Abstract
Little is known about how metastatic cancer cells arrest in small capillaries and traverse the vascular wall during extravasation in vivo. Using real-time intravital imaging of human tumor cells transplanted into transparent zebrafish, we show here that extravasation of cancer cells is a highly dynamic process that involves the modulation of tumor cell adhesion to the endothelium and intravascular cell migration along the luminal surface of the vascular wall. Tumor cells do not damage or induce vascular leak at the site of extravasation, but rather induce local vessel remodeling characterized by clustering of endothelial cells and cell-cell junctions. Intravascular locomotion of tumor cells is independent of the direction of blood flow and requires β1-integrin-mediated adhesion to the blood-vessel wall. Interestingly, the expression of the pro-metastatic gene Twist in tumor cells increases their intravascular migration and extravasation through the vessel wall. However, in this case, Twist expression causes the tumor cells to switch to a β1-integrin-independent mode of extravasation that is associated with the formation of large dynamic rounded membrane protrusions. Our results demonstrate that extravasation of tumor cells is a highly dynamic process influenced by metastatic genes that target adhesion and intravascular migration of tumor cells, and induce endothelial remodeling.
Keywords: Cancer metastasis, Extravasation, Zebrafish
Introduction
For a tumor cell to disseminate in the body it must perform several important steps, including invasion of surrounding tissues, intravasation into the blood vessel, survival in the circulation, extravasation from the blood stream, and proliferation at a secondary site in a foreign environment (Nguyen et al., 2009). The recent development of fluorescent protein techniques and intravital imaging methods has significantly expanded our understanding of this dynamic process in vivo (Sahai, 2007; Kienast et al., 2010). The majority of these studies have focused on the initial steps of metastasis (tumor cell invasion and intravasation) because they are more amenable to high-resolution in vivo imaging using existing animal models. Consequently, late steps of the metastatic cascade, such as tumor cell extravasation, are not fully appreciated and poorly understood. In fact, most of the data is inferred from low-resolution in vivo studies or endpoint assays that indirectly monitor tumor cell extravasation by quantifying secondary tumor formation in mouse or chick CAM models, days or weeks after tumor cell inoculation into the circulation (Luzzi et al., 1998; Podsypanina et al., 2008; Sahai, 2007; Townson and Chambers, 2006). However, given that the circulation is a hostile environment, it seems plausible that successful metastatic cells would rapidly exit the vessel within a few hours or days to gain access to ECM-rich tissues and other survival factors. This suggests that circulating tumor cells that can efficiently exit the blood system might have a distinct advantage during the metastatic cascade of events (Bos et al., 2009; Gupta et al., 2007).
Several studies have attempted to visualize cellular mechanisms of tumor cell extravasation in vivo using mouse or chick CAM human tumor xenograft assays. These studies led to the conclusion that tumor cell extravasation is a complex process that might involve passive or active tumor cell movement within the vessel lumen, adhesion of tumor cells to the vascular wall and transendothelial passage of tumor cells using yet unknown mechanisms (Colmone et al., 2008; Kienast et al., 2010; Luzzi et al., 1998; Sipkins et al., 2005; Voura et al., 2004; Yamauchi et al., 2006). The extravasation kinetics were found to vary significantly depending on the cancer cell type used and extravasation site (organ) studied, suggesting that it might be regulated not only by the metastatic potential of the cell, but also by the unique characteristics of the local endothelium (Colmone et al., 2008; Schluter et al., 2006; Kienast et al., 2010). Indeed, leukemic cells were found to home preferably to SDF-1-positive areas within the bone marrow endothelium before their extravasation (Sipkins et al., 2005). It has also been postulated that tumor cells could affect the local structure and viability of the vessel endothelium at the site of extravasation; however, the exact nature of these events remains unknown (Heyder et al., 2006; Weis et al., 2004). All together, these studies suggest that extravasation of tumor cells involves a dynamic interplay between the invading tumor cell and the vessel endothelium. However, the inability to observe these dynamic cell interactions in high-resolution detail in real time has hindered our understanding of how tumor cells modulate the endothelium and physically penetrate the vascular wall during extravasation.
Here, we investigate in high-resolution detail the dynamic behavior of metastatic human cancer cells undergoing extravasation using intravital confocal microscopy and optically transparent Tg(fli1:egfp) transgenic zebrafish that uniformly express GFP throughout their vasculature. This line has been extensively used to image tumor-induced and developmental angiogenesis, and is uniquely suited for high-resolution multicolor confocal imaging of the interface between tumor cells and the blood vessel wall (Isogai et al., 2003; Stoletov et al., 2007; Stoletov and Klemke, 2008). In addition, the zebrafish embryonic vascular system is fully functional and, unlike the vasculature of adult mammals, is precisely patterned, which allows for efficient detection of extravasating tumor cells and tumor-induced changes in the vascular system over time (Isogai et al., 2003). The Tg(fli1:egfp) embryos are also completely transparent and the immune system is not fully developed, which allows for successful xenotransplantation of human tumor cells for several days (Haldi et al., 2005; Lee et al., 2009; Nicoli et al., 2007; Stoletov et al., 2007). Thus, compared with traditional mouse and chick CAM models of cancer progression, the zebrafish model system is highly amenable to direct observation of interactions between tumor cells and the vasculature at the cell level, and even at subcellular levels, using standard confocal microscopy. Using this model system, we found that extravasation of cancer cells is a dynamic process that involves intravascular migration of tumor cells after arrest in small capillaries, endothelial cell clustering around arrested tumor cells and alterations in endothelial cell-cell junction architecture. Furthermore, we demonstrate that these processes are modulated by tumor cell expression of the prometastatic genes Twist, VEGFA and ITGB1 (which encodes β1 integrin).
