Abstract
Substantial evidence indicates bioenergetic dysfunction and mitochondrial impairment contribute either directly and/or indirectly to the pathogenesis of numerous neurodegenerative disorders. Treatment paradigms aimed at ameliorating this cellular energy deficit and/or improving mitochondrial function in these neurodegenerative disorders may prove to be useful as a therapeutic intervention. Creatine is a molecule that is produced both endogenously, and acquired exogenously through diet, and is an extremely important molecule that participates in buffering intracellular energy stores. Once creatine is transported into cells, creatine kinase catalyzes the reversible transphosphorylation of creatine via ATP to enhance the phosphocreatine energy pool. Creatine kinase enzymes are located at strategic intracellular sites to couple areas of high energy expenditure to the efficient regeneration of ATP. Thus, the creatinekinase/phosphocreatine system plays an integral role in energy buffering and overall cellular bioenergetics. Originally, exogenous creatine supplementation was widely used only as an ergogenic aid to increase the phosphocreatine pool within muscle to bolster athletic performance. However, the potential therapeutic value of creatine supplementation has recently been investigated with respect to various neurodegenerative disorders that have been associated with bioenergetic deficits as playing a role in disease etiology and/or progression which include; Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. This review discusses the contribution of mitochondria and bioenergetics to the progression of these neurodegenerative diseases and investigates the potential neuroprotective value of creatine supplementation in each of these neurological diseases. In summary, current literature suggests that exogenous creatine supplementation is most efficacious as a treatment paradigm in Huntington’s and Parkinson’s disease but appears to be less effective for ALS and Alzheimer’s disease.
Keywords: Alzheimer’s, Huntington’s, Parkinson’s, Amyotrophic lateral sclerosis, Mitochondria, Apoptosis, Bioenergetics, Reactive oxygen species
Introduction
Neurological diseases are generally marked by degeneration of neurons within distinct areas of the brain. Neuronal loss and/or dysfunction can lead to a wide variety of neurological diseases which is dependent on the location of neuronal loss, specific pathogenesis, and the course of disease progression (Beal 1996). Despite these variances, there is increasing evidence to suggest that this class of diseases also share some similar fundamental biochemical processes that contribute to the pathogenesis and clinical phenotype of otherwise different neurological diseases. These overlapping processes which include excitotoxicity, oxidative stress, energy depletion, and mitochondrial dysfunction have been implicated in several disorders such as Huntington’s disease (Browne et al. 1997, 1999), Parkinson’s disease (PD, Beal 1996; Thomas and Beal 2007), amyotrophic lateral sclerosis (ALS; Beal 1996; Bogdanov et al. 1998b; Hervias et al. 2006) and mitochondrial diseases (O’Gorman et al. 1997a; Simon and Johns 1999). Although not all of these characteristics are present in each neurological disorder, it is believed that certain aspects of the aforementioned concepts ultimately converge to contribute to the overall loss of neurons in selected regions by apoptosis and/or necrosis. Mitochondria are not only fundamental to cellular bioenergetics, but also key mediators of apoptosis and can be linked either directly and/or indirectly to many of these common deleterious processes involved in neurodegeneration. Thus, improvement in mitochondrial function and cellular bioenergetics represents a potential target for therapeutic intervention in neurological disease. This review will summarize the importance of mitochondrial dysfunction in neurodegeneration and focus on the potential therapeutic efficacy of creatine monohydrate to improve overall cellular bioenergetics in various neurological disorders.
Mitochondrial Involvement in Neurodegeneration
Mitochondria are critical organelles involved in regulating the energy status of the cell through oxidative phosphorylation. Oxidative phosphorylation produces a usable form of energy (ATP) for a variety of cellular processes and this is particularly important in highly metabolic tissues with large ATP requirements such as heart, skeletal muscle, and brain (Adhihetty et al. 2003). Although the primary role of mitochondria are to supply and regulate energy for the cell, they have also been shown to be involved in neurodegenerative processes including excitotoxicity (Peng and Greenamyre 1998), reactive oxygen species production (Green and Reed 1998), dysregulated cellular calcium homeostasis (Steeghs et al. 1997), and apoptosis (Green and Reed 1998). Additionally, toxins that specifically target the electron transport chain of mitochondria such as 3-nitroproprionic acid and/or malonate, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) have been shown to reproduce the pathogenesis and disease progression of Huntington’s and Parkinson’s disease, respectively (Beal 1996; Thomas and Beal 2007). In the past, mitochondria have only been associated with playing a critical role in supplying and maintaining cellular energy metabolism and commonly described by textbooks as the “powerhouse of the cell.” However, it is now clear that when mitochondria become dysfunctional, they are also key participants in the pathogenesis of numerous diseases and this review will highlight their involvement in neurodegenerative diseases.
Mitochondrial Involvement in Apoptosis
Neuronal cell loss represents a convergent endpoint for many neurological diseases. The involvement of mitochondria, and mitochondrially mediated signaling in apoptotic cell death, is now well recognized, and a large body of current literature in neurodegeneration is currently focused on this research topic. Mitochondria are involved in apoptosis because, (1) they contain several pro-apoptotic proteins which can contribute toward cell death upon release into the cytosol and, (2) they are the primary producers of reactive oxygen species that can serve to initiate the release pro-apoptotic mitochondrial proteins (Fig. 1). Mitochondrial pro-apopotic proteins include cytochrome c, apoptosis-inducing factor (AIF), endonuclease G (Endo G), Smac/Diablo, and Omi/Htra2 (Primeau et al. 2002; Adhihetty and Hood 2003). However, the specific signal transduction pathways and/or mechanisms leading to the release of these pro-apoptotic proteins from the mitochondrion are still being elucidated.
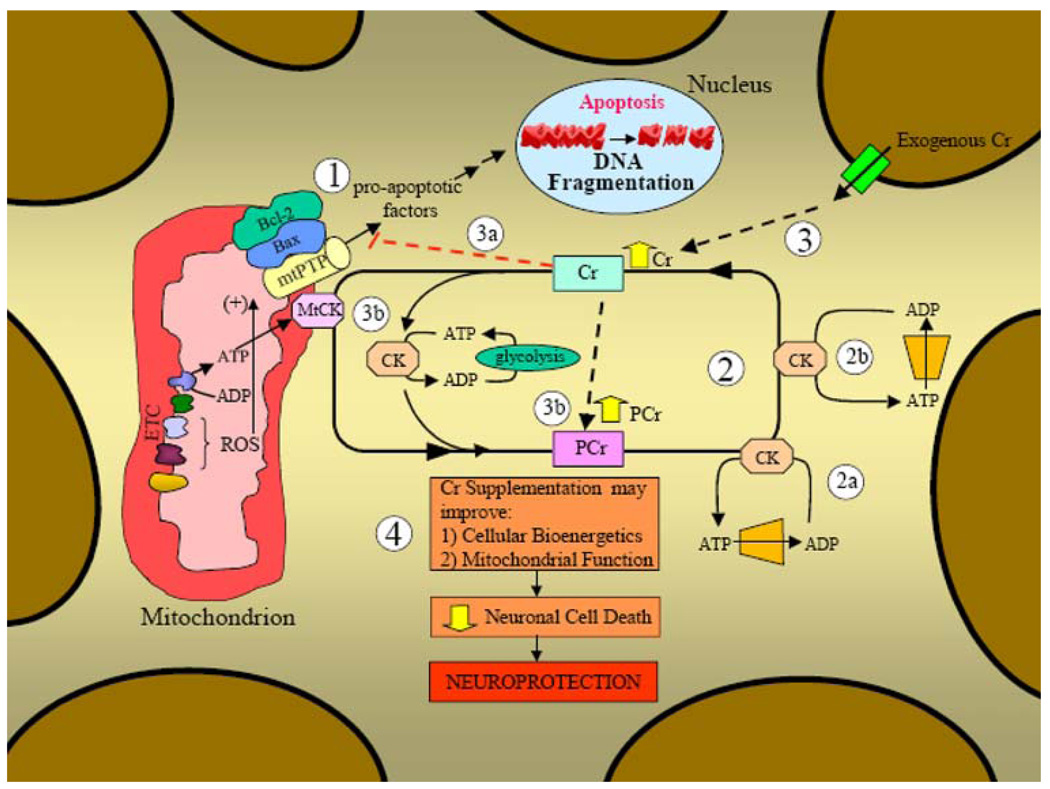
Fig. 1.
