Abstract
A need for antigen-processing and presentation to B cells is not widely appreciated. However, cross-linking of multiple B cell receptors (BCRs) by T-independent antigens delivers a potent signal that induces antibody responses. Such BCR cross-linking also occurs in germinal centers where follicular dendritic cells (FDCs) present multimerized antigens as periodically arranged antigen-antibody complexes (ICs). Unlike T cells that recognize antigens as peptide-MHC complexes, optimal B cell-responses are induced by multimerized FDC-ICs that simultaneously engage multiple BCRs. FDC-FcγRIIB mediates IC-periodicity and FDC-BAFF, -IL-6 and -C4bBP are co-stimulators. Remarkably, specific antibody responses can be induced by FDC-ICs in the absence of T cells, opening up the exciting possibility that people with T cell insufficiencies may be immunized with T-dependent vaccines via FDC-ICs.
Introduction
Certain antigens (Ag) can engage B cells such that specific antibodies (Abs) are induced in the absence of T cell help. These so-called T cell-independent (TI) Ags are further classified into TI type 1 and 2. The TI-1 Ags, such as LPS, are B cell mitogens, which function by nonspecifically or polyclonally activating most B cells. The so-called TI-2 Ags are characterized by multiple identical epitopes that are spaced regularly approximately 100 – 700 Angstroms apart. This periodicity allows simultaneous engagement of multiple B cell receptors (BCRs) with each epitope and the collective signal is sufficiently strong to induce TI Ab responses [1–6]. Moreover, as reviewed by Bachmann and Zinkernagel [7], certain viral Ags and self Ags exhibiting repeating epitopes (in the range of 50 –100 Angstroms apart) induce TI responses. A good example is vesicular stomatitis virus (VSV). When disaggregated, Ab responses to VSV Ags are T cell dependent (TD) but TI IgM responses are induced when intact virus with periodically arranged epitopes are injected [7].
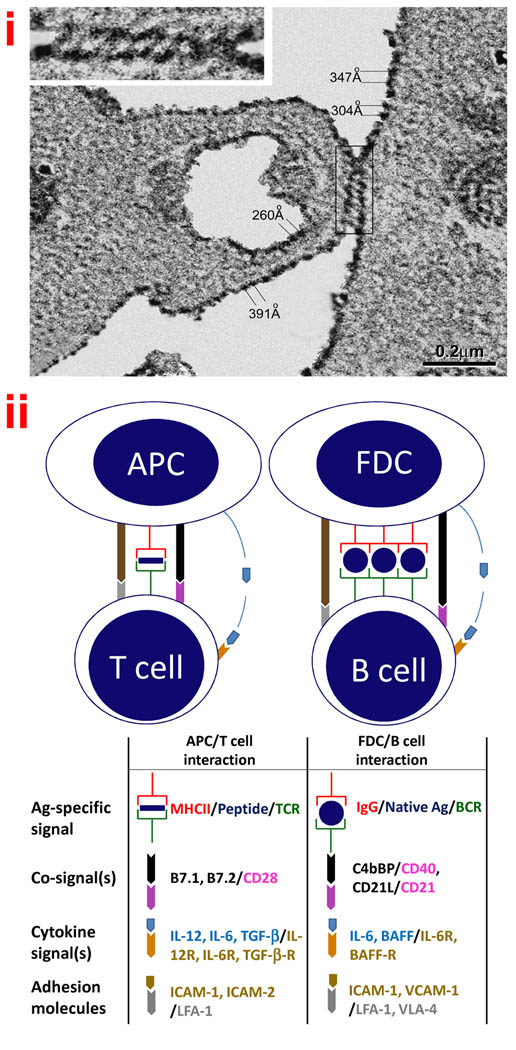
Follicular dendritic cells (FDCs) are a novel cell type localized to the follicles of secondary lymphoid tissues where they form interactive networks or reticula of non-mobile antigen-bearing cells. Immobile FDCs in these FDC-reticula engage the mobile B and T cells and other mobile cells trafficking through the follicles. The basic features and functions of FDCs are summarized in Box 1. FDCs trap immune complexes (ICs), and Ag in FDC-ICs are intact and persist in a form that can be recognized by specific antibodies for months or even years [9,31]. When FDCs from the draining lymph nodes of horseradish peroxidase (HRP) immunized mice were examined using scanning EM, it was apparent that HRP-ICs are periodically arranged on FDC membranes. The pattern suggested an orderly, spiraling, arrangement of the attached ICs around FDC processes [32]. More recently, HRP-ICs were loaded in vitro and FDCs were examined at higher resolution using transmission EM. Again it was apparent that the ICs are periodically arranged and the distance between the HRP deposits ranged from 200 to 500 Angstroms, which resembles the arrangement of epitopes on TI-2 antigens. This periodic arrangement is illustrated in Figure 1 panel i.
Box 1. Features and functions of follicular dendritic cells.
When defined as cells that retain immune complexes (ICs) long-term, FDCs exist in all jawed vertebrates, including amphibians, reptiles and fish [8].
FDCs are localized to the follicles of all secondary lymphoid tissues, where they retain antigens for months in the form of ICs [9].
The origin of FDCs remains unclear. There are data supporting a hematopoietic origin but even more supporting a stromal cell origin [10].
