Abstract
Hypertrophic scarring after burns is an unsolved problem and remains as devastating today as it was in the 40s and it may be that the main reason for this is the lack of an accepted, useful animal model. The female, red Duroc pig was described as a model of hypertrophic scarring nearly 30 years ago but then vanished from the literature. This seemed strange since the authors reported that 12 of 12 pigs developed thick scar. In the mid 90s we explored the model and found that, indeed, the red Duroc pig does make thick scar. Other authors have established that the Yorkshire pig does not heal in this fashion so there is the possibility of a same species control. We have continued to explore the Duroc/Yorkshire model and herein describe our experiences. Is it a perfect model of hypertrophic scarring? No. Is it a useful model of hypertrophic scarring? Time will tell. We have now obtained gene expression data from the Duroc/Yorkshire model and analysis is underway.
Hypertrophic scarring is a significant negative outcome of a burn injury. Hypertrophic scars (HSs) are hard, raised, red, itchy, tender, and contracted.1,2 These scars are ugly and uncomfortable and may diminish, but never completely go away. The resulting disfigurement and scarring affects quality of life, which, in turn, can lead to lowered self-esteem, social isolation, prejudicial societal reactions, and job discrimination.3–7 Scarring may also have profound rehabilitation consequences including loss of physical function, impairment, disability, and difficulties pursuing recreational and vocational pursuits.6,8,9
Literally hundreds of studies of collagen, fibroblasts, and growth factors in HSs have been performed over the past decades and yet the pathophysiology and treatment of this process are still essentially unknown.10–18 It can be argued that the reason that the etiology of human HS is unknown is the absence of a useful animal model.18–22 Mice, rats, rabbits, dogs, and cats have all failed to produce scar analogous to human HS. Repetitive literature searches have yielded few references to validated animal models of HS. Morris et al.21 reported a scar model in the rabbit ear following a small full-thickness wound, but this is quite different from the human situation that involves large, partial-thickness wounds so the relationship is somewhat questionable. Since originally reported, this model has been adopted by one other group.23 Xiang et al.24 created 2×5 cm full-thickness wounds on rabbit ears but again the full-thickness model seems to differ from the human condition. Models that include human HS tissue implanted into athymic rats and mice have also been described.22,25–31 Since originally reported, these athymic models have been adopted by two other groups in two studies,32,33 but this model seems very dissimilar to the clinical situations leading to HS formation in humans. Furthermore, the human transplanted tissue is established scar so any early morphogenic changes are missed. Yang et al.34 implanted normal human skin into nude mice and created wounds in the transplanted skin. But again the model seems quite dissimilar to the human situation. The authors recently reported that wounding the transplanted skin is not necessary for the development of hypertrophic scar.35 Aksoy et al.18 described a hypertrophic scar model in the albino, male guinea pig after excision of the panniculus carnosus and application of thermal injury or coal tar. Again, this seems quite dissimilar to the human condition and we could find no further use of this model. It would appear that no animal model of hypertrophic scarring has acquired the status of “gold standard” and become widely adopted.
Nearly 30 years ago, Silverstein and colleagues36,37 reported that deep partial thickness wounds in 12 of 12 female red Duroc pigs healed with “hypertrophic” scarring but was never published in a peer-reviewed journal. We could find no further studies of hypertrophic scarring utilizing this model. A manuscript by Aksoy et al.18 referenced an abstract presented at the 1981 meeting of the Plastic Surgery Research Council,38 which stated, “We initially performed several investigative studies in red Duroc pigs, including … various types of dermatome excisions … all without success”; however, no description of the methods nor actual results were provided. Furthermore, the abstract referenced a personal communication from B. Pruitt that the model was not reproduced in later studies. A review article by Kischer et al.26 also reported that the model had never been reproduced but gave no references.
We contacted Dr. Erk but it would appear that the details of the abstract are no longer available. We also discussed the abstract with Dr. Pruitt39,40 who could not recall this personal communication but did say “the scars were initially impressive, with time, spontaneous diminution occurred. Dr. Silverstein became discouraged when others criticized the model on the basis of that diminution ….” We also discussed the model with Dr. Silverstein who stated “While my scars did diminish somewhat over time, they never totally flattened out” and second “when Dr. Salisbury later tried the model on minipigs the wounds did not heal with thick scar.”41,42 Dr. Salisbury commented “We couldn’t duplicate Paul’s findings in the Duroc pig.”43 Dr. Robson also recalled that “over time the scars melted away.”44 Clearly there was some problem with this model but it seemed strange that the first 12 of 12 Durocs demonstrated thick scar at 5 months postwounding but then subsequent Durocs did not.
As the acquisition of tissue from human partial-thickness wounds in a systematic and controlled fashion is difficult, and our understanding of hypertrophic scarring is so minimal, we decided to attempt to validate an animal model. This Duroc model had appeal owing to the similarity of porcine skin to human skin, the similarity of the wounds to human wounds that result in HS, and the initial reported occurrence of thick scar in 12 of 12 animals. We have attempted to summarize our efforts in this review. We have also addressed the similarities and differences of pig and human skin and possible reasons that the Duroc model was not further utilized after the original description.
Summary of our validation of the female red Duroc pig model of hypertrophic scarring
To validate an animal model, biologic findings in that model must be compared with the findings in the human condition. We reviewed the literature for human HS and enumerated the characteristic features. We felt that each criterion must be confirmed in the Duroc tissue to validate the model including (1) clinical appearance, (2) histologic appearance, (3) proteins and other biomolecules, (4) nerve fiber counts, (5) mast cell counts, (6) presence of collagen nodules, and (7) presence of myofibroblasts (Table 1).45–51
Table 1.
