Abstract
Runx proteins are tissue-specific transcriptional scaffolds that organize and assemble regulatory complexes at strategic sites of target gene promoters and at intranuclear foci to govern activation or repression. During interphase, fidelity of intranuclear targeting supports the biological activity of Runx1 and Runx2 proteins. Both factors regulate genes involved in cell cycle control and cell growth (e.g., rRNA genes), as well as lineage commitment. Here, we have examined the subcellular regulatory properties of the third Runx member, the tumor suppressor protein Runx3, during interphase and mitosis. Using in situ cellular and biochemical approaches we delineated a subnuclear targeting signal that directs Runx3 to discrete transcriptional foci that are nuclear matrix associated. Chromatin immunoprecipitation results show that Runx3 occupies rRNA promoters during interphase. We also find that Runx3 remains associated with chromosomes during mitosis and localizes with nucleolar organizing regions (NORs), reflecting an interaction with epigenetic potential. Taken together, our study establishes that common mechanisms control the subnuclear distribution and activities of Runx1, Runx2 and Runx3 proteins to support RNA polymerase I and II mediated gene expression during interphase and mitosis.
Keywords: Runx3, Cancer, Transcription Factor, Mitosis, Nucleolus, rRNA
INTRODUCTION
Runt related (Runx) proteins are tissue-restricted and cancer-related transcription factors that regulate cell proliferation and growth, as well as differentiation (Coffman, 2003; Kagoshima et al, 2007; Miyazono et al, 2004; Stein et al, 2004b; Young et al, 2007a; Young et al, 2007b). Both human and mouse genomes contain three Runx genes (Runx1, Runx2 and Runx3) (van Wijnen et al, 2004) that encode the α-subunit [polyomavirus enhancer-binding protein 2 (PEBP2α) /core-binding factor alpha (CBFα)] that forms a heterodimeric complex with the non-DNA binding partner CBFβ. The expression of Runx proteins is very tightly regulated both spatially and temporally throughout development and adulthood and they control diverse biological processes (Ito, 2008).
Runx1 and Runx2 are scaffolding proteins that assemble regulatory complexes at specific intranuclear sites to mediate activation or repression of target genes (Stein et al, 2004b; Stein et al, 2005; Lian et al, 2006; Harrington et al, 2002; Saltman et al, 2005; Zeng et al, 1998). During interphase these proteins are associated with the nuclear matrix through a 31–35 amino acid nuclear matrix targeting signal (NMTS) in the carboxy terminus (Zeng et al, 1997; Zaidi et al, 2001; Tang et al, 1999). Fidelity of nuclear matrix association is important for the biological activity of several transcription factors (Okorokov et al, 2002; Bushmeyer and Atchison, 1998; Nickerson, 2001; Stein et al, 2004a). Defective subnuclear targeting of Runx1 and Runx2 leads to defects in hematopoiesis and bone differentiation, respectively (Stein et al, 2004a; Vradii et al, 2005).
During mitosis the architectural organization of regulatory machinery in the nucleus undergoes major changes and the nuclear scaffold is disassembled. We have shown that Runx1 and Runx2 proteins remain associated with the nucleolar organizing regions (NORs) of acrocentric chromosomes during mitosis, and this association requires the DNA binding domain (Bakshi et al, 2008; Young et al, 2007a; Young et al, 2007b). Discrete Runx2 foci at NORs colocalize with UBF1, a principal regulator of Pol I gene transcription that is required for rRNA synthesis. Consistent with the anti-proliferative role of Runx2 in osteoblasts (Pratap et al, 2003; Galindo et al, 2005; Zaidi et al, 2007), binding of Runx2 to the rDNA promoter downregulates rRNA transcription (Young et al, 2007a). These previous studies from our group together indicate that Runx2 may regulate cell proliferation by coordinating cell cycle kinetics with control of cell growth.
Runx3, the smallest protein of the Runx family, has been shown to play an important role in neuronal (Inoue et al, 2003; Inoue et al, 2002; Inoue et al, 2008; Levanon et al, 2002) and T cell development (Woolf et al, 2003) and its loss leads to gastric tumorigenesis(Bae and Choi, 2004; Friedrich et al, 2006; Ito et al, 2005; Li et al, 2004; Wei et al, 2005; Yamamura et al, 2006). As observed for Runx1 and Runx2, Runx3 is predominantly a nuclear protein that is punctately organized. However, mechanisms associated with Runx3 subnuclear localization remain to be investigated. In the present study, we identify and characterize the NMTS of Runx3 and show that subnuclear targeting of Runx3 contributes to its transcriptional activity. Using immunofluorescence microscopy and chromatin immunoprecipitation assays, we demonstrate that Runx3 is retained on mitotic chromosomes in large discrete foci, co-localizes with UBF1 and occupies rDNA promoters. Taken together, we have established that common mechanisms control subnuclear localization and activity of Runx proteins in interphase and mitosis.
MATERIALS AND METHODS
Cell Culture and Transient Transfections
All cell types were grown in 37°C incubators in a 5% carbon dioxide atmosphere in DMEM with L-Glutamine and 10% FBS. HeLa cells were transfected using FuGENE-6 (Roche, Indianapolis, IN) according to the instructions of the manufacturer.
Biochemical Fractionation
HeLa cells were processed for biochemical fractionation 24 h after transfection, as previously described (Zaidi et al, 2001). Fractions were resolved by 10% SDS PAGE and subjected to western blotting using anti-Flag (Sigma-Aldrich, St. Louis, MO), anti-Cdk2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or anti-Lamin B (Santa Cruz) antibodies.