Creatine kinase/phosphocreatine energy shuttle pathway and the role of mitochondria in apoptotic cell death in neurons. (1) Mitochondrial involvement in apoptosis. Mitochondria are intimately involved in apoptotic cell death since, (1) they contain pro-apoptotic proteins which can lead to cell death upon release into the cytosol (DNA fragmentation is a hallmark feature of apoptosis), and (2) they produce the majority of the potentially damaging reactive oxygen species (ROS). ROS can damage intracellular components (i.e., alter DNA, proteins, lipids) and have also been shown to increase the susceptibility to apoptosis by modulating components of the channel that traverses the inner and outer mitochondrial membrane termed the mitochondrial permeability transition pore (mtPTP). Upon a significant apoptotic insult, mitochondria can release pro-apoptotic factors to the cytosol to induce cellular death. The octameric form of the mitochondrial creatine kinase (MtCK) interacts with components of the mtPTP to suppress pore opening and potentially reduce apoptotic susceptibility. Creatine has been shown to maintain the mtCK in an octameric conformation (as opposed to dimeric) which inhibits mtPTP opening. (2) Creatine kinase/phosphocreatine energy shuttle pathway. Creatine (Cr) is taken up by the neuron via specific creatine transporters and transphosphorylated to the high-energy phosphocreatine (PCr) by either mitochondrial creatine kinases (MtCKs) or cytosolic creatine kinases (CKs). MtCK is located in the intermembrane space of mitochondria and is coupled to ATP production by mitochondrial oxidative phosphorylation to produce PCr which is then exported from the organelle. Cytosolic CK transphosphorylates ATP generated via glycolysis to PCr. This PCr can then contribute to the overall cellular PCr pool. The PCr pool is coupled to localized CKs in areas of high ATP demand such as (2a) organelle transport and/or ATP dependent cell signaling or (2b) at the cell membrane by ATP-dependent pumps and/or ATP-dependent receptors. This localized distribution of CKs allows for the efficient production of ATP from ADP to fuel ATP-dependent processes in various portions of the cell. (3) Effect of exogenous creatine supplementation (dashed lines). Exogenous creatine is transported through the cell membrane via creatine transporters to increase the overall creatine pool. (3a) Increased creatine levels may further stabilize the octameric form of mtCK to suppress opening of the mtPTP and this may reduce mitochondrially mediated apoptosis. (3b) Elevated levels of creatine within the cell can be transphosphorylated by mitochondrial and cytosolic creatine kinases (MtCKs and CKs, respectively) to enhance the overall PCr pool. Increased PCr levels can be utilized by localized creatine kinases to transphosphorylate ADP to ATP for various ATP-dependent processes within the cell (2a) and at the cell membrane (2b). (4) Potential neuroprotective effects of creatine. Creatine supplementation may improve both the bioenergetic and mitochondrial deficits associated with some neurological diseases (more efficacious in Parkinson’s and Huntington’s disease). These beneficial effects of creatine may ultimately reduce neuronal cell death and provide neuroprotection in certain neurodegenerative disorders
Mitochondria are comprised of outer and inner membranes separated by an intermembrane space. In order for mitochondrial pro-apoptotic protein release to occur, a continuum between the inner and outer mitochondrial membranes is required. This is mediated by the formation of specialized permeability transition pores that facilitate pro-apoptotic release from mitochondria which can eventually lead to DNA fragmentation, a hallmark feature of apoptosis (Adhihetty et al. 2003; Fig. 1). The most characterized mitochondrial pore is termed the mitochondrial permeability transition pore (mtPTP). The mtPTP consists of several components which include: the mitochondrial matrix protein, Cyclophilin D (Cyp D), an inner membrane protein, adenine nucleotide translocase (ANT), and the outer membrane protein, the voltage-dependent anion channel (VDAC) (Adhihetty et al. 2003). Although these proteins represent key components of the mtPTP, there are a variety of factors that can both facilitate the congregation of these specific proteins to form the basic structure of the mtPTP and/or cause a change in the status of the mtPTP and place it in an open conformation (see details below regarding creatine kinase). The signaling pathways mediating the closed and/or open conformation of the mtPTP have yet to be fully established. However, current literature does suggest a variety of factors can facilitate either mtPTP formation/opening and these include: (1)an accumulation of Ca2+, (2) a reduction in the membrane potential (ΔΨ), (3) an increase in inorganic phosphate, (4) a reduction in adenine nucleotides (ATP and ADP), and (5) elevations in oxidative stress. Once mtPTP formation and opening has occurred due to any combination of these factors, pro-apoptotic proteins can be released into the cytosol to initiate cell death pathways (Di Lisa et al. 2006). Although CypD, ANT and VDAC have traditionally been considered intricate components necessary for mtPTP formation, there is recent compelling evidence that suggests CypD is the only essential component (Kokoszka et al. 2004; Baines et al. 2005; Csukly et al. 2006). Despite the finding that VDAC and ANT may be indispensable for mtPTP formation, these proteins may still serve as regulatory pore proteins when interacting with Bcl-2 family members (see below) and further investigation is warranted.
The Bcl–2 family of proteins regulates the conformational status of the mtPTP. There are pro-apoptotic (i.e., Bax, Bak, Bok) and anti-apoptotic family members (i.e., Bcl–2, Bcl-XL, Bcl-w; Adams and Cory 1998 for review; Fig. 1). These pro- and anti-apoptotic proteins can neutralize or titrate the function of one another by forming heterodimers. Thus, the relative proportion of pro- and anti-apoptotic proteins are an important factor determining cell fate when faced with a pro-apoptotic stimulus (Primeau et al. 2002).
Mitochondrial oxidative phosphorylation involves a series of electron transfers within the inner mitochondrial membrane. The inefficient transfer of electrons can produce a variety of unstable and potentially damaging reactive oxygen species (ROS; Fig. 1). As a result, mitochondria are the primary ROS producers in the cell, with mitochondrial ROS levels being 5- to 10-fold greater than within cytosol (2). Mitochondrial ROS are proposed to initiate early apoptotic triggering events. ROS can directly induce cytochrome c dissociation from the inner mitochondrial membrane and cause its subsequent release from the organelle (2). Additionally, ROS can directly interact with, and facilitate mtPTP opening (Fig. 1). However, the accumulation of ROS within the matrix is somewhat limited by mitochondrial antioxidant enzymes, including phospholipid hydroperoxide glutathione peroxidase (PHGPx), glutathione peroxidase (GPx), and Mn-superoxide dismutase (Mn-SOD; Adhihetty and Hood 2003).
ROS can also indirectly influence the apoptotic pathway by activating mitogen-activated protein kinases (MAPKs) and various redox-sensitive transcription factors involved in the expression of both anti- and pro-apoptotic gene expression (Adhihetty and Hood 2003). Since oxidative stress is a common feature of many neurological disorders and mitochondria are the primary producers of ROS within the cell, this further fortifies the importance of mitochondria as a central and convergent component integral in neurological disease.
Creatine Monohydrate-Structure, Function, and Role in Cellular Energy Status
Creatine is a guanidino-compound found primarily in meat products and is produced endogenously by the liver, kidneys, and pancreas (Juhn and Tarnopolsky 1998a). The production of creatine requires the amino acids arginine and glycine. Additionally, the amino acid methionine is required to supply a methyl group to the overall structure. Creatine is initially synthesized by the conjugation of arginine and glycine by the rate-limiting enzyme l-arginine:glycine amidinotransferase (transamidinase) to produce guanidinoacetate. This product is subsequently methylated by S-adenosyl-methionine which is catalyzed by the important guanidinoacetate-methyltransferase (GAMT) to produce the end product of creatine (Tarnopolsky and Beal 2001). The importance of creatine to normal neurological function is clearly demonstrated in patients that have an inborn deficiency of GAMT which leads to an abnormally low biosynthesis of creatine. Patients with GAMT deficiency exhibit clear developmental delays, extrapyramidal movement disorders and seizures (Stockler and Hanefeld 1997; Stockler et al. 1997; van der Knaap et al. 2000). To further substantiate the importance of creatine in normal neurological function, creatine monohydrate supplementation improves a variety of the neurological impairments in patients afflicted with this rare disorder (Stockler and Hanefeld 1997; van der Knaap et al. 2000). Thus, these studies clearly illustrate the importance of creatine in the maintenance of normal neural cell bioenergetics and function.
Creatine is taken up into brain, heart, and skeletal muscle tissue by a sodium-dependent transporter and inward movement is enhanced by the presence of insulin (Sora et al. 1994; Steenge et al. 1998). Some concern has been raised on whether chronic exogenous supplementation of creatine could potentially result in a compensatory downregulation of creatine transporters in the cell. However, data have shown that the creatine transporter is not downregulated following 2 months of a high physiological dose of creatine in humans (10 g/day; Juhn and Tarnopolsky 1998b). Dietary creatine is found in meat containing products (i.e., 5 g of creatine is found in 1.1 kg of beef) and the typical North American diet provides approximately 1 g of creatine per day (Juhn and Tarnopolsky 1998a). Creatine supplementation has been used in both healthy individuals and patients with neurological disease. The safety of creatine supplementation has been reviewed extensively and it has been concluded that creatine supplementation does not have deleterious effects in humans (Juhn and Tarnopolsky 1998b; Persky and Brazeau 2001; Wyss and Schulze 2002; Baker and Tarnopolsky 2003). Creatine supplementation has been generally regarded as safe and has been used for extended periods of times by athletes and in some patient populations with few reported side effects. However, there are a few reports that creatine supplementation can cause an elevation in creatinine levels and that this may have adverse effects on, and/or be a consequence of renal function or dysfunction, respectively (Mihic et al. 2000; Robinson et al. 2000; Benzi and Ceci 2001). In contrast, other studies have shown that extended creatine supplementation does not alter either renal function and/or glomerular filtration (Poortmans et al. 1997; Poortmans and Francaux 2000; Mihic et al. 2000). Although there is some concern over long-term, chronic high-dose creatine supplementation, the majority of evidence suggests that creatine supplementation is certainly tolerable and a safe dietary supplement (Tarnopolsky and Beal 2001; Wyss and Schulze 2002).
Creatine exists in the cell as both free creatine (Cr) and phosphocreatine (PCr) which together comprise the total creatine pool (Fig. 1). In tissues with high energy requirements, such as skeletal muscle and brain, PCr serves as a short-term energy buffer in which adenosine diphosphate (ADP) is phosphorylated to adenosine triphosphate (ATP). This phosphoryl group transfer is catalyzed by the important creatine kinase (CK) enzyme (Tarnopolsky and Beal 2001). Thus, CK is an important mediator of cellular homeostasis since it can reversibly convert creatine into phosphocreatine to create a large pool of phosphocreatine for temporal and spatial ATP buffering (Andres et al. 2008; Fig. 1). There are two different isoenzymes of CK which exist in most tissues, and are localized to different cellular regions. Cytosolic CK is found as a dimer and is often associated with subcellular structures within the cell while either an octameric or dimeric form is found within the cristae and intermembrane space of mitochondria (MtCK; Eppenberger et al. 1967; Schlattner et al. 2006). Additionally, there are two tissue-specific MtCK isoenzymes which are termed sarcomeric mtCK (sMtCK) found in striated muscle and ubiquitous MtCK (uMtCK) has been found in most other tissues, including neural tissue (Wallimann and Hemmer 1994; Boero et al. 2003). Despite being localized in different regions of the cell, both cytosolic and mitochondrial CK isoenzymes contribute to the overall pool of phosphocreatine within the cell to create an efficient energy buffering system. As mentioned previously, MtCk can exist either as a dimeric or an octameric form in mitochondria and the conformational status has been shown to dictate the overall function and/or interactions of MtCK with other mitochondrial proteins. The octameric form facilitates the functional coupling of VDAC (also known as porin), an outer membrane protein, and the inner membrane protein, ANT, to suppress mtPTP opening, which may serve to reduce mitochondrial apoptotic susceptibility (O’Gorman et al. 1997b; Dolder et al. 2003). Interestingly, the octameric MtCK is particularly vulnerable to oxidative stress and upon a sufficient oxidative insult, the octameric mtCK is converted to the dimeric MtCK form. This octameric-to-dimeric MtCK shift has been shown to negatively impact mitochondrial calcium homeostasis, facilitates the opening of the mtPTP and leads to impaired mitochondrial respiration (O’Gorman et al. 1997a). Additionally, within skeletal muscle, the dimeric sMtCK can crystallize to form paracrystalline inclusions which are a common feature of many mitochondrial cytopathies and also occur following chronic creatine depletion in muscle tissue. The physiological consequences of paracrystalline inclusions have not been fully established. However, evidence does suggest that the dimeric sMtCK present in paracrystalline structures is enzymatically non-functional which likely contributes to dysfunctional cellular energy homeostasis.
