Abstract
Energy balance during lactation critically influences survival and growth of a mother’s offspring, and hence, her reproductive success. Most experiments have investigated the influence of a single factor (e.g., ambient temperature [Ta] or litter size) on the energetics of lactation. Here, we determined the impact of multiple interventions, including increased conductive heat loss consequent to dorsal fur removal, cold exposure (Ta of 5°C versus 23°C), and differential lactational load from litters of different sizes (2 or 4 pups), on maternal energy balance and offspring development of Siberian hamsters (Phodopus sungorus). Lower Ta, fur removal, and larger litters were associated with increased maternal food consumption. Females exposed to multiple challenges (e.g., both fur loss and lower Ta) ate substantially more food than those exposed to a single challenge, with no apparent ceiling to elevated food intake (increases up to 538%). Thus, energy intake of dams under these conditions does not appear to be limited by feeding behavior or the size of the digestive tract. Housing at 5°C attenuated pup weight gain and increased pup mortality to more than 5 times that of litters housed at 23°C. Increases in the dam’s conductive heat loss induced by fur removal did not affect pup weight gain or survival, suggesting that effects of low Ta on pup weight gain and survival reflect limitations in the pups’ ability to ingest or incorporate energy.
Keywords: maternal care, thermoregulation, fur, food intake, Siberian hamster
INTRODUCTION
Lactation is the most energetically expensive event in most female mammals’ lifetimes. The demanding challenge imposed by lactation is particularly pronounced for small mammals nursing altricial young [1]. For these species, not only is the dam initially the sole source of nutrition for the litter, but before offspring develop an insulative pelage and effective thermoregulatory capabilities, contact with the dam is essential for offspring to maintain normal body temperatures [2]. Food intake of dams increases markedly during lactation, often by more than 100% in rodents housed at moderate ambient temperatures (Tas) [1]. For many species, the energetic demands of reproduction increase as Tas decline in autumn and winter, presumably contributing to the evolution of spring-summer breeding seasons and winter thermoregulatory adaptations that conserve energy [3].
During lactation, rodent dams have higher metabolic rates [4] and are less able to contend with elevated Tas [5]. These factors, coupled with the extra heat generated by milk synthesis, increases in prolactin, progesterone and corticosterone secretion, and huddling with the litter, predispose dams to hyperthermia [e.g., 6,7]. Perhaps as a consequence of incipient hyperthermia, the mitochondrial activity of brown adipose tissue, the major source of non-shivering thermogenesis, is substantially lower during lactation, indicating decreased endogenous maternal heat generation [8]. Rodent dams nurse their litters in discrete bouts interspersed with time away from the pups. Leon [9] concluded that maternal termination of individual nursing bouts is driven by the dam’s need to dissipate heat accumulated during contact with pups. This thermoregulatory influence on nursing behavior consequently affects the duration of mother-offspring interaction, and presumably, pup growth and development.
The Siberian hamster (Phodopus sungorus) provides a favorable model for comparative studies of thermoregulation and lactation. This small nocturnal rodent, native to steppes of Siberia and northern Mongolia, contends with low Tas for most of the year [10]. Ground surface temperatures in January average −25°C and mean daily Ta exceeds 15°C on only 22 days per year [10,11]. Consequently, Siberian hamsters have a restricted breeding season, which coincides with higher Ta’s (typically, between April and September) [10]. As in other species, food intake increases during lactation [12] and body temperatures are elevated [13]. Siberian hamsters, in common with rats, appear to alleviate maternal hyperthermia during lactation by periodically terminating contact with the litter [14].
Insulation provided by fur contributes significantly to mammalian energy balance. Relatively few studies have assessed the impact of pelage insulation on the behavior and physiology of rodents [summarized in 15], and even fewer have addressed the interaction between thermal insulation and the energetics of lactation. Presumably, the fur coat increases maternal heat retention and thus, energy savings during lactation when energy demands are highest; however, increased insulation also promotes hyperthermia during nursing, which could decrease the duration of nursing bouts and thereby influence pup development. Removal of the ventral fur of lactating rats increased nest time with the litter [16], raising the possibility that increased maternal heat dissipation during lactation can increase nursing duration and benefit the pups. Król et al. (2007) reported that removal of the dorsal fur elevated maternal milk production and accelerated litter weight gain in mice.
