Abstract
A sedentary lifestyle is a major risk factor for cardiovascular disease, and rates of inactivity and cardiovascular disease are highly prevalent in our society. Cardiovascular disease is often associated with overactivity of the sympathetic nervous system, which has both direct and indirect effects on multiple organ systems. Although it has been known for some time that exercise positively affects the brain in terms of memory and cognition, only recently have changes in how the brain regulates the cardiovascular system been examined in terms of physical activity and inactivity. This brief review will discuss the evidence for physical activity-dependent neuroplasticity related to control of sympathetic outflow. It will focus particularly on recent studies from our laboratory and others that have examined changes that occur in the rostral ventrolateral medulla (RVLM), considered one of the primary brain regions involved in the regulation and generation of sympathetic nervous system activity.
Keywords: exercise, inactivity, blood pressure regulation
physical inactivity is a major risk factor for cardiovascular disease (106), the leading cause of death in the United States (62, 84). Despite this relationship, rates of physical inactivity are prevalent in our society (107), and recent studies indicate that more people are dying as a result of physical inactivity than any other preventable risk factor (12). Inactivity-related diseases also continue to produce an increasing economic burden on our health care system. Direct costs for cardiovascular disease associated with inactivity have been estimated at more than 23 billion dollars per year (110). Similarly, the cost of inactivity-related diseases, such as hypertension has been estimated to be over 63 billion dollars (106). In spite of this substantial investment, as many as two-thirds of hypertensive patients have poorly controlled blood pressure (18). It is critical then that we continue to further identify the mechanisms by which major cardiovascular risk factors such as physical inactivity contribute to cardiovascular disease. New insights into mechanisms that predispose individuals to cardiovascular disease could lead to new therapeutic strategies designed to combat cardiovascular disease and thereby lessen the burden on the population and our health care system.
Cardiovascular diseases are often characterized by overactivity of the sympathetic nervous system, and this is apparent in both humans and laboratory animal models (4, 29, 31, 35, 52, 90, 100, 112). Sympathetic nervous system activity is influenced by multiple sites within the brain (22, 35). Several of these brain regions have been shown to be altered in models of physical activity and inactivity, including (but not limited to) the nucleus tractus solitarius, paraventricular nucleus of the hypothalamus, and the rostral ventrolateral medulla (41, 68, 71, 74, 77, 80, 81). These alterations occur across a variety of animal models in which physically active animals (treadmill or spontaneous running) have been compared with animals under “normal” cage conditions and termed sedentary (68, 74, 80, 81). In addition, animals under “normal” cage conditions have also been compared with hindlimb unloaded rats, a model of spaceflight or bedrest inactivity (41). These alterations can be termed “physical activity-dependent neuroplasticity” and could contribute importantly in the prevention or development of cardiovascular disease in physically active or sedentary individuals, respectively (74). Because the rostral ventrolateral medulla (RVLM) has been implicated in several disease states associated with sympathetic overactivity, it is highly likely that the RVLM is involved in altered sympathetic regulation in sedentary vs. physically active individuals and the predisposition toward chronic disease associated with an inactive lifestyle.
Sympathetic Overactivity and Cardiovascular Disease
When sedentary subjects (humans or animals) are compared with physically active “controls,” the sedentary groups may exhibit enhancement in risk factors for cardiovascular disease, including increased resting and baroreflex-mediated sympathoexcitation (19, 23, 27, 33, 58, 67, 79, 89, 113). Similarly, studies in laboratory animals suggest that remaining sedentary increases vascular reactivity (16, 50, 97), decreases insulin sensitivity (59), increases visceral adiposity (60), and other markers for cardiovascular disease (14). While differences in resting blood pressure and sympathetic nervous system activity are often not observed between sedentary and physically active humans (86, 87, 93, 94, 105), there is strong evidence that inactivity-related diseases such as hypertension, obesity, and diabetes are associated with overactivity of the sympathetic nervous system (29, 35, 52, 90, 100). Furthermore, increasing physical activity under these conditions of sympathetic overactivity, such as heart failure, has been shown to lower resting sympathetic nerve activity in humans (24, 32, 89) and laboratory animals (113). Overactivity of the sympathetic nervous system can produce detrimental effects on both cardiovascular and noncardiovascular target organs and thus may contribute to a variety of disease states (31). In addition, there is growing evidence that the mechanisms by which physical activity produces beneficial effects (and physical inactivity produces detrimental effects) may extend beyond those associated with more traditional risk factors (51). One possibility involves alterations in central neural networks that regulate sympathetic outflow (51, 74). Collectively, the current evidence suggests that remaining sedentary may increase the propensity for cardiovascular disease via effects on brain regions important in sympathetic nervous system regulation.
