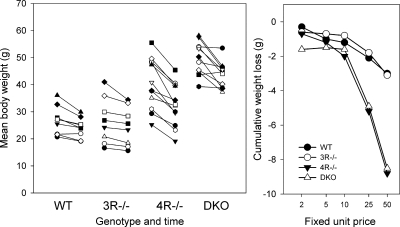
The body weights of these animals during the operant protocol are shown in Fig. 3. The left panel shows the individual weights at the beginning and end of the cost series, and the right panel shows the mean group cumulative changes in weight at each cost. DKO animals were uniformly obese at the start of the study, the MC4R−/− were variably obese, and MC3R−/− and WT had comparably low weights. All animals lost some weight during the FUP series, but the greatest absolute loss was in the MC4R−/− and DKO animals (right). One-way ANOVAs on the cumulative changes were significant (P < 0.001) at FUP50. Interestingly, the greatest individual weight losses were not consistently associated with the highest initial weights (left).
Fig. 3.
Weight changes of mice during the demand series. Left: weights are shown of individual mice (different symbols) of each genotype at the beginning of the study (first 2 days of FUP2), and at the end of the study (last 2 days of FUP50). The lines thus join the start and end weights for each mouse. Right: as group means, the cumulative body weight changes from initial weight until the end of each demand schedule are shown.
These differences in body weight were reflected in dissected body fat (Table 1). The mean fat from the combined subcutaneous and visceral depots rose from a mean of 3% body weight in WT to 11.6% in MC4R−/− and 21.7% in DKO. The thoracic cavity also contained visible fat in MC4R−/− and DKO: while not a large mass compared with the other pads, thoracic fat mass (<0.02 g in WT and MC3R−/−) was 0.06 ± 0.01 g (means ± SE) in MC4R−/− and 0.13 ± 0.02 g in DKO.
Table 1.
Fat content of mice from experiment 1
| Genotype, n | Body wt, g | Dissected Fat, g | Fat, % Body wt |
|---|---|---|---|
| WT (4) | 25.3±.9a | 0.8 ± 0.1a | 3.0±.4a |
| MC3R−/− (4) | 20.6 ± 1.9a | 1.1±.5a | 4.9 ± 1.7a |
| MC4R−/− (8) | 40.4 ± 9.8b | 4.9±.9a | 11.5 ± 1.7b |
| DKO (7) | 50.4 ± 2.2c | 11.1 ± 1.7b | 21.5 ± 2.4c |
Values are expressed as means ± SE; number of animals (n) is shown in parentheses. Statistical difference. a–dP < 0.05) between groups is indicated by different letter superscripts.
Experiment 2: food demand in diet-induced obese mice.
Prior to the diet regimen, mice designated for HF and LF groups had similar weights (24.2 ± 0.4 and 24.2 ± 0.3 g, respectively; means ± SE). At the end of 9 wk, the HF and LF groups weighed 45.6 ± 0.7 g and 35.3 ± 1.5 g, respectively (P < 0.001).