Summary of comparison between thick scar of the female Duroc pig and human hypertrophic scar
| Shallow female Duroc wounds | Deep female Duroc wounds | Human wounds that develop hypertrophic scar | |
|---|---|---|---|
| Healed at 3 weeks | Yes | No | No |
| Shallow female Duroc scar | Deep female Duroc scar | Human hypertrophic scar | |
| Scar raised above surrounding skin | No | Up to 2 mm | Up to 1 cm |
| Color of scar is red | No | No | Yes |
| Scar is thicker than uninjured skin | No | Yes | Yes |
| Disorganized collagen | No | Yes | Yes |
| Whorls/nodules | No | Yes | Yes |
| Elevated mast cell counts | No | Yes | Yes |
| Myofibroblasts | Yes at 1, 2, and 3 weeks | Yes at 1, 2, and 3 weeks | Yes |
| Shallow female Duroc |
Deep female Duroc wounds |
Human hypertrophic scar | |||
|---|---|---|---|---|---|
| Early | Late | Early | Late | ||
| TGFβ1 | |||||
| Protein | ↑ | ↑ | |||
| mRNA | ↑ | ↑ | ↑ | ||
| IGF-1 | |||||
| Protein | ↑ | ↑ | ↑ | ↑ | |
| mRNA | ↑ | ↑ | ↑ | ↑ | |
| Decorin | |||||
| Protein | ↓ | ↓ | ↓ | ||
| Versican | |||||
| Protein | ↑ | ↑ | ↑ | ||
| NO | ↓ | ↓ | ↓ | ||
| Nerve fiber counts | ↑ | ↑ | |||
NO, nitric oxide.
Wound model
Our hypotheses regarding hypertrophic scarring include that both the deep dermis and the cones of the dermis47 play a role in hypertrophic scarring. Therefore, our wounds are deep partial thickness leaving the deepest portion of the dermis and the deep aspect of the cones. When the study requires the thickest scars, we leave very little dermis. When the study involves the deep aspects of the cones, we leave more of the deep dermis. At present, we are not studying full-thickness wounds where no dermis remains.
As previously reported,50 tangential wounds are created with a Padgett dermatome (Integra LifeSciences Corporation, Plainsboro, NJ) on the backs of 7-week-old female, red Duroc pigs. The dermatome is set to 0.015 in., 0.020 in., or 0.030 in. It is known that actual wound depth obtained with a dermatome is quite variable.52 Furthermore, for the deeper wounds two or more passes of the dermatome are necessary that introduces further error in wound depth. Therefore, we refer to total dermatome setting rather than wound depth. Eight 7×7 cm wounds are created on the back of each Duroc and the total dermatome settings are divided into six groups: 0.015 in., 0.030 in., 0.045 in., 0.060 in., 0.075 in., and 0.090 in. The wounds are allowed to heal without application of topical agents or dressings. Tissue samples are routinely harvested at 1, 2, and 3 weeks and 3 and 5 months after wounding. At 20 weeks, the pigs are returned to the producer.
Clinical appearance of Duroc wounds
At 3 weeks, shallow partial thickness wounds are usually healed, but deep partial thickness wounds are not. The gross clinical appearance of cutaneous scar on the female red Duroc after shallow partial thickness wounds is essentially the same as uninjured skin. The gross appearance of deep dermal wounds is raised, hard, hyperpigmented, and contracted, similar to human HS.47 It is not, however, as raised as in man. Whereas human HSs may reach 1–2 cm elevation above the surrounding skin, the scar elevation in the Duroc is at most 1–2 mm. Furthermore, the Duroc scars are not as red as in man.
Histologic appearance
After shallow injury, the histology of the healed wound at 3 weeks is essentially the same as uninjured skin. After deep partial thickness wounds, scars on the Duroc pig may reach 11 mm in thickness from hypodermis to stratum corneum compared with the normal skin thickness of ~3 mm (Figure 1).47,51 However, the shape of the scars is quite different from human HS and, as indicated above, only 1–2 mm of this scar extends above the surrounding uninjured skin, the remainder projects inward (Figure 2). There are large numbers of disorganized collagen fibers in the scar, in some places formed into whorls and nodules.47,51
Figure 1.
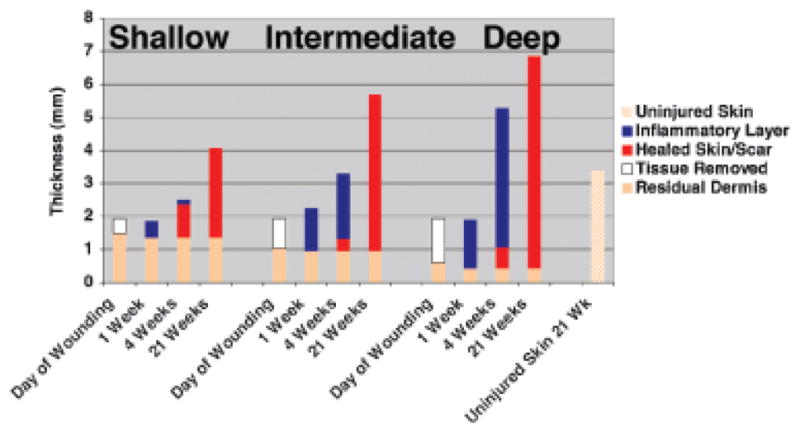
Thickness of residual dermis, removed tissue, inflammatory layer, healed skin/scar, and uninjured in shallow, intermediate, and deep Duroc wounds.
