Abstract
The transient receptor potential vanilloid type 1 (TRPV1) channel is a ligand-gated cation channel expressed by sensory nerves. P2Y receptors are G protein-coupled receptors that are also expressed by TRPV1-positive sensory neurons. Therefore, we studied interactions between P2Y receptors and TRPV1 function on kidney projecting sensory neurons. Application of Fast Blue (FB) to nerves surrounding the renal artery retrogradely labeled neurons in dorsal root ganglia of rats. Whole cell recording was performed on FB-labeled neurons maintained in primary culture. Capsaicin was used to activate TRPV1. Four types of kidney projecting neurons were identified based on capsaicin responses: 1) desensitizing (35%), 2) nondesensitizing (29%), 3) silent (3%), and 4) insensitive (30%). Silent neurons responded to capsaicin only after ATP (100 μM) pretreatment. ATP reversed desensitization in desensitizing neurons. Insensitive neurons never responded to capsaicin. UTP, a P2Y purinoceptor 2 (P2Y2)/P2Y4 receptor agonist, reversed capsaicin-induced TRPV1 desensitization. 2-methyl-thio-ATP (2-Me-S-ATP), a P2Y1 receptor agonist, did not change desensitization. MRS 2179 and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), drugs that block P2Y1 receptors, did not block ATP-induced resensitization of TRPV1. Suramin, a P2Y2 receptor antagonist, blocked resensitization caused by UTP. Immunocytochemical studies showed that FB-labeled neurons coexpressed P2Y2 receptors and TRPV1. We conclude that P2Y2 receptor activation can maintain TRPV1 function perhaps during sustained episodes of activity of kidney projecting sensory neurons.
Keywords: dorsal root ganglia, capsaicin, desensitization, retrograde labeling
mammalian transient receptor potential (TRP) channels consist of seven related subfamilies of ion channels that are involved in a variety of physiological and pathophysiological functions (31). The TRP vanilloid 1 (TRPV1) channel, a member of the TRPV subfamily, is a Ca2+-permeable, nonselective cation channel. TRPV1 is expressed predominantly on small- to medium-diameter dorsal root ganglion (DRG) neurons and corresponding C- and Aδ-sensory fibers (29). TRPV1 is a molecular integrator for multiple types of sensory input (6). TRPV1 is activated or regulated by membrane depolarization, noxious heat, vanilloid and endocannabinoid compounds, extracellular protons, and inflammatory mediators (34). Capsaicin is a vanilloid compound that is a TRPV1 agonist used to study TRPV1 function, and prolonged or repeated applications of capsaicin induce TRPV1 desensitization (39). Desensitization of TRPV1 is a Ca2+-dependent process, which is inhibited or enhanced by TRPV1 phosphorylation or dephosphorylation, respectively (9, 21, 26). ATP is an important signaling molecule in the nervous system (11). Extracellular ATP potentiates TRPV1 activity via metabotropic ATP P2Y receptors (33, 41), but the involvement of P2Y receptors in modulation of TRPV1 desensitization has not been established.
Besides the sensory function, TRPV1-positive sensory neurons also have an efferent function. Activation of TRPV1 leads to Ca2+-dependent peptide release from some primary afferent neurons (28). At least 12 different types of transmitters are present in capsaicin-sensitive sensory neurons. CGRP and substance P (SP) are potent vasodilators released from sensory nerves in response to TRPV1 activation by capsaicin (4). Recent evidence supports a role for TRPV1 in control of blood flow and blood pressure via release of vasoactive neuropeptides from sensory nerves supplying the kidney (45, 46). This can initiate centrally directed afferent nerve activity and changes in renal blood flow and kidney function (16, 48). TRPV1 can also participate in a local efferent function of perivascular sensory nerves (19, 35). TRPV1 activation causes a local depolarization of sensory nerve endings (generator potential) and an increase in nerve terminal calcium. The generator potential may not reach action potential threshold and fail to generate afferent nerve activity (39). However, the increase in nerve terminal calcium causes neuropeptide release producing local vasodilation (19, 39). Therefore, TRPV1-positive sensory nerve fibers have the potential to regulate local blood flow in the absence of centrally directed afferent nerve activity.
The kidney contributes to blood pressure regulation and salt sensitivity (36). Recent studies showed that TRPV1-positive sensory nerves in the kidney enhance renal excretory function (49). Impaired renal TRPV1 activity increases salt sensitivity and development of salt-sensitive hypertension (25). Therefore, studies of TRPV1 and its regulation are important to our understanding of blood pressure regulation and salt-sensitive hypertension. Individual DRG contain sensory afferent neurons that supply multiple visceral organs (6, 20, 27). Retrograde tracing techniques have been used to identify the sources of afferent and efferent nerves supplying the kidney in rats (10, 14). Calcium channel function has been studied in sympathetic neurons supplying the kidney (43), but the functional properties of kidney projecting sensory neurons have not been studied. We used a retrograde labeling technique to identify kidney projecting sensory neurons, enabling us to study interactions between P2Y receptors and TRPV1 function in sensory neurons supplying the kidney.
