Abstract
Objective
We sought to estimate how serosorting may affect HIV prevalence and individual risk among MSM in Seattle, Washington, and how the results vary under different assumptions of HIV testing frequency, heterogeneity in sexual behavior, and condom use.
Methods
We developed a deterministic mathematical model of HIV transmission dynamics. Data from the 2003 random digit dial study of MSM conducted in Seattle, Washington (n = 400) are used to parameterize the model.
Results
Predicted population-level HIV prevalence as well as an individual’s risk of HIV acquisition decreases when the odds of serosorting are increased in the mathematical model. In our model based on observed levels of serosorting, we predict an HIV prevalence of 16%. In contrast, if serosorting were eliminated in the population, we predict that HIV prevalence would increase to 24.5%. However, our findings depend on rates of condom use, mean anal sex contact rates, and HIV testing in the population.
Conclusions
Under realistic scenarios of sexual behavior and testing frequency for MSM in the US, serosorting can be an effective harm reduction strategy.
Keywords: HIV/AIDS, mathematical modeling, homosexual men, HIV testing
Introduction
Men who have sex with men (MSM) are the group most affected by HIV in the United States and many other nations, and have dramatically changed their sexual behavior in response to the epidemic. Part of that change has involved preferentially selecting sex partners with concordant HIV status, and preferentially using condoms with partners of discordant status (1–4). These practices have been termed serosorting. Some research suggests that serosorting is increasing (5–8), and may explain stable or declining HIV infection rates among MSM even as rates of bacterial sexually transmitted infections have risen dramatically (9).
Serosorting is controversial. A 2007 paper used a static model to suggest that serosorting might actually increase one’s risk of HIV acquisition because of the high transmission probability during early HIV infection (10) when most men are undiagnosed. In contrast, case-control and cohort studies suggest that serosorting affords men partial protection against HIV infection (7, 11–15).
Using population-based behavioral surveillance data and mathematical modeling, we sought to evaluate the population- and individual-level effects of serosorting among MSM in Seattle, Washington, and to define factors influencing how serosorting affects HIV transmission dynamics (16).
Methods
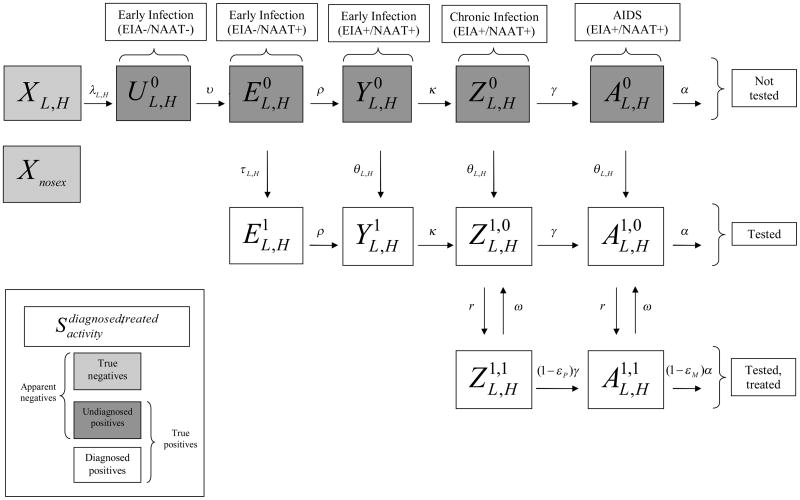
We developed a deterministic, continuous-time model of HIV transmission. The population is divided into compartments (Figure 1) whose size through time is specified with a system of ordinary differential equations. The model is not intended to capture the full temporal trajectory of the HIV epidemic among MSM, but rather to obtain equilibrium prevalence under each behavioral scenario we consider. We explain the model’s general structure and assumptions here, and provide additional technical detail in the online appendix.
Figure 1.
Flow chart of mathematical model
Data sources
The model is parameterized using sexual behavior data from the 2003 random-digit-dial (RDD) study of Seattle MSM (n = 400) (16).
Model framework
Compartments are defined by anal sexual activity level (none, low, high) and true HIV serostatus. HIV-infected men are further subdivided by disease stage and detectability by 2nd generation enzyme immunoassay (EIA) or nucleic acid amplification testing (NAAT); the five resulting categories are acute NAAT−/EIA−; early NAAT+/EIA−; early NAAT+/EIA+; chronic; and AIDS. The last four stages are divided by diagnosis status; diagnosed men are further subdivided by antiretroviral therapy (ART) status. These attributes define 25 compartments, including one for men forgoing all anal sex, used only to calculate prevalence. The compartments comprise three types with distinct roles vis-à-vis serosorting: true negatives, undiagnosed positives and diagnosed positives. We refer to men’s true serostatus (true negative, true positive) and apparent serostatus (apparent negative, diagnosed positive; the former includes true negatives and undiagnosed positives).