The present study investigated the combined influences of several environmental and thermoregulatory factors on the energy balance of lactating female hamsters and their offspring. Specifically, we 1) manipulated the degree of conductive heat loss during lactation by reducing the amount of insulative protection provided by the dam’s fur, 2) modified the thermal gradient experienced by the dam and her offspring by varying Ta, and 3) imposed differential lactational loads by adjusting litter size. We report that each of these interventions influences maternal food intake, but only cold exposure alters pup survival and body mass. When compared to findings in house mice, the present data suggest that the benefits of fur removal on offspring development are dependent on the species investigated and/or large litter sizes.
METHODS
Animals and housing conditions
One hundred twenty-two female Siberian hamster offspring from a colony originally derived from animals obtained from Katherine Wynne-Edwards (Queen’s University, Kingston, ON) were gestated and maintained in either a warm or cold room (Ta = 23°C and 5°C, respectively) illuminated for 14 h/day (onset of darkness at 1800 h, PST). Hamsters were housed in polypropylene cages (25 × 14 × 12 cm) provisioned with a reduced amount of Tek-Fresh laboratory animal bedding (Harlan Teklad, Madison, WI). Purina rodent chow 5015 and tap water were available ad libitum. All animal procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley and conformed to the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Design
Adult female hamsters, acclimated to 5°C or 23°C from birth, were paired with adult males and subsequently gave birth to 2 successive litters. Males were removed from cages when females displayed substantial weight gain associated with their second pregnancy. The data reported are from dams 6–8 months of age caring for their second litters. During lactation three variables were manipulated: Ta (5°C or 23°C), amount of thermal insulation of the dam (presence or absence of dorsal pelage) and number of pups nursed (2 or 4 pups for lactating dams, 0 for virgin controls). On lactational day 3 (L3; day of birth = L1) pups were redistributed to create litter sizes of 2 or 4 pups; mean litter size before redistribution was 7, but preliminary work indicated that larger litters led to unacceptable levels of cannibalism in dams housed at 5°C. On L6, dams were subjected to either complete shaving of the dorsal fur (shaved) or removal of a 4 cm2 dorsal fur patch (controls). Previous studies in nulliparious Siberian hamsters established that complete dorsal fur removal significantly increased daily food intake, whereas removal of a 4 cm2 dorsal fur patch did not affect food consumption at Tas of 5°C or 23°C [15]. Body mass (BM) of dams and pups was recorded on L6 and at 3-day intervals thereafter. A pre-weighed amount of food was reweighed every 3 days to generate mean daily food intake (FI) values for that interval. Pups were weaned on L22 and dams sacrificed by CO2 inhalation followed by cervical dislocation; fur mass on the previously shaved skin was recorded at autopsy. Additional singly-housed females, 4–8 months of age, were treated as above except they were not mated (virgins). Nineteen dams lost pups, of which 17 were housed at 5°C and 2 at 23°C; these dams were excluded from all analyses except those pertaining to pup survival. Thus, BM, FI, and fur regrowth were assessed in the remaining 103 females (dams and virgins) and their litters. One dam (of the 103 remaining females) was sacrificed on L23; terminal measures for this hamster were excluded from analyses.
Measurement of fur regrowth
For fur removal, hamsters were anesthetized with isoflurane vapors, and the dorsal skin shaved with an electric razor. At sacrifice, this area was re-shaved, and hairs gathered and weighed on a Sartorius R200D balance (±0.02 mg). To correct for inter-individual variations in patch size, the length and width of the shaved patches were measured to the nearest 0.1 mm with calipers, and the area of shaved skin calculated. Fur regrowth rate was calculated as the mass of the hair removed at the terminal shaving divided by time between shavings (mg fur/cm2 skin/16 days between shaving).
Statistical Analyses
To assess the effects of Ta and shaving, each dependent variable was split into 3 two-way ANOVAs (repeated measures ANOVAs for FI and BM measures): virgins, dams with 2 pups, and dams with 4 pups. Significant ANOVAs permitted 4 planned comparisons for each time point with Fisher’s protected least significant differences test (Fisher’s PLSD): a) shaved versus control females at 23°C, b) the same comparison at 5°C, c) shaved females at 5°C versus 23°C, and d) control females at 5°C versus 23°C.
The effects of litter size at each Ta and shave condition were assessed by 4 one-way ANOVAs (repeated measures ANOVAs for FI and BM): control and shaved females at 23°C and control and shaved females at 5°C. Significant ANOVAs in this analysis were followed by pair-wise comparisons within each time point using the post hoc Tukey-Kramer correction.
The influences of Ta, shaving, and litter size on pup survival were assessed with Fisher’s exact test. All statistical tests were performed using Statview 5.0 (SAS Institute, Cary, NC). Statistically significant differences are reported as p < 0.05 regardless of actual p values below this threshold.
