Abstract
To determine whether skin surface cooling increases left ventricular preload and contractility to a greater extent in older compared with young adults we studied 11 young (28 ± 2 yr; means ± SE) and 11 older (64 ± 3 yr) adults during normothermia (35°C water perfused through a tube-lined suit) and cooling (15°C water perfused for 20 min) using standard and tissue Doppler echocardiography. Cooling significantly decreased skin surface temperature in young (Δ2.8 ± 0.3°C) and older (Δ3.0 ± 0.3°C) adults and increased rate-pressure product, an index of myocardial oxygen demand, in older (6,932 ± 445 to 7,622 ± 499 mmHg·beats/min for normothermia and cooling, respectively), but not young (7,085 ± 438 to 7,297 ± 438 mmHg·beats/min) adults. Increases in blood pressure (systolic and mean blood pressure) during cooling were greater (P < 0.05) in older than in young adults. Cooling increased preload in older (left ventricular end-diastolic volume from 106 ± 7 to 126 ± 9 ml and left ventricular internal diameter in diastole from 4.69 ± 0.12 to 4.95 ± 0.14 cm; both P < 0.01), but not young adults (left ventricular end-diastolic volume from 107 ± 7 to 111 ± 7 ml and left ventricular internal diameter in diastole from 4.70 ± 0.10 to 4.78 ± 0.10 cm). Indices of left ventricular contractility (ejection fraction, myocardial acceleration during isovolumic contraction, and peak systolic mitral annulus velocity) were unchanged during cooling in both young and older adults. Collectively, these data indicate that cooling increases left ventricular preload, without affecting left ventricular contractility in older but not young adults. Greater increases in preload and afterload during cooling in older adults contribute to greater increases in indices of myocardial oxygen demand and may help explain the increased risk of cardiovascular events in cold weather.
Keywords: tissue Doppler imaging, echocardiography, age
cardiovascular-related mortality increases in the cold winter months, particularly in older adults (10, 12, 23, 24, 28, 40). Associations between increased cardiovascular-related mortality and cold temperatures may be mediated, in part, by acute physiological responses to cold, which include peripheral and visceral vasoconstriction, elevated plasma norepinephrine, and diuresis (33, 38). Recently, we reported an augmented increase in arterial blood pressure during acute cold exposure (nonpainful and noninternal temperature lowering) in older adults (15). This augmented pressor response to cold stress in older adults was strongly associated with increased levels of central arterial stiffness before cooling and an increase in rate-pressure product during cooling (15). This effect appears to be confined to older adults as neither rate-pressure product nor cardiac minute work increase during similar cold exposure in young adults (36, 38).
Myocardial oxygen demand is affected by a number of factors (17) that may be altered by acute (i.e., cold stress) and chronic processes (i.e., aging). For instance, cold stress that does not induce shivering or decrease internal temperature does result in increased blood pressure, central venous pressure, and pulmonary capillary wedge pressure (7, 8, 37–39, 41). When pulmonary capillary wedge pressure is plotted against stroke volume or stroke work there is no apparent effect of cold stress on the Frank-Starling relation in young adults (36). However, cold-induced increases in afterload may obscure the ability to detect the effect of increased inotropy on the Frank-Starling relation as afterload and inotropy shift the curve in opposite directions. Thus, it is possible that cold-induced increases in left ventricular filling pressure offset increases in afterload to maintain stroke volume in young adults, but that the same increase in left ventricular filling pressure is unable to maintain stroke volume in older adults due to reductions in left ventricular compliance and diastolic function with age (1, 16, 25, 32, 35) without a compensatory increase in inotropy.