Transmission
Transition from true negative into acute NAAT−/EIA− infection occurs via transmission, a potential consequence of anal sex with a true positive. We define “act” as an instance of anal sex, “contact” as the set of acts between two men, “contact rate” as the rate of new contacts, and “partner” as either member of a contact. The contact rate between true negatives and true positives is determined by the apparent negative and diagnosed positive contact rates, level of serosorting, and fraction of apparent negatives who are true positives. We derive all but the last parameter from our data on respondents’ activity levels and respondents’ and their partners’ apparent serostatus.
The RDD study asked men to enumerate their contacts in the prior year. We defined low-activity men as those reporting 1 contact in that period and high-activity men as those reporting >1. High-activity men reported a mean of 10 contacts. Lacking data on partner’s activity level, we assume random mixing by activity level. We also assume that men who have ever tested positive report themselves as positive, and all others report themselves as negative.
In our baseline run, we assume levels of serosorting within various types of contacts consistent with our data, as measured by two odds ratios: (1) the odds that a low-activity diagnosed positive’s partner is diagnosed positive is 68 times greater than the odds that a low-activity apparent negative’s partner is diagnosed positive; and (2) the comparable odds ratio for high-activity men is 12. While population size and composition change over time, our approach ensures that these two directly measured components of serosorting remain constant, as do overall contact rates by compartment. By focusing on counts of anal sex contacts by serostatus, we include two processes within our definition of serosorting: use of serostatus in selecting sex partners and in selecting sex act (anal vs. other). For clarity, we define differential adoption of condom use by perceived serostatus as status-based condom use. These two definitions are often combined; we model them separately to disentangle their effects on HIV risk.
Within serodiscordant contacts, transmission probability depends on the seropositive’s stage and treatment status, number of acts per contact, seropositive’s role (insertive/receptive), and condom use. Since there are no published estimates of penile-anal transmission probabilities by infection stage, we derived stage- and role-specific estimates by taking relative stage-specific estimates for heterosexuals (17) and scaling them according to overall MSM anal sex estimates (18). Acts per contact and role by serostatus are derived from the RDD (Table 1).
Table 1.
Model inputs, Seattle MSM serosorting model.
| Parameter | Value | Source |
|---|---|---|
| Population Dynamics | ||
| Proportion of men with no anal sex contacts in the prior year | 0.39 | Derived from(16) |
| Proportion of low-activity men | 0.26 | Derived from(16) |
| Proportion of high-activity men | 0.35 | Derived from(16) |
| Duration of sexual activity (1/μ years) | 50 | |
| HIV Disease Dynamics | ||
| Duration of acute RNA−/Ab− infection (1/υ days) | 7 | (19) |
| Duration of acute RNA+/Ab− infection (1/ρ days) | 35 | (17, 19, 20) |
| Duration of early RNA+/Ab+ infection (1/κ days) | 48 | (17, 19, 20) |
| Duration of chronic infection (1/γ years) | 10 | (21, 22) |
| Duration of AIDS (1/α years) | 2 | (17, 22) |
| HIV Treatment | ||
| Proportion of men with chronic infection or AIDS who are on treatment | 0.67 | Derived from(16) |
| Rate of withdrawal from treatment (ω yr−1) | 0.02 | (23) |
| Reduction in rate of disease progression from chronic infection to AIDS as a result of ART (εP) | 0.6 | (22) |
| Reduction in rate of death after an AIDS diagnosis as a result of ART (εM) | 0.6 | (22) |
| Reduction in infectiousness as a result of ART (εT) | 0.6 | (24–26) |
| HIV Transmission | ||
| Transmission probability per receptive UAI act, primary infection ( ) | 0.02 | (17, 18) |
| Transmission probability per receptive UAI act, chronic infection ( ) | 0.008 | (17, 18) |
| Transmission probability per receptive UAI act, AIDS ( ) | 0.01 | (17, 18) |
| Transmission probability per insertive UAI act, primary infection ( ) | 0.008 | (17, 18) |
| Transmission probability per insertive UAI act, chronic infection ( ) | 0.0007 | (17, 18) |
| Transmission probability per insertive UAI act, AIDS ( ) | 0.001 | (17, 18) |
| Sexual Risk Behavior | ||
| Mean number of anal sex contacts per year (c), base model | ||
| Low-activity men (cL) | 1 | Derived from(16) |
| High-activity men (cH) | 10 | Derived from(16) |
| Mean number of anal sex acts per perceived concordant anal sex contacts reported by HIV-negative men, base modela | ||
| Low-activity men ( ) | 32 | Derived from(16) |
| High-activity men ( ) | 22 | Derived from(16) |
| Mean number of anal sex acts per perceived discordant anal sex contacts reported by HIV-negative men, base modela | ||
| Low-activity men ( ) | 14 | Derived from(16) |
| High-activity men ( ) | 6 | Derived from(16) |
| Proportion of anal sex acts in which the negative partner is insertive, for perceived discordant contact, base modela | ||
| Low-activity men ( ) | 0.64 | Derived from(16) |
| High-activity men ( ) | 0.41 | Derived from(16) |
