Abstract
The integration of adult-born neurons into the circuitry of the adult hippocampus suggests an important role for adult hippocampal neurogenesis in learning and memory, but its specific function in these processes has remained elusive. In this article, we summarize recent progress in this area, including advances based on behavioural studies and insights provided by computational modelling. Increasingly, evidence suggests that newborn neurons might be involved in hippocampal functions that are particularly dependent on the dentate gyrus, such as pattern separation. Furthermore, newborn neurons at different maturation stages may make distinct contributions to learning and memory. In particular, computational studies suggest that, before newborn neurons are fully mature, they might function as a pattern integrator by introducing a degree of similarity to the encoding of events that occur closely in time.
The discovery of neurogenesis in the brain of adult mammals overturned the long-held dogma that the adult brain has no capacity for generating new neurons1. Although the idea met with scepticism for a long time, it is now generally accepted that new neurons are continuously added in discrete regions of the brain throughout adult life in various species, including humans2. Adult neurogenesis, originating from neural progenitor cells (NPCs), has been consistently observed in two regions of the adult brain: the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. Neurons born in the SVZ migrate through the rostral migratory stream and become granule neurons and periglomerular neurons of the olfactory bulb. Neurons born in the SGZ differentiate and integrate into the local neural network as granule cells of the dentate gyrus.
The adult-born neurons can be identified by many approaches, such as by incorporation of nucleotide analogues (for example, bromodeoxyuridine (BrdU)), by virus-mediated labelling and by genetically engineered reporter genes3-5. Research in the past decade has led to a greater understanding of the processes involved in adult neurogenesis, including the proliferation of NPCs, the fate determination of NPC progenies, the differentiation, morphogenesis and maturation of adult-born neurons, and the eventual integration of adult-born neurons into the neural networks of both the olfactory bulb and the hippocampus6. Furthermore, each of these processes is subject to regulation by numerous intrinsic and extrinsic factors7. Despite this progress, many questions regarding the functional importance of adult neurogenesis remain unanswered. However, the putative functions of adult-born olfactory neurons are being revealed by many studies that are in progress8-11, and recent research has shed light on the role of adult hippocampal neurogenesis in hippocampus-mediated functions.
In the adult brain, the hippocampus (FIG. 1) is a crucial structure for the formation of certain types of memory, such as episodic memory and spatial memory12. Through its interactions with brain structures associated with emotion, the hippocampus is also implicated in emotional behaviour13. Does the integration of new neurons into the existing hippocampal circuit influence hippocampus-related behaviours? If so, how? Correlations between the rate of adult hippocampal neurogenesis and the emotional status of animals have been shown in several studies. However, there is no direct evidence that adult neurogenesis is required for emotional regulation, although it can mediate the efficacy of antidepressants under certain conditions13,14. By contrast, our understanding of the functions of hippocampal neurogenesis in learning and memory has advanced considerably in the past few years. In this Review, we briefly describe the basic processes involved in adult hippocampal neurogenesis and consider how these processes are regulated by neural activity and how adult-born neurons respond to environmental and behavioural stimuli. We next examine the potential role of hippocampal neurogenesis in learning and memory as proposed by various computational models and based on recent findings from behavioural studies (see Supplementary information S1 (table)). We discuss these observations in the context of the functions of the hippocampus, and of the dentate gyrus in particular.
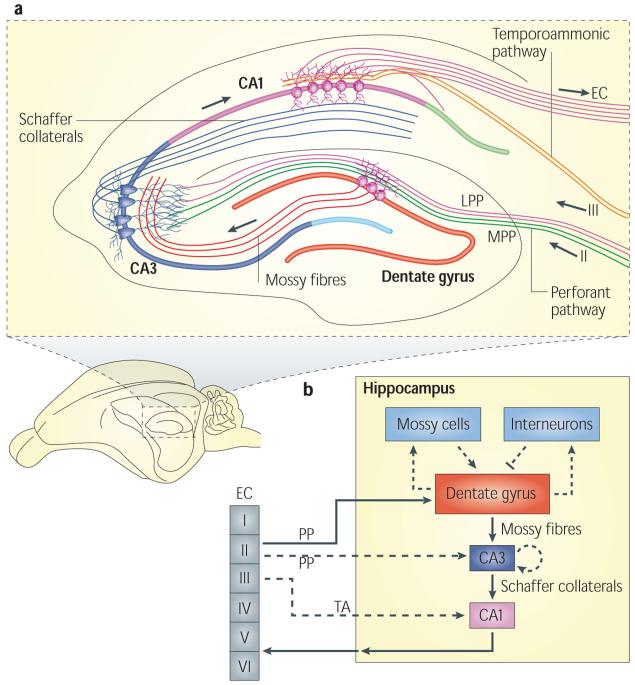
Figure 1. The neural circuitry in the rodent hippocampus.
a | An illustration of the hippocampal circuitry. b | Diagram of the hippocampal neural network. The traditional excitatory trisynaptic pathway (entorhinal cortex (EC)–dentate gyrus–CA3–CA1–EC) is depicted by solid arrows. The axons of layer II neurons in the entorhinal cortex project to the dentate gyrus through the perforant pathway (PP), including the lateral perforant pathway (LPP) and medial perforant pathway (MPP). The dentate gyrus sends projections to the pyramidal cells in CA3 through mossy fibres. CA3 pyramidal neurons relay the information to CA1 pyramidal neurons through Schaffer collaterals. CA1 pyramidal neurons send back-projections into deep-layer neurons of the EC. CA3 also receives direct projections from EC layer II neurons through the PP. CA1 receives direct input from EC layer III neurons through the temporoammonic pathway (TA). The dentate granule cells also project to the mossy cells in the hilus and hilar interneurons, which send excitatory and inhibitory projections, respectively, back to the granule cells.
Maturation of adult-born DGCs
Adult neurogenesis begins with the proliferation of NPCs in the SGZ (FIG. 2). Most progeny of NPCs differentiate into dentate granule cells (DGCs), whereas a small population become glia15. The newly born DGCs bear little resemblance to their mature counterparts and must undergo a lengthy process of morphological and physiological maturation16. The kinetics of DGC maturation are species dependent17. Here, we discuss the maturation of adult-born DGCs in mice because most of the relevant studies have been carried out in this species.
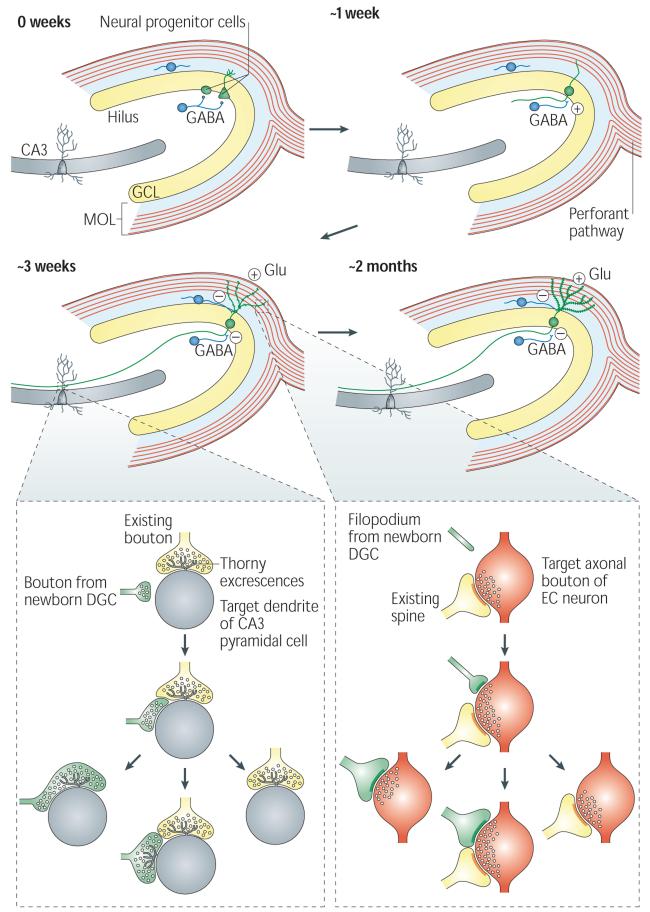
Figure 2. Adult hippocampal neurogenesis.