Accordingly, the primary aim of the present study was to determine whether skin surface cooling increases left ventricular contractility and preload to a greater extent in older compared with young adults. To address this aim we tested three hypotheses. Hypothesis 1: cold stress improves cardiac systolic function in older but not young adults. The rationale for this hypothesis is that during cold stress, greater systolic pressure development would be required to overcome reduced levels of arterial compliance in older adults coupled with reduced diastolic function in older adults. Hypothesis 2: cold stress would not be severe enough to alter diastolic relaxation as can occur in times of myocardial ischemia (2, 19). Hypothesis 3: cold stress increases indices of left ventricular filling pressure more in older than young adults. The rationale for this hypothesis is that age-associated decreases in venous compliance (22) could result in greater translocation of blood volume to the heart during cold stress (6), resulting in greater increases in indices of left ventricular filling pressure in older compared with young subjects. Addressing these hypotheses may provide insight into the mechanism(s) underlying altered age-associated responses to cold stress and associations between decreased outdoor temperatures and increased cardiovascular-related mortality in humans.
METHODS
Subjects
Twenty-two subjects participated in this study. Eleven were 20–34 yr old (young), and eleven were 58–76 yr old (older). All subjects were healthy, normotensive, nonsmokers, nonobese (body mass index < 30 kg/m2; Table 1), and unmedicated. Subjects were sedentary to recreationally active (2 or fewer bouts of aerobic exercise per week lasting < 30 min in duration each). Studies were conducted in the morning or early afternoon during the summer months of a northern temperate climate. Because of the time of year of the testing (summer), it is unlikely that subjects were cold acclimatized and thus blood pressure responses to cold exposure should not be affected (42). The Institutional Review Board at the Pennsylvania State University College of Medicine approved the experiments, and written consent was obtained from all subjects prior to testing. Subjects were studied at least 4 h postprandial and after a 12-h abstinence from caffeine.
Table 1.
Subject characteristics
| Variable | Young | Older |
|---|---|---|
| Sex | 8 male/3 female | 8 male/3 female |
| Age, yr | 28 ± 2 (20–34) | 64 ± 3 (58–76)* |
| Height, cm | 173.8 ± 2.1 (161.3–182.8) | 173.0 ± 2.3 (160.0–182.9) |
| Body mass, kg | 70.8 ± 3.7 (47.6–86.9) | 75.8 ± 3.4 (56.7–95.3) |
| BMI, kg/m2 | 23.3 ± 0.9 (18.6–27.5) | 25.3 ± 0.9 (19.0–29.3) |
| Body surface area, m2 | 1.9 ± 0.1 (1.58–2.06) | 1.8 ± 0.1 (1.63–2.15) |
| Systolic BP, mmHg | 114 ± 2 (102–123) | 126 ± 2 (118–137)* |
| Diastolic BP, mmHg | 65 ± 1 (59–69) | 73 ± 2 (63–82)* |
| Mean BP, mmHg | 85 ± 1 (78–88) | 93 ± 2 (87–101)* |
| Heart rate, beats/min | 62 ± 3 (50–79) | 54 ± 2 (49–65)* |
Values are means ± SE (range, minimum–maximum value); n = 11/group. BMI, body mass index; BP, blood pressure.
P < 0.05 compared to young.
Experimental Protocol
During the experiments, subjects were positioned supine while wearing a high-density tube-lined suit (Med-Eng Systems, Ottawa, ON, Canada). This water-perfused suit covered the entire body except the hands, feet, and head. Two-way zippers in the torso of the suit permitted echocardiography measures with minimal exposure of the skin to ambient air. Skin surface temperature was changed by altering the temperature of the water perfusing the suit, using an external pump connected in series with an external reservoir tank with an onboard heater. Skin surface cooling was accomplished by adding ice to the reservoir tank to achieve the desired water temperature. All subjects performed a cooling trial consisting of a 6-min baseline period (water temperature, 35°C) followed by a 20-min cooling period (water temperature, 15°C). A subgroup of young (n = 5) and older subjects (n = 5) also performed a control trial that was identical to the cooling trial, except water temperature was maintained at 35°C throughout. At least 30 min elapsed before each trial (water perfusion temperature, 35°C). During trials, blood pressure and heart rate were measured at 2-min intervals, and oral and skin surface temperatures were averaged and recorded at 5-min intervals. Echocardiograms were performed at baseline as well as in the final 8 min of each trial.