| Proportion of acts of insertive anal sex with perceived concordant partners during which HIV-negative men use condoms, base model a | ||
| Low-activity men ( ) | 0.54 | Derived from(16) |
| High-activity men ( ) | 0.34 | Derived from(16) |
| Proportion of acts of insertive anal sex with perceived disconcordant partners during which HIV-negative men use condoms, base model a | ||
| Low-activity men ( ) | 0.36 | Derived from(16) |
| High-activity men ( ) | 0.14 | Derived from(16) |
| Proportion of acts of receptive anal sex with perceived concordant partners during which HIV-negative men use condoms, base modela | ||
| Low-activity men ( ) | 0.54 | Derived from(16) |
| High-activity men ( ) | 0.34 | Derived from(16) |
| Proportion of acts of receptive anal sex with perceived disconcordant partners during which HIV-negative men use condoms, base modela | ||
| Low-activity men ( ) | 0.29 | Derived from(16) |
| High-activity men ( ) | 0.22 | Derived from(16) |
| Effectiveness of condoms (εC) | 0.8 | (32) |
| HIV Testing Frequency | ||
| Second generation enzyme immunoassay (EIA), base model (range) | ||
| Low-activity men (θL yr−1) | 1 (1–2) | Derived from(16) |
| High-activity men (θH yr−1) | 2 (1–4) | Derived from(16) |
| Nucleic acid amplification testing (NAAT), base model (range) | ||
| Low-activity men (τL yr−1) | 0 (0–2) | |
| High-activity men (τH yr−1) | 0 (0–4) | |
| Sexual Mixing | ||
| Serosorting odds ratio derived from reports by low-activity men(ORL) | 68 | Derived from(16) |
| Serosorting odds ratio derived from reports by high-activity men(ORH) | 12 | Derived from(16) |
| Serosorting odds ratio in contacts between low-activity and high- activity men (ORL,H) | 60 | |
For these parameters, our data provide us with two estimates: one for low-activity men and high-activity men. However, our model requires three parameters defined on activity levels for actor pairs (HH, HL, LL). The method we use to derive these three parameters from the two observed data points is identical for each parameter class and is described in the online appendix.
Transition through disease stage
We assume men average 7 days from infection to detectable HIV-RNA (19) and 35 additional days to detectable anti-HIV antibody (20). Primary infection concludes 48 days later, for a 3-month total duration of heightened infectiousness (17). Without treatment, chronically infected individuals develop AIDS after 10 years (21). With treatment, AIDS progression reduces by 0.6 (22).
Diagnosis
Our base model assumes low-activity men average one EIA test/year, and high-activity men average two (16).
Treatment
67% of HIV-positive RDD participants reported taking ART (16). We assume that men discontinue ART because of treatment failure or side effects at 0.02/year (23). Recent reports indicate that ART reduced HIV transmission by 80–100% among serodiscordant African couples (24, 25) and by 60% among MSM (26); we adopted this latter, more conservative, estimate.
Entry and exit
Men remaining truly negative average 50 years in the sexually active population. Upon developing AIDS, men survive on average 2 years if untreated (17, 22); treatment reduces AIDS mortality by 0.6 (22). For convenience, entries by activity level equal exits
Initial conditions
At model outset, three low-activity men and three high-activity men are HIV-positive; in each group, one is EIA−/NAAT−, one EIA−/NAAT+ and undiagnosed, and one EIA−/NAAT+ and diagnosed. All other men are HIV-negative, distributed according to the activity class distribution described in Table 1.
Parameterization
Our baseline run was parameterized by our data; this yielded an endemic HIV prevalence within the prevalence range estimated for our population, which we considered confirmatory evidence for model and data quality. We then ran the model varying parameter values reflecting hypothetical scenarios of interest.
The resulting system of equations is expressed in the Appendix. The system is coded and solved using Berkeley Madonna 8.3.14 (Berkeley Madonna, Inc., University of California, Berkeley). This research was defined as exempt by the University of Washington Human Subjects Division (07-9056-X/C).
Results
Our baseline model considers the epidemic using our observed data, including observed serosorting and status-based condom use levels. Equilibrium prevalence is 16.0%, within the range estimated for Seattle MSM (13%; 95% confidence interval [CI]: 10%–17%). By eliminating serosorting (i.e. maintaining overall contact rates for apparent negatives and for diagnosed positives, but making contact and sex act selection random by apparent serostatus), equilibrium prevalence rises to 24.6%. Serosorting appears to result in lower incidence of HIV among this population.
With serosorting, 22% of contacts are apparently serodiscordant, compared to 50% without serosorting. The proportion of apparent negative-negative contacts in which one partner is actually undiagnosed positive is lower in the model with serosorting (2.3%) than without (4.8%), reflecting difference in HIV incidence (1.1% vs. 1.7% per year, respectively). However, the proportions of infections resulting from all undiagnosed men and from undiagnosed, recently-infected (<3 months) men are higher in the model with serosorting (48.6% and 30.6% respectively) than without (26.3% and 16.5% respectively). This last pattern holds qualitatively for all additional models described below.