Morphologically, FDCs are slightly larger than lymphocytes and possess fine dendritic processes that intimately interact with neighboring cells. They have irregular, sometimes bilobed, euchromatic nuclei (sometimes there are multiple nuclei) containing distinct nucleoli. FDCs possess a scanty cytoplasm with few mitochondria, a rough endoplasmic reticulum, a Golgi apparatus and vesicles [11].
Morphological types include one with filiform or finger-like processes, and one with ‘beaded’ dendrites. The released beads are called “iccosomes”, which are immune complex-coated bodies or ‘somes’ [11].
Monoclonal Abs useful for identifying FDCs include: FDC-M1 & FDC-M2 for murine FDCs and DRC-1, HJ2 & KI-M4 for human FDCs [8,12,13].
Other phenotypic markers include: FcγRIIB/CD32, FcεRII/CD23, CR1/2/CD21/35,ICAM-1/CD54, VCAM-1/CD106, MadCAM-1, 8D6/CD320, CD40, TLR(2, 3 and 4), & lymphotoxin receptor [8,10,14–16].
FDC-cytokines and –chemokines include: CXCL13, IL-6, IL-7, IL-15, BAFF, and TNF-α [8,10,17–20],
FDCs are critically involved in germinal center development, immunoglobulin class switching, memory B cell generation, selection of somatically mutated B cells with high affinity receptors, affinity maturation, induction of recall responses, and regulation of serum IgG & IgE levels [9,21–25].
In addition to their role in humoral immunity, FDCs are associated with certain diseases including HIV/AIDS, prion diseases, follicular lymphomas, and chronic inflammatory autoimmune diseases [8,26–28].
- Presentation of Ag to B cells by FDCs, contrasts with Ag presentation to T cells [Figure 1 panel ii]:
- FDCs express distinctive co-stimulatory signals including C3b fragments to bind the B cell co-receptor complex, C4 binding protein that binds FDC-C4b and engages B cell CD40 [17].
- Ag in FDC-ICs is presented to B cells days after primary immunization to induce GC reactions whereas presentation to T cells is an early event [24].
Figure 1. Antigen processing and presentation by follicular dendritic cells (FDCs).
(i) Periodicity of Ags on FDCs
Purified FDCs were incubated with horseradish peroxidase ICs (HRP-ICs), washed, and prepared for transmission EM. Note measurements between IC deposits and cross-linking of ICs in the EM. The “zippering” of membranes may help explain long-term retention of Ags in ICs on FDCs (With kind permission from Springer Science and Business Media: Cell and Tissue Research, Ultrastructural study of highly enriched follicular dendritic cells reveals their morphology and the periodicity of immune complex binding, 332(1), 2008, 89–99, Sukumar S, El Shikh ME, Tew JG, Szakal AK, Figure 6).
(ii) Comparison of Ag presentation to T vs. B cells
Note the similarities between the two models and that major differences relate to how T and B cell receptors for Ag engage Ags differently [see Box 1 for details].
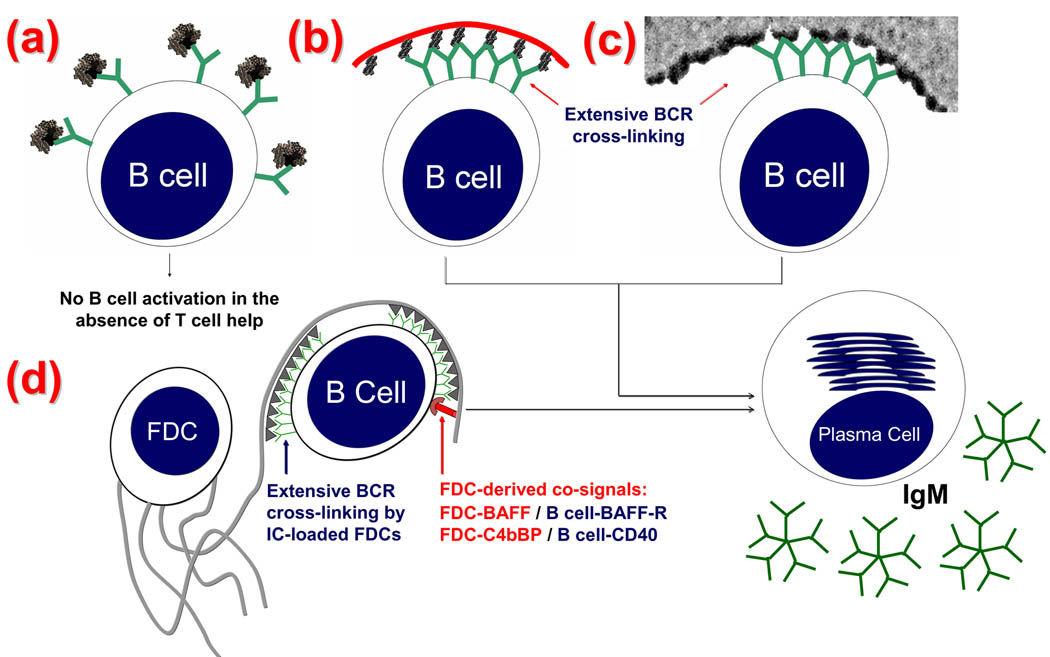
Specific receptors on T cells and B cells recognize Ags differently and the well-known features of Ag presentation to T cells by antigen presenting cells (APCs) is illustrated in Figure 1 panel ii and the model for Ag presentation to B cells by FDCs is placed beside it for comparison. The similarity between engagement of BCRs by a TI-2 Ag and multimerized Ag on FDCs is illustrated (diagrammatically) in Figure 2. Note the lack of B cell cross-linking by a TD Ag in (a) vs simultaneous cross-linking of multiple BCRs with a TI-2 Ag in (b), and HRP (a TD Ag) on FDCs in (c). An illustration of how FDC-ICs work in conjunction with FDC-derived co-signals is illustrated in (d). Engagement of BCRs by free Ag can cause B cell activation however an immunogen is converted into ICs (i.e. multiply bound by Ab) as soon as Ab is produced in a primary response, and instantaneously in recall responses by Ab persisting from previous immunizations. IgG-ICs co-ligate BCR with FcγRIIB and inhibit B cell activation via the tyrosine-based inhibitory motif (ITIM). In contrast, IgG-ICs trapped by FDC-FcγRIIB do not engage B cell-FcγRIIB and consequently ITIM-mediated signaling is minimized [15,29,33–36]. Thus, one might reason that Ag in FDC-ICs would approach the efficiency of free Ag in ability to stimulate B cells. However, Ag in FDC-ICs are far more potent inducers of Ab responses than free Ag and this has been demonstrated both in vitro [25,29] and in vivo [24]. This difference is not explained by FDC-trophic factors (i.e. cytokines) alone because free Ag, in the presence of FDCs with their trophic factors, is not as immunogenic as Ag in FDC-ICs [25,29]. These relationships prompted the hypothesis that Ag in ICs on FDCs is “processed” in a way that promotes immunogenicity when it is “presented” to B cells by FDCs.
