Abstract
Ischemia-reperfusion (I/R)-induced acute kidney injury (AKI) results in prolonged impairment of peripheral (i.e., nonrenal) vascular function since skeletal muscle resistance arteries derived from rats 5 wk post-I/R injury, show enhanced responses to ANG II stimulation but not other constrictors. Because vascular superoxide increases ANG II sensitivity, we hypothesized that peripheral responsiveness following recovery from AKI was attributable to vascular oxidant stress. Gracilis arteries (GA) isolated from post-I/R rats (∼5 wk recovery) showed significantly greater superoxide levels relative to sham-operated controls, as detected by dihydroeithidium, which was further augmented by acute ANG II stimulation in vitro. Hydrogen peroxide measured by dichlorofluorescein was not affected by ANG II. GA derived from postischemic animals manifested significantly greater constrictor responses in vitro to ANG II than GA from sham-operated controls. The addition of the superoxide scavenging reagent Tempol (10−5 M) normalized the response to values similar to sham-operated controls. Apocynin (10−6 M) and endothelial denudation nearly abrogated all ANG II-stimulated constrictor activity in GA from post-AKI rats, suggesting an important role for an endothelial-derived source of peripheral oxidative stress. Apocynin treatment in vivo abrogated GA oxidant stress and attenuated ANG II-induced pressor responses post-AKI. Interestingly, gene expression studies in GA vessels indicated a paradoxical reduction in NADPH oxidase subunit and AT1-receptor genes and no effect on several antioxidant genes. Taken together, this study demonstrates that AKI alters peripheral vascular responses by increasing oxidant stress, likely in the endothelium, via an undefined mechanism.
Keywords: ischemia, vascular regulation, oxidant stress
ischemia-reperfusion (I/R) of the kidneys in rats is a common model to study the pathogenesis and reversibility of acute kidney injury (AKI). I/R injury results in a profound loss of renal function and significant damage to renal tubular cells. An interesting aspect of this model is the increasingly recognized effects that renal injury has on distant organs. Renal injury is associated with the liberation of a number of cytokines that may result in cardiovascular instability and affect outcome of patients with AKI (11). In animal models, AKI induced by I/R results in immune-mediated damage in the pulmonary system and brain (15, 24, 27). Similarly, Kelly (23) demonstrated compromised cardiac contractility and cardiac myocyte apoptosis following renal I/R.
Despite the typical recovery of renal tubular structure indicative of this model, there are permanent alterations in renal microvascular structure that may promote renal hypoxia and alter renal hemodynamics, thereby predisposing the development of chronic kidney disease (CKD) (4, 40). In addition to renal anomalies, we have also demonstrated chronic alterations in vascular control and blood pressure regulatory mechanisms of postischemic recovered animals. For example, rats following recovery from bilateral I/R injury maintain normal blood pressure values after 5 wk of recovery; however, these animals will develop hypertension when challenged with elevated sodium intake (3, 4, 31, 32, 40). In addition, we have demonstrated that conscious rats between 4 and 8 wk of recovery from I/R have enhanced pressor responses to ANG II stimulation using doses as low as 10 ng·kg −1·min −1 (3, 4). Increased vasoconstrictor reactivity was observed in cremaster muscle arterioles in situ and isolated skeletal muscle arteries following 5 wk of recovery from I/R compared with sham controls. Interestingly, contractility of post-AKI vessels was enhanced in response to ANG II, but not in response to stimulation with norepinephrine (NE), increased Po2, acetylcholine, and nitroprusside (3).
The mechanisms underlying this enhanced ANG II activity in the post-AKI state are not clear. Previously we showed that both plasma renin activity and basal ANG II levels are not different from sham-operated controls following 5 wk of recovery from AKI (3, 40). Because ANG II activity is known to be enhanced by vascular oxidative stress (8, 36), we hypothesized that AKI alters vascular oxidant generation, which contributes to increased vascular ANG II sensitivity in postischemic animals. The following studies were therefore conducted to determine the role of oxidant stress in the enhanced peripheral vasoconstrictor reactivity to ANG II following recovery from a standard rat model of AKI.
METHODS
Animals.
Care of the rats before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols received prior approval by the Institutional Animal Care and Use Committees at Indiana University and the Medical College of Wisconsin, where some studies were initiated.
Male Sprague-Dawley rats ∼250 g were housed in pairs in standard shoe-box cages with 12:12-h light-dark cycle. Animals were acclimated to a lab diet (cat. no. AIN76A; Dyets, Bethlehem, PA) with a defined 0.4% Na content. Food and water were available ad libitum.
Induction of AKI.
To induce AKI, rats were anesthetized with ketamine (100 mg/kg ip) and pentobarbital sodium (25 mg/kg ip) placed on a heated surgical table. Following a midline incision, the blood supply to the kidneys was interrupted by applying microvascular clamps on the renal pedicles of both kidneys for a period of 40 min (40). The clamps were then released, and reperfusion was visualized. Additional rats were subjected to sham surgery where the kidneys were exposed but not touched. Rats were allowed to recover from either sham or postischemic surgery for a period of between 5 and 6 wk, and animals were then prepared for analysis of microvascular reactivity or evaluation of blood pressure responses to ANG II. In one group of studies, post-AKI animals were treated in vivo between weeks 4 and 5 with apocynin (15 mmol/l; Sigma) in the drinking water for a total of 7 days, while a control group of rats continued on normal drinking water ad libitum. In some studies, similarly treated animals were prepared, and tissues were isolated for subsequent RNA analysis.
Measurement of renal function.
For measurement of serum creatinine, tail blood samples (0.5 ml) were collected into heparinized tubes and plasma obtained by centrifugation. Serum and urine creatinine were determined using Beckman Creatinine Analyzer II.
Preparation of isolated resistance vessels.
