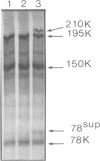
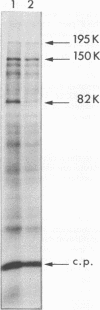
Abstract
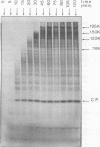
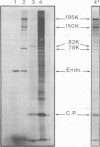
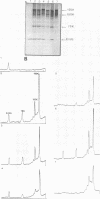
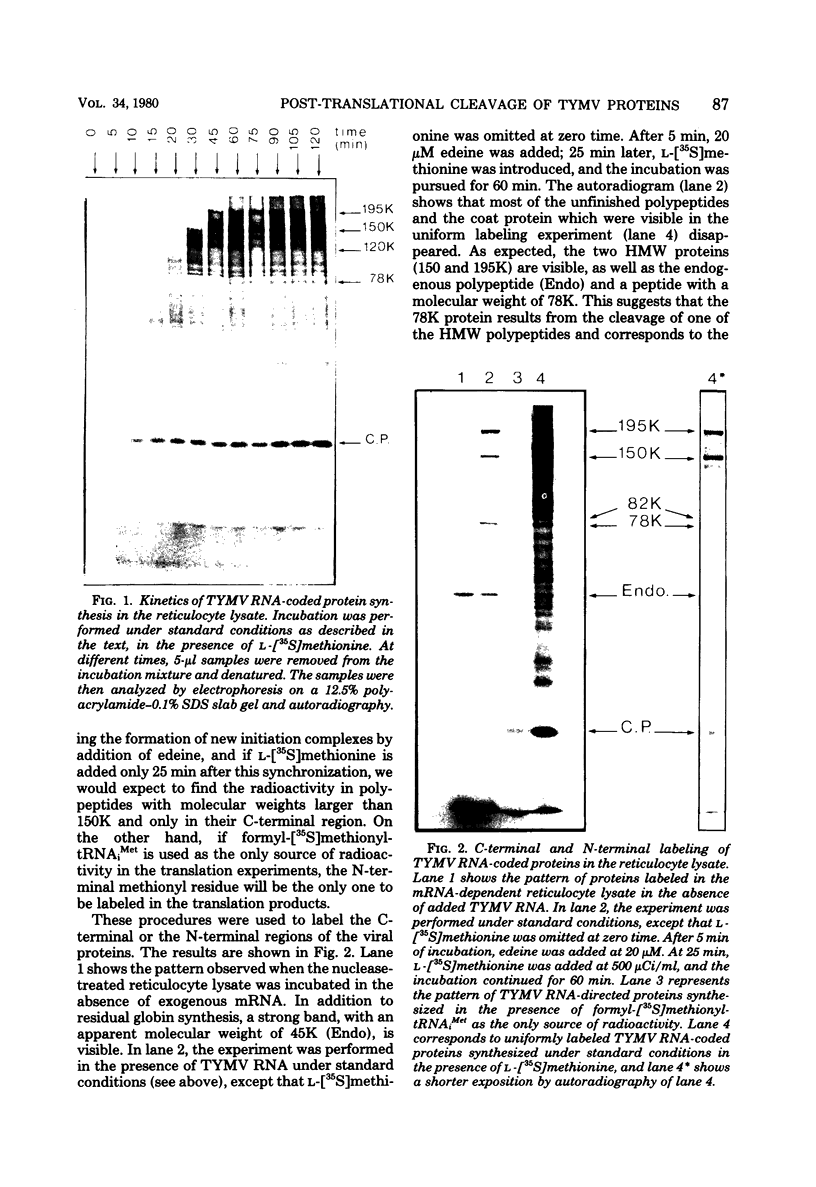
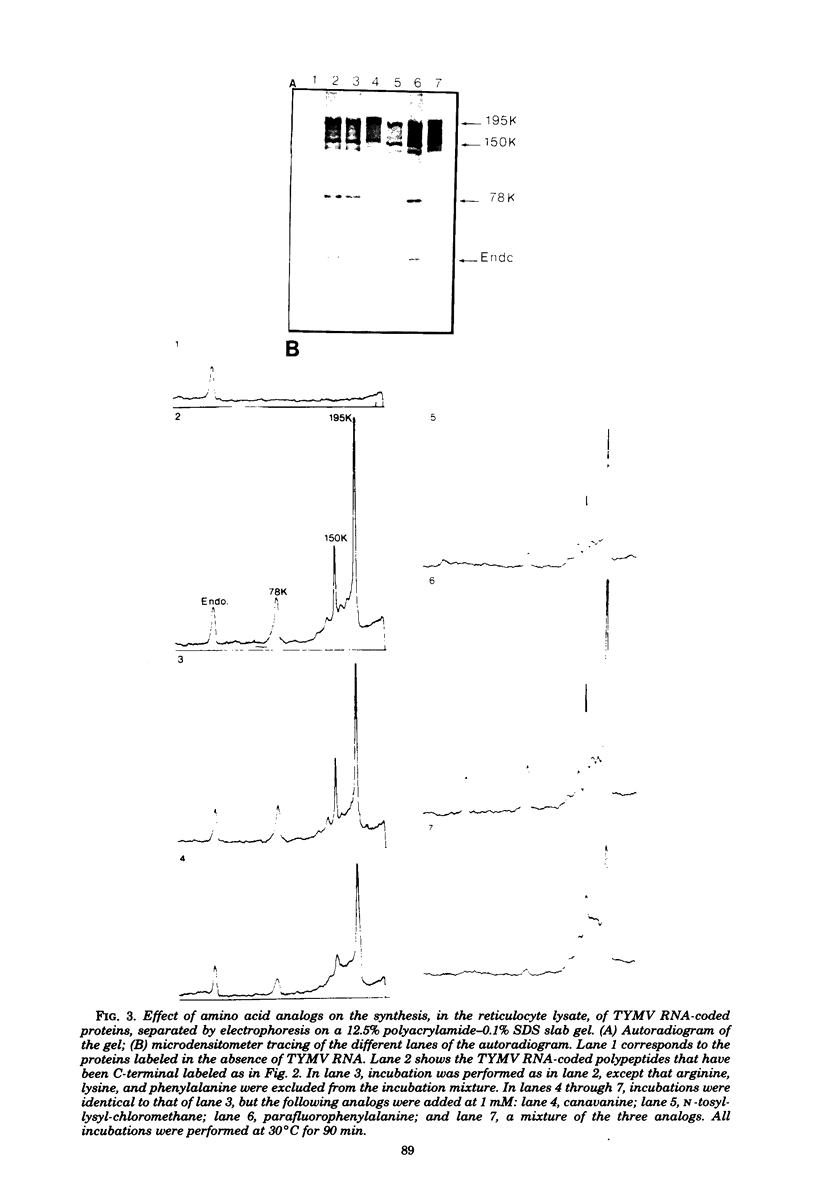
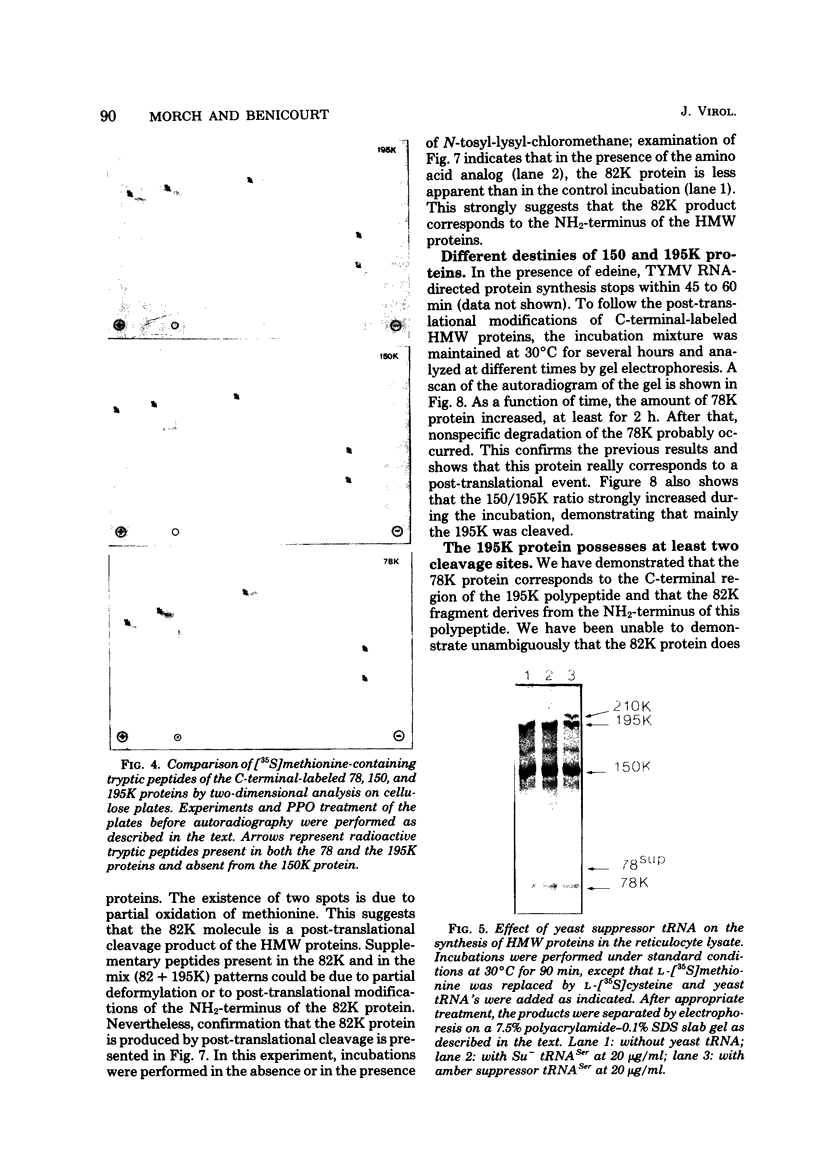
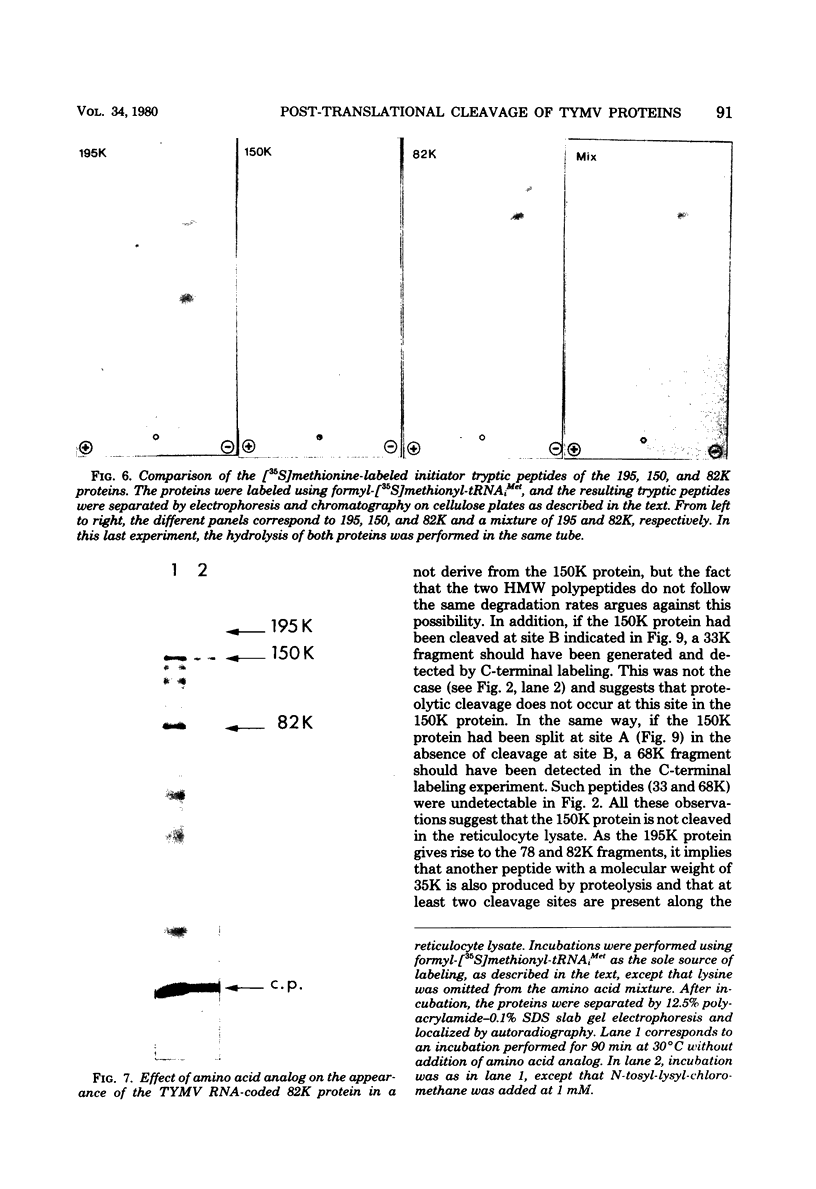
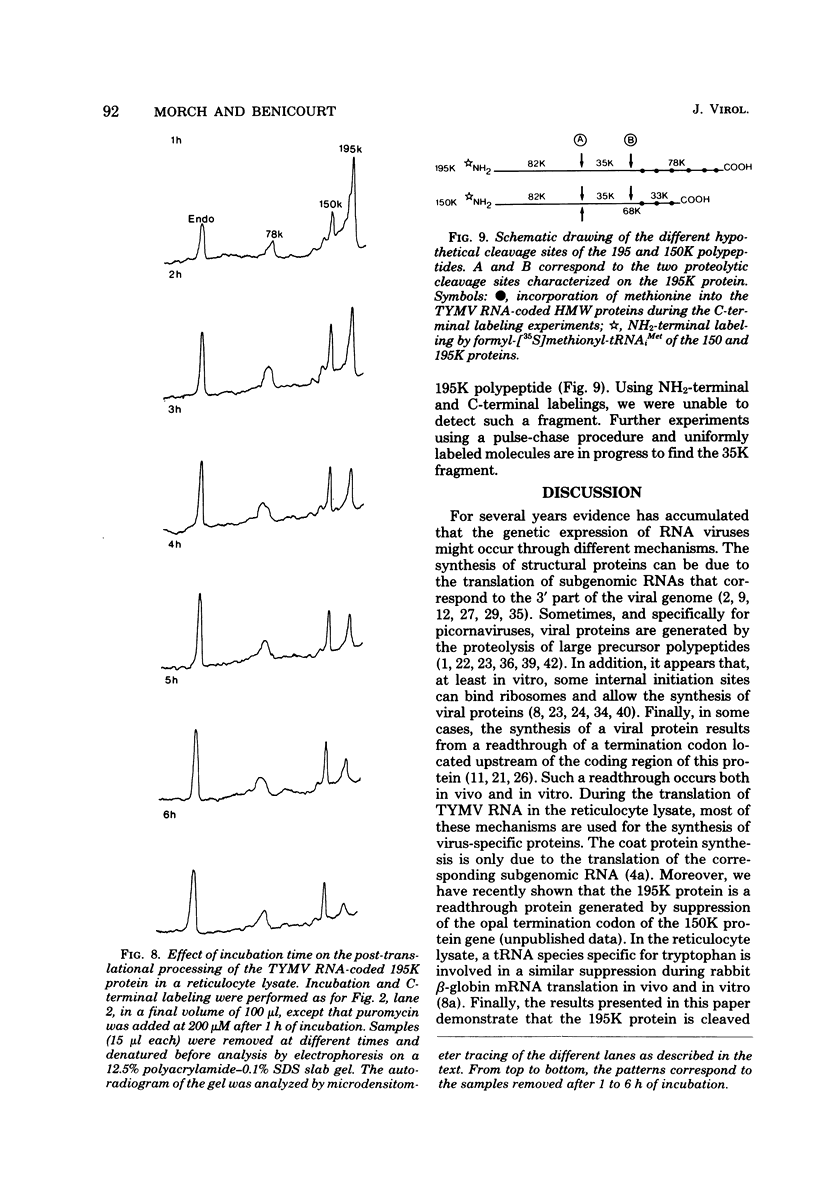
In a reticulocyte lysate, turnip yellow mosaic virus genomic RNA directs the synthesis of two proteins with molecular weights of 150,000 (150K) and 195K. We present evidence that the larger protein is processed in vitro, after its completion, in at least three fragments. The NH2-terminal fragment (82K) and the COOH-terminal fragment (78K) have been well characterized by different methods. The fact that the 150K protein is not cleaved in vitro, although it contains the regions that are processed in the 195K protein, could be of fundamental biological significance for the expression of the viral genes: a single polypeptide chain could be processed in several ways, leading to different peptides with distinct biological activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology. 