The phosphocreatine and CK energy pathway represents an extremely efficient energy buffering system for two reasons. First, phosphocreatine has a slightly higher diffusion capacity than ATP, making PCR transport a more efficient energy delivery system to different cellular locations. Second, the subcellular localization of cytosolic and mitochondrial CK couples areas of energy generation with energy production (Fig. 1). Thus, the CK-phosphocreatine essentially serves as a spatial “energy shuttle” or “energy circuit” within the cell (Jacobus and Lehninger 1973; Bessman and Geiger 1981; Saks et al. 1978; Wallimann et al. 1992). The PCr buffering system is responsible for half of the energy requirements during short-term (i.e., 10 s) muscle contraction. In fact, the beneficial impact of creatine supplementation and widespread usage originally occurred as a result of its well-defined functional improvements in muscle tissue to ultimately enhance athletic performance. For example, it has been shown that exogenous supplementation of creatine utilizing various loading strategies can prolong the duration of short-term muscle contractions by bolstering the cellular energy pool (Mahoney et al. 2002). Additionally, supplementation with creatine has not only been shown to enhance bioenergetics but also to increase lean body mass in human subjects (Mihic et al. 2000). This implies that the PCr/Cr system may not only be involved in energy metabolism but may also activate signaling pathways responsible for upregulating myofibrillar protein synthesis. The clear benefits of dietary creatine supplementation on skeletal muscle function have made it one of the most popular and scientifically proven ergogenic aids. In addition to athletic performance, creatine supplementation has also been shown to improve the beneficial adaptations of resistance exercise alone in elderly subjects and this may serve to reduce sarcopenia with aging (Tarnopolsky and Safdar 2008).
The importance of the creatine kinase system for brain function has been shown using genetically altered mice that lacked the brain isoform of the cytosolic CK which showed deficits in open field behavior, slower learning and a loss in hippocampal mossy fiber connections (Jost et al. 2002). Mice lacking both the cytosolic and the mitochondrial CK within the brain, not surprisingly, exhibited a more severe phenotype compared to the single cytosolic CK knockout (Streijger et al. 2005). Taken together, these studies clearly demonstrate the importance of the CK system for normal brain function. Since many neurological diseases are characterized by deteriorations in cellular bioenergetics and metabolism, this led to the hypothesis that exogenous creatine may prove to be useful as a therapeutic intervention in neurodegenerative disorders.
Creatine and its Protection in Neurodegenerative Disorders
Given the role of creatine in cellular energy homeostasis, the therapeutic efficacy of creatine supplementation is most promising in the neurological disorders that have marked impairment in energy metabolism. Although many of the molecular mechanisms are not well understood, creatine supplementation has been proposed and/or proven to be partially efficacious in a variety of animal/cellular models of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, ALS, and Huntington’s. Although the detailed mechanisms of the creatine-induced neuroprotection for each disease is slightly different, the basic overall premise is similar in that creatine supplementation is proposed to improve the overall bioenergetics and/or mitochondrial deficits associated with each particular neurodegenerative disease. This review will now focus on the evidence for and/or potential protective effect of creatine supplementation in each of the specific neurodegenerative diseases.
Alzheimer’s Disease
The molecular features of Alzheimer’s disease (AD) include: significant loss of neurons, deposition of extracellular plaques, and intracellular neurofibrillary tangles (Selkoe 1999; Small and McLean 1999; Hoyer 2004). One of the earliest detectable defects in AD patients has been shown to be impaired energy metabolism and mitochondrial electron transport chain dysfunction (Parker 1991; de la Monte et al. 2000; Maurer et al. 2000; Valla et al. 2001; Fig. 2). In addition, another biochemical signature of AD is a higher level of oxidatively damaged mitochondrial DNA, lipids, and proteins when compared to control individuals (Markesbery 1997; Butterfield and Lauderback 2002; Smith et al. 1991; Castegna et al. 2002a, b; Fig. 2). Furthermore, some data have suggested that mtDNA mutation rates are elevated in neural tissue of AD patients, but this is still under debate (Onyango and Khan 2006). Taken together, these studies strongly suggest that defects in overall cellular bioenergetics, mitochondrial dysfunction, and mitochondrially mediated oxidative stress contribute to the progression of AD. In addition, elevated oxidative stress in AD is thought to contribute to the significant reduction in creatine kinase activity levels in AD brain homogenates (Hensley et al. 1995; David et al. 1998; Aksenov et al. 2000). As discussed earlier, the CK enzyme is sensitive to oxidative stress due to the presence of a highly sensitive cysteine residue that can be easily modified by an oxidative insult. Thus, these data suggest that AD brain tissue may be under significant energetic duress due to a reduction in CK activity as a result of elevated oxidative stress. In fact, one study has shown that AD patients have reduced levels of brain PCr at the onset of symptoms and decreased oxidative metabolism in later stages (Pettegrew et al. 1994). Recently, creatine deposits have been identified in a transgenic model of AD using Fourier transform infrared microspectroscopy (FTIR; Gallant et al. 2006). A variety of potential explanations for this creatine deposition have been proposed, however, the most plausible explanation appears to be that the oxidative stress associated with AD impairs both cytosolic and mitochondrial CK to substantially reduce the formation of PCr (Burklen et al. 2006). It is hypothesized that the limited PCr pool available in the neural tissue of AD patients is expeditiously depleted to support ADP to ATP conversion, and this generates an excess of Cr. Since oxidative-induced damage may have impaired the ability of CK to convert Cr to PCr, an excess of Cr accumulates to ultimately form deposition sites in the cell (Burklen et al. 2006; Fig. 2). Another study supporting this explanation for creatine deposits showed that amyloid precursor protein (APP) directly interacts with and binds to mitochondrial CK (Li et al. 2006). It has been proposed that functional APP may serve as a chaperone to target mitochondrial CK and other proteins from the cytoplasm to the mitochondria. This chaperoning role is likely disrupted by the loss in APP function, as commonly occurs in AD patients, potentially reducing its chaperone-like function and leading to lower mitochondrial CK levels (Fig. 2). This would ultimately decrease PCr synthesis at the mitochondria and could also contribute toward an excess of Cr deposition. The finding of creatine deposits in the brain of AD patients suggests that creatine supplementation as a potential therapeutic intervention may be futile and only result in further deposition of creatine with a lack of functional bioenergetic improvement. However, creatine supplementation has been shown to improve mental concentration, memory, and learning in normal healthy subjects and it is possible that these benefits may also occur in early stage AD patients (Watanabe et al. 2002; Rae et al. 2003). Although creatine supplementation would not improve cellular bioenergetics if the CK system is inactivated due to oxidative stress at later stages of AD, it is possible that creatine supplementation may increase cellular bioenergetics at early stages of AD when the CK system might be reduced yet still functioning (Fig. 2). Additionally, another beneficial function of creatine is that it has been shown to confer protection against the oxidative-induced inactivation of the CK isoenzymes (Aksenov et al. 2000). Specifically, creatine has been shown to prevent the oxidative-mediated conversion of octameric MtCK to the dysfunctional dimeric MtCK. Thus, elevating creatine levels by exogenous supplementation may result in a protection of the CK system and potentially delay the ROS-induced inactivation of CK as occurs in AD patients (Aksenov et al. 2000).
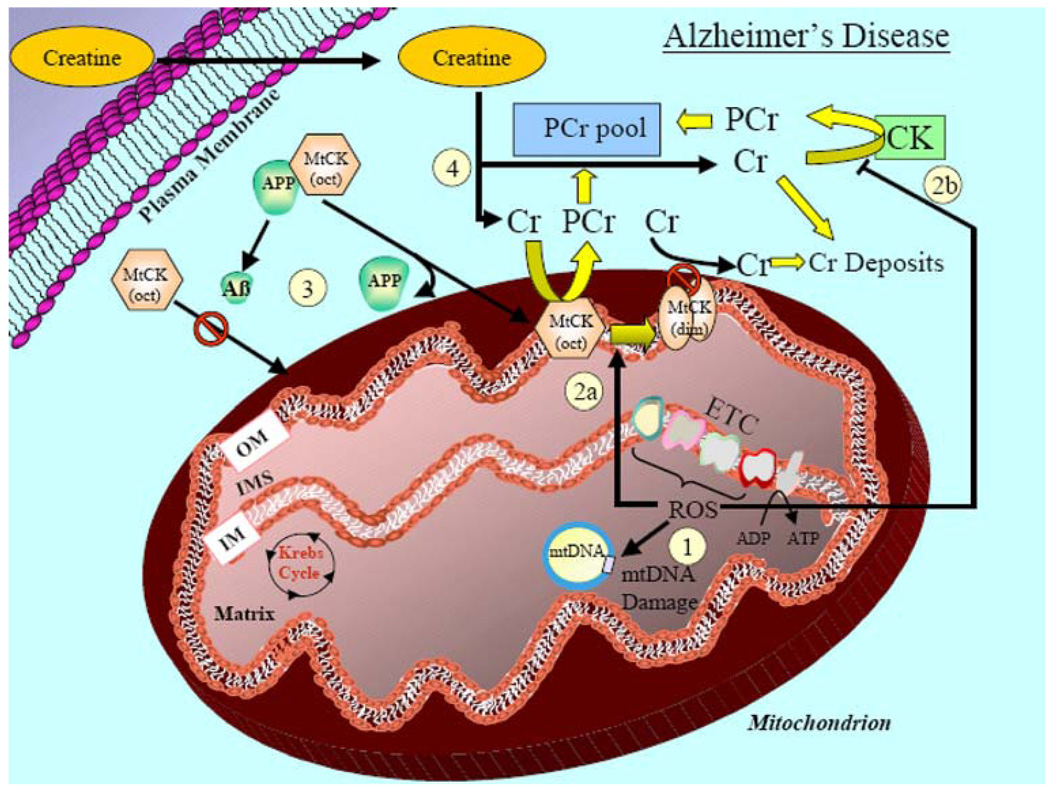
Fig. 2.