Measurements
Blood pressure and heart rate.
Blood pressure was determined noninvasively using a semiautomated device (Dinamap) over the brachial artery. Heart rate was determined from a three-lead ECG. Rate-pressure product was calculated as systolic blood pressure times heart rate.
Temperature.
Oral temperature was measured in the sublingual sulcus by using a thermocouple. Subjects refrained from speaking and oral breathing during the trials, and the head and neck were not directly exposed to cold. Adopting such precautions helps assure that measurements of oral temperatures are representative of internal temperature (5). Mean skin surface temperature was measured as a weighted average of four skin sites on the chest, arm, thigh, and leg (26).
Echocardiography.
Transthoracic echocardiography was performed using a digital ultrasound system (model iE33; Philips Ultrasound, Bothell, WA) and a 5.0–1.0 MHz probe. Left atrial diameter was measured in the parasternal long-axis view, and then adequate M-mode imaging was obtained from the parasternal view between the mitral valve and papillary muscle. The ultrasound beam was positioned perpendicular to the intraventricular septum and left ventricle posterior wall, allowing a clear view of the left ventricle diameter during diastole and systole. Left ventricular internal diameter was measured at the furthest endocardium endpoints (27). Left ventricular internal end-diastolic diameter was measured, and left ventricular end-diastolic volume, stroke volume, and ejection fraction were calculated by the echocardiography device (30). Left ventricular internal diameter in diastole and left ventricular end-diastolic volume were used as indices of preload. Ejection fraction was used as an index of systolic function. Indices of diastolic function were early diastolic mitral flow (E), late diastolic mitral flow (A), and E/A ratio. All measurements were averaged over 3–5 cardiac cycles.
Tissue-Doppler imaging analysis was performed on echographic data collected in the apical four-chamber view, while the pulsed Doppler sample volume was placed at the septal and lateral mitral annulus. The spectral Doppler signal filters were adjusted to obtain Nyquist limits of 15–20 cm/s using the lowest wall filter settings and the minimum optimal gain. The sample volume was placed at the base of the myocardium at the border of the intraventricular septal and lateral walls. Three major components of regional myocardial velocities were recorded: 1) the positive systolic velocity when the mitral ring moved toward the cardiac apex (Sm), which is a sensitive marker of regional myocardial systolic function and lower loading dependence (11, 14); 2) the first rapid negative diastolic velocity (Em) when the mitral annulus moves toward the base away from the apex; and 3) the second negative diastolic velocity (Am). Em and Am are sensitive markers of regional diastolic function (29). The ratio of E/Em was used as an index of diastolic function. Myocardial acceleration during isovolumic contraction (ACC) was calculated as the difference between baseline and peak velocity divided by their time interval from Q wave to onset of systolic ejection and was used as another index of systolic function (i.e., myocardial contractility) (34). Sm, Em, Am, and ACC were analyzed using Prosolv Software version 3.0.47. All tissue-Doppler data were averaged from two different sites (septal and lateral walls) at the mitral annulus over three to five cardiac cycles.
Statistical Analysis
Group differences (young compared with older) in subject characteristics were determined by independent t-tests. The effect of age and cooling were determined using a two-way, mixed-model, repeated-measures ANOVA with Student Newman-Keuls post hoc analysis performed when significance was identified. A similar analysis was also performed on the time-control data. Statistical significance was established at P < 0.05. All data are presented as means ± SE.
RESULTS
Subject Characteristics
In addition to age differences, older subjects' blood pressure was higher, and their heart rates were lower than young subjects at rest (i.e., during normothermic conditions; Table 1).