Results
Human tumor cell lines have different extravasation abilities
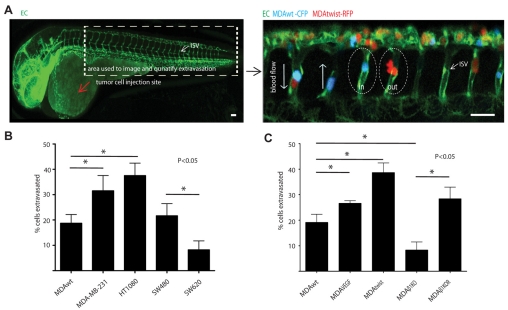
We first determined whether known high (HT1080, MDA-MB-231, SW620) and low (MDA-MB-435 and SW480) metastatic tumor cells showed a difference in the number of cells that can exit the blood stream after direct injection into the circulation (Koop et al., 1996). These cell lines display different metastatic potential in chicken, mice and zebrafish models of cancer metastasis, as displayed by their different abilities to form secondary metastatic colonies (Stoletov et al., 2007; Hewitt et al., 2000; Zijlstra et al., 2002). However, their potential to extravasate has not been previously investigated. After injection into the pericardium, human tumor cells or control 10-15 μm beads were observed to enter the blood circulation 3-5 hours after injection, where they typically lodged in small vessels (5-8 μm) in the head and tail regions (Fig. 1A). We focused our attention on cells that had arrested in the tail intersegmental vessels (ISV), because, as previously shown, these vessels are readily amenable to confocal imaging, their cellular structure is well characterized and they display a highly patterned morphology (2-7 endothelial cells per vessel, ~300 μm in length, 5-14 μm in diameter) (Fig. 1A) (Blum et al., 2008). In this model, beads were observed to lodge in the ISV after injection, but never extravasated (0 out of >100 animals, ~500 beads observed). HT1080 cells displayed the greatest extravasation potential compared with the MDA-MB-231 or MDA-MB-435 cells (Fig. 1B). These findings correlated with their relative metastatic potential as measured in a chick CAM model of metastasis (HT1080>MDA-MB-231>MDA-MB-435) (Zijlstra et al., 2002). By contrast, the low metastatic human colon adenocarcinoma cell line SW480, which was derived from the primary tumor, showed significantly higher extravasation potential compared with the high metastatic SW620 cell line that was derived from the lymph node of the same cancer patient (Hewitt et al., 2000). It is notable that although SW620 cells show a significantly higher ability to form metastatic sites when injected into mice, they are less invasive and migratory in 2D and 3D in vitro culture systems, which might limit their ability to extravasate (Hewitt et al., 2000; Kubens and Zänker, 1998). The higher metastatic potential of SW620 cells is probably attributable to their higher proliferation rate and/or resistance to apoptosis, which is independent of their invasive and extravasation potentials (Hewitt et al., 2000; Kubens and Zänker, 1998). These attributes could better support SW620 survival in the circulation. Also, these cells might proliferate and form microtumors after arrest in the vascular bed at the secondary site, without the need to extravasate into the surrounding tissue, as has been reported for other cells (Al-Mehdi et al., 2000). In any case, our findings indicate that human tumor cells with different metastatic potential exit the vascular system with different efficiencies and that extravasation in this model is an active process and not a simple passive event.
Fig. 1.
Tumor cell extravasation correlates with metastatic potential. (A) Left panel shows whole-mount fluorescence image of a 2 d.p.f. Tg(fli1:EGFP) zebrafish embryo showing the perivascular tumor cell injection site (red arrow) and the ISV vessels in the tail region where cells typically arrest (box). Right panel is a multi-color confocal image of CFP- or RFP-expressing MDA tumor cells arrested in the ISV of Tg(fli1:EGFP) embryos and either inside or outside (extravasated) the vessel lumen. Image was taken 24 hours post injection (h.p.i.) with a 20× objective. (B) The percentage of extravasated tumor cells quantified 24 h.p.i. for several human tumor cell lines: MDA-MB-435 (MDAwt) MDA-MB-231, HT1080, SW480 and SW620. (C) The percentage of extravasated tumor cells quantified 24 h.p.i. for variants of MDA-MB-435 cell line used in the manuscript: MDAwt (wild type); MDA-MB-435 cells expressing the metastatic genes VEGFA (MDAVEGF) or Twist (MDAtwist). In addition, extravasation was measured for MDAwt cells depleted of the metastatic gene ITGB1 (MDAβ1KO) or these cells reconstituted with ITGB1 (MDAβ1KOR). Results are means ± s.e.m. Scale bars: 200 μm.
β1 integrin, twist and VEGF modulate tumor cell extravasation
Although numerous genes have been linked to the metastatic process, their role in extravasation is poorly understood. Therefore, we determined whether the expression of the metastatic genes Twist, ITGB1 and VEGFA could directly alter the ability of MDA cells to extravasate. Increased expression of these genes occurs in various human cancers and has been previously associated with increased metastasis and poor patient survival (Crawford and Ferrara, 2008; Matsumoto and Claesson-Welsh, 2001; Morozevich et al., 2009; Wang et al., 2004; Weis et al., 2004; Yang et al., 2004). Additionally, the integrin β1 subunit is critically involved in tumor cell pulmonary arrest in the mouse human tumor xenograft model (Wang et al., 2004). MDA cells were engineered to overexpress Twist (MDAtwist) or the pro-angiogenic growth factor VEGFA (MDAVEGF). To examine the role of β1 integrins, we used a standard shRNA knockdown reconstitution strategy. Overexpression of Twist and VEGFA significantly increased the percentage of extravasated tumor cells, whereas, silencing of ITGB1 gene expression (MDAβ1KO) potently reduced this process compared with levels in mock-transfected control cells (MDAwt, Fig. 1C). As expected, reconstitution of MDAβ1KO cells with β1 integrin (MDAβ1KOR) restored their ability to extravasate (Fig. 1C). Importantly, all cells displayed similar proliferation rates in zebrafish over the short 24 hour period of observation, indicating that the changes in extravasation were not due to changes in cell number (Al-Mehdi et al., 2000). These findings indicate that there is a correlation between the ability of cells to extravasate and the expression of specific genes (Twist, VEGFA and ITGB1) that contribute to metastasis of cancer cells.