Immunofluorescence Microscopy
HeLa cells grown on gelatin coated coverslips were transfected with the indicated plasmid constructs. After 24 h, coverslips were processed for immunofluorescence microscopy as previously described (Javed et al, 2000) using mouse monoclonal anti-Flag antibody (1:5000) (Sigma-Aldrich), followed by incubation with Alexa 488 conjugated secondary antibody (1:800) (Molecular Probes, Eugene, OR). For co-localization experiments, coverslips were also incubated with rabbit polyclonal anti-UBF antibody (1:500) (Santa Cruz) followed by incubation with Alexa 568 conjugated secondary antibody (1:800) (Molecular Probes). All images were taken using a Zeiss Axioplan digital microscope and analyzed using Metamorph software (Universal Imaging, Downingtown, PA).
Luciferase Reporter Assay
HeLa cells were co-transfected with 100 ng expression constructs and 500 ng Firefly luciferase reporter and 10 ng Renilla luciferase reporter as a control for transfection efficiency. Cells were harvested 24 h after transfection in 250 µl of 1x Reporter Lysis Buffer (Promega, Madison, WI) and the lysates were processed for luciferase assay. Luciferase activity was assessed with a dual Luciferase Assay Kit according to the manufacturer’s instructions.
Chromatin Immunoprecipitation (ChIP)
IEC-6 rat gastric epithelial cells were washed once with PBS and then crosslinked for 10 min by adding PBS with 1% formaldehyde. Immediately following crosslinking, the reaction was quenched by adding 2.5 M glycine. Cells were washed twice with PBS and resuspended in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris pH 8.0). Samples were sonicated to shear DNA, and immunoprecipitated with anti-Runx3 antibody (Santa Cruz) or normal IgG (Santa Cruz). DNA was isolated from immunoprecipitate using phenol/chloroform extraction and RT-PCR was performed. Primers used are: rrDNA3.1, (F) 5’-GCTCGTATTCCCGTCCAGT and (R) 5’-AGCACCACATCGATCCAAC; tubulin3’UTR, (F) 5’-TGAGGAGGAGGTGGCTTAG and (R) 5’-ACAGGACAGCAAATGCACAG.
RESULTS
Subnuclear targeting of Runx3 to the nuclear matrix contributes to its biological activity in interphase cells
Runx proteins have important biological activities that control cell fate determination, as well as cell growth and differentiation (Lian et al, 2004). Runx1 and Runx2 are targeted to subnuclear foci associated with the nuclear matrix, which is a nuclear scaffold where regulatory machinery for gene expression is organized. We investigated whether Runx3 is also targeted to the nuclear matrix compartment. Transiently expressed Runx3 exhibits a focal nuclear pattern and these foci are retained upon removal of soluble proteins, chromatin and associated components as reflected by loss of DAPI signal (Fig. 1A). To confirm nuclear matrix association we performed biochemical subcellular fractionation of HeLa cells transiently expressing Runx3 followed by western blotting with a Flag antibody (Fig. 1B). While Cdk2 is enriched in the cytoplasmic fraction, Runx3 is predominantly detected in the nuclear matrix fraction together with Lamin B, a marker for the nuclear lamina. Hence, similar to other Runx proteins, Runx3 is an integral component of the nuclear matrix.
Fig. 1. Runx3 resides in distinct nuclear matrix associated subnuclear domains.
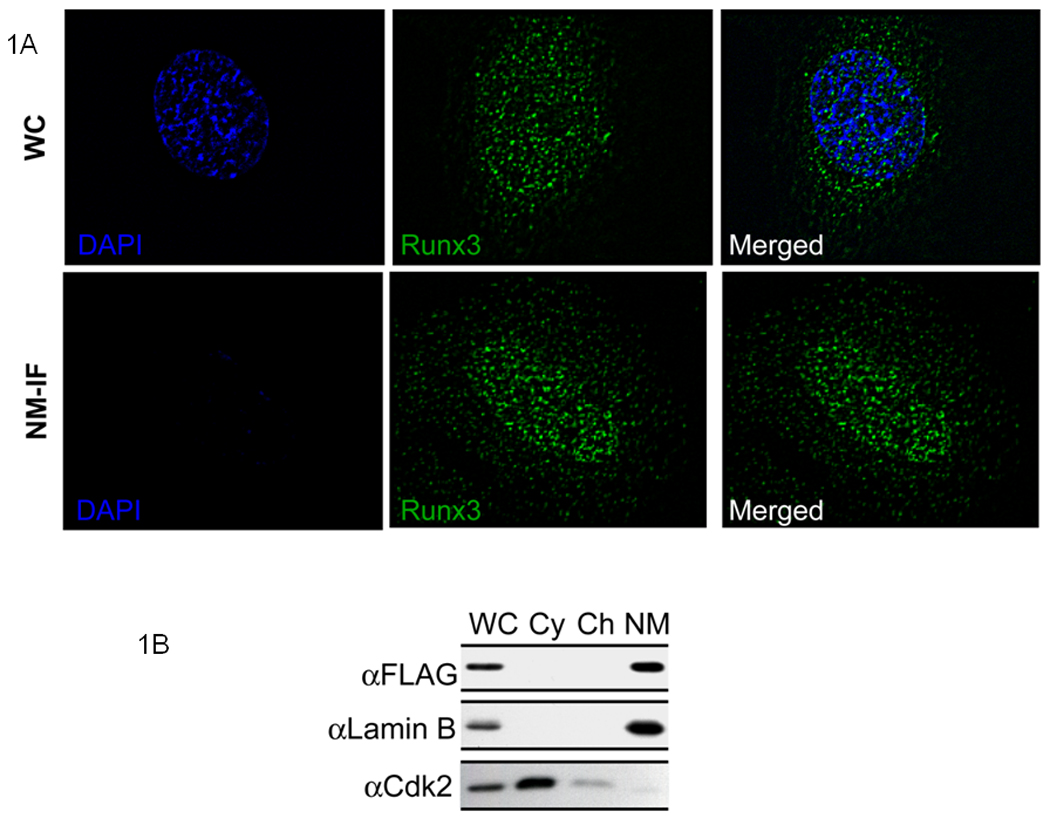
A) HeLa cells were grown on gelatin- coated coverslips and were transfected with 500 ng of full length Runx3 construct. Twenty four hours later, cells were processed for NM-IF preparation and in situ immunofluorescence microscopy. The coverslips were incubated with monoclonal antibody against FLAG tag (1:5000) followed by incubation with Alexa 488 conjugated secondary antibody (1:800) to detect Runx3. Images were taken using a Zeiss Axioplan digital microscope and Metamorph software was used for bio imaging.