The small muscular branch of the femoral artery supplying the gracilis muscle was freed from surrounding tissue and allowed to equilibrate in situ for 30 min with the application of warm physiological salt solution. After the equilibration period, the artery was carefully excised. The gracilis artery was located using a dissecting microscope and carefully isolated to separate the vessel from the surrounding parenchymal tissue. GA were cannulated with glass micropipettes filled with cold bicarbonate buffer (physiological salt solution) consisting of (in mM): 123 NaCl, 4.4 KCl, 2.5 CaCl2, 1.2 MgSO4, 20 NaHCO3, 1.2 KH2PO4, and 11 glucose. Both ends of the vessel were secured with 10-0 nylon Ethilon monofilament suture, and the vessel was maintained at an intraluminal pressure of 20 mmHg for 30 min. Each preparation was transferred to the stage of an inverted microscope (magnification, ×200) attached to a video camera, video monitor, VCR recorder, and a video measuring device (Boeckeler VIA-100). The external bathing medium was continuously superfused with heated buffer solution (pH = 7.4 ± 0.05, Po2 = 140 ± 10 mmHg) aerated with a gas mixture of 21% O2-5% CO2, –74% N2 and maintained at 37°C. Under these conditions, Po2 values during 21% O2 are 140 Torr (12). After a 1-h equilibration period, every vessel was pressurized to 80 mmHg, and arterial reactivity was assessed in response to ANG II (10−8 M) and NE (10−8 M (both from Sigma) in the presence and absence of the xanthine oxidase inhibitor allopurinol (10−6 M; Sigma) or the NADPH oxidase inhibitor apocynin (10−6 M; Sigma). In separate studies, the sequencing of the pharmacological inhibitors was reversed to control for an ordering effect of allopurinol and apocynin on constrictor responses. In some studies, the endothelium was removed in arterioles by bolus injection of 3 ml of air through the vessel as described previously (12). Effective denudation was determined by elimination of the dilation to ACh (10−4 M).
Evaluation of apocynin treatment on acute pressor responses.
In some studies, at 5 wk of recovery from I/R injury, animals were anesthetized with ketamine HCl (13 mg/kg im) and thiopental (100 mg/kg ip). Rats were placed on a heated surgical board to maintain body temperature at 37°C. The trachea was cannulated to facilitate respiration, and catheters were placed in the femoral artery and vein to facilitate measurement of blood pressure and for infusion, respectively. Blood pressure was monitored using a Biopac data acquisition system, and animals were allowed to recover for 30–60 min while being infused with 1% BSA in saline at a rate of 1 ml·kg−1·h−1. In these anesthetized preparations, blood pressure responses were evaluated by infusion of ANG II at doses of 50, 100, and 200 ng·kg−1·min−1 (Sigma) for 15 min.
Fluorescence detection of reactive oxygen species.
The cell-permeable dye dihydroethidine (Molecular Probes, Eugene, OR) was used to evaluate the production of superoxide (O2·−): briefly, arterioles were cannulated and maintained at 37°C in a 3-ml chamber at an equilibration pressure of 60 cmH2O for 30 min. Vessels were exposed to dihydroethidium (DHE; 5 × 10−6 M) for 30 min and then washed and examined under fluorescence microscopy equipped with a krypton/argon laser fluorescent microscope (Nikon Eclipse model TE 200). Fluorescence was detected with a 585-nm long-pass filter, and digital images were recorded. The baseline measurement of fluorescence in the absence of flow was used as a control to adjust for laser settings. Those settings were maintained constant throughout the remainder of the experiment. The fluorescence intensity of the central portion of the vessel was obtained in the absence of flow and every 5 min during 10 min of exposure to ANG II (10−8 M). Acquired images were analyzed for fluorescence intensity in arbitrary units per minute using NIH Image software, and the rate of superoxide generation was calculated as the change in arbitrary units of fluorescent intensity per minute over the 30-min period normalized to the value in the absence of ANG II (33).
Separate studies were used to evaluate the role of NADPH oxidase on superoxide production during AKI. DHE fluorescence was determined in vessels isolated from AKI in the presence and absence of apocynin (10−6 M) and ANG II (10−8 M). In these studies, vessels from the same animal were exposed to ANG II in the presence and absence of apocynin or vehicle (10 min). Vessels were loaded with 5 × 10−6 M DHE for 30 min at 37°C in HEPES buffer (pH 7.4). The vessels were rinsed three times and mounted on slides for the measurement of DHE as described above.
To evaluate the in vitro production of peroxide, vessels were loaded with 5 × 10−6 M 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA). DCF-DA oxidizes rapidly to the highly fluorescent 2′,7′-dichlorofluorescein in the presence of reactive oxygen species (ROS) (33). DCF-DA fluorescence was excited by light at a 488-nm and visualized using fluorescence microscopy as described above.
Malondialdehyde levels in rat plasma.
The malondialdehyde (MDA) level in a plasma sample was measured using HPLC with UV detection as described previously with modifications (9, 34). Briefly, 0.2 ml serum was mixed with 40 μl of 6 M NaOH. The mixture was incubated at 60°C for 30 min to hydrolyze the protein-bound MDA. Protein was precipitated with 100 μl of 35% (vol/vol) perchloric acid and centrifuged at 10,000 g for 10 min. Supernatant was transferred to a clean glass tube, and 25 μl of 2,4-dinitrophenylhydrazine (5 mM solution in 2 M hydrochloric acid) was added. After being incubated for 15 min at room temperature, the mixture was extracted with 1 ml of n-hexane twice. The organic phases were combined and dried under nitrogen flow. The extractant was reconstituted in 200 μl of 20% acetonitrile. An aliquot of 50 μl-reconstituted sample was injected onto a Waters Alliance 2690 HPLC system with a Waters 996 Photodiode Array detector (Waters, Milford, MA). MDA was separated on a reverse-phase LC-18-DB column (3 μM, 150 × 4.6 mm; Supelco, Bellefonte, PA) and detected with a UV detector at a wavelength of 290 nm.
RNA isolation and real-time PCR.