Figure 2.

Shape of thick scar from deep wounds.
Proteins and other biomolecules
TGFβ1
TGFβ1 is a suspected mediator of fibrosis in a number of organs, including skin, lung, liver, and kidney.53–56 TGFβ1 protein is found in human HS54 and TGFβ1 mRNA is elevated in HS compared with uninjured tissue.57
After shallow wounds, TGFβ1 localization in the healing wounds was not immunohistochemically evident at any time. In contrast, in deep partial thickness wounds TGFβ1 was detectable at early time points in cells of the central scar mass and then declined to weakly detectable levels in the extracellular matrix in the depths of the wound at 5 months, probably in the residual dermis,47,49 which correlates with the findings reported for human HS.54 In both shallow and deep wounds, TGFβ1 mRNA was elevated at early time points and then declined to the levels of uninjured skin at 5 months,49 which also corresponds to the reports for human HS.57
IGF-1
Ghahary and others have suggested that IGF-1 may be involved in wound repair and associated with fibrosis.58–60 The authors reported that (1) IGF-1 protein was increased 78% in HS over uninjured skin61 and (2) IGF-1 mRNA is elevated early after wounding.
In shallow wounds, IGF-1 localization at early times was found in the epidermis, neo-vessels, and inflammatory cells. After 30 days, the staining was restricted to the epidermis and endothelial cells as in uninjured skin. In deep wounds at 10 days, a very strong expression was found throughout the wound that declined to 150 days when the expression was found only in a few fibroblasts47,49, which correlates with the published reports for human HS.61 IGF-1 mRNA as detected by in situ hybridization in shallow wounds at 10 days was found in inflammatory cells and by 30 days was essentially gone. In deep wounds, the expression at 10 days was overwhelming throughout the wound bed. This was greatly diminished at 5 months but still greater than in uninjured skin.47,49 With qRTPCR, in shallow wounds IGF-1 mRNA was elevated at early times and declined to the levels of uninjured skin. In deep wounds, the levels were significantly elevated at day 10 and declined thereafter. There was a second lesser rise at 3 months. Levels were still elevated, however, at 5 months compared with shallow wounds.49 These findings also correlate with reported findings for human HS.62 The second rise at 3 months also correlates with the findings of Gallant et al.63 who has also extensively evaluated scarring in red Duroc pigs.63–66
Decorin
Decorin is the most abundant proteoglycan in normal dermis67 and has been reported to promote formation of correct collagen fibrils.68 It is possible, then, that in the absence of decorin, the structure and organization of collagen fibrils become distorted into whorls and nodules. It has been reported that decorin expression is reduced in HSs.69,70
In shallow Duroc wounds, decorin localization was reduced at 10 days but returned to normal at 30 days. In deep wounds, decorin staining was consistently reduced even at 5 months.47,50 This pattern is similar to that reported in human HS.69,70
Versican
Versican is highly expressed in fast growing tissue and cells, occupies the space between the collagen fibrils, and interferes with the assembly of fibrils into bundles and fibers.70–72 This large proteoglycan has 12–15 chondroitin sulfate chains, which are largely responsible for the water-holding capacity of connective tissue.73 Versican has been found to be virtually undetectable in normal dermis70 and increased in HS.70,74
In shallow wounds at all time points, we found no versican immunostaining. In deep wounds, versican appeared at 30 days in the scar tissue layer and the staining increased thereafter to 150 days.47,50 These findings are similar to those reported for human HS.70,72
Nitric oxide (NO)
NO production both by inducible NO synthase and constitutive endothelial NO synthase plays many important roles in wound healing from the early inflammatory phase through the process of scar remodeling.75 In addition, it has been suggested that NO may be involved in hypertrophic scarring.76
NO levels in deep Duroc wounds were higher than in shallow wounds at 10 days and then declined to levels significantly lower than in shallow wounds at 5 months.50 These low levels are similar to those reported for human HS.76
Nerve fiber counts
Recently, the nerve system of skin has been implicated in wound healing, perhaps via the biological effects of neuropeptides including the proliferation of epithelial, vascular, and connective tissue.77–82 The debilitating itching and pain associated with HS implicates sensory nerves within this aberrant healing process. Several investigators have studied nerves in HS. Crowe et al.83 demonstrated an increase in the number of neuropeptide-containing nerves in human HS compared with uninjured skin. Zhang and Laato84 reported that HSs are characterized by extensive nerve fasciculi identified by immunofluorescence with antineurofilament antibodies.
In the thick scar of the female Duroc pig, nerve density is increased,48 which correlates with the findings in human HS.83,84
Mast cells, collagen nodules, and myofibroblasts
Mast cell counts are increased in Duroc scar as they are in human HS.51 Collagen nodules are present although not as common as in human HSs.51 Myofibroblasts are present at 1, 2, and 3 weeks but are quite sparse at 3 and 5 months.51
Summary of findings from other groups on healing in the female, red Duroc pig
The female, red Duroc pig model has been studied by two other groups of investigators. Gallant and colleagues63–66 have found or confirmed the following:
Wounds created with dermatomes have a variable depth, which must be considered when obtaining biopsies.
Red Duroc pigs form hypercontracted, hyperpigmented, fibroproliferative scars with collagen nodules as compared with Yorkshires.
There is no difference between female and castrated male Durocs.
