Abstract
Objective:
To examine the predictors of antiretroviral adherence among HIV-infected adults, with a particular focus on advancing age, neuropsychological dysfunction, and substance abuse.
Design:
Prospective observational design.
Methods:
Participants were 148 HIV-infected adults between the ages of 25 and 69 years, all on a self-administered antiretroviral regimen. Medication adherence was tracked over a one-month period using an electronic monitoring device (medication event monitoring system caps). All participants completed a comprehensive battery of neuropsychological tests as well as a structured psychiatric interview.
Results:
The mean adherence rate for the entire cohort was 80.7%, with older patients (⩾ 50 years) demonstrating significantly better medication adherence than younger patients (87.5 versus 78.3%). Logistic regression analyses found that older patients were three times more likely to be classified as good adherers (defined as ⩾ 95% adherent). Neurocognitive impairment conferred a 2.5 times greater risk of poor adherence. Among the older patients, those who were classified as poor adherers performed significantly worse on neuropsychological testing, particularly on measures of executive function and psychomotor speed. Current drug abuse/dependence, but not current alcohol abuse/dependence, was also associated with sub-optimal medication adherence.
Conclusion:
Although older age is associated with higher rates of antiretroviral adherence, older participants who were cognitively impaired showed disproportionate difficulty in adequately adhering to their medication regimen. As such, efforts to detect neuropsychological dysfunction, particularly among older patients, and a thorough assessment of substance abuse, appear to be essential for the effective treatment of HIV-infected adults.
Keywords: aging, alcohol abuse, drug abuse, electronic monitoring, medication adherence, neuropsychology
Introduction
The advent of highly active antiretroviral therapy (HAART) has resulted in improved virological, immunological, and clinical outcomes, including reduced mortality rates, in individuals infected with HIV [1]. Meticulous adherence to HAART is necessary, however, to achieve an optimal clinical and virological response [2-4], with some studies suggesting that adherence rates of at least 90–95% are required for optimal viral suppression [4,5]. Sub-optimal adherence to HAART can lead to increased viral replication and the development of drug-resistant HIV strains [6,7], with obvious adverse personal and public health consequences.
A variety of factors may predict medication adherence among HIV-infected adults. Central to the present study is the finding that neurocognitive deficits have been associated with decreased adherence among HIV-positive adults [8-11]. Chesney and colleagues [9] examined self-reported factors influencing adherence among participants in HIV clinical trials, and found that 66% of those surveyed endorsed ‘simply forgot’ as a reason for missing antiretroviral doses. However, that study did not objectively assess cognitive function. Of those studies that have used objective measures of neuropsychological function to quantify cognitive dysfunction, one relied upon patient self-report to index adherence [8] and one employed pill counts [11]. A recent study from our laboratory avoided those methodological confounds by employing standardized measures of neuropsychological function, coupled with the electronic monitoring of medication adherence. Although electronic monitoring devices such as medication event monitoring system (MEMS) caps also have their limitations [12], they have been shown to be more accurate than patient self-report or pill counts, both of which run the risk of overestimating adherence rates [13-16]. We found that deficits in executive function, memory, and attention were associated with poorer adherence to HAART regimens among HIV-positive individuals [10]. Furthermore, we found that cognitively compromised patients on more complex medication regimens showed disproportionate difficulty in achieving adequate adherence.
Two trends in the changing demographics of the HIV pandemic are of particular relevance to medication adherence. First, the number of new cases of HIV infection among middle-aged and older adults is growing [17]. The pronounced improvement in HIV-associated mortality and morbidity is also adding to the ranks of the older HIV-infected population. These trends underscore the need for a better understanding of the impact of aging on medication adherence in HIV/AIDS. Second, substance abuse continues to be a major risk factor for contracting HIV. Intuitively, it would seem that substance abuse might have a deleterious impact on adherence, and recent studies seem to bear this out [18-20].