B) Subcellular fractionation of HeLa cells transiently expressing full length Runx3. HeLa cells expressing full length Runx3 construct were subjected to biochemical fractionation 24 h after transfection. Fractions were resolved by 10% SDS PAGE gel followed by western blotting. Monoclonal FLAG antibody was used to detect Runx3. Lamin B and Cdk2 were used as controls to indicate proper fractionation.
A specific carboxy terminal targeting signal directs Runx3 to subnuclear sites in interphase
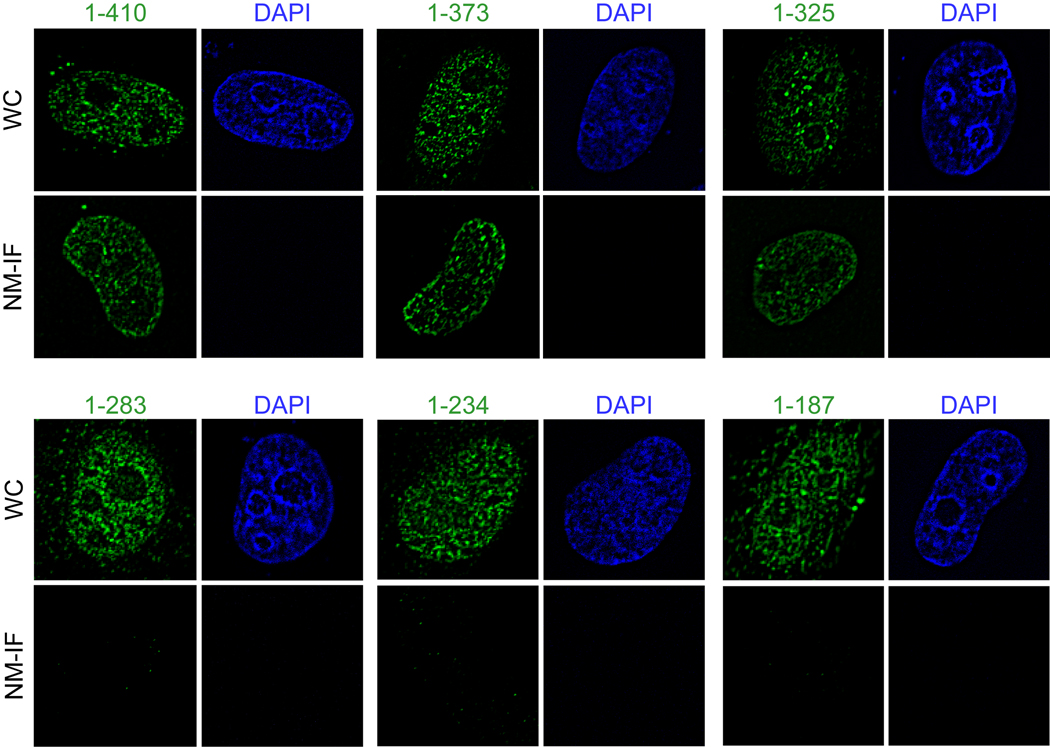
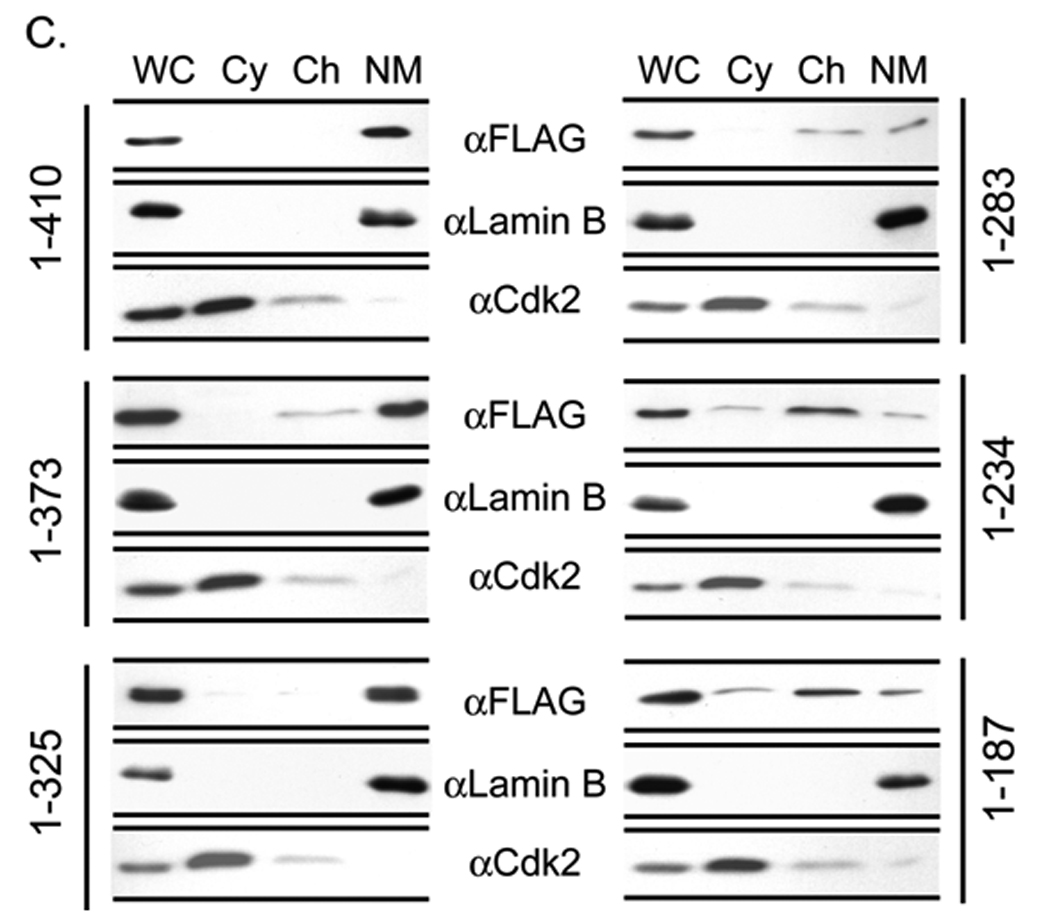
Both Runx1 and Runx2 contain similar nuclear matrix targeting signals that support the trafficking of these regulatory factors to functional sites within the nucleus (Zeng et al, 1997; Zaidi et al, 2001). The C-terminus of Runx3 has a segment of about 40 amino acids (residue 280–327) that shares significant homology with these nuclear matrix targeting signals. To address whether this sequence directs Runx3 to the nuclear matrix, we utilized a panel of epitope tagged C-terminal deletion constructs of Runx3 that were transfected into HeLa cells (Fig. 2A). Western blot analysis shows that all deletion proteins are expressed at comparable levels (Fig. 2B). Subcellular fractionation combined with western blotting of HeLa cells transiently expressing Runx3 deletion mutants show that deletion of amino acid (aa) residues 283–325 causes a dramatic change in the partitioning between chromatin and nuclear matrix fractions (Fig. 2C). We subsequently assessed the nuclear matrix association of the proteins at single cell level by immunofluorescence microscopy. Consistent with the subcellular fractionation results, deletion mutants of Runx3 that retain aa 283–325 are associated with the nuclear matrix, whereas deletion of this peptide motif causes loss of association of the protein with the nuclear matrix (Fig. 3). Taken together, these results indicate that the domain containing aa 283–325 represents the nuclear matrix targeting signal of Runx3.
Fig. 2. Delineation of the nuclear matrix targeting signal (NMTS) of Runx3.
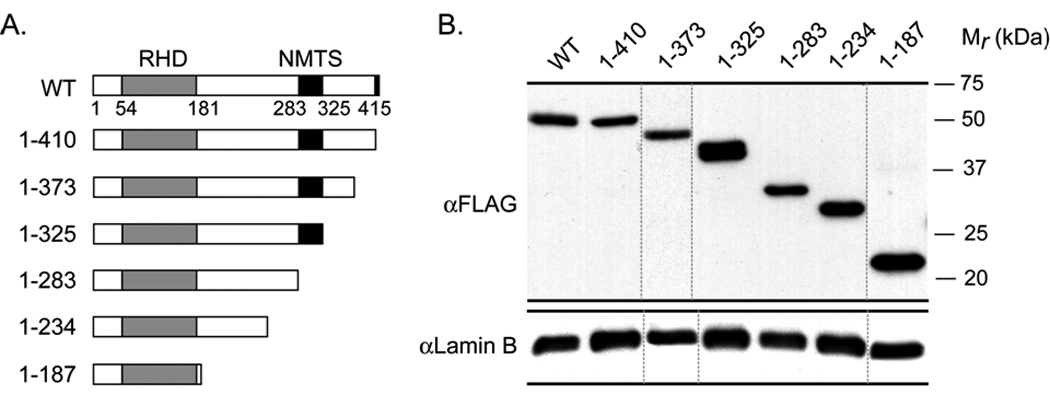
A) Schematic representation of full length Runx3 and its deletion mutants.