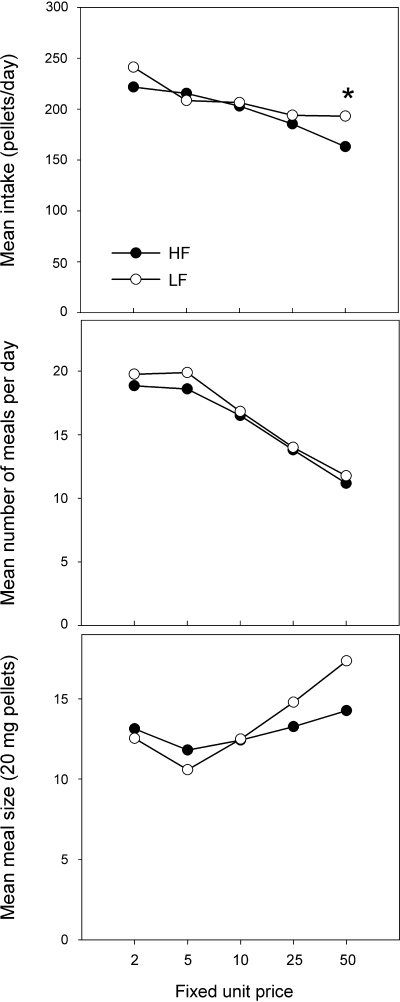
The demand for food as a function of unit cost is shown in Fig. 4, top. There were differences in intake as a function of unit price (P < 0.001) with intake declining as unit price increased. The only significant difference between the DIO (previously HF) and LF mice in terms of total pellets earned per day was during FUP50 (P < 0.05), as DIO mice received fewer pellets than LF mice. The meal parameters are also shown in Fig. 4. The number of meals (Fig. 4, middle) taken per day did not differ as a function of phenotype but declined as the unit prices increased (P < 0.001 for both phenotypes). Mean meal size (Fig. 4, bottom) did not change significantly across the unit cost series in HF mice. In contrast, the mean meal size of the LF mice increased as unit cost increased (P < 0.05).
Fig. 4.
Daily (23 h) food intake (top), as number of 20-mg pellets eaten, in diet-induced obese high-fat (HF), and low-fat (LF) mice exposed to increasing fixed unit prices (nose pokes) per pellet. Each datum is the average of 4 days at each price step. Intake data were further analyzed for number of meals (middle), and mean meal size (bottom). *P < 0.05 HF vs. LF.
Experiment 3: action of CCK.
In the first study, using the dessert protocol, mean intake of Crunchies in the baseline period did not differ as a function of genotype. The mean ± SE numbers of Crunchies eaten were WT 8.5 ± 0.4, MC4R−/− 7.1 ± 0.7, and DKO 7.2 ± 0.6. After vehicle injection, mean intakes of the WT and MC4R−/− groups were within 5% of their baseline, but the intake of the DKO group was reduced significantly, both from their baseline and from the absolute intake of the other two groups (P < 0.05) to 4.4 ± 0.5 and 5.4 ± 0.8 Crunchies (64% of their baseline). Intakes following peptide injections were thus analyzed both as a percentage of baseline and as a percentage of vehicle (Table 2). The anorectic effect of CCK was at least as great in the MC4R−/− and DKO mice as in WT. CCK reduced food intake from vehicle and baseline in each group (P < 0.05). While the suppression of intake in the MC4R−/− and DKO groups was slightly greater than for the WT, only the DKO was significant (P < 0.05) relative to WT and only when expressed as a percentage of baseline.
Table 2.
Effect of CCK-8 (6 μg/kg) on intake of Crunchies by undeprived mice
| Genotype, n | Test Intake, % Baseline | Test Intake, % Vehicle |
|---|---|---|
| WT (5) | 49 ± 5 | 46 ± 5 |
| MC4R−/− (5) | 28 ± 9 | 25 ± 8 |
| DKO (6) | 29 ± 4* | 47 ± 7 |
Values are expressed as means ± SE; number of animals (n) is shown in parentheses.
P < 0.05 vs. corresponding WT datum.