A biphasic pattern of gene expression for bone morphogenetic protein, types I and III collagen, heat shock protein 47, decorin, fibromodulin, osteopontin, TIMPs 1–3. This pattern was not seen in their Yorkshire pigs. Our own work with IGF-1 also demonstrated this biphasic response.49
First generation cross pigs exhibit an intermediate healing phenotype.
Wound depth is influential in wound healing as deep partial-thickness wounds behave quite differently from full-thickness wounds.
There are some differences in our “Duroc model” and that used by these authors. Our wounds are 6×6 up to 8×8 cm compared with the 2×2 cm wounds the authors reported. In addition, we follow the wounds for 20 weeks compared with the author’s 10 weeks. It is possible that wound geometry and time after wounding may alter the outcomes. Furthermore, the authors use a dermatome setting of 1.8 mm or 0.072 in., whereas we set the dermatome at 0.5 mm or 0.020 in. This difference could introduce differences in actual wound depth. It may be that there are Duroc models of scarring rather than one Duroc model.
Nevertheless, the authors reported gross and histologic findings similar to ours with the exception of the following. The authors reported that at 10 weeks the wounds were no longer raised or overtly fibrotic. As mentioned above, the thick scars in the Durocs are far less raised than HS in man. Usually the deep partial thickness wounds in the Durocs are raised ~1 mm compared with 1–2 cm for HS in man.
In summary, the authors have conducted extensive studies of healing in the Duroc pig and confirmed many findings. In addition, they added an extensive array of gene expression data. The comparable findings between the two studies are similar and seem to demonstrate that healing in the Duroc differs from that of the Yorkshire and is more similar to fibroproliferative scarring.
Liang et al.85 repeated the histologic studies and found thick scar at 5 months and that the cones exist in Duroc skin and are severely amputated in deep wounds.
Pig as an animal model of cutaneous wound healing
Given the difficulty working with a large animal model, it is worth evaluating the benefits of porcine models. The skin of the pig is known to be similar to human skin. We will summarize some of the similarities and differences.47,86–98
Pigs can sustain sunburn, as can man, and both species rely on fat, not fur, for insulation. Both have sparse hair over most of the body. The epidermis of both is thick with distinct rete pegs and dermal papillae and the dermal–epidermal ratio varies from 10:1 to 13:1. The total turnover time of the epidermis in both is ~30 days and the epidermis of both contains Langerhans cells. Both contain elastic fibers. The collagenous tissue framework of the dermis and the adipose chambers of the hypodermis are quite similar. Immunohistochemical staining of human and porcine skin shows similar patterns for keratins 10 and 16, collagen IV, fibronectin, and vimentin. Collagen structure in porcine skin is remarkably similar to human collagen and evokes a minimal immune response in man. Both skins have cones in the dermis.47
There are dissimilarities however. Porcine epidermis includes only three layers (germinativum, granulosum, and corneum) and stratum corneum is thicker and very compact. Over the body surface, the pig has only apocrine sweat glands that are not involved in thermal regulation. Eccrine sweat glands in the pig are found only in the snout, lips, and “carpal organ.” The epidermis of the pig is high in alkaline phosphatase, whereas the endothelium of the surface capillaries of the pig contains no alkaline phosphatase. The hairs of the pig have practically no medulla and, in active colored hair follicles, melanocytes are present not only in the bulb, above the critical level, but a special population of them is also found in the matrix. The sebaceous glands of the pig contain much alkaline phosphatase and no glycogen. The pig displays a rapid and marked mast cell sensitivity to stress.
As a result of these general similarities, as mentioned by Rothschild99,100 the pig has assumed an important place in wound healing research and has been used in many studies, with many from the laboratories of Eriksson, Hart, Nanney, and Davis. Most studies used the young female Yorkshire pig. We found only two groups using the Duroc101–103 and two using the Yucatan minipig104,105 before the studies referenced in this manuscript.
Why was the female, red Duroc pig model of hypertrophic scarring abandoned?
It is strange that the first authors reported thick scar in 12 of 12 pigs or 100% and then did not study the model further. It is also strange that the first 12 of 12 pigs produced thick scar and subsequent Durocs studied by other authors did not. It seems clear from the history we obtained by personal communication that the problem has to do with the thickness of the scar at 5 months postwounding. We suspect that this has to do with wounding and wound depth. Healing with thick scar only occurs with deep wounds. Full-thickness wounds are quite easy to make. But then all of the dermis is removed and our HS hypothesis is that the deep dermis is required. Leaving a small portion of the dermis is difficult. It may be that the model was discarded because too much dermis was left in the wounds resulting in normotrophic healing. In addition, at that time very few antibodies and reagents were available for pig tissues. And finally for other reasons, as described below, the model is not perfect.
Is the female, red Duroc model of hypertrophic scarring a perfect model?
No. In addition to the problem of wound creation described above, Duroc pigs are not easy to obtain, as they are not common in the food industry. In addition, they are large (average 200# at 5 months of age) and expensive. Furthermore, their life span is 6–8 years and they achieve sexual maturity in 5–6 months so it is difficult to compare the biologic clock of the pig with the human.
Summary
Porcine models may be our best hope for recreating human wounds using animal models. We and others have recently revived the female red Duroc pig model of hypertrophic scarring that Silverstein and colleagues36,37 originally described in 12 of 12 Duroc pigs in the 1970s. As the scars, in our experience with 12 animals, are not as thick as human HSs or as raised above the surrounding skin, we refer to the scar in Durocs as “thick” rather than as “hypertrophic.” Nevertheless, our data combined with the work of Gallant and colleagues suggest that many similarities exist between the thick scars of the female red Duroc pig and human HS. As porcine microarrays are now available, and given the devastating nature of hypertrophic scarring, our ignorance as to cause and treatment, and the paucity of accepted animal models, we have now used the Duroc/Yorkshire model to obtain gene expression data in shallow and deep wounds at 1, 2, and 3 weeks and 3 and 5 months. Analysis is underway.