MATERIALS AND METHODS
Animals.
All animal use protocols were reviewed and approved by the All University Committee for Animal Use and Care at Michigan State University. Normal Wistar rats (male, 250–350 g, Charles River Laboratories, Portage, MI) were used in the present study. All rats were provided with food and tap water ad libitum in a temperature-controlled room with a 12:12-h light-dark cycle.
Retrograde labeling.
Rats were anesthetized using isoflurane inhalation. A left flank incision was made. The renal nerve bundles were dissected free of the renal artery and surrounding tissue and crushed with the sharp end of a needle. A small sheet of parafilm was placed beneath usually 1–2 nerve bundles to avoid leakage of dye. Fast Blue (FB; 1 μl of a 2% solution in 2% acetic acid) (Polysciences, Warrington, PA) was applied to the nerve bundles for 0.5 h, after which, the excess dye was blotted off, and the nerves were washed with sterile normal saline. The nerves were placed back on the surface of the renal artery. Ticarcillin (1 ml) solution was applied locally for bacterial prophylaxis, and the incision was closed with 3.0 silk. Carprofen (Rimadyl, 5 mg/kg im) was given once daily for 3 days after the surgery to relieve surgical pain.
Isolation of sensory neurons.
Six days after surgery and FB labeling, rats were deeply anesthetized using pentobarbital sodium (50 mg/kg ip), and a pneumothorax was performed, and the abdominal aorta was severed. The left dorsal root ganglia (T10-L2) were rapidly removed from the animal. Then they were moved to Hanks' balanced salt solution containing papain (1.1 mg/ml, activity = 33 units/mg; Worthington, Lakewood, NJ) and dithioerythritol (1 mg/ml) (Calbiochem, Los Angeles, CA) and incubated in a 37°C shaking bath for 15 min. Ganglia were moved to HBSS containing collagenase type I (1 mg/ml, activity = 125 units/mg; Worthington, Lakewood, NJ) and trypsin inhibitor (0.75 mg/ml) (Sigma, St. Louis, MO) at 37°C for 10 min. The ganglia were triturated using long-neck fire-polished pipettes with sequentially smaller tip sizes. After trituration, enzyme activity was blocked by the addition of tissue culture medium (DMEM; Sigma) containing 4% FBS, and the suspension was centrifuged for 3 min at 900 g. The supernatant was discarded, and 5 ml of culture medium was added before centrifuging again for 3 min. Finally, the supernatant was discarded, and the pellet containing neurons was resuspended using DMEM supplemented with 4% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml). The neurons were plated on eight poly-l-lysine (50 μg/ml)-coated round coverslips (∼500 neurons/coverslip) in DMEM with 4% FBS and antibiotics and incubated at 37°C in 5% CO2 overnight before electrophysiological studies.
Immunohistochemical localization of TRPV1, CGRP, and P2Y receptors in kidney projecting DRG neurons.
DRG neurons were fixed using Zamboni's solution for 20 min at room temperature. Then, the cells were washed with 0.1 M PBS and then incubated in PBS with blocking serum diluted in Triton-X100 (0.2%) for 1 h. Tissues were then incubated overnight at 4°C in diluted primary antibodies. Polyclonal goat anti-TRPV1 (1:200), polyclonal rabbit anti-P2Y1 (1:400), or polyclonal goat anti-P2Y purinoceptor 2 (P2Y2; 1:200) (Santa Cruz Biotechnology, Santa Cruz, CA) were used. The next day, the cells were washed with PBS and incubated 1 h at room temperature with diluted secondary antibodies conjugated with FITC or Cy3 (Jackson ImmunoResearch, West Grove, PA). Cells then were washed in PBS, mounted on slides, and examined using a Nikon TE2000-U inverted microscope. Photographs were taken using a SPOT Insight Color Mosaic camera (Mager Scientific) with MetaImaging Series software. Controls with no primary antibodies were used to ensure that binding is specific.
Whole cell voltage clamp.