Physical (In)activity-Dependent Neuroplasticity in Sympathetic Nervous System Regulation
Over the past 10 years, increasing evidence indicates that physical activity alters neuronal structure and function in brain regions involved in learning and memory (30, 56, 82, 109). This physical activity-dependent neuroplasticity has generally been thought to be restricted to higher brain regions such as the hippocampus (109). However, recent work suggests that physical activity- and inactivity-dependent changes occur in brain regions important in blood pressure regulation (41, 55, 68, 73, 74, 80, 81, 111, 113). A significant amount of the evidence for central nervous system mechanisms has come from models in which cardiovascular disease is already evident (e.g., hypertension and heart failure) (41, 55, 68, 73, 111, 113). Although these studies are highly clinically relevant and suggest reversal of changes associated with disease states, there is also increasing evidence that in the absence of overt disease, physical activity or inactivity alone can produce central alterations that influence sympathetic nervous system regulation (74, 80, 81). Our recent study comparing spontaneous wheel running to “normal” cage activity emphasized this point and suggested that a sedentary lifestyle alone or in combination with other cardiovascular risk factors may contribute to cardiovascular disease via influences on central structures involved in regulation of the sympathetic nervous system (75). We have focused our efforts on the RVLM as a key brain region involved in the generation of sympathetic outflow.
Role of the RVLM in Health and Disease
The RVLM is considered one of the most important brain regions involved in control of basal and reflex changes in activity of the sympathetic nervous system (20, 21, 35, 38). Under normal conditions, bulbospinal neurons in the RVLM play a key role in the integration and generation of central sympathetic drive via projections to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord (21, 36, 37). The activity of RVLM neurons is regulated by both excitatory and inhibitory neurotransmitters (21, 38). Although a number of neurotransmitters have been localized in the RVLM (85, 102), glutamate and GABA appear to be the primary excitatory and inhibitory neurotransmitters, respectively. Sympathoexcitatory neurons in the RVLM have been classified into two major groups based on the presence (C1 neurons) or absence (non-C1 neurons) of phenylethanolamine-N-methyl transferase (PNMT), the enzyme responsible for the synthesis of epinephrine (21, 38, 65, 69, 104). All C1 neurons are generally considered sympathoexcitatory and comprise up to 70% of barosensitive RVLM neurons that project to the spinal cord (91, 104). Specific lesioning of C1 cells reduces reflex sympathoexcitation and diminishes responsiveness of the RVLM to microinjections of glutamate (63, 64, 92). However, generalized inhibition of the RVLM is required to eliminate a variety of cardiovascular reflexes and decrease blood pressure and sympathetic nerve activity to levels observed after complete spinal cord transection or ganglionic blockade (20, 36, 48). These data imply that the RVLM and, in particular, both C1 and non-C1 neurons within the RVLM, are critically important in the maintenance and activation of sympathetic nervous system activity and control of blood pressure (20, 21, 38).