Acknowledgments
This work was funded by (1) Washington State Council of Firefighters Burn Foundation; (2) National Institute on Disability and Rehabilitation Research/Office of Special Education and Rehabilitation Services/US Department of Education; (3) National Institutes of Health; and the Northwest Burn Foundation. In addition, the authors are indebted to Dr. Edward E. Tredget, MD, for invaluable advice on how to validate the model.
References
- 1.Ehrlich HP, Kelley SF. Hypertrophic scar: an interruption in the remodeling of repair—a laser Doppler blood flow study. Plast Reconstr Surg. 1992;90:993–8. [PubMed] [Google Scholar]
- 2.Rudolph R. Wide spread scars, hypertrophic scars, and keloids. Clin Plast Surg. 1987;14:253–60. [PubMed] [Google Scholar]
- 3.Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA. Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J Trauma. 1983;23:1001–4. doi: 10.1097/00005373-198311000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Fauerbach JA, Heinberg LJ, Lawrence JW, Bryant AG, Richter L, Spence RJ. Coping with body image changes following a disfiguring burn injury. Health Psychol. 2002;21:115–21. [PubMed] [Google Scholar]
- 5.Fauerbach JA, Heinberg LJ, Lawrence JW, Munster AM, Palombo DA, Richter D, Spence RJ, Stevens SS, Ware L, Muehlberger T. Effect of early body image dissatisfaction on subsequent psychological and physical adjustment after disfiguring injury. Psychosom Med. 2000;62:576–82. doi: 10.1097/00006842-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Brych SB, Engrav LH, Rivara FP, Ptacek JT, Lezotte DC, Esselman PC, Kowalske KJ, Gibran NS. Time off work and return to work rates after burns: systematic review of the literature and a large two-center series. J Burn Care Rehabil. 2001;22:401–5. doi: 10.1097/00004630-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Abdullah A, Blakeney P, Hunt R, Broemeling L, Phillips L, Herndon DN, Robson MC. Visible scars and self-esteem in pediatric patients with burns. J Burn Care Rehabil. 1994;15:164–8. doi: 10.1097/00004630-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Engrav LH, Covey MH, Dutcher KD, Heimbach DM, Walkinshaw MD, Marvin JA. Impairment, time out of school, and time off from work after burns. Plast Reconstr Surg. 1987;79:927–34. doi: 10.1097/00006534-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN, LeMaster J, Beard S, Bernstein N, Lewis SR, Rutan TC, Winkler JB, Cole M, Bjarnason D, Gore D. The quality of life after major thermal injury in children: an analysis of 12 survivors with greater than or equal to 80% total body, 70% third-degree burns. J Trauma. 1986;26:609–19. [PubMed] [Google Scholar]
- 10.Linares HA. From wound to scar. Burns. 1996;22:339–52. doi: 10.1016/0305-4179(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 11.Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84:827–37. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Murray JC. Keloids and hypertrophic scars. Clin Dermatol. 1994;12:27–37. doi: 10.1016/0738-081x(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 13.Su CW, Alizadeh K, Boddie A, Lee RC. The problem scar. Clin Plast Surg. 1998;25:451–65. [PubMed] [Google Scholar]
- 14.Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol. 2004;202:476–85. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- 15.Rahban SR, Garner WL. Fibroproliferative scars. Clin Plast Surg. 2003;30:77–89. doi: 10.1016/s0094-1298(02)00069-x. [DOI] [PubMed] [Google Scholar]
- 16.Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 17.Robson MC. Proliferative scarring. Surg Clin North Am. 2003;83:557–69. doi: 10.1016/S0039-6109(02)00197-4. [DOI] [PubMed] [Google Scholar]
- 18.Aksoy MH, Vargel I, Canter IH, Erk Y, Sargon M, Pinar A, Tezel GG. A new experimental hypertrophic scar model in guinea pigs. Aesthet Plast Surg. 2002;26:388–96. doi: 10.1007/s00266-002-1121-z. [DOI] [PubMed] [Google Scholar]
- 19.Greenhalgh DG. Models of wound healing. J Burn Care Rehab. 2005;26:293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- 20.Mast BA. The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical & clinical aspects. Philadelphia: W.B. Saunders Co; 1992. p. 353. [Google Scholar]