Mitochondrial involvement in Alzheimer’s disease (AD) and the effect of creatine supplementation. (1) Mitochondrial ROS-induced damage. AD is associated with a greater level of mitochondrially mediated oxidative damage to intracellular proteins and lipids, and this elevated oxidative stress can induce damage to mitochondrial DNA. (2) ROS-induced inactivation of mitochondrial and cytosolic creatine kinase. (2a) Elevated ROS can evoke an octameric-to-dimeric conformational shift in mitochondrial creatine kinase (MtCK-oct) leading to an inactive form of the enzyme (MtCK-dim). (2b) Elevated ROS can also cause damage to, and reduce the activity of the cytosolic creatine kinase (CK). The decreased activity of both the mitochondrial and cytosolic creatine kinases results in less generation of phosphocreatine. This leads to an excess accumulation of creatine and the formation of creatine deposits in the cytosol. (3) Cleavage of amyloid precursor peptide (APP) to Aβ inhibits chaperone-like activity to reduce mtCK in mitochondria. APP can bind to MtCK and is proposed to have chaperone-like activity that facilitates translocation of MtCK from the cytosol to mitochondria. In AD, APP is cleaved to form Aβ fragments that are incapable of this chaperone-like activity. (4) Exogenous creatine supplementation. Improvements in cellular bioenergetics in AD with exogenous creatine supplementation is dependent on (a) the extent of inactivation of the mitochondrial and cytosolic CK and, (b) the levels of reduced mitochondrial CK due to suppressed translocation to the mitochondria. Given that creatine deposition sites have found in late-stage AD, creatine supplementation would most likely be efficacious only during early stages of AD when mitochondrial and cytosolic CK might be suppressed but still active to be capable of generating and enhancing the PCr pool
An additional neuroprotective mechanism of creatine supplementation might be through the activation of the AMPK signaling pathway in a similar manner to that shown in skeletal muscle cells (Ceddia and Sweeney 2004). The AMPK pathway is important in regulating mitochondrial content and function in a PGC–1α-dependent pathway in various tissues and in response to different stimuli (Zong et al. 2002). Thus, creatine supplementation may improve cellular bioenergetics by activating AMPK to ultimately improve overall mitochondrial content and/or function. However, the supplemental efficacy of creatine in activating the AMPK pathway has only been shown in skeletal muscle cells. It is currently unknown whether creatine supplementation exerts similar AMPK effects in neural tissue and/or cells. This certainly represents an intriguing and potentially promising neuroprotective signaling pathway/mechanism of creatine given the prominent role of AMPK in mitochondrial regulation and the known mitochondrial dysfunction/impairments in AD and other neurodegenerative disorders.
Despite the potential neuroprotective effect of creatine in delaying AD progression by improving overall cellular bioenergetics (as discussed above), very few studies have systematically investigated the therapeutic value of creatine in AD. This may be due, in part, to the presence of creatine deposits in the neural tissue of AD patients. The creatine deposits exhibited in late stage AD patients implies that creatine supplementation at that point would appear to be a futile exercise but creatine supplementation during early stages of AD may provide some therapeutic value and this certainly should be investigated. One study did show that creatine supplementation is protective against glutamate and β-amyloid toxicity in rat hippocampal neurons (Brewer and Wallimann 2000). However, further creatine studies in AD, and particularly early stages of AD, are warranted given that creatine can improve bioenergetics and mitochondrial function, both of which have been shown to be impaired in neural tissue of AD patients.
Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disorder marked by progressive bradykinesia, rigidity, tremor, and gait abnormalities. At the molecular level, PD is marked by the neuronal loss and/or dysfunction of dopaminergic neurons located in the substantia nigra which ultimately leads to suppressed activation and/or function of neurons in the motor cortex (Beal 2003). A variety of molecular mechanisms have been shown to contribute to the dopaminergic neuronal loss and/or dysfunction in PD and once again mitochondrial dysfunction and oxidative stress are believed to be central in the pathogenesis of this disease (Beal 1995). Specifically, studies have indicated that deficiencies exist within complex I of the mitochondrial electron transport chain in platelets of patients with early PD and in the substania nigra pars compacta region of postmortem tissue of more advanced PD patients (Bindoff et al. 1989; Parker et al. 1989; Schapira et al. 1990; Krige et al. 1992; Fig. 3). To further emphasize the contribution of mitochondria in PD, it has been shown that the neurotoxin, 1-methyl–4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), impairs mitochondrial function and leads to Parkinsonism in humans and animal models. Specifically, MPTP exposure and/or treatment impairs complex I of the electron transport chain and it is highly selective for dopaminergic neurons in the substantia nigra region within the brain (Fig. 3). The fact that MPTP exposure leads to neurological degradation was discovered accidentally when there was an outbreak of Parkinsonism in young individuals in southern California. An investigation into this strange occurrence showed that it was due to the presence of MPTP as a contaminant in the production of synthetic opiates which these individuals were using as a recreational drug. This unfortunate event led to the development of MPTP treatment as a useful research paradigm to mimic PD in animal models. It has now been convincingly shown that inadvertent MPTP exposure and/or MPTP-treatment in humans and animal models, respectively, causes a significant impairment of neuronal energy metabolism, ATP production, and ultimately leads to the selective loss of dopaminergic neurons from the substantia nigra region. Thus, MPTP-treatment in rodents is commonly used as a neurotoxin model to investigate the molecular mechanisms associated with PD pathogenesis. To further support mitochondrial involvement and potentially impaired bioenergetics in PD, numerous nuclear-encoded genes are either directly and/or indirectly involved with mitochondria and have been shown to contribute to the pathogenesis of PD. These nuclear-encoded genes that have either direct and/or indirect affects on mitochondria in PD include: α-synuclein, parkin, DJ-1, PINK1, LRRK2, and HTRA2 (Thomas and Beal 2007). The details of how each of these genes is involved with mitochondria and/or mitochondrial function and PD progression is beyond the scope of this review (see Thomas and Beal 2007 for review). However, taken together, it is certainly clear that mitochondrial dysfunction and bioenergetic impairment contribute to PD pathogenesis.
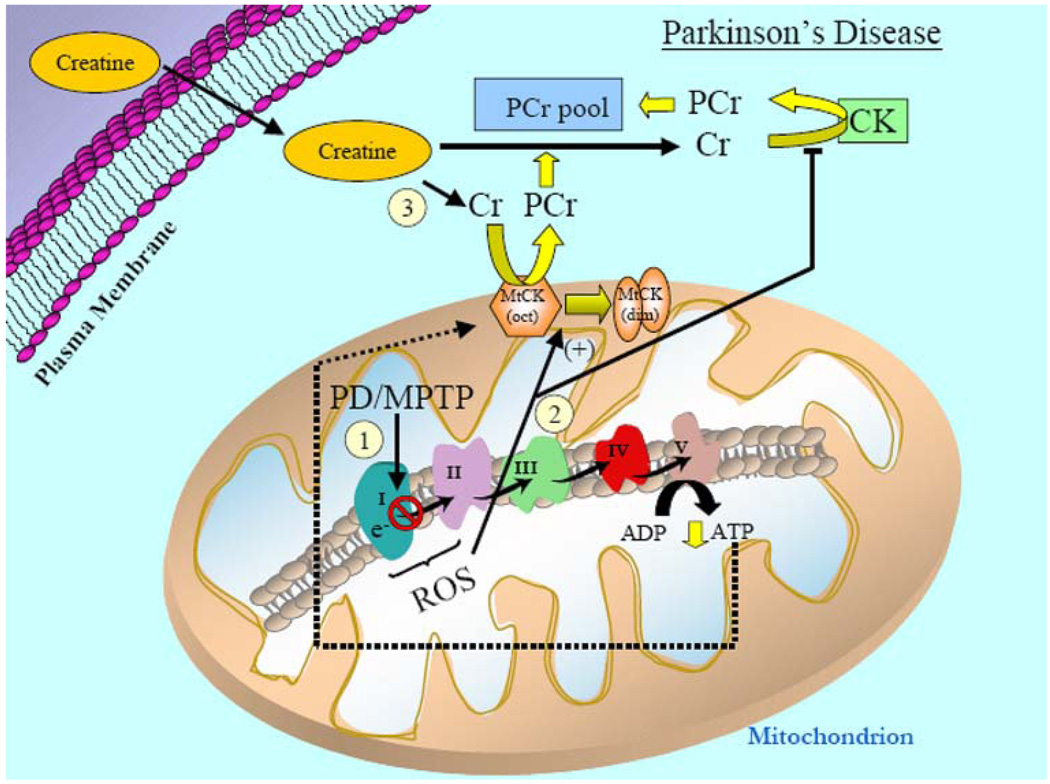
Fig. 3.