A growing body of literature indicates that the RVLM is also involved in pathophysiological increases in the activity of the sympathetic nervous system (11, 46, 47, 78, 98, 102). For example, elevations in arterial pressure in various models of hypertension, including obesity-related hypertension, are dependent on neuronal activity in the RVLM (11, 46, 47, 78, 98). The increased output of the RVLM in hypertension is likely dependent on increased excitatory input from other brain regions, as well as an increase in sensitivity to excitatory input (3, 4, 11, 17, 45, 45, 108). Interestingly, the increase in sensitivity to excitation may occur together or separately from the increased tonic excitatory input depending on the model or risk factor studied. For example, in some (but not all) models of hypertension, augmented sympathoexcitation is attributed to increased tonic excitatory glutamatergic input to the RVLM (3, 4, 11, 17, 45, 45, 108). In addition, pressor and sympathoexcitatory responses to direct activation of the RVLM are enhanced in some models (11, 17, 108) but not in others (78, 95, 96, 102, 103). Interestingly, Dahl salt-resistant rats that remain normotensive after being fed a high-salt diet, exhibit enhanced pressor responses to glutamate in the RVLM yet exhibit little or no response to blockade of ionotropic glutamate receptors (47). We have observed similar results when comparing sedentary vs. physically active rats (76), suggesting that increased sensitivity to excitation may occur in the absence of an overt change in tonic excitatory input. Furthermore, these data suggest that the influence of the different risk factors (e.g., dietary salt and inactivity) may occur via similar or disparate mechanisms within the RVLM, and in combination, produce additive or synergistic effects on sympathetic outflow and the development of cardiovascular disease.
Proposed Role of the RVLM in Physical (In)activity-Dependent Changes in Sympathetic Nerve Activity Regulation
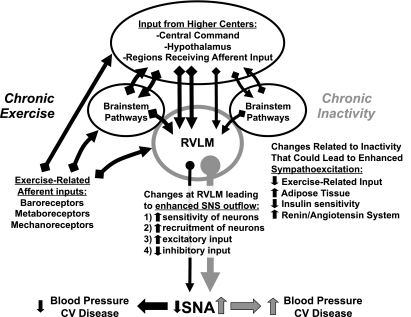
Figure 1 contains a generalized schematic of the wide variety of inputs to the RVLM and its crucial role in the regulation of sympathetic activity and blood pressure. It also demonstrates how this regulation may be affected by exercise or physical inactivity. As the RVLM receives a variety of cardiovascular and exercise-related inputs (20, 21, 38), it is not surprising that it has been shown to be activated during acute bouts of dynamic exercise (49), in response to muscle contraction (7, 9), and stimulation of group III and IV afferents (72, 99). Pressor and sympathoexcitatory responses occurring under these conditions appear to be mediated by release of glutamate acting on ionotropic receptors in the RVLM (5, 8, 54). Along with feedback from exercising muscle (53), higher brain centers also contribute to the cardiovascular response to acute exercise via direct and indirect projections to brain stem pathways that likely involve the RVLM (25, 26, 28, 42, 57, 83).
Fig. 1.
Schematic diagram illustrating the role of the rostral ventrolateral medulla (RVLM) in the integration and generation of sympathetic outflow. Potential influences of chronic exercise vs. chronic inactivity are presented that could affect sympathetic nerve activity (SNA) and its direct and indirect effects on blood pressure and cardiovascular (CV) disease.
Although glutamate and other neurotransmitters in the RVLM appear to be involved in the cardiovascular response to acute exercise (61, 88), the influence of repeated bouts of exercise (i.e., regular physical activity) and the recurring, often cyclical activation of these exercise-related inputs on the RVLM are likely to be important in terms of neuroplastic changes. Conversely, in the absence of these repetitive inputs (i.e., a sedentary lifestyle/“normal” cage activity), similar or additional mechanisms may contribute to altered regulation of sympathetic outflow and blood pressure observed in sedentary subjects. Recently, our laboratory and others have demonstrated that compared with physically active animals, sedentary animals (which lack repetitive exercise-related inputs) exhibit enhanced blood pressure (66) and sympathoexcitatory responses (76) to direct glutamatergic activation of the RVLM. These data suggest that alterations occurring at the RVLM may be responsible for enhanced sympathoexcitation in sedentary animals (See 1–4 on Fig. 1 under changes at RVLM). Furthermore, responses to ANG II microinjection in the RVLM are not enhanced in sedentary vs. physically active animals (10), suggesting that enhanced excitation to glutamate is not due to a generalized effect on neuronal excitability, as appears to occur in animals on excess dietary salt (1, 2).