Effect of Skin Surface Cooling on Temperature and Systemic Hemodynamics
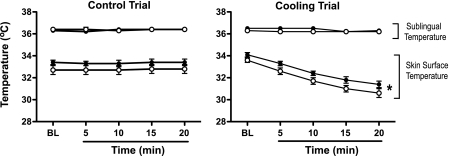
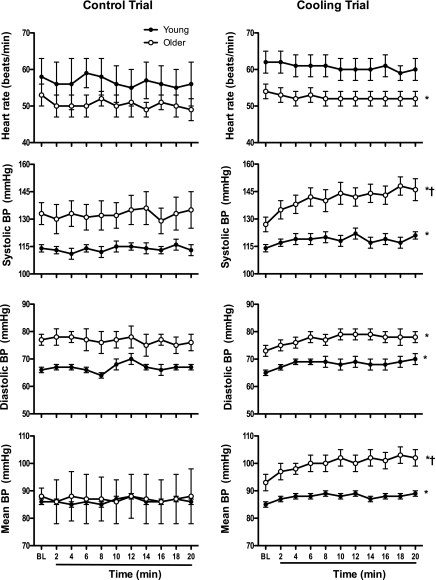
Cooling decreased (P < 0.05) skin surface temperature without affecting sublingual temperature in young or older adults (Fig. 1). Cooling increased blood pressure in young and older adults, although responses were greater (P < 0.05) in older adults (systolic and mean blood pressure; Fig. 2). Stroke volume increased (P < 0.05), heart rate decreased (P < 0.05), and cardiac output was unchanged in older adults during cooling (Table 2 and Fig. 2). Heart rate trended (P < 0.1) lower in the young group during cooling, but this trend was not observed in stroke volume or cardiac output (Table 2 and Fig. 2).
Fig. 1.
Sublingual and mean skin surface temperature responses to control (left; 5 young and 5 older) and cooling (right; 11 young and 11 older) trials in young (●) and older adults (○). Data are presented at baseline (BL) and minutes 5, 10, 15, and 20 of the respective intervention. Values are means ± SE. *P < 0.05 main effect of the intervention in young and older groups.
Fig. 2.
Blood pressure (BP) and heart rate responses during control (left; 5 young and 5 older) and cooling trials (right; 11 young and 11 older) in young (●) and older adults (○). Values are means ± SE. *P < 0.05 main effect (time); †P < 0.05 group by time interaction.
Table 2.
Hemodynamic measurements during the cooling trial
| Young |
Older |
|||
|---|---|---|---|---|
| Variable | Baseline | Cooling | Baseline | Cooling |
| Left atrial diameter, cm | 2.86 ± 0.13 | 2.86 ± 0.14 | 3.21 ± 0.14 | 3.27 ± 0.14 |
| IDd, cm | 4.70 ± 0.10 | 4.78 ± 0.10 | 4.69 ± 0.12 | 4.95 ± 0.14*† |
| End-diastolic volume, ml | 107 ± 7 | 111 ± 7 | 106 ± 7 | 126 ± 9*† |
| End-systolic volume, ml | 29 ± 4 | 33 ± 4 | 26 ± 4 | 29 ± 3 |
| Stroke volume, ml | 79 ± 4 | 78 ± 5 | 80 ± 5 | 97 ± 7*† |
| Cardiac output, l/min | 5.3 ± 0.4 | 5.1 ± 0.3 | 4.5 ± 0.4 | 5.1 ± 0.5 |
| Ejection fraction, % | 72 ± 1 | 70 ± 2 | 72 ± 2 | 76 ± 2 |
| Diastolic Function | ||||
| E, cm/s | 84.87 ± 2.96 | 81.45 ± 4.20 | 70.00 ± 4.67* | 67.18 ± 5.92* |
| A, cm/s | 41.21 ± 3.53 | 36.73 ± 2.26 | 61.64 ± 5.24* | 56.36 ± 5.42* |
| E/A | 2.24 ± 0.24 | 2.31 ± 0.20 | 1.22 ± 0.13* | 1.28 ± 0.13* |
| E/Em | 6.67 ± 0.45 | 6.42 ± 0.50 | 8.92 ± 0.90 | 10.49 ± 1.68*‡ |
| Myocardial Oxygen Demand | ||||
| Rate pressure product mmHg·beats/min | 7085 ± 438 | 7297 ± 438 | 6932 ± 445 | 7622 ± 499† |
Values are means ± SE; n = 11/group. IDd, diameter of left ventricle at the end-diastole; E, early diastolic mitral flow; A, late diastolic mitral flow; Em, peak early diastolic mitral annulus velocity.