1977 Aug;81(1):160–169. doi: 10.1016/0042-6822(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Benicourt C., Haenni A. L. Differential translation of turnip yellow mosaic virus mRNAs in vitro. Biochem Biophys Res Commun. 1978 Oct 30;84(4):831–839. doi: 10.1016/0006-291x(78)91659-5. [DOI] [PubMed] [Google Scholar]
- Benicourt C., Haenni A. L. In vitro synthesis of turnip yellow mosaic virus coat protein in a wheat germ cell-free system. J Virol. 1976 Oct;20(1):196–202. doi: 10.1128/jvi.20.1.196-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Bénicourt C., Morch M. D. Organization of the genome of turnip yellow mosaic virus deduced from its messenger properties [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1095–1097. doi: 10.1042/bst0071095a. [DOI] [PubMed] [Google Scholar]
- Bénicourt C., Péré J. P., Haenni A. L. Translation of TYMV RNA into high molecular weight proteins. FEBS Lett. 1978 Feb 15;86(2):268–272. doi: 10.1016/0014-5793(78)80577-8. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Ehrenfeld E. Translation of poliovirus RNA in vitro: detection of two different initiation sites. J Mol Biol. 1975 Nov 15;98(4):761–780. doi: 10.1016/s0022-2836(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Glanville N., Ranki M., Morser J., Käriäinen L., Smith A. E. Initiation of translation directed by 42S and 26S RNAs from Semliki Forest virus in vitro. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3059–3063. doi: 10.1073/pnas.73.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., McCrae M. A., Joklik W. K. Studies on the amino acid sequence content of proteins specified by the gag and pol genes of avian sarcoma virus B77. Virology. 1978 Aug;89(1):272–284. doi: 10.1016/0042-6822(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Monstein H. J., Weissmann C. The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta. 1974 Dec 6;374(2):238–251. doi: 10.1016/0005-2787(74)90366-9. