Abstract
Chloride intracellular channel 5 (CLIC5) and other CLIC isoforms have been implicated in a number of biological processes, but their specific functions are poorly understood. The association of CLIC5 with ezrin and the actin cytoskeleton led us to test its possible involvement in gastric acid secretion. Clic5 mutant mice exhibited only a minor reduction in acid secretion, Clic5 mRNA was expressed at only low levels in stomach, and Clic5 mutant parietal cells were ultrastructurally normal, negating the hypothesis that CLIC5 plays a major role in acid secretion. However, the mutants exhibited gastric hemorrhaging in response to fasting, reduced monocytes and granulocytes suggestive of immune dysfunction, behavioral and social disorders suggestive of neurological dysfunction, and evidence of a previously unidentified metabolic defect. Wild-type and mutant mice were maintained on normal and high-fat diets; plasma levels of various hormones, glucose, and lipids were determined; and body composition was studied by quantitative magnetic resonance imaging. Clic5 mutants were lean, hyperphagic, and highly resistant to diet-induced obesity. Plasma insulin and glucose levels were reduced, and leptin levels were very low; however, plasma triglycerides, cholesterol, phospholipids, and fatty acids were normal. Indirect calorimetry revealed increased peripheral metabolism and greater reliance on carbohydrate metabolism. Because Clic5 mutants were unable to maintain energy reserves, they also exhibited increased susceptibility to fasting-induced torpor, as indicated by telemetric measurements showing episodes of reduced body temperature and heart rate. These data reveal a requirement for CLIC5 in the maintenance of normal systemic energy metabolism.
Keywords: gastric hemorrhage, leptin
chloride intracellular channel 5 (CLIC5) is a member of the CLIC family of proteins (1) and was originally isolated from bovine kidney on the basis of its binding to indanyloxyacetic acid, a Cl− channel inhibitor (34, 44). Later cloning studies (4, 33, 47) identified two splice variants, termed CLIC5A and CLIC5B, with the latter corresponding to the original bovine p64 (34) and avian p62 (6) proteins, which exhibited Cl− channel activity when purified and reconstituted in lipid vesicles (4, 33). Although it is clear that CLIC5 and other members of the CLIC family do, under some circumstances, mediate ion channel activity (1, 51), their molecular, cellular, and physiological functions are complex and not well understood. CLICs exist as both soluble cytosolic and membrane-associated forms (1, 40), and CLIC5 and several other CLICs associate with actin and other cytoskeletal components (4, 5, 51). The latter property might position them well for reversible insertion into the appropriate membranes and/or serve structural or signaling functions unrelated to ion channel activity (1).
Cell biological and expression studies have implicated CLICs in a wide variety of functions, including assembly or maintenance of the cytoskeleton (5), apoptosis (54), activation and proliferation of microglial cells (41), and regulation of the ryanodine receptor (15). The physiological functions of CLIC proteins are beginning to be explored using gene disruption models. In Caenorhabditis elegans, a CLIC protein (EXC-4) has been implicated in development and maintenance of the excretory canal (3); and, in an apparently similar process involving impaired tubulogenesis, CLIC4-null mutant mice exhibit deficiencies in angiogenesis and the formation of collateral vessels (13, 58). A naturally occurring mutant of Clic5 (jitterbug) has impaired hearing and balance, runs in circles (21), and is hyperactive. CLIC5 is expressed at the base of the stereocilia of sensory hair cells, where it appears to associate with radixin and the actin cytoskeleton, and complete deafness occurs in Clic5 mutants by 7 mo of age as a result of degeneration of the stereocilia (21).
To identify possible Cl− channels that might function in gastric HCl secretion, we performed microarray analyses of stomach mRNA from mice lacking Na+/H+ exchanger-4 (NHE4; Slc9a4), which have very few parietal cells (23). The initial data indicated that CLIC5 and CLIC6 were sharply reduced, consistent with the hypothesis that one or both of these putative ion channels might contribute to Cl− secretion by the parietal cell. CLIC6 had already been proposed as a possible gastric Cl− channel (49); however, a null mutant mouse model that would allow testing of its role in acid secretion is not yet available. CLIC5A had been shown to be associated with both ezrin and the actin cytoskeleton in placental microvilli (4, 5). Furthermore, ezrin is known to play a major role in regulation of gastric acid secretion (64), and CLIC5 has been implicated in HCl secretion by osteoclasts (16).
Although a modest reduction in stomach acid was observed in Clic5 mutant (Clic5−/−) mice, the results of the current study indicate that CLIC5 does not play a direct role in gastric acid secretion. However, the results showed that loss of CLIC5 causes a severe metabolic phenotype, which has not been reported previously and is likely related, at least in part, to hyperactivity. Here we show that Clic5−/− mice are hyperphagic, smaller and leaner than their wild-type littermates, and highly resistant to diet-induced obesity. Furthermore, their impaired capacity for storing energy renders these mice more susceptible to fasting-induced gastric hemorrhaging and torpor. These studies are consistent with a novel role for CLIC5 in systemic energy metabolism.
MATERIALS AND METHODS
Animals.
Mice harboring a deletion mutation in exon 5 of the Clic5 gene (termed jitterbug or jbg) were obtained from Jackson Laboratories. The mutation causes a frameshift that eliminates an extended COOH-terminal region present in both splice variants of CLIC5 and that is highly conserved in other CLICs. Because it appears to be a null mutation, we refer to the mutants as Clic5−/− mice, although possible effects of a truncated form of CLIC5 in some tissues cannot be ruled out. The original background was C3H/HeJ, but they bred very poorly and had very small litters (3 offspring or fewer). To improve breeding efficiency and litter sizes, the mice were backcrossed for one generation onto C57BL/6, and mutant and wild-type littermates on the mixed background were used for all experiments. Despite the single backcross, the phenotype was robust, and sex-matched littermate controls were used in almost all experiments to further minimize variability due to background effects between the two genotypes. PCR genotyping of tail DNA was performed by amplifying across the deletion mutation, using the primers described by Gagnon et al. (21). Mice were housed in a specific pathogen-free barrier facility and fed standard laboratory chow, with access to food and water ad libitum, except when on special diets or fasted. When fasted, mice were placed in individual cages with wire bottoms to prevent autocoprophagia, with free access to water. Mice were euthanized via CO2 asphyxiation followed by pneumothorax. All animal experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Microarray and PCR analysis.
RNA samples for microarray analyses were isolated from stomachs of adult mice lacking the NHE4 Na+/H+ exchanger 4 (23) and wild-type controls (n = 5 mice of each genotype) using TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol, but with one additional extraction and two additional precipitation steps. Microarray analyses were carried out by the University of Cincinnati Genomics and Microarray Laboratory core facility by using microarrays spotted with 70-mer oligonucleotide probes and cDNA samples labeled with Cy3 and Cy5 fluorophores exactly as described previously (9). The complete data set and additional methods have been deposited in NCBI's Gene Expression Omnibus (accession no. GSE19099). For quantitative real-time PCR, 4 μg of total RNA was reverse transcribed using oligo(dT) and Superscript II reverse transcriptase (Invitrogen), the cDNA was diluted fourfold in water, and 2 μl was used in a 25-μl final reaction volume with primer concentrations of 500 nM. Real-time PCR was run on a DNA Engine Opticon II (MJ Research) thermalcycler using iQ SYBR Green (Bio-Rad). For each gene, samples were run at least in duplicate, and mean values were normalized to the expression of mRNA encoding the L32 ribosomal subunit. For all primer sets (Supplementary Table 1; supplemental data for this article are available online at the American Journal of Physiology–Regulatory, Integrative and Comparative Physiology website) annealing was done at 58°C for 30 s, followed by 30 s of extension at 68°C, which resulted in amplification of a single product as verified by melting curve analysis and gel electrophoresis. PCR analysis of the tissue distribution of wild-type and mutant Clic5 mRNAs was performed using cDNA prepared from the indicated tissues, with 35 cycles of amplification and the WEX4 primers described previously (21).