P < 0.05 vs. young;
P < 0.05 vs. baseline;
P < 0.1 vs. baseline.
Effect of Skin Surface Cooling on Cardiac Function
Cardiac structure and function were generally well preserved with age during normothermic conditions (Tables 2 and 3). However, there were several age-related differences, which included impaired indices of diastolic function (E, A, Em, E/A, and E/Em) in older compared with young adults (Tables 2 and 3).
Table 3.
Tissue Doppler imaging measurements during the cooling trial
| Young |
Older |
|||
|---|---|---|---|---|
| Variable | Baseline | Cooling | Baseline | Cooling |
| ACC, cm/s2 | 132.45 ± 9.13 | 127.55 ± 10.65 | 137.81 ± 10.14 | 143.94 ± 9.36 |
| Sm, cm/s | 8.03 ± 0.39 | 7.91 ± 0.28 | 7.14 ± 0.30* | 6.95 ± 0.35* |
| Em, cm/s | 13.04 ± 0.60 | 12.95 ± 0.46 | 8.42 ± 0.79* | 7.73 ± 1.17* |
| Am, cm/s | 6.78 ± 0.38 | 6.68 ± 0.39 | 7.49 ± 0.87 | 7.91 ± 0.85 |
Values are means ± SE; n = 11/group. ACC, myocardial acceleration during isovolumic contraction; Sm, peak systolic mitral annulus velocity; Am, peak late diastolic mitral annulus velocity.
P < 0.05 vs. young.
Cooling increased preload (left ventricular internal diameter in diastole and left ventricular end-diastolic volume) and E/Em tended (P < 0.1) to increase in older but not young adults (Table 2). Stroke volume increased (P < 0.05) without a concomitant change in left ventricular end-systolic volume in older but not young adults, during cooling (Table 2). Diastolic function was unchanged by cooling in both young and older adults despite the presence of differences before cooling (Tables 2 and 3). Indices of left ventricular contractility (ejection fraction, ACC, and Sm) were unchanged by cooling in young and older adults. Rate-pressure product increased significantly during cooling in older but not young adults (Tables 2 and 3).
Responses to Control Trial
Baseline measurements during control and cooling trials did not differ within young or older adults, although some between-group differences were present (Tables 4 and 5). Values did not change over the duration of the control trial in either young or older adults for any of the measures except for a small decrease (P < 0.05) in heart rate in older adults (Tables 4 and 5 and Figs. 1 and 2).
Table 4.
Hemodynamic measurements during the control trial
| Young |
Older |
|||
|---|---|---|---|---|
| Variable | Baseline | Control | Baseline | Control |
| Left atrial diameter, cm | 2.73 ± 0.23 | 2.80 ± 0.29 | 3.55 ± 0.21 | 3.50 ± 0.21 |
| IDd, cm | 4.70 ± 0.07 | 4.66 ± 0.07 | 4.72 ± 0.24 | 4.86 ± 0.19 |
| End-diastolic volume, ml | 100 ± 4 | 104 ± 5 | 112 ± 16 | 116 ± 14 |
| End-systolic volume, ml | 25 ± 2 | 30 ± 2 | 28 ± 11 | 31 ± 4 |
| Stroke volume, ml | 75 ± 3 | 74 ± 4 | 84 ± 6 | 83 ± 11 |
| Cardiac output, ml | 4.6 ± 0.3 | 4.5 ± 0.2 | 4.3 ± 0.5 | 4.3 ± 0.7 |
| Ejection fraction, % | 76 ± 1 | 72 ± 1 | 73 ± 4 | 74 ± 2 |
| Diastolic Function | ||||
| E, cm/s | 81.17 ± 7.01 | 82.02 ± 4.72 | 71.29 ± 6.93* | 68.17 ± 6.88* |
| A, cm/s | 37.24 ± 6.56 | 40.59 ± 5.97 | 65.67 ± 5.56* | 59.14 ± 6.54* |
| E/A | 2.39 ± 0.38 | 2.17 ± 0.28 | 1.12 ± 0.15* | 1.22 ± 0.18* |
| E/Em | 6.31 ± 0.43 | 6.81 ± 0.19 | 11.11 ± 1.37* | 10.07 ± 0.99* |
| Myocardial Oxygen Demand | ||||
| Rate pressure product, mmHg·beats/min | 6654 ± 688 | 6539 ± 640 | 7091 ± 427 | 6560 ± 607 |
Values are means ± SE; n = 5/group.