The current study sought to examine the synergistic effects of older age, cognitive impairment, and substance abuse on medication adherence in HIV. The effective pharmacological treatment of HIV/AIDS involves strict adherence to a demanding and often complex medication regimen, with respect to both the number of pills to be taken per day and the specific dosing instructions (e.g. take with food, with water, on an empty stomach, or in temporal order relative to other prescribed medications). In general, older individuals, particularly those with cognitive impairment, may have greater difficulty in adhering to complex medication regimens [21]. There is evidence suggesting that patients over the age of 50 years who meet diagnostic criteria for AIDS perform significantly worse on neuropsychological testing than do those with less advanced disease [22]. Although in general, older age has been associated with better adherence to HAART [10,23,24], because cognitive status is associated with successful adherence, we predict that maintaining a high level of medication adherence will be particularly difficult for cognitively impaired older HIV infected adults. Similarly, we predict worse medication adherence in HIV-infected substance abusers.
Methods
Research participants
A total of 148 HIV-seropositive adults were enrolled in the present study. Preliminary data based on a subset of these individuals were reported in a previous publication by our group [10]. Participants were recruited using fliers posted in infectious disease clinics at two university-affiliated medical centers and from community agencies in the Los Angeles area specializing in providing service to HIV-infected individuals. HIV infection status was confirmed with enzyme-linked immunosorbent assay and Western blot. Sixty-two per cent of participants met Centers for Disease Control and Prevention diagnostic criteria for AIDS [25]. At the time of testing, all participants were on self-administered HAART. The mean age of the sample was 44.2 years (7.7) with a range of 25–69 years. As suggested by several recent National Institutes of Health-sponsored conferences on aging and AIDS, we adopted the age of 50 years as a cut-off point to define ‘older’ subjects. Using that cut-off point 26% of participants were classified as older. Additional demographic data are presented in Table 1.
Table 1.
Demographic characteristics of participants (N=148).
| Demographic characteristic | Mean | SD |
|---|---|---|
| Age | 44.2 | 7.7 |
| Education | 13.4 | 2.3 |
| Estimated premorbid intelligence | 103.7 | 10.9 |
| Beck Depression Inventory (2nd ed) | 15.0 | 11.0 |
| Beck Anxiety Inventory | 12.0 | 9.9 |
| CD4 cell counta | 391 | 245 |
|
| ||
| % of participants | ||
|
| ||
| Female | 17 | |
| Age 50 years or over | 26 | |
| Ethnicity | ||
| African American | 70 | |
| White | 17 | |
| Hispanic | 9 | |
| Other | 4 | |
| AIDS diagnosis | 62 | |
CD4 cell counts were obtained on 125 participants.
Neuropsychological measures
Participants completed a battery of standardized neuropsychological tests that assessed learning and memory, executive function, psychomotor speed, motor speed, attention, and verbal fluency (see Table 2) [26-32]. Test scores were converted to demographically corrected T scores with a mean of 50 and a standard deviation of 10, using published normative data. Deficit scores were then calculated using an algorithm developed by Heaton and colleagues [26] that assigns an impairment rating to T scores as follows: T > 39 = 0; 39 ⩾ T ⩾ 35 = 1; 34 ⩾ T ⩾ 30 = 2; 29 ⩾ T ⩾ 25 = 3; 24 ⩾ T ⩾ 20 = 4; T < 20 = 5. Participants were classified as ‘impaired’ on a particular cognitive domain if the average deficit score for the neuropsychological tests comprising that domain was greater than or equal to 0.5. Subjects were classified as having global neuropsychological impairment if their average deficit score across all tests was greater than or equal to 0.5. In addition to neuropsychological testing, a structured clinical interview composed of the mood, psychotic-spectrum, and substance use modules of the structured clinical interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV [33] was administered to all participants.
Table 2.