The proliferation of neural progenitor cells (NPCs) with two different morphologies gives rise to adult-born dentate granule cells (DGCs) (shown in green). The fate-committed, adult-born DGCs undergo several stages of development, with gradual changes in morphological and physiological characteristics. About 1 week after birth, the adult-born DGC extends its dendrite into the granule cell layer (GCL) and molecular layer (MOL) and projects the axon into the hilus toward CA3. The DGC receives excitatory GABA (γ-aminobutyric acid)-ergic input, presumably from local interneurons (shown as blue cells). During the third week after birth, the DGC receives glutamatergic input (Glu) from the perforant pathway. At this stage, the GABA input changes from being excitatory to being inhibitory19. Both efferent and afferent synapses of the adult-born DGCs begin to form around this time24-26.
At around 2 months of age, the basic structural and physiological properties of the adult-born DGCs are indistinguishable from those of mature DGCs. The inset panels illustrate the competitive nature of synapse formation24-25. Left inset: a small bouton (shown in green) from the axon of an adult-born DGC contacts the dendritic shaft (shown in grey) of a CA3 pyramidal neuron at a site near the thorny excrescences that contact an existing axonal bouton (shown in yellow). During the subsequent development of the new synapse, the bouton from the newborn DGC either replaces the existing axonal bouton or forms a new thorny excrescence nearby, or retracts. Right inset: the filopodium (shown in green) from an adult-born DGC dendrite extends to an axonal bouton (shown in red) that is associated with another spine (shown in yellow), which leads to the eventual formation of either a monosynaptic bouton targeting spines from the adult-born DGC or a multisynaptic bouton, or leads to retraction.
During the first week after birth, after committing to the neuronal lineage, the adult-born DGCs undergo their initial differentiation and migrate a short distance into the inner granule cell layer of the dentate gyrus, where they extend limited cellular processes but do not seem to be synaptically integrated into the network. Notably, these cells are tonically activated by ambient GABA (γ-aminobutyric acid)16,18,19.
During the second week after birth, the adult-born DGCs become more neuron-like: they grow polarized processes, with dendrites extending towards the molecular layer and axons (that is, mossy fibres) growing through the hilus towards CA3 (REFS 16,20) (FIG. 2). Nevertheless, these immature DGCs are still considerably different from mature DGCs. For example, they have a higher membrane resistance and different firing properties18. Moreover, at this stage, the adult-born DGCs lack glutamatergic input, which is consistent with the absence of dendritic spines in the molecular layer (in which the glutamatergic fibres are located) at this time16,18 (FIG. 2). However, these immature DGCs receive synaptic GABAergic input, presumably from local interneurons, and the resulting responses have slow rising and decay kinetics18,19,21,22.
The GABAergic input results in neuronal depolarization owing to the presence of the Na+–K+–2Cl− cotransporter, solute carrier family 12 member 2 (SLC12A2; also known as NKCC1) on the DGC membrane18,19. The GABA-mediated activity seems to be important for the survival and maturation of adult-born DGCs: knockdown of SLC12A2 by RNA interference reduced the number of adult-born DGCs surviving the second week after birth23, caused defects in the formation of GABA- and glutamate-mediated synapses and reduced dendrite arborizations19. By contrast, administration of a GABA receptor agonist promotes dendrite growth of adult-born DGCs19. The GABA-dependent depolarization is mediated by cyclic AMP response element-binding protein (CREB)23. The knockdown of SLC12A2 results in loss of CREB phosphorylation (the active form of CREB) in adult-born DGCs, and forced activation of CREB can normalize the impaired maturation of newborn DGCs caused by SLC12A2 knockdown23.
During the third week after birth, adult-born DGCs start to form afferent and efferent connections with the local neuronal network (FIG. 2). By approximately day 16, spines begin to appear on the dendrites of adult-born DGCs, forming synapses with the afferent axon fibres in the perforant pathway that come from the entorhinal cortex16,24. Initially, filopodia are frequently present on dendrites24. The majority of the filopodia on the adult-born DGCs target axon boutons that already synapse with existing spines on other DGCs by forming multiple synaptic boutons, suggesting that network integration of the adult-born DGCs is influenced by local synaptic activity24. Similarly, the mossy fibre boutons of adult-born DGCs initially form synapses either with the dendritic shaft of CA3 pyramidal neurons in a region near existing thorny excrescences or directly with existing thorny excrescences that already contain a bouton25,26. This suggests that efferent synapse formation of adult-born DGCs can also be affected by existing synapses. Thus, the development of both afferent and efferent synapses from newly generated DGCs seems to involve targeting to pre-existing synaptic partners, which suggests a role for circuit activity in the integration of adult-born DGCs.
The timing of synaptic integration coincides with the transition of GABAergic input from being excitatory to being inhibitory and with the onset of glutamatergic synaptic inputs18,19. The NMDA (N-methyl-d-aspartate) receptor is a glutamate receptor that has been implicated in neuronal development and plasticity27. Between 2 and 3 weeks of age, the survival of adult-born DGCs depends on NMDA-receptor mediated cell-autonomous activity18,19,28. The adult-born DGCs at this stage still have characteristics — such as high membrane resistance and high resting potentials18 — that could contribute to increased excitability29,30, although their action potentials have kinetic characteristics similar to those of mature DGCs18.
Around 4–6 weeks of age, together with the gradual maturation of their physiology and connectivity16,18,31, the adult-born DGCs exhibit stronger synaptic plasticity than mature DGCs, as indicated by their lower threshold for the induction of long-term potentiation (LTP) and their higher LTP amplitude29. This enhanced plasticity is mediated by NMDA receptor subunit NR2B29. A transcription factor, krupple-like factor 9, seems to be essential for the survival of adult-born DGCs during this time32. Although the structural modification of dendritic spines and axonal boutons continues to occur as the adult-born DGCs become older16,24,25, the basic physiological properties and synaptic plasticity at 8 weeks of age are indistinguishable from those of mature DGCs. As discussed below, the unique physiological characteristics of adult-born DGCs before 6 weeks of age enable these neurons to be discretely regulated by network activity and possibly to make distinct contributions to learning and memory.
Regulation of adult neurogenesis
One of the implications of a role for adult neurogenesis in learning and memory is that neurogenesis can be regulated by numerous factors associated with an animal’s behavioural and cognitive states. Indeed, an animal’s experiences, including hippocampus-dependent learning, environmental enrichment and voluntary running, can affect the rate of neurogenesis. These experiences, which are associated with enhanced cognition, presumably do so by stimulating the hippocampal neural network.