Intravital imaging of tumor cell behavior after arrest and during extravasation
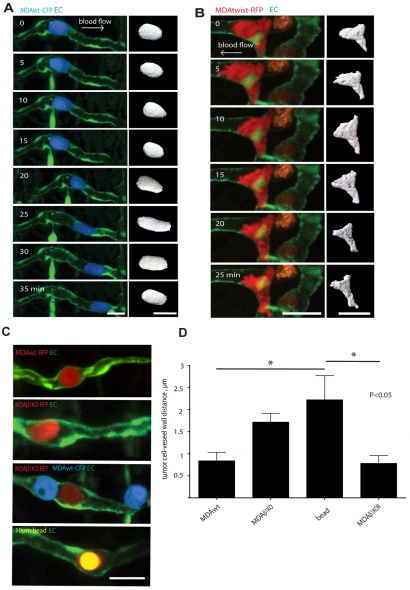
To gain insight into how tumor cells interact with endothelial cells and exit through the vascular wall, we imaged the behavior of MDA tumor cells after arrest in the vasculature and during extravasation through the vessel wall. We observed that circulating beads and tumor cells almost always lodged next to endothelial nuclei that project into the vessel lumen of the small intersegmental vessels, which can be seen as bright areas of strong nuclear EGFP expression (Isogai et al., 2003; Blum et al., 2008). Based on these observations, we conclude that the initial arrest in the ISVs is due to size restriction and not due to the specific adhesive mechanisms between the tumor cell and the endothelium. Strikingly, we found that vessel-arrested MDA cells did not always remain passively lodged in ISVs as did Sepharose beads, but rather rapidly migrated along the surface of the ISV lumen (24%, 16 out 65 cells were migratory, maximum velocity 2.01 μm/minute, mean migration velocity 1.26±0.56 μm/minute) (Fig. 2A, supplementary material Movies 1 and 2). In many cases, these migratory cells were observed to move against blood flow using a rounded morphology, compressing and flattening endothelial nuclei as they navigated the narrow contours of the vessel wall (Fig. 2B, supplementary material Movies 3 and 4). These findings demonstrate that a subset of arrested tumor cells migrate within the vessel lumen, apparently by generating forces that allow movement against blood flow and through the narrow vessel confines.
Fig. 2.
Tumor cells arrest in small vessels then actively migrate along the luminal surface of the vascular endothelium. (A) MDAwt cell expressing CFP and migrating within the ISV lumen for the indicated times (supplementary material Movie 1). Right panel shows 3D isosurface rendering of the tumor cell body for each time point (supplementary material Movie 2). (B) MDAtwist cell expressing RFP and migrating in the lumen of an ISV for the indicated times (supplementary material Movie 4). Right panel shows 3D isosurface rendering of the tumor cell body for each time point (supplementary material Movie 5). (C) Multi-color confocal images of MDAwt and MDAβ1KO cells labeled with CFP or RFP that have arrested in the ISV lumen. Lower panel shows a control 10 μm fluorescent Sepharose bead (yellow) arrested in the ISV. (D) Quantification of distance from tumor cell to vessel wall for MDAwt, MDAβ1KO cells, and Sepharose beads as shown in representative Fig. 2C above. Results are means ± s.e.m. Scale bars: 20 μm.
The ability of tumor cells to undergo intravascular migration after arrest could be an important mechanism adopted by metastatic cells to survey the endothelium for a suitable site of extravasation. Many metastatic genes including ITGB1, Twist and VEGFA increase the migration of cancer cells (Matsumoto and Claesson-Welsh, 2001; Morozevich et al., 2009; Yang et al., 2004). However, although it is generally believed that increased migration contributes to cancer cell dissemination, active intravascular migration after vessel arrest has not been previously investigated. Therefore, we determined whether intravascular migration of MDA cells would be altered by metastatic gene expression. Indeed expression of Twist in MDA cells increased both tumor cell extravasation (Fig. 1C) and intravascular migration (47%, 26 of 57 cells; Fig. 2B, supplementary material Movies 4 and 5) compared with control cells (24%). Interestingly, although control and MDAtwist cells migrated at the same speed (1.26±0.56 and 1.18±0.43 μm/minute, respectively), MDAtwist cells displayed more membrane protrusions (Fig. 2B, supplementary material Movies 4 and 5). This was associated with their ability to rapidly adapt their shape to the narrow contours of the inner luminal surface and to move through small vessel branch points (2-5 μm) (Fig. 2B, supplementary material Movies 4 and 5) (Sahai, 2007). By contrast, arrested MDAβ1KO cells and Sepharose beads were not protrusive, remained completely immotile in the vessel lumen (0 motile MDAβ1KO cells out of 9 imaged; Fig. 2C, supplementary material Movies 6 and 7) and did not extravasate efficiently. High-magnification imaging of arrested tumor cells revealed that MDAβ1KO cells and Sepharose beads were not as closely associated with the luminal surface of the vessel wall as were MDAwt or MDAβ1KOR cells, suggesting that they do not readily adhere to the vessel surface in the absence of β1 integrins (Fig. 2C,D). These findings demonstrate that some tumor cells actively migrate in the vessel lumen after arrest and this process can be modulated by expression of ITGB1 and Twist. These findings also support the notion that the ability of tumor cells to successfully extravasate is associated with their ability to adhere to the endothelium and migrate along the vascular wall.
Twist induces a switch from an integrin-dependent to an integrin-independent mode of tumor cell extravasation
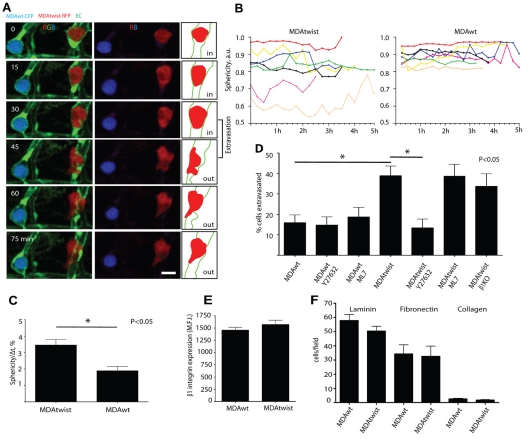
To investigate in more detail the mechanisms of Twist-induced tumor cell extravasation, we co-injected and simultaneously imaged the behavior of MDAtwist (RFP, red) and MDAwt (CFP, blue) cells after their final arrest in the ISVs. MDAtwist cells were observed to rapidly change their shape, and produced large rounded protrusions. By contrast, MDAwt cells remained relatively rounded and displayed less protrusive activity (Fig. 3A-C, supplementary material Movies 8-10). Importantly, Twist-induced extravasation was not dependent on β1 integrins because silencing of ITGB1 expression in MDAtwist cells did not inhibit this process (Fig. 3D). It is notable that exogenous Twist expression in MDAwt cells did not alter cell-surface expression of endogenous β1 integrin, nor did it inhibit their adhesion to ECM proteins indicating that functional β1 integrins are still present on these cells (Fig. 3E,F). These findings suggest that the switch to β1-independent extravasation occurred downstream of the integrin receptor or through modulation of its ligand on the surface of the endothelium. Alternatively, these cells may use a different non-β1-integrin-mediated pathway to extravasate.