B) Deletions of Runx3 are expressed at comparable levels in HeLa cells. Total protein extracts were prepared from HeLa cells 24 h after transfection with full length Runx3 or its deletion mutants. Equal amounts of protein were loaded in each lane. Western blot using FLAG antibody reveals that all Runx2 deletion mutants are expressed at similar levels. Lamin B was used as loading control.
C) Subcellular fractionation of HeLa cells transiently expressing full length Runx3 or its deletion mutants. HeLa cells expressing various deletion constructs of Runx3 were subjected to biochemical fractionation 24 h after transfection. Fractions were resolved by 10% SDS PAGE followed by western blotting using a monoclonal FLAG antibody. Lamin B and Cdk2 were used as controls to indicate proper fractionation.
Fig. 3. Delineation of the nuclear matrix targeting signal (NMTS) of Runx3.
HeLa cells were grown on gelatin- coated coverslips and were transfected in parallel with 500 ng of each of the Runx3 deletion constructs. Twenty four hours later, cells were processed for NM-IF preparation and in situ immunofluorescence microscopy. The coverslips were incubated with monoclonal antibody against FLAG tag (1:5000) followed by incubation with Alexa 488 conjugated secondary antibody (1:800) to detect expressed proteins. Images were taken using a Zeiss Axioplan digital microscope and Metamorph software was used for bio imaging.
We analyzed the ability of Runx3 and all six deletion mutants to activate a luciferase reporter under control of multimerized Runx binding element fused to a minimal promoter. Full length Runx2 and Runx3 robustly activate the reporter plasmid, but Runx3 mutants that lack the NMTS (i.e. 1–187, 1–234 and 1–283) fail to significantly activate the luciferase reporter above empty vector level. Mutants 1–373 and 1–410 activate the reporter similar to WT protein. Mutant 1–325 failed to activate the reporter even though it has the NMTS indicating that residues located C-terminal of Runx3 NMTS are required for Runx3 mediated transactivation (Fig. 4).
Fig. 4. Transcriptional activity of Runx3 is regulated by its association with nuclear matrix.