FB-labeled sensory neurons were visualized using epifluorescence optics attached to Olympus IX70 inverted microscope at UV excitation (360 nm). Membrane currents were recorded using the conventional whole cell configuration of the patch-clamp technique (18). Whole-cell recordings were made with 4–6 MΩ electrodes mounted on the head stage of an Axopatch 1D patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). The membrane was voltage clamped at −70 mV, and currents were filtered at 2 kHz and sampled at 5 kHz by an A/D converter (Digidata 1322A, Molecular Devices) in conjunction with a personal computer and stored on the hard disk of the PC. Membrane currents were expressed as current amplitude over cell capacitance (pA/pF) to normalize current amplitudes from neurons of different sizes. The standard pipette solution contained (in mM): 122.5 K-aspartate, 20 KCl, 1 MgCl2, 2 MgATP, 10 HEPES, and 10 EGTA (pH 7.3 with KOH). The external solution contained (in mM): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 12 glucose, and 10 HEPES (pH 7.4 with NaOH). Drugs were applied via a pipette with a tip diameter of 100 μm, positioned within 100 μm of the cell body. Solution changes through the drug pipette were gated using solenoid-controlled valves, and drug solution changes occurred within 500 ms.
Statistical analysis.
Data are means ± SE. Differences among groups were analyzed using one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. Differences were considered statistically significant at P < 0.05, and n values refer to the number of neurons from which the data were obtained.
RESULTS
Localization of kidney projecting neurons in DRG.
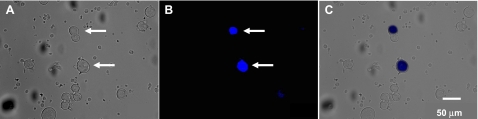
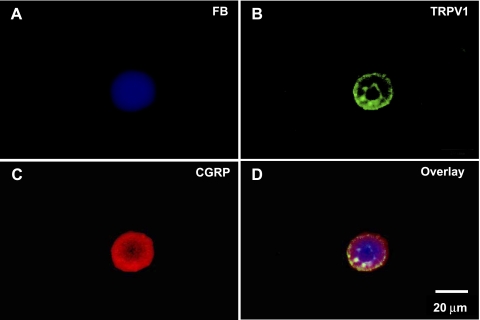
DRG neurons (T10-L2 segments) and nodose ganglion neurons were isolated for primary culture, and FB-labeled sensory neurons were visualized under fluorescence microscope (Fig. 1). About 5% of the DRG neurons ipsilateral to the FB application side were FB-labeled, but no FB-labeled neurons were detected in contralateral DRG. Immunocytochemical studies revealed that TRPV1 and CGRP were colocalized in all FB-labeled, kidney projecting DRG neurons (Fig. 2).
Fig. 1.
Isolated dorsal root ganglion (DRG) neurons retrogradely labeled by Fast Blue (FB). A: DRG neurons under bright field illumination 1 wk after FB application. B: FB-labeled DRG neurons revealed using UV epifluorescence illumination, same field as A. Arrows indicate FB-labeled neurons. C: overlay of A and B.
Fig. 2.
Colocalization of transient receptor potential vanilloid 1 (TRPV1) and CGRP on kidney projecting sensory neurons using fluorescence microscopy. A: kidney projecting sensory neuron labeled by FB (blue). B: TRPV1- immunostaining (green) on the same neuron. C: CGRP-immunostaining (red) on the same neuron. D: overlay picture combining A, B, and C.
Subtypes of kidney projecting sensory nerves identified by capsaicin responses.
Four types of kidney projecting sensory neurons could be discriminated on the basis of properties of capsaicin (1 μM)-induced inward currents. The first type of neuron exhibited a desensitizing capsaicin response (Fig. 3A) when capsaicin was applied for 6 s at 2-min intervals; this response occurred in 66 of 189 neurons (35%) studied. The second type of neuron exhibited a nondesensitizing capsaicin response (Fig. 3B), in which repeated capsaicin applications did not cause obvious TRPV1 desensitization. In these neurons, capsaicin was applied at least 6 times at 2-min intervals, and there was no decline in current amplitude. This type of response was detected in 55 of 189 neurons (29%). The third type of neuron exhibited a “silent” capsaicin-induced response (Fig. 3C). Capsaicin only caused an inward current after these neurons were pretreated with extracellular ATP; this response occurred in 11 of 189 neurons (6%). The fourth type was TRPV1 negative (Fig. 3D), in which capsaicin did not evoke an inward current, even after ATP pretreatment; this group composed 30% of the 189 neurons studied.
Fig. 3.
Four types of kidney projecting sensory neurons based on capsaicin reactivity. Traces are representative recordings obtained from each type of neuron. A: repeated applications of capsaicin (CAP; 1 μM) cause TRPV1 desensitization. B: in the second type of neuron, TRPV1 is not desensitized by repeated applications of capsaicin. C: in the third type of neuron, capsaicin does not induce a TRPV1 response until after pretreatment with ATP (100 μM). D: capsaicin does not cause a TRPV1 response, even after ATP pretreatment. Responses are representative of recordings obtained from 189 kidney projecting sensory neurons.