Figure 2. Model of FDC-dependent but T-independent B cell activation and Ig production.
(a): Monomeric proteins generally express a single copy of each epitope and are unable to cross-link multiple BCRs and activate B cells in the absence of T cell help. (b): TI-2 Ags contain numerous periodically arranged epitopes (black protrusions) attached to a flexible backbone (red curve) (e.g., polyacrylamide). This allows extensive simultaneous cross-linking of BCRs (Y-shaped green). Multiple BCR cross-linking delivers a signal leading to B cell activation and proliferation. (c): A transmission electron EM showing HRP (a TD Ag) retained on the FDC surface in IC clusters 200–500 Å apart. (d): FDCs accessory activity includes secondary or co-signals that promote B cell activation and Ig production. Specifically, FDCs are decorated with the complement-derived CD21L which engage B cell CD21. Binding CD21 in the CD21–CD19–CD81 B cell co-receptor complex delivers a positive co-signal for B-cell activation and differentiation [29]. FDC-derived BAFF ligates BAFF receptors on B cells, and FDC-derived C4b-Binding Protein (C4BP) ligates B cell-CD40, which is a classic co-signal in B cell activation [17] (Reproduced with permission, from “T-independent antibody responses to T-dependent antigens: A novel follicular dendritic cell-dependent activity” J Immunol. 2009 Mar 15;182(6):3482-91. Copyright 2009. The American Association of Immunologists, Inc.).
FDCs can obviate the need for T cells
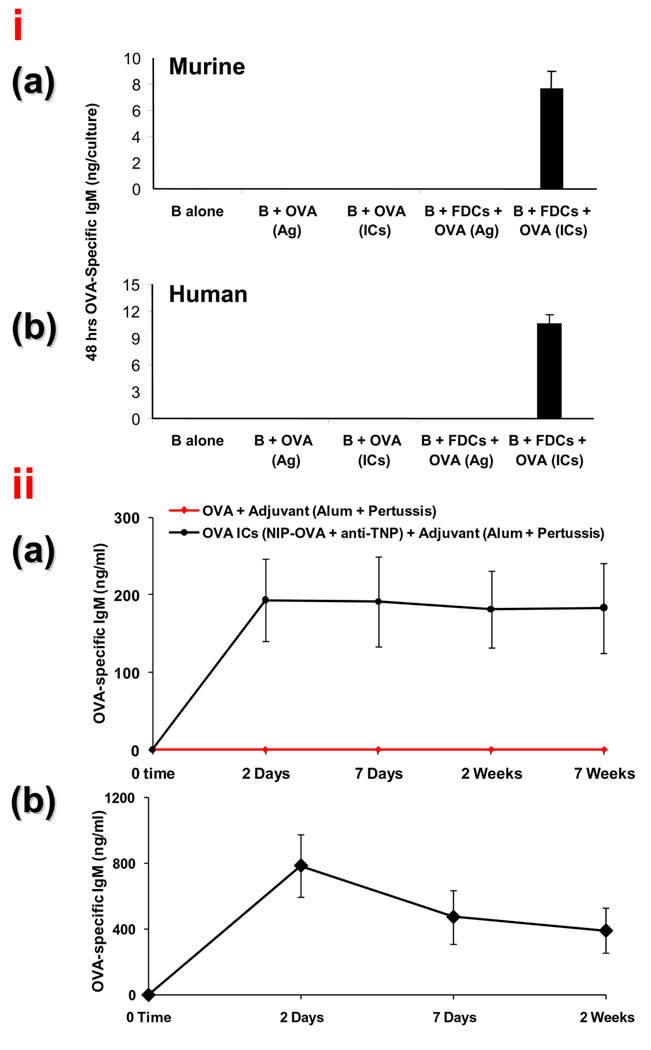
The arrangement of TD Ags on FDCs with periodicity like TI-2 epitopes prompted reasoning that TD Ags on FDCs should induce rapid immune responses in the absence of T cells. This was investigated using purified naïve B cells incubated in vitro with purified IC bearing FDCs and 48 hrs later specific IgM anti-ovalbumin (OVA) responses were apparent (Figure 3, panel i; illustration (a) for murine B cells and illustration (b) for human B cells). Ag is bound to FDCs by Ab molecules rather than MHC molecules and Ag-presentation is not MHC or species restricted. Thus, ICs on murine FDCs also induced purified naïve human B cells to produce OVA specific IgM [17,29]. Note that FDCs were unable to induce Ig production with free Ag, which would have unfettered access to BCRs, but only with ICs that can be multimerized on their surfaces. Moreover, these TI but FDC-dependent IgM responses were inhibited when FDC-FcγRIIB was blocked to interfere with the ability to bind and multimerize ICs [17].