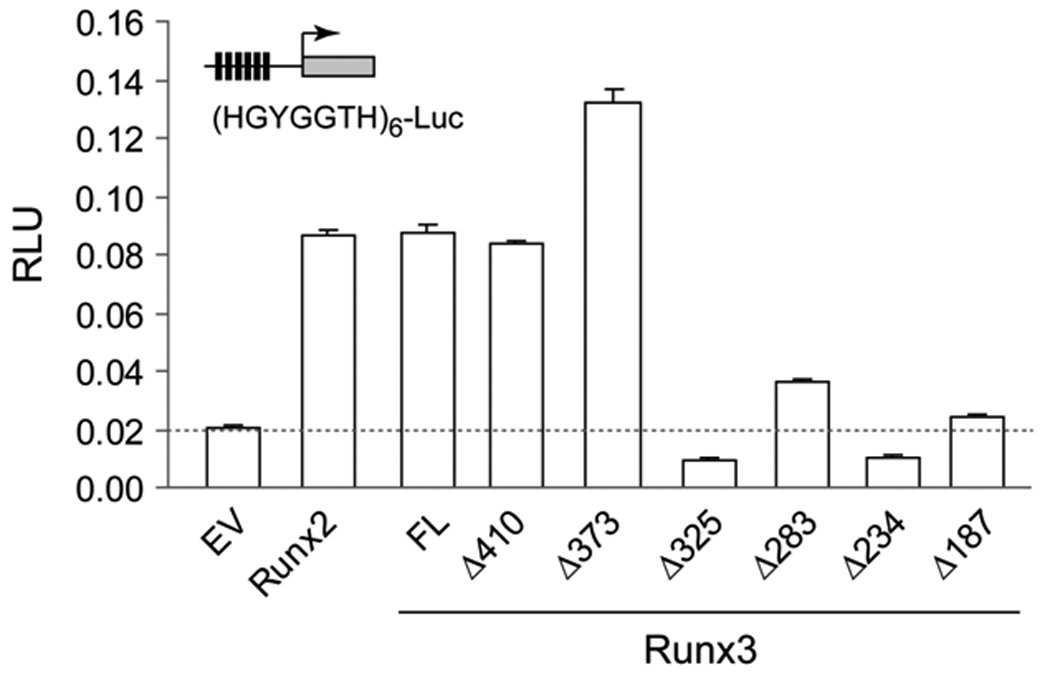
HeLa cells grown on six- well plates were co-transfected with 100 ng of the indicated Runx3 expression constructs, 500 ng Firefly luciferase reporter, and 10 ng Renilla luciferase reporter as a control for transfection efficiency. Transfection with a Runx2 expression vector served as a positive control. Cells were harvested 24 h after transfection in 250 µl of 1x Reporter Lysis Buffer and the lysates were processed for luciferase assays. Firefly values were normalized with respect to Renilla luciferase values. Transfections were carried out in triplicate. Error bars represent SD between triplicates.
Runx3 associates with mitotic chromosomes and occupies the rRNA promoter
When cells prepare for mitosis, the chromosomes condense, subnuclear organization is altered and the nuclear envelope disassembles. Cell fate determining transcription factors such as Runx1 and Runx2 remain associated with mitotic chromosomes (Young et al, 2007a; Young et al, 2007b). Because Runx1 and Runx2 epigenetically regulate phenotypic genes and ribosomal RNA genes to maintain lineage commitment and growth of progeny cells, we analyzed the subcellular distribution of Runx3 during mitosis. In transiently transfected cells, Runx3 foci remain associated with chromosomes during mitosis. These foci co-localize with UBF1, a principal component of the RNA polymerase I (Pol I) controlled rRNA transcription machinery and a defining marker of the nucleolar organizing regions (NORs) in mitotic chromosomes. This association of Runx3 and UBF1 becomes prominent during prophase and persists through anaphase, but is no longer apparent when the nuclear architecture reassembles during telophase (Fig. 5). Thus, the association of Runx3 with NORs suggests that Runx3 is an epigenetic regulator of gene expression similar to Runx1 and Runx2.
Fig. 5. Runx3 localizes to the nucleolar organizing regions on mitotic chromosomes.
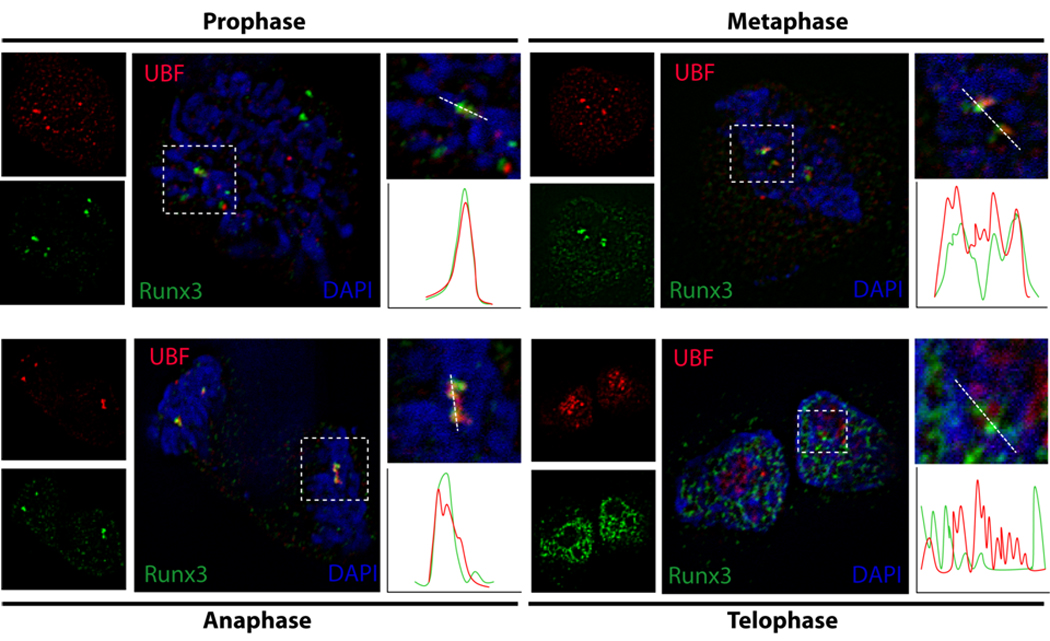
Immunofluorescence microscopy for Runx3 (green) and UBF1 (red) with DAPI staining (blue) and merged images showing co localization. HeLa cells were grown on gelatin-coated coverslips and transfected for 24 h with 500 ng of full length Runx3. Cells were processed for in situ immunofluorescence microscopy. The coverslips were incubated with a monoclonal antibody against FLAG tag and a polyclonal UBF1 antibody followed by incubation with anti mouse Alexa 488 conjugated and anti rabbit Alexa 568 conjugated secondary antibody (1:800) to detect Runx3 and UBF1 respectively. Images of cells in various stages of mitosis were taken using Zeiss Axioplan digital microscope and Metamorph software was used for bioimaging.