- 21.Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, Mustoe TA. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg. 1997;100:674–81. doi: 10.1097/00006534-199709000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Polo M, Kim YJ, Kucukcelebi A, Hayward PG, Ko F, Robson MC. An in vivo model of human proliferative scar. J Surg Res. 1998;74:187–95. doi: 10.1006/jsre.1997.5251. [DOI] [PubMed] [Google Scholar]
- 23.Ha X, Li Y, Lao M, Yuan B, Wu CT. Effect of human hepatocyte growth factor on promoting wound healing and preventing scar formation by adenovirus-mediated gene transfer. Chin Med J (England) 2003;116:1029–33. [PubMed] [Google Scholar]
- 24.Xiang J, Wang ZY, Jia SX, Jin SW, Lu SL, Liao ZJ. Establishment of an animal model with hypertrophic scar. Zhonghua Shao Shang Za Zhi. 2004;20:281–3. [PubMed] [Google Scholar]
- 25.Kischer CW, Pindur J, Shetlar MR, Shetlar CL. Implants of hypertrophic scars and keloids into the nude (athymic) mouse: viability and morphology. J Trauma. 1989;29:672–7. doi: 10.1097/00005373-198905000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Kischer CW, Sheridan D, Pindur J. Use of nude (athymic) mice for the study of hypertrophic scars and keloids: vascular continuity between mouse and implants. Anat Rec. 1989;225:189–96. doi: 10.1002/ar.1092250303. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Smith P, Pu LL, Kim YJ, Ko F, Robson MC. Exogenous transforming growth factor beta modulates collagen I and collagen III synthesis in proliferative scar xenografts in nude rats. J Surg Res. 1999;87:194–200. doi: 10.1006/jsre.1999.5757. [DOI] [PubMed] [Google Scholar]
- 28.Polo M, Smith PD, Kim YJ, Wang X, Ko F, Robson MC. Effect of TGF-beta2 on proliferative scar fibroblast cell kinetics. Ann Plast Surg. 1999;43:185–90. [PubMed] [Google Scholar]
- 29.Shetlar MR, Shetlar CL, Kischer CW, Pindur J. Implants of keloid and hypertrophic scars into the athymic nude mouse: changes in the glycosaminoglycans of the implants. Connect Tissue Res. 1991;26:23–36. doi: 10.3109/03008209109152161. [DOI] [PubMed] [Google Scholar]
- 30.Shetlar MR, Shetlar CL, Hendricks L, Kischer CW. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med. 1985;179:549–52. doi: 10.3181/00379727-179-rc3. [DOI] [PubMed] [Google Scholar]
- 31.Robb EC, Waymack JP, Warden GD, Nathan P, Alexander JW. A new model for studying the development of human hypertrophic burn scar formation. J Burn Care Rehab. 1987;8:371–5. doi: 10.1097/00004630-198709000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Reiken SR, Wolfort SF, Berthiaume F, Compton C, Tompkins RG, Yarmush ML. Control of hypertrophic scar growth using selective photothermolysis. Lasers Surg Med. 1997;21:7–12. doi: 10.1002/(sici)1096-9101(1997)21:1<7::aid-lsm2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 33.Wolfort SF, Reiken SR, Berthiaume F, Tompkins RG, Yarmush ML. Control of hypertrophic scar growth using antibody-targeted photolysis. J Surg Res. 1996;62:17–22. doi: 10.1006/jsre.1996.0166. [DOI] [PubMed] [Google Scholar]
- 34.Yang DY, Li SR, Li G, Liu JY, Wang ZX, Wu JL, Chen YQ. Establishment of an animal model of human hyperplastic scar in nude mice. Zhonghua Shao Shang Za Zhi. 2004;20:82–4. [PubMed] [Google Scholar]
- 35.Yang DY, Li SR, Wu JL, Chen YQ, Li G, Bi S, Dai X. Establishment of a hypertrophic scar model by transplanting full-thickness human skin grafts onto the backs of nude mice. Plast Reconst Surg. 2007;119:104–9. doi: 10.1097/01.prs.0000244828.80490.62. [DOI] [PubMed] [Google Scholar]
- 36.Silverstein P, Goodwin MN, Raulston GL, Pruitt B. Hypertrophic scar in the experimental animal. In: Longacre JJ, editor. The ultrastructure of collagen; its relation to the healing of wounds and to the management of hypertrophic scar. Springfield, IL: Thomas; 1976. pp. 213–36. [Google Scholar]
- 37.Silverstein P, Goodwin MN, Raulston GL., Jr Hypertrophic scarring, etiology and control of a disabling complication in burned soldiers. Ann Res Prog Rep US Army Instit Surg Res Sec. 1972;37:1–5. [Google Scholar]
- 38.Erk Y, Rose F, Spira M. The development of an animal model for the study of hypertrophic scar. Paper presented at the Plastic Surgery Research Council; Springfield, IL. 1981. [Google Scholar]
- 39.Pruitt BA. Personal communication. 1998.
- 40.Pruitt BA. Personal communication. 2005.
- 41.Silverstein P. Personal communication. 2002.
- 42.Silverstein P. Personal communication. 2005.
- 43.Salisbury RE. Personal communication. 2005.
- 44.Robson MC. Personal communication. 2005.