Gastric acid measurements.
Gastric acid secretion was measured as described by Gawenis et al. (23). Mice were fasted overnight, given a single subcutaneous dose of 2 μg/g body wt histamine, and euthanized 20 min later. The stomachs were removed, everted into 3 ml oxygenated physiological saline, and shaken to suspend the contents. The pH was then measured. Data from mutant mice in which there was evidence of gastric bleeding were analyzed separately from those in which gastric bleeding was not apparent.
Histology and electron microscopy.
For routine pathology, freshly-excised tissue was rinsed in PBS, fixed in 10% neutral buffered formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin. To visualize glycogen, liver sections were stained with periodic acid and Schiff's reagent. For electron microscopy, 2-mm sections of stomach and liver were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde for 24 h, postfixed in 1% osmium tetroxide in PBS for 2 h, dehydrated through an ethanol series and propylene oxide, and embedded in Spurr's resin (Electron Microscopy Sciences, Hatfield, PA). Thick sections (1–2 μm) were stained with toluidine blue for light microscopy. Thin sections were stained with lead citrate and uranyl acetate for transmission electron microscopy. Morphometry was performed as described previously (39).
Food consumption, body composition, and indirect calorimetry.
Metabolic measurements were performed by the Mouse Metabolic Phenotyping Core at the University of Cincinnati using mice that were housed individually in a room maintained at 22°C, on a 12;12-h light-dark cycle. Food consumption was measured for 4 days by using the DietMax System from AccuScan Instruments (Columbus, OH). Body composition of unanesthetized mice was measured by quantitative magnetic resonance imaging using EchoMRI (Echo Medical Systems, Houston, TX) as described by Lo et al. (36). Indirect calorimetry was performed by the PhysioScan System from AccuScan Instruments as described previously (36, 48). Volumes of O2 consumed and CO2 produced were determined as ml·kg body wt−1·min−1, and heat production was determined as calories·kg body wt−1·h−1.
High-fat and normal fat diets.
For studies of the effects of high-fat content in the diet, adult female mice were fed a high-fat diet (5.24 kcal/g, 60% calories from fat, 20% calories from carbohydrate, cat. no. D12492; Research Diets) for 4 mo beginning at 7 wk of age. A separate cohort of female mice (n = 3 wild-type and 3 mutants) was maintained on regular chow (3.1 kcal/g, 16% calories from fat, 55% calories from carbohydrates, cat. no. 7022 NIH-07; Harlan Laboratories). All mice had access to food and water ad libitum and were weighed at least twice a week.
Analysis of blood and plasma.
For analysis of clotting factors and differential white blood cell counts, blood was collected via cardiac puncture and submitted to Antech Diagnostics (Oak Brook, IL). For measurements of plasma glucose, hormones, and lipids, blood was taken in the midafternoon (during the light cycle) from both unfasted mice and from mice that were fasted for 5 h. Blood was collected from the tail vein into heparinized capillaries, transferred to EDTA-coated microfuge tubes, centrifuged, and stored at −80°C until use. Assays for insulin, leptin, and glucagon were done using the Mouse Endocrine Lincoplex Kit from Millipore. Assays for plasma phospholipids (lecithin, lysolecithin, and sphingomyelin) and nonesterified fatty acids were performed using kits from Wako Diagnostics, cholesterol was determined using the Infinity Total Cholesterol kit from Thermo Fisher Scientific, triglyceride levels were determined using a kit from Randox Laboratories, and glucose was measured using a kit from Diagnostic Chemicals.
Telemetric measurements of body temperature and heart rate.
Mice were anesthetized via inhalation of 2% isoflurane and O2, and were given buprenorphine (0.1 mg/kg) for analgesia. A 3-cm incision of the skin and muscle was made for intraperitoneal implantation of the ETA-F20 transmitter (DSI, St. Paul, MN). The biopotential leads were tunneled subcutaneously using a 16-gauge trochar positioned with the negative lead 1 cm to the right of the xyphoid process and the positive lead at the clavicle ∼1 cm to the left of the sternum, according to the manufacturer's instructions. The mice were allowed to recover for 7 days. Temperature and ECG recordings took place continuously while mice were housed in separate cages in a temperature-regulated room (∼22°C) with a 12:12-h light-dark cycle (7 AM–7 PM). ECG patterns were used to determine heart rate. Collection and analysis of data were performed using the PowerLab system (AD Instruments, Colorado Springs, CO).
Intestinal fat absorption and urinary ketone analysis.
To determine whether mutant mice absorb dietary fat through the intestine, four wild-type and four mutant mice were placed on a high-fat diet containing 5% sucrose polybehenate for 4 days, 10 fecal pellets were collected from each mouse on days 3 and 4, and extracts were subjected to gas chromatography as described by Jandacek et al. (31). The magnitude of intestinal fat absorption was calculated by comparing the ratio of the nonabsorbable polybehenate/absorbable fats in the feces with the original ratio present in the food. Semiquantitative measurements of urinary ketone bodies (acetoacetic acid) were performed using Ketone Care test strips (Home Diagnostics, Ft. Lauderdale, Fl).
Statistics.
Comparisons of two groups were made using a two-tailed Student's t-tests except for analysis of the plasma profiles, which were done using a Mann-Whitney log rank test. For morphometric analyses of secretory membranes in gastric parietal cells, data were analyzed using SAS version 9.1 and SigmaPlot 2000 as described previously (39). All data are presented as the means ± SE, with n as the number of animals used. P < 0.05 was considered significant.
RESULTS
Identification of CLIC5 as a candidate protein involved in gastric acid secretion.
Our original objective was to identify Cl− channels involved in gastric acid secretion. To do this, we performed microarray analyses of stomach RNA from mice lacking NHE4, which exhibit a severe reduction in the number of parietal cells (23). We reasoned that the expression of mRNAs encoding parietal cell-specific proteins would be sharply reduced. Consistent with this expectation, a number of ion transporters and channels known to function in parietal cells were downregulated (Supplementary Table 2). These included the gastric H+,K+-ATPase α- and β-subunits that mediate H+ secretion; AE2, the major basolateral Cl−/HCO3− exchanger (24); aquaporin 4, the basolateral water channel (61); and KCNE2, a component of the apical K+ channel (45). Expression of mRNAs encoding several additional K+ channels and other ion transporters with no reported function in the parietal cell were also downregulated.
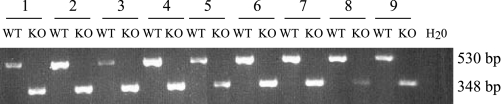
Among the known or putative Cl− channels, both CLIC5, which can associate with ezrin (4, 5), a major regulator of gastric acid secretion (64), and CLIC6, which is expressed at high levels in parietal cells (40) and has been proposed as a possible gastric Cl− channel (49), were identified as being downregulated (Supplementary Table 2). Quantitative RT-PCR analysis of stomach RNA from NHE4-null and wild-type mice confirmed the reduction in expression of Clic6 but not Clic5 mRNA (Supplementary Fig. 1), suggesting that the apparent reduction in expression of Clic5 mRNA was a false positive. Nevertheless, PCR analysis showed that Clic5 mRNA was expressed in stomach and many other tissues (Fig. 1), and its known ability to associate with ezrin (4, 5) made it a potential candidate as a regulator of acid secretion. The availability of a mouse model carrying a deletion mutation in a critical region of the Clic5 gene (21) made it feasible to test the possible role of CLIC5 in gastric acid secretion.
Fig. 1.
PCR analysis of the tissue distribution of Clic5 mRNA in wild-type (WT) and Clic5 mutant (KO) mice. Total RNA was extracted from tissues of WT and KO mice and reverse transcribed, and the resulting cDNA was amplified as described in materials and methods to yield 530- and 348-bp products for WT and KO alleles, respectively. The following tissues were analyzed: 1, duodenum; 2, jejunum; 3, ileum; 4, colon; 5, stomach; 6, heart; 7, kidney; 8, liver; 9, brain.