Figure 3. T independent B cell activation by IC-bearing FDCs.
(i) Purified OVA-IC-bearing FDCs induced a 48 h OVA-specific IgM response by purified naïve B cells in vitro in the absence of T cells
Murine (a) and human (b) B cells stimulated with FDCs bearing OVA-ICs produced OVA-specific IgM in 48 h. Control conditions that failed to produce detectable responses included FDCs with B cells stimulated with free OVA that would have had free access to BCRs (Reproduced with permission, from “T-independent antibody responses to T-dependent antigens: A novel follicular dendritic cell-dependent activity” J Immunol. 2009 Mar 15;182(6):3482-91. Copyright 2009. The American Association of Immunologists, Inc.).
(ii) Nude mice challenged with OVA ICs, but not with OVA, mount OVA-specific immune responses in 48 h.
Nude mice were challenged with alum-precipitated OVA or OVA-ICs with B. pertussis. Serum anti-OVA IgM levels were monitored over time. As expected, anti-OVA was not detectible in animals immunized with OVA in adjuvant (red tracking baseline). In marked contrast, OVA-specific IgM was present in just 48 h with IC immunized mice and was maintained for weeks. (b), Heterozygous nu/+ mice with a competent T cell compartment also responded to ICs by producing a potent OVA-specific IgM within 48 hrs (Reproduced with permission, from “T-independent antibody responses to T-dependent antigens: A novel follicular dendritic cell-dependent activity” J Immunol. 2009 Mar 15;182(6):3482-91. Copyright 2009. The American Association of Immunologists, Inc.).
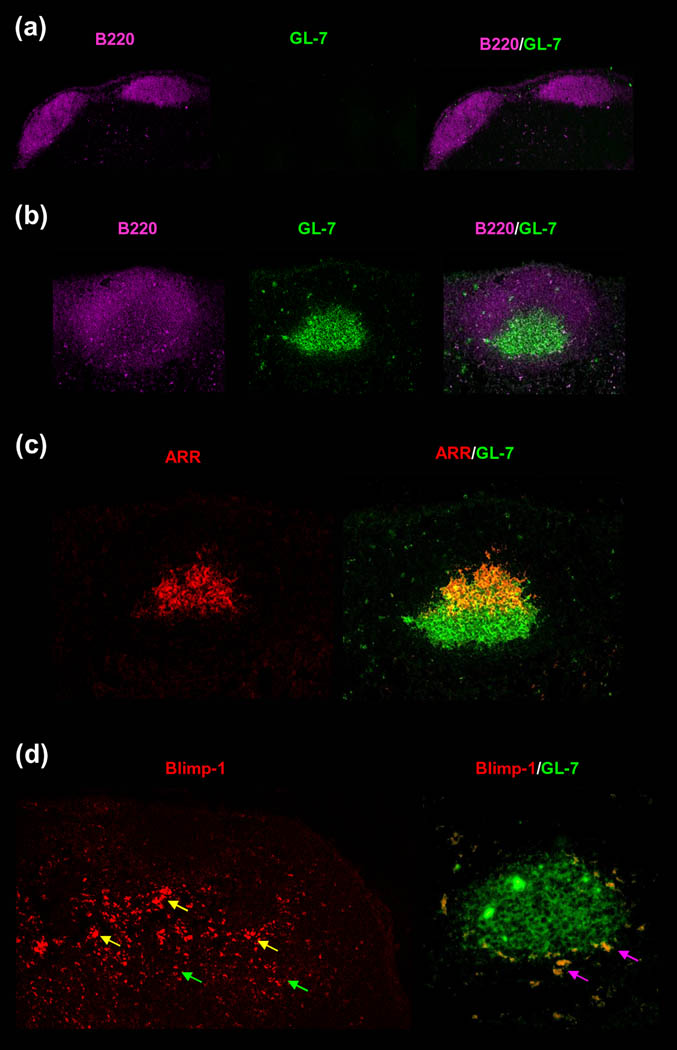
Similarly, specific IgM responses were studied in vivo in athymic nude (nu/nu) mice injected with OVA-ICs. To minimize any residual T cell activity they were also injected with anti-Thy-1. OVA-IC-challenged nude mice produced OVA-specific IgM within 48 h after OVA-IC challenge and the response was maintained for weeks (Figure 3 iia). Moreover, FDC-reticula adjacent to robust 48 hr GCs and plasmablasts were apparent in the follicles of the IC injected nu/nu mice (Figure 4). In marked contrast, free OVA in adjuvant induced no GCs, plasmablasts, or IgM in nude mice (Figure 3iia and Figure 4a) [17]. Illustration Figure 3 iib represents the phenotypically normal nu/+ mice that responded to ICs with a potent 48 hr IgM response although the levels declined as the response switched to IgG. In contrast, an IgG response was not detectible in nude mice nor was it measurable in vitro when Ag was presented to purified naïve B cells by FDCs in the absence of T cells [17].