In some studies, rat GA were microdissected and immersed in RNA Later (Ambion). Total RNA was isolated from whole kidney by using a Qiagen total RNA microisolation kit optimized for use with fibrous tissue, according the manufacturer's recommendations. Individual primer sets specific for NADPH oxidase subunits and AT1-receptors (See results, Table 1) were purchased from SA Biosciences (Frederick, MD). In addition, the rat “oxidative stress and antioxidant defense” RT2 Profiler PCR Array (cat. no. PARN-065) and RT2 Real-Time SYBR Green/ROX PCR kit was also purchased from SA Biosciences. Real-time PCR was performed on ABI Prism 7900 HT (Applied Biosystems) according to the manufacturer's instructions. RT reactions were performed on 20 ng total RNA for each sample, and the total product was divided evenly per a 96-well array plate. An identical amount of RT reaction product was utilized for individual real-time PCR reactions unless otherwise indicated. The samples were derived from gracilis arterioles of three sham and three postischemic rats at 35 days postsurgery.
Table 1.
Summary of real-time RT-PCR expression data for gracilis arteries from NADPH oxidase (Nox) and ANG II receptor genes in response to renal I/R injury
| Unigene ID | RefSeq | Symbol | Gene Name | Log2, ratio |
|---|---|---|---|---|
| Rn.98491 | NM_023965 | Cybb-gp91 | Gp91-phox | −2.80* |
| Rn.5856 | NM_024160 | Cyba-p22 | p22-phox | −1.08 NS |
| Rn.9815 | NM031009 | AT1b | Angiotensin receptor, type 1b | −2.78*† |
| Rn.220465 | NM_053683 | NADPH oxidase 1 | Nox1 | −5.04* |
Values are natural log-of-ratio-derived ΔΔCt values; negative values represent suppressed expression vs. sham-operated control.
Genes considered differentially expressed as defined as ratio > mean ± 2*SD using the distribution of ratios derived from the RT2 profiler array assayed identically and described in Table 2.
Gene had low level expression and was subsequently reassayed with 10× higher RT product than was used for all genes in Tables 1 and 2. Under these conditions, the expression was consistently detectable in the samples derived from the sham-operated control samples but not in all samples from experimental samples. For calculation purposes, the undetectable samples were considered at Ct = 40 for the computation of ΔΔCt values.
Statistical analysis.
All data are expressed as means ± SE. Responses to ANG II were assessed with repeated-measures ANOVA to determine the effect of the treatment on the response. Further analysis was completed with Student's t-test for a single dose of ANG II. To compare multiple treatment groups receiving a single dose of a drug, one-way ANOVA was used. Differences in the means after one-way ANOVA were determined with post hoc analysis using a Student-Newman-Keuls post hoc test. P < 0.05 was considered to be statistically significant.
For data analysis of PCR, the ΔΔCt method was used with the aid of a Microsoft Excel spreadsheet containing algorithms provided by the manufacturer. Fold changes were then calculated and expressed as log-normalized ratios of values from postischemic/sham-operated tissues.
RESULTS
The effect of recovery from AKI on vascular sensitivity was addressed in rats ∼ 5 wk following recovery from sham surgery or I/R injury as previously reported (3). I/R injury resulted in an early loss of renal function indicated by serum creatinine values rising to between 2.5 and 4.2 mg/dl 24 h following surgery and returning to values of sham-operated controls at the time of analysis (i.e., 5 wk postsurgery).
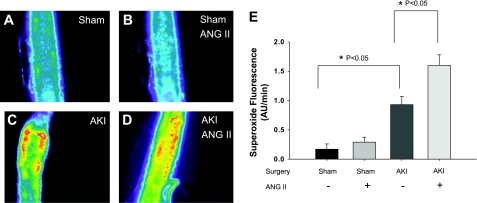
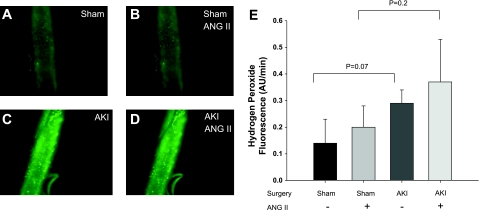
Since previous studies indicate vasoconstrictor responses to ANG II were enhanced in GA (3), we evaluated whether GA from postischemic animals had increased levels of oxidative stress. DHE fluorescence was used to measure superoxide generation in vessels isolated from sham-operated or postischemic animals and in response to ANG II (10−8 M) administration in vitro. Representative images derived from fluorescent microscopy are shown in Fig. 1, A–D, and the relative fluorescence values are summarized in Fig. 1E. Importantly, there was evidence of increased superoxide production in vessels from AKI compared with sham control animals as indicated by a significant ∼ 2.5-fold increase in DHE signal intensity measured in isolated skeletal muscle resistance arteries (P = 0.018). We also sought to determine the potential effect of ANG II on the stimulation of superoxide in vessels from sham and post-AKI rats; ANG II had no effect on the DHE fluorescence in vessels from sham-operated control rats. The DHE signal in vessels from post-AKI rats was significantly higher after ANG II relative to pre-ANG II treatment (Fig. 1E; P = 0.003; n = 10). To determine whether H2O2 generation is elevated post-AKI, we used DCF-DA fluorescence. The H2O2 tended to be increased but was not significant in GA from post-AKI vessels in both the basal and ANG II-induced state compared with sham animals (Fig. 2). ANG II did not significantly increase H2O2. In separate studies, the superoxide dismutase mimetic Tiron (10−5 M) and the peroxide scavenger polyethylene glycol catalase (500 U/ml;) reduced the DHE (ratio vs. baseline: 0.38 ± 0.08; P = 0.02) and DCF (ratio vs. baseline: 0.48 ± 0.09; P = 0.05) fluorescence in arteries from AKI rats demonstrating the specificity of these probes for superoxide and H2O2, respectively.
Fig. 1.