RESULTS
Pup Survival
Pup survival was strongly influenced by Ta. Specifically, pup mortality was more common among litters housed at 5°C than 23°C, occurring in 17 of 51 litters housed at 5°C (33.3%) and in only 2 of 34 litters (5.9%) housed at 23°C (p<0.05). Neither litter size nor dam fur removal significantly affected pup survival (p>0.05). All remaining analyses were conducted on litters in which all pups survived to weaning.
Pup Body Mass
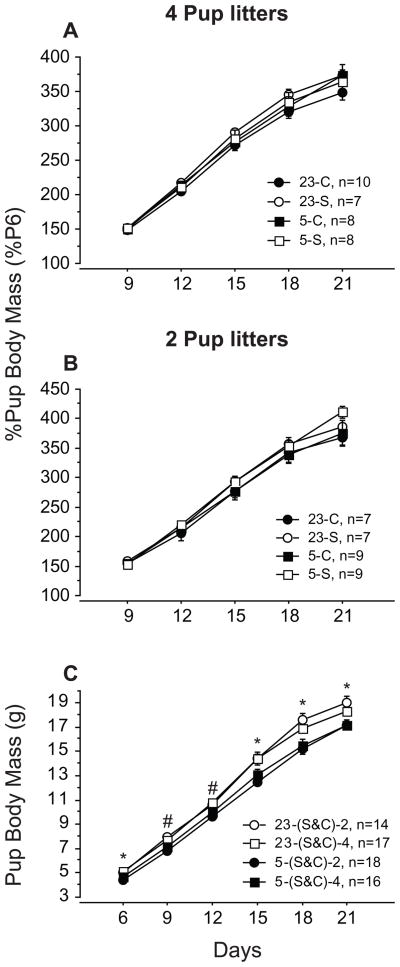
Slight, but significant, differences in pup body mass (BM) between groups were evident before dams were shaved. To assess the effects of fur removal, the data were expressed as percent mean BM relative to baseline values on the day of shaving (L6). Fur removal did not affect pup BM gains; all groups displayed the same pattern of increases over the course of lactation (Fig. 1A, B; p>0.05). Data from shaved and control hamsters were combined to assess effects of Ta and litter size on pup BM. Actual BM was used in these analyses rather than % baseline BM because Ta and litter size manipulations were employed before baseline measures were recorded at L6. Pups reared at 5°C consistently weighed less (by ~ 7–14%) than those reared at 23°C (Fig. 1C; p<0.05). Litter size did not affect pup BM (Fig 1C, p>0.05).
Figure 1.
Percent (±s.e.) of baseline body mass (postnatal day 6; % P6) of pups in litters of 4 (A) or 2 (B) nursed by control (C; closed symbols) and shaved (S; open symbols) dams at 23°C (circles) or 5°C (squares). (C) Mean BM (±s.e.) of pups reared in litters of 2 (circles) or 4 (squares) at 23°C (open symbols) or 5°C (closed symbols) after collapsing across shave treatments. *Pups reared in litters of 2 and 4 at 23°C differ significantly from those reared at 5°C. #Pups reared in litters of 2 at 23°C differ significantly from those reared at 5°C; this comparison falls short of significance for litters of 4 (p<0.11). All statistical comparisons made with Fisher’s PLSD test.
Food Intake and Body Mass of Dams and Virgins
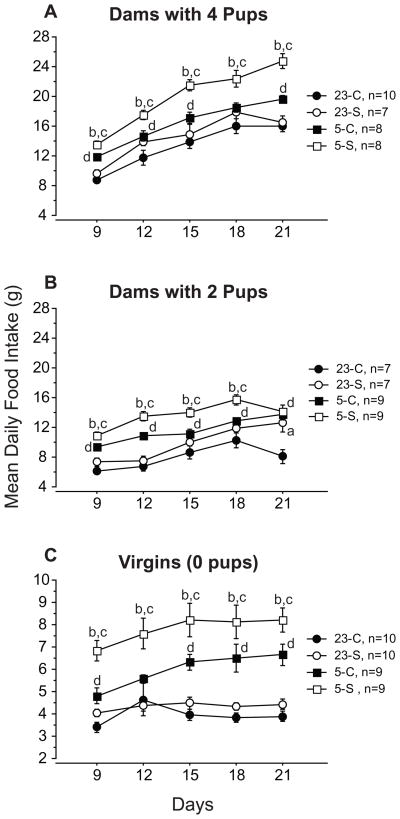
Food intake (FI) was significantly influenced by all 3 manipulated variables. Lower Ta and larger litter size each were associated with increased FI across all other treatment variables (Figs. 2 and 3; p<0.05). Dams and virgins housed at 5°C ate more food than their counterparts at 23°C (denoted by letters c and d in each panel of Fig. 2 for shaved and control hamsters, respectively; p<0.05); hamsters with larger litters ate more food than those caring for smaller litters or no pups (4 pups > 2 pups > 0 pups; Fig. 3; p<0.05). Effects of fur removal varied with Ta: shaved dams and shaved virgins housed at 5°C consumed more food than did controls (denoted by letter b in each panel of Fig. 2; p<0.05), but shaving did not affect food intake of hamsters housed at 23°C (p>0.05).