The in situ interaction of Runx3 with ribosomal DNA repeats to control rRNA transcription was biochemically validated by chromatin immunoprecipitation (ChIP) analysis. We used PCR primers that selectively amplify the ribosomal RNA promoter and IEC-6 gastric epithelial cells that endogenously express Runx3 as established by western blotting. ChIP results show that rRNA promoter fragments are selectively amplified in Runx3 immunoprecipitates, while the tubulin 3’ UTR (which serves as a negative control) is not. We conclude that endogenous Runx3 occupies ribosomal rRNA promoters in gastric epithelial cells consistent with its in situ localization with NORs.
DISCUSSION
In this study, we have delineated a subnuclear targeting signal (aa 283–325) that directs the tumor suppressor protein Runx3 to discrete transcriptional foci that are nuclear matrix associated. We also establish that Runx3 remains associated with chromosomes during mitosis and localizes at NORs. Thus, our study provides a molecular basis for the tight association of Runx3 with components of nuclear architecture.
Apart from subnuclear compartmentalization, Runx proteins exhibit dynamic shuttling between nuclear and cytoplasmic compartments. For example, we have shown that the presence of Runx2 in the nucleus is controlled by the balance between microtubule dependent import and CRM1 dependent (i.e., leptomycin-sensitive) nuclear export in human osteosarcoma cells (Pockwinse et al, 2006). In our study, we observed that both full length Runx3 and a deletion mutant spanning 1–187 are both present in the nucleus of HeLa cervical carcinoma cells as established by IF and biochemical fractionation followed by western blotting. Earlier studies have shown that the 1–187 deletion mutant of Runx3 localizes to the cytoplasm of SNU16 gastric cancer cells (Ito et al, 2005). However, endogenous Runx3 protein is also present in the cytoplasm and enters the nucleus only on stimulation of these cells by TGFβ (Ito et al, 2005; Yano et al, 2006). Thus, there appear to be cell type-specific differences in the nuclear localization of Runx3 between SNU16 cells and HeLa cells. It is possible that Runx3 expressed in HeLa cells may not be subjected to nuclear export or that the protein is not sequestered in the cytoplasm.
The targeting of Runx3 to the nuclear matrix may have significant biological consequences as the transcriptional activity of the closely related Runx1 and Runx2 proteins is regulated by association with the nuclear matrix. The nuclear matrix targeting signals of Runx1 and Runx2 overlap their transcriptional activation domains, and the NMTS supports the in situ integration of cell signaling and transcriptional responses at target genes. Compromised subnuclear targeting of these proteins has been linked to defects in cellular maturation in both myeloid and bone lineages, as well as to increased tumorigenic potential of cells (Zeng et al, 1997; Zaidi et al, 2001; Vradii et al, 2005; Zaidi et al, 2007; Bakshi et al, 2008; McNeil et al, 1999; Barseguian et al, 2002). We propose that disruption of Runx3 subnuclear targeting may abrogate target gene activation and have important pathological implications for the development of gastric tumors.
During mitosis, the nuclear envelope and matrix are disassembled, chromatin condenses to form distinct chromosomes, and a large number of transcription factors are displaced from their target promoters or are degraded during mitotic gene silencing. Our previous studies have shown that lineage specific transcription factors that include Runx2 remain associated with chromosomes during all phases of mitosis through sequence specific DNA binding (Zaidi et al, 2003; Young et al, 2007a; Young et al, 2007b). A large number of cell cycle control and lineage commitment genes are mitotically regulated by Runx2, thus providing a critical regulatory function in progeny cells for post mitotic gene expression in G1 (Young et al, 2007b). On the mitotic chromosomes, Runx2 not only associates with Pol II dependent genes but also represses Pol I dependent transcription of ribosomal RNA genes (Young et al, 2007a). We report here that Runx3 is retained on the chromosomes of mitotic cells where it associates with UBF1, a principal component of the rRNA transcription machinery and remains associated with the ribosomal gene promoter as shown by ChIP assays. Loss of growth regulation and enhanced protein synthesis are hallmarks of transformation. Downregulation of Runx1 and Runx2 modulates rRNA synthesis and protein synthesis capacity (Bakshi et al, 2008; Young et al, 2007a). The association of Runx3 with mitotic chromosomes and specifically with the promoter of ribosomal RNA genes raises the possibility that loss of Runx3-mediated tumor suppression may deregulate epigenetic control and protein synthesis during gastric tumor development.
Fig. 6. Runx3 occupies the rDNA promoter in vivo.