Individual-level risk of acquiring HIV from an unprotected anal sex act with an apparent negative is also lower in the baseline serosorting scenario. At equilibrium, transmission probabilities during unprotected receptive anal intercourse (URAI) and unprotected insertive anal intercourse (UIAI) with a randomly chosen apparent negative (weighted by those men’s activity levels) in the serosorting scenario are 0.00028 and 0.00008, respectively. Comparable rates in the absence of serosorting are 0.00060 and 0.00016, approximately twice as high. UAI with a diagnosed positive is far more risky than with an apparent negative; under serosorting, the probability of acquiring HIV from URAI and UIAI with a randomly chosen diagnosed positive equals 0.0050 and 0.00046, respectively (17.9 and 6.1 times the apparent negative rates). The rates did not change in the no-serosorting scenario.
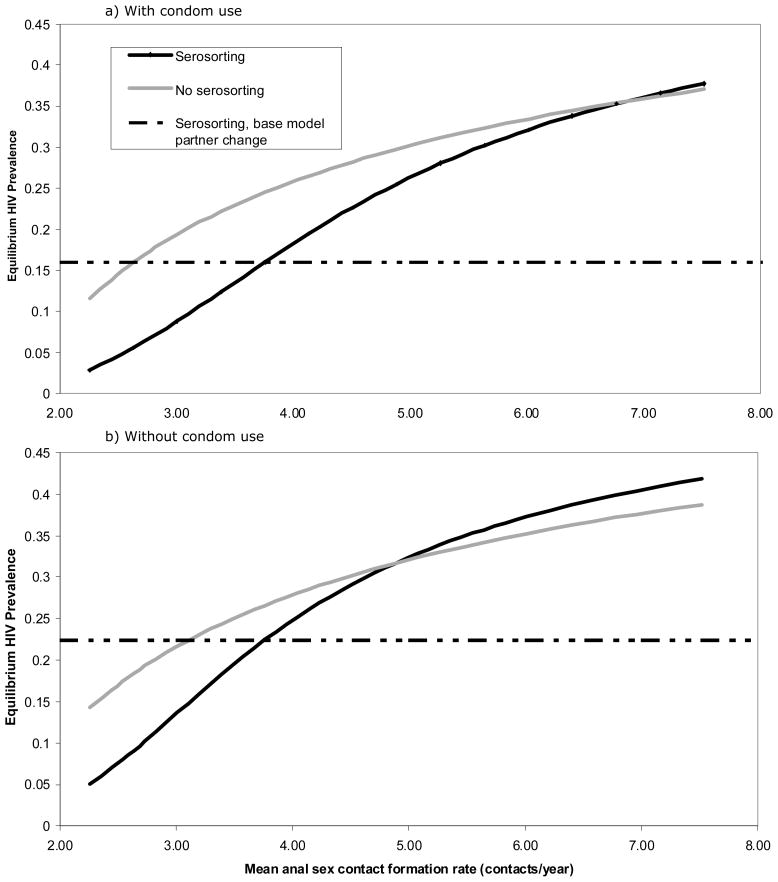
Figure 2 shows equilibrium prevalence for various contact rates (produced by increasing each group’s contact rate by an equal fraction). We model scenarios with (Figure 2a) and without (Figure 2b) condom use in perceived seroconcordant contacts (see Table 1 for condom usage rates in baseline model). Figure 2a includes our two baseline scenarios (mean contact rate = 3.76; equilibrium prevalence = 16.0% with serosorting and 24.6% without). Equilibrium prevalence remains lower for serosorting than equivalent no-serosorting scenarios when contact rates are <7.0/year; men would need an 86% increase above observed contact rate to abrogate the protective effects of serosorting.
Figure 2.
Equilibrium HIV prevalence for a variety of mean anal sex contact rates, with and without condom use in perceived seroconcordant contacts
We conducted additional simulations incorporating no condom use in perceived concordant contacts (Figure 2b). Equilibrium prevalence remains lower for serosorting than equivalent no-serosorting scenarios when contact rates are <4.9/year. Under these assumptions, men would need a 30% increase in contact rate above that observed in Seattle to abrogate the protective effects of serosorting.