Neuropsychological tests by domain and normative data utilized.
| Domain/test | Normative data | |
|---|---|---|
| Speed of information processing | ||
| Symbol digit modalities test | Smith (1982) | [27] |
| Trail making test part A | Heaton et al. (1991) | [26] |
| Learning and memory | ||
| CVLT trials 1–5 | Delis et al. (1987) | [28] |
| CVLT short-delay free recall | Delis et al. (1987) | [28] |
| CVLT long-delay free recall | Delis et al. (1987) | [28] |
| Verbal fluency | ||
| Controlled oral word association test | Selnes et al. (1991) | [29] |
| Boone et al. (1990) | [30] | |
| Attention and working memory | ||
| Paced serial addition test | Stuss et al. (1988) | [31] |
| Executive functioning | ||
| Short category test, booklet format | Wetzel and Boll (1987) | [32] |
| Trail making test part B | Heaton et al. (1991) | [26] |
| Stroop color word test – Interference | Selnes et al. (1991) | [29] |
| Boone et al. (1990) | [30] | |
| Motor functioning | ||
| Grooved pegboard dominant hand | Heaton et al. (1991) | [26] |
| Grooved pegboard non-dominant hand | Heaton et al. (1991) | [26] |
CVLT, California verbal learning test.
Measurement of adherence
Medication adherence was determined using MEMS caps to measure HAART adherence over a 4-week period. MEMS caps employ a pressure-activated microprocessor in the medication bottle cap that automatically records the date, time, and duration of bottle opening. MEMS cap data was later retrieved from the cap using a specially designed communication module connected to a PC serial port. For the majority of subjects (61%), MEMS caps were employed to track adherence to protease inhibitors. For those participants on a protease-sparing regimen, MEMS caps were used to track adherence to another antiretroviral medication (e.g. nucleoside reverse transcriptase inhibitors or nonnucleoside reverse transcriptase inhibitors). Participants who took at least 95% of their prescribed doses were classified as good adherers. Participants who took less than 95% were classified as poor adherers.
Procedure
After providing written informed consent, participants were administered neuropsychological tests and the psychiatric interview. Trained psychometrists, supervised by a board-certified neuropsychologist (C.H.H.), conducted all neuropsychological testing. Psychiatric interviewing was conducted by psychology fellows under the supervision of a licensed clinical psychologist with expertise in diagnostic interviewing (S.A.C.). Participants received instruction in how to use the MEMS caps and were scheduled to return one month later. At the follow-up visit, MEMS caps were collected and data downloaded. Participants received US$80.00 for participating in the study, which was approved by the institutional review boards of both UCLA and the West Los Angeles VA Medical Center.
Statistical analyses
Logistic regression was used to determine the predictive relationship between older age, cognitive dysfunction, and medication adherence. Age was handled as a dichotomous variable, with older age defined as at least 50 years of age. Medication adherence was also dichotomized on the basis of whether participants were able to adhere to at least 95% of prescribed doses. Chi-squared analyses were used to determine the relationship between age and adherence, as well as the association between adherence and specific neuropsychological domains.
Results
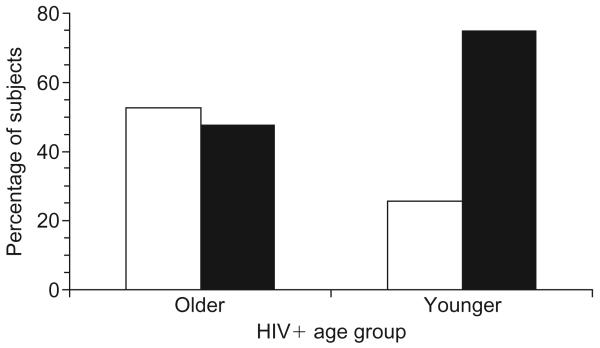
The mean adherence rate for the entire cohort was 80.7%, with older subjects achieving a mean adherence rate of 87.5% and younger subjects achieving a mean adherence rate of 78.3%, a difference that is statistically significant, F(1,146) = 6.3, P = 0.01. Subjects were then classified as either good adherers if they adhered to 95% or better of prescribed doses, or poor adherers if they failed to attain a 95% adherence rate. As can be seen in Fig. 1, 53% of older subjects were classified as good adherers, whereas only 26% of younger subjects were able to attain a 95% adherence rate, a difference that is statistically significant [χ2(148) = 9.5, P = 0.004]. Using a more liberal 90% cut-off point to define good adherence, 71% of older subjects were found to be adherent versus only 37% of younger subjects, a difference which again is statistically significant, [χ2(148) = 13.0, P = 0.001].