Hippocampus-dependent learning is one of the major regulators of hippocampal neurogenesis33. For example, learning of hippocampus-dependent tasks but not hippocampus-independent tasks increases the number of adult-born DGCs at around 1 week of age33-36. However, proliferation of NPCs in the SGZ immediately after learning is not affected by learning33. Similarly, hippocampal neurogenesis is exquisitely regulated by spatial navigation learning in the Morris water maze (MWM). For example, learning in the MWM promotes the survival of DGCs that were born 7 days before the onset of MWM training and, at the same time, induces apoptosis in DGCs that were born 3 days before37. Of note, at the onset of MWM training, DGCs born 1 week earlier have begun to form GABAergic synapses with the local network and enter the hyper-excitable stage during MWM learning (see above), and can therefore potentially be influenced by learning or recruited to memories. Furthermore, the late phase of MWM training, when animals show little improvement in performance, is associated with increased apoptosis in the dentate gyrus and a decrease in the number of DGCs that were born during the early phase of training37,38. At the same time, such asymptotic training induces the proliferation of NPCs in the SGZ37,38. Blocking apoptosis in the late phase of learning prevents the enhanced survival of DGCs born 7 days before MWM training and the induction of NPC proliferation, and impairs the performance in the MWM37, suggesting that learning regulates these events interdependently. Together, these findings suggest that learning selectively adds and removes adult-born DGCs according to their maturity and functional relevance.
Living in an enriched environment, which presumably provides a larger number of opportunities for learning than standard laboratory housing39, can also enhance hippocampal neurogenesis by increasing the survival of adult-born DGCs40. Environmental enrichment for as little as 1 week is sufficient to increase the survival of the adult-born DGCs that are younger than 3 weeks of age but not of older ones, suggesting that the environmental-enrichment effect is dependent on the maturation state of adult-born DGCs41. In addition, environmental enrichment improves performance in learning and memory tasks such as the MWM and object recognition tests40,42. However, it is currently debated whether hippocampal neurogenesis is necessary for these behavioural effects of environmental enrichment42,43.
Increasing evidence suggests that physical exercise not only improves the physical health of individuals but also improves cognition and other brain functions44,45. In rodents, voluntary running significantly increases the proliferation of NPCs in the SGZ of both young and aged animals46,47. Moreover, voluntary running for about 3 weeks following BrdU birth-dating enhances the survival of BrdU-labelled adult-born DGCs48,49. In addition, voluntary running increases the amplitude of LTP in the dentate gyrus and improves the performance of animals in the MWM47,50, indicating that increased neurogenesis correlates with increased network activity and improved cognition. However, whether increased neurogenesis is responsible for cognitive improvement remains to be tested. Voluntary running also interacts with other stimuli, such as stress and social interactions, to regulate neurogenesis49,51. Although physical exercise can induce angiogenesis and expression of neurotrophic factors such as brain-derived neurotrophic factor in the brain45, the molecular mechanisms responsible for exercise-induced neurogenesis remain undetermined.
Adult hippocampal neurogenesis can also be modulated by artificial induction of network activity. For example, induction of LTP in the dentate gyrus by high-frequency stimulation of the perforant pathway can increase the proliferation of NPCs in the SGZ and enhance the survival of adult-born DGCs at 1–2 weeks of age52,53. Electroconvulsive shock, which induces a brief seizure in the brain and has therapeutic effects in many emotional disorders, also increases both the proliferation of NPCs and neurogenesis in the SGZ54,55.
Finally, adult hippocampal neurogenesis and neuronal integration can be affected by aberrant circuit activity under pathological conditions, as observed in human patients and animal models of neurological disorders (reviewed in REF. 6). For example, a drug-induced seizure increases NPC proliferation56, causes morphological abnormalities in adult-born DGCs57, induces ectopic migration of adult-born DGCs58 and accelerates the integration of adult-born DGCs59. It is currently unknown whether the altered hippocampal neurogenesis further exacerbates the pathological conditions.
Responsiveness of adult-born DGCs
If behavioural stimuli and network activity can modulate the survival and integration of adult-born DGCs, how do adult-born DGCs respond to such stimuli and activity? This question is generally addressed by examining the expression of immediate early genes (IEGs). IEGs, such as FBJ osteosarcoma oncogene (Fos), activity regulated cytoskeletal-associated protein (Arc), early growth response 1 (Egr1; also known as Zif268), and Homer1A, play key parts in regulating synaptic plasticity. Their expression is tightly coupled to neuronal activity associated with learning and memory and is widely used to study population activity of neurons in various brain regions60. Using BrdU to birth-date adult-born DGCs, it was found that neuronal activity (whether at physiological or aberrant levels) does not induce IEG expression in adult-born DGCs until they reach a certain stage of maturation — around 3–4 weeks of age in mice17,61 and about 2 weeks of age in rats17,52.
Neural stimuli seem to preferentially activate adult-born DGCs. Compared with their mature counterparts, a higher proportion of 5-month-old adult-born DGCs expressed IEGs when rats were allowed to encounter and explore a novel environment62. Moreover, the experiences an animal undergoes when a population of adult-born DGCs are young may influence the responsiveness of these neurons later on. For example, MWM learning by a mouse when a set of adult-born DGCs was at least 4–6 weeks of age led to preferential activation of these cells during memory retrieval in the MWM when the DGCs were 10 weeks old63. Interestingly, this time frame coincides with the stage of enhanced synaptic plasticity of adult-born DGCs.
Furthermore, it has been suggested that the experiences of a mouse when adult-born DGCs are about 1–3 weeks of age — a stage when newly generated DGCs are not capable of expressing IEGs — can enhance the experience-specific responsiveness of these DGCs when they are older41,64. In one study, mice were exposed to an enriched environment when BrdU-labelled adult-born DGCs were about 1–2.5 weeks of age; the mice were subsequently exposed to different experiences and, 6 weeks after BrdU labelling, the expression of IEGs in BrdU-labelled DGCs was examined41. A higher proportion of BrdU-labelled adult-born DGCs responded to re-exposure to the enriched environment than to MWM training41.
Finally, activation of adult-born DGCs by memory retrieval tasks seems to depend on cognitive demand64. In one study, mice were trained in the MWM when BrdU-labelled adult-born DGCs were 9 days old; 30 days later, they were subjected to various paradigms in the MWM and the expression of IEGs in BrdU-labelled DGCs was examined64. A higher proportion of BrdU-labelled DGCs expressed IEGs in mice that were subjected to a single trial with the hidden platform in the original training location (matched situation), than in mice subjected to a single trial in the absence of the platform (mismatched situation), suggesting that the activation of adult-born DGCs is situation-specific64. In addition, the proportion of IEG-expressing BrdU-labelled DGCs could be further increased (by more than twofold) if mice underwent nine trials (instead of one trial) with the platform in the original training location, but not with the platform moved to a new location.
Together, these findings suggest that, compared with their mature counterparts, adult-born DGCs may be specifically activated by an animal’s experiences and thus can make unique contributions to learning and memory.