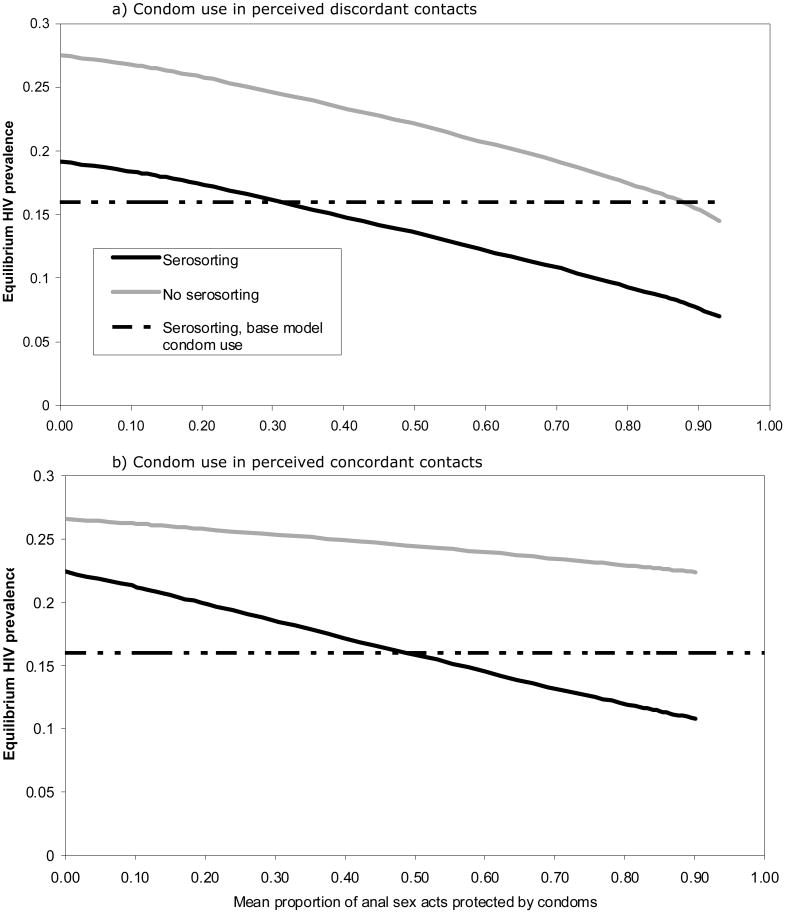
We consider various levels of status-based condom use in perceived discordant and concordant contacts (Figure 3a and 3b). In the baseline model, 31% and 49% of sexual acts were protected with condoms in perceived discordant and concordant contacts, respectively. (These counterintuitive rates of condom use may be due to hidden heterogeneity in men’s risk tolerance, i.e. risk-prone men are likely both to have discordant partnerships and forgo condoms.) Equilibrium prevalence is consistently higher in the no-serosorting scenarios for all levels of condom use in either perceived discordant or perceived concordant contacts. In this scenario, serosorting’s protective efficacy results primarily from preferential selection of apparent negative partners by apparent negative men. However, as condom use in perceived concordant contacts decreases, the difference in prevalence between the serosorting and no-serosorting scenarios decreases as well, from a 12% difference at 90% coverage to a 5% difference at 10% coverage. Thus, the more condoms are used in perceived concordant negative contacts, the bigger the protective effect of serosorting. Given reported contact rates, were men not to serosort, they would need to increase condom use to 90% in perceived discordant contacts to achieve the prevalence observed in the baseline serosorting scenario. No level of condom use among perceived concordant contacts would achieve the same result.
Figure 3.
Equilibrium HIV prevalence for various levels of condom use in perceived serodiscordant and perceived seroconcordant contacts.
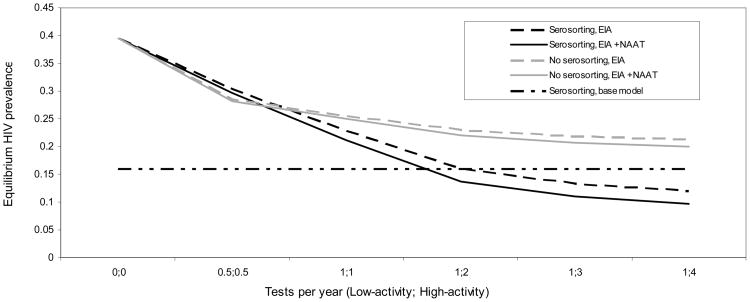
We note five observations of the effect of testing frequency on the relationship between serosorting and HIV equilibrium prevalence. First, in the absence of any testing (i.e. no serosorting, status-based condom use or ART, since status is unknown) equilibrium prevalence is 40%. Second, equilibrium prevalence declines as testing frequency increases (Figure 4). However, there are diminishing marginal returns with increasing testing frequency for high-activity men. Third, in scenarios where all men test once every two years, equilibrium prevalence under assumptions of serosorting is greater than under no serosorting; however, with more frequent testing, equilibrium prevalence is consistently lower under assumptions of serosorting compared with no serosorting. Fourth, the protective effect of serosorting increases with more frequent testing. Finally, test type leads to differential effects on equilibrium prevalence, both with and without serosorting. Under serosorting, the addition of NAAT to EIA testing yields an 8% reduction in equilibrium prevalence when all men test once per year, and a 20% reduction when low-activity men test once per year and high-activity men test four times per year. Under no serosorting, these estimates are 2% and 6%, respectively. Our estimates of equilibrium prevalence were similar under the assumption that low-activity men tested twice per year rather than once in all presented scenarios (data not shown).
Figure 4.
Equilibrium HIV prevalence by testing frequency and type.
Under our base model behavioral assumptions, the proportion of infections stemming from undiagnosed men for EIA alone is 62.5%, 48.6%, 41.8%, and 37.9% when low-activity men test once per year and high-activity men test 1, 2, 3, and 4 times per year, respectively. Comparable numbers for EIA plus NAAT are 59.2%, 42.4%, 32.1%, and 29.1%.