Fig. 1. Medication adherence in younger and older HIV-infected adults.
□ Good adherence (percentage adherence > 95%); ■ poor adherence (percentage adherence < 95%).
Logistic regression analysis was performed to determine the predictive relationship between older age, cognitive dysfunction, and medication adherence, with good medication adherence conservatively defined as at least 95% of prescribed doses taken. The results of logistic regression revealed that age (β = 1.12, P = 0.005) and global neuropsychological performance (β = 0.92, P = 0.02) were significantly associated with medication adherence [χ2(2,148) = 14.9, P = 0.0006]. Older individuals were three times more likely to be good adherers than younger subjects [odds ratio (OR) 3.1, 95% confidence interval (CI) 1.40–6.76]. Neuropsychologically compromised subjects were 2.5 times more likely to be poor adherers (OR 2.5, 95% CI 1.19–5.35).
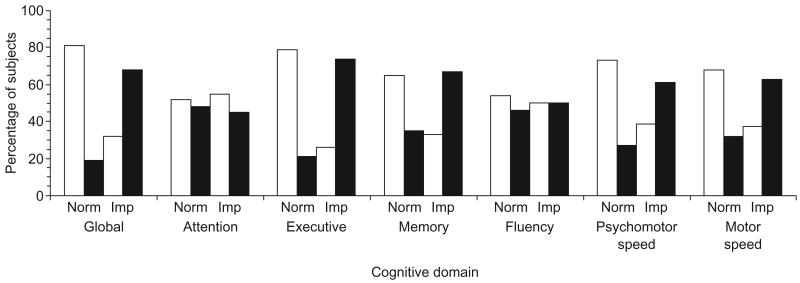
In an effort to determine what differentiated older subjects who were able to adhere adequately to their medication regimen from those who could not, the next analysis examined the association between neuropsychological status and medication adherence rates. As depicted in Fig. 2, 65% of the older subjects who were classified as good adherers scored within the normal range on the composite measure of global cognitive status. In contrast, only 17% of older subjects classified as poor adherers were cognitively normal, with 83% meeting the study criteria for global cognitive impairment. This difference in global neuropsychological impairment as a function of the adherence group is statistically significant [χ2(38) = 7.4, P = 0.009].
Fig. 2. Medication adherence in older HIV-infected adults as a function of cognitive impairment.
□ Good adherence (percentage adherence > 95%); ■ poor adherence (percentage adherence < 95%). Imp, Cognitively impaired; Norm, cognitively normal. Global cognition is a composite of all the individual cognitive domains.
A series of chi-squared analyses were then performed to identify which component cognitive processes might be driving this relationship. Poor adherence was associated with impairment in executive function [χ2 (38) = 10.6, P = 0.001], memory [χ2(38) = 3.7, P = 0.05], and psychomotor slowing [χ2(38) = 4.3, P = 0.04] (see Fig. 2). Of those older subjects with executive dysfunction, 74% were classified as poor adherers. In contrast, only 21% of older subjects who performed normally on tests of executive function were found to be poor adherers. A similar relationship emerged between the psychomotor slowing factor and medication adherence. Among those patients classified as poor adherers, 78% performed within the impaired range on the neuropsychological measures of psychomotor speed. Finally, 67% of older subjects with memory impairment were classified as poor adherers. The remaining cognitive domains were not significantly associated with medication adherence.