Fig. 3.
Twist promotes tumor cell extravasation by increasing tumor cell intravascular migration and membrane-protrusion dynamics dependent on ROCK kinase activity. (A) Comparison of membrane protrusion dynamics of MDAtwist and MDAwt cells. Middle panel, red and blue channels showing individual tumor cell shapes. Right panel shows schematic outlines of the blood vessel and MDAtwist tumor cell. (B) Intravascular shape index (sphericity, round=1.0) for individual MDAtwist and MDAwt cells for the indicated times. Each line in B represents relative change in sphericity over the indicated time period for individual MDAwt or MDAtwist tumor cells. (C) Average speed of shape index change for MDAwt and MDAtwist cells. (D) Effect of ROCK and MLCK inhibition on Twist-induced tumor cell extravasation. (E) β1 integrin expression on MDAwt and MDAtwist cells (MFI, mean fluorescence intensity units) as determined by FACS analysis. (F) Average numbers of MDAwt and MDAtwist cells attached to various extracellular matrixes (3 hour time point) per 40× field. Results are means ± s.e.m. Scale bars, 20 μm.
We also examined the role of β1 integrins in VEGF-mediated extravasation because VEGFR activation can modulate integrin functions including cell adhesion and migration (Matsumoto and Claesson-Welsh, 2001). In this case, silencing of expression of ITGB1 in VEGF-secreting MDA cells significantly reduced VEGF-induced extravasation (from 27±2.1% to 16±2.3%), indicating that this process is β1-integrin dependent. Thus, extravasation of tumor cells might occur in an integrin-dependent or integrin-independent mode depending on which metastatic gene is expressed in these cells.
Protrusion of tumor cell membrane has been shown to be regulated by ROCK kinase and myosin contraction (Wyckoff et al., 2006). Recently, Twist was shown to modulate expression of the metastatic gene RHOC, which is an upstream regulator of ROCK activity (Ma et al., 2007; Narumiya et al., 2009). Additionally, ROCK is strongly involved in regulation of rearrangement and migration of the endothelial cell cytoskeleton (Fischer et al., 2009). To investigate whether ROCK activity is necessary for MDAwt and MDAtwist tumor cell extravasation, we used the well-characterized ROCK kinase inhibitor, Y27632 (Wyckoff et al., 2006). Treatment of animals with Y27632 dramatically decreased MDAtwist-induced extravasation, whereas inhibition of MLCK activity with M7, which also inhibits myosin contractility, independent of ROCK activation, did not have any significant effect (Fig. 3D). Both compounds worked efficiently in our model as demonstrated by their ability to alter the sphericity of MDAtwist tumor cells (supplementary material Fig. S1). Importantly, inhibition of ROCK or MLCK activity did not prevent MDAwt cell extravasation, indicating that ROCK specifically mediates Twist-induced extravasation (Fig. 3D). Together these findings indicate that tumor cells can use different cytoskeletal signaling mechanisms to regulate extravasation in vivo.
Extravasating tumor cells induce clustering of endothelial cells
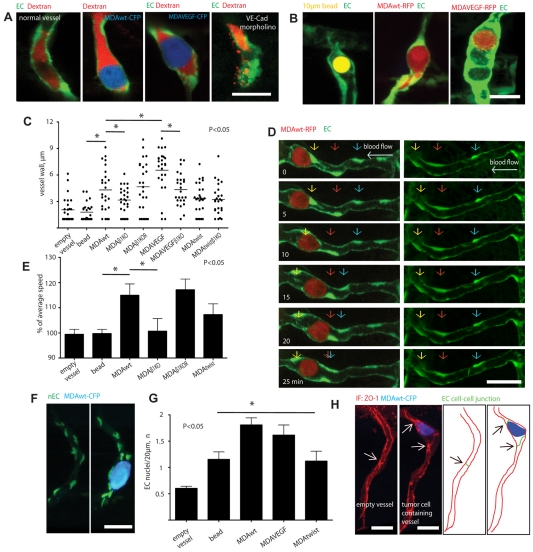
We next investigated how arrested tumor cells alter endothelial cell behavior because this process has not been examined in vivo. Previous in vitro work using cultured endothelial cells suggests that the vessel endothelium presents a non-dynamic and static barrier to arrested tumor cells and that tumor cells induce endothelial cell death and vascular leakage during their extravasation (Heyder et al., 2006; Weis et al., 2004). However, during the course of numerous time-analyses of arrested tumor cells, we never observed loss of endothelial cells at sites of extravasation, nor did we detect vascular leak in animals injected with Dextran-Rhodamine (Fig. 4A-B). Importantly, ISVs are not uniquely resistant to vascular leak because morpholino knockdown of VE-cadherin, which is necessary for the maintenance of the endothelial cell-cell junction integrity, induced a strong vascular leak, which corroborates previous findings (Fig. 4A) (Montero-Balaguer et al., 2009). Instead, time-lapse imaging revealed an increase in the movement and clustering of endothelial cell nuclei around arrested tumor cells. These vessels also showed a thickening of the vascular wall surrounding arrested tumor cells (Fig. 4A-E, supplementary material Movie 3). By contrast, the arrest of 10-15 μm Sepharose beads did not induce these responses in the ISVs (Fig. 4B,C,E; supplementary material Movie 7).
Fig. 4.