Figure 4. Robust GCs and plasmablast responses induced in 48 h in nude mice by OVA-ICs in association with IC-retaining FDC-reticula.
Nude mice were challenged with OVA-specific rabbit serum or normal rabbit serum plus alum-precipitated OVA and B. pertussis. Two days later, mid-sagittal sections from draining LNs were triple labeled with GL-7-FITC (green, GC-B cells), B220-Cy5.5 (magenta, pan-B cell marker), and Rhodamine Red X-goat anti-rabbit IgG (red, OVA-ICs in FDC-reticulum). GC-associated plasmablasts were labeled with anti-Blimp-1-PE (red). (a), Injection of OVA-adjuvant into nude mice failed to induce detectible GCs. The B cell follicles labeled well with B220 (magenta) but not with the GC B cell marker GL-7 (green); thus, the overlay (B220/GL-7) remained magenta. (b), Injection of OVA-ICs into nude mice induced robust GCs. The GL-7bright B cells (green) correspond with an area of B220dim labeling (magenta). The overlay (B220/GL-7) illustrates GL-7bright GC B cells surrounded by a zone of resting B220bright B cells. (c), Labeling with anti-rabbit IgG revealed well-developed, crescent-shaped, antigen retaining reticulum (ARR) (red). In the overlay of the ARR and the GL-7, the GL-7+ GC B cells (green) were seen capped with the ARR (red) and the light zone of the GC where the FDC-ARR (red) overlapped with the GC B cells (green) appeared orange-yellow. (d), Blimp-1+ plasmablasts were found at the paracortical borders of multiple GCs (yellow arrows), and some migrated toward the medullary cords deeper in the node (green arrows). These cells were also GL-7+ (green) giving an orange overlay (magenta arrows) (Reproduced with permission, from “T-independent antibody responses to T-dependent antigens: A novel follicular dendritic cell-dependent activity” J Immunol. 2009 Mar 15;182(6):3482-91. Copyright 2009. The American Association of Immunologists, Inc.)
Influence of costimulatory factors produced by T cells and FDCs on TI responses
Mice challenged with TI-2 Ags can exhibit GCs and IC-retaining FDC-reticula, and this occurs in the absence of cognate T cells [37,38]. Nevertheless, the finding that natural and synthetic multivalent Ags can induce Ig secretion in the absence of T cells does not mean that T cells do not play a role in TI responses (reviewed [4]). Indeed involvement of T cells and T-cell-derived cytokines, including IL-2, IL-3, IL- 4, IL-5, IL-6, IL-10, IFN-γ, and GM-CSF, has been reported [3,4]. Bachmann, Zinkernagel and others have expanded this relationship to TI Ab response to multivalent viral Ags [7]. Note that the 48 hr anti-OVA response was much higher in phenotypically normal nu/+ mice (Figure 3-iib) than in nu/nu mice (Figure 3-iia).
Like APCs for T cells, FDCs express costimulatory molecules including C4bBP, CD21L, B cell activation factor of the TNF family (BAFF) and IL-6. Blocking the effect of costimulatory molecules by use of soluble receptors, neutralizing Abs or knockout animals for FDC-C4BP, -CD21L, -IL-6, or -BAFF inhibits Ab responses [17,18,39,40]. Clearly, Ag-presentation by FDCs involves secondary signals as well as a primary signal by multimerized-Ag.
Typically ~48 hrs is needed before primed T cells are able to provide cognate help and it is unlikely that cognate T helper cells are involved in 48 hr TI responses. It is important to appreciate that IC activated FDCs produce BAFF and IL-6 and that these cytokines can in turn rapidly induce cytokine production by T cells [41–43]. Moreover, T-cell subsets like γδ and NK T cells mediate non-MHC class II-restricted non-cognate help and might be a source of B cell activation factors within 48 hrs.
Presentation of ICs by FDCs in TD responses
Typically GC events including Ig class switching and somatic hypermutation (SHM) are TD. We sought to determine if Ag processing and presentation by FDCs is important in these GC events. Accordingly, the ability of naïve B cells to produce IgM and class switch to IgG with high affinity in vitro was studied using memory T cells and FDCs. The immunogen was either an IC that would be multimerized and presented by FDCs, or free Ag that would have free access to BCRs but would not be multimerized and presented by FDCs [25]. In cultures where FDCs presented ICs, the Ig switched from IgM to IgG with high affinity in the second week and almost all IgG induced by FDC-ICs was of high affinity in the second week. However, class switching was minimal and high affinity IgG was not obtained when FDC-ICs were replaced with free Ag plus FDCs even though memory T cells were present [25]. These data support the concept that Ag presented by FDCs that can simultaneously cross-link multiple B cell receptors is important for class switching and affinity maturation.