Mitochondrial involvement in Parkinson’s disease (PD) and the potential therapeutic efficacy of creatine supplementation. (1) PD is characterized by impaired activity of complex I in the ETC of mitochondria. In support of a mitochondrially based etiology in PD, administration of the mitochondrial-specific neurotoxin, MPTP, in animal models, inhibits complex I of the ETC and leads to PD-like pathogenesis. Suppression and/or inhibition of complex I of the ETC lowers overall mitochondrial ATP production and consequently diminishes the ATP (dashed lines) that can be potentially utilized for PCr generation by the MtCK. 2) Additionally, impaired ETC function increases ROS production to induce an octameric-to-dimeric shift of MtCK rendering it an inactive state and elevated ROS will also reduce cytosolic CK activity. (3) Data have shown that creatine supplementation is an efficacious treatment paradigm for PD animal models, but the exact molecular mechanisms are not fully elucidated. However, exogenous creatine appears to improve overall cellular bioenergetics and mitochondrial function by enhancing the PCr pool and this reduces the neuronal cell loss associated with PD pathogenesis. Given the promising results of creatine in animal models of PD, a phase III clinical study of creatine supplementation in PD patients is now underway
Given the mitochondrial involvement and suppressed energy metabolism (i.e., reduced ATP) exhibited in the pathogenesis of idiopathic PD, treatment paradigms aimed at restoring the bioenergetic deficit and/or improving mitochondrial function would likely be efficacious as a therapeutic intervention to combat PD progression (Fig. 3). Since the major energy source within the brain is ATP, and this energy pool is tightly coupled to the creatine kinase system, it was hypothesized that creatine supplementation might be a beneficial treatment paradigm in PD. Our data show that oral supplementation (1%) with creatine resulted in significant protection against MPTP-induced dopamine depletion in mice (Matthews et al. 1999). Additionally, we found that creatine supplementation protected against the loss of both Nissl and tyrosine hydroxylase immunostained neurons in the substantia nigra region (Matthews et al. 1999). A randomized, double-blind, futility phase II clinical trial of creatine in early PD patients in 2006 (NINDS NET-PD Investigators) indicated that creatine supplementation is NOT futile and should be considered for Phase III clinical trials (NINDS NET-PD Investigators 2006). Currently, a double-blind, placebo-controlled, phase III clinical study investigating the efficacy of creatine supplementation is underway and it is one of the largest PD clinical trials to date. The study will involve 1,720 people with early-stage PD at 51 medical centers in the United States and Canada, and will be conducted over the next 5–7 years. This phase III creatine trial is the first large study in a series of NINDS-sponsored clinical trials called NIH exploratory trials in PD (NET-PD). NINDS has organized a large network of sites with the intent of researchers being able to interact and track the progress of PD patients over an extended period of time. The ultimate goal of these clinical trials is to determine the most effective and lasting treatment paradigm for PD. NET-PD is organized in a developmental research process that focuses on effective laboratory research that has the potential to be translated into pilot studies in PD patients. The encouraging results of the Phase II clinical trials of creatine in PD patients illustrates the potential of creatine supplementation to slow the clinical progression of PD and provide long-term benefits for people who are currently afflicted with PD.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS, often called Lou Gehrig’s disease) is a progressive, usually fatal, neurodegenerative disease caused by the degeneration of motor neurons in the central nervous system (Hervias et al. 2006). The disorder leads to muscle weakness and atrophy throughout the body as both the upper and lower motor neurons degenerate, and reduced motor signals are transmitted to peripheral muscles. ALS is one of the most common neuromuscular diseases worldwide and it afflicts approximately 1–2 people per 100,000 each year (Hervias et al. 2006). People of all races and ethnic backgrounds are equally affected and the onset of the disease is usually between 40 and 60 years of age. ALS can be categorized into a genetically linked form, termed familial ALS, and a spontaneous form in which there is no known common genetic predisposition, termed sporadic ALS (Phukan et al. 2007). Familial ALS accounts for approximately 5–10% of all ALS cases and is caused by a variety of genetic mutations. Of these genetic abnormalities, the most common (1 in 10) is linked to a mutation in copper/zinc superoxide dismutase (SOD1), a cytosolic enzyme responsible for scavenging and reducing free radicals in the cell (Hervias et al. 2006; Fig. 4). Using mutant SOD1 transgenic mice, studies have shown that mitochondrial swelling and vacuolization are early pathological features associated with this model of ALS (Gurney et al. 1994; Wong et al. 1995; Fig. 4). Mice with the G93A human SOD1 mutation have altered electron transport chain enzyme activities and expression of the mutant enzyme in vitro causes a loss in mitochondrial membrane potential and elevations in cytosolic calcium levels (Carri et al. 1997; Fig. 4). The exact mechanism leading to these mitochondrial alterations have yet to be fully understood. However, there is evidence that suggests a portion of SOD1 (primarily within the cytosol) may be localized to the mitochondria in both familial ALS patients and transgenic models. Once translocated to the mitochondrial fraction, evidence suggests that SOD1 forms protein aggregates, and that this may contribute to mitochondrial dysfunction although the exact mechanisms have yet to be fully established (Hervias et al. 2006; Fig. 4). Additionally, biochemical and morphological abnormalities of mitochondria have also been found in postmortem spinal cords of ALS patients (Hervias et al. 2006). Taken together, these studies indicate that mitochondrial dysfunction contributes toward the pathogenesis of ALS. Impaired mitochondrial function could lead to reduced ATP levels and potentially contribute to motor neuron cell death. Thus, buffering cellular energy with exogenous creatine supplementation might exert neuroprotective effects and provide an effective treatment paradigm for ALS.
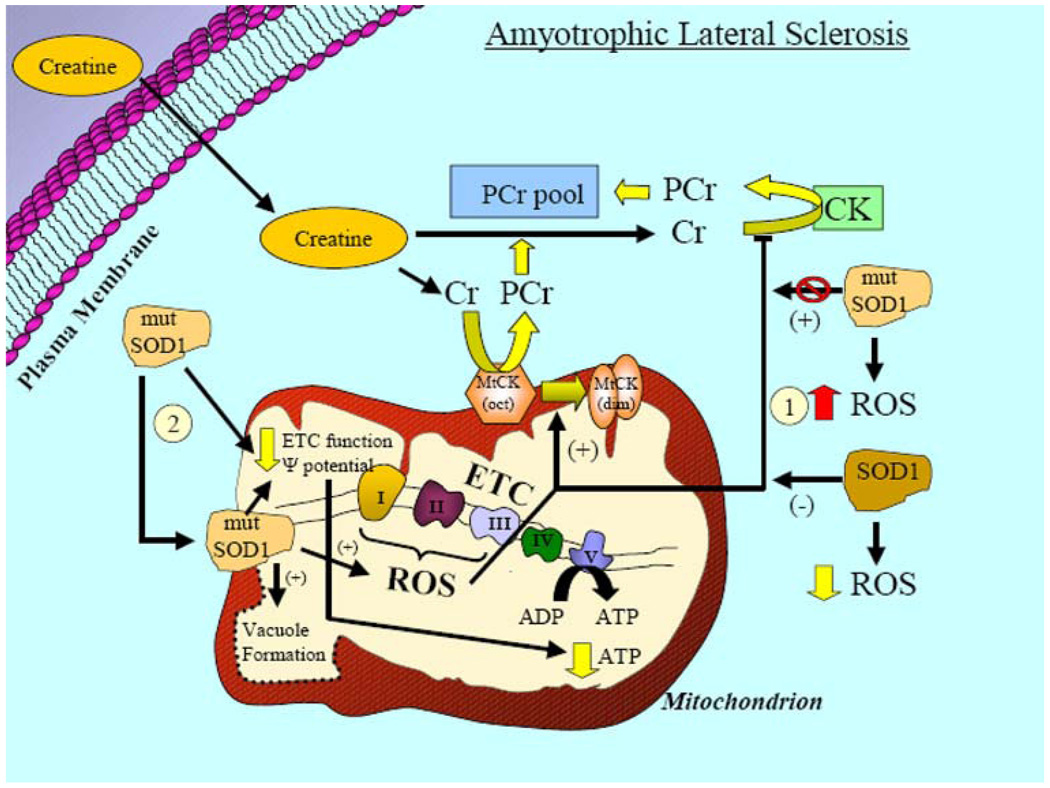
Fig. 4.
Schematic illustrating the relationship of mitochondria to amyotrophic lateral sclerosis (ALS) and the potential benefit of creatine supplementation. The most common form of familial ALS (FALS) is caused by numerous different mutations in the metallo-proteinase, superoxide dismutase I (SODI). SODI is an antioxidant that is ubiquitously expressed in the cytosolic fraction of cells and catalyzes the dismutation of superoxide (a highly reactive free radical) to the less reactive hydrogen peroxide (H2O2) and water. (1) Elevations in ROS may occur due to mutations in SOD1, causing reduced SOD1 antioxidant activity. Although somewhat counterintuitive, data have shown that this does not play a major role in the pathogenesis associated with SOD1 mutations. (2) Alternatively, the effects of mutant SOD1 are due to toxic gain-of-function properties. While SOD1 is primarily located in the cytosolic portion of the cell, mutant SOD1 has been found in the intermembrane space and the matrix of mitochondria. Although the detailed molecular mechanisms are not completely understood, mitochondrial mutant SOD1 is associated with impaired ETC function, reduced mitochondrial membrane potential, elevated mitochondrial ROS production, mitochondrial swelling, and intra-mitochondrial vacuole formation. These alterations likely contribute to reduced ATP generation by mitochondria and creatine supplementation in ALS may serve to buffer these mutant SOD1-induced impairments in intracellular bioenergetics. Creatine supplementation was shown to be neuroprotective in animal models of ALS (G93A), but despite promise, was ineffective in clinical trials with ALS patients
We have shown that oral administration of creatine produced a dose-dependent improvement in motor performance and extended survival in G93A mice (Klivenyi et al. 1999). Additionally, creatine supplementation protected against the loss of neurons in both the substantia nigra and motor cortex and also reduced the level of oxidative damage in our G93A mice (Klivenyi et al. 1999). Based on the clear neuroprotective effects seen in our transgenic model of ALS, there appeared to be much promise for creatine supplementation as an effective therapeutic intervention to treat ALS patients. Despite these findings, two independent human clinical trials testing the efficacy of creatine in ALS patients demonstrated no evidence for a beneficial effect of creatine on survival and/or disease progression in patients with ALS (Groeneveld et al. 2003; Shefner et al. 2004). It is currently unclear as to why there were such stark differences in the efficacy of creatine when comparing a transgenic ALS model versus ALS patients. Nonetheless, based on these clinical trials, it appears that creatine supplementation is not an effective treatment paradigm for ALS patients despite the extremely promising results that we found with creatine in our transgenic ALS model.