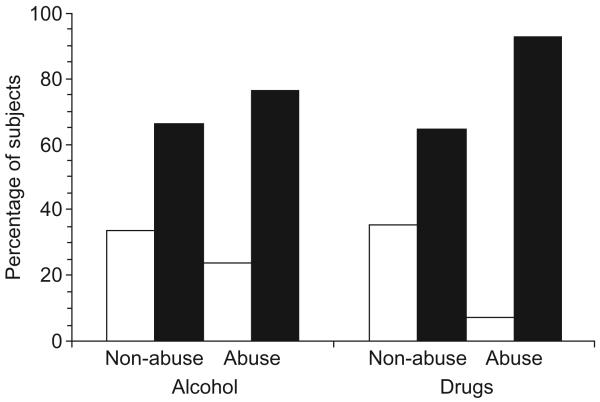
Current drug abuse or dependence was also associated with poor medication adherence. As can be seen in Fig. 3, 13 of the 14 subjects (93%) who met DSM-IV diagnostic criteria for current drug abuse or dependence were classified as poor adherers, whereas only 65% of the non-drug abusing subjects were poor adherers, a difference that is statistically significant [χ2(1,144) = 4.6, P = 0.04]. Cocaine was the most common drug of abuse, with nine out of 14 (64%) meeting diagnostic criteria for cocaine abuse or dependence. Although 77% of subjects who met DSM-IV diagnostic criteria for alcohol abuse/dependence were poor adherers, current alcohol abusers were not statistically more likely to be poor adherers than were subjects who did not meet diagnostic criteria for current alcohol abuse/dependence [χ2(1,144) = 0.73, P = 0.58]. Because of a low base rate of substance abuse among older subjects in this sample, we were unable to determine whether the concomitant presence of both older age and drug/alcohol abuse resulted in disproportionate difficulties with medication adherence.
Fig. 3. Medication adherence among HIV-infected adults who meet Diagnostic and Statistical Manual of Mental Disorders IV diagnostic criteria for current drug abuse/dependence or current alcohol abuse/dependence.
□ Good adherence (percentage adherence > 95%); ■ poor adherence (percentage adherence < 95%).
Discussion
This study, which combined objective measures of neuropsychological function with an objective measure of medication adherence (MEMS caps), found that older age is associated with significantly better medication adherence. HIV-infected adults aged 50 years or over were three times more likely to achieve a 95% adherence rate than were younger subjects. Among the older HIV-infected participants, poor adherers were significantly more likely to demonstrate neuropsychological compromise. Finer-grained analyses of the neuropsychological data revealed that deficits in executive function memory and psychomotor speed account for this association.
Although we have conceptualized cognitive dysfunction as causing poor adherence, it is equally plausible that poor adherence results in a number of untoward clinical outcomes, including neuropsychological impairment. Given the lack of longitudinal follow-up in the present study, we cannot presently disentangle the causative pathway between these two factors. In all likelihood, a bi-directional relationship exists, with cognitive impairment adversely affecting patients' ability to adhere to their medication regimen, which in turn results in further disease progression and a worsening of cognitive function.
Current drug abuse was also associated with poor medication adherence. Indeed, 13 of the 14 HIV-positive participants who met DSM-IV diagnostic criteria for drug abuse or dependence were classified as poor adherers. Although these data underscore the deleterious effects of active drug abuse on medication adherence, the mechanism by which this occurs is unclear. One possibility is that drug abuse potentiates HIV-associated neuropsychological compromise, which then results in difficulties with adherence. Another explanation would argue that the often chaotic lifestyle associated with illicit drug abuse is antithetical to the structure necessary to adhere to a complex medication regimen. We are currently engaged in a larger-scale study focused on the interplay of drug abuse, neuropsychological dysfunction, and medication adherence; research that should help shed light on this increasingly important topic.