Loss of CLIC5 leads to fasting-induced gastric hemorrhaging but has little effect on gastric pH or histology.
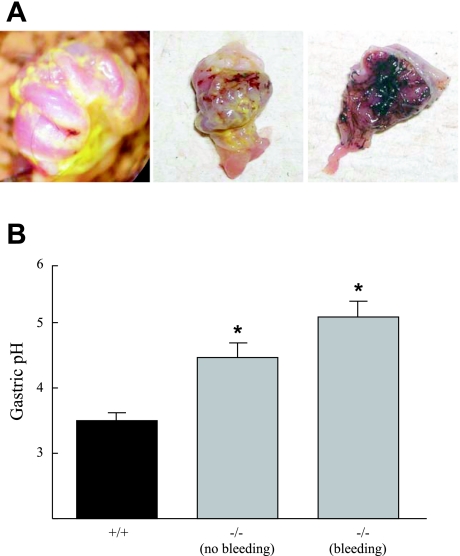
After overnight fasting, histamine-stimulated acid secretion was reduced in Clic5−/− mice; however, the reduction was modest and interpretation of these measurements was confounded by gastric hemorrhaging (Fig. 2A), which was observed in > 50% of the Clic5−/− mice. As shown in Fig. 2B, the pH of stomach contents of mutant mice that did not exhibit gastric bleeding was higher than that of wild-type mice (pH 4.5 ± 0.2 in mutant vs. 3.5 ± 0.1 in wild type, P = 0.007), but less than that of mutant mice with mild-to-moderate bleeding (5.1 ± 0.25), suggesting that the apparent reduction in acid secretion may be a secondary effect.
Fig. 2.
Gastric hemorrhaging and mild hypochlorhydria in KO mice. A: everted stomachs of KO mice subjected to overnight fasting reveal hemorrhaging of the glandular stomach with varying levels of severity, from focal bleeding (left), mild but more diffuse bleeding (center), to severe hemorrhaging (right). B: measurements of gastric pH after overnight fasting and stimulation with histamine revealed mild hypochlorhydria in KO (−/−) mice relative to WT (+/+) mice. KO mice were separated based on the presence of blood on the lumenal surface of the stomach (bleeding) or the absence of any detectable hemorrhaging (no bleeding). For mice with no signs of hemorrhage: n = 5 (WT) and 8 (KO) mice; for mice in which bleeding was detected: n = 4. *P < 0.05 compared with WT based on a Student's t-test.
Quantitative PCR analysis of stomach RNA from Clic5−/− and wild-type stomachs revealed no significant changes in mRNAs encoding the gastric H+-K+-ATPase α-subunit, CLIC1, or CLIC6, but showed a small but significant reduction in CLIC4 (Supplementary Fig. 2), which has been implicated in vascular dysfunction (13, 58). Because of the metabolic phenotype described below, mRNAs for the peptide hormone ghrelin and ghrelin O-acyltransferase, the enzyme that mediates acylation of ghrelin (32), were also analyzed. Because CLIC4 has been reported in mitochondria (19), we analyzed mRNA for Cox4i1, a cytochrome-c oxidase subunit; however, none of these mRNAs was significantly altered.
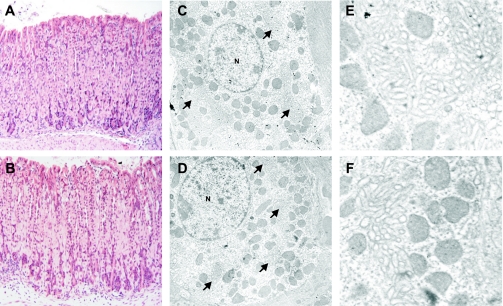
Despite severe hemorrhaging in some fasted Clic5−/− mice, we saw no histological evidence of intravascular coagulation, and no significant histological or ultrastructural abnormalities were found in any sections of the stomach. Analysis of hematoxylin and eosin-stained sections by light microscopy revealed abundant parietal cells in both wild-type and mutant stomachs (Fig. 3, A and B). Ultrastructural analyses revealed morphologically normal tubulovesicles, canalicular membranes, microvilli, and mitochondria (Fig. 3, C–F), suggesting that Clic5−/− parietal cells are capable of modulating their membranes appropriately between the resting and stimulated state. Electron microscopy and morphometry of sections from fasted mice also revealed normal volume densities of the mitochondria, basolateral membranes, tubulovesicles, and canalicular membranes; and the length and width of the parietal cell microvilli appeared normal in the mutant mice (data not shown).
Fig. 3.
Morphology of gastric glands and parietal cells in WT and KO mice. Hematoxylin and eosin staining shows normal structure of the gastric glands in stomachs of WT (A) and KO mice (B). Electron microscopy revealed normal tubulovesicles, canalicular membranes (arrows), mitochondria, and nuclei (N) in parietal cells from WT (C and E) and KO (D and F) mice. E (WT) and F (KO): increased magnification of normal canalicular membranes.
Analysis of blood samples revealed significant reductions in numbers of neutrophils, eosinophils, and monocytes (Table 1); however, both the intrinsic and extrinsic clotting pathways appeared to be intact in the mutant mice (Supplementary Table 3), indicating that the observed hemorrhaging was not due to a coagulation disorder. The incidence of gastric bleeding in the mutants showed a strong correlation with reduced body temperature (described in more detail below), weight loss during fasting, and the duration of the fast. When fasted, mutant mice rapidly lost body weight. After 4 h, they lost ∼6% of their starting weight, and by 8 h they had lost > 9% (Supplementary Fig. 3). In contrast, the wild-type mice lost only 2% and 6% of their body weight at those time points. These data suggest that fasting puts an immediate and severe metabolic stress on Clic5−/− mice. Since a metabolic disorder had not been reported previously for Clic5 mutants or other Clic mutant mice, we focused our attention on the analysis of this phenotype.
Table 1.
White blood cell counts (WBC) from wild-type (WT) and Clic5 mutant mice
| WT | KO | P Value | |
|---|---|---|---|
| WBC, 103/μl | 7.3 ± 1.5 | 4.2 ± 0.8 | 0.11 |
| Neutrophils | 2,825 ± 641 | 1,118 ± 203 | 0.04* |
| Lymphocytes | 3,884 ± 921 | 2,897 ± 559 | 0.39 |
| N/L | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.11 |
| Monocytes | 218 ± 45 | 90 ± 11 | 0.03* |
| Eosinophils | 323 ± 63 | 84 ± 15 | 0.01* |
| Basophils | 0 | 0 | 1 |
Data are means ± SE. Blood samples from 3-mo-old WT and mutant (KO) males (n = 4 mice of each genotype) were analyzed. Reductions in the mean number of total WBC, lymphocytes, and the ratio of neutrophils to lymphocytes (N/L) were not significant. The number of monocytes, neutrophils, and eosinophils were significantly reduced in Clic5 mutant mice; no basophils were detected in any of the mice.
P < 0.05 based on a Student's t-test.
Mutant mice are more active, smaller, and consume more food than wild-type mice.
Beginning at 2 wk of age, homozygous mutant mice were easily distinguishable by their head bobbing and awkward gait. Although we did not perform quantitative analyses of locomotor activity, it was clear from observations of the mutants that they were hyperactive. An additional phenotype, not reported previously, was that male Clic5 mutants were highly aggressive and had to be separated from other males. By weaning and at all later ages both male and female mutants were hyperactive and exhibited movement abnormalities (see discussion), were smaller than their wild-type littermates (Fig. 4A) and, as discussed in more detail below, were also leaner.
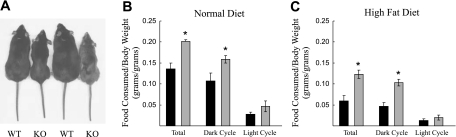
Fig. 4.