Acute kidney injury (AKI) induces alterations in superoxide levels in gracilis arteries (GA). Isolated GA were incubated with dihydroethidium (DHE) and evaluated via fluorescent microscopy. Representative fluorescent images were obtained from vessels derived from sham-operated rats (A and B) and post-AKI rats (C and D). Images were also evaluated prior to (A and C) and following (B and D) stimulation with ANG II (10−8 M). E: summarized data describing the rate of accumulation of superoxide fluorescence production in arbitrary units (AU) of fluorescent intensity per minute in the presence and absence of ANG II. *Significantly different vs. sham-operated or AKI controls (P < 0.05).
Fig. 2.
AKI induces alterations in hydrogen peroxide levels in GA, which were incubated with dichlorofluoroscein and evaluated via fluorescent microscopy. Representative fluorescent images were obtained from vessels derived from sham-operated rats (A and B) and post-AKI rats (C and D). Images were also evaluated prior to (A and C) and following (B and D) stimulation with ANG II (10−8 M). E: summarized data describing the rate of accumulation of peroxide production in AU of fluorescent intensity per minute. Values are means ± SE. There were no differences between groups.
In addition, we sought to evaluate whether recovery from AKI was associated with a generalized increase in circulating oxidant stress. Recovery from AKI was evident by resolution of serum creatinine to levels of sham-operated controls in the cohort of experimental animals evaluated for oxidant stress. There was a significant 23% increase in plasma MDA levels in postischemic animals relative to corresponding sham-operated controls (sham, 11.5 ± 0.4 nmol/ml−1; post-I/R, 14.2 ± 0.7 nnmol/ml−1; P < 0.05 by Student's t-test). These data indicate that oxidant stress markers in the circulation and the vascular wall of skeletal muscle resistance arteries are increased in AKI animals compared with controls.
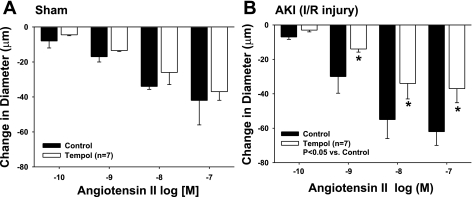
Rat skeletal muscle resistance arteries (∼140 μm) were isolated and suspended on micropipettes, and contractile responses were evaluated in vitro as a function of increasing ANG II concentrations. There was no difference in the baseline diameters of the GA of sham (148 μm ± 3) and AKI (142 μm ± 10) animals. Similar to our previous work (3), the ANG II-induced response of vessels isolated from post-I/R rats was significantly enhanced relative to vessels isolated from sham-operated control rats (Fig. 3, A vs. B). Because ANG II activity may be influenced by ROS, we also measured responses in the presence of the superoxide dismutase mimetic, Tempol (10−5 M). Tempol had no effect on ANG II-induced constriction of vessels isolated from sham-operated control animals (Fig. 3A). In contrast, Tempol significantly attenuated the constrictor responses to ANG II in arteries of AKI rats, bringing the response to levels similar to that observed in sham-operated controls (compare Fig. 3, B to A).
Fig. 3.
Responses to ANG II of isolated GA from sham-operated (A) or postischemic (B) rats. GA were subjected to increasing doses of ANG II in the presence and absence of Tempol (n = 7, 10−4 M). Data are means ± SE and are expressed as absolute change from baseline. There was no effect of Tempol on ANG II responses in sham rats. *P < 0.05 in Tempol treated vs. physiological salt solution control for AKI rats.
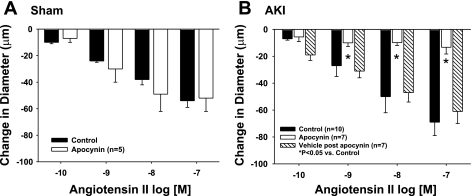
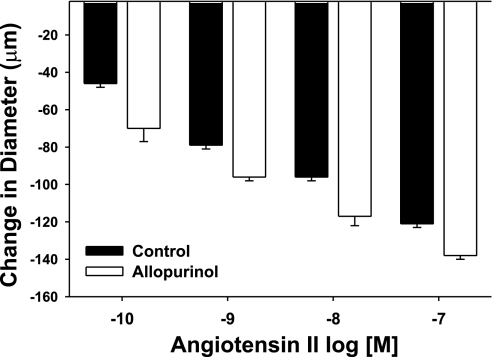
To evaluate the source of oxidant stress in vessels from AKI rats, vascular reactivity studies were performed in vessels from AKI animals in the presence of the putative NADPH-oxidase inhibitor apocynin and the xanthine oxidase inhibitor allopurinol. Apocynin had no detectable effect on vessels derived from sham-operated controls (Fig. 4A) but completely blocked ANG II-induced constriction in GA from post-AKI rats (Fig. 4B, black bars vs. white bars). The effect of apocynin was reversible, as the enhanced ANG II-induced constriction was restored after a 30-min apocynin washout period (Fig. 4B, striped bar). In contrast, there was no effect of allopurinol on constrictor responses to ANG II in GA from post-AKI rats (Fig. 5). These results suggest that ANG II-induced constriction shifts to an NADPH-dependent, oxidant-dependent state following recovery from AKI.
Fig. 4.
Effect of AKI on ANG II-induced constriction and the effects of apocynin in isolated GA from postischemic rats. Response to increasing levels of ANG II are shown (white bars) or with increasing levels of ANG II and the NADPH oxidase inhibition with apocynin (1 μM, black bars). Results are shown for vessels obtained from sham-operated controls (A) and post-AKI animals (B). Stippled bars in B represent the restored activity following washout of apocynin, demonstrating restoration of the ANG II response. Data are means ± SE expressed as the absolute change from baseline. *Significant difference from vehicle (n = 4; P < 0.05).
Fig. 5.