GABA tonically suppresses the activity of RVLM neurons, primarily by activation of GABAA receptors (6, 13, 43, 70, 101). We hypothesized that enhanced sympathoexcitation to glutamate microinjections observed in sedentary animals could be due to reduced GABAergic inhibition of RVLM neurons. If GABAergic tone were reduced in the RVLM of sedentary rats, we would have expected that responses to blockade of GABAA receptors would also be reduced compared with physically active animals. However, we have observed that blockade of GABAA receptors in the RVLM produced enhanced (not reduced) responses in sedentary rats compared with treadmill-exercised rats (76). We interpreted these data to suggest that enhanced sympathoexcitation in sedentary rats was not due to reduced GABAergic inhibition of the RVLM, but that these neurons may receive more excitatory input or be more sensitive to persisting excitatory glutamatergic input following removal of GABAergic tone. In additional experiments, blockade of ionotropic glutamate receptors alone in the RVLM did not reveal differences in tonic glutamatergic excitation of RVLM neurons in sedentary or physically active rats (76). These data indicate that individual RVLM neurons may be more sensitive to excitatory glutamatergic input and/or that more RVLM neurons participate in glutamatergically mediated sympathoexcitation in sedentary animals (See 1 and 2 on Fig. 1). Our observation of enhanced sympathoexcitatory responses to glutamate microinjections in sedentary animals supports either possibility (76); however, previous studies indicate an increased number of activated RVLM neurons (determined by c-Fos labeling) in sedentary vs. physically active animals under various conditions, including acute exercise (44) and acute stress (34). These findings suggest that more RVLM neurons may be recruited to produce sympathoexcitation following sedentary vs. physically active conditions. In either case, we speculate that the increased sensitivity to glutamate in the absence of a change in tonic input is due to the lack of repetitive activation of glutamate receptors in the RVLM of sedentary compared with physically active animals. Furthermore, we contend that neuroplasticity in glutamate receptor-mediated neurotransmission in the RVLM may be at least one important target by which physical activity and inactivity influence regulation of sympathetic outflow. It is possible that these alterations occur in the absence of any overt structural plasticity since Nelson and colleagues (80, 81) have reported no alterations in dendritic branching in Golgi-Cox impregnated neurons examined in the region of the RVLM of sedentary vs. spontaneously exercising rats. Whether enhanced sympathoexcitation observed in sedentary animals is due to increased activation of individual RVLM neurons, increased recruitment of spinally projecting RVLM neurons, or both is unknown.
Perspectives and Significance
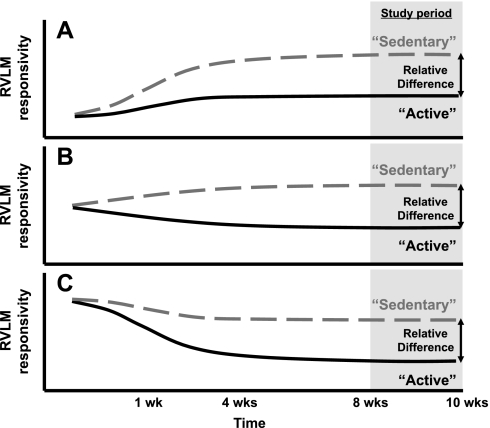
One subtle yet important question is whether the relative difference between sedentary and physically active groups is due to the effects of being sedentary, the effects of being physically active, or a combination of both. Figure 2 illustrates this point graphically. The gray area represents the time period from our previous study, in which enhanced responses to activation of the RVLM were observed in sedentary vs. physically active animals (76). For simplicity, the y-axis is labeled “RVLM responsivity”, and examples A, B, and C represent some of the basic possibilities by which these differences could develop over time. In example A, maintaining sedentary conditions (dashed line) may increase RVLM sensitivity while physically active conditions (solid line) may attenuate or prevent this increase. In example B, maintaining sedentary conditions may slightly increase or not affect RVLM sensitivity; whereas, allowing animals to exercise may reduce RVLM sensitivity. Finally, in example C, RVLM sensitivity may decrease over time in animals that are allowed to exercise but to a lesser extent in sedentary animals. Certainly, these possibilities are not mutually exclusive, and more complex possibilities may occur. A fundamental contention is that by examining the development of these differences over time, it may be possible to separate out the potential mechanisms by which physical activity vs. inactivity contribute to the relative differences observed. In addition, examination of a variety of factors, including the type of exercise (aerobic vs. resistance), duration, intensity, environmental conditions, and individual responsiveness to exercise or inactivity are important, clinically relevant issues. Like other laboratories (39, 40, 60), we continue to promote the development of experimental designs and models that examine this fundamental question by treating the sedentary condition as a contributing factor to chronic disease and physically active conditions as the “normal” healthy control (15, 75). We feel that this reflects more than a semantic difference but rather reflects an important and distinct paradigm shift as to what should be considered a “normal” or “control” group.