Computational models of adult-born DGC function
As adult neurogenesis has become more widely appreciated as an important form of hippocampal plasticity, its potential to affect learning and memory has been increasingly recognized65-67. This interest is largely due to the position of the dentate gyrus within the hippocampal circuit and its presumed role in memory formation. The dentate gyrus, which receives direct inputs from the entorhinal cortex and sends projections to the CA3 region (FIG. 1), is traditionally considered the information gateway to the hippocampus. Following the seminal work of David Marr, who suggested that the hippocampus stores memories in associative networks68, several computational studies recognized that highly separated inputs are required to encode different memories in the CA3 and assigned this role — a process known as pattern separation — to the dentate gyrus (BOX 1)69-72. Recent experimental evidence also supports the pattern separation function of the dentate gyrus in information processing73-75. However, studies of dentate gyrus pattern separation have generally not taken neurogenesis into consideration.
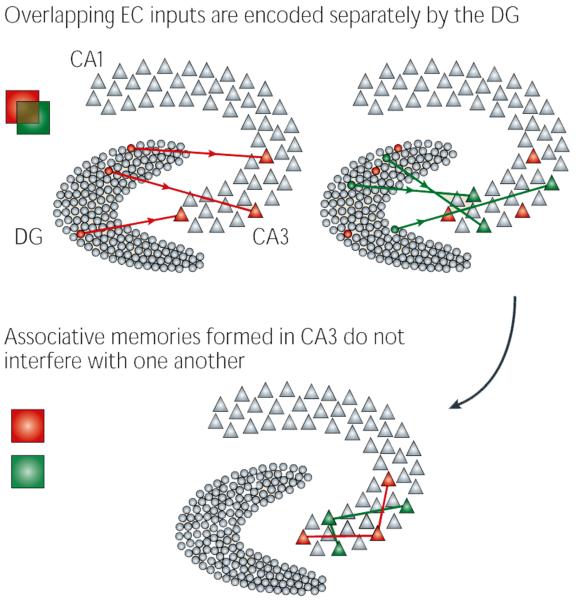
Box 1 | Pattern separation.
Pattern separation is a computational process that has long been associated with the dentate gyrus (DG). Pattern separation occurs when the output firing patterns of a network are less similar to one another than the input firing patterns. Similarity measures such as correlation and cosine similarity (also known as normalized dot product) can be used to quantify separation. If the firing patterns of the output neurons for a set of events are more separated from each other than the firing patterns of its input neurons, the network can be considered a separator (see the figure). This separation can occur either through rate modulation of neurons within a population or by the firing of a unique set of neurons to each input.
There are many reasons why the dentate gyrus is thought to have such a pattern separation function. First, the anatomy of the region seems to be ideally suited for separation: the dentate gyrus contains five to ten times more neurons than its principal input, the entorhinal cortex (EC)115. In this way, the dentate gyrus is similar to support vector machine algorithms in machine learning, by which information is projected into higher-dimension spaces to facilitate discrimination116. Second, it seems to have a sparse coding scheme: dentate granule cells (DGCs) receive substantial feedforward and feedback inhibition from local interneurons117 and in vivo recordings suggest that DGCs are rarely active during behaviour74,118. Together, these findings suggest that DGCs are finely tuned, making it possible that even similar inputs activate distinct populations of DGCs70,72,119. Finally, individual DGC mossy fibres are capable of depolarizing downstream CA3 pyramidal neurons120, suggesting that, despite their sparse activation, they can drive memory encoding in the hippocampus69.
Why is pattern separation important? In the hippocampus, it has been proposed to be an essential step in information processing to avoid interference between memories72. In associative networks, as the CA3 is thought to be, memory cues activate a set of neurons that then, by virtue of connections within the network, activate those neurons that represent the stored memory itself — a process known as pattern completion121. Such a scheme requires that memories be stored ‘far apart’. If two events are encoded too similarly, the stored memories may converge into a single, inappropriate memory, rendering future recall impossible122.
Following the long tradition of hippocampal modelling, computational approaches have been increasingly used to identify and understand the role that adult neurogenesis may have in the hippocampal network76. These modelling studies have shown that directing learning to adult-born DGCs can have several different effects depending on the specific architecture of the network model and the form of neurogenesis that is being tested. These models have taken different forms, ranging from ‘top-down’, abstract neural network models to ‘bottom-up’, biologically-derived models of the dentate gyrus and hippocampus. Nevertheless, a common thread has emerged across many of these models: neurogenesis allows plasticity to be mostly localized to newborn immature DGCs, preserving the information that is represented by mature DGCs (FIG. 3).
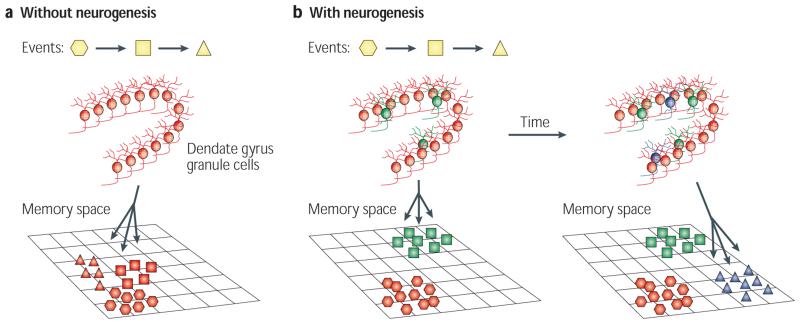
Figure 3. Computational theories of neurogenesis.
a | Without neurogenesis, new events (represented by different shapes) are limited by the set of sparse ‘codes’ (combinations of active neurons) provided by mature granule cells in the dentate gyrus. This can lead to the dentate gyrus not having the flexibility to encode new memories well80,82 or to interference between memories formed in the hippocampus (shown as a cluster of memories in a projection of the high-dimension hippocampal ‘memory space’)81. b | New neurons (shown in green) provide new sparse codes for encoding new information, while older memories are preserved because they are represented by older neurons (shown in red). This can facilitate the formation of new memories while avoiding catastrophic interference, saving older memories80-82 (shown in the left panel as two separate clusters of memories in a projection of the high-dimension hippocampal memory space). The three-way arrow indicates that new neurons can change how memories are encoded in the hippocampal network. Neurons born at different times (shown in green and blue in the right panel) represent different inputs, and the sparse codes generated at a particular time are clustered together (active neurons in a population are similar in composition to one another), separately from sparse codes that were generated at a different time, essentially encoding time into new memories83,84,86.
New neurons and memory capacity: addition or replacement?
The greatest distinction in how neurogenesis is implemented in these different computational models is whether new neurons replace existing neurons (‘replacement models’) or whether they are added to the network (‘addition models’). In replacement models, old DGCs are simply removed and replaced by new DGCs with random (or naive) synapses. In simple feedforward architectures, replacement of neurons in this manner accelerates learning considerably because the random connections made by the new neurons are flexible and can therefore participate in various network learning paradigms (for example, neurogenesis allows the network to avoid local minima, which are a problem with some learning rules)77,78. A possible downside indicated by these models is that this learning improvement is accompanied by the forgetting of older memories through the loss of information that was encoded by the removed neurons, although targeted cell death could mitigate this problem79. In contrast to the abstract networks in these replacement models, the replacement model described by Becker80 considers neurogenesis in a model of the full hippocampal loop (entorhinal cortex–dentate gyrus–CA3–CA1–entorhinal cortex). Becker’s model shows that, if the role of the dentate gyrus is limited to encoding (with no involvement in retrieval), the storage and subsequent recall of highly similar items by the full hippocampal circuit are considerably improved if there is substantial neuronal replacement in the dentate gyrus, without disrupting the retrieval of older memories80.