Figure 2.
Mean (±s.e.) daily food intake of control (closed symbols) and shaved (open symbols) dams with litters of 4 pups (A), or 2 pups (B) during the first 21 days of lactation and of virgins (C) housed at 23°C (circles) or 5°C (squares). 23-C and 23-S refer to control and shaved dams kept at 23°C, respectively; 5-C and 5-S designate control and shaved dams held at 5°C, respectively. a = significant difference between shaved and control females at 23°C. b = significant difference between shaved and control females at 5°C. c = significant difference between shaved females at 23°C versus 5°C. d = significant difference between control hamsters at 23°C versus 5°C. All statistical comparisons were performed with Fisher’s PLSD test. Note the different scale for the abscissa of panel C.
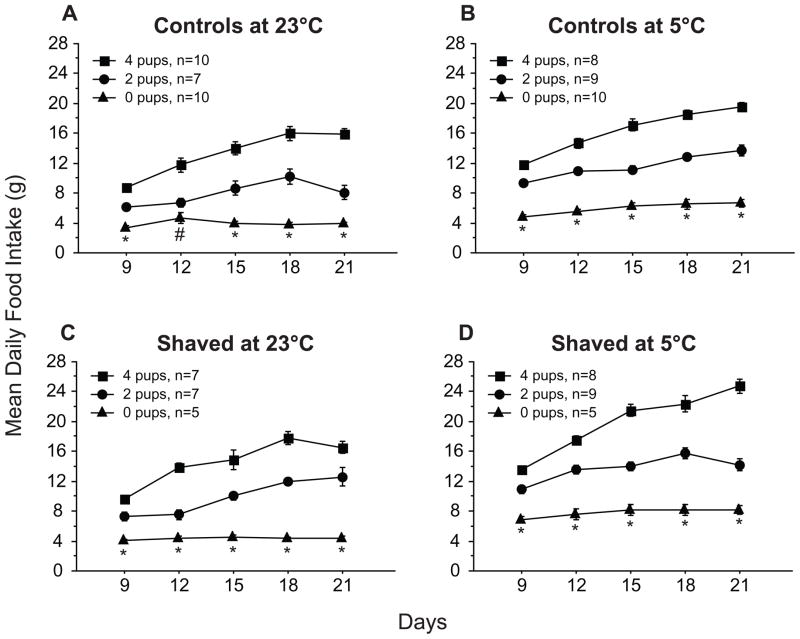
Figure 3.
Mean (±s.e.) daily food intake of control (A and B) and shaved (C and D) dams with litters of 4 (squares) or 2 pups (circles) and virgins (0 pups; triangles) at 23°C (A and C) or 5°C (B and D). *All groups differ significantly from each other. #Dams with 4 pups differ significantly from virgins and dams with 2 pups. All statistical comparisons made with the Tukey-Kramer test.