Tumor cells induce vessel remodeling in response to metastatic gene expression, but do not induce vascular leakage. (A) Single optical sections of MDAwt or MDAVEGF cells expressing CFP and arrested in ISVs with or without reduced VE-cadherin expression (VE-Cad morpholino), which serve as a positive control for vascular leakage (Montero-Balaguer et al., 2009). Vessel-wall integrity and leakage around arrested tumor cells (blue) was assessed by injection of Rhodamine-Dextran (MW=2×106, red) into the circulation. (B) Representative images of vascular wall changes of ISVs with an arrested Sepharose bead (left), MDAwt cell (center) and MDAVEGF cell (right). (C) The thickness of the vessel wall was measured around arrested Sepharose beads or MDA cells expressing the indicated genes. (D) Left panel, representative image of MDAwt-RFP cell-induced movement of endothelial cell nuclei at the indicated times (supplementary material Movie 3). Right panel shows movement of endothelial cell nuclei in a normal vessel without an arrested tumor cell. Colored arrows indicate individual nuclei positions at the indicated times. (E) Nuclei speed was measured in ISVs with arrested Sepharose beads or MDA cells expressing the indicated genes. Each bar represents increase or decrease (percentage) above the average endothelial nuclei movement speed for a particular condition. (F) Representative image of endothelial cell nuclei clustering around a MDAwt cell expressing CFP (blue) and arrested in the ISV of a Tg(fli1:nEGFP) embryo in which GFP is expressed exclusively in the nucleus. (G) Quantification of endothelial cell nuclei clustering around ISV-arrested Sepharose beads or MDA cells expressing the indicted genes. (H) Confocal image of anti-ZO1 immunofluorescence staining (red) of cell-cell junctions (arrows) of an ISV with an arrested MDAwt cell (blue). Right panels show schematically EC cell-cell junctions and the tumor cell. Arrows indicate individual cell-cell junctions. Scale bars: 20 μm.
We next determined whether expression of ITGB1, Twist and VEGFA altered the clustering of endothelial cells at sites of tumor cell arrest. For this purpose, we directly monitored the position of individual endothelial cell nuclei in the vicinity of tumor cells using a zebrafish transgenic line that specifically expresses GFP in the nucleus (Siekmann and Lawson, 2007). This approach was previously used by other groups to track individual endothelial cell position within the ISVs (Siekmann and Lawson, 2007). Tracking of endothelial cell nuclei revealed that MDAwt and MDAVEGF tumor cells induced rapid nuclei movement that resulted in clustering of individual endothelial cells at the tumor cell-vascular interface (Fig. 4D-G). This did not result from an increase in endothelial cell proliferation because the total number of endothelial nuclei was the same in vessels containing control and tumor cells (4.2±1.3 and 4.0±0.9 nuclei, respectively). Importantly, the dynamic rearrangement of the endothelium was not simply due to the passive arrest of the tumor cell in the vessel, but was regulated by tumor cell adhesion to the vascular wall, because depletion of β1 integrin completely abrogated this response (Fig. 4C,E). In support of this, MDAtwist cells, which do not use β1 integrins to extravasate, did not significantly increase vascular wall thickness or clustering of endothelial cell nuclei when compared with control Sepharose beads (Fig. 4C,E,G). By contrast, vessel walls around MDAVEGF cells displayed dramatically increased thickness of the vessel wall, which was dependent on the expression of β1 integrin (Fig. 4C,G). The tumor-cell-induced alteration in the vascular wall was associated with an increase in the number of endothelial cell-cell junctions as revealed by ZO-1 staining (2.8±0.3 junctions/100 μm of vessel length) compared with empty ISVs without tumor cells (1.3±0.13 junctions/100 μm of vessel length) (Fig. 4H). These findings indicate that tumor cells induce endothelial cell clustering and remodeling of their cell junctions at sites of extravasation.
Discussion
Most of our understanding of extravasation of tumor cells has been gleaned from histological sections of secondary tumors isolated from various organs of mice or chickens. This static picture is usually taken days or weeks after the injection of tumor cells into the circulation, and therefore does not report on the ability of cells to penetrate the vessel wall. A large drawback to the standard imaging methods is the inability to visualize extravasation of tumor cells in vivo in real time, which typically occurs in small capillaries deep within the tissue. Consequently, this step in the metastatic cascade has been difficult to distinguish from the later metastatic events such as transition of tumor cells from dormancy and secondary colony formation. Our work here shows that the optical transparency and the highly patterned vascular system of zebrafish provide a unique model to visualize human tumor cell extravasation in real time in a live animal. This approach revealed that the process is complex and involves dynamic interaction between tumor cells and endothelial cells.
We found that expression of the pro-metastatic genes Twist, VEGFA and ITGB1 affects intravascular migration of arrested tumor cells, and their capability to remodel the vasculature and exit the circulation. These findings suggest that genetic or epigenetic factors that influence migration and vasculature remodeling of tumor cells could provide a metastatic advantage by increasing the number of cells that exit the vasculature after arrest. This could have a deleterious outcome because more tumor cells would gain access to extravascular tissues rich in ECM proteins and other survival factors, increasing their chances of survival and forming secondary tumors. Indeed, these genes have already been shown to be important metastatic signatures associated with poor patient outcome. Given that Twist, ITGB1 and VEGFA also support survival and proliferation of tumor cells, as well as motility, these genes would arm the tumor cells for both extravasation and secondary tumor growth (Matsumoto and Claesson-Welsh, 2001; Morozevich et al., 2009; Shibue and Weinberg, 2009; Yang et al., 2004).
The precise mechanism that drives intravascular tumor cell migration after arrest is not yet known, but appears to involve the actin-myosin contractile system and formation of dynamic rounded membrane protrusions. A striking finding is that tumor cells can migrate with or against blood flow and navigate through narrow vessel lumen openings and vessel branch points. These findings suggest that tumor cells generate strong traction forces and membrane protrusions that propel the cell forward while deforming and stretching the endothelium. We speculate that motile tumor cells are surveying the endothelial surface for a suitable site to extravasate and/or remodel the endothelium to create new sites suitable for vessel penetration.