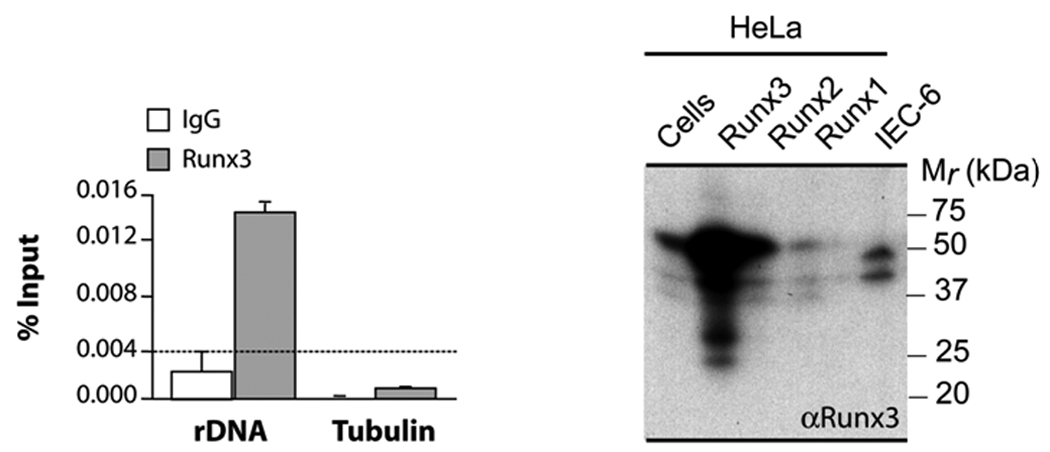
IEC-6 cells that express endogenous Runx3 (right panel) were processed for chromatin immunoprecipitation. Immunoprecipitation was carried out using Runx3 antibody (Santa Cruz) and normal rabbit IgG (Santa Cruz). The immunoprecipitated DNA samples were amplified using rat rDNA promoter primer (rrDNA_3.1) or tubulin 3’ UTR primer which serves as a negative control. Samples immunoprecipitated by normal rabbit IgG served as control for nonspecific immunoprecipitation of genomic DNA (left panel).
ACKNOWLEDGEMENTS
We thank the members of our research group for stimulating discussions throughout the course of these studies. We are appreciative of editorial assistance from Judy Rask and Marta DeSourdis for assistance with the preparation of this manuscript.
Contract Grant Sponsor: National Institutes of Health grant P01 CA082834, P01 AR048818 and R01 AR039588. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004;23:4336–4340. doi: 10.1038/sj.onc.1207286. [DOI] [PubMed] [Google Scholar]
- Bakshi R, Zaidi SK, Pande S, Hassan MQ, Young DW, Lian JB, van Wijnen AJ, Stein JL, Stein GS. The leukemogenic t(8;21) fusion protein AML1-ETO controls ribosomal RNA genes and associates with nucleaolar organizing regions at mitotic chromosomes. 2008 doi: 10.1242/jcs.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barseguian K, Lutterbach B, Hiebert SW, Nickerson J, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Multiple subnuclear targeting signals of the leukemia-related AML1/ETO and ETO repressor proteins. Proc Natl Acad Sci U S A. 2002;99:15434–15439. doi: 10.1073/pnas.242588499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushmeyer SM, Atchison ML. Identification of YY1 sequences necessary for association with the nuclear matrix and for transcriptional repression functions. J Cell Biochem. 1998;68:484–499. [PubMed] [Google Scholar]
- Coffman JA. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int. 2003;27:315–324. doi: 10.1016/s1065-6995(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ, Rad R, Langer R, Voland P, Hoefler H, Schmid RM, Prinz C, Gerhard M. Lack of RUNX3 regulation in human gastric cancer. J Pathol. 2006;210:141–146. doi: 10.1002/path.2042. [DOI] [PubMed] [Google Scholar]
- Galindo M, Pratap J, Young DW, Hovhannisyan H, Im HJ, Choi JY, Lian JB, Stein JL, Stein GS, van Wijnen AJ. The bone-specific expression of RUNX2 oscillates during the cell cycle to support a G1 related anti-proliferative function in osteoblasts. J Biol Chem. 2005;280:20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, Stein JL, van Wijnen AJ, Wang Y-L, Stein GS. Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J Cell Sci. 2002;115:4167–4176. doi: 10.1242/jcs.00095. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ozaki S, Ito K, Iseda T, Kawaguchi S, Ogawa M, Bae SC, Yamashita N, Itohara S, Kudo N, Ito Y. Runx3 is essential for the target-specific axon pathfinding of trkc-expressing dorsal root ganglion neurons. Blood Cells Mol Dis. 2003;30:157–160. doi: 10.1016/s1079-9796(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ozaki S, Shiga T, Ito K, Masuda T, Okado N, Iseda T, Kawaguchi S, Ogawa M, Bae SC, Yamashita N, Itohara S, Kudo N, Ito Y. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci. 2002;5:946–954. doi: 10.1038/nn925. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shiga T, Ito Y. Runx transcription factors in neuronal development. Neural Develop. 2008;3:20. doi: 10.1186/1749-8104-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, Hiong KC, Peh BK, Han HC, Ito T, Teh M, Yeoh KG, Ito Y. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–7750. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- Javed A, Guo B, Hiebert S, Choi J-Y, Green J, Zhao S-C, Osborne MA, Stifani S, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Groucho/TLE/R-Esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J Cell Sci. 2000;113:2221–2231. doi: 10.1242/jcs.113.12.2221. [DOI] [PubMed] [Google Scholar]
- Kagoshima H, Shigesada K, Kohara Y. RUNX regulates stem cell proliferation and differentiation: insights from studies of C. elegans. J Cell Biochem. 2007;100:1119–1130. doi: 10.1002/jcb.21174. [DOI] [PubMed] [Google Scholar]
- Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, Kremer E, Otto F, Brenner O, Lev-Tov A, Groner Y. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QL, Kim HR, Kim WJ, Choi JK, Lee YH, Kim HM, Li LS, Kim H, Chang J, Ito Y, Youl LK, Bae SC. Transcriptional silencing of the RUNX3 gene by CpG hypermethylation is associated with lung cancer. Biochem Biophys Res Commun. 2004;314:223–228. doi: 10.1016/j.bbrc.2003.12.079. [DOI] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- McNeil S, Zeng C, Harrington KS, Hiebert S, Lian JB, Stein JL, van Wijnen AJ, Stein GS. The t(8;21) chromosomal translocation in acute myelogenous leukemia modifies intranuclear targeting of the AML1/CBFalpha2 transcription factor. Proc Natl Acad Sci U S A. 1999;96:14882–14887. doi: 10.1073/pnas.96.26.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene. 2004;23:4232–4237. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- Nickerson JA. Experimental observations of a nuclear matrix. J Cell Sci. 2001;114:463–474. doi: 10.1242/jcs.114.3.463. [DOI] [PubMed] [Google Scholar]