The use of objective techniques to track medication adherence and to assess neurocognitive status represents a significant methodological improvement over past studies in this research domain. It is well accepted that self-report techniques, particularly when used to assess ‘socially undesirable’ behaviors such as failing to adhere to physicians' instructions, lack precision, and frequently underestimate the behavior in question [13,34]. The lack of concordance between self-reported cognitive status and objective indices of neuropsychological performance is also well established [35,36]. As such, studies that have relied either on patients' descriptions of their cognitive status or patients' self-report of their medication adherence rates are possibly weakened by this confound. Electronic monitoring technology, however, is not without its critics. First, MEMS caps simply record pill bottle opening and not the actual ingestion of medication. Second, it has been argued that MEMS caps may underestimate actual adherence rates, particularly among patients who remove an additional dose from their pill bottle with the intent of taking it at a later time. Termed ‘pocket dosing’, such behavior can result in the misleading appearance of poor adherence. To minimize the impact of this, in the present study we explicitly discouraged participants from removing extra doses from their pill bottle. Nonetheless, it is possible that these data underestimate actual adherence rates.
The use of the age of 50 years to define older age is admittedly arbitrary. Most of the aging literature employs a lower bound of 65 years, and often even older, to define older age. The decision to adopt this cut-off point was based on several National Institutes of Health-sponsored working groups that offered this as a compromise position, in large part driven by the logistical difficulty in assembling a sufficiently large cohort of HIV-infected adults in their 60s and 70s. It is recognized, however, that age-related declines in cognition do not typically start among individuals in their 50s, although our group has reported preliminary data to suggest that the deleterious effects of age may be detectable among patients in their 50s with more advanced disease (i.e. AIDS). Another viable criticism could stem from the decision to use a 95% threshold to define good adherence. Whereas several widely cited papers have found that such high adherence rates are necessary to achieve optimal viral suppression [4], and slow the rate at which drug-resistant strains of the virus emerge [6], others have failed to find differences in disease progression between patients who only adhere to 70-90% of doses compared with those who attain an adherence rate of at least 90% of prescribed doses [13,37].
These questions aside, it does appear that as a group HIV-infected individuals over the age of 50 years are better able to adhere to their HAART regimens than are younger adults. Further research is required to determine the decade at which the beneficial impact of older age on adherence begins to attenuate. It is also evident that neuropsychological dysfunction and substance abuse are associated with greater difficulty with medication adherence. Indeed, cognitively impaired older subjects demonstrated disproportionate difficulty with adherence. Given the ‘graying’ of the HIV-infected populace, further research targeting this critical line of research appears to be warranted.
Acknowledgements
The authors would like to thank Robert Schug and Sara Chovan for research assistance.
Sponsorship: This study was supported by a grant from the National Institute of Mental Health (RO1 MH58552) to C.H.H., with supplemental funds provided by the National Institute on Drug Abuse.