In contrast to the replacement models, more recent models have considered the possibility that the granule cell layer continues to grow through the addition of new neurons. By not removing existing neurons and directing plasticity towards new neurons, these models have suggested that neurogenesis allows the mature neurons within the dentate gyrus network to remain specialized for the same memories for long periods of time. Wiskott and colleagues described how limiting synaptic plasticity to synapses made by new neurons in the network can keep the network from suffering catastrophic interference, a process by which the attractor structure of the network breaks down81. This interference is avoided because the attractors using old neurons are not affected by new information that is being stored in the network (although the old attractors are still useful for encoding familiar components of new memories). Similarly, in the full hippocampal model by Weisz and Argibay82 — which, like the Becker model, incorporates neurogenesis into a simulation of the entorhinal cortex–dentate gyrus–CA3–CA1–entorhinal cortex loop but treats neuro genesis as an additive process — after a certain memory load was reached, new information could not be effectively encoded if no neurogenesis took place; however, if neurogenesis was incorporated into the model (simulated as a one-off 30% increase in dentate gyrus size), the network could effectively store and retrieve the new memories. Aimone and colleagues83 investigated the long-term effects of this addition. Their model showed that if all DGCs ‘grow’ into the network through neurogenesis (and thus move from a high-plasticity, immature state to a lower-plasticity, mature state), a network ultimately results in which most DGCs permanently encode their past ‘experience’83. Such an arrangement could bias the dentate gyrus to preferentially use subsets of mature DGCs during the encoding of new information in familiar contexts. The experience-specific modification of adult-born DGC activity described above is consistent with this hypothesis41.
Pattern separation in models of neurogenesis
Although most computational models that investigate the role of neurogenesis in hippocampal function have not explicitly addressed the pattern separation function of the dentate gyrus, many of these results have implications that are relevant to the role of neurogenesis in pattern separation. In replacement models, the complete replacement of neurons within a layer could be considered a potent form of separation that ensures that different memories are always represented by distinct DGC populations without the potential for overlap. For example, the benefits of neurogenesis on the ability for the Becker model to store and recall similar memories could be attributed to the substantial pattern separation between the entorhinal cortex signals in the model. Similarly, the results that emerge from the Wiskott and Aimone addition models (which involve a reduction of interference or a specialization of neurons) can be considered ‘separation effects’ in the sense that new memories are more likely to involve new neurons that were not available for older memories. In these cases, however, the contribution of new neurons to pattern separation occurs over a long timescale — probably days to weeks — because neurogenesis ensures that the populations of immature DGCs change continuously over several days. What occurs over shorter timescales that are relevant to hippocampal processing, such as minutes and hours, during which the population of immature neurons probably does not change substantially? One prediction from Aimone’s model is that immature DGCs make a distinct contribution to the separation properties of the dentate gyrus83. Although mature DGCs are presumably very selective in their responses (that is, tightly tuned), allowing them to contribute to pattern separation by making the stimulus inputs independent of one another, the physiological properties of immature DGCs probably make them less selective (that is, broadly tuned), allowing them to fire in response to multiple events. We refer to this effect as pattern integration and propose that this added similarity between the representations of encoded memories might make a crucial contribution to the global pattern separation function of the dentate gyrus. Importantly, this pattern integration effect, which prevents new memories from becoming completely separated during encoding, is distinct from the pattern completion that occurs during memory retrieval. Incidentally, the full hippocampal model of Weisz and Argibay also showed that neurogenesis decreases pattern separation because new neurons in their model are more likely to participate indiscriminately in the encoding of new memories82.
The different potential effects of adult-born DGCs on pattern separation at different timescales has led to an additional hypothesis — namely, that adult-born DGCs contribute to the encoding of temporal information80,83-85. According to our model83, events (stimuli) that occur close to each other in time will be associated with each other owing to pattern integration by immature adult-born DGCs, but the memories of events that occur far apart in time (and that thus will be represented by different immature DGC populations) will be better separated84. Modelling results showed that immature DGCs make an important contribution in this respect and that the pattern integration effect decreases over several days, ensuring that memories that are formed at different times are encoded distinctly83. From a different mechanistic perspective, Becker and Wojtowicz proposed that temporal information could be encoded by ‘waves’ of new DGCs, whereby DGCs arising from the same NPC encode different features of the same temporal context86.
In summary, the varied approaches to using computational models to study the role of neurogenesis in hippocampal function have suggested several potential functions for adult-born DGCs in the dentate gyrus. Despite differences in design and assumptions, these models point towards similar conclusions. First, how neurogenesis might affect learning and memory will depend on the timescale that is being studied; the effect of immature DGCs on the encoding of individual events is likely to be distinct from the contribution of persistent neurogenesis over a lifetime. Second, the specific plasticity-related and physiological properties of immature DGCs probably have an effect on the function of the entire dentate gyrus and hippocampal circuit. As a result, conditions that affect immature DGCs are predicted to have a substantial effect on overall hippocampal function.
Behavioural studies of adult-born DGC function
In the past decade, accumulating evidence has suggested a correlation between the number of adult-born DGCs and an animal’s cognitive ability (see above). Nevertheless, studies that directly investigated the effect of depletion of adult-born DGCs on an animal’s cognitive ability have generated inconsistent results (see Supplementary information S1 (table)), making it difficult to formulate basic principles regarding the function of adult neurogenesis in learning and memory. In this section, we describe the experimental observations, discuss possible causes for the inconsistencies and propose potential strategies to resolve them.
A common strategy for studying the function of adult neurogenesis is to examine the consequences of neurogenesis ablation on cognitive performance. Several methods have been developed to suppress the production of new neurons in the adult brain (BOX 2). Most of the studies evaluated cognitive performance by using common behavioural paradigms designed to examine the role of the hippocampus in learning and memory (see Supplementary information S1 (table)). These studies have revealed that neurogenesis is required for some but not other hippocampus-dependent tasks, and is not required for tasks that do not involve the hippocampus87-89. For example, the ablation of adult neurogenesis in rats by methylazoxymethanol acetate (MAM) treatment results in deficits in hippocampus-dependent trace conditioning tasks but not in hippocampus-independent delay conditioning tasks89,90. Among several other hippocampus-dependent tasks, MAM treatment prevents the improvement of long-term recognition memory by environmental enrichment, but it does not alter contextual fear conditioning or spatial navigation learning in the MWM42,90. Similar task-dependent involvement of hippocampal neurogenesis has been observed in other studies in both rats and mice87,88,91-94. Therefore, the involvement of adult neurogenesis in learning and memory seems to depend on the demands in each task, similar to the task-specific responsiveness of adult-born DGCs discussed above64.
Box 2 | Methodologies for ablating neurogenesis.
The early approaches for ablating neurogenesis such as anti-mitotic drug treatments (for example, methylazoxymethanol acetate and temozolomide) and irradiation89,95 were traditionally used in cancer therapy. These methods take advantage of the fact that the proliferative neural progenitor cells (NPCs) are more sensitive than differentiated neurons to these insults, which disrupt cell cycle progression. Although these methods are effective in reducing neurogenesis, they have considerable side effects in animals, such as general health deterioration and inflammation123,124.