Additional sensitivity analyses (not shown) revealed that higher estimates of transmission probability during primary stage decreased the benefits of serosorting. Increasing the primary/chronic ratio of transmission probabilities by 10% decreased serosorting’s effect on endemic prevalence from 8.6% to 7.5%, while decreasing the ratio by 10% increases the difference to 9.3%. A longer duration of heightened primary infection also decreases the benefit: HIV prevalence would be 7.3% higher without serosorting if the primary stage lasted 4 months, whereas the change in prevalence would be 9.9% if the primary stage lasted 2 months. Lastly, shortening the duration of sexual activity from 50 years to 30 years did not change the estimated effect of serosorting.
Discussion
Our model suggests that serosorting (differential selection of sex partners or sex acts -- anal vs. oral -- by perceived HIV status), combined with status-based condom use, is highly protective at both the individual and population level among MSM. The protective effect of these behaviors remains true under a wide range of scenarios, at least relative to partner selection or condom use without regard to HIV status. However, in the Seattle MSM scenario, almost half of infections are transmitted by men undiagnosed for HIV. These findings have implications both for understanding HIV transmission and for prevention.
The finding that serosorting is protective is consistent with most epidemiologic studies evaluating HIV acquisition risk among MSM. These studies have consistently found that the greatest risk of acquiring HIV through UAI is associated with having HIV-infected partners, followed by partners of unknown HIV status and lowest with partners thought to be HIV uninfected (11, 13, 14). However, our results differ from those reported in an earlier modeling study (10) that found that serosorting could be detrimental. In large measure, these divergent findings reflect the different assumptions underlying the models. The authors of the previously published model assumed that the prevalence of recent (<6 months) seroconversion among all negative disclosers was 4% and that no apparent negatives used condoms with men they believed to be HIV-negative (10). In our model, prevalence of recent HIV is around 1.2% if one defines recent as <6 months, or about 0.5% given our assumption of a 3-month primary stage. Moreover, many HIV-negative MSM use condoms with apparently negative partners. Had we assumed no condom use in apparent seroconcordant contacts and that 4% seronegative disclosers were acutely infected (by artificially increasing mean contact rate to 5.64 – a 67% increase), our model would also have found that serosorting is detrimental.
To place our findings into context, if Seattle MSM were to abandon serosorting as a practice and choose partners and sex acts randomly by apparent serostatus, they would need to reduce contact rates by 30% or increase condom use with apparently discordant contacts by 50% to prevent an increase in transmission.
Our findings have several prevention implications. First, they should not be construed to suggest that efforts to actively promote serosorting are justified. It is imperative to bear in mind that our scenarios considered serosorting relative to not serosorting, with all other behaviors held equal. That is, for an HIV-negative man to choose an apparently HIV-negative partner for UAI is highly protective relative to choosing a partner without regard to HIV status. However, UAI with an apparently HIV-negative man is more risky than consistent condom use, and may be more risky than either protected anal intercourse or oral sex with an apparently HIV-positive man. Also, concordant UAI between apparent negatives within a stable relationship with “negotiated safety” is safer than concordant UAI between causal partners (12). Risk is complex, and the implications of increased serosorting, for both individual and population, depend on what behaviors the practice replaces. Attempts to promote serosorting as a harm reduction strategy, particularly among HIV negative men, need to consider the hierarchy of risk and the extent to which an increase in serosorting represents a move up or down that hierarchy. This process is more straightforward when considering promotion of serosorting among diagnosed positives than apparent negatives. In the former case, adoption of serosorting cannot increase transmission of HIV to seronegatives regardless of which behavior it is replacing, while in the former case it can. Recent studies have indeed tended to observe larger increases in serosorting among diagnosed positive MSM than among apparent negatives (6, 7). Note, however, that promotion of serosorting among positives is not without potential trade-offs, given the possibility of superinfection or transmission of other STIs (28, 29).
Second, recognition of serosorting as an informal risk reduction strategy should prompt efforts to promote more frequent HIV testing and the widespread use of more sensitive assays. Although serosorting appears to generally reduce HIV prevalence, it also increases the proportion of new HIV infections transmitted by undiagnosed individuals, including from those recently infected. Consequently, serosorting may be a detrimental practice at very low testing frequencies. Our findings suggest that increasing testing frequency among highly sexually active MSM from once every two years to four times per year, coupled with the use of NAATs, could reduce equilibrium prevalence of HIV by over 50%. Although NAAT testing has not been widely adopted in the US, recent studies suggest that a combined antigen-antibody EIA that is already available in Europe and that could easily replace existing assays in the US would likely identify the vast majority of antibody-negative infections (27, 28).