P < 0.05 vs. young.
Table 5.
Tissue Doppler imaging measurements during the control trial
| Young |
Older |
|||
|---|---|---|---|---|
| Variable | Baseline | Cooling | Baseline | Cooling |
| ACC, cm/s2 | 119.00 ± 12.57 | 129.40 ± 19.17 | 153.00 ± 17.02 | 141.20 ± 9.36 |
| Sm, cm/s | 7.96 ± 0.51 | 8.13 ± 0.51 | 6.73 ± 0.47 | 7.01 ± 0.23 |
| Em, cm/s | 12.82 ± 0.46 | 12.07 ± 0.74 | 6.85 ± 0.99* | 7.05 ± 0.90* |
| Am, cm/s | 7.62 ± 0.36 | 7.33 ± 0.78 | 9.79 ± 0.30* | 9.42 ± 0.18* |
Values are means ± SE; n = 5/group.
P < 0.05 vs. young.
DISCUSSION
The primary new finding of this study is that left ventricular contractility (ejection fraction, Sm, or ACC) does not increase during cold stress in older adults, despite the presence of a greater increase in left ventricular preload (left ventricular end-diastolic volume and left ventricular internal diameter in diastole) and rate-pressure product in this group. Because left ventricular preload increased in older adults during cooling, despite impaired diastolic function (E, A, E/A, and Em), it is probable that cooling caused a greater increase in left ventricular filling pressure in older compared with young adults.
Skin surface cooling results in an augmented increase in blood pressure in older compared with young adults. The augmented pressor response to skin surface cooling observed in the present study is consistent with our recent observations (15). In this previous study, we suggested that age-associated increases in central arterial stiffness might contribute to the augmented pressor response to skin surface cooling in older adults (15). In the present study, we extend these prior findings by demonstrating that the augmented pressor response to skin surface cooling in older adults is not mediated by an increase in cardiac output. Collectively, these data suggest an augmented vasoconstrictor response may occur in vascular beds, other than the skin, during cooling in older compared with young adults (31).
Left ventricular filling pressure may increase more in older than young adults during skin surface cooling. Skin surface cooling increases central venous pressure and pulmonary capillary wedge pressure in young adults (8, 39). These increases in indices of left ventricular filling pressure during skin surface cooling in young adults may be predicted to result in increased left ventricular end-diastolic diameter. However, in the present study, we observed no such increase in left ventricular end-diastolic volume during skin surface cooling in young adults. This finding is consistent with previous studies using traditional echocardiography (36). Using thoracic impedance measures some (6), but not all studies (13) observed an increase in central blood volume during cold stress in young adults. Thus, it is possible that increases in pulmonary capillary wedge pressure during cooling are insufficient to cause detectable increases in echocardiography-derived, left ventricular, end-diastolic volumes in young adults. In contrast, we observed large increases in left ventricular end-diastolic volume (∼25 ml) during skin surface cooling in older adults. As diastolic function is impaired with age (e.g., decrease in E and Em) it would appear that greater increases in left ventricular filling pressure occurred during cooling in older compared with young adults to explain the observed increase in left ventricular end-diastolic volume and left ventricular internal diameter in diastole. The mechanism(s) by which this occurs is unclear, but may include age-related effects on pulmonary arterial compliance and pressure (20).