Huntington’s Disease
Huntington’s disease (HD), also called Huntington’s chorea, is an inherited autosomal dominant progressive neurological disorder that afflicts approximately 3–7 per 100,000 individuals and the onset usually occurs between 40 and 50 years of age (Beal and Ferrante 2004). HD is characterized by abnormal “dance-like” body movements, termed chorea, and a lack of coordination, but it has also been shown to alter certain mental abilities and some aspects of behavior (Beal and Ferrante 2004). Huntington’s disease is caused by a trinucleotide expansion (CAG) in the huntington gene which results in an abnormal polyglutamine repeat stretch in the huntingtin protein. Huntingtin protein is ubiquitously expressed, predominantly in the cytosol, of both nervous and peripheral tissues. The normal function of huntingtin is currently unknown but based on its protein–protein interactions it has been hypothesized to be involved in intracellular transport, autophagy, transcription, signal transduction, and mitochondrial function (Beal and Ferrante 2004). Mutant huntingtin has been proposed to confer toxic effects to neural tissue by several different methods which include: transcriptional dysregulation, proapoptotic signaling, oxidative injury, excitotoxicity, inflammatory reactions, malfunctioning proteolysis, metabolic dysfunction, and mitochondrial dysfunction (Ryu and Ferrante 2005; Fig. 5).
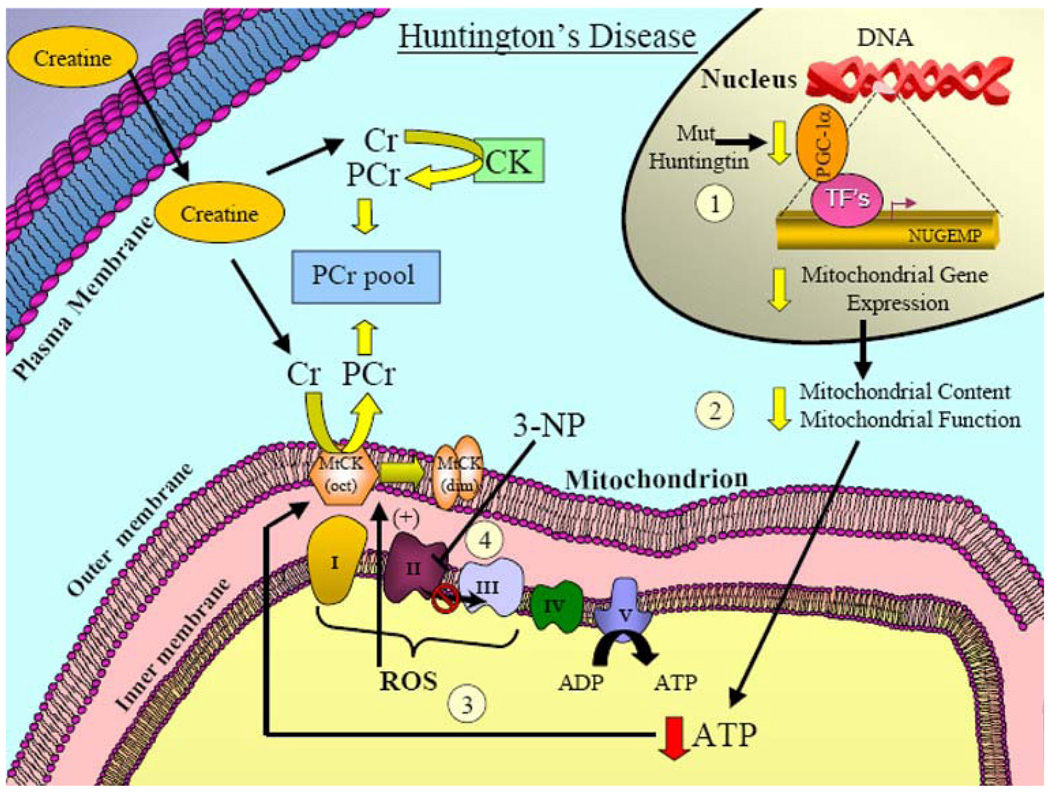
Fig. 5.
Mitochondrially associated mechanisms involved in Huntington’s disease and the potential effect of creatine supplementation. Mutant huntingtin has been proposed to confer toxic effects to neural tissue by a variety of different mechanisms. (1) With respect to mitochondrial dysfunction, mutant huntingtin has been shown to impair the levels of an important cofactor, PGC-1α, which is involved in regulating the expression nuclear genes encoding mitochondrial proteins (NUGEMPs). (2) Reductions in NUGEMPs can potentially lead to a decrease in the overall content and function in mitochondria. (3) As a consequence, these changes can lead to a reduction in ATP levels and enhanced ROS production. (4) Evidence has also shown that ETC complex activities are impaired in postmortem HD brain tissue. Additionally, in animal models, administration of the neurotoxin, 3-NP, inhibits complex II of the ETC, and leads to Huntington’s-like pathogenesis which underscores the importance of mitochondrial dysfunction in HD. Creatine supplementation reduces lesion volume in the mitochondrial toxin (3-NP) models of HD and provides significant neuroprotection. Given the promising neuroprotective effects of creatine found in animal and phase II clinical studies, a phase III clinical trial in HD patients is currently ongoing
Various transgenic models of HD have been developed which can be placed into three broad categories: (1) mice that contain only a fragment of exon-1 of the human huntingtin gene containing polyglutamine mutations (in addition to both alleles of murine wild-type huntingtin, Hdh), (2) mice with pathogenic CAG repeats inserted into the existing CAG expansion in murine Hdh (knock-in mice), and (3) mice that express the full-length human HD gene (plus murine Hdh). Substantial evidence using these various transgenic HD models have suggested that an important interplay between energy metabolism dysfunction, mitochondrial abnormalities and excitotoxicity occurs in the pathogenesis of HD (Beal et al. 1993; Brouillet et al. 1993;Schulz and Beal 1995; Beal 1996, 2000a, b, c; Palfi et al. 1996; Browne et al. 1999; Grunewald and Beal 1999; Tarnopolsky and Beal 2001). We and others have shown that: (1) lactate levels are elevated in the cerebral cortex and basal ganglia of patients with HD, (2) there is a reduced phosphocreatine/inorganic phosphate in the resting muscle of HD patients and, (3) there are reductions in mitochondrial electron transport enzymes in HD postmortem tissue (Jenkins et al. 1993; Brouillet et al. 1995; Gu et al. 1996; Browne et al. 1997; Koroshetz et al. 1997; Fig. 5). Additionally, in support of both energetic defects and mitochondrial dysfunction as contributors to HD, animal models using mitochondrial neurotoxins have convincingly shown that electron transport chain inhibition can lead to similar behavioral and neuropathological phenotypes associated with HD (Alston et al. 1977; Bogdanov et al. 1998a; Beal et al. 1993; Brouillet et al. 1993, 1995; Henshaw et al. 1994;Schulz and Beal 1995; Palfi et al. 1996). Administration of a naturally occurring plant toxin, 3-nitroproprionic acid (3-NP), an irreversible, and/or malonate, a reversible inhibitor, of succinate dehydrogenase (complex II) of the electron transport chain have both been shown to produce HD-like symptoms (Candlish et al. 1969; Ludolph et al. 1991; Fig. 5). These neurotoxin treatments are now commonly used as an experimental animal model to mimic HD pathogenesis (Alston et al. 1977; Ludolph et al. 1991, 1992; Beal et al. 1993; Brouillet et al. 1995; Palfi et al. 1996). Furthermore, recent studies have illustrated that the coactivator PGC-1α, an important mediator of mitochondrial biogenesis, is downregulated in HD patients and animal models of HD (Cui et al. 2006; Weydt et al. 2006; Fig. 5). These studies were the first to demonstrate that HD pathogenesis is related to impaired PGC-1α expression, and this leads to suppressed mitochondrial function and/or mitochondrial gene expression (Fig. 5). Taken together, these data provide a mechanistic link between PGC-1α, mutant HD, and mitochondrial dysfunction, and strongly implies that impaired energy metabolism contributes to HD pathogenesis.
These studies indicate that mitochondrial impairment and energy dysfunction certainly play a role in HD pathogenesis. This would suggest that any therapeutic treatment that might serve to buffer intracellular energy stores may delay the onset and/or progression of HD pathogenesis. Given the role of creatine in cellular bioenergetics, it was hypothesized that creatine supplementation in animal models of HD might improve cellular bioenergetics and provide neuroprotection. We have shown that administration of creatine in our transgenic models of HD improved motor performance, extended survival, attenuated the loss in body weight and brain weight, reduced neuron atrophy, and lowered the number of Huntington-positive aggregates (Ferrante et al. 2000; Andreassen et al. 2001; Dedeoglu et al. 2003). Additionally, we have also shown that creatine supplementation reduces lesion volume in our mitochondrial toxin models of HD and provides significant neuroprotection (Matthews et al. 1998). Given the positive results of creatine supplementation in both our neurotoxin HD model and our transgenic model, we conducted a 16-week, randomized, double-blind, placebo-controlled phase II clinical trial of the safety, and tolerability of 8 g/day creatine in HD patients (64) and assessed serum biomarkers (Hersch et al. 2006). This study illustrated that creatine supplementation suppressed serum levels of 8-hydroxy-2′-deoxyguanosine (8OH2’dG), an indicator of oxidative-induced damage to DNA and the creatine dosage was both tolerable and safe to patients (Hersch et al. 2006). Given the efficacy of creatine in this phase II clinical trial, and the neuroprotective effects illustrated in both our neurotoxin and transgenic HD models, a double-blind, placebo-controlled phase III clinical trial has been approved (2006) and is currently ongoing at various centers. Taken together, these data provide strong support for creatine to be a particularly effective neuroprotective agent in HD which may ultimately improve and/or extend the quality of life for individuals afflicted with HD.