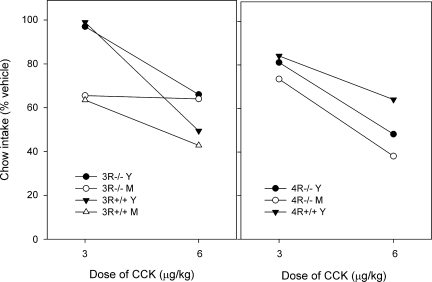
The deprivation-chow protocol was used in the second study, and the results are shown in Table 3 and Fig. 5. Table 3 shows the absolute intakes, which differed significantly across groups (P < 0.001). Post hoc tests showed that the intake of young (3–4 mo) and middle-aged (10–11 mo) MC4R−/− differed significantly (P < 0.02) from each other, but neither differed from the corresponding MC4R+/+ (for which only young mice were available). Two-way ANOVA showed that intakes were lower in MC3R−/− than in MC3R+/+ groups and were lower in young than middle-aged cohorts. The higher dose of 6 μg/kg suppressed intake relative to the vehicle condition in all groups; the lower dose of 3 μg/kg suppressed in all groups except for young MC3R−/− and MC3R+/+. Mean intakes as a percentage of vehicle are shown in Fig. 5. At the lower dose, the young MC3R−/− and MC3R+/+ differed from all other groups (one-way ANOVA; P < 0.01), while at the higher dose, there was no group difference in percentage of suppression. Since the MC4R−/− groups weighed about 2 times more than the MC4R+/+ control (Table 3), for a given dose, they received about twice the absolute amount of CCK. Thus, approximately the same absolute amounts of CCK were received by the MC4R+/+ after 6 μg/kg and MC4R−/− after 3 μg/kg, respectively; the percent intakes were comparable in these conditions (Fig. 5, right).
Table 3.
Effect of CCK-8 on chow intake by deprived mice
| Genotype | Age, mo | n | Body Weight, g | Intake After Vehicle, g | Intake After CCK, 3 μg/kg | Intake After CCK, 6 μg/kg |
|---|---|---|---|---|---|---|
| MC3R−/− | 3–4 | 5 | 21.9 ± 1.2 | 0.53±.04 | 0.53±.07 | 0.38±.13 |
| MC3R−/− | 7–8 | 5 | 26.0 ± 0.9 | 0.72±.09 | 0.48±.10 | 0.47±.06 |
| MC3R+/+ | 3–4 | 4 | 18.9 ± 1.2 | 0.69±.06 | 0.68±.07 | 0.35±.11 |
| MC3R+/+ | 7–8 | 10 | 23.6±.08 | 0.81±.04 | 0.52±.05 | 0.35±.06 |
| MC4R−/− | 3–4 | 7 | 42.5 ± 1.2 | 0.50±.05 | 0.40±.05 | 0.24±.03 |
| MC4R−/− | 10–11 | 5 | 57.1 ± 4.6 | 0.76±.03 | 0.57±.08 | 0.28±.03 |
| MC4R+/+ | 3–4 | 7 | 25.6 ± 0.6 | 0.67±.04 | 0.56±.05 | 0.41±.02 |
Values are expressed as means ± SE. Body weights are the mean of 3 wk, predeprivation.
Fig. 5.
Effect of CCK-8 on intakes of chow in 30-min tests after 18-h food deprivation. Intakes are expressed as mean % intake after injection of vehicle. Error bars are not shown for clarity, but standard error averages about 10% of the mean. Left: data from young adult (Y: 3–4 mo) and middle-aged (M: 7–8 mo) MC3R−/− and MC3R+/+ mice. Right: data from Y and M (10–11 mo) MC4R−/− and Y MC4R+/+ mice. The numbers of mice per group were 4–10, as shown in Table 3.
The effect of CCK-8 on c-Fos-ir (immunoreactive) in PVN is shown in Table 4. c-Fos was induced strongly in WT, MC4R−/−, and DKO mice, but less so in MC3R−/− (P < 0.05), with this latter group not significantly different from saline-treated controls. This dose of CCK induced very strong c-Fos in the AP and NTS of all four genotypes, and accurate counting was not possible.
Table 4.
c-Fos-positive cells in paraventricular hypothalamus following CCK (6 μg/kg)
| Group (n) | c-Fos-Positive Cells |
|---|---|
| WT (6) | 235 ± 50* |
| MC3R−/− (6) | 122 ± 26 |
| MC4R−/− (4) | 269 ± 18* |
| DKO (6) | 218 ± 38* |
Values are expressed as means ± SE; number of animals (n) is shown in parentheses.