The mechanism(s) underlying the apparent increase in myocardial oxygen demand in older but not young adults during skin surface cooling likely involves increased left ventricular diameter and developed pressure rather than changes in heart rate (18). Our initial hypothesis that left ventricular contractility would increase in older adults during skin surface cooling was based on the observation that older adults have increased left ventricular and central arterial stiffness (1, 3, 4). Increased left ventricular stiffness could reduce left ventricular filling at a given left ventricular filling pressure. However, we found that indexes of cardiac contractility, obtained using traditional echocardiography (ejection fraction) as well as tissue Doppler imaging (Sm and ACC) did not increase during skin surface cooling in either young or older adults. Importantly, tissue Doppler imaging indices of contractility are particularly sensitive to changes in cardiac contractility independent of preload (9). Consistent with prior studies using a similar cooling protocol (15, 38), we did not observe increases in heart rate during skin surface cooling. Thus, it would appear that the apparent increase in myocardial oxygen demand (i.e., rate-pressure product) in older adults during skin surface cooling is best explained by an increase in left ventricular developed pressure and an increase in preload (increased left ventricular end-diastolic diameter and internal diameter in diastole) rather than changes in heart rate (Table 2 and Fig. 2).
The observed increase in an index of myocardial oxygen demand during skin surface cooling in older adults may have important health implications as risk of myocardial infarction and all-cause cardiovascular-related mortality increase in the cold winter months, particularly in older adults (10, 12, 23, 24, 28, 40). A recent study (40) highlighted this point by showing that relative risk for myocardial infarction increased 7% per 10°C decrease in same-day temperature. Cold can act as a powerful trigger for angina symptoms (17) and potentially influence critical determinants of myocardial oxygen supply and demand. The present study provides strong evidence suggesting increased myocardial oxygen demand during cooling in older adults, but future studies will be needed to identify whether critical determinants of myocardial oxygen supply, such as coronary blood flow, are increased by such cold stress in older adults.
Two main study limitations exist. First, it is possible that the augmented pressor response observed during cooling in older adults resulted in a baroreflex-mediated reduction in sympathetic outflow to the heart to reduce left ventricular contractility. Although this is possible, impaired cardiovagal baroreflex responsiveness in older adults (21) could partially diminish this effect. Second, the observed responses may be specific to the cooling paradigm employed. Therefore, other results may be obtained if the cooling paradigm was altered.
Perspective and Significance
In conclusion, the primary new finding of this study is that older adults demonstrate greater increases in estimated myocardial oxygen demand (rate-pressure product) during cold stress, which is related to increased left ventricular preload (left ventricular internal diameter in diastole and left ventricular end-diastolic volume) and arterial blood pressure, but not left ventricular contractility (ejection fraction, Sm, or ACC). This finding was contrary to our proposed hypothesis that older individuals would increase left ventricular contractility to maintain cardiac output in the face of increased afterload during skin surface cooling. These findings may provide insight into mechanisms underlying associations between cold outdoor temperatures and increased incidence of myocardial infarction and all-cause cardiovascular-related mortality, particularly in older adults.
GRANTS
National Institutes of Health Grants HL-92309, AG-24420, M01-RR-10732, and C06-RR-016499 supported this research.
DISCLOSURES
No conflicts of interest, financial, or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Richard Klabunde for providing feedback regarding the manuscript.