- 45.Matsumura H, Engrav LH, Reichenbach D, Gibran NS, Maser B. Cones, fat domes and hypertrophic scarring in the female, red, Duroc pig. Paper presented at the Plastic Surgery Research Council; Loma Linda. 1998. [Google Scholar]
- 46.Matsumura H, Engrav LH, Yunusov MD, Reichenbach D, Gibran NS, Maser B. Cones, fat domes, and hypertrophic scarring in the female, red Duroc pig. Paper presented at the American Burn Association; Lake Buena Vista, FL. 1999. [Google Scholar]
- 47.Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, Isik FF, Carrougher GJ, Muangman P, Yunusov MY, Yang T. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns. 2003;29:649–64. doi: 10.1016/s0305-4179(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 48.Liang Z, Engrav LH, Muangman P, Muffley LA, Zhu KQ, Carrougher GJ, Underwood RA, Gibran NS. Nerve quantification in female red Duroc pig (FRDP) scar compared to human hypertrophic scar. Burns. 2004;30:57–64. doi: 10.1016/j.burns.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhu KQ, Engrav LH, Tamura RN, Cole JK, Muangman P, Carrougher GJ, Gibran NS. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burns. 2004;30:518–30. doi: 10.1016/j.burns.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Zhu KQ, Engrav LH, Armendariz RT, Muangman P, Klein MB, Carrougher GJ, Deubner H, Gibran NS. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female, Duroc scar and human hypertrophic scar. Burns. 2005;31:5–10. doi: 10.1016/j.burns.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, Isik FF, Gibran NS, Engrav LH. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669–77. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang P, Engrav LH, Gibran NS, Honari S, Kiriluk DB, Cole JK, Fleckman P, Heimbach DM, Bauer GJ, Matsumura H, Warner P. Dermatome setting for autografts to cover INTEGRA. J Burn Care Rehab. 2002;23:327–32. doi: 10.1097/00004630-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 54.Ghahary A, Shen YJ, Scott PG, Tredget EE. Immunolocalization of TGF-beta 1 in human hypertrophic scar and normal dermal tissues. Cytokine. 1995;7:184–90. doi: 10.1006/cyto.1995.1025. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka K, Takemura T, Murakami K, Okada M, Hino S, Miyamoto H, Maki S. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68:154–63. [PubMed] [Google Scholar]
- 56.Zhang K, Phan SH. Cytokines and pulmonary fibrosis. Boil Signals. 1996;5:232–9. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- 57.Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE. Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human postburn hypertrophic scar tissues. J Lab Clin Med. 1993;122:465–73. [PubMed] [Google Scholar]
- 58.Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122 (Suppl 6):289S–93S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- 59.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–63. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 60.Rumalla VK, Borah GL. Cytokines, growth factors, and plastic surgery. Plast Reconstr Surg. 2001;108:719–33. doi: 10.1097/00006534-200109010-00019. [DOI] [PubMed] [Google Scholar]
- 61.Ghahary A, Shen YJ, Wang R, Scott PG, Tredget EE. Expression and localization of insulin-like growth factor-1 in normal and post-burn hypertrophic scar tissue in human. Mol Cell Biochem. 1998;183:1–9. doi: 10.1023/a:1006890212478. [DOI] [PubMed] [Google Scholar]
- 62.Ghahary A, Shen YJ, Nedelec B, Scott PG, Tredget EE. Enhanced expression of mRNA for insulin-like growth factor-1 in post-burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mol Cell Biochem. 1995;148:25–32. doi: 10.1007/BF00929499. [DOI] [PubMed] [Google Scholar]
- 63.Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen. 2004;12:305–19. doi: 10.1111/j.1067-1927.2004.012311.x. [DOI] [PubMed] [Google Scholar]
- 64.Gallant-Behm CL, Hart DA. Genetic analysis of skin wound healing and scarring in a porcine model. Wound Repair Regen. 2006;14:46–54. doi: 10.1111/j.1743-6109.2005.00087.x. [DOI] [PubMed] [Google Scholar]
- 65.Gallant-Behm CL, Olson ME, Hart DA. Cytokine and growth factor mRNA expression patterns associated with the hypercontracted, hyperpigmented healing phenotype of red duroc pigs: a model of abnormal human scar development? J Cutan Med Surg. 2006;9:165–77. doi: 10.1007/s10227-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 66.Gallant-Behm CL, Tsao H, Reno C, Olson ME, Hart DA. Skin wound healing in the first generation (F1) offspring of Yorkshire and red Duroc pigs: evidence for genetic inheritance of wound phenotype. Burns. 2006;32:180–93. doi: 10.1016/j.burns.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–23. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Boil Chem. 1996;271:31767–70. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 69.Sayani K, Dodd CM, Nedelec B, Shen YJ, Ghahary A, Tredget EE, Scott PG. Delayed appearance of decorin in healing burn scars. Histopathology. 2000;36:262–72. doi: 10.1046/j.1365-2559.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 70.Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-beta in human post-burn hypertrophic and mature scars. Histopathology. 1995;26:423–31. doi: 10.1111/j.1365-2559.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 71.Evanko SP, Vogel KG. Ultrastructure and proteoglycan composition in the developing fibrocartilaginous region of bovine tendon. Matrix. 1990;10:420–36. doi: 10.1016/s0934-8832(11)80150-2. [DOI] [PubMed] [Google Scholar]
- 72.Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Chemical characterization and quantification of proteoglycans in human post-burn hypertrophic and mature scars. Clin Sci (Colch) 1996;90:417–25. doi: 10.1042/cs0900417. [DOI] [PubMed] [Google Scholar]
- 73.Ogston AG. The biological functions of the glycosaminoglycans. In: Balazs EA, editor. Chemistry and molecular biology of the intercellular matrix. Vol. 3. New York: Academic Press; 1970. pp. 1231–40. [Google Scholar]