KO mice are smaller than their WT littermates and are hyperphagic. A: at 8 mo of age, female KO mice were significantly leaner than their WT littermates. B: when normalized to body weight (g food consumed/g body wt), adult KO mice (grey bars) consumed more food over 24 h than WT mice (black bars), which was largely due to increased food consumption during the dark cycle (7 PM to 7 AM); n = 5 male and n = 2 female littermate pairs. C: after 2 wk on the high-fat diet, adult male mutant mice (grey bars, n = 4) continued to consume more food than male WT mice (black bars, n = 4). *P < 0.05 using a Student's t-test.
Female mutant mice rarely exceeded 25 g and male mutants typically did not exceed 30 g, even when older than 1 yr of age. In addition to lower body weights, nose-to-tail lengths at ∼3 mo of age were reduced in CLIC5 mutants (Clic5−/−, 7.94 ± 0.17 cm; wild type, 8.89 ± 0.14 cm; P < 0.001). Despite their smaller size, Clic5−/− mice consumed more food than wild-type controls (Clic5−/−, 5.27 ± 0.26 g/day; wild type, 4.75 ± 0.11 g/day; P < 0.05). When normalized to body weight, the mutants consumed 48% more food (normal fat diet) than wild-type controls (Fig. 4B), equivalent to 0.62 kcal/g over a 24-h period vs. 0.42 kcal/g in wild-type controls (P = 0.002). An even greater fold difference between the two genotypes in food consumption was observed when the mice were placed on a high-fat diet, with Clic5−/− and wild-type mice consuming 0.12 ± 0.01 and 0.06 ± 0.01 g·g body weight−1·day−1 (Fig. 4C), equivalent to 0.64 and 0.31 kcal·g−1·day−1, respectively (P < 0.007). On both the normal and high-fat diets, the differences were largely attributable to food consumption during the dark cycle, when mice were more active, although similar differences were also apparent during the light cycle.
Clic5 mutant mice have very little fat and are refractory to weight gain on a high-fat diet.
Total intestinal fat absorption, as measured by the sucrose polybehenate method, was 99.4 ± 0.1% in wild-type mice and 99.3 ± 0.4% in mutant mice (n = 4 mice of each genotype; P = 0.8), indicating that fat absorption across the intestine was normal in mutant mice. Despite the normal fat absorption, quantitative magnetic resonance imaging studies of body composition (Table 2) showed that total body fat was severely reduced in male mutant mice maintained on normal chow. In fact, the significant difference in body weight was due primarily to the difference in body fat (0.5 ± 0.1% of body weight in mutant, 16.9 ± 3.9% in wild type); there was very little difference in lean body weight (24.9 ± 1.2 g in mutant, 26.6 ± 1.6 g in wild type). As discussed below, females also exhibited reduced body fat, but it was not as severe as in male mice.
Table 2.
Body composition of male mice on a normal diet
| Body Weight, g | Fat Mass, g | %Fat Mass | Lean Mass, g | %Lean Mass | Water Mass, g | |
|---|---|---|---|---|---|---|
| WT | 35.6 ± 2.8 | 6.3 ± 1.3 | 16.9 ± 3.9 | 26.6 ± 1.6 | 75.3 ± 3.3 | 24.1 ± 1.4 |
| KO | 27.2 ± 1.3* | 0.1 ± 0.0* | 0.5 ± 0.1* | 24.9 ± 1.2 | 91.7 ± 0.4* | 22.5 ± 1.1 |
Data are means ± SE. Sibling pairs of male WT and Clic5 KO mice (11–25 wk old; n = 5 of each genotype) were maintained on regular chow. Quantitative magnetic resonance imaging was used to determine the fat mass, lean mass, and water mass of each mouse.
P < 0.05 WT vs. KO.
To determine whether Clic5−/− mice could gain weight and increase body fat, female mice were placed on a high-fat diet for 4 mo beginning at 7 wk of age, and an additional cohort of female mice was fed a normal fat diet. Only females were used for these long-term feeding studies because males were highly aggressive toward cage mates and had to be housed separately, and females appeared to have higher fat levels, suggesting that they might be more susceptible to diet-induced weight gain. At the end of 4 mo, the weight of female mice on normal chow was 26.5 ± 1.2 g for wild-type vs. 23.4 ± 0.9 g for mutant mice, whereas wild-type mice on the high-fat diet weighed 42.2 ± 3.9 g vs. 21.7 ± 0.6 g for mutant mice (P < 0.05) (Table 3) and had 43.9 ± 2.9% body fat vs. 7.6 ± 0.7% for mutant mice (P < 0.05). As observed with male mice on a normal diet, female mutant mice fed a normal diet exhibited no significant difference in lean body weight when compared with wild-type controls. Surprisingly, there was no significant difference between the weights of Clic5−/− mice maintained on the high-fat diet compared with those on the normal fat diet. Also, the percent lean mass was slightly higher for mutants fed the high-fat diet than for wild-type mice on the normal fat diet. After 4 mo on the high-fat diet, mutant female mice increased their fat mass to levels similar to those of wild-type females on the normal diet (Table 3).
Table 3.
Body composition of female mice on a normal or high-fat diet (HFD) for 4 mo
| Body Weight, g | Fat Mass, g | %Fat Mass | Lean Mass, g | %Lean Mass | Water Mass, g | |
|---|---|---|---|---|---|---|
| WT normal | 26.5 ± 1.3 | 2.2 ± 1.0 | 7.8 ± 3.4 | 21.8 ± 0.4 | 82.6 ± 2.9 | 19.8 ± 0.4 |
| KO normal | 23.4 ± 0.9 | 0.6 ± 0.3 | 2.5 ± 1.2 | 20.6 ± 0.7 | 88.1 ± 0.8 | 18.7 ± 0.6 |
| WT HFD | 42.2 ± 3.9† | 18.9 ± 3.1† | 43.9 ± 2.9† | 21.5 ± 0.9 | 51.6 ± 2.3† | 19.4 ± 0.8 |
| KO HFD | 21.7 ± 0.6*† | 1.7 ± 0.1*† | 7.6 ± 0.7*† | 18.1 ± 0.6*† | 83.5 ± 0.4*† | 16.4 ± 0.6*† |
Data are means ± SE. WT and Clic5 KO female mice were maintained on either regular chow (normal) or placed on a HFD for 4 mo beginning at 7 wk of age. Quantitative magnetic resonance imaging was used to determine the fat mass, lean mass, and water mass of each mouse. Mice on the normal diet: n = 3 wild-type and 3 Clic5 KO mice; on the HFD: n = 4 wild-type and 4 Clic5 mutant mice.
P < 0.05 WT vs. KO and
P < 0.05 WT vs. WT or KO vs. KO.
Urinary ketone (acetoacetate) excretion was minimal for both wild-type and Clic5−/− mice on normal and high-fat diets (data not shown), suggesting that carbohydrate stores were sufficient (i.e., the mice are not starving) and that any ketones produced as a byproduct of fat metabolism were being utilized.
Lipid accumulation is reduced in livers of Clic5−/− mice fed a high-fat diet.
Although both light and electron microscopy revealed relatively normal liver histology, mutant mice on the high-fat diet failed to accumulate hepatic lipid to the same extent as wild-type controls (Fig. 5). Wild-type mice on the high-fat diet exhibited grossly visible lipid deposition in the liver, and microscopy revealed the presence of a wide range of lipid droplet sizes in hepatocytes, some of which were very large. While lipid droplets were visible in the livers of the Clic5−/− mice, they tended to be small and were associated with both hepatocytes and hepatic stellate cells (Fig. 5). Glycogen was visible both by electron microscopy and by light microscopy after periodic acid and Schiff's reagent staining of livers of both wild-type and mutant mice (data not shown), although it appeared to be increased in wild-type mice. Neither genotype exhibited any evidence of fibrosis or inflammation in the liver. Interestingly, three of the six livers analyzed from adult mutant mice maintained on the high-fat diet showed foci of hematopoiesis (Fig. 5), which were not observed in any sections from wild-type controls.