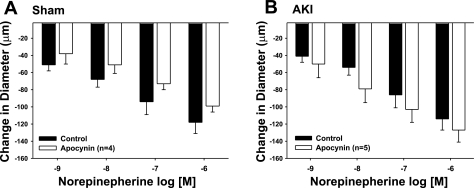
Effect of apocynin on norepinephrine-induced constriction in isolated GA from postischemic (AKI) rats. Response to increasing levels of norepinephrine are shown alone (white bars) or with apocynin (1 μM, black bars). Results are shown for vessels obtained from sham-operated controls (A) and postischemic animals (B). Data are means ± SE expressed as the absolute change from baseline. There was no effect of apocynin on norepinepherine responses in either group.
We next addressed whether the prominent apocynin effect in vessels from post-AKI rats was selective for ANG II-induced constriction or was a generalized phenomenon. In a previous study, we demonstrated that the postischemic pressor response of GA to NE was not different from sham-operated controls (3). Similarly, additional studies confirmed that the NE-induced constriction in GA was not different in vessels from sham-operated and postischemic animals (compare Fig. 6, A vs. B, open bars) and that apocynin did not influence the NE response in either group (Fig. 6, black bars).
Fig. 6.
Effect of allopurinol on ANG II-induced contriction in isolated GA from postischemic (AKI) rats. Response to increasing levels of ANG II are shown (white bars) or with increasing levels of ANG II and with allopurinol (1 μM, black bars). Allopurinol had no significant effect on the response to ANG II.
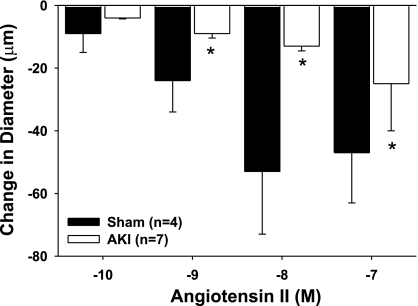
To distinguish the role of endothelial or smooth muscle cells, ANG II-induced vasoconstriction was evaluated in endothelium-denuded microvessels from sham-operated or postischemic rats. The dose-response profile observed in denuded vessels from sham-operated control rats was similar to that observed in normal, endothelium-intact vessels from control rats (compare Fig. 7, black bars, with Fig. 3A, dark bars). Interestingly, the ANG II response in denuded vessels obtained from post-AKI rats was not enhanced, but rather was significantly attenuated (Fig. 7, open bars). These studies suggest that the endothelium contributes increased ANG II sensitivity post-AKI.
Fig. 7.
Effect of endothelial denudation on ANG II-induced constriction in isolated GA from postischemic rats. Response to increasing levels of ANG II are shown in endothelial-denuded vessels obtained from sham-operated controls (black bars) and postischemic animals (white bars). Data are means ± SE expressed as the absolute change from baseline. *Significant difference from sham-operated controls (n = 4, P < 0.05).
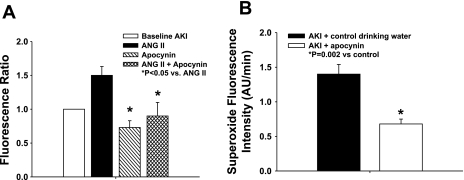
We confirmed the contribution of NADPH oxidase activity to ANG II-induced superoxide production in AKI by measuring DHE fluorescence in vessels in the presence or absence of apocynin. Fig. 8A demonstrates the change in DHE fluorescence signal, measured as a ratio to control vessels, derived from post-AKI rats. ANG II (10−8 M) stimulation augmented the DHE fluorescence signal in post-AKI animals; apocynin treatment (10−6 M) in vitro significantly lowered DHE fluorescence in post-AKI animals with or without stimulation by ANG II.
Fig. 8.
Effect of apocynin on superoxide fluorescence in skeletal muscle resistance arteries from AKI rats. A: isolated GA were incubated with DHE and evaluated via fluorescent microscopy in the presence and absence of apocynin (10−5 M) and ANG II (10−8 M). Results are presented as a ratio from baseline. *Significantly different vs. ANG II (P < 0.05). B: isolated GA were incubated from AKI rats and AKI rats treated with in vivo apocynin in the drinking water (15 mmol/l) between days 28 and 35; DHE fluorescence measurements were conducted at day 35 post-AKI. *Significantly different vs. AKI drinking water only control (n = 4 group, P < 0.05).
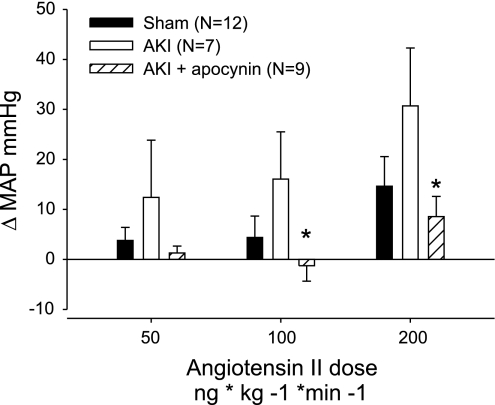
DHE fluorescence was also measured in vessels from postischemic animals at day 35 (5 wk). Apocynin treatment in vivo between days 28 and 35 post-I/R resulted in a ∼50% reduction in DHE signal relative to post-AKI rats maintained on normal drinking water (Fig. 8B). These data suggest that the NADPH oxidase system plays a role in the increased oxidant stress observed in peripheral blood vessels of postischemic animals. Treatment with apocynin in vivo reduced ANG II pressor responses in postischemic animals; Fig. 9 demonstrates the pressor effect of ANG II in anesthetized rats, which was significantly attenuated by treatment with apocynin.
Fig. 9.
Effect of apocynin treatment in vivo on acute pressor response to ANG II. Rats were allowed to recover from AKI for 35 days; apocynin treatment occurred on days 28–35 and pressor responses evaluated are based on increasing doses of ANG II as indicated. Data are presented as change in pressure from baseline values. *Significant difference in apocynin-treated vs. nonapocynin-treated AKI by Student's t-test (P < 0.05).