SHM is important for affinity maturation and SHM is a late GC event beginning 9 –11 days after primary challenge. A late specific signal (~a week after priming) is delivered by Ag-persisting on FDCs and promotes SHM [24]. To test the importance of FDC-ICs, GC reactions were elicited in vitro where pure populations of cells and cell factors can easily be used and manipulated. In vitro the B cells and T cells cluster around FDCs, as occurs in the light zone of GCs in vivo, and the B cells proliferate, become GL7 positive, and some express blimp 1 recapitulating what is seen in GC light zones [17,44]. In vitro GC reactions were set up using FDCs, CGG specific memory T cells, and B cells. B cells were obtained from mice immunized 6 days earlier with (4-hydroxy-3-nitrophenyl) acetyl-chicken gamma globulin (NP-CGG). The 6 days allowed NP-specific B cells to clonally expand, class switch, and to begin producing IgG anti-(4-hydroxy-5-iodo-nitrophenyl)acetyl (NIP) under physiological conditions in vivo. NIP has higher affinity for anti-NP Abs than NP, and NIP is generally used to measure NP responses [45]. However, as shown by others, and confirmed by us, B cells taken 6 days after primary immunization are not mutated [46–48] but are primed and poised for SHM. These 6-day B cells were then cultured for 7 additional days in vitro and production of specific anti-NIP Ab and mutations in the VH186.2 gene segment were determined. These in vivo activated B cells could produce specific Ab in vitro and this was enhanced by the addition of FDCs. However, SHM was not obtained unless the FDCs present were bearing the specific ICs that can simultaneously cross-link multiple B cell receptors while signaling the B cells [24].
In short, immunogen binds the initial Ab produced in primary responses and the ICs formed are trapped by FDCs. The FDC-ICs promote GC reactions and FDC-ICs persist in GCs where they provide a second Ag specific signal late in the GC reaction that induces SHM around day 9. It is important to appreciate that ICs are efficiently cleared by phagocytic cells (t½ of ~ 30 min) and that by a week after priming, Ag persisting in vivo is intact in ICs on FDCs, where the multimerized specific immunogen can readily encounter GC B cells and induce SHM [31].
Several days are required to generate IgG-ICs that can be trapped and periodically arranged by FDCs in vivo. We reasoned that IgG-IC-bearing FDCs might be rate-limiting and explain why SHM is a late event. To test this hypothesis, irradiated mice were reconstituted with naïve B cells together with memory T cells and given either preformed IgG-ICs or Ag alone for a control. B cells were harvested 7 days after immunogen challenge and mutations in the variable heavy gene segment were determined. Mutations in the variable heavy chain gene segment from the IgG-IC-injected mice were at a frequency of 12 mutations/1000 bases. In marked contrast, no mutations were found after 7 days in clones from control mice given memory T cells and free Ag. This frequency of 12 mutations/1000 bases in 7 days is comparable with results obtained 14 days after a typical primary immunization. Mice injected with Ag would be expected to have mutations by 14 days but they are not apparent by day 7. Thus, time needed for extensive SHM was reduced by ~1 week when naive B cells were challenged in vivo with Ag in ICs that would be immediately multimerized and presented by FDCs [24].
SHM occurs in mice where Ig secretion, and thus the amount of ICs trapping on FDCs, is markedly reduced [49]. These results raise a question about the need for ICs (at least at high levels) in promoting SHM. The present data do not exclude the possibility that SHM may occur in the absence of IC-bearing FDCs. However, the data provide strong support for the concept that SHM is promoted by IgG-ICs that FDCs readily process and present in the GC microenvironment.
In addition to the primary signal by multimerized Ag, FDCs provide B cell co-stimulatory and adhesion molecules including CD21L, IL-6, CD320/8D6, ICAM-1, and VCAM-1 [18,40,44,50–52]. Blocking FDC-CD21L-B cell CD21 interactions inhibits IgG responses and production of activation-induced cytidine deaminase (AID) in B cells which is critical for Ig class switching and SHM. [25]. Blocking IL-6 from FDCs inhibits both IgG responses and SHM [18]. Blocking adhesion molecules including ICAM-1 or LFA-1 inhibits FDC-B cell clustering and B cell activation [44,52]. Moreover, like APCs for T cells, FDCs express Toll-like receptors (TLRs) and are activated by interaction with TLR ligands [14]. Activated FDCs express higher levels of functional molecules including FcγRIIB, ICAM-1, VCAM-1, BAFF and IL-6 [14,15,18]. Both specific Ab responses and SHM are dramatically enhanced when FDCs are activated by TLR ligands suggesting that adjuvant activity likely involves FDC-activation and not just APC-activation for T cells [14,24].
Concluding comments
FDCs have received little attention and in vitro studies are challenging because FDCs are rare and fragile. Nevertheless, in our opinion, an understanding of FDCs is critical to an understanding of GCs, B lymphocyte maturation, and optimal Ab responses that are critical to effective vaccination. GCs are a fundamental feature of the immune system and presentation of FDC-Ags to B cells has potential applications. For example, generation of TI immune responses to TD Ags could facilitate immunizing patients with congenital or acquired T cell insufficiencies [53,54] including: HIV infected [55], aged [56], diabetic [57], uremic [58] and neonates [59,60]. This T cell bypass strategy could minimize failures attributable to defective Ag-presentation to T cells, limited MHC repertoires, or suppression by regulatory T cells. The FDC-IC pathway makes it possible to generate specific Ab in less than 48 hrs. Rapid responses may be crucial for protecting people traveling in areas with endemic infections, from biological warfare, or rapidly spreading epidemics. The ability of FDCs to productively present Ag to B cells should advance the development of new mAbs including human mAbs. The ability of IL-4 and IL-5 to induce class-switching in vitro allows both IgM & IgG mAbs to be rapidly generated. Moreover, mAb against agents too toxic to inject in vivo may be inducible in vitro. On the other hand, FDC-ICs probably promote autoimmune diseases [61,62].