Fig. 5.
Morphology of the liver in WT and KO mice. Hematoxylin and eosin staining shows normal liver morphology in adult WT (A) and KO (B) mice that were maintained on a normal diet. However, after 4 mo on a high-fat diet, sections from WT mice (C) clearly showed the presence of lipid in hepatocytes (black arrows), while lipid accumulation appeared to be significantly reduced in the liver from KO mice (D). In panels A–D the white space surrounding the hematoxylin-stained nuclei indicates where lipid was localized prior to dehydration of the sections. E: toluidine blue staining of plastic sections revealed large, well-formed lipid droplets (black arrows) in hepatocytes from WT mice maintained on a high-fat diet. F: in contrast, very few, and much smaller, lipid droplets (black arrow) were visible in liver sections from KO mice maintained on a high-fat diet. Foci of hematopoiesis (white arrow) were visible in some liver sections from KO mice maintained on a high-fat diet, although they were never detected in liver sections from WT mice.
Clic5 mutants exhibit reduced plasma levels of insulin, glucose, and leptin, but normal lipid levels.
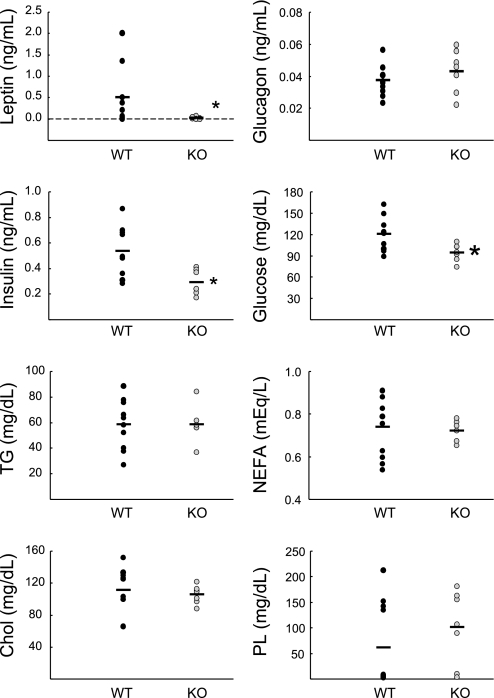
In the absence of fasting, levels of plasma leptin were dramatically reduced in Clic5 mutant mice that were fed a normal diet, consistent with their profound lack of white adipose tissue, and plasma levels of insulin and glucose were also significantly lower (Fig. 6). Levels of glucagon, triglycerides, nonesterified fatty acids, cholesterol, and phospholipids were normal (Fig. 6). When mice maintained on a normal diet were fasted for 5 h, insulin and glucose were significantly lower in the mutants and glucagon levels were significantly increased compared with wild-type controls (Fig. 7). As observed in unfasted animals, leptin levels were very low, and the levels of plasma triglycerides, fatty acids, cholesterol, and phospholipids in mutant mice were similar to those of wild-type mice.
Fig. 6.
Plasma profile of nonfasted WT and KO mice. Plasma hormones and lipids from male WT and heterozygous mice (black circles, n = 7 WT and 4 heterozyotes) and KO mice (grey circles, n = 7) were measured without fasting. Values for WT and heterozygous mice were not significantly different, so they were pooled. Leptin levels were severely reduced in the mutant mice (0.04 ± 0.02 ng/ml), and 2 of the 7 KO mice had no detectible levels of leptin. Plasma triglycerides (TG), nonesterified fatty acids (NEFA), total cholesterol (Chol), and phospholipids (PL) were normal in the KO mice. *P < 0.05 using a Mann-Whitney rank sum test.
Fig. 7.
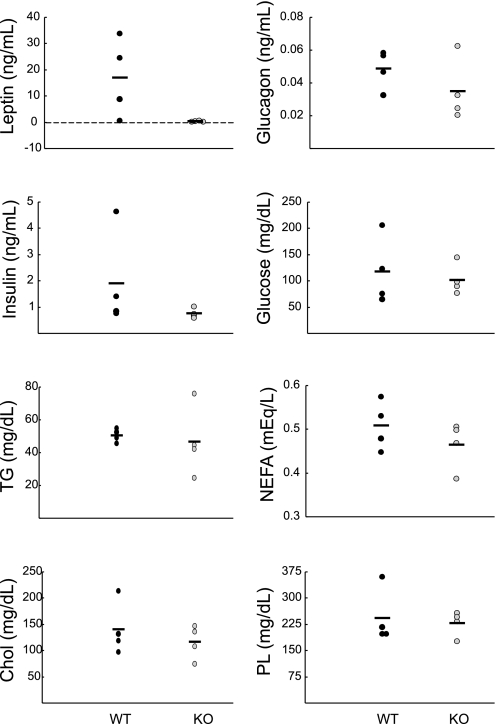
Plasma profile of fasted WT and KO mice. Plasma hormones and lipids from male WT (black circles, n = 5) and male KO mice (grey circles, n = 5) were measured after 5 h of fasting. In KO mice, leptin levels were 0.14 ± 0.14 ng/ml, with 3 of the 5 KO mice having undetectable levels of leptin. TG, NEFA, Chol, and PL were normal in mutant mice. *P < 0.05 using a Mann-Whitney rank sum test.
When plasma samples from mice fed a high-fat diet were analyzed (Fig. 8), mean leptin levels in wild-type mice (16.9 ± 7.5 ng/ml) were substantially increased relative to those of wild-type mice on the normal fat diet (0.70 ± 0.65 ng/ml; see Fig. 6); however, leptin levels in mutant mice fed a high-fat diet remained very low (0.27 ± 0.14 ng/ml on high-fat diet; 0.03 ± 0.02 on normal diet). Plasma levels of insulin and glucagon were slightly lower in Clic5 mutants than in wild-type mice, although the differences were not statistically significant, and levels of glucose, phospholipids, cholesterol, triglycerides, and fatty acids were essentially the same in both genotypes (Fig. 8).
Fig. 8.
Plasma profile of WT and KO mice on a high-fat diet. Plasma hormone and lipid levels from unfasted female WT (black circles, n = 4) and KO mice (grey circles, n = 4) that had been on a high-fat diet for 4 mo were measured. Due to the high variability, neither the differences in leptin nor insulin levels achieved statistical significance. Levels of TG, NEFA, Chol, and PL were normal in mutant mice.
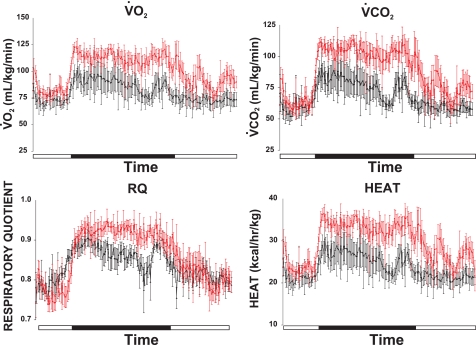
Mutant mice have increased peripheral metabolism.
Measurements of oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) revealed that, relative to wild-type controls, Clic5−/− mice fed a normal diet had increased peripheral metabolism, which was more pronounced during the dark cycle (Fig. 9). Although the respiratory quotient (RQ) of the mutant mice was similar to wild-type controls during the light cycle, it was elevated during the dark cycle, indicating increased utilization of carbohydrate over lipid for energy. Heat production in Clic5−/− mice was also elevated during the dark cycle, as would be expected on the basis of their increased food intake and hyperactivity (Fig. 9).
Fig. 9.