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Fritsch C., Briand J. P., Richards K. E., Jonard G., Hirth L. Physical and functional heterogeneity in TYMV RNA: evidence for the existence of an independent messenger coding for coat protein. Nucleic Acids Res. 1976 Nov;3(11):3043–3061. doi: 10.1093/nar/3.11.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Identification of a viral protein involved in post-translational maturation of the encephalomyocarditis virus capsid precursor. J Virol. 1975 Apr;15(4):918–928. doi: 10.1128/jvi.15.4.918-928.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Matthews R. E. Some properties of TYMV nucleoproteins isolated in cesium chloride density gradients. Virology. 1974 Jul;60(1):54–64. doi: 10.1016/0042-6822(74)90365-1. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of fragmented viral RNA in vitro: initiation at multiple sites. FEBS Lett. 1979 Apr 1;100(1):195–199. doi: 10.1016/0014-5793(79)81162-x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Neeleman A., van Vloten-Doting L., Bosch L. Translation of turnip yellow mosaic virus RNA in vitro: a closed and an open coat protein cistron. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4437–4441. doi: 10.1073/pnas.73.12.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Ricard B., Barreau C., Renaudin H., Mouches C., Bové J. M. Messenger properties of TYMV-RNA. Virology. 1977 Jun 1;79(1):231–235. doi: 10.1016/0042-6822(77)90347-6. [DOI] [PubMed] [Google Scholar]
- Salomon R., Bar-Joseph M., Soreq H., Gozes I., Littauer U. Z. Translation in vitro of carnation mottle virus RNA. Regulatory function of the 3'-region. Virology. 1978 Oct 15;90(2):288–298. doi: 10.1016/0042-6822(78)90313-6. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. The molecular biology of poliovirus. Sci Am. 1975 May;232(5):25–31. [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Complete translation of encephalomyocarditis virus RNA and faithful cleavage of virus-specific proteins in a cell-free system from Krebs-2 cells. FEBS Lett. 1978 Mar 1;87(1):7–11. doi: 10.1016/0014-5793(78)80121-5. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]