- Okorokov AL, Rubbi CP, Metcalfe S, Milner J. The interaction of p53 with the nuclear matrix is mediated by F-actin and modulated by DNA damage. Oncogene. 2002;21:356–367. doi: 10.1038/sj.onc.1205112. [DOI] [PubMed] [Google Scholar]
- Pockwinse SM, Rajgopal A, Young DW, Mujeeb KA, Nickerson J, Javed A, Redick S, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Doxsey SJ. Microtubule-dependent nuclear-cytoplasmic shuttling of Runx2. J Cell Physiol. 2006;206:354–362. doi: 10.1002/jcp.20469. [DOI] [PubMed] [Google Scholar]
- Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi J-Y, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Cell growth regulatory role of Runx2 during proliferative expansion of pre-osteoblasts. Cancer Res. 2003;63:5357–5362. [PubMed] [Google Scholar]
- Saltman LH, Javed A, Ribadeneyra J, Hussain S, Young DW, Osdoby P, Amcheslavsky A, van Wijnen AJ, Stein JL, Stein GS, Lian JB, Bar-Shavit Z. Organization of transcriptional regulatory machinery in osteoclast nuclei: compartmentalization of Runx1. J Cell Physiol. 2005;204:871–880. doi: 10.1002/jcp.20329. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, van Wijnen AJ, Javed A, Montecino M, Zaidi SK, Young DW, Choi JY, Pratap J. Combinatorial organization of the transcriptional regulatory machinery in biological control and cancer. Adv Enzyme Regul. 2005;45:136–154. doi: 10.1016/j.advenzreg.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, van Wijnen AJ, Stein JL, Javed A, Montecino M, Zaidi SK, Young D, Choi JY, Gutierrez S, Pockwinse S. Nuclear microenvironments support assembly and organization of the transcriptional regulatory machinery for cell proliferation and differentiation. J Cell Biochem. 2004a;91:287–302. doi: 10.1002/jcb.10777. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004b;23:4315–4329. doi: 10.1038/sj.onc.1207676. [DOI] [PubMed] [Google Scholar]
- Tang L, Guo B, Javed A, Choi J-Y, Hiebert S, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Zhou GW. Crystal structure of the nuclear matrix targeting signal of the transcription factor AML-1/PEBP2αB/CBFα2. J Biol Chem. 1999;274:33580–33586. doi: 10.1074/jbc.274.47.33580. [DOI] [PubMed] [Google Scholar]
- van Wijnen AJ, Stein GS, Gergen JP, Groner Y, Hiebert SW, Ito Y, Liu P, Neil JC, Ohki M, Speck N. Nomenclature for Runt-related (RUNX) proteins. Oncogene. 2004;23:4209–4210. doi: 10.1038/sj.onc.1207758. [DOI] [PubMed] [Google Scholar]
- Vradii D, Zaidi SK, Lian JB, van Wijnen AJ, Stein JL, Stein GS. Point mutation in AML1 disrupts subnuclear targeting, prevents myeloid differentiation, and effects a transformation-like phenotype. Proc Natl Acad Sci, USA. 2005;102:7174–7179. doi: 10.1073/pnas.0502130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, Le X, Yao J, Wu TT, Huang S, Xie K. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809–4816. doi: 10.1158/0008-5472.CAN-04-3741. [DOI] [PubMed] [Google Scholar]
- Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, Levanon D, Groner Y. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K, Ida H, Bouillet P, Strasser A, Bae SC, Ito Y. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DW, Hassan MQ, Pratap J, Galindo M, Zaidi SK, Lee SH, Yang X, Xie R, Javed A, Underwood JM, Furcinitti P, Imbalzano AN, Penman S, Nickerson JA, Montecino MA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007a;445:442–446. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- Young DW, Hassan MQ, Yang X-Q, Galindo M, Javed A, Zaidi SK, Furcinitti P, Lapointe D, Montecino M, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic retention of gene expression patterns by the cell fate determining transcription factor Runx2. Proc Natl Acad Sci USA. 2007b;104:3189–3194. doi: 10.1073/pnas.0611419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Javed A, Choi J-Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin gene. J Cell Sci. 2001;114:3093–3102. doi: 10.1242/jcs.114.17.3093. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Pande S, Pratap J, Gaur T, Grigoriu S, Ali SA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc Natl Acad Sci U S A. 2007;104:19861–19866. doi: 10.1073/pnas.0709650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Pockwinse SH, Javed A, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic partitioning and selective reorganization of tissue specific transcription factors in progeny cells. Proc Natl Acad Sci USA. 2003;100:14852–14857. doi: 10.1073/pnas.2533076100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, McNeil S, Pockwinse S, Nickerson JA, Shopland L, Lawrence JB, Penman S, Hiebert SW, Lian JB, van Wijnen AJ, Stein JL, Stein GS. Intranuclear targeting of AML/CBFα regulatory factors to nuclear matrix-associated transcriptional domains. Proc Natl Acad Sci USA. 1998;95:1585–1589. doi: 10.1073/pnas.95.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, van Wijnen AJ, Stein JL, Meyers S, Sun W, Shopland L, Lawrence JB, Penman S, Lian JB, Stein GS, Hiebert SW. Identification of a nuclear matrix targeting signal in the leukemia and bone-related AML/CBFα transcription factors. Proc Natl Acad Sci USA. 1997;94:6746–6751. doi: 10.1073/pnas.94.13.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]