Indirect calorimetry of WT and KO mice. Measurements of O2 consumption (V̇o2), CO2 production (V̇co2), the respiratory quotient (RQ), and heat production were measured over 24 h as described in materials and methods. Black bars indicate the dark cycle (7 PM-7 AM). The groups included mice of both sexes, ranging from 3 to 6 mo old, and individual pairs were sex-matched littermates (n = 5 male and n = 2 female mice of each genotype).
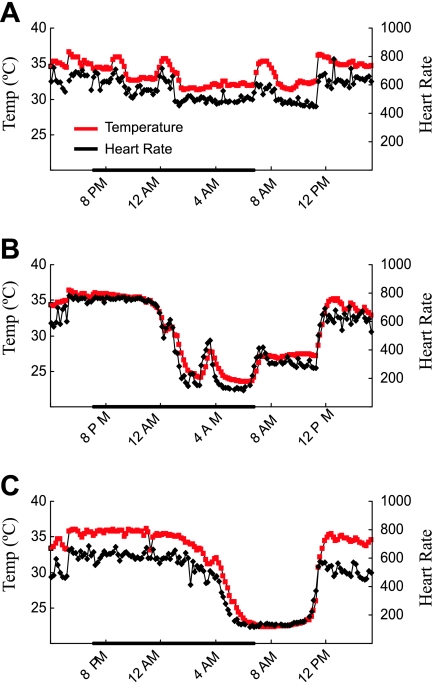
Mutant mice are more susceptible to fasting-induced torpor.
In our initial studies of gastric acid secretion we noticed that the body temperature of Clic5 mutants subjected to fasting often appeared to be reduced and that this coincided with a quiescent state that contrasted with their normal hyperactive state. The frequent reduction in body temperature, confirmed with a rectal thermometer, indicated that Clic5 mutants were more susceptible to torpor, a hypometabolic state in which body temperature and heart rate are dramatically reduced (26). To further document this trait, temperature and heart rate were analyzed by telemetry. Although all mice are capable of entering torpor in response to fasting (25), Clic5−/− mice subjected to fasting entered torpor more readily, and exhibited greater reductions in body temperature and heart rate than wild-type siblings (Fig. 10). However, when very lean wild-type female mice were compared with weight-matched mutant female mice, which required using relatively young wild-type and much older mutant mice, the lean wild-type mice appeared to undergo similar bouts of torpor in response to fasting (data not shown).
Fig. 10.
. KO mice are more susceptible to fasting-induced torpor than WT mice. Because fasting Clic5−/− mice were frequently observed to enter torpor, mice were implanted with radiotelemeters to measure core body temperature (red line) and heart rate (black line) to further analyze susceptibility to fasting-induced torpor. The graphs show one 24-h period (from 4 PM to 4 PM); mice were fasted from 6 PM to 11 AM, and the dark bar indicates the dark cycle (7 PM to 7 AM). A representative tracing is shown for a WT male mouse (A) and a female littermate (B). During torpor, the WT mouse reduced its temperature to 31.2°C and its heart rate to 448 beats/min, while the mutant mouse reduced its body temperature to 23.5°C and 117 beats/min. Note the simultaneous fluctuation of body temperature and heart rate. Although it is normal for mice to rouse from torpor periodically during the dark cycle, some mutant mice did not. C: tracing of a female KO mouse undergoing a sustained bout of torpor, with body temperature reduced to 22.3°C and heart rate to 112 beats/min.
DISCUSSION
Gastric acid secretion was significantly reduced in Clic5−/− mice; however, the reduction was modest. Some Clic5 mutants were able to reduce their gastric pH to as low as pH 3, and there was no evidence of histological or ultrastructural changes similar to those observed in previous studies of mice with defective acid secretion (23, 24, 46, 52). These observations and the relatively low expression of Clic5 relative to Clic6 mRNA in the stomach argue against a major role for CLIC5 as either a gastric Cl− channel or a regulator of acid secretion. The fact that Clic5−/− mice with gastric bleeding exhibited a greater reduction in stomach acid than mutant mice with no evidence of bleeding suggests that the modest reduction in stomach pH might be secondary to systemic effects of Clic5 disruption, such as the metabolic phenotype discussed below.
Fasting-induced gastric hemorrhaging was the most striking stomach phenotype in Clic5 mutants. This is likely due, at least in part, to the absence of significant body fat reserves, as this condition is known to contribute to metabolic stress ulcers (62). Also, both food restriction and food deprivation are sufficient to cause gastric ulceration in rodents (63). Interestingly, mice lacking the thioredoxin binding protein, which exhibit impaired fatty acid utilization, also experience gastric hemorrhaging upon fasting (42). An increase in physical activity stimulated by food restriction (17) has been shown to exacerbate the severity of metabolic stress ulcers by contributing to increased energy expenditure. Thus, both the low-fat stores and hyperactivity in Clic5 mutant mice are likely to be significant factors in the high incidence of gastric hemorrhaging.
Studies in rats have shown that the incidence of gastric ulceration is also increased as core body temperature decreases (50). In the present study, neither baseline internal body temperature (wild type, 34.3 ± 0.04°C; Clic5 mutant, 34.1 ± 0.41°C, determined by telemetry), nor surface temperature (wild type, 36.2 ± 0.3°C; Clic5 mutant, 36.0 ± 0.1°C, determined by placing a temperature probe on the surface between the shoulder blades) differed between the two genotypes. However, during fasting, body temperature of Clic5−/− mice dropped sharply. It was not uncommon to measure core body temperatures of < 30°C in awake, fasted Clic5−/− mice when maintained in the laboratory in the middle of the afternoon, and during torpor core body temperature dropped to as low as 22°C (ambient temperature). Although other factors could also be involved in the gastric bleeding, such as potential vascular effects discussed below, the fasting-induced gastric bleeding is likely to be exacerbated by low energy reserves, increased energy expenditure due to hyperactivity, and reduced body temperature as the mice enter torpor.
Laboratory mice can enter torpor, which serves as an energy conservation mechanism, in response to food restriction (25). During torpor, energy expenditure may be reduced by as much as 70% and the body temperature, accordingly, may drop to as low as 20°C. Both the entrance into and the exit from torpor are carefully regulated processes, with no pathological consequences. Although all wild-type mice may enter torpor, a number of factors can contribute, including reduced caloric intake, low energy reserves, regular light-dark cycles, and low ambient temperature (26, 55). Clic5 mutant mice have very little body fat, and fasting can be fatal for these mice. Therefore, it would appear to be advantageous for Clic5−/− mice to more readily enter torpor upon fasting to conserve energy. It should be noted that lean wild-type controls that were matched with respect to body weight exhibited similar bouts of torpor. Thus, the increased susceptibility to torpor appears to be due to the lean phenotype rather than being a more direct consequence of the loss of CLIC5.
Clic5 mutant mice are hyperphagic regardless of whether food intake is normalized to body weight. Nevertheless, they remain significantly leaner than their wild-type littermates. This may be due directly to their increased peripheral metabolism, which, as discussed below, is likely to be caused, at least in part, by their apparent hyperactivity. However, Clic5 mutant mice can maintain their body weight and grow under normal conditions, indicating that they are not in negative energy balance. Previous studies have shown that atrophy of the thymus, spleen, and lymph nodes is a rapid consequence of inadequate nutrition (28); however, the weights of these organs as a percent of body weight were normal in Clic5 mutants (data not shown). Additionally, both male and female mutant mice are fertile and produce viable offspring, which further suggests that they do not suffer from a chronic nutritional deficit. Although the mutant mice have a much greater caloric intake than their wild-type littermates, which exhibited a reduction in caloric intake when placed on the high-fat diet, possibly due to the elevation in leptin levels, the caloric intake of Clic5−/− mice on the high-fat diet is essentially the same as on the normal diet. This suggests that this high level of caloric intake is required to maintain basal metabolism and stable body weights.