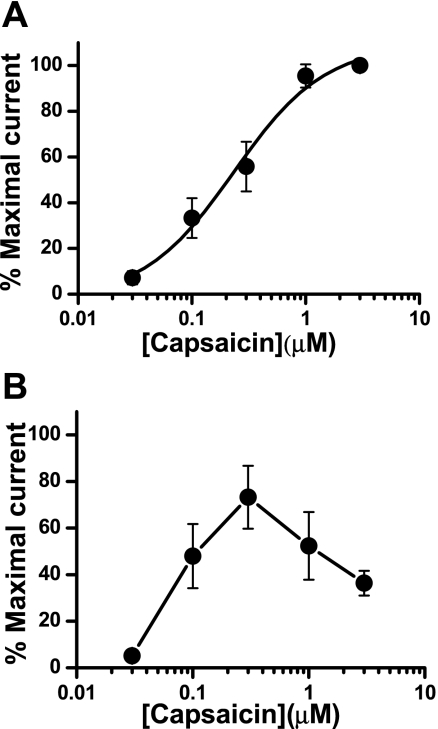
Capsaicin concentration response curve in kidney projecting sensory neurons.
We next constructed capsaicin concentration-response curves (0.03–3 μM) for TRPV1 activation in kidney projecting sensory neurons. There were two types of capsaicin-responsive neurons. In the first type, repeated capsaicin application did not desensitize TRPV1. The amplitude of capsaicin-induced TRPV1 currents increased in a concentration-dependent manner (n = 16, EC50 = 0.23 μM) (Fig. 4A). In the second type of neuron, TRPV1 desensitization occurred at higher capsaicin concentrations (n = 9) (Fig. 4B). Further studies of TRPV1 function were done using capsaicin at a concentration of 1 μM, as this was near the maximum of the concentration response curve for both types of neurons.
Fig. 4.
Concentration response curves for capsaicin-induced activation of TRPV1 on kidney projecting sensory neurons. A: concentration-response curve in neurons (n = 16 neurons from 9 rats) exhibiting nondesensitizing TRPV1 responses. B: concentration response obtained from neurons (n = 9 neurons from 7 rats), exhibiting desensitizing TRPV1 responses. Data are expressed as means ± SE and are currents normalized to the maximum response obtained in each neuron.
ATP resensitizes TRPV1 after capsaicin-induced desensitization.
Previous studies showed that extracellular ATP potentiates capsaicin-induced TRPV1 currents via an action at metabotropic P2Y1 or P2Y2 receptors (33, 41). We speculated that ATP might also modulate TRPV1 desensitization-resensitization, and this effect would be mediated by P2Y1 or P2Y2 receptors. Sensory neurons express P2X receptors, which mediate rapidly desensitizing inward currents (23). We detected rapidly desensitizing ATP-induced currents in some kidney projecting sensory neurons. However, these currents completely decayed during ATP application and did not overlap with currents caused by capsaicin in our protocol. In our studies, we focused on the neurons exhibiting rapidly desensitizing capsaicin responses. Capsaicin was applied for 6 s three consecutive times at 2-min intervals. In control neurons, the capsaicin current declined in amplitude during each application, and the current caused by the third capsaicin application was significantly smaller than the first (n = 6, P < 0.01, Fig. 5, A and B). However, ATP (100 μM) applied for 6 s prior to the third capsaicin application fully restored the capsaicin response (Fig. 5, C and D). In the absence of ATP, TRPV1 was desensitized again, and TRPV1 currents evoked by capsaicin were significantly smaller than first response (not shown, n = 7, P < 0.01). ATP pretreatment did not affect the amplitude of capsaicin-induced currents in the nondesensitizing neurons (n = 5, not shown).
Fig. 5.
Capsaicin-induced TRPV1 desensitization and reversal caused by ATP on kidney projecting sensory neurons. A: representative trace shows that repeated capsaicin applications induce TRPV1 desensitization. B: mean data of experiments illustrated in A. Data are expressed as means ± SE; n = 6 neurons from 4 rats. C: representative trace shows that ATP (100 μM) reversed capsaicin-induced TRPV1 desensitization. D: mean data of experiments illustrated in C. Data are expressed as means ± SE; n = 7 neurons from 6 rats. CAP1, CAP2, and CAP3 represent the sequence of capsaicin applications *P < 0.01 vs. first capsaicin application. #P < 0.01 vs. second capsaicin application.