Fig. 2.
Hypothetical possibilities to describe the relative differences in the responsiveness of the RVLM in sedentary vs. physically active animals. Examples are represented over time and include attenuated increases in active animals over time (A), increases in sedentary and decreases in active animals over time (B), or attenuated decreases in sedentary animals over time (C). The responsiveness of the RVLM is based on results of different studies that suggest the RVLM is activated to a greater extent in sedentary vs. physically active animals (34, 44, 66, 76).
The evidence reviewed strongly supports the need to identify the mechanisms by which a sedentary lifestyle contributes to enhanced sympathoexcitation at the level of the RVLM and the impact of these alterations on sympathetic control of blood pressure. Important questions remain including how RVLM neurons individually and, as a population process, integrate, and produce sympathetic drive. Other brain regions via projections to the RVLM and spinal cord are also likely to play important roles as well. Understanding these mechanisms is important because of the obvious impact of physical inactivity on the health of the population. In fact, physical inactivity is considered by some as “the biggest health care problem of the 21st century” (12). The knowledge gained from future studies may ultimately allow the development of novel therapeutic strategies for cardiovascular diseases associated with a sedentary lifestyle.
GRANTS
The author has been supported by grants from the Heartland Affiliate of the American Heart Association (Beginning Grant In Aid 0265264Z and Grant In Aid 0650161Z) and the National Institutes of Health (R21 HL089364).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
The author would like to thank the faculty and technical support staff at Wayne State University School of Medicine and the University of Missouri-Columbia. In particular, at Wayne State University School of Medicine, I thank Dr. Donal O'Leary, Dr. Noreen Rossi, Dr. Tadek Scislo, and Nicholas Mischel for helpful comments on this manuscript. I would also like to acknowledge support from Drs. Eileen Hasser and Cheryl Heesch at the University of Missouri-Columbia and Dr. Ida Llewellyn-Smith at Flanders University.