CLIC4, another CLIC isoform, is involved in angiogenesis and in the generation of collateral blood vessels (13, 58), and its mRNA was mildly reduced in stomachs of Clic5−/− mice. Although there are no reported metabolic or bleeding abnormalities in CLIC4-null mice, the investigators noted that they are smaller than their wild-type littermates (58). Furthermore, studies have shown that inhibition of angiogenesis using TNP-470 prevents diet-induced obesity (10), and it is known that both monocyte activation (30) and metabolic influences (60), particularly reduced leptin, affect angiogenesis. Clic5 mutants have less than half the normal number of monocytes and have significantly reduced leptin levels. It is possible, therefore, that loss of CLIC5 may have direct or indirect effects on angiogenesis and that this contributes to the bleeding and/or metabolic phenotypes; however, a role for CLIC5 in angiogenesis has not been reported and the metabolic changes are the most likely explanation for the bleeding disorder.
Although it is clear that energy metabolism is altered in Clic5−/− mice, plasma hormone and lipid profiles suggest that certain aspects of basal metabolism are normal and that the transport, de novo synthesis, and storage of lipids is not impaired by the loss of CLIC5. Under both normal and high-fat dietary conditions, Clic5−/− mice had moderately reduced levels of both insulin and glucose relative to wild-type mice, suggestive of increased insulin sensitivity. Plasma triglycerides, cholesterol, free fatty acids, and phospholipids were also normal under both dietary conditions and even after a 5-h fast in mice maintained on the normal fat diet. Female Clic5 mutants did not gain weight on the high-fat diet; however, they did increase their percent body fat (2.5% of body weight initially vs. 7.6% after 4 mo). Although Clic5−/− mice did not develop fatty livers, as in wild-type controls, lipid deposits were seen in hepatocytes and also in hepatic stellate cells, which are involved in storage of lipid soluble factors such as vitamin A (59). In fact, up to 80% of vitamin A (as retinyl esters) is stored in hepatic stellate cells (7). Interestingly, administration of retinoic acid to mice has been shown to upregulate uncoupling proteins in white adipose tissue and skeletal muscle (8, 38), reduce body weight (8), increase insulin sensitivity (18), and decrease the expression of adipokines (8). Some of these changes are similar to those seen in Clic5 mutants.
Leptin levels were very low in Clic5−/− mice regardless of their diet, but did rise somewhat when the mice were placed on a high-fat diet (from 0.04 to 0.27 ng/ml), which was consistent with the modest increase in percent body fat. It is likely that the very low levels of leptin is a physiological response due to the low amounts of white adipose tissue, increased energy expenditure, and decreased energy reserves rather than a direct effect of CLIC5 deficiency on the production or secretion of leptin. Leptin-deficient (ob/ob) mice are severely obese (29) and have high plasma insulin, glucose, and lipids (35), in contrast to the relatively normal levels in Clic5−/− mice. This suggests that these aspects of the metabolic phenotype are not secondary to reduced leptin. Nevertheless, the low leptin levels in Clic5−/− mice may contribute to a number of their phenotypic characteristics, including hyperphagia, torpor, predominance of carbohydrate over fat metabolism, susceptibility to gastric lesions, and impaired lymphopoiesis.
Leptin-deficient mice are more susceptible to torpor due to the perceived negative energy balance (22), and low leptin levels have been shown to be permissive for torpor (20). Thus, the low levels of circulating leptin in Clic5−/− mice likely contribute to their increased susceptibility to torpor. In studies utilizing indirect calorimetry, treatment of leptin-deficient (ob/ob) mice with leptin led to a decrease in RQ but had no effect on RQ in mice lacking the leptin receptor, suggesting that leptin stimulates fat metabolism (27). In our own studies, RQ was greater in Clic5−/− mice than in wild-type controls (Fig. 9), indicating a greater reliance on carbohydrate metabolism, which is consistent with their low leptin levels. Leptin, given as intraperitoneal injections or released in response to cholecystokinin, has been shown to have gastroprotective effects (11), suggesting that the low levels in Clic5−/− mice may be an additional factor contributing to the gastric bleeding. Finally, Clic5−/− mice had significant reductions in the numbers of neutrophils, eosinophils, and monocytes and also exhibited evidence of hematopoiesis in the adult liver. Ob/ob mice have significant impairment of lymphopoiesis, including both B-cells and those from the myeloid lineage (granulocytes and monocytes) (14), and the total number of circulating lymphocytes is decreased in mice lacking the leptin receptor (2). Thus, it is possible that both the decreased number of circulating white blood cells and the foci of hematopoiesis in the livers of adult Clic5−/− mice are secondary to reduced leptin levels.
Clic5−/− mice exhibit a number of behavioral and social abnormalities, which have not been studied in detail in either the current or previous studies. Not only do they run in circles (21) but they also run or walk backward rather than forward when trying to escape restraint, often do back flips, and engage in hindlimb clasping when held by the tail. For example, when placed in a round container, Clic5−/− mice circle backward, whereas wild-type mice move forward and attempt to jump over the edge of the container, and when placed in a container to be weighed, many Clic5 mutants perform rapid and repeated back somersaults. Similar behavioral abnormalities are often seen in mice treated with agents that have neurological effects (12, 43, 57). Female Clic5−/− mice exhibit normal fertility and parturition, but typically scatter their pups and are unable to care for them. Male Clic5−/− mice appear to have normal fertility, but are extremely aggressive and violent toward other male mice of any genotype. Unlike wild-type mice, male Clic5−/− mice often exhibit aggression toward their male siblings and must be housed individually from the time they are weaned; psychological stimulation with nestlets does not appear to reduce the severity of this phenotype. Some of the observed behaviors, including spinning and head bobbing, are undoubtedly affected by the vestibular defect (21), but other behaviors, such as the aggressiveness of Clic5−/− males, are unlikely to be due to this defect.
The behavioral and social abnormalities suggest that loss of CLIC5 causes a neurological defect, which could also be involved in the metabolic phenotype. The mechanisms by which CLIC5-deficiency might cause a neurological defect are unclear; however, CLIC5B is associated with the Golgi apparatus, where it interacts with AKAP350 (47). Thus, it is possible that its absence affects processing and trafficking of membrane components important in neuronal function. Alternatively, neurological effects might be related to its functional associations with the actin cytoskeleton or ezrin, radixin, and moesin proteins (4, 5, 51), which have important roles in neuronal function. Possible neuronal functions have been suggested for CLIC4, a related family member (37, 53).
Perspectives and Significance
CLIC proteins have been studied for over 20 yr, but a clear understanding of their molecular, cellular, and physiological functions is lacking. Although CLICs can exist as soluble cytosolic forms, there is strong evidence that they also form membrane-embedded Cl− channels. Given the expression of CLIC5 in stomach, we hypothesized a role in gastric Cl− channel activity, which would have provided the first direct correlation between molecular, cellular, and physiological functions for a CLIC isoform. We found no evidence of a direct role for CLIC5 in acid secretion; however, gastric hemorrhaging was a major stomach phenotype. Further investigation, using quantitative magnetic resonance imaging, indirect calorimetry, telemetric measurements of body temperature, and analysis of plasma samples, indicated that the gastric bleeding was not a direct consequence of the loss of CLIC5 in stomach, but was secondary to perturbations of metabolism. These included increased susceptibility to torpor, extreme leanness, hyperphagia, resistance to weight gain on a high-fat diet, very low leptin levels, and increased peripheral metabolism. Although the gastric hemorrhaging can be understood in terms of the metabolic phenotype, the mechanisms underlying the metabolic changes remain unclear. The fact that the metabolic phenotype is accompanied by severe behavioral and social abnormalities suggests that neuronal mechanisms could be involved and that the metabolic phenotype could be secondary to hyperactivity. On the other hand, intracerebroventricular administration of ghrelin or agouti-related protein causes both increased food intake and decreased spontaneous locomotor activity (56), indicating that food intake and activity levels can be regulated simultaneously by the central nervous system. Whether metabolism and food intake are affected directly by loss of CLIC5, rather than being largely secondary to hyperactivity, and whether the observed phenotypes are due to the loss of CLIC5 activity in the central nervous system, in peripheral tissues, or in both types of tissues remains to be determined.