- 74.Kischer CW, Shetlar MR. Collagen and mucopolysaccharides in the hypertrophic scar. Connect Tissue Res. 1974;2:205–13. doi: 10.3109/03008207409152245. [DOI] [PubMed] [Google Scholar]
- 75.Schwentker A, Billiar TR. Nitric oxide and wound repair. Surg Clin North Am. 2003;83:521–30. doi: 10.1016/S0039-6109(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 76.Wang R, Ghahary A, Shen YJ, Scott PG, Tredget EE. Nitric oxide synthase expression and nitric oxide production are reduced in hypertrophic scar tissue and fibroblasts. J Invest Dermatol. 1997;108:438–44. doi: 10.1111/1523-1747.ep12289708. [DOI] [PubMed] [Google Scholar]
- 77.Ansel JC, Armstrong CA, Song I, Quinlan KL, Olerud JE, Caughman SW, Bunnett NW. Interactions of the skin and nervous system. J Investig Dermatol Symp Proc. 1997;2:23–6. doi: 10.1038/jidsymp.1997.6. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura M, Nishida T, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp Eye Res. 1997;65:321–9. doi: 10.1006/exer.1997.0345. [DOI] [PubMed] [Google Scholar]
- 79.Haegerstrand A, Dalsgaard CJ, Jonzon B, Larsson O, Nilsson J. Calcitonin gene-related peptide stimulates proliferation of human endothelial cells. Proc Natl Acad Sci USA. 1990;87:3299–303. doi: 10.1073/pnas.87.9.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiedermann CJ, Auer B, Sitte B, Reinisch N, Schratzberger P, Kahler CM. Induction of endothelial cell differentiation into capillary-like structures by substance P. Eur J Pharmacol. 1996;298:335–8. doi: 10.1016/0014-2999(95)00818-7. [DOI] [PubMed] [Google Scholar]
- 81.Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985;315:61–3. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- 82.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–8. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 83.Crowe R, Parkhouse N, McGrouther D, Burnstock G. Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br J Dermatol. 1994;130:444–52. doi: 10.1111/j.1365-2133.1994.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang LQ, Laato M. Innervation of normal and hypertrophic human scars and experimental wounds in the rat. Ann Chir Gynaecol. 2001;90 (Suppl 215):29–32. [PubMed] [Google Scholar]
- 85.Liang Z, Xie CY, Lin HB, Guo ZD, Yang WG. Pathomorphological observation of the hypertrophic scar induced by injury to conical structure in female red Duroc pig. Zhonghua Shao Shang Za Zhi. 2006;22:29–32. [PubMed] [Google Scholar]
- 86.Moritz AR, Henriques FC., Jr Studies of thermal injury II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- 87.Montagna W, Yun J. The skin of the domestic pig. J Invest Dermatol. 1964;43:11–21. [PubMed] [Google Scholar]
- 88.Marcarian HQ, Calhoun ML. Microscopic anatomy of the integument of adult swine. Am J Vet Res. 1966;27:765–72. [PubMed] [Google Scholar]
- 89.Nicolaides N, Fu HC, Rice GR. The skin surface lipids of man compared with those of eighteen species of animals. J Invest Dermatol. 1968;51:83–9. doi: 10.1038/jid.1968.96. [DOI] [PubMed] [Google Scholar]
- 90.Forbes PD. Vascular supply of the skin and hair in swine. In: Montagna W, Dobson RL, editors. Advances in the biology of skin: hair growth. Vol. 9. Oxford: Pergamon; 1969. pp. 419–32. [Google Scholar]
- 91.Douglas WR. Of pigs and men and research. Space Life Sci. 1972;3:226–34. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- 92.Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975;16:434–40. [PubMed] [Google Scholar]
- 93.Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39–52. doi: 10.1159/000401274. [DOI] [PubMed] [Google Scholar]
- 94.Morris GM, Hopewell JW. Epidermal cell kinetics of the pig: a review. Cell Tissue Kinet. 1990;23:271–82. doi: 10.1111/j.1365-2184.1990.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 95.Wollina U, Berger U, Mahrle G. Immunohistochemistry of porcine skin. Acta Histochem. 1991;90:87–91. doi: 10.1016/S0065-1281(11)80166-2. [DOI] [PubMed] [Google Scholar]
- 96.Monteiro-Riviere NA, Stromberg MW. Ultrastructure of the integument of the domestic pig (Sus scrofa) from one through fourteen weeks of age. Anat Histol Embryol. 1985;14:97–115. doi: 10.1111/j.1439-0264.1985.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 97.Vardaxis NJ, Brans TA, Boon ME, Kreis RW, Marres LM. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat. 1997;190 (Part 4):601–11. doi: 10.1046/j.1469-7580.1997.19040601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 99.Rothschild MF. Porcine genomics delivers new tools and results: this little piggy did more than just go to market. Genet Res. 2004;83:1–6. doi: 10.1017/s0016672303006621. [DOI] [PubMed] [Google Scholar]
- 100.Rothschild MF. From a sow’s ear to a silk purse: real progress in porcine genomics. Cytogenet Genome Res. 2003;102:95–9. doi: 10.1159/000075732. [DOI] [PubMed] [Google Scholar]
- 101.Gallant CL, Olson ME, Hart DA. Full- and partial-thickness skin wounds in red Duroc pigs leads to hypercontracted, hyperpigmented scars. Paper presented at the Wound Healing Society; Baltimore, MD. 2002. [Google Scholar]
- 102.Perez J, Garcia PM, Bautista MJ, Millan Y, Ordas J, Martin de las Mulas J. Immunohistochemical characterization of tumor cells and inflammatory infiltrate associated with cutaneous melanocytic tumors of Duroc and Iberian swine. Vet Pathol. 2002;39:445–51. doi: 10.1354/vp.39-4-445. [DOI] [PubMed] [Google Scholar]
- 103.Gallant CL, Hart DA, Olson ME. Skin wound healing in red Duroc pigs: potential role of growth factors and cytokines in scar formation. Paper presented at the Wound Healing Society; Baltimore, MD. 2002. [Google Scholar]
- 104.Yao F, Visovatti S, Johnson CS, Chen M, Slama J, Wenger A, Eriksson E. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2001;9:371–7. doi: 10.1046/j.1524-475x.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 105.Adcock D, Paulsen S, Jabour K, Davis S, Nanney LB, Shack RB. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2001;108:233–40. doi: 10.1097/00006534-200107000-00038. [DOI] [PubMed] [Google Scholar]