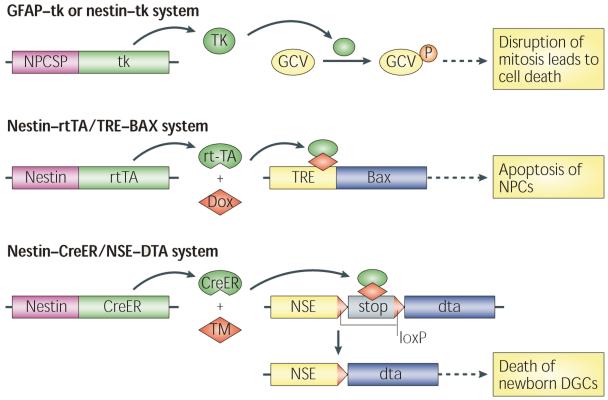
More precise, cell type-specific targeting of neurogenesis could be achieved through the development of numerous transgenic mouse models by expressing suicide genes driven by NPC-specific promoters (NPCSPs), such as nestin and glial fibrillary acidic protein (GFAP) promoters (see the figure). In an example of such a method, expression of thymidine kinase (tk) from herpes simplex virus in NPCs facilitates the incorporation of the nucleotide analogue ganciclovir (GCV) into DNA during replication, which disrupts DNA replication and leads to cell death (top panel)88,125. In the nestin–rtTA/TRE–BAX (nestin–reverse tetracycline-controlled transactivator/tetracycline response element–BAX) mice (middle panel), the expression of the pro-apoptotic protein BAX is induced by doxycycline (Dox), which activates the apoptosis pathway in NPCs87. In the nestin–CreER/NSE–DTA (nestin–CRE recombinase-modified oestrogen receptor/neuron-specific enolase 2–diphtheria toxin fragment A) mice (bottom panel), nestin–CreER targets the expression of a tamoxifen (TM)-inducible form of Cre in NPCs and a CRE-inducible DTA is engineered into the locus of the Nse gene97. TM-induced CRE activity leads to the recombination of loxP sites (red triangles) and removal of the stop cassette upstream of the dta gene, thus allowing the expression of dta from the NSE promoter. This strategy allows tamoxifen-dependent ablation of NPC progeny that are committed to a neuronal lineage.
Our increasing understanding of the molecular mechanisms of adult neurogenesis allows the development of new techniques for blocking neurogenesis. WNT signalling is important for neuronal fate determination at the early stages of neurogenesis. A lentivirus-mediated overexpression of a dominant-negative WNT protein that blocks WNT signalling suppresses the production of adult-born dentate granule cells (DGCs) in the subgranular zone94. TLX is an orphan nuclear receptor that is of key importance for the proliferation of NPCs. Induced deletion of Tlx in the adult mouse brain by cytomegalovirus CreER also suppresses adult neurogenesis93. Polycomb 3 homologue (PC3; also known as CBX8) is a factor that promotes terminal differentiation in the CNS. Ectopic expression of PC3 in NPCs results in a smaller NPC population, which is accompanied by accelerated differentiation and impaired morphogenesis of adult-born DGCs126.
Even among studies that used the same task to investigate the cognitive effects of hippocampal neurogenesis, the data can be inconsistent (see Supplementary information S1 (table)). The reference memory version of the MWM is one of the most frequently used tests for the functional assessment of neurogenesis. Surprisingly, an apparent deficit in spatial-navigation learning, as measured by the latency or the distance travelled to reach the hidden platform, was only detected in two mouse studies using genetic ablation of neurogenesis87,93, but not in studies using other knockdown methods to prevent neurogenesis88,90,94-96. However, many studies showed impairments in the long-term but not the short-term retention of spatial memory as a consequence of reduced adult neurogenesis92,94,96,97. Similarly, inconsistent data have been obtained regarding the role of adult neurogenesis in contextual fear conditioning, which is another commonly used hippocampus-dependent learning and memory task. Deficits in this task have been repeatedly observed in rodents in which neurogenesis was almost completely eliminated by irradiation88,98,99 and in two lines of transgenic mice with genetic ablation of neurogenesis88,97. However, such defects were not detected in several other rodent studies in which neurogenesis was suppressed using different methods87,90,92,93.
Some of these results support the hypotheses that have arisen from computational studies. For example, the observations of impaired long-term spatial memory retention in some studies are consistent with the prediction from computational studies that the addition of newborn neurons helps to maintain the stability of old memories during the encoding of new information80-82. Similarly, the involvement of neurogenesis in tasks such as trace conditioning and contextual fear conditioning support the hypothesis that newborn neurons are involved in associating events that occur within a short time span83,84,86. However, these roles for neurogenesis are not corroborated by other studies (see Supplementary information S1 (table)).
Possible factors underlying contradictory findings
Many factors in the details of experimental design might have contributed to these discrepancies, including the species and strains of animals tested, the efficiency and side effects of ablation methods, the specific parameters in the design of behavioural paradigms and the parameters for evaluation of the behavioural phenotype (see Supplementary information S1 (table)). For example, recent studies revealed that contextual learning was defective in irradiated rats and in some but not other strains of mice17,88. Even in the same mouse strain, contextual fear conditioning deficits were detected if mice were treated with a high dose but not a low dose of irradiation, with the high dose of irradiation causing a greater reduction of neurogenesis100. Blocking neurogenesis (the rate of which declines exponentially with ageing101,102) at different ages can result in substantially different behavioural phenotypes103,104.
The designs of behavioural paradigms are another source of discrepancies in the aforementioned studies. For example, prolonged pre-training procedures may mask defective performance in behavioural tasks90,93. In addition, different studies use different parameters to evaluate behavioural phenotypes, which are not equally sensitive for revealing learning and memory deficits. For example, a recent study105 showed that ablation of neuro genesis by temozolomide treatment in mice did not alter path length or latency to find the hidden platform during the learning phase of the MWM task; however, it did affect the ability of the animals to efficiently adopt a precise, place-specific strategy to localize the hidden platform, a function that is presumably dependent on the hippocampus. It is therefore likely that the most commonly used parameters in the MWM, such as path length and latency to find the platform, may not be sensitive enough to reveal the subtle defects in learning caused by loss of adult neurogenesis.
In addition to the differences in the experimental details discussed above, there are two issues that are worth particular consideration when comparing data from different studies. First, a lack of adult-born DGCs at different maturation stages may lead to different behavioural phenotypes. As discussed in previous sections, newly generated DGCs that are less than 1 week old have not yet made connections with the local network and would probably not be involved in learning and memory. Indeed, rats that had received a 6-day MAM treatment were not impaired in eye blink trace conditioning when tested 2 days after treatment, in contrast to rats that had undergone a 14-day treatment scheme89. Furthermore, adult-born DGCs aged between 1 and 6 weeks may make distinct contributions to learning and memory owing to their enhanced excitability and plasticity, which could also account for the memory impairment in rats treated with MAM for 14 days89. Using pharmacogenetic approaches to reduce the number of adult-born DGCs at particular maturation stages in mice, a recent study detected deficits in long-term spatial memory and extinction of spatial preference and contextually evoked fear by specifically ablating adult-born DGCs of approximately 1–4 weeks of age — that is, before they were fully mature92. The transient nature of this transgenic model also allowed the authors to show that the phenotype was restored after replenishment of the affected DGC population, further corroborating the notion that the behavioural deficits were caused by reduced adult neurogenesis92. Finally, the survival of adult-born DGCs was compromised around 4–6 weeks after their birth in mice lacking kruppel-like factor 9. These mice were impaired in contextual discriminative learning, implying a role for adult-born DGCs at 4–6 weeks of age in pattern separation32. Together, these observations suggest that adult-born DGCs at the immature stage may make a distinct contribution to learning and memory.