Conclusion
Exogenous creatine supplementation appears to primarily exert its beneficial effects by increasing the PCr pool to improve overall cellular bioenergetics. Additionally, some evidence suggests that creatine may also enhance mitochondrial function and reduce the susceptibility to mitochondrially mediated apoptosis. These beneficial effects of creatine supplementation were initially recognized in muscle tissue where they were shown to prolong contractility and enhance overall athletic performance. The convincing functional improvements in muscle tissue led to the hypothesis that creatine may be useful as a therapeutic intervention to target neurological diseases with metabolic/bioenergetic dysfunction as part of their disease etiology. The evidence presented in this review suggests that creatine supplementation improves bioenergetic deficits and may exert neuroprotective effects in Parkinson’s and Huntington’s disease. However, current evidence suggests that creatine supplementation is not efficacious in the treatment of AD and ALS (refer to Andres et al. 2008 for an additional/alternative review of the neuroprotective effect of creatine). Further clinical studies investigating the role of creatine in Parkinson’s and Huntington’s disease over the next several years will certainly provide insight and potentially substantiate the neuroprotective role of creatine in these neurological diseases. In addition to the clinical trials of creatine supplementation in Parkinson’s and Huntington’s disease, more creatine studies utilizing animal and cell culture models of these diseases are warranted to fully understand and appreciate the exact mechanisms by which creatine exerts its neuroprotective effects. Further insight of the molecular details and/or mechanisms of the neuroprotective effects of creatine supplementation in these diseases may potentially offer novel cellular targets and/or processes for future therapeutic interventions.
Acknowledgements
The administrative assistance of Greta Strong, the editorial advice provided by Anna-Maria Joseph and the generous contribution of portions of the mitochondrial illustrations provided by Dr. David Hood’s laboratory (York University, Toronto, ON, Canada) during the preparation of this manuscript are gratefully acknowledged. This work was supported by the NINDS, NIA, HDSA, and the Department of Defense. The authors have attempted to include all relevant topics/articles but realize that certain interesting aspects may have been excluded due to space constraints and we apologize for the inability to include these areas.
References
- Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. doi:10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, Hood DA. Mechanisms of apoptosis in skeletal muscle. Basic and applied myology. 2003;13:171–179. [Google Scholar]
- Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology. 2003;88:99–107. doi: 10.1113/eph8802505. doi:10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. Journal of Neurochemistry. 2000;74:2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. doi:10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- Alston TA, Mela L, Bright HJ. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:3767–3771. doi: 10.1073/pnas.74.9.3767. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, et al. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiology of Disease. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. doi:10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Research Bulletin. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. doi:10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. doi:10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baker SK, Tarnopolsky MA. Targeting cellular energy production in neurological disorders. Expert Opinion on Investigational Drugs. 2003;12:1655–1679. doi: 10.1517/13543784.12.10.1655. doi:10.1517/13543784.12.10.1655. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, et al. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. Journal of Neuroscience. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of Neurology. 1995;38:357–366. doi: 10.1002/ana.410380304. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, free radicals, and neurodegeneration. Current Opinion in Neurobiology. 1996;6:661–666. doi: 10.1016/s0959-4388(96)80100-0. doi:10.1016/S0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria and the pathogenesis of ALS. Brain. 2000a;123(Pt 7):1291–1292. doi: 10.1093/brain/123.7.1291. doi:10.1093/brain/123.7.1291. [DOI] [PubMed] [Google Scholar]
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends in Neurosciences. 2000b;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. doi: 10.1016/S0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- Beal MF. Limited-time exposure to mitochondrial toxins may lead to chronic progressive neurodegenerative diseases. Movement Disorders. 2000c;15:434–435. doi: 10.1002/1531-8257(200005)15:3<434::AID-MDS1002>3.0.CO;2-Q. doi:10.1002/1531-8257(200005)15:3 <434::AID-MDS1002>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Annals of Neurology. 2003;53 Suppl 3:S39–S47. doi: 10.1002/ana.10479. doi:10.1002/ana.10479. [DOI] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ. Experimental therapeutics in transgenic mouse models of Huntington’s disease. Nature Reviews. Neuroscience. 2004;5:373–384. doi: 10.1038/nrn1386. doi:10.1038/nrn1386. [DOI] [PubMed] [Google Scholar]
- Benzi G, Ceci A. Creatine as nutritional supplementation and medicinal product. Journal of Sports Medicine and Physical Fitness. 2001;41:1–10. [PubMed] [Google Scholar]
- Bessman SP, Geiger PJ. Transport of energy in muscle: The phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Bindoff LA, Birch-Machin M, Cartlidge NE, Parker WD, Jr, Turnbull DM. Mitochondrial function in Parkinson’s disease. Lancet. 1989;2:49. doi: 10.1016/s0140-6736(89)90291-2. doi:10.1016/S0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- Boero J, Qin W, Cheng J, Woolsey TA, Strauss AW, Khuchua Z. Restricted neuronal expression of ubiquitous mitochondrial creatine kinase: Changing patterns in development and with increased activity. Molecular and Cellular Biochemistry. 2003;244:69–76. doi:10.1023/A:1022409101641. [PubMed] [Google Scholar]
- Bogdanov MB, Ferrante RJ, Kuemmerle S, Klivenyi P, Beal MF. Increased vulnerability to 3-nitropropionic acid in an animal model of Huntington’s disease. Journal of Neurochemistry. 1998a;71:2642–2644. doi: 10.1046/j.1471-4159.1998.71062642.x. [DOI] [PubMed] [Google Scholar]
- Bogdanov MB, Ramos LE, Xu Z, Beal MF. Elevated “hydroxyl radical” generation in vivo in an animal model of amyotrophic lateral sclerosis. Journal of Neurochemistry. 1998b;71:1321–1324. doi: 10.1046/j.1471-4159.1998.71031321.x. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. Journal of Neurochemistry. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. doi:10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, et al. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. Journal of Neurochemistry. 1993;60:356–359. doi: 10.1111/j.1471-4159.1993.tb05859.x. doi:10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, et al. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. doi:10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, et al. Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Annals of Neurology. 1997;41:646–653. doi: 10.1002/ana.410410514. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington’s disease. Brain Pathology. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, et al. The Creatine Kinase/Creatine Connection to Alzheimer’s Disease: CK-Inactivation, APP-CK Complexes and Focal Creatine Deposits. Journal of Biomedicine and Biotechnology. 2006;2006:35936. doi: 10.1155/JBB/2006/35936. doi:10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. doi:10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Candlish E, La CJ, Unrau AM. The biosynthesis of 3-nitropropionic acid in creeping indigo (Indigofera spicata) Biochemistry. 1969;8:182–186. doi: 10.1021/bi00829a026. doi:10.1021/bi00829a026. [DOI] [PubMed] [Google Scholar]
- Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, et al. Expression of a Cu, Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Letters. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. doi:10.1016/S0014-5793(97)01051-X. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: Dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. Journal of Neurochemistry. 2002a;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. doi:10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radical Biology and MedicineSS. 2002b;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. doi:10.1016/S0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. Journal of Physiology. 2004;555:409–421. doi: 10.1113/jphysiol.2003.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csukly K, Ascah A, Matas J, Gardiner PF, Fontaine E, Burelle Y. Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. Journal of Physiology. 2006;574:319–327. doi: 10.1113/jphysiol.2006.109702. doi:10.1113/jphysiol.2006.109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. doi:10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- David S, Shoemaker M, Haley BE. Abnormal properties of creatine kinase in Alzheimer’s disease brain: Correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Research. Molecular Brain Research. 1998;54:276–287. doi: 10.1016/s0169-328x(97)00343-4. doi:10.1016/S0169-328X(97)00343-4.ssss. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Laboratory Investigation. 2000;80:1323–1335. doi: 10.1038/labinvest.3780140. doi:10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. Journal of Neurochemistry. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondria and ischemiareperfusion injury of the heart: Fixing a hole. Cardiovascular Research. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. doi:10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Dolder M, Walzel O, Speer U, Schlattner T, Wallimann T. Inhibition of the mitochondrial transition by creatine kinase substrates. Requirement for microcompartmentation. Journal of Biological Chemistry. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. doi:10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Eppenberger HM, Dawson DM, Kaplan NO. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. Journal of Biological Chemistry. 1967;242:204–209. [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. Journal of Neuroscience. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant M, Rak M, Szeghalmi A, Del Bigio MR, Westaway D, Yang J, et al. Focally elevated creatine detected in amyloid precursor protein (APP) transgenic mice and Alzheimer disease brain tissue. Journal of Biological Chemistry. 2006;281:5–8. doi: 10.1074/jbc.C500244200. doi:10.1074/jbc.C500244200. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. doi:10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Groeneveld GJ, Van Kan HJ, Kalmijn S, Veldink JH, Guchelaar HJ, Wokke JH, et al. Riluzole serum concentrations in patients with ALS: Associations with side effects and symptoms. Neurology. 2003;61:1141–1143. doi: 10.1212/01.wnl.0000090459.76784.49. [DOI] [PubMed] [Google Scholar]
- Grunewald T, Beal MF. Bioenergetics in Huntington’s disease. Annals of the New York Academy of Sciences. 1999;893:203–213. doi: 10.1111/j.1749-6632.1999.tb07827.x. doi:10.1111/j.1749-6632.1999.tb07827.x. [DOI] [PubMed] [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Annals of Neurology. 1996;39:385–389. doi: 10.1002/ana.410390317. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. doi:10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Henshaw R, Jenkins BG, Schulz JB, Ferrante RJ, Kowall NW, Rosen BR, et al. Malonate produces striatal lesions by indirect NMDA receptor activation. Brain Research. 1994;647:161–166. doi: 10.1016/0006-8993(94)91412-5. doi:10.1016/0006-8993(94)91412-5. [DOI] [PubMed] [Google Scholar]