REFERENCES
- 1.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aroesty JM, McKay RG, Heller GV, Royal HD, Als AV, Grossman W. Simultaneous assessment of left ventricular systolic and diastolic dysfunction during pacing-induced ischemia. Circulation 71: 889–900, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68: 50–58, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond 93: 298–306, 1922 [Google Scholar]
- 5.Brengelmann GL. Dilemma of body temperature measurement In: Man in Stressful Environments: Thermal and Work Physiology, edited by Shiraki K, Yousef MK. Springfield, IL: Charles C. Thomas, 1987 [Google Scholar]
- 6.Cai Y, Jenstrup M, Ide K, Perko M, Secher NH. Influence of temperature on the distribution of blood in humans as assessed by electrical impedance. Eur J Appl Physiol 81: 443–448, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol 103: 1284–1289, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol 289: H2429–H2433, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dalsgaard M, Snyder EM, Kjaergaard J, Johnson BD, Hassager C, Oh JK. Isovolumic acceleration measured by tissue Doppler echocardiography is preload independent in healthy subjects. Echocardiography 24: 572–579, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, Cottel D, Marecaux N, Amouyel P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 100: E1–E7, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 97: 1970–1977, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Dilaveris P, Synetos A, Giannopoulos G, Gialafos E, Pantazis A, Stefanadis C. CLimate Impacts on Myocardial infarction deaths in the Athens TErritory: the CLIMATE study. Heart 92: 1747–1751, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol 286: R199–R205, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gorcsan J, III, Strum DP, Mandarino WA, Gulati VK, Pinsky MR. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation 95: 2423–2433, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innelli P, Sanchez R, Marra F, Esposito R, Galderisi M. The impact of aging on left ventricular longitudinal function in healthy subjects: a pulsed tissue Doppler study. Eur J Echocardiogr 9: 241–249, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation 79: 1015–1020, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Klabunde RE. Cardiovascular Physiology Concepts Philadelphia, PA: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 19.Kudaiberdieva G, Timuralp B, Ata N, Unalir A, Gorenek B, Cavusoglu Y, Goktekin O, Birdane A. Cold exposure and left ventricular diastolic performance in coronary artery disease. Angiology 54: 187–193, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119: 2663–2670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Monahan KD, Dinenno FA, Seals DR, Halliwill JR. Smaller age-associated reductions in leg venous compliance in endurance exercise-trained men. Am J Physiol Heart Circ Physiol 281: H1267–H1273, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol 105: 288–293, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Panagiotakos DB, Chrysohoou C, Pitsavos C, Nastos P, Anadiotis A, Tentolouris C, Stefanadis C, Toutouzas P, Paliatsos A. Climatological variations in daily hospital admissions for acute coronary syndromes. Int J Cardiol 94: 229–233, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 19: 531–533, 1964 [DOI] [PubMed] [Google Scholar]
- 27.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30: 474–480, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11, 1976 [DOI] [PubMed] [Google Scholar]
- 31.Thompson-Torgerson CS, Holowatz LA, Kenney WL. Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev 36: 122–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tighe DA, Vinch CS, Hill JC, Meyer TE, Goldberg RJ, Aurigemma GP. Influence of age on assessment of diastolic function by Doppler tissue imaging. Am J Cardiol 91: 254–257, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Toner MM, McArdle WD. Physiological Adjustments of Man to the Cold, Human Performance Physiology and Environmental Medicine at Terrestrial Extremes Carmel, IN: Cooper Publishing Group, 1988 [Google Scholar]
- 34.Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, Redington AN. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 105: 1693–1699, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wierzbowska-Drabik K, Krzeminska-Pakula M, Chrzanowski L, Plewka M, Waszyrowski T, Drozdz J, Kurpesa M, Trzos E, Kasprzak JD. Age-dependency of classic and new parameters of diastolic function. Echocardiography 25: 149–155, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol 587: 3383–3392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103: 1257–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120: 735–742, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. J Appl Physiol 88: 393–400, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Young AJ. Homeostatic responses to prolonged cold exposure: human cold acclimatization. In: Handbook of Physiology (Section 4): Environmental Physiology, edited by Fregly MJ, Blatteis CM. New York, NY: Oxford University Press, 1996 [Google Scholar]