We next evaluated whether recovery from AKI was associated with an increase in the molecular expression of genes associated with the NADPH oxidase system or antioxidant defense mechanisms by comparing the mRNA expression of genes in the GA of sham-operated and postischemic rats. These studies utilized both specific primer sets corresponding to different NADPH oxidase genes as well as a pathway-based real-time PCR array (RT2 profiler) corresponding to oxidant- and antioxidant-related genes.
Paradoxically, both NADPH oxidase genes measured (Nox2/gp91 and Nox1), as well as AT1b mRNA expression, was reduced in GA from postischemic rats (Table 1). Evaluation of the oxidant stress pathway-targeted commercial PCR array demonstrated that 63 of 84 test genes were detectable in rat GA. However, only four of these genes (UCP-3, myoglobin, scl38a, and Zmynd17) were significantly reduced in post-AKI vessels, while no genes were significantly enhanced. Genes included on this PCR array that are related to the NADPH oxidase system were not affected by I/R injury (Table 2).
Table 2.
Summary of genes, Nox genes, and differentially expressed genes from oxidant-stress PCR array at 35 days post-IR injury in gracilis arteries
| Unigene ID | RefSeq | Symbol/Abbreviation | Name | Log2, ratio |
|---|---|---|---|---|
| Rn.40511 | NM_021588 | Mb | Myoglobin | −3.75* |
| Rn.17990 | NM_130748 | Slc38a4 | Solute carrier protein 38a4 | −2.21*† |
| Rn.25565 | XM_214130 | Zmynd17 | Zinc Finger MYND type containing 17 | −1.77*† |
| Rn.9902 | NM_013167 | UCP3 | Uncoupling protein 3 | −1.34* |
| Rn.162651 | XM_231042 | Noxa1 | NADPH oxidase activator 1 | ND |
| Rn.137764 | XM_220221 | Noxo1 | NADPH oxidase organizer 1 | ND |
| Rn.14744 | NM_053524 | Nox4 | NADPH oxidase 4 | 0.63 NS |
Values expressed as natural log of ratio-derived ΔΔCt values; positive values represent enhanced expression vs. sham-operated control; negative values represent suppressed expression vs. sham-operated control. ND, not detectable consistently in both experimental and control samples.
Genes considered differentially expressed as defined as ratio > mean ±2*SD; derived from the distribution of ratios averaged over 3 separate comparisons; the value was ± 1.34.
Despite inclusion on this pathway-focused array, there are no substantive reports indicating the role of these genes in mediating oxidant stress.
DISCUSSION
In early AKI, data from animal models suggest that kidney injury compromises function in distant organs (15, 24, 27). These studies were undertaken to understand the complex nature of intensive care unit patients with AKI and the recognition that kidney injury may lead to overall hemodynamic instability, perhaps via activated humoral pathways. In addition, recent studies using animal models have demonstrated that IR-induced AKI has a persistent effect on kidney structure resulting in predisposition to CKD (40). More recently, a number of reports now suggest a link between AKI and the development of CKD and hypertension in both pediatric and aging populations (2, 19).
The results of the present study suggest that, within the confines of a well-described rat model of AKI, the persistent effects of AKI are not limited to the kidney but extend to the peripheral vasculature. The purpose of this study was to delineate mechanisms leading to impaired peripheral vascular function in post-AKI rats, which have undergone a functional renal recovery but prior to the development of classic manifestations of CKD and hypertension. There are two major novel findings of this study. First, ROS are persistently elevated in the vascular wall of skeletal muscle resistance arteries of rats following AKI. Second, the mechanism of enhanced ANG II vasoconstrictor activity in skeletal muscle resistance arteries from AKI rats likely involves endothelium-derived superoxide.
Mechanisms of enhanced ANG II responsiveness.
Recent evidence suggests that elevated vascular superoxide production is responsible for reduced vasodilation and decreased nitric oxide bioavailability during AKI (21). Other studies indicate treatment with antioxidant supplements reduce oxidative stress during hypertension and kidney disease (1, 18). In the present study, we sought to determine the role of ROS generation in contributing to the enhanced reactivity of isolated GA to ANG II. DHE and DCF fluorescence were used to assess oxidant generation in the vascular wall of GA from AKI and sham-operated control rats. GA demonstrated increased dihydroethidine and DCF fluorescence in AKI compared with sham animals and an augmented dihydroethidine response to ANG II in vessels from AKI rats. These data suggest that ROS generation is increased after recovery from AKI.
Previous studies demonstrated that ROS contribute to ANG II signaling in the vascular smooth muscle cells (36). Consistent with these studies, we found that ANG II responses in AKI vessels were restored to sham levels in the presence of the superoxide dismutase mimetic, Tempol. Consistent with other studies in human coronary and porcine femoral arteries (38), we found no effect of Tempol on responses of GA to ANG II in rats exposed to sham surgery (Fig. 1) and suggest that the enhanced sensitivity of post-AKI rats is attributable to increased sustained vascular ROS following AKI.
The renin angiotensin aldosterone system is activated in renal failure, and angiotensin receptor blockade is an effective treatment of kidney disease and hypertension. When ANG II acts through the AT1-receptor, it stimulates generation of ROS, thereby promoting endothelial dysfunction. However, in a previous study, AKI did not alter microvascular responses to endothelium-dependent dilations to acetylcholine after recovery (3). Cardiovascular disease states are known to reduce endothelial nitric oxide (NO) and other vasodilators by elevating the production of ROS, namely superoxide (25). Alternatively, other endothelial-derived dilator substances are known to compensate for the lack of NO release in response to agonists (16, 28, 44). While the mechanism of this maintained dilation was beyond the focus of the present investigation, previous studies have demonstrated the role of the H2O2 in maintaining peripheral vascular endothelial function in cardiovascular disease (33). Coupled with evidence in the present study, indicating H2O2 generation is modestly elevated in GA of AKI animals (Fig. 2), it is possible that Tempol does not completely block the ANG II response because of the added vasodilatory effect of H2O2 in the presence of Tempol. However, future studies are required to determine the nature of this discrepancy.