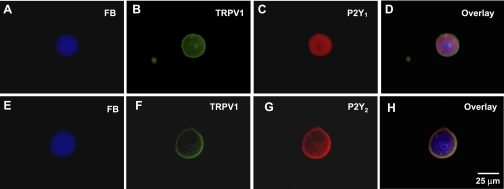
Localization of P2Y1 and P2Y2 receptors to kidney projecting sensory neurons.
We next used immnocytochemical methods to identify P2Y receptors expressed by FB-labeled sensory neurons. We found that FB-labeled neurons coexpressed TRPV1, P2Y1, and P2Y2 receptors (Fig. 6). There was also a differential distribution of P2Y1 and P2Y2 labeling in kidney projecting sensory neurons. P2Y1 labeling was predominantly intracellular (Fig. 6, C and D), while P2Y2 labeling was predominantly in the cell membrane (Fig. 6, G and H).
Fig. 6.
Colocalization of TRPV1 and P2Y receptor subtypes on kidney projecting sensory neurons. A, E: kidney projecting sensory neurons labeled by FB. B, F: TRPV1-immunostaining in the same neurons shown in A and E. C: P2Y1 receptor immunostaining in the same neuron shown in A and B. G: P2Y2 receptor immunostaining in the same neuron shown in E and F. D, H: Overlay pictures.
P2Y2 receptors mediate TRPV1 resensitization.
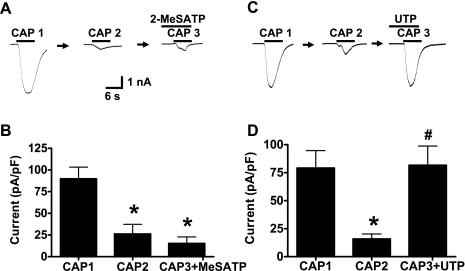
To identify the ATP receptor subtypes responsible for ATP-induced TRPV1 resensitization, we first examined the effect of 2-Me-S-ATP. 2-Methyl-thio-ATP (2-Me-S-ATP) is a P2Y1 receptor agonist (42). Capsaicin was applied twice to kidney projecting sensory neurons, which were then treated for 6 s with 2 Me-S-ATP (100 μM) prior to a third capsaicin application. 2-Me-S-ATP did not restore the capsaicin response (n = 6; P < 0.01) (Fig. 7, A and B). UTP is an agonist at P2Y2 and P2Y4 receptors (41). UTP (50 μM) restored capsaicin-induced TRPV1 currents in neurons that were previously desensitized to capsaicin (n = 6) (Fig. 7, C and D).
Fig. 7.
ATP-induced TRPV1 resensitization is mediated by P2Y2 receptors on kidney projecting sensory neurons. A: representative recordings showing that the P2Y1 receptor agonist, 2-Me-S-ATP (100 μM) does not reverse capsaicin-induced TRPV1 desensitization. B: mean data of experiments illustrated in A. Data are expressed as means ± SE; n = 6 neurons from 4 rats. C: representative trace shows that UTP (50 μM) reverses capsaicin-induced TRPV1 desensitization. D: mean data of experiments illustrated in C. Data are expressed as means ± SE; n = 6 neurons from 6 rats. *P < 0.01 vs. first application of capsaicin. #P < 0.01 vs. second application of capsaicin.
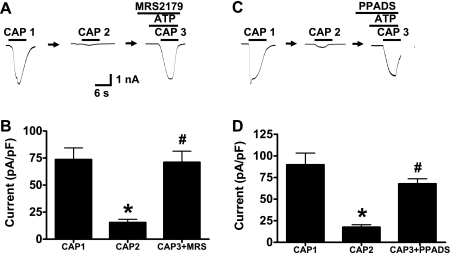
MRS 2179 is a selective P2Y1 receptor antagonist (42). After pretreatment with MRS 2179 (10 μM) for 2 min, ATP reversed TRPV1 desensitization as the amplitude of capsaicin-induced currents in the presence of ATP and MRS 2179 was not different from the initial response (n = 6) (Fig. 8, A and B). PPADS (10 μM) blocks P2Y1 but not P2Y2 receptors. PPADS did not block ATP-induced reversal of TRPV1 desensitization (Fig. 8, C and D).
Fig. 8.
ATP-induced TRPV1 resensitization is not blocked by P2Y1 receptor or non-P2Y2 receptor antagonist. A: representative recordings showing that the P2Y1 receptor antagonist, MRS 2179 (10 μM), does not block ATP-induced TRPV1 resensitization. B: mean data of experiments illustrated in A. Data are expressed as means ± SE; n = 6 neurons from 3 rats. C: representative trace shows that pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (10 μM) does not block ATP-induced TRPV1 resensitization. D: mean data of experiments illustrated in C. Data are expressed as means ± SE; n = 4 neurons from 2 rats. *P < 0.01 vs. first application of capsaicin. #P < 0.01 vs. second application of capsaicin.