Ectopic GCs containing autoreactive B cells in association with well-developed auto-Ag-retaining FDC-reticula are frequently found [26]. Once induced, FDC-ICs might sustain autoimmune B cell responses with minimal or no T cell-help. Finally, these implications of Ag-presentation by FDCs are important and we look forward to further exploration.
Acknowledgement
This work was supported by NIH grant AI-17142. Confocal microscopy and transmission electron microscopy was performed at the Virginia Commonwealth University -Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463-02) to the department of Anatomy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dintzis HM, et al. Molecular determinants of immunogenicity: the immunon model of immune response. Proc.Natl.Acad.Sci.U.S.A. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dintzis RZ, et al. Studies on the immunogenicity and tolerogenicity of T-independent antigens. J.Immunol. 1983;131:2196–2203. [PubMed] [Google Scholar]
- 3.Mond JJ, et al. T cell-independent antigens type 2. Annu.Rev.Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. 655–692. [DOI] [PubMed] [Google Scholar]
- 4.Vos Q, et al. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol.Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. 154–170. [DOI] [PubMed] [Google Scholar]
- 5.Goroff DK, et al. Polyclonal activation of the murine immune system by an antibody to IgD. XI. Contribution of membrane IgD cross-linking to the generation of an in vivo polyclonal antibody response. J.Immunol. 1991;146:18–25. [PubMed] [Google Scholar]
- 6.Pecanha LM, et al. Dextran-conjugated anti-Ig antibodies as a model for T cell-independent type 2 antigen-mediated stimulation of Ig secretion in vitro. I. Lymphokine dependence. J.Immunol. 1991;146:833–839. [PubMed] [Google Scholar]
- 7.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu.Rev.Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. 235–270. [DOI] [PubMed] [Google Scholar]
- 8.El Shikh ME, et al. Encyclopedia of Life Sciences. Chichester: John Wiley & Sons, Ltd; 2009. Follicular Dendritic Cells (B Lymphocyte Stimulating) [Google Scholar]
- 9.Tew JG, et al. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol.Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 10.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin.Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szakal AK, et al. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu.Rev.Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- 12.Kranich J, et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J.Exp.Med. 2008;205:1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PR, et al. The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur.J.Immunol. 2002;32:1888–1896. doi: 10.1002/1521-4141(200207)32:7<1883::AID-IMMU1888>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.El Shikh ME, et al. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J.Immunol. 2007;179:4444–4450. doi: 10.4049/jimmunol.179.7.4444. [DOI] [PubMed] [Google Scholar]
- 15.El Shikh ME, et al. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur.J.Immunol. 2006;36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]
- 16.Balogh P, et al. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat.Rec. 2002;268:160–168. doi: 10.1002/ar.10148. [DOI] [PubMed] [Google Scholar]
- 17.El Shikh ME, et al. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J.Immunol. 2009;182:3482–3491. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, et al. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int.Immunol. 2009;21:745–756. doi: 10.1093/intimm/dxp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 20.Thacker TC, et al. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J.Virol. 2009;83:150–158. doi: 10.1128/JVI.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tew JG, et al. Follicular dendritic cells as accessory cells. Immunol.Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 22.Sukumar S, et al. Differential T cell-mediated regulation of CD23 (Fc epsilonRII) in B cells and follicular dendritic cells. J.Immunol. 2006;176:4811–4817. doi: 10.4049/jimmunol.176.8.4811. [DOI] [PubMed] [Google Scholar]
- 23.Schnizlein CT, et al. Follicular dendritic cells in the regulation and maintenance of immune responses. Immunobiology. 1984;168:391–402. doi: 10.1016/S0171-2985(84)80125-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Immune complex-bearing follicular dendritic cells deliver a late antigenic signal that promotes somatic hypermutation. J.Immunol. 2008;180:281–290. doi: 10.4049/jimmunol.180.1.281. [DOI] [PubMed] [Google Scholar]
- 25.Aydar Y, et al. The influence of immune complex-bearing follicular dendritic cells on the IgM response, Ig class switching, and production of high affinity IgG. J.Immunol. 2005;174:5358–5366. doi: 10.4049/jimmunol.174.9.5358. [DOI] [PubMed] [Google Scholar]
- 26.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat.Rev.Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 27.Burton GF, et al. Follicular dendritic cells (FDC) in retroviral infection: host/pathogen perspectives. Immunol.Rev. 1997;156:185–197. doi: 10.1111/j.1600-065x.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 28.Li L, et al. Novel follicular dendritic cell molecule, 8D6, collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line, L3055. Blood. 2004;104:815–821. doi: 10.1182/blood-2004-01-0292. [DOI] [PubMed] [Google Scholar]
- 29.Tew JG, et al. Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol. 2001;22:361–367. doi: 10.1016/s1471-4906(01)01942-1. [DOI] [PubMed] [Google Scholar]
- 30.Sukumar S, et al. Ultrastructural study of highly enriched follicular dendritic cells reveals their morphology and the periodicity of immune complex binding. Cell Tissue Res. 2008;332:89–99. doi: 10.1007/s00441-007-0566-4. [DOI] [PubMed] [Google Scholar]