References
- 1.Ory MG, Mack KA. Middle-aged and older people with AIDS. Res Aging. 1998;20:653–664. [Google Scholar]
- 2.Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society - USA Panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 3.Durant J, Clevenbergh P, Halfon P, Delgiudice P, Porsin S, Simonet P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353:2195–2199. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Swindells S, Mohr J, Brester M, Vergis R, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Hecht FM, Charlebois ED, Zalopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 6.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquired Immune Defic Syndr. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wainberg MA, Friedland G. Public health implications of anti-retroviral therapy and HIV drug resistance. JAMA. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 8.Albert SM, Weber CM, Todak G, Polanco C, Clouse R, McElhiney M, et al. An observed performance test of medication management ability in HIV: relation to neuropsychological status and medication adherence outcomes. AIDS Behav. 1999;3:121–128. [Google Scholar]
- 9.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Swickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 10.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Adherence to antiretroviral medication among HIV-infected adults: effects of cognitive dysfunction, regimen complexity, and age. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol. 2001;23:149–163. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- 12.Burke LE. Electronic measurement. In: Burke LE, Okene IS, editors. Compliance in healthcare research. Futura Publishing Co. Inc.; Armonk, NY: 2001. pp. 117–138. [Google Scholar]
- 13.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Burney KD, Krishnan K, Ruffin MT, Zhang D, Brenner DE. Adherence to single daily dose of aspirin in a chemoprevention trial. An evaluation of self-report and microelectronic monitoring. Arch Fam Med. 1996;5:297–300. doi: 10.1001/archfami.5.5.297. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien MK, Petrie K, Raeburn J. Adherence to medication regimens: updating a complex medical issue. Med Care Rev. 1992;49:435–454. doi: 10.1177/002570879204900403. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? JAMA. 1993;269:2779–2781. [PubMed] [Google Scholar]
- 17.Levy JA. HIV/AIDS and injecting drug use in later life. Res Aging. 1998;20:776–797. [Google Scholar]
- 18.Catz SL, Heckman TG, Kochman A, DiMarco M. Rates and correlates of HIV treatment adherence among late middle-aged and older adults living with HIV disease. Psychol, Health Med. 2001;6:47–58. [Google Scholar]
- 19.Kresina TF, Flexner CW, Sinclair J, Correia MA, Stapleton JT, Adeniyi-Jones S, et al. Alcohol use and HIV pharmacotherapy. AIDS Res Hum Retroviruses. 2002;18:757–770. doi: 10.1089/08892220260139495. [DOI] [PubMed] [Google Scholar]
- 20.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC, Weissman S. The survival experience of older and younger adults with AIDS. Res Aging. 1998;20:665–685. [Google Scholar]
- 22.Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore LH. Age differences and neurocognitive performance in HIV-infected adults. NZ J Psychol. 1999;28:94–101. [Google Scholar]
- 23.Becker SL, Dezil CM, Burtcel B, Kawabata H, Hodder S. Young HIV-infected adults are at greater risk for medication nonadherence. MedGenMed. 2002;4(3) World wide web address: www.medscape.com/viewarticle/438510. [Accessed 15 October 2003] [PubMed]
- 24.Wutoh AK, Brown CM, Kumoji EK, Daftary MS, Jones T, Barnes NA, et al. Antiretroviral adherence and use of alternative therapies among older HIV-infected adults. J Natl Med Assoc. 2001;93:243–250. [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Revised classification system for HIV infection and expanded surveilance case definition for AIDS among adolescents and adults. MMWR. 1993;41:1–10. [PubMed] [Google Scholar]
- 26.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- 27.Smith A. Symbol Digit Modalities Test (SDMT) Manual (Revised) Western Psychological Association; Los Angeles, CA: 1982. [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. The Psychological Corporation; New York, NY: 1987. [Google Scholar]
- 29.Selnes OA, Jacobson L, Machado AM, Becker JT. Normative data for a brief neuropsychological screening battery. Percept Motor Skills. 1991;73:539–550. doi: 10.2466/pms.1991.73.2.539. [DOI] [PubMed] [Google Scholar]
- 30.Boone KB, Miller BL, Lesser IM, Hill E. Performance on frontal lobe tests in healthy, older individuals. Dev Neuropsychol. 1990;6:215–223. [Google Scholar]
- 31.Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. Clin Neuropsychologist. 1988;2:246–250. [Google Scholar]
- 32.Wetzel L, Boll TJ. Short Category Test, booklet format. Western Psychological Services; Los Angeles, CA: 1987. [Google Scholar]
- 33.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 34.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinkin CH, van Gorp WG, Satz P, Marcotte T, Durvasula RS, Wood S, et al. Actual versus self-reported cognitive dysfunction in HIV-1 infection: memory-metamemory dissociations. J Clin Exp Neuropsychol. 1996;18:431–443. doi: 10.1080/01688639608408999. [DOI] [PubMed] [Google Scholar]
- 36.Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. J Clin Exp Neuropsychol. 1999;21:737–756. doi: 10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- 37.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Robertson M, Zalopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]