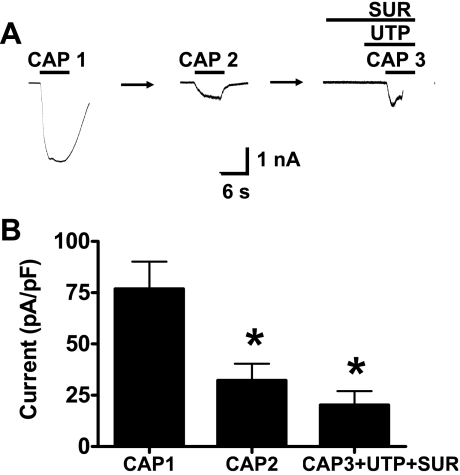
Suramin blocks P2Y2, but not P2Y4, receptors (8). After capsaicin-induced desensitization of TRPV1, neurons were pretreated with suramin (30 μM) for 10 s and UTP for 6 s. Under these conditions, UTP did not reverse TRPV1 desensitization (n = 6) (Fig. 9, A and B).
Fig. 9.
Suramin (SUR), a P2Y2 receptor antagonist, blocks UTP-induced resensitization of TRPV1. A: representative recording showing that suramin (30 μM) blocks the resensitization of TRPV1 caused by UTP. B: mean data of experiments illustrated in A. Data are expressed as means ± SE; n = 6 neurons from 5 rats. *P < 0.01 vs. first application of capsaicin.
DISCUSSION
Localization of kidney projecting sensory neurons.
The kidney in rats is supplied by sensory nerves predominantly from ipsilateral DRG (12, 14). Our data are consistent with these findings as we only found FB-labeled neurons in ipsilateral DRG at the lower thoracic and upper lumbar spinal levels. We did not detect FB-labeled neurons in contralateral DRG.
P2Y2 receptors modulate TRPV1 function in kidney projecting sensory neurons.
Extracellular ATP modulates sensory neuron function either through ionotropic P2X receptors or metabotropic P2Y receptors (32, 36). Activation of P2Y1 or P2Y2 receptors potentiates capsaicin-induced TRPV1 currents, which contributes to hyperalgesia (33, 41). Sensory neurons in the DRG are heterogenous, and the target tissues or organs of those neurons modify the phenotype and function of sensory neurons (27). This heterogeneity makes it difficult to explore the characteristics of a receptor as the properties can vary among neurons supplying different target tissues. For example, some studies show that extracellular ATP potentiates TRPV1-mediated thermal sensation via an action at P2Y1 receptors (41). However, others have found that P2Y2 receptors mediate ATP-induced thermal hyperalgesia (33). Using retrograde labeling, we identified the subpopulation of sensory neurons supplying the kidney. Therefore, we minimized variability that might occur across DRG neurons supplying different target tissues by studying only those neurons that supply the kidney.
Our data show that brief pretreatment of neurons with ATP reversed TRPV1 desensitization in kidney projecting sensory neurons. We used a pharmacological approach to determine which P2Y receptor mediated TRPV1 resensitization. Our agonist studies revealed that UTP, a P2Y2/P2Y4 receptor agonist but not 2-Me-S-ATP, a P2Y1 receptor agonist, restored TRPV1 function after desensitization. Although 2-Me-S-ATP activates P2Y1 receptors, it can also activate other P2 receptors. Therefore, we used a selective P2Y1 antagonist (MRS 2179) and PPADS, an antagonist that blocks P2Y1 but not P2Y2 receptors (42). Neither MRS 2179 or PPADS blocked ATP-induced TRPV1 resensitization. Finally, we found that suramin, an antagonist that blocks P2Y2 but not P2Y4 receptors (8) blocked UTP-induced resensitization of TRPV1. Suramin can block multiple P2 receptors, and results obtained with this drug need to be interpreted cautiously. However, when suramin is used in conjunction with other P2Y receptor agonists and antagonists, it can provide some information about the contribution of P2Y2 receptors to a particular response. Therefore, we conclude that P2Y2 receptors link to modulation of TRPV1 in kidney projecting sensory neurons. This response could occur via P2Y2-linked activation of PKC, which is known to reverse capsaicin-induced desensitization of TRPV1 via channel phosphorylation (29). If this is true, resensitization occurs within just a few seconds, so P2Y2 receptors and TRPV1 channels must be closely localized in the plasma membrane of kidney projecting sensory neurons. These pharmacological data are supported by our immunocytochemical data. Although kidney projecting sensory neurons express both P2Y1 and P2Y2 receptors, only P2Y2 receptors are localized to the plasma membrane where they could respond to extracellular ATP. This result needs to be confirmed by studies directly assessing membrane localization of P2Y2 receptors.