REFERENCES
- 1.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci Basic Clin 108: 17–21, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ally A. Ventrolateral medullary control of cardiovascular activity during muscle contraction. Neurosci Biobehav Rev 23: 65–86, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Amano M, Kubo T. Involvement of both GABAA and GABAB receptors in tonic inhibitory control of blood pressure at the rostral ventrolateral medulla of the rat. Naunyn Schmiedebergs Arch Pharmacol 348: 146–153, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Bauer RM, Iwamoto GA, Waldrop TG. Discharge patterns of ventrolateral medullary neurons during muscular contraction. Am J Physiol Regul Integr Comp Physiol 259: R606–R611, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Bauer RM, Iwamoto GA, Waldrop TG. Ventrolateral medullary neurons modulate pressor reflex to muscular contraction. Am J Physiol Regul Integr Comp Physiol 257: R1154–R1161, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Bauer RM, Waldrop TG, Iwamoto GA, Holzwarth MA. Properties of ventrolateral medullary neurons that respond to muscular contraction. Brain Res Bull 28: 167–178, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Becker LK, Santos RAS, Campagnole-Santos MJ. Cardiovascular effects of angiotensin II and angiotensin-(1–7) at the RVLM of trained normotensive rats. Brain Res 1040: 121–128, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension 26: 1117–1120, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 43: 1–2, 2009 [PubMed] [Google Scholar]
- 13.Blessing WW. Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla. Am J Physiol Heart Circ Physiol 254: H686–H692, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol 102: 381–390, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Booth FW, Lees SJ. Physically active subjects should be the control group. Med Sci Sports Exerc 38: 405–406, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Bowles DK, Woodman CR, Laughlin MH. Coronary smooth muscle and endothelial adaptations to exercise training. Exerc Sport Sci Rev 28: 57–62, 2000 [PubMed] [Google Scholar]
- 17.Carvalho THF, Bergamaschi CT, Lopes OU, Campos RR. Role of endogenous angiotensin II on glutamatergic actions in the rostral ventrolateral medulla in Goldblatt hypertensive rats. Hypertension 42: 707–712, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatments of high blood pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 45: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Dampney RAL. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol 42: 197–227, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Dampney RAL. Medullary pathways regulating sympathetic outflow: the need for more lateral thinking. Am J Physiol Regul Integr Comp Physiol 286: R446–R448, 2004 [DOI] [PubMed] [Google Scholar]
- 23.De Angelis K, Wichi RB, Jesus WRA, Moreira ED, Morris M, Krieger EM, Irigoyen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol 96: 2174–2178, 2004 [DOI] [PubMed] [Google Scholar]
- 24.de Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, Middlekauff H, Negrao CE, Pereira Barretto AC. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail 8: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Degtyarenko AM, Kaufman MP. Bicuculline and strychnine supress the mesencephalic locomotor region-induced inhibition of group III muscle afferent input to the dorsal horn. Neuroscience 118: 779–788, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Degtyarenko AM, Kaufman MP. MLR-induced inhibition of barosensory cells in the NTS. Am J Physiol Heart Circ Physiol 289: H2575–H2584, 2005 [DOI] [PubMed] [Google Scholar]
- 27.DiCarlo SE, Bishop VS. Exercise training attenuates baroreflex regulation of nerve activity in rabbits. Am J Physiol Heart Circ Physiol 255: H974–H979, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neuobiology of exercise. Obesity 14: 345–356, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Esler M, Rumantir M, Kaye D. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol 28: 986–989, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124: 71–79, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail 9: 630–636, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension 23: 294–301, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HEW, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120: 269–281, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York, New York: Oxford University, 1990, p. 145–167 [Google Scholar]
- 37.Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li YW. Role of medulla oblongata in generation of sympathetic and vagal outflows. Prog Brain Res 107: 127–144, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Guyenet PG, Stornetta RL. The presympathetic cells of the rostral ventrolateral medulla (RVLM): anatomy, physiology and role in the control of circulation. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Norwell, MA: Kluwer Academic, 2004, p. 187–218 [Google Scholar]