In endothelial cells, NADPH oxidase is a particularly prominent source of superoxide formation (29) and appears to be the primary source of abnormal ROS formation during hypertension (6, 28, 36, 41). This data is consistent with that of others in which overexpression of vascular NADPH oxidase resulted in increased superoxide production and an increased sensitivity to ANG II (36). While superoxide itself may act a vasoconstrictor (22), there was no change in baseline diameter of sham-operated vessels vs. AKI vessels, nor was there any change in the presence of the superoxide dismutase mimetic Tempol. Since exogenous generation of ROS can potentiate endothelium-dependent contractions to agonists (45), these data suggest that ROS production in GA from AKI may potentiate the ANG II constriction via ROS signaling.
In previous studies, we showed that the angiotensin AT1-receptor blocker losartan eliminated the ANG II response in GA of AKI animals (3). Since ANG II stimulates NADPH oxidase activity and expression in vascular smooth muscle cells (14, 39) and endothelium (37), we tested the role of NADPH oxidase enzyme system in contributing to enhanced ANG II responses after recovery from acute renal injury. In these studies, the NADPH oxidase inhibitor apocynin eliminated the ANG II constrictor response in AKI animals (Fig. 4). Since NADPH oxidase is a prominent source of superoxide formation in endothelial cells, these results support the role of NADPH oxidase generation of ROS in the enhanced ANG II constrictor responses observed after recovery from AKI. This conclusion should be interpreted cautiously since 1) the pharmacologic inhibition with apocynin is limited (17), and 2) there are other enzymatic sources that can generate ROS (i.e., NO synthase, xanthine oxidase, mitochondria, and cyclooxygenase). Although speculative, the repressed expression of UCP-3, a protein involved in mitochondrial antioxidant defense (13), suggests that increased superoxide may derive from a mitochondrial source. While such investigation is beyond the scope of the present study, there was no effect of the xanthine oxidase (another prominent source of oxidant stress in vascular cells) inhibitor allopurinol on the ANG II constrictor response in GA of AKI rats (Fig. 5), supporting the conclusion that enhanced ANG II constrictions are dependent on the NADPH oxidase generation of ROS.
It is important to point out that removal of endothelium reduced the ANG II constrictor response in AKI animals (Fig. 5), suggesting that the endothelium contributes to enhanced ANG II responsiveness in the peripheral vasculature following recovery from ischemic injury. This finding is supported by the paradoxical reduction in AT1-receptor gene expression in GA of post-AKI animals (Table 1). This is also consistent with reports demonstrating that ANG II may elicit the release of endothelial-derived contractile factors, such as endothelin-1 during hypertension and other cardiovascular diseases (42). Endothelin-1 is released predominately from endothelial cells (10). Since endothelin-1-dependent vasoconstrictions can be reduced by the NADPH oxidase inhibitor apocynin (20), the enhanced ANG II vasoconstrictor effects during AKI may be entirely endothelium- and NADPH oxidase-dependent.
A simple interpretation of our functional data led us to believe that increased endothelial expression of NADPH oxidase subunits results in increased ROS generation in skeletal muscle resistance arteries of post-AKI. However, such a mechanism is not supported by our measurement of gene expression in GA, which showed a paradoxical decrease in the expression of Nox2/gp91 and Nox1 genes. We also evaluated several antibodies and were unable to detect alterations in microvessel NADPH oxidase protein expression in post-AKI rats using immunohistochemical approaches (not shown). Although our use of an antioxidant pathway array is limited to the identification of transcripts, we failed to identify other potential alterations in gene expression related to the NADPH oxidase system or genes or antioxidant genes. Therefore, it is difficult to precisely identify the molecular mechanisms mediating vascular ROS generation; nevertheless, given the observation that apocynin blocked the superoxide signal in GA of AKI animals, it is possible that NADPH oxidase activity is increased independently of gene and/or protein expression.
It is curious that the ANG II constriction is nearly abrogated in the presence of apocynin and in denuded resistance arteries from post-AKI rats. Thus, is appears that AKI induces a switch to a completely NADPH oxidase-dependent state from an NADPH oxidase-independent state. The effect of apocynin is thought to be dependent on ROS generation based on both in vivo and in vitro data, and a 30-min washout of apocynin restored enhanced ANG II response. The apocynin effect cannot be explained by a nonspecific effect on constrictor mechanisms post-AKI, since NE responses were not altered by apocynin.
While the mechanism for the abrogated response to ANG II in denuded vessels is not known, it is reasonable to suggest that there is compensation at the level of the vasculature to downregulate ANG II constrictor mechanisms in the face of increased oxidant stress, thus enabling the maintenance of normal tone. We suggest that this may be the basis for the paradoxical decrease in the expression of the AT1-receptor, and perhaps also for the repression the Nox1 and Nox2 genes. However, the interpretation of these results is further confounded by the relatively high dose of ANG II used to elicit vasoconstriction compared with the circulating levels measured in the AKI model in previous studies (3), making it important to determine the mechanism of ANG II signaling in future studies of AKI.
A major unanswered question relates to how renal injury imparts altered sensitivity to the peripheral vasculature. This is currently unclear, but is likely mediated by humoral factors. Inflammatory cytokines may contribute to altered ANG II-mediated ROS signaling in the vascular wall after I/R injury. For example, circulating cytokines associated with I/R injury, such as TNF-α, CRP, and IL-6 induce endothelial oxidative stress (26, 30, 35, 43). Interestingly, Wassmann et al. (43) demonstrated that the proinflammatory cytokine IL-6 induced an upregulation of the AT1-receptor, thereby facilitating ANG II vasoconstriction and oxidative stress vascular smooth muscle cells an unlikely mechanism in this study given the reduced AT1b-receptor expression in gracilis arterioles of AKI rats (Table 1). While altered cytokine production has not yet been evaluated in the postrecovery phase of AKI, previous studies suggest that the kidney remains in a persistent proinflammatory state for at least 5 wk in this model (5). Therefore, the connection between kidney damage and humoral influences as a mediator of peripheral oxidant stress/ANG II axis may represent an important feature of understanding the distant effects of AKI.