Functional states of TRPV1 in kidney projecting sensory neurons.
One of the characteristic properties of TRPV1 is capsaicin-induced desensitization. One factor affecting TRPV1 desensitization is capsaicin concentration (40). Our data in kidney projecting sensory neurons also show that TRPV1 desensitization by capsaicin is concentration dependent, as, for example, concentrations of capsaicin, <1 μM produce little TRPV1 desensitization.
When we used the maximum concentration of capsaicin (1 μM), we found that TRPV1 occupies one of several function states in kidney projecting sensory neurons. Capsaicin did not cause an inward current in 30% of kidney projecting neurons; it is likely that this latter group of cells does not express TRPV1. In 35% of kidney projecting sensory neurons, brief applications of a maximum concentration of capsaicin caused TRPV1 desensitization; this is the conventional capsaicin-induced response. In this group of neurons, TRPV1 function is regulated by its phosphorylation state, as described above, and by phosphatidylinositol 4,5-biphosphate (PIP2), which can enhance or inhibit TRPV1 function (26). In 29% of kidney projecting neurons, repeated applications of maximum concentrations of capsaicin produced stable amplitude inward currents. In these neurons, it is possible that constitutive intracellular signaling activity is shifted toward a state that promotes maintained TRPV1 activity (high phosphorylation, low phosphatase activity, and high PIP2 levels) (29, 31, 37). In 6% of neurons, capsaicin only elicited an inward current after ATP pretreatment, suggesting that there may be a high constitutive phosphatase/phosphorylation ratio in these neurons. Studies in heterologous expression systems have shown that there are potential multiple intracellular signaling pathways by which TRPV1 function can be modulated (2, 7, 22). Multiple mechanisms might contribute to different TRPV1 responses upon repeated applications of capsaicin on kidney projecting sensory neurons. It is also important to note that even brief applications (<6 s) were capable of either resensitizing TRPV1 in the first group of neurons or sensitizing TRPV1 to capsaicin. This result suggests that TRPV1 function in kidney projecting sensory neurons is dynamic, and its function can be modulated on a moment-to-moment basis.
Perspectives and Significance
TRPV1 is widely expressed in the nervous system, functioning as a molecular integrator for multiple types of sensory input. However, TRPV1-expressing sensory nerves can also have an efferent function. We showed that TRPV1 is expressed by sensory nerves supplying the renal artery and that these nerves coexpress CGRP. This is similar to previous data showing that TRPV1 is expressed by sensory nerves supplying the vasculature (1) and TRPV1 activation causes vasodilation through release of CGRP and SP from sensory nerves (45). TRPV1 activation in the kidney increases glomerular filtration rate and sodium/water excretion (24, 48) and TRPV1 agonists prevent ischemia/reperfusion-induced renal injury (42). TRPV1 in the kidney is also activated by high sodium levels, and this leads to increased sodium excretion, which prevents increases in blood pressure. This mechanism is compromised in Dahl salt-sensitive rats and likely contributes to the sodium-dependent hypertension in this strain of rat (46). Our data show that TRPV1 function in kidney projecting sensory neurons is modulated by extracellular ATP, and this modulation occurs within seconds in the nerve cell body. There are several potential sources for ATP in dorsal root ganglia that could serve to modulate TRPV1 function there. ATP could be released by glial cells in ganglia during periods of high neuronal activity (5). The increased extracellular ATP acting at P2Y2 receptors on dorsal root ganglion neurons could help to maintain TRPV1 function. Inflammatory cells and endothelial cells lining arterioles in dorsal root ganglia are also potential sources of extracellular ATP (5). Previous work showed that ATP acting at P2Y receptors can sensitize sensory neurons to the excitatory effects of capsaicin (41). It has been suggested that ATP released near the nerve terminals of these sensory neurons might also sensitize TRPV1 expressed by the same nerve terminals (5, 41). It is reasonable to speculate that a similar interaction might occur at the peripheral ends of kidney projecting sensory neurons, where locally released ATP could modulate TRPV1 function on sensory nerves and, therefore, modulate release of vasodilator peptides. Confirmation of this speculation will require additional studies that test directly the effects of ATP release from sympathetic nerves on sensory nerve function in the renal vasculature.