- 39.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Hamilton MT, Hamilton DR, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev 32: 161–166, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasser EM, Moffitt JA. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Ann NY Acad Sci 940: 454–468, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Hayes SG, Kaufman MP. MLR stimulation and exercise pressor reflex activate different renal sympathetic fibers in decerebrate cats. J Appl Physiol 92: 1628–1634, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Horiuchi J, Killinger S, Dampney RAL. Contribution to sympathetic vasomotor tone of tonic glutamatergic inputs to neurons in the RVLM. Am J Physiol Regul Integr Comp Physiol 287: R1335–R1343, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Ichiyama RM, Gilbert AB, Waldrop TG, Iwamoto GA. Changes in the exercise activation of diencephalic and brainstem cardiorespiratory areas after training. Brain Res 947: 225–233, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension 40: 552–559, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acid in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 35: 413–417, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 37: 687–691, 2001 [PubMed] [Google Scholar]
- 48.Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol Regul Integr Comp Physiol 273: R487–R494, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Iwamoto GA, Wappel SM, Fox GM, Buetow KA, Waldrop TG. Identification of diencephalic and brainstem cardiorespiratory areas activated during exercise. Brain Res 726: 109–122, 1996 [PubMed] [Google Scholar]
- 50.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol 86: 441–449, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julius S, Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure, and diabetes. Blood Press 7: 5–13, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst 43: 231–240, 1993 [DOI] [PubMed] [Google Scholar]
- 55.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 11: 342–348, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Kramer JM, Plowey ED, Beatty JA, Little HR, Waldrop TG. Hypothalamus, hypertension, and exercise. Brain Res Bull 53: 77–85, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Krieger EM, Da Silva GJJ, Negrao CE. Effects of exercise training on baroreflex control of the cardiovascular system. Ann NY Acad Sci 940: 338–347, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol 102: 1341–1347, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Lillaney R, Maher TJ, Chaiyakul P, Ally A. Changes in extracellular glutamate and pressor response during muscle contraction following AMPA-receptor blockade in the RVLM and CVLM. Brain Res 844: 164–173, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics—2010 Update. A Report From the American Heart Association [Online]. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol 277: R1063–R1075, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol 285: H2734–H2748, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Madden CJ, Sved AF. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobiol 23: 739–749, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martins-Pinge MC, Becker LK, Garcia MR, Zoccal DB, Neto RV, Basso LS, de Souza HC, Lopes OU. Attenuated pressor responses to amino acids in the rostral ventrolateral medulla after swimming training in conscious rats. Auton Neurosci 122: 21–28, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 18: 575–582, 1991 [DOI] [PubMed] [Google Scholar]
- 68.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res 448: 205–222, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Miyawaki T, Goodchild AK, Pilowsky PM. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res 924: 56–62, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM following hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol 283: R604–R614, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Morrison SF, Reis DJ. Reticulospinal vasomotor neurons in the RVL mediate the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 256: R1084–R1097, 1989 [DOI] [PubMed] [Google Scholar]
- 73.Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J Appl Physiol 104: 616–624, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 34: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Mueller PJ. Influence of sedentary versus physically active conditions on regulation of plasma renin activity and vasopressin. Am J Physiol Regul Integr Comp Physiol 295: R727–R732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 102: 803–813, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Mueller PJ, Cunningham JT, Patel KP, Hasser EM. Proposed role of the paraventricular nucleus in cardiovascular deconditioning. Acta Physiol Scand 177: 27–35, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Muratani H, Ferrario CM, Averill DB. Ventrolateral medulla in spontaneously hypertensive rats: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol 264: R388–R395, 1993 [DOI] [PubMed] [Google Scholar]
- 79.Negrao CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol Regul Integr Comp Physiol 265: R365–R370, 1993 [DOI] [PubMed] [Google Scholar]
- 80.Nelson AJ, Iwamoto GA. Reversibility of exercise-induced dendritic attenuation in brain cardiorespiratory and locomotor areas following exercise training. J Appl Physiol 101: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol 99: 2312–2322, 2005 [DOI] [PubMed] [Google Scholar]