Another major reason for data discrepancy in the field is that the behavioural paradigms used in most studies do not directly assess the function of the dentate gyrus, despite the fact that adult-born neurons differentiate and integrate into the neural network exclusively as DGCs. Instead, the majority of studies of adult neurogenesis investigate the role of adult-born DGCs in hippocampus-dependent functions. It is possible that learning and memory in some common hippocampus-dependent behavioural tasks do not rely on the dentate gyrus, as direct inputs from the entorhinal cortex to CA3 and CA1 might underlie the tasks (FIG. 1). Recent studies suggest that the monosynaptic pathway (entorhinal cortex–CA1–entorhinal cortex) is sufficient for MWM learning under certain conditions106. In fact, this monosynaptic pathway is necessary for forming precise spatial representations, as suggested by physiological studies in rats107. Additionally, the absence of NMDA receptor-mediated plasticity in the dentate gyrus does not affect the performance of mice in the MWM task or in the contextual fear conditioning task75. Therefore, examining the role of hippocampal neurogenesis using such tasks may result in inconsistent observations.
Previous studies suggest that pattern separation might be a role of the dentate gyrus in both animals and humans73,75,108. Does adult neurogenesis have a role in pattern separation, and if so, how? Computational modelling suggests that immature adult-born DGCs might function as pattern integrators by responding indiscriminately to events that occur closely in time83. A lack of neurogenesis may allow each event to be encoded more distinctly, which could be beneficial when a large amount of information needs to be learned in a short time. Indeed, mice in which neurogenesis was suppressed by two independent approaches performed better than controls in a spatial working memory task in an eight-arm radial maze with high interference (for example, the sample arms and/or goal arm being used repeatedly and interchangeably) and long delays between the sample trial and the test trial109. In this case, it is possible that a lack of pattern integration mediated by adult-born DGCs may have allowed each learning trial to be encoded more distinctly, resulting in less intra-trial or inter-trial interferences and eventually the observed performance improvement.
The role of adult-born DGCs in pattern separation has recently been examined directly in behavioural tasks for spatial pattern discrimination. Chronic ablation of neurogenesis by either irradiation or lentivirus-mediated overexpression of dominant-negative Wnt in mice impaired performance in two spatial discrimination tasks when two stimuli were presented with limited spatial separation but not when two stimuli were widely separated in space91. Although the function of the dentate gyrus in these two specific tasks awaits validation, this finding provides the first evidence for a possible involvement of adult-born DGCs in pattern separation. How does this observation relate to the pattern integration function of adult-born DGCs? One possible explanation is that the integrative ability of adult-born DGCs facilitates the association of the target stimuli with other elements in the context and leads to better pattern separation and eventually better spatial discrimination ability.
In summary, despite all of these inconsistent results, accumulating evidence suggests that adult-born DGCs do make contributions to learning and memory, consistent with computational theories that newborn neurons in the networks are likely to be selected for encoding new information. To resolve the controversies discussed above, future studies need to take into consideration both the design of behavioural paradigms (for example, by directly targeting the function of the dentate gyrus and carefully selecting the experimental timeline) and characteristics of the experimental animals (for example, the species and age).
Conclusions and future directions
Neurogenesis in the hippocampus represents a form of cellular plasticity in the adult brain that had not been previously recognized. Activity-dependent regulation of neurogenesis and experience-dependent participation of adult-born DGCs in information processing both imply a relationship between adult neurogenesis and learning and memory. Despite mixed results, behavioural evaluation of rodents with reduced adult neurogenesis has consistently suggested an involvement of adult-born DGCs in learning and memory. Nevertheless, the precise function of adult-born DGCs in cognitive processes remains elusive. Although many hypotheses have been proposed by computational modelling, most of them have not been explicitly tested in experimental studies.
Emerging data have already suggested that hippocampal neurogenesis is involved in pattern separation91, which can be modulated by pattern integration83. The observation that adult-born DGCs between 2 and 6 weeks of age are hyper-excitable18,29,30 might mean that these DGCs are particularly suited to mediating pattern integration. It will be intriguing to investigate whether these adult-born DGCs have a distinct influence on pattern separation and integration in a maturation stage-dependent manner. Furthermore, the long-lasting nature of these properties (that is, hyper-excitability and enhanced plasticity) and the constant turnover of immature DGCs suggest that adult-born DGCs could have a role in temporal association and separation during learning and memory. Experimental evidence is needed to support this hypothesis. Moreover, adult-born DGCs at 4–6 weeks of age show enhanced plasticity29, which makes them more suited to encoding new information, as predicted by computational studies (see above). These hypotheses are consistent with the finding that adult-born DGCs are preferentially activated upon memory retrieval once they reach a certain stage of maturation62,63. Further studies are needed to clarify the functional importance of this preferential activation and recruitment and whether it is dependent on the maturation stage of adult-born DGCs.
An important consideration when interpreting both IEG expression and behavioural studies is that it remains unclear whether adult neurogenesis is involved in the encoding, the consolidation or the recall of memory. Combining optogenetic techniques110 with retrovirus-mediated labelling of adult-born DGCs28 could provide a system to examine the physiology of adult-born DGCs in awake, behaving animals. Furthermore, some computational models suggest that addition of new neurons to the network increases memory capacity in the hippocampus80-82. Intriguingly, a recent study in mice suggested that adult neurogenesis facilitated memory reorganization that led to a gradual reduction of the hippocampus-dependence of memories and the permanent storage of these memories in extra-hippocampal regions111. Finally, both computational and experimental studies are needed to investigate whether (and if so, how) adult-born DGCs are involved in other dentate gyrus-mediated functions, such as conjunctive encoding112. In summary, future studies using a combination of molecular, genetic, behavioural and physiological approaches will be helpful in testing these hypotheses and elucidating the involvement of adult-born DGCs in cognition.
Hippocampal neurogenesis in humans is affected by various neurological disorders, including depression, epilepsy, cerebral ischaemia, Alzheimer’s disease and Parkinson’s disease, many of which are associated with cognitive decline6. Recently, several non-invasive imaging techniques have been developed for monitoring neurogenesis in human subjects113,114. Although their robustness and reproducibility await further testing, these techniques might allow the function of neurogenesis to be investigated in humans under various physiological or pathological conditions. Combined with sophisticated cognitive tasks and psychiatric examinations in humans, such studies have the potential to reveal functional mechanisms of adult neurogenesis that cannot be addressed in animal models, and will hopefully lead to improvement in therapies for these neurological disorders.
Supplementary Material
Acknowledgments
We thank M. L. Gage for editorial comments. This work is funded by the James S. McDonnell Foundation, the Lookout Fund, the Kavli Institute for Brain and Mind, the NSF Temporal Dynamics of Learning Center, the US National Institutes of Health (NS-050217) and National Institute on Aging (AG-020938).
Glossary
- Filopodia
Thin, long and highly motile protrusions that are the predecessors of spines in an early stage of spine formation.
- Thorny excrescences
The complex spines on the dendrites of CA3 pyramidal neurons in the stratum lucidum. These spines form multiple synapses with mossy fibres of dentate granule cells.