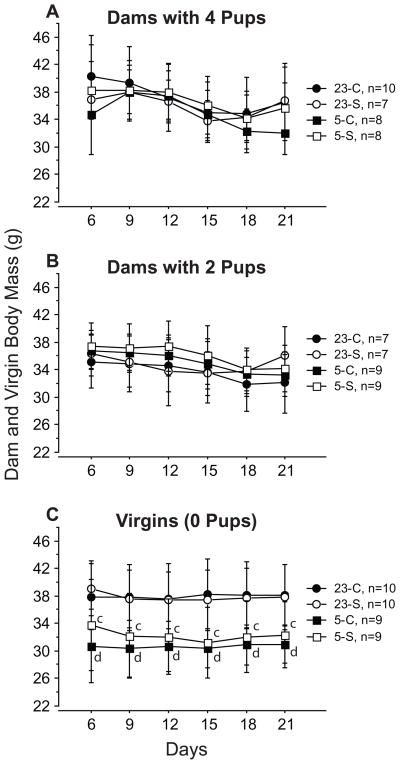
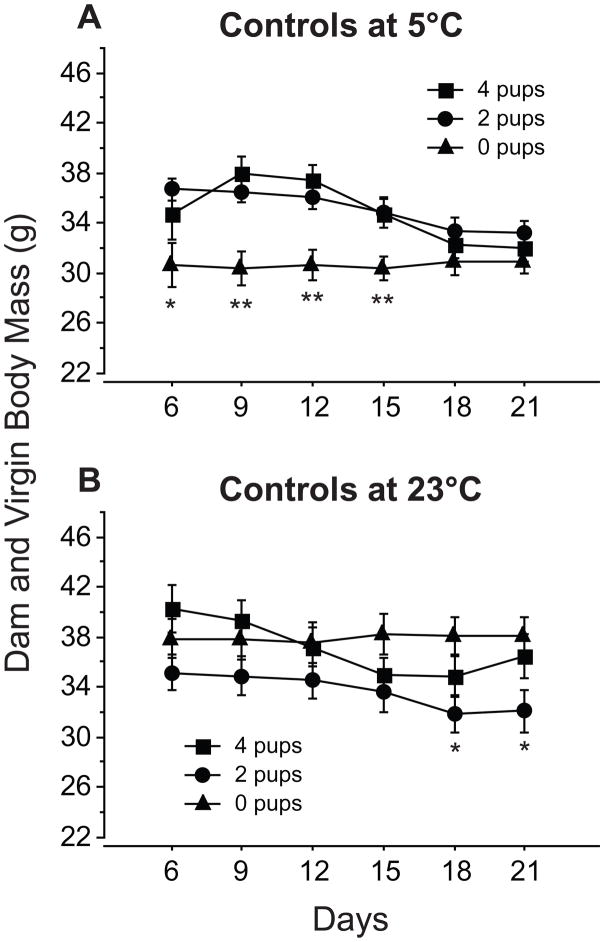
Ta did not affect BM of dams (Fig. 4A, B; p>0.05). Virgins housed at 5°C, however, weighed less than their counterparts at 23°C throughout the experiment (Fig. 4C; p<0.05). At 5°C, virgins initially weighed less than dams caring for litters of 2 or 4 pups, irrespective of pelage status, whereas at 23°C, no differences were detected in the initial BM of virgins and dams with differing litter sizes (Fig. 4). Dams tended to lose weight as lactation progressed, but this effect was not significant under all conditions. Dams maintained at 5°C no longer differed from similarly housed virgins by L18 (Fig. 5A). Despite similar decreases in dams maintained at 23°C, litter size did not reliably affect BM: control dams with 2 pups weighed less than virgins at L18 and L21 (p<0.05), but there were no differences in BM detected for other litter size comparisons maintained at this Ta (4 pups vs. 2 pups and 4 pups vs. 0 pups; p>0.05; Fig. 5B). Fur removal did not alter the BM of virgins or dams (Fig. 4; p>0.05).
Figure 4.
Mean (±s.e.) body mass of control (closed symbols) and shaved (open symbols) dams with litters of 4 pups (A), or 2 pups (B) during the first 21 days of lactation and of virgins (C) housed at 23°C (circles) or 5°C (squares). Significance symbols and statistical comparisons as in Fig. 2.
Figure 5.
Mean (±s.e.) body mass of control dams caring for 4 (squares) or 2 pups (circles) and virgins (0 pups; triangles) housed at 5°C (A) or 23°C (B). Virgins differ significantly from dams with 2 pups* or from dams with 2 and 4 pups**. The BM of dams caring for litters of 2 or 4 pups did not differ significantly from each other in any comparisons. Sample sizes as in Fig. 3. All statistical comparisons made with the Tukey-Kramer test.
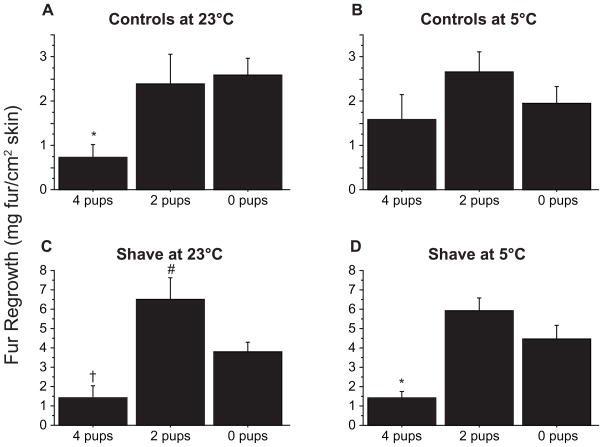
Fur Regrowth in Shaved Dams
Because we did not correct for back curvature, the reported values slightly underestimate the size of the shaved surface. The underestimates are similar for hamsters that received the same treatment, but greater for the complete dorsal shave than for the 4 cm2 control shave. Consequently, we did not compare differences across types of shaving. Ta did not affect hair regrowth in either shaved or control hamsters (p>0.05; not illustrated). Fur regrowth was reduced, however, in dams nursing 4 pups compared to that for shaved virgins and shaved dams with 2 pups (Fig. 6), but this effect was not significant under all conditions (see Fig. 6B, C).