- 82.O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res 176: 362–366, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Padley JR, Kumar NN, Li Q, Nguyen TB, Pilowsky PM, Goodchild AK. Central command regulation of circulatory function mediated by descending pontine cholinergic inputs to sympathoexcitatory rostral ventrolateral medulla neurons. Circ Res 100: 284–291, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine Position Stand. Exercise and hypertension. Med Sci Sports Exerc 36: 533–553, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Pilowsky PM, Abbott SB, Burke PG, Farnham MM, Hildreth CM, Kumar NN, Li Q, Lonergan T, McMullan S, Spirovski D, Goodchild AK. Metabotropic neurotransmission and integration of sympathetic nerve activity by the rostral ventrolateral medulla in the rat. Clin Exp Pharmacol Physiol 35: 508–511, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Ray CA, Carter JR. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol 298: H229–H234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ray CA, Hume KM. Sympathetic neural adaptation to exercise training in humans: insights from microneurography. Med Sci Sports Exerc 30: 387–391, 1998 [DOI] [PubMed] [Google Scholar]
- 88.Reidman DA, Maher TJ, Chaiyakul P, Ally A. Modulation of extracellular glutamate and pressor response to muscle contraction during NMDA-receptor blockade in the rostral ventrolateral medulla. Neurosci Res 36: 147–156, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Schlaich MP, Lambert E, Kaye D, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler M. Sympathetic augmentation in hypertension. Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997 [DOI] [PubMed] [Google Scholar]
- 92.Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol 279: R729–R742, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Seals DR. Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension 17: 36–43, 1991 [DOI] [PubMed] [Google Scholar]
- 94.Sheldahl LM, Ebert TJ, Cox B, Tristani FE. Effect of aerobic training on baroreflex regulation of cardiac and sympathetic function. J Appl Physiol 76: 158–165, 1994 [DOI] [PubMed] [Google Scholar]
- 95.Smith JK, Barron KW. Cardiovascular effects of l-glutamate and tetrodotoxin microinjected into the rostral and caudal ventrolateral medulla in normotensive and spontaneously hypertensive rats. Brain Res 506: 1–8, 1990 [PubMed] [Google Scholar]
- 96.Smith JK, Barron KW. GABAergic responses in ventrolateral medulla in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 258: R450–R456, 1990 [DOI] [PubMed] [Google Scholar]
- 97.Spier SA, Laughlin MH, Delp MD. Effects of acute and chronic exercise on vasoconstrictor responsiveness of rat abdominal aorta. J Appl Physiol 87: 1752–1757, 1999 [DOI] [PubMed] [Google Scholar]
- 98.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Stornetta RL, Morrison SF, Ruggiero DA, Reis DJ. Neurons of rostral ventrolateral medulla mediate somatic pressor reflex. Am J Physiol Regul Integr Comp Physiol 256: R448–R462, 1989 [DOI] [PubMed] [Google Scholar]
- 100.Straznicky N, Lambert E, Lambert G, Masuo K, Esler M, Nestel P. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 90: 5998–6005, 2005 [DOI] [PubMed] [Google Scholar]
- 101.Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol Regul Integr Comp Physiol 249: R672–R680, 1985 [DOI] [PubMed] [Google Scholar]
- 102.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep 5: 262–268, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Sved AF, Ito S, Yajima Y. Role of excitatory amino acid inputs to the rostral ventrolateral medulla in cardiovascular regulation. Clin Exp Pharmacol Physiol 29: 503–506, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Sved AF, Mancini DL, Graham JC, Schreihofer AM, Hoffman GE. PNMT-containing neurons of the C1 cell group express c-fos in response to changes in baroreceptor input. Am J Physiol Regul Integr Comp Physiol 266: R361–R367, 1994 [DOI] [PubMed] [Google Scholar]
- 105.Svedenhag J, Martinsson A, Ekblom B, Hjemdahl P. Skeletal muscle sympathetic activity at rest in trained and untrained subjects. Acta Physiol Scand 120: 499–504, 1984 [DOI] [PubMed] [Google Scholar]
- 106.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2006 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 113: e85–e151, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Tsuchihashi T, Kagiyama S, Ohya Y, Abe I, Fujishima M. Antihypertensive treatment and the responsiveness to glutamate in ventrolateral medulla. Hypertension 31: 73–76, 1998 [DOI] [PubMed] [Google Scholar]
- 109.Van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med 10: 128–140, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Wang G, Pratt M, Macera CA, Zheng ZJ, Heath G. Physical activity, cardiovascular disease, and medical expenditures in U.S. adults. Ann Behav Med 28: 88–94, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension 53: 370–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 48: 1005–1011, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev 32: 107–111, 2004 [DOI] [PubMed] [Google Scholar]