- Morris water maze (MWM)
A spatial learning paradigm in which an animal must learn a fixed location of a platform using distal spatial cues. Animals are released from a variable start point in each trial to encourage them to use a spatial strategy to solve the task.
- BrdU birth-dating
The thymidine analogue bromodeoxyuridine (BrdU) is injected into adult animals and incorporated into cells synthesizing DNA in preparation for division, which are visualized using immunocytochemistry. Because the in vivo half-life of BrdU is ~2 hours, it only labels dividing cells in a short time window.
- Sparse coding
A type of neural code in which each event is encoded by the strong activation of a small set of neurons.
- Attractor
A stable point in a dynamic system. Attractors are typically found in neural networks with strong feedback connections and are determined by the weights of the recurrent connections between units (neurons) in the network. Depending on the initial conditions and external inputs, the network will evolve towards one of these stable states.
- Pattern integration
The ability of immature dentate granule cells to provide an association between events owing to their indiscriminate responses to inputs.
- Pattern completion
A process by which a stored neural representation is reactivated by a cue that consists of a subset of that representation.
- Trace conditioning
A form of classical conditioning in which the conditioned stimulus occurs before the unconditioned stimulus with a stimulus-free period (the ‘trace interval’ or ‘conditioning interval’) between the two.
- Delay conditioning
A form of classical conditioning in which the onset of the conditioned stimulus precedes the onset of the unconditioned stimulus, with an overlap between the presentation of the conditioned stimulus and the presentation of the unconditioned stimulus.
- Recognition memory
The ability to correctly remember something that has been previously encountered. It is a subcategory of declarative memory.
- Contextual fear conditioning
A form of conditioning in which animals associate the conditioning context (the ‘neutral’ conditioned stimulus) with an aversive stimulus — for example, a foot shock.
- Spatial discrimination
The ability to discriminate separate locations in space.
- Conjunctive encoding
A form of information encoding in which a neuron requires the concurrent activity of multiple input neurons. In the hippocampus, dentate granule cells can associate spatial information from the medial entorhinal cortex with non-spatial information from the lateral entorhinal cortex to form a multi-dimensional representation of an event.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/gene
Arc | Egr1 | Fos | Homer1A
UniProtKB: http://www.uniprot.org
CREB | SLC12A2
FURTHER INFORMATION
Fred H. Gage’s homepage: http://www.salk.edu/faculty/gage.html
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
Competing interests statement
The authors declare competing financial interests: see web version for details.
References
- 1.Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Enikolopov G, Overstreet Wadiche L. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 81–100. [Google Scholar]
- 4.Kuhn HG, Peterson DA. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 25–47. [Google Scholar]
- 5.Zhao C. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 101–117. [Google Scholar]
- 6.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 8.Grubb MS, Nissant A, Murray K, Lledo PM. Functional maturation of the first synapse in olfaction: development and adult neurogenesis. J. Neurosci. 2008;28:2919–2932. doi: 10.1523/JNEUROSCI.5550-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nature Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 10.Breton-Provencher V, Lemasson M, Peralta MR, III, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Sahay A, Hen R. Hippocampal neurogenesis and depression. Novartis Found. Symp. 2008;289:152–160. doi: 10.1002/9780470751251.ch12. discussion 160–164, 193–195. [DOI] [PubMed] [Google Scholar]
- 14.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. The first systematic characterization of the process of adult hippocampal neurogenesis regarding the morphological and physiological maturation of adult-born DGCs.
- 19.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings NB, Seth MI, Tanapat P, Rydel TA, Gould E. Granule neurons generated during development extend divergent axon collaterals to hippocampal area CA3. J. Comp. Neurol. 2002;452:324–333. doi: 10.1002/cne.10386. [DOI] [PubMed] [Google Scholar]
- 21.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 22.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J. Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nature Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 25.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nature Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faulkner RL, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc. Natl Acad. Sci. USA. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 28.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 29.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. This study demonstrated the enhanced plasticity of developing adult-born DGCs by a systematic characterization of retrovirus-labelled DGCs at different time points.
- 30.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 31.Ambrogini P, et al. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 32.Scobie KN, et al. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J. Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 34.Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 35.Leuner B, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J. Neurosci. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupret D, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrossy MD, et al. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- 39.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 40.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. The first study to show the regulation of the survival of adult-born DGCs by experience in mice.
- 41.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. The authors showed that the experiences of mice when the adult-born DGCs are in a hyper-excitable stage affect the subsequent responsiveness of these DGCs to various inputs.
- 42.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 43.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 44.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 45.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 47.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus. 2009;19:1002–1007. doi: 10.1002/hipo.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl Acad. Sci. USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- 52.Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J. Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol. Learn. Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Madsen TM, Greisen MH, Nielsen SM, Bolwig TG, Mikkelsen JD. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98:33–39. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- 55.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jessberger S, et al. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 59.Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J. Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guzowski JF, et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur. J. Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J. Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 64.Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc. Natl Acad. Sci. USA. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn. Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 66.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology (Bethesda) 2004;19:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- 68.Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 69.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 70.O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 71.Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 72.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 73.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 75.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 76.Aimone JB, Wiskott L. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 101–117. [Google Scholar]
- 77.Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- 78.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 79.Crick C, Miranker W. Apoptosis, neurogenesis, and information content in Hebbian networks. Biol. Cybern. 2006;94:9–19. doi: 10.1007/s00422-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 80.Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- 81.Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- 82.Weisz VI, Argibay PF. A putative role for neurogenesis in neuro-computational terms: inferences from a hippocampal model. Cognition. 2009;112:229–240. doi: 10.1016/j.cognition.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. A bottom-up computational model of adult hippocampal neurogenesis. The authors proposed a role for adult-born DGCs with enhanced excitability in pattern integration through their broad tuning properties.
- 84.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 85.Friedman WJ. Comment on “Potential role for adult neurogenesis in the encoding of time in new memories”. Hippocampus. 2007;17:503–504. doi: 10.1002/hipo.20280. [DOI] [PubMed] [Google Scholar]
- 86.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn. Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. The first study to show the functional importance of adult neurogenesis. The authors discovered that rats with reduced adult neurogenesis were impaired in learning conditioned response in an eye blink trace conditioning paradigm.
- 90.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. The first study to suggest an involvement of adult hippocampal neurogenesis in pattern separation, a proposed function for the dentate gyrus.
- 92.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. This study showed that adult-born DGCs contribute to learning and memory before their full maturation, at a stage when they have enhanced excitability.
- 93.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 94.Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 96.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 98.Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur. J. Neurosci. 2008;27:1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- 99.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 100.Ko HG, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol. Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging. 31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 103.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 104.Rola R, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Garthe A, Behr J, Kempermann G. Adultgenerated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 107.Brun VH, et al. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 108.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 109.Saxe MD, et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl Acad. Sci. USA. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 111.Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. The first study to show a role for adult hippocampal neurogenesis in system consolidation.
- 112.Kesner RP. A behavioral analysis of dentate gyrus function. Prog. Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- 113.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog. Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baker JL. Is there a support vector machine hiding in the dentate gyrus? Neurocomputing. 2003;52–54:199–207. [Google Scholar]
- 117.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog. Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 118.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 119.Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nature Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 121.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 122.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl Acad. Sci. USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dupret D, et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur. J. Neurosci. 2005;22:778–783. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- 124.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 125.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 126.Farioli-Vecchioli S, et al. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.