Perspectives and Significance
The present study evaluates chronic, persistent changes in vascular sensitivity in an animal model of AKI. There is increased interest in understanding some of the factors that lead to increased morbidity and mortality in patients following AKI. Although kidney injury can lead to long-term complications including reduced kidney function, CKD, and end-stage renal disease, nonrenal cardiovascular complications may represent an important long-term care issue for AKI patients. Increased cardiovascular risk independent of kidney function per se appears to be present even after recovery from kidney injury. The present investigation suggests that the increased risk may be perpetuated by altered vascular function that is mediated by a generalized oxidant stress far removed from the initial injury, the pathogenesis of which may contribute to long-term complications from AKI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-063114 (to D. P. Basile). S. A. Phillips is supported by National Heart, Lung, and Blood Institute Grant HL-085614.
DISCLOSURES
No conflicts of interest, financial, or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors greatly appreciate helpful discussions with Dr. Jefferson Frisbee about this manuscript. The authors also thank Drs. James Klaunig and Lisa Kamendulis, at the Center of Environmental Health at the Indiana University School of Medicine, for assistance with the MDA assay.
REFERENCES
- 1.Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Askenazi D, Feig D, Graham N, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to ANG II following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 289: R1770–R1776, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, Fredrich K, Alausa MT, Vio C, Liang M, Greene AL, Cowley AW., Jr Identification of persistently altered gene expression in kidney following functional recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 288: R953–R963, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Beswick R, Dorrance A, Leite R, Webb R. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brandes R, Kreuszer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003 [DOI] [PubMed] [Google Scholar]
- 9.de Peyster A, Rodriguez R, Shuto R, Goldberg B, Gonzales R, Pu X, Klaunig JE. Effect of oral methyl-t-butyl ether (MTBE) on the male mouse reproductive tract and oxidative stress in the liver. Reprod Toxicol 26: 246–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emori T, Hirata Y, Ohta K, Shichiri M, Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun 160: 93–100, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Feltes C, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron 109: 80–84, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fredricks KT, Liu Y, Lombard JH. Response of extraparechymal resistance arteries of rat skeletal muscle to reduced Po2. Am J Physiol Heart Circ Physiol 267: H706–H715, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Goglia F, Skulachev V. A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. FASEB J 17: 1585–1591, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hanna I, Taniyama Y, Szocs K, Rocic P, Greiendling K. NADPH oxidase derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal: 899–914, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 293: F30–F40, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hatoum O, Binion D, Miura H, Telford G, Otterson M, Gutterman D. Role of hydrogen peroxide in ACh induced dilation of human submucosal intestinal microvessels. Am J Physiol Heart Circ Physiol 288: H48–H54, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but is an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of TEMPOL in Dahl salt-sensitive rats. Hypertension 41: 697–702, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Just A, Whitten CL, Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. Am J Physiol Renal Physiol 294: F719–F728, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kakoki M, Hirata Y, Hayakawa H, Suzuki E, Nagata D, Nishimatsu H, Kimura K, Goto A, Omata M. Effects of vasodilatory antihypertensive agents on endothelial dysfunction in rats with ischemic acute renal failure. Hypertens Res Clin Exp 23: 527–533, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol Heart Circ Physiol 257: H33–H37, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Laurindo F, Pedro M, Barbeiro H. Vascular free radical release: ex vivo and in vivo evidence for a flow dependent endothelial mechanism. Circ Res 74: 700–709, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Fan L, Christie M, Shah A. Acute tumor necrosis factor-α signaling via NADPH oxidase in microvascular endothelial cells: role of p47 phox phosphorylation and binding to TRAF4. Mol Cell Biol 25: 2320–2330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura H, Bosnjak ZJ, Ning G, Saito T, Miura M, Gutterman D. Role for hydrogen peroxide in flow induced dilation of human coronary arterioles. Circ Res 92: 31–40, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mohazzab H, Kaminski P, Wolin M. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol 266: H2568–H2572, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Orschal J, Khalil R. Interleukin 6 impairs endothelium dependent NO cGMP mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 286: R1013–R1023, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Pechman K, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium sensitive hypertension following recovery from acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Pechman K, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis and sodium sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips SA, Hatoum O, Gutterman D. The mechanism of flow induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292: H93–H100, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pilz J, Meineke I, Gleiter G. Measurement of free and bound malondialdehyde in plasma by high performance liquid chromatography as the 2,4 dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl 742: 315–325, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Qamirani E, Ren Y, Kuo L, Hein T. C reactive protein inhibits endothelium dependent NO mediated dilation in coronary arterioles by activating p38 kinase and NADPH oxidase. Arterioscler Thromb Vasc Biol 25: 995–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rajagopalan PR, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 22: 1845–1851, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Schuijit MP, Beril T, deVries R, Saxena PR, Sluiter W, van Kats JP, Dansler AHJ. Superoxide does not mediate the acute vasoconstrictor effects of angiotensin II: a study in human and porcine arteries. J Hypertens 21: 2335–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Seshiah P, Weber DS, Rocic P, Valppu L, Taniyama Y, Greiendling K. Angiotensin II stimulation of NADPH oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ungvari Z, Csiszar A, Huang A, Kaminski P, Wolin M, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C dependent activation of NADPH oxidase. Circulation 108: 1253–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Vanhoutte PM, Feletou M, Taddei S. Endothelium dependent contractions in hypertension. Br J Pharmacol 144: 449–458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin 6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender specific compensation for the lack of NO in the mediation of flow induced arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2456–H2461, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension 41: 143–148, 2003 [DOI] [PubMed] [Google Scholar]