GRANTS
This work was supported by National Institutes of Health Grants HL P01 HL70687 (to J. J. Galligan), R01HL073287 (to D. H. Wang) and American Heart Association Midwest Affiliate Predoctoral Fellowship, 0715734Z (to H. Wang).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Arulmani U, Heiligers JP, Garrelds IM, Sanchez-Lopez A, Willems EW, Villalon CM, Saxena PR. Effects of sumatriptan on capsaicin-induced carotid haemodynamic changes and CGRP release in anaesthetized pigs. Cephalalgia 24: 717–727, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bossowska A. Distribution of primary afferent neurons associated with the porcine inferior mesenteric ganglion (IMG). Folia Histochem Cytobiol 40: 367–372, 2002 [PubMed] [Google Scholar]
- 4.Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev 38: 179–226, 1986 [PubMed] [Google Scholar]
- 5.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol 194: 333–392, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23: 617–624, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. Cloned and transfected P2Y4 receptors: Characterization of a suramin and PPADS-insensitive response to UTP. Br J Pharmacol 119: 1301–1303, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Arch 431: 828–837, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Donovan MK, Wyss JM, Winternitz SR. Localization of renal sensory neurons using the fluorescent dye technique. Brain Res 259: 119–122, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Ferguson M, Bell C. Substance P-immunoreactive nerves in the rat kidney. Neurosci Lett 60: 183–188, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson M, Ryan GB, Bell C. Localization of sympathetic and sensory neurons innervating the rat kidney. J Auton Nerv Sys 16: 279–288, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Fredholm BB. Purinoceptors in the nervous system. Pharmacol Tox 76: 228–239, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Gattone VH, 2nd, Marfurt CF, Dallie S. Extrinsic innervation of the rat kidney: a retrograde tracing study. Am J Physiol Renal Fluid Electrolyte Physiol 250: F189–F196, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57: 125–130, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Gontijo JR, Smith LA, Kopp UC. CGRP activates renal pelvic substance P receptors by retarding substance P metabolism. Hypertension 33: 493–498, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Lozano-Cuenca J, Villalon CM, de Vries R, Garrelds IM, Avezaat CJ, van Kats JP, Saxena PR, MaassenVanDenBrink A. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn-Schmiedeberg's Arch Pharmacol 375: 29–38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res 99: 119–31, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kandel ERSJ, Jessell TM. Principles of Neural Science New York: McGraw-Hill, 2000, p. 431–433 [Google Scholar]
- 21.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 17: 3525–3537, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar J, Szabo T, Kovacs L, Blumberg PM, Biro T. Distinct features of recombinant rat vanilloid receptor-1 expressed in various expression systems. Cell Mol Life Sci 60: 2228–2240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377: 432–435, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wang DH. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol Res 57: 239–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Wang DH. Role of TRPV1 channels in renal haemodynamics and function in Dahl salt-sensitive hypertensive rats. Exper Physiol 93: 945–953, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 4835–4843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res 41: 355–363, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Maggi CA. The pharmacological modulation of neurotranmitter release. In: Capsaicin in the Study of Pain, edited by Wood J. New York: Harcourt Brace & Company, 1993, p. 161–189 [Google Scholar]
- 29.Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium 35: 471–478, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 19: 1844–1854, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280: 13424–13432, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Montell C. The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 23: 6058–6062, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilius B, Voets T, Peters J. TRP channels in disease. Sci STKE 2005: re8, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Ralevic V, Kendall DA. Cannabinoid modulation of perivascular sympathetic and sensory neurotransmission. Curr Vasc Pharmacol 7: 15–25 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rettig R, Uber A. Hypertension and the kidney: new insights from results of renal transplantation studies. Nephrol Dial Transplant 10Suppl 9: 9–16, 1995 [PubMed] [Google Scholar]
- 37.Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol 37: 153–163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawynok J, Sweeney MI. The role of purines in nociception. Neuroscience 32: 557–569, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 38: 378–384, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999 [PubMed] [Google Scholar]
- 41.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors (P2Y1) as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA 98: 6951–6956, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda K, Tsuji F, Hirata T, Takaoka M, Matsumura Y. Preventive effect of TRPV1 agonists capsaicin and resiniferatoxin on ischemia/reperfusion-induced renal injury in rats. J Cardiovasc Pharmacol 51: 513–520, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Vari RC, Emaduddin M, Schofield GG. Renal function and Ca2+ currents after dye-labeling identification of renal sympathetic neurons. Am J Physiol Regul Integr Comp Physiol 277: R1513–R1521, 1999 [DOI] [PubMed] [Google Scholar]
- 44.von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol 362: 310–323, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Wang DH. The vanilloid receptor and hypertension. Acta pharmacologica Sinica 26: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension 47: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exper Physiol 94: 31–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie C, Sachs JR, Wang DH. Interdependent regulation of afferent renal nerve activity and renal function: role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J Pharmacol Exper Ther 325: 751–757, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Wang Y, Wang DH. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension 46: 992–997, 2005 [DOI] [PubMed] [Google Scholar]