Figure 6.
Mean (+s.e.) mg fur regrowth of control (A and B) and shaved (C and D) dams caring for 4 pups, 2 pups, or virgins (0 pups) housed at 23°C (A and C) or 5°C (B and D). *Dams with 4 pups differ significantly from virgins and dams with 2 pups. †Dams with 4 pups differ significantly from dams with 2 pups but not virgins. #Dams with 2 pups differ significantly from virgins and dams with 4 pups. All statistical comparisons made with the Tukey-Kramer test.
DISCUSSION
Energy intake of female Siberian hamsters was significantly impacted by the combined challenges of lactation, decreased ambient temperature (Ta), increased litter size, and loss of fur. Importantly, the effects of these perturbations were additive: hamsters consumed more food when contending with 2 or more concurrent challenges than after a single challenge. We failed to detect a ceiling for food intake during these challenges; on the fifteenth day of lactation, shaved dams nursing 4 pups in the cold consumed 21.5 g of food compared to 4.0 g by control virgins housed in the warm. In addition to the effects on dam energy intake, offspring survival and body mass (BM) gain were both impaired in the lower Ta. Collectively, these findings indicate that factors influencing dam energy balance significantly affect mother and pup physiology.
Increased food intake was sufficient for dams to defend BMs typical of lactating hamsters kept at 23°C and, for all challenges except cold exposure, to support normal increases in pup BM. As in laboratory mice [17], dams defended their BM at low Tas, whereas increments in pup BM lagged behind those of pups reared in moderate Tas. The decreased BM of cold-exposed pups was evident at L6 and persisted through weaning on L22. Although both maternal fur removal and lower Ta challenge the dams’ thermoregulatory abilities, only the latter imposes a direct challenge to the pups. As this manipulation was the only one to affect pup BM, restraint on pup weight gain in the cold may reflect direct effects of Ta on pups, rather than a decrement in maternal care or milk production; direct measures of maternal behavior and milk output are needed to evaluate this hypothesis. Nonetheless, our data indicate that shaved dams in the cold compensate for increased conductive heat loss without incurring BM loss. Their pups, however, may not be capable of increasing milk intake or altering metabolic processes to meet increased energy demands, and consequently weigh less than pups kept at moderate Tas.
Similar to the pup BM results, low Ta increased pup mortality, irrespective of whether dams were caring for 2 or 4 pups, and whether fully shaved or not. Increased pup mortality was also noted in litters reared by Syrian hamsters housed at 10°C [18] and laboratory mice moved from 21°C to 8°C on postnatal day 10 [17]. Cold exposure appears to trigger litter reduction in dams. Alternatively, cold exposure may present a thermoregulatory challenge that cannot be met by some of the pups.
The sustained maximum rate of energy intake is thought to constrain the behavior (e.g., foraging) and reproductive performance (e.g., lactation) of rodents [19,20]. Three hypotheses have been proposed to explain limits to the sustained maximum rate of energy intake during lactation [reviewed in 19,20]. The “central limitation hypothesis” posits that energy intake is limited by the processing capacity of the alimentary canal and related digestive organs. The “peripheral limitation hypothesis” considers that energy availability to pups is limited by the output capacity of the mammary glands. The “heat dissipation hypothesis” proposes that maternal hyperthermia from digestion, milk production, and contact with the litter limits the duration of nursing and consequently, energy transfer to the pups. The substantial increases in food consumption upon multiple thermal challenges to Siberian hamster dams in the present study does not support the central limitation hypothesis, in agreement with previous studies [17,21,22].