P < 0.05 differs from saline-treated controls (36 ± 5; n = 4).
DISCUSSION
Food demand.
MC4R−/− and DKO mice were obese and hyperphagic relative to WT, but only under the specific economic conditions of the low cost for food. The elastic nature of their demand functions for food was not replicated in DIO B6 mice. The hyperphagia of MC4R−/− and DKO mice, when present, was due mainly to a large meal size. This suggests that the disruption of the MC4R gene, and independently of obesity, is sufficient to disrupt normal physiological controls of and/or environmental effects on food intake and meal size.
These findings in MC4R−/− mice are consistent with but expand considerably on previous reports from our laboratory. Thus, using a FUP3 schedule, we found that MC4R−/− ate ∼33% more food per day than WT, and with a larger meal size (19). Hyperphagia and increased meal size were also found using progressive ratio schedules of pellet cost; under progressive schedules, MC4R−/− paid a higher average cost per pellet than WT (7.7 vs. 5.6 lever presses under one condition; see Ref. 19 and Table 2). However, under a procurement-consummatory dual cost foraging protocol (18), intake of MC4R−/− was identical to that of WT and MC3R−/− across a range of procurement costs but low (FUP 5, 10) per pellet costs. Together, with the present data, these findings suggest that MC4R−/− are hyperphagic when proximate or unit costs per pellet are less than ∼10 responses. If unit costs increase further, including when part of the cost is distal or “approach”, then the mice are no longer hyperphagic and may eat less than WT. As a result they lose weight (see Ref. 18 and Fig. 3), but that weight loss does not appear to influence their food-directed operant behavior. In this regard, the first meal is smaller in MC4R−/− after food deprivation (6), and from Table 3, it can be derived that young MC4R+/+ ate 26.2 g/kg body weight/30 min compared with 11.8 g/kg in MC4R−/−. It follows that MC4R−/− may be relatively insensitive to afferent information about adiposity, consistent with the observation by Marsh et al. (13) that MC4R−/− mice did not show anorexia to either peripheral or central injection of leptin.
To our knowledge, this is the first detailed report of intake and feeding patterns in DKO, but our data agree with Chen et al. (7) that they are significantly more obese than MC4R−/−, a difference that is due almost exclusively to more adipose tissue (In Table 1, note that the fat pads dissected do not account for all body fat.). At 26 wk of age, Chen et al. (7) reported that female and male DKOs were 27 and 13% heavier, respectively, relative to MC4R−/− counterparts. The sex difference in weight in MC4R−/− was no longer evident in DKO. Our body weight data closely confirm those observations (Fig. 3). We also noted considerable pericardial fat in the DKO mice.
Our finding that food demand was similar in WT and MC3R−/− extends our previous data (18) to meal analysis. Chen et al. (7) had reported modestly lower free food intake in MC3R−/− relative to WT. Chen reported that, despite normal body weight, MC3R−/− have increased adipose tissue mass. Our data, from a small number of animals (Table 1), are consistent with this view but do not reach statistical significance.
DIO and control mice in experiment 2 showed generally similar, although flatter demand functions than the WT in experiment 1. Interestingly, despite their obese state at the start of the FUP series, the DIO mice showed the same total intake and parameters as the nonobese (LF) group. Note that for both groups, this involved a change to a common grain-based, low-carbohydrate diet, and so it suggests that the diet and the economy determined intake more than the weight of the animal. Further, the DIO mice lost weight as FUP increased, but there was no tendency to eat less than LF except at the highest cost. Clearly, the demand curves in DIO mice are inelastic or flat compared with the steep functions in MC4R−/− and DKO.
Effects of CCK-8.