Although the mechanisms are not understood, the process of tumor cell extravasation was found to be β1-integrin dependent or independent based on the metastatic gene being overexpressed. The integrin-dependent mode of cell movement and extravasation was used by wild-type MDA cells and was associated with a rounded morphology, decreased cell membrane protrusion, remodeling of the vascular endothelium and augmented by VEGFA expression. In this case, the thickening of the vascular wall and dynamic clustering of endothelial cell nuclei at the site of extravasation results in an increased number of endothelial cell-cell junctions, which would increase the number of potential sites for tumor cell extravasation (Engelhardt and Wolburg, 2004). By contrast, Twist-induced tumor cell extravasation was independent of β1 integrins. Correspondingly, in this case, tumor cell extravasation was not associated with vascular remodeling. The integrin-independent mode of extravasation used by MDAtwist cells might be facilitated by strong membrane-protrusive forces that directly penetrate the endothelium without the need for substrate adhesion. A highly protrusive cell phenotype similar to that displayed by MDAtwist cells has been associated with an amoeboid-like mode of tumor cell migration, which can be independent of integrin adhesions and ECM degradation (Friedl, 2004; Pinner and Sahai, 2008; Wolf et al., 2003). Interestingly, Twist has been shown to regulate the small GTPase RhoC, which in turn regulates ROCK, myosin contractility and formation of rounded protrusions (Ma et al., 2007; Narumiya et al., 2009). Our findings that the ROCK inhibitor Y27632 inhibited Twist-induced extravasation, support a mechanism by which a Twist-ROCK-myosin contraction mediates cell extravasation through an amoeboid-like mechanism that occurs independently of endothelial cell clustering. In MDAwt cells, β1 integrins mediate extravasation through an alternative mechanism, because Y27632 did not prevent this process. In this case, movement and extravasation of tumor cells might be mediated more through integrin-adhesion mechanisms that induce actin polymerization leading to invadapodia formation and/or cell locomotion and vascular-remodeling events (Friedl, 2004; Pinner and Sahai, 2008; Wolf et al., 2003). Interestingly, VEGFA-mediated tumor cell extravasation also relies on expression of β1 integrin and is associated with vascular remodeling. In this case, β1-integrin-mediated contact between tumor cells and endothelial cells might assure efficient diffusion of VEGF from the tumor cell to the endothelium surface where it activates the VEGFR and its associated downstream signals that facilitate vascular remodeling (Matsumoto and Claesson-Welsh, 2001). The induction of vascular-remodeling events combined with strong integrin-mediated membrane adhesion to the endothelium surface might work cooperatively to facilitate penetration of the vascular wall, possibly through binding to endothelial VCAM (Haddad et al., 2010). In the case where tumor cells express Twist and secrete VEGF, cell extravasation might rely more heavily on force generation, leading to membrane protrusion and intravascular migration, and might not rely as much on direct adhesion of tumors and endothelial cells or vascular remodeling events. Confirmation of our results using mammalian models of cancer cell metastasis would be an appropriate next step before applying them to human clinical therapies.
In summary, our findings provide evidence that extravasation of tumor cells is influenced by specific metastatic gene signatures that alter the actin-myosin cytoskeleton and induce vasculature remodeling. These findings challenge the widespread belief that tumor cell extravasation is a simple passive process and not a crucial determinant in the metastatic cascade. In light of these findings, a detailed understanding and confirmation of the regulatory mechanisms that govern tumor cell extravasation in zebrafish and mammals could provide valuable clinical markers that predict metastatic potential and provide novel therapeutic targets designed to block the spread of cancer in patients.
Materials and Methods
Cell lines and constructs
Stable fluorescent tumor cell line MDA-MB-435 (MDA; human breast adenocarcinoma and HT1080 human fibrosarcoma) were generated as previously described (Stoletov et al., 2007). Stable fluorescent tumor cell lines MDA-MB-231 human breast adenocarcinoma, SW480 and SW620 human colon adenocarcinoma cell lines were generated using pCFP-N1 or pDsRed-N1 expression vectors (Invitrogen). To generate MDAtwist cells, mouse Twist was cloned into pLentiCMVMCS. Twist-encoding viral particles were used for infection of MDA (RFP) cells, whereas MDAwt (CFP) cells were infected with empty vector virus. Twist was overexpressed 4.2-fold above the endogenous level as measured by western blotting (Yang et al., 2004). To generate ITGB1-knockdown MDA cells (MDAβ1KO), a lentiviral vector expressing ITGB1-specific shRNA was used, as described previously (Watanabe et al., 2008; Qin et al., 2003). Recombinant human VEGF adenoviral vector (Cell Biolabs) was used at multiplicity of 100 for VEGF expression in MDA cells as described by the manufacturer. Unselected population of virus-infected cell lines were used for all the experiments. To exclude the possibility that increased or decreased extravasation of a particular tumor cell line was due to its increased or decreased cell-proliferation rate, MDAwt, MDAtwist, MDAβ1KO, MDAβ1KOR, MDAVEGF and HT1080 cells were injected intravenously into zebrafish embryos and counted at 0 and 24 h.p.i. All the cell lines tested showed only a minor increase (less than 5% in 24 hours) in the total cell number that was independent of the cell type when injected intravenously in zebrafish embryos. Significantly, during our time-lapse analysis (>300 cells imaged) we never detected apoptotic tumor cell death and fragmentation within the vasculature. 10-15 μm fluorescent Sepharose beads were purchased from Molecular Probes. Anti VE-Cadherin morpholino was used at concentration of 0.5 ng/embryo, as previously described (Montero-Balaguer et al., 2009).