Król et al. [19] reported increases in both pup and litter masses, as well as milk energy output, of shaved compared to unshaved, lactating laboratory mice maintained at 21°C. Their data support the heat dissipation hypothesis; increased heat conductance after shaving removes thermal constraints on nursing behavior, allowing for greater milk production and longer nursing bouts. We, however, did not detect more rapid weight gain in pups reared by shaved Siberian hamster dams maintained at 5°C or 23°C. Although definitive conclusions require measurements of milk output, our data are more congruent with the peripheral limitation hypothesis, whereby maternal energy output to the pups was maximal and could not be increased by removing thermal constraints. This species may have developed means of counteracting maternal hyperthermia (e.g., increased number of short nursing bouts) or tolerating the high heat load [13], thereby diminishing thermal constraints evident in some other species. Alternatively, lactational constraints in this species may be imposed by the pups’ ability to ingest and/or incorporate food. Finally, it is possible that methodological factors may account for the divergent findings in mice and Siberian hamsters, including shaving procedure (repeated shavings of lactating mice compared to one-time fur removal of hamsters early in lactation) and differences in litter size (litter sizes of ~11 for mice and 4 or 2 for hamsters in the present study). The mean litter size of hamsters in the present study before culling was 7. Hence, the culled litters of 2 and 4 pups are below the mean number of young nursed by this species under normal laboratory conditions. Reduced litter size presumably decreases the heat load on dams during nursing bouts, thereby diminishing maternal hyperthermia and increasing the duration of nursing bouts, an effect not amplified by fur removal. Additionally, smaller litters may reduce sibling competition for the dam’s milk and maternal care, resulting in greater resources for each pup during thermal challenges. Benefits from increased maternal heat dissipation may require a large lactational load and thus, be inconsequential for dams nursing small litters. Litter sizes of Siberian hamster dams in the wild are not known and may or may not match those used in the current experiment. It remains for future studies to establish typical litter sizes of Siberian hamster dams in nature, and whether heat dissipation of the dam limits pup development under field conditions.
Fur regrowth was suppressed in dams nursing 4 but not 2 pups, compared to that of females with no pups (virgins). None of the other energetic manipulations affected fur regrowth. Fur growth is regulated by several humoral factors [see 23 and references therein]. Differences in prolactin secretion, a major regulator of seasonal changes in pelage color of Siberian hamsters [24,25], can affect other pelage characteristics. Prolactin suppresses fur depth, fur density, and guard hair length in meadow voles (Microtus pennsylvanicus) [26] and hair growth during development in laboratory mice [27]. Decreased fur density, possibly mediated by increased prolactin secretion during lactation, may facilitate dam heat dissipation during nursing of larger litters.
Lactation poses a complex energetic challenge to female mammals; energetic demands are markedly increased, and the risk of hyperthermia is accentuated. These two factors push the thermoregulatory adaptation of pelage insulation in opposite directions, and maintaining the proper balance may be essential for successful rearing of young. The present results demonstrate that Siberian hamster fur confers significant energetic savings to females lactating in the cold, but the thermal insulation provided by the dam’s dorsal pelage does not limit pup growth, at least for litters of 2 and 4 pups. Increased litter size and cold exposure pose energetic challenges to the dam, and the latter can negatively impact offspring development and survival. Females meet the combined energetic demands of these factors by augmenting food intake. Limitations on Siberian hamster pup development under the present conditions appear to reside within the peripheral organs important for lactation or with the pups’ ability to ingest or incorporate energy.