GRANTS
This work was supported by National Institutes of Health Grants DK-050594, HL-061974, DK-059630, and ES-06096.
DISCLOSURES
No conflicts of interest, financial, or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dana Lee for assistance in the metabolic phenotyping studies and Maureen Bender for expert animal husbandry.
REFERENCES
- 1.Ashley RH. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins. Mol Membr Biol 20: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6: 1170–1180, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Berry KL, Bulow HE, Hall DH, Hobert OA. C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science 302: 2134–2137, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Berryman M, Bretscher A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol Biol Cell 11: 1509–1521, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berryman M, Bruno J, Price J, Edwards JC. CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. J Biol Chem 279: 34794–34801, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Blair HC, Schlesinger PH. Purification of a stilbene sensitive chloride channel and reconstitution of chloride conductivity into phospholipid vesicles. Biochem Biophys Res Commun 171: 920–925, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science 250: 399–404, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Bonet ML, Ribot J, Felipe F, Palou A. Vitamin A and the regulation of fat reserves. Cell Mol Life Sci 60: 1311–1321, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford EM, Sartor MA, Gawenis LR, Clarke LL, Shull GE. Reduced NHE3-mediated Na+ absorption increases survival and decreases the incidence of intestinal obstructions in cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 296: G886–G898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 94: 1579–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Duda A, Pierzchalski P, Bielanski W, Hahn EG. Leptin in gastroprotection induced by cholecystokinin or by a meal. Role of vagal and sensory nerves and nitric oxide. Eur J Pharmacol 374: 263–276, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Cadet JL, Braun T, Freed WJ. The dopamine D-2 antagonist, Ro 22–1319, inhibits the persistent behavioral syndrome induced by iminodipropionitrile (IDPN) in mice. Exp Neurol 96: 594–600, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res 105: 89–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA 105: 2017–2021, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulhunty AF, Pouliquin P, Coggan M, Gage PW, Board PG. A recently identified member of the glutathione transferase structural family modifies cardiac RyR2 substate activity, coupled gating and activation by Ca2+ and ATP. Biochem J 390: 333–343, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JC, Cohen C, Xu W, Schlesinger PH. c-Src control of chloride channel support for osteoclast HCl transport and bone resorption. J Biol Chem 281: 28011–28022, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exner C, Hebebrand J, Remschmidt H, Wewetzer C, Ziegler A, Herpertz S, Schweiger U, Blum WF, Preibisch G, Heldmaier G, Klingenspor M. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry 5: 476–481, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Felipe F, Bonet ML, Ribot J, Palou A. Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes 53: 882–889, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem 274: 36488–36497, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Freeman DA, Lewis DA, Kauffman AS, Blum RM, Dark J. Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 287: R97–R103, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, Gillespie PG, Johnson KR. The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci 26: 10188–10198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci USA 96: 14623–14628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280: 12781–12789, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl−/HCO3− exchanger are achlorhydric. J Biol Chem 279: 30531–30539, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol 291: R1303–R1309, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141: 317–329, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hogberg H, Engblom L, Ekdahl A, Lidell V, Walum E, Alberts P. Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity 14: 673–682, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104: 1051–1059, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950 [DOI] [PubMed] [Google Scholar]
- 30.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 80: 829–837, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 15: 741–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landry D, Sullivan S, Nicolaides M, Redhead C, Edelman A, Field M, al-Awqati Q, Edwards J. Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J Biol Chem 268: 14948–14955, 1993 [PubMed] [Google Scholar]
- 34.Landry DW, Akabas MH, Redhead C, Edelman A, Cragoe EJ, Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science 244: 1469–1472, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice]. ScientificWorldJournal 7: 666–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol 294: R803–R810, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Maeda K, Haraguchi M, Kuramasu A, Sato T, Ariake K, Sakagami H, Kondo H, Yanai K, Fukunaga K, Yanagisawa T, Sukegawa J. CLIC4 interacts with histamine H3 receptor and enhances the receptor cell surface expression. Biochem Biophys Res Commun 369: 603–608, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Mercader J, Ribot J, Murano I, Felipe F, Cinti S, Bonet ML, Palou A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 147: 5325–5332, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Miller ML, Judd LM, Van Driel IR, Andringa A, Flagella M, Bell SM, Schultheis PJ, Spicer Z, Shull GE. The unique ultrastructure of secretory membranes in gastric parietal cells depends upon the presence of H+, K+-ATPase. Cell Tissue Res 309: 369–380, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Nishizawa T, Nagao T, Iwatsubo T, Forte JG, Urushidani T. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J Biol Chem 275: 11164–11173, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Novarino G, Fabrizi C, Tonini R, Denti MA, Malchiodi-Albedi F, Lauro GM, Sacchetti B, Paradisi S, Ferroni A, Curmi PM, Breit SN, Mazzanti M. Involvement of the intracellular ion channel CLIC1 in microglia-mediated beta-amyloid-induced neurotoxicity. J Neurosci 24: 5322–5330, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, Yoshida T, Nakamura H, Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J 20: 121–123, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors. Physiol Behav 66: 355–363, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Redhead CR, Edelman AE, Brown D, Landry DW, al-Awqati Q. A ubiquitous 64-kDa protein is a component of a chloride channel of plasma and intracellular membranes. Proc Natl Acad Sci USA 89: 3716–3720, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem 281: 23740–23747, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanks RA, Larocca MC, Berryman M, Edwards JC, Urushidani T, Navarre J, Goldenring JR. AKAP350 at the Golgi apparatus. II. Association of AKAP350 with a novel chloride intracellular channel (CLIC) family member. J Biol Chem 277: 40973–40980, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab 293: E316–E326, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Shin JM, Munson K, Vagin O, Sachs G. The gastric H+, K+ -ATPase: structure, function, and inhibition. Pflügers Arch 457: 609–622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigman HH, Gillich A. Role of hypothermia in the production of gastric ulcers in a rat spinal cord transection model. Dig Dis Sci 26: 60–64, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian “chloride intracellular channels” CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J 274: 6306–6316, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, Shull GE. Stomachs of mice lacking the gastric H,K-ATPase alpha-subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem 275: 21555–21565, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Suginta W, Karoulias N, Aitken A, Ashley RH. Chloride intracellular channel protein CLIC4 (p64H1) binds directly to brain dynamin I in a complex containing actin, tubulin and 14–3-3 isoforms. Biochem J 359: 55–64, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem 279: 4632–4641, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol Regul Integr Comp Physiol 297: R769–R774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschöp M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology 145: 4645–4652, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Tanii H, Hayashi M, Hashimoto K. Effects of neurotropic agents with a selectivity for α-adrenoceptors on nitrile-induced dyskinetic syndrome in mice. Pharmacol Biochem Behav 36: 317–320, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol 174: 1084–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vicente CP, Guaragna RM, Borojevic R. Lipid metabolism during in vitro induction of the lipocyte phenotype in hepatic stellate cells. Mol Cell Biochem 168: 31–39, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res 49: 554–560, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Wang KS, Komar AR, Ma T, Filiz F, McLeroy J, Hoda K, Verkman AS, Bastidas JA. Gastric acid secretion in aquaporin-4 knockout mice. Am J Physiol Gastrointest Liver Physiol 279: G448–G453, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi I, Stephan FK. Body fat reserves attenuate gastric ulcers induced by restricted feeding in rats. Physiol Behav 59: 931–936, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Yi I, Stephan FK. The effects of food deprivation, nutritive and non-nutritive feeding and wheel running on gastric stress ulcers in rats. Physiol Behav 63: 219–225, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, Yao X. Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J Biol Chem 278: 35651–35659, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.