Animal preparation and injection of human tumor cells
Animals were maintained according to the University of California San Diego animal welfare guidelines as described (Nusslein-Volhard and Dahm, 2002). Zebrafish embryos were obtained using standard mating conditions and staged by h.p.f. At 48 h.p.f., embryos were de-chorionized if necessary, anesthetized with 0.003% tricaine and positioned on their right side on a wet agarose pad (~4 mm). Human tumor cells were lifted from culture dishes using non-enzymatic cell-lifting solution (Gibco) and washed twice with PBS at room temperature. Cells were resuspended in PBS at a concentration of 106 cells per 50 μl and kept on ice before injection. Thirty to 100 tumor cells or 10-15 μm beads (Molecular Probes) suspended in PBS were injected into the common cardinal vein (CCV) using an Eppendorf FemtoJet injector equipped with a 0.75 mm borosilicate glass needle (no filament, L=50 mm, diameter of the needle opening=20 μm). Tumor cells were injected approximately at the site where CCV opens into the heart (Fig. 1A). The approximate injection parameters were: injection pressure=300 p.s.i., holding pressure=10 p.s.i., injection time=0.2 mseconds. Injected embryos were washed once with E3 embryonic medium, transferred to 10 cm dishes containing 10 ml E3 and kept at 35.5°C. Injected tumor cells could normally be seen entering the vasculature 15-30 minutes after injection and starting to arrest in the ISVs 2-3 hours after injection. Generally, 5-30 cells per embryo were arrested in the ISVs. Embryos that contain ISV-arrested cells were selected using Leica MZ-FLIII stereo fluorescent microscope and used for further experiments. Normally, tumor-cell-injected embryos were euthanized at the end of experiments (~3.5 d.p.f.) by tricaine overdose (Nusslein-Volhard and Dahm, 2002). For ROCK and MLCK pharmacological inhibition, Y27632 and ML7 (Calbiochem) were added directly to the water starting at 3 h.p.i. for 21 hours at 30 and 40 μM final concentrations of E3 medium, respectively. DMSO was used as a vehicle control. E3 medium plus drug was changed every 12 hours.
Live imaging
A thin layer (3-4 mm) of 1.5% agarose (Sigma) containing 0.01% tricaine, pH 7.4, was made on a 2.2 cm circular coverslip (Fisher). A round trench (0.4 mm) was cut in the middle of the agarose layer using 1 ml plastic pipette tip and filled with E3 medium, 0.01% tricaine, pH 7.4. Anesthetized embryos (E3 medium, 0.01% tricaine, pH 7.4) were positioned in the middle of the trench using fine forceps. Embryos were adjusted to lie on their side as close as possible to the coverslip. E3 medium from the trench was removed and changed to 0.8% agarose containing 0.01% tricaine, pH 7.4. Agarose was allowed to solidify and coverslip was put inside humidified o-ring imaging chamber (Molecular Probes). The agarose layer inside the chamber was then covered with a thin (<2 mm) layer of E3 embryonic medium containing 0.01% tricaine, pH 7.4. The imaging chamber was wet-sealed using another coverslip and small drop of E3 medium. The temperature was maintained at 35.5°C using a 20×20 Technologies heating unit. For static (3D) images, z-stacks were taken with a Nikon C1-si confocal microscope with a step number between 20 and 50 and step size of 0.5-2.0 μm. For time-lapse (4D) images, z-stacks were taken every 5-15 minutes for a total time of up to 13 hours with a step number between 10 and 40 and step size of 0.5-2.0 μm. During imaging, z-stack position was adjusted every 1-2 hours to correct for embryo movement.
Generation of ITGB1-knockdown cells
To generate MDAβ1KO cells, a lentiviral vector was prepared as described previously (Qin et al., 2003). Plasmids containing shRNA encoding human integrin β1 (ITGB1) and the human U6 promoter were amplified by PCR: 5′-CACCGATTTAGGTGACACTATAG-3′, 5′-AAAAAGGACTGACCACAGTTATTACGACACTCCTCAAGCTTCAAGAGTGCCGTAACAACTGTGGTCAATCCGGTGTTTCGTCCTTTCCACAA-3′, and cloned into the lentiviral vector FG12 (Watanabe et al., 2008). Viruses were collected from culture supernatant 3 days after calcium-phosphate-dependent co-transfection of HEK293T cells with FG12, pCI-vesicular stomatitis virus G-protein, and packaging plasmid pCMV dR8.91. To achieve knockdown of ITGB1, MDA-MB-435 cells were incubated for 16 hours with 8 μg/ml Polybrene (Sigma) and lentivirus-containing medium. To rescue expression of ITGB1 after knockdown (MDAβ1KOR), silent mutations were introduced in ITGB1 (expressed in MDA-MB-435 cells using FG12 lentiviral vector) by substituting a DNA fragment from overlap extension PCR. ITGB1 gene expression was downregulated approximately 34-fold in MDAβ1KO or MDAtwistβ1KO when compared with mock-infected cells as determined by FACS (Watanabe et al., 2008). Reconstitution of ITGB1 gene expression restored normal β1 integrin expression levels. Control cells (MDAwt) were mock infected. Primer sequences used were: 5′-AAATCAGTGAATGGGAACAACGA-3′, 5′-GTTAACGACTGTAGTGACAGCGCTTTTATAAATAGGATTTTCACCCGTGTCCCATTT-3′, 5′-AAGCGCTGTCACTACAGTCGTTAACCCGAAGTATGAGGGAAAATGAGTACTG-3′. The sequence 5′-TTTCTCGAGCCCTGGCATGAATTACAACA-3′ was used as an RNAi target sequence. To test ITGB1 expression levels on MDAwt and MDAtwist, FACS analysis was used as previously described (Watanabe et al., 2008). Mean fluorescence intensity (MFI) units ± s.e.m. are shown in Fig. 3E. To test the adhesion of MDAwt and MDAtwist to various extracellular matrixes MDAwt and MDAtwist cells were lifted from culture dishes using non-enzymatic cell-lifting solution and allowed to attach for 3 hours under normal culture conditions on extracellular matrixes shown (laminin, fibronectin, collagen). Average numbers of cells per field (40×) ± s.e.m. are shown in Fig. 3F.