Electrophysiological, biochemical, and behavioral studies suggest that activation of MC4Rs in the NTS/AP and/or dorsal motor nucleus of the vagus potentiates the effect of vagally mediated satiation signals such as CCK (2, 20, 21). This is consistent with our demonstration of a large meal phenotype in MC4R−/− and DKO at low food costs. It follows that the satiating effect of CCK-8 should be reduced in MC4R−/− and DKO, but probably not in MC3R−/−, relative to WT. That result has been reported in MC4R−/− in two publications (6, 8), both using a food deprivation and chow protocol similar to our own. In contrast, using a 12-h fast and a liquid diet, we found that the intake in MC4R−/− was suppressed by various doses of CCK-8, at least as effectively as in WT (17).
In the study by Fan et al. (8), mice were 9 wk of age, and so obesity presumably had not fully developed. The dose they used (3 nmol/kg, close to our lower dose) reduced 30 min of food intake in MC4R−/− by ∼40% relative to saline, less than in WT (∼70%), and is not significant. Their feeding test lasted 3 h, and at later times, the suppressant effect of CCK had completely disappeared in MC4R−/− but not in the WT. Thus, at the short interval we used (30 min), Fan's MC4R−/− mice were, in fact, partially responsive to CCK. Blevins et al. (6) reported a dose-response analysis, with mice receiving 0.75, 2.5, or 7.5 nmol CCK-8/kg lean body mass, and a 30-min food intake test. They found a suppression of food intake in WT at all 3 doses, but in MC4R−/−, only the highest dose produced a significant reduction relative to vehicle injection. However, at the highest dose, the percentage of suppressions was not markedly different in WT and MC4R−/− (43 and 36%, respectively). Our doses were not based on lean body mass, but Blevins (J. E. Blevins, personal communication, June 2009) graciously provided data that the lean body mass of his WT and MC4R−/− mice averaged 78% and 63% of body weight, respectively. Thus, the highest dose that his mice received transforms to 5.85 and 4.72 nmol/kg (∼5.5 and 4.5 μg/kg) in WT and MC4R−/− respectively, a dose that is intermediate between our two doses. In our study, there is no evidence in either young or middle-aged MC4R−/− for reduced sensitivity to CCK (Fig. 5). This was also evident in our nondeprived (Crunchies) protocol (Table 2), which showed that DKO were also responsive to CCK-8. None of the published or present protocols indicate that MC4R−/− mice are completely unresponsive to exogenous CCK, but in some protocols, there may be a reduced responsiveness. Consistent with this finding, we were unable to find differences in c-Fos expression in AP/NTS of either MC4R−/− or DKO compared with WT mice. Finally, in a study in which we stimulated endogenous release of CCK and other meal-related factors, we found that oral preloads suppressed subsequent intake equivalently in WT and MC4R−/− (17).
The relative sparseness of MC3R in the hindbrain, as well as the above results in DKO, would lead us to expect that MC3R−/− mice would show normal satiation responses to CCK-8. This was, in fact, reported by Fan et al. (8) using mice 5–10 mo of age. However, our results revealed an unexpected insensitivity to CCK-8 (Fig. 5). In the young cohorts, both the MC3R+/+ and −/− genotypes were unresponsive to the lower dose of CCK-8 and −/− showed a smaller effect than +/+ at the higher dose. In middle-aged cohorts, the low dose was comparably effective in MC3R+/+ and −/−, but again at the higher dose, the −/− were less responsive. In a preliminary study, using even younger mice (∼2 mo) and a higher dose of CCK-8 (9 μg/kg), we found complete insensitivity in MC3R−/−. In summary, our data show more evidence for insensitivity to CCK-8 in MC3R−/− than in MC4R−/− and that this may be age-dependent. Thus, putative insensitivity to CCK is not a viable explanation for the large meal phenotype in MC4R−/− because MC3R−/− should show comparably increased meal size, but they do not.