Our work relies on self-reports of perceived partner status. Unlike many other cases where self-reports are inherently limiting, here they are appropriate because serosorting occurs on the basis of individuals’ beliefs about their potential partners’ serostatus rather than the actual status. We did not, however, include the possibility that individuals might knowingly misrepresent their serostatus (29). In our RDD study, 2 of 37 (5%) HIV-positive men and 4 of 189 (2%) HIV-negative men who had anal sex in the prior year reported misrepresenting their HIV status. This misrepresentation would reduce the protective effect of serosorting. On the other hand, non-disclosure of HIV status would most likely increase the protective effect of serosorting. In our model we assume that non-disclosers’ actual HIV status is representative of the HIV distribution in the population, and non-disclosers are chosen as partners at the same rate as HIV-positive and negative individuals. If instead we assumed that non-disclosers were refused as partners, and not replaced by others, the odds ratio of serosorting would increase, as would the protective effect. However, to properly test the effect of non-disclosure, a serosorting model would need a two-step process: 1) HIV status disclosure, and 2) the decision whether to engage in an anal sex act. This could be difficult since data are not usually collected on potential partners that were refused.
In modeling the effect of testing frequency on HIV we did not consider the motivation for testing; while some MSM test on a regular basis regardless of risk, others may be spurred to test by specific risk events and symptoms of acute HIV infection (30). In our model, we only consider the former; future work should consider the latter as well. Doing so would likely require use of an agent-based model.
We further assumed random mixing among men by activity class, partly for lack of data and partly for model simplicity, although in truth some level of assortative mixing is likely. We expect that including assortative mixing in the model might slightly decrease the effectiveness of serosorting by creating a “core group” of assortatively mixing high-activity men whose presence increases the size of the epidemic and thus of the proportion of apparent negatives who are actually acutely infected at any time.
Additionally, we considered only a lower bound estimate for reduction of HIV transmission associated with ART, and did not consider transmission effects of early treatment. Because serosorting increases the proportion of infections stemming from acutely infected individuals, early ART may further increase the protective effects of serosorting at the population- and individual-levels. Future modeling work should explore this topic.
Despite the paucity of existing data on the benefits of serosorting, some public health programs and community-based organizations now promote the practice (31). Our data and model suggest that under realistic scenarios of sexual behavior and testing frequency for MSM in the US, serosorting can be an effective harm reduction strategy. However, the impact of serosorting promotion remains uncertain, and will depend on what behaviors MSM abandon as they adopt the practice. In contrast, the benefits of increasing the frequency of HIV testing and using the most sensitive available HIV tests are straightforward, and these interventions should be widely promoted.
Acknowledgments
1) We would like to thank Bob Wood, Martina Morris, and the members of the Public Health – Seattle & King County and University of Washington Sociobehavioral and Prevention Research Core working group for their support of this project. A previous version of this paper was presented at the Epidemics Conference in Asilomar, CA: December 1 – 3, 2008 and at the Population Association of America Annual Meeting in Detroit, MI: April 30 – May 2, 2009.
2) SC conceived the project in collaboration with the UW CFAR/Public Health – Seattle King County working group. SC and TM wrote the modeling code and ran the analyses. SG provided methodological expertise and oversaw the project. MG provided the data, and TM parameterized the model. All authors collectively contributed to drafting and editing the final article.
3) In accordance with U.S. Health and Human Services Guidelines, a certificate of exemption was obtained from the University of Washington Human Subjects Division 07-9056-X/C.
Sources of funding: University of Washington, Center for AIDS Research Emerging Opportunity Grant (NIH AI27757) and NIH K99 HD057553. SMG was supported in part by the National Institutes of Health (NIH DA022116). TWM was supported by the National Institutes of Health (NIH T32 AI07140). Data collection was supported by NIH K23 AI001846.
Footnotes
There are no conflicts of interest.
References
- 1.Kippax S, Race K. Sustaining safe practice: twenty years on. Soc Sci Med. 2003;57(1):1–12. doi: 10.1016/s0277-9536(02)00303-9. [DOI] [PubMed] [Google Scholar]
- 2.Dawson JM, Fitzpatrick RM, Reeves G, Boulton M, McLean J, Hart GJ, et al. Awareness of sexual partners’ HIV status as an influence upon high-risk sexual behaviour among gay men. Aids. 1994;8(6):837–41. [PubMed] [Google Scholar]
- 3.Golden MR, Brewer DD, Kurth A, Holmes KK, Handsfield HH. Importance of sex partner HIV status in HIV risk assessment among men who have sex with men. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2004;36(2):734–742. doi: 10.1097/00126334-200406010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Koblin BA, Chesney MA, Husnik MJ, Bozeman S, Celum CL, Buchbinder S, et al. High-risk behaviors among men who have sex with men in 6 US cities: baseline data from the EXPLORE Study. Am J Public Health. 2003;93(6):926–32. doi: 10.2105/ajph.93.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Crawford JM, Hospers HJ, Prestage GP, Grulich AE, Kaldor JM, et al. “Serosorting” in casual anal sex of HIV-negative gay men is noteworthy and is increasing in Sydney, Australia. Aids. 2006;20(8):1204–6. doi: 10.1097/01.aids.0000226964.17966.75. [DOI] [PubMed] [Google Scholar]