- 31.Mandel TE, et al. The follicular dendritic cell: long term antigen retention during immunity. Immunol.Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 32.Szakal AK, et al. Isolated follicular dendritic cells: cytochemical antigen localization, Nomarski, SEM, and TEM morphology. J.Immunol. 1985;134:1349–1359. [PubMed] [Google Scholar]
- 33.Aydar Y, et al. FcgammaRII expression on follicular dendritic cells and immunoreceptor tyrosine-based inhibition motif signaling in B cells. Eur.J.Immunol. 2004;34:98–107. doi: 10.1002/eji.200324147. [DOI] [PubMed] [Google Scholar]
- 34.Aydar Y, et al. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J.Immunol. 2003;171:5975–5987. doi: 10.4049/jimmunol.171.11.5975. [DOI] [PubMed] [Google Scholar]
- 35.Qin D, et al. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J.Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 36.Tew JG, et al. In vitro evidence indicating a role for the Fc region of IgG in the mechanism for the long-term maintenance and regulation of antibody levels in vivo. Cell Immunol. 1976;26:141–152. doi: 10.1016/0008-8749(76)90358-0. [DOI] [PubMed] [Google Scholar]
- 37.Lentz VM, Manser T. Cutting edge: germinal centers can be induced in the absence of T cells. J.Immunol. 2001;167:15–20. doi: 10.4049/jimmunol.167.1.15. [DOI] [PubMed] [Google Scholar]
- 38.de Vinuesa CG, et al. Germinal centers without T cells. J.Exp.Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaspal FM, et al. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur.J.Immunol. 2006;36:1665–1673. doi: 10.1002/eji.200535339. [DOI] [PubMed] [Google Scholar]
- 40.Qin D, et al. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J.Immunol. 1998;161:4549–4554. [PubMed] [Google Scholar]
- 41.Huard B, et al. T cell costimulation by the TNF ligand BAFF. J.Immunol. 2001;167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 42.Heijink IH, et al. Interleukin-6 promotes the production of interleukin-4 and interleukin-5 by interleukin-2-dependent and -independent mechanisms in freshly isolated human T cells. Immunology. 2002;107:316–324. doi: 10.1046/j.1365-2567.2002.01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huard B, et al. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int.Immunol. 2004;16:467–475. doi: 10.1093/intimm/dxh043. [DOI] [PubMed] [Google Scholar]
- 44.Kosco MH, et al. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J.Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 45.Reth M, et al. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur.J.Immunol. 1978;8:393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- 46.Decker DJ, et al. Defining subsets of naive and memory B cells based on the ability of their progeny to somatically mutate in vitro. Immunity. 1995;2:195–203. doi: 10.1016/s1074-7613(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 47.Jacob J, et al. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J.Exp.Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furukawa K, et al. Junctional amino acids determine the maturation pathway of an antibody. Immunity. 1999;11:329–338. doi: 10.1016/s1074-7613(00)80108-9. [DOI] [PubMed] [Google Scholar]
- 49.Haberman AM, Shlomchik MJ. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat.Rev.Immunol. 2003;3:757–764. doi: 10.1038/nri1178. [DOI] [PubMed] [Google Scholar]
- 50.Aydar Y, et al. Age-related depression of FDC accessory functions and CD21 ligand-mediated repair of co-stimulation. Eur.J.Immunol. 2002;32:2817–2826. doi: 10.1002/1521-4141(2002010)32:10<2817::AID-IMMU2817>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 51.Kopf M, et al. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J.Exp.Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda K, et al. Expression of the intercellular adhesion molecule-1 on high endothelial venules and on non-lymphoid antigen handling cells: interdigitating cells, antigen transporting cells and follicular dendritic cells. Cell Tissue Res. 1995;279:47–54. doi: 10.1007/BF00300690. [DOI] [PubMed] [Google Scholar]
- 53.Buckley RH. Primary cellular immunodeficiencies. J.Allergy Clin.Immunol. 2002;109:747–757. doi: 10.1067/mai.2002.123617. [DOI] [PubMed] [Google Scholar]
- 54.Grunebaum E, et al. Human T cell immunodeficiency: when signal transduction goes wrong. Immunol.Res. 2006;35:117–126. doi: 10.1385/ir:35:1:117. [DOI] [PubMed] [Google Scholar]
- 55.Cowley S. The biology of HIV infection. Lepr.Rev. 2001;72:212–220. doi: 10.5935/0305-7518.20010028. [DOI] [PubMed] [Google Scholar]
- 56.Fulop T, et al. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 57.Spatz M, et al. Impaired primary immune response in type-1 diabetes. Functional impairment at the level of APCs and T-cells. Cell Immunol. 2003;221:15–26. doi: 10.1016/s0008-8749(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 58.Moser B, et al. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: a comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem.Biophys.Res.Commun. 2003;308:581–585. doi: 10.1016/s0006-291x(03)01389-5. [DOI] [PubMed] [Google Scholar]
- 59.Garcia AM, et al. T cell immunity in neonates. Immunol.Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 60.Velilla PA, et al. Defective antigen-presenting cell function in human neonates. Clin.Immunol. 2006;121:251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009;30:130–142. doi: 10.1016/j.immuni.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Schroder AE, et al. Antigen-dependent B cell differentiation in the synovial tissue of a patient with reactive arthritis. Mol.Med. 1997;3:260–272. [PMC free article] [PubMed] [Google Scholar]