Acknowledgments
We are grateful to Kim Pelz for technical assistance and to the Office of Laboratory Animal Care for animal husbandry. This research was supported by grants MH-61171 from the NIMH and NS-58135 from the NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bronson FH. Mammalian Reproductive Biology. Chicago: University of Chicago Press; 1989. [Google Scholar]
- 2.Alberts JR. Huddling by rat pups: ontogeny of individual and group behavior. Dev Psychobiol. 2007;49:22–32. doi: 10.1002/dev.20190. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 1 San Diego: Academic Press; 2009. pp. 507–538. [Google Scholar]
- 4.Zhang XY, Wang DH. Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles Lasiopodomys brandtii. J Exp Biol. 2007;210:512–521. doi: 10.1242/jeb.02659. [DOI] [PubMed] [Google Scholar]
- 5.Knecht EA, Toraason MA, Wright GL. Thermoregulatory ability of female rats during pregnancy and lactation. Am J Physiol. 1980;239:R470–475. doi: 10.1152/ajpregu.1980.239.5.R470. [DOI] [PubMed] [Google Scholar]
- 6.Kittrell EM, Satinoff E. Diurnal rhythms of body temperature, drinking and activity over reproductive cycles. Physiol Behav. 1988;42:477–484. doi: 10.1016/0031-9384(88)90180-1. [DOI] [PubMed] [Google Scholar]
- 7.Adels LE, Leon M. Thermal control of mother-young contact in Norway rats: factors mediating the chronic elevation of maternal temperature. Physiol Behav. 1986;36:183–196. doi: 10.1016/0031-9384(86)90094-6. [DOI] [PubMed] [Google Scholar]
- 8.Trayhurn P, Douglas JB, McGuckin MM. Brown adipose tissue thermogenesis is ‘suppressed’ during lactation in mice. Nature. 1982;298:59–60. doi: 10.1038/298059a0. [DOI] [PubMed] [Google Scholar]
- 9.Leon M. Mother-young reunions. Prog Psychobiol Physiol Psychol. 1979;9:301–334. [Google Scholar]
- 10.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp Zool Soc Lond. 1987;57:167–187. [Google Scholar]
- 11.Weiner J, Górecki A. Standard metabolic-rate and thermoregulation in 5 species of Mongolian small mammals. J Comp Physiol [B] 1981;145:127–132. [Google Scholar]
- 12.Stamper JL, Zucker I, Lewis DA, Dark J. Torpor in lactating Siberian hamsters subjected to glucoprivation. Am J Physiol. 1998;274:R46–51. doi: 10.1152/ajpregu.1998.274.1.R46. [DOI] [PubMed] [Google Scholar]
- 13.Scribner SJ, Wynne-Edwards KE. Disruption of body temperature and behavior rhythms during reproduction in dwarf hamsters (Phodopus) Physiol Behav. 1994;55:361–369. doi: 10.1016/0031-9384(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 14.Scribner SJ, Wynne-Edwards KE. Thermal constraints on maternal behavior during reproduction in dwarf hamsters (Phodopus) Physiol Behav. 1994;55:897–903. doi: 10.1016/0031-9384(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 15.Kauffman AS, Cabrera A, Zucker I. Energy intake and fur in summer- and winter-acclimated Siberian hamsters (Phodopus sungorus) Am J Physiol. 2001;281:R519–527. doi: 10.1152/ajpregu.2001.281.2.R519. [DOI] [PubMed] [Google Scholar]
- 16.Leon M, Croskerry PG, Smith GK. Thermal control of mother-young contact in rats. Physiol Behav. 1978;21:790–811. doi: 10.1016/0031-9384(78)90021-5. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MS, Speakman JR. Limits to sustained energy intake. V. Effect of cold-exposure during lactation in Mus musculus. J Exp Biol. 2001;204:1967–1977. doi: 10.1242/jeb.204.11.1967. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JE, Wade GN. Effects of ambient temperature and body fat content on maternal litter reduction in Syrian hamsters. Physiol Behav. 1991;49:135–139. doi: 10.1016/0031-9384(91)90244-i. [DOI] [PubMed] [Google Scholar]
- 19.Król E, Murphy M, Speakman JR. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J Exp Biol. 2007;210:4233–4243. doi: 10.1242/jeb.009779. [DOI] [PubMed] [Google Scholar]
- 20.Hammond KA, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- 21.Hammond KA, Kristan DM. Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus) Physiol Biochem Zool. 2000;73:547–556. doi: 10.1086/317757. [DOI] [PubMed] [Google Scholar]
- 22.Rogowitz GL. Limits to milk flow and energy allocation during lactation of the hispid cotton rat (Sigmodon hispidus) Physiol Zool. 1998;71:312–320. doi: 10.1086/515923. [DOI] [PubMed] [Google Scholar]
- 23.Paul MJ, George NT, Zucker I, Butler MP. Photoperiodic and hormonal influences on fur density and regrowth in two hamster species. Am J Physiol. 2007;293:R2363–2369. doi: 10.1152/ajpregu.00520.2007. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. II. Role of prolactin. J Exp Zool. 1984;230:97–103. doi: 10.1002/jez.1402300113. [DOI] [PubMed] [Google Scholar]
- 25.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I. Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- 26.Smale L, Lee TM, Nelson RJ, Zucker I. Prolactin counteracts effects of short day lengths on pelage growth in the meadow vole, Microtus pennsylvanicus. J Exp Zool. 1990;253:186–188. doi: 10.1002/jez.1402530208. [DOI] [PubMed] [Google Scholar]
- 27.Craven AJ, Nixon AJ, Ashby MG, Ormandy CJ, Blazek K, Wilkins RJ, Pearson AJ. Prolactin delays hair regrowth in mice. J Endocrinol. 2006;191:415–425. doi: 10.1677/joe.1.06685. [DOI] [PubMed] [Google Scholar]