- 6.Elford J, Bolding G, Sherr L, Hart G. High-risk sexual behaviour among London gay men: no longer increasing. Aids. 2005;19(18):2171–4. doi: 10.1097/01.aids.0000194133.28135.03. [DOI] [PubMed] [Google Scholar]
- 7.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008;49(2):212–8. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 8.Osmond D, Pollack L, Paul JP, Catania JA. Changes in Prevalence of HIV Infection and Sexual Risk Behavior in Men Who Have Sex With Men: San Francisco, 1997–2002. American Journal of Public Health. 2007;97(6) doi: 10.2105/AJPH.2005.062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong HM, Kellogg T, Klausner JD, Katz MH, Dilley J, Knapper K, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect. 2006;82(6):461–6. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler D, Smith D. Serosorting can potentially increase HIV transmissions. AIDS. 2007;21(9):1218–1220. doi: 10.1097/QAD.0b013e32814db7bf. [DOI] [PubMed] [Google Scholar]
- 11.Koblin BA, Husnik MJ, Colfax G, Huang YJ, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. Aids. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 12.Jin F, Crawford J, Prestage GP, Zablotska I, Imrie J, Kippax SC, et al. Unprotected anal intercourse, risk reduction behaviours, and subsequent HIV infection in a cohort of homosexual men. Aids. 2009;23(2):243–52. doi: 10.1097/QAD.0b013e32831fb51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiede H, Jenkins RA, Carey JW, Hutcheson R, Thomas KK, Stall RD, et al. Determinants of Recent HIV Infection Among Seattle-Area Men Who Have Sex with Men. Am J Public Health. 2009 doi: 10.2105/AJPH.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder SP, Vittinghoff E, Heagerty PJ, Celum CL, Seage GR, Judson FN, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2005;39(1):82–89. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 15.Philip S, Donnell D, Yu X, Vittinghoff E, Buchbinder S. Serosorting, but not seropositioning, is associated with decreased risk of HIV seroconversion in the EXPLORE study cohort. 15th Conference on Retroviruses and Opportunistic Infections; February 4, 2008; Boston, MA. Feb 4, 2008. [Google Scholar]
- 16.Brewer DD, Golden MR, Handsfield HH. Unsafe sexual behavior and correlates of risk in a probability sample of men who have sex with men in the era of highly active antiretroviral therapy. Sex Transm Dis. 2006;33(4):250–5. doi: 10.1097/01.olq.0000194595.90487.ed. [DOI] [PubMed] [Google Scholar]
- 17.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 18.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 19.Busch MP, Lee LLL, Satten GA, Henrard DR, Farzadegan H, Nelson KE, et al. Time-course of detection of viral and serologic markers preceding human-immunodeficiency-virus type-1 seroconversion: Implications for screening of blood and tissue donors. Transfusion. 1995;35(2):91–97. doi: 10.1046/j.1537-2995.1995.35295125745.x. [DOI] [PubMed] [Google Scholar]
- 20.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17(13):1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Longini IM, Jr, Clark WS, Byers RH, Ward JW, Darrow WW, Lemp GF, et al. Statistical analysis of the stages of HIV infection using a Markov model. Stat Med. 1989;8(7):831–43. doi: 10.1002/sim.4780080708. [DOI] [PubMed] [Google Scholar]
- 22.Survival after introduction of HAART in people with known duration of HIV-1 infection. The CASCADE Collaboration. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355(9210):1158–9. [PubMed] [Google Scholar]
- 23.Chou R, Fu R, Huffman LH, Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta-analyses. Lancet. 2006;368(9546):1503–15. doi: 10.1016/S0140-6736(06)69638-4. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan P, Kayitenkore K, Chomba E, Karita E, Mwananyanda L, Vwalika C, et al. Reduction of HIV transmission risk and high risk sex while prescribed ART: results from discordant couples in Rwanda and Zambia. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- 25.Reynolds S, Makumbi F, Kagaayi J, Nakigozi G, Galiwongo R, Quinn TC, et al. ART reduced the rate of sexual transmission of HIV among HIV-discordant couples in rural Rakai, Uganda. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- 26.Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18(1):81–8. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel P, Bennett B, Sullivan T, Parker M, Sullivan P, Group CAS. Detection of acute HIV infections using a fourth generation antigen/antibody assay. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- 28.Stekler J, Swenson PD, Coombs RW, Dragavon J, Brennan C, Devare S, et al. Limitations of rapid HIV antibody testing in a population with high incidence of HIV infection. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- 29.Golden MR, Wood RW, Buskin SE, Fleming M, Harrington RD. Ongoing risk behavior among persons with HIV in medical care. AIDS Behav. 2007;11(5):726–35. doi: 10.1007/s10461-007-9244-5. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez T, Finlayson T, Drake A, Behel S, Cribbin M, Dinenno E, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors--United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003–April 2005. MMWR Surveill Summ. 2006;55(6):1–16. [PubMed] [Google Scholar]
- 31.San Francisco - Department of Public Health. Social Marketing Campaign Evaluation Report: Disclose HIV; 2008.
- 32.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;(1):CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]