The c-Fos-ir data give no indication for a reduced CCK activation in AP/NTS in any genotype relative to WT, but they do suggest a reduced activation in PVN of MC3R−/−. If peripheral CCK-8 is activating the PVN exclusively via the NTS/AP as a relay, this result would suggest that the relay has reduced effect in MC3R−/− either because the synaptic transmission is reduced or the target neurons are tonically inhibited, for example, because inhibitory POMC arcuate afferents have increased firing rate due to loss of functional MC3R autoreceptors. If this tonic inhibitory signal were carried through MC4Rs, then DKO mice would be insensitive to any increase in arcuate firing due to the MC3R−/− deletion. In any event, the reduced sensitivity of MC3R−/− to both CCK anorexia and c-Fos expression in PVN, at least warrant the speculation of a functional connection. This hypothesis requires further investigation, as well as the corollary that overexpression of MC3R might increase sensitivity to CCK.
Perspectives and Significance
Obesity and its related metabolic disorders have become an intractably large financial burden to modern societies in terms of health care costs and loss of productivity. There has been considerable focus on predisposing genetic factors, including polymorphisms of MC4R, but it is likely that most obesity is more directly linked to the ubiquitous availability of energy-dense foods. The present demonstration that a model of genetic obesity may be highly responsive to economic cost suggests that changes to the food environment can be effective in curbing food intake and/or choice in both normal and genetically disadvantaged obese populations. It is unrealistic to believe that a purely physiological manipulation such as gene or pharmacotherapy will be effective in the face of a “toxic” food environment. It is more realistic to ask how the obesity epidemic might be reversed by physiological manipulations in conjunction with radical changes of approach by the health delivery and food industries. The present study is thus presented as an early and small step in the preclinical analysis of this type of approach.
GRANTS
This work was funded in part by National Institutes of Health Grants RO1DK064712 and RO1DK057080.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Dennis Huszar and Millennium Pharmaceuticals/Gene Logic for providing the breeding stock MC4R−/− mice, Dr. Lex VanDerPloeg, and Merck Research for providing the breeding stock MC3R−/− mice, and both David White at Gene Logic and Merck Research for allowing us to generate and study the MC3R−/− × MC4R−/− double knockout mice.
REFERENCES
- 1.Adan RAH, Tiesjema B, Hillebrand JJG, la Fleur SE, Kas MJH, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol 149: 815–827, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atalayer D, Rowland NE. Meal patterns of mice under systematically varying approach and unit costs for food in a closed economy. Physiol Behav 98: 85–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Behav Rev 26: 393–428, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR, Sutton Townsend L GM, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav 89: 517–524, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. Am J Physiol Regul Integr Comp Physiol 296: R476–R484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan X, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26: 97–102, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Fan W, Ellacott KLJ, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7: 335–336, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Haskell-Luevano C, Schaub JW, Andreasen A, Haskell KR, Moore MC, Koerper LM, Rouzard F, Baker HV, Millard WJ, Walter G, Litherland SA, Xiang Z. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FASEB J 23: 642–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Li BH, Rowland NE. Dexfenfluramine induces fos-like immunoreactivity in discrete brain regions in rats. Brain Res Bull 31: 43–48, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Lubrano-Berthelier C, Cavazos M, Dubern B. Molecular genetics of human obesity-associated MC4R mutations. Ann NY Acad Sci 994: 49–57, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4-receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21: 119–122, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Rinaman L, Verbalis JG, Stricker Hoffman GE EM. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol 338: 475–90, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Rowland NE, Robertson KL. Effect of two types of environmental enrichment for singly housed mice on food intake and weight gain. Lab Animal 34: 29–32, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Vaughan CH, Haskell-Luevano C, Andreasen A, Rowland NE. Effects of oral preload, CCK or bombesin administration on short term food intake of melanocortin 4-receptor knockout (MC4RKO) mice. Peptides 27: 3226–3233, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiol Behav 84: 129–133, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides 27: 2829–2835, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28: 4957–4966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247–R258, 2005 [DOI] [PubMed] [Google Scholar]