- Hensley K, Butterfield DA, Mattson M, Aksenova M, Harris M, Wu JF, et al. A model for beta-amyloid aggregation and neurotoxicity based on the free radical generating capacity of the peptide: Implications of “molecular shrapnel” for Alzheimer’s disease. Proceedings of the Western Pharmacology Society. 1995;38:113–120. [PubMed] [Google Scholar]
- Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. doi:10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- Hervias I, Beal MF, Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle and Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. doi:10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: Therapeutic implications. Advances in Experimental Medicine and Biology. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Jacobus WE, Lehninger AL. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. Journal of Biological Chemistry. 1973;248:4803–4810. [PubMed] [Google Scholar]
- Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Jost CR, Van Der, Zee CE, In ‘t Zandt HJ, Oerlemans F, Verheij M, Streijger F, et al. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. European Journal of Neuroscience. 2002;15:1692–1706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Juhn MS, Tarnopolsky M. Oral creatine supplementation and athletic performance: A critical review. Clinical Journal of Sport Medicine. 1998a;8:286–297. doi: 10.1097/00042752-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Juhn MS, Tarnopolsky M. Potential side effects of oral creatine supplementation: A critical review. Clinical Journal of Sport Medicine. 1998b;8:298–304. doi: 10.1097/00042752-199810000-00007. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Medicine. 1999;5:347–350. doi: 10.1038/6568. doi:10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. doi:10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Annals of Neurology. 1997;41:160–165. doi: 10.1002/ana.410410206. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Annals of Neurology. 1992;32:782–788. doi: 10.1002/ana.410320612. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Li X, Burklen T, Yuan X, Schlattner U, Desiderio DM, Wallimann T, et al. Stabilization of ubiquitous mitochondrial creatine kinase preprotein by APP family proteins. Molecular and Cellular Neurosciences. 2006;31:263–272. doi: 10.1016/j.mcn.2005.09.015. doi: 10.1016/j.mcn.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Canadian Journal of Neurological Sciences. 1991;18:492–498. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, Seelig M, Ludolph AG, Sabri MI, Spencer PS. ATP deficits and neuronal degeneration induced by 3-nitropropionic acid. Annals of the New York Academy of Sciences. 1992;648:300–302. doi: 10.1111/j.1749-6632.1992.tb24562.x. doi:10.1111/j.1749-6632.1992.tb24562.x. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Parise G, Tarnopolsky MA. Nutritional and exercise-based therapies in the treatment of mitochondrial disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:619–629. doi: 10.1097/00075197-200211000-00004. doi:10.1097/00075197-200211000-00004. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology and Medicine. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. doi:10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, et al. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. Journal of Neuroscience. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Experimental Neurology. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. doi:10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. doi:10.1016/S0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine and Science in Sports and Exercise. 2000;32:291–296. doi: 10.1097/00005768-200002000-00007. doi:10.1097/00005768-200002000-00007. [DOI] [PubMed] [Google Scholar]
- NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. doi:10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- O’Gorman E, Piendl T, Muller M, Brdiczka D, Wallimann T. Mitochondrial intermembrane inclusion bodies: The common denominator between human mitochondrial myopathies and creatine depletion, due to impairment of cellular energetics. Molecular and Cellular Biochemistry. 1997a;174:283–289. doi: 10.1023/A:1006881113149. [PubMed] [Google Scholar]
- O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Letters. 1997b;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. doi:10.1016/S0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- Onyango IG, Khan SM. Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Current Alzheimer Research. 2006;3:339–349. doi: 10.2174/156720506778249489. doi:10.2174/156720506778249489. [DOI] [PubMed] [Google Scholar]
- Palfi S, Ferrante RJ, Brouillet E, Beal MF, Dolan R, Guyot MC, et al. Chronic 3-nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington’s disease. Journal of Neuroscience. 1996;16:3019–3025. doi: 10.1523/JNEUROSCI.16-09-03019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of Neurology. 1989;26:719–723. doi: 10.1002/ana.410260606. doi:10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD., Jr Cytochrome oxidase deficiency in Alzheimer’s disease. Annals of the New York Academy of Sciences. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Peng TI, Greenamyre JT. Privileged access to mitochondria of calcium influx through N-methyl-D-aspartate receptors. Molecular Pharmacology. 1998;53:974–980. [PubMed] [Google Scholar]
- Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacological Reviews. 2001;53:161–176. [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR. Alterations of cerebral metabolism in probable Alzheimer’s disease: A preliminary study. Neurobiology of Aging. 1994;15:117–132. doi: 10.1016/0197-4580(94)90152-x. doi:10.1016/0197-4580(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurology. 2007;6:994–1003. doi: 10.1016/S1474-4422(07)70265-X. doi:10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR. Effect of short-term creatine supplementation on renal responses in men. European Journal of Applied Physiology and Occupational Physiology. 1997;76:566–567. doi: 10.1007/s004210050291. doi: 10.1007/s004210050291. [DOI] [PubMed] [Google Scholar]
- Poortmans JR, Francaux M. Adverse effects of creatine supplementation: Fact or fiction? Sports Medicine. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. doi:10.2165/00007256-200030030-00002. [DOI] [PubMed] [Google Scholar]
- Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Canadian Journal of Applied Physiology. 2002;27:349–395. doi: 10.1139/h02-020. [DOI] [PubMed] [Google Scholar]
- Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proceedings. Biological Sciences. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. doi:10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TM, Sewell DA, Casey A, Steenge G, Greenhaff PL. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. British Journal of Sports Medicine. 2000;34:284–288. doi: 10.1136/bjsm.34.4.284. doi:10.1136/bjsm.34.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Ferrante RJ. Emerging chemotherapeutic strategies for Huntington’s disease. Expert Opinion on Emerging Drugs. 2005;10:345–363. doi: 10.1517/14728214.10.2.345. doi:10.1517/14728214.10.2.345. [DOI] [PubMed] [Google Scholar]
- Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EI. Role of creatine phosphokinase in cellular function and metabolism. Canadian Journal of Physiology and Pharmacology. 1978;56:691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Journal of Neurochemistry. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. doi:10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochimica et Biophysica Acta. 2006;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Beal MF. Neuroprotective effects of free radical scavengers and energy repletion in animal models of neurodegenerative disease. Annals of the New York Academy of Sciences. 1995;765:100–110. doi: 10.1111/j.1749-6632.1995.tb16565.x. doi:10.1111/j.1749-6632.1995.tb16565.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. doi:10.1038/19866. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, et al. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- Simon DK, Johns DR. Mitochondrial disorders: Clinical and genetic features. Annual Review of Medicine. 1999;50:111–127. doi: 10.1146/annurev.med.50.1.111. doi:10.1146/annurev.med.50.1.111. [DOI] [PubMed] [Google Scholar]
- Small DH, McLean CA. Alzheimer’s disease and the amyloid beta protein: What is the role of amyloid? Journal of Neurochemistry. 1999;73:443–449. doi: 10.1046/j.1471-4159.1999.0730443.x. doi:10.1046/j.1471-4159.1999.0730443.x. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. doi:10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Richman J, Santoro G, Wei H, Wang Y, Vanderah T, et al. The cloning and expression of a human creatine transporter. Biochemical and Biophysical Research Communicationss. 1994;204:419–427. doi: 10.1006/bbrc.1994.2475. doi:10.1006/bbrc.1994.2475. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, de HA, Heerschap A, Ruitenbeek W, et al. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. doi:10.1016/S0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. American Journal of Physiology. 1998;275:E974–E979. doi: 10.1152/ajpendo.1998.275.6.E974. [DOI] [PubMed] [Google Scholar]
- Stockler S, Marescau B, De Deyn PP, Trijbels JM, Hanefeld F. Guanidino compounds in guanidinoacetate methyltransferase deficiency, a new inborn error of creatine synthesis. Metabolism. 1997;46:1189–1193. doi: 10.1016/s0026-0495(97)90215-8. doi:10.1016/S0026-0495(97)90215-8. [DOI] [PubMed] [Google Scholar]
- Stockler S, Hanefeld F. Guanidinoacetate methyltransferase deficiency: A newly recognized inborn error of creatine biosynthesis. Wiener Klinische Wochenschrift. 1997;109:86–88. [PubMed] [Google Scholar]
- Streijger F, Oerlemans F, Ellenbroek BA, Jost CR, Wieringa B, Van Der, Zee CE. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behavioural Brain Research. 2005;157:219–234. doi: 10.1016/j.bbr.2004.07.002. doi:10.1016/j.bbr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Annals of Neurology. 2001;49:561–574. doi: 10.1002/ana.1028. [PubMed] [Google Scholar]
- Tarnopolsky MA, Safdar A. The potential benefits of creatine and conjugated linoleic acid as adjuncts to resistance training in older adults. Applied Physiology, Nutrition, and Metabolism. 2008;33:213–227. doi: 10.1139/H07-142. doi:10.1139/H07-142. [DOI] [PubMed] [Google Scholar]
- Thomas B, Beal MF. Parkinson’s disease. Human Molecular Genetics. 2007;16(Spec no. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: Superficial laminar cytochrome oxidase associated with disease duration. Journal of Neuroscience. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Verhoeven NM, Maaswinkel-Mooij P, Pouwels PJ, Onkenhout W, Peeters EA, et al. Mental retardation and behavioral problems as presenting signs of a creatine synthesis defect. Annals of Neurology. 2000;47:540–543. doi: 10.1002/1531-8249(200004)47:4<540::aid-ana23>3.3.co;2-b. doi:10.1002/1531-8249(200004)47:4<540::AID-ANA23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Molecular and Cellular Biochemistry. 1994;133–134:193–220. doi: 10.1007/BF01267955. doi:10.1007/BF01267955. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neuroscience Research. 2002;42:279–285. doi: 10.1016/s0168-0102(02)00007-x. doi:10.1016/S0168-0102(02) 00007-X. [DOI] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metabolism. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. doi:10.1016/j.cmet.2006. 10.004. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. doi:10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Wyss M, Schulze A. Health implications of creatine: Can oral creatine supplementation protect against neurological and atherosclerotic disease? Neurosciences. 2002;112:243–260. doi: 10.1016/s0306-4522(02)00088-x. doi:10.1016/S0306-4522(02)00088-X. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. doi:10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]