Immunofluorescence staining
Embryos at 72 h.p.f. (24 hours after tumor cell injection) were fixed in 4% paraformaldehyde for 2 hours at room temperature. Embryos were then washed three times for 10 minutes in PBST (0.1% Tween20 in PBS) and once (10 minutes) in PBSTX (PBS, 0.1% Tween20, 0.1% Triton X-100). Embryos were then blocked in PBSTX, 10% BSA, 1% NGS for 24 hours. Embryos were incubated with primary antibodies (in PBSTX, 1% BSA, 0.1% NGS) overnight at 4°C. Embryos were then washed five times for 30 minutes in PBSTX, 1% BSA, 0.1% NGS and then incubated with the secondary antibody (in PBSTX, 1% BSA, 0.1% NGS) overnight at 4°C. Embryos were finally washed several times (30 minutes to overnight) in PBST. All steps were performed at RT except for antibody incubations. The following antibodies were used: mouse anti-human ZO-1, 1:200 (Zymed); Alexa Fluor 568 goat anti-mouse IgG, 1:1000; Alexa Fluor 633 goat anti-mouse IgG (Invitrogen), 1:1000. z-stacks were acquired with a Nikon C1-si confocal microscope (~50 optical sections per stack at a step size of 0.25 μm, 60× objective).
Quantification of tumor cell extravasation
For quantification of tumor cell extravasation (Fig. 1B-C, Fig. 3D), embryos injected with tumor cells (or Sepharose beads) were immobilized in a small drop of E3 medium containing 0.01% tricaine in a humidified o-ring imaging chamber. Only the embryos that contained at least 5 ISV-arrested cells were used for analysis. Immobilized embryos were imaged using a Nikon C1-si confocal microscope and 20× objective. Tumor cell extravasation was scored based on analysis of single (1-2 μm) optical sections. A particular cell was considered extravasated if at least 50% of its body was outside of the vessel lumen and in the surrounding tissue. For simplicity, only the ISV-arrested cells were included in the analysis. Data was displayed as a percentage of tumor cells that are outside of the vessel to the total number of cells in ISVs. For Fig. 1B, 10-16 animals were analyzed for each tumor cell type. For Fig.1 C, numbers of animals were 56 for MDAwt, 31 for MDAVEGF, 40 for MDAtwist, 23 for MDAβ1KO and 16 for MDAβ1KOR. For Fig. 3D numbers of animals were 24 for MDAwt, 18 for MDAwt/Y27632, 24 for MDAwt/ML7, 25 for MDAtwist, 10 for MDAtwist/Y27632, 17 for MDAtwist/ML7, 19 for MDAtwistβ1KO.
Time-lapse analysis of intravascular tumor cell migration
For time-lapse analysis of intravascular tumor cell migration (Fig. 2A,B) embryos injected with tumor cells were anesthetized and embedded in 0.8 or 1.5% agarose as described above. Imaging field (512×512, 60×) was selected to include ISV-arrested tumor cell and most of the ISV. z-stacks (10-40 steps) were taken every 5 minutes for a total time of up to 5 hours with a step size of 0.5-1 μm. If necessary, the imaging field was adjusted manually during the imaging procedure to correct for embryo movement. Acquired 4D datasets were analyzed using Imaris Track function to measure tumor cell track speed. Imaris Contour Surface function was used to manually isolate migrating tumor cell body which was then 3D rendered for each time point using Imaris Isosurface function (Fig. 2A,B). If any signs of animal distress were observed, time-lapse imaging was stopped.
Quantification of tumor cell intravascular attachment
For quantification of tumor cell intravascular attachment (Fig. 2D), tumor cell injected embryos were immobilized in small 0.8% agarose drops on the coverslip and sealed in the o-ring imaging chamber as described above. z-stacks (10-20 steps) of ISV arrested tumor cells or control beads were acquired using 60× objective with a step of 0.5 μm. Measurements were done based on a single optical section that contains maximum tumor cell or control Sepharose bead diameter. For each tumor cell or control bead, a line passing through its center was drawn and a distance between tumor cell or bead surface and inner vessel wall along this line was measured. At least 22 (5-10 animals) independent measurements (one per each tumor cell or bead) were done for each MDA mutant cell or bead.
Quantification of intravascular tumor cell dynamics
For quantification of intravascular tumor cell dynamics (Fig. 3B,C) embryos injected with tumor cells were anesthetized and embedded in 0.8 or 1.5% agarose as described above. z-stacks (10-40 steps) were taken every 5 minutes for a total time of up to 5 hours with a step of 0.5-1 μm. Single, immotile ISV-arrested MDAwt or MDAtwist tumor cells were digitally isolated at each time point using Imaris Contoursurface function. A separate Isosurface was then built for each cell based on Contoursurface-isolated pixels and tumor cell sphericity was measured at each time point. Seven individual tumor cells of each type were used for analysis. Sphericity of particular 3D object (such as tumor cell) ranges from 0 (line) to 1 (sphere) and shows how this shape deviates from a sphere. For Fig. 3B, absolute cell sphericity measurements for each cell are displayed at each time point. For Fig. 3C, average percentage of cell sphericity change for MDAwt or MDAtwist per 5 minutes is shown. To exclude the possibility that the change in the tumor cell shape was induced by tumor cell passage trough the narrow part of the vessel lumen, only the finally arrested immotile tumor cells were used for analysis. Seven tumor cells of each type (MDAwt and MDAtwist) from nine independent animals were analyzed.
Injection of fluorescent Dextran
For intravital analysis of blood vessel wall integrity (Fig. 4A) embryos were anesthetized with 0.001% tricaine 24 hours after injection of tumor cells. Dextran-Texas-Red (0.5 μl) in PBS (5 mg/ml, molecular mass, 2↔106 Da; Molecular Probes) was injected into the CCV using an Eppendorf FemtoJet injector equipped with a 0.75 mm borosilicate glass needle (L=50 mm, diameter of the needle opening=2-5 μm). The approximate injection parameters were: injection pressure=50 p.s.i., holding pressure=5 p.s.i., injection time=0.1 mseconds. Injected embryos were washed once with E3 medium and transferred to a Petri dish with pre-warmed (35.5°C) E3 medium for 15 minutes. Embryos were transferred to the temperature controlled o-ring imaging chamber in a small drop of E3 medium containing 0.01% tricaine. z-stacks (10-20 steps) of ISV-arrested tumor cells or control beads were acquired using 60× objective with a step of 0.5 μm. Red-channel-intensity measurements (561 nm laser line, range 0-4095) were recorded along the vessel walls of tumor cells (MDAwt, MDAVEGF, MDAtwist) or control Sepharose bead (10-15 μm) based on the single optical sections that contain maximum tumor cell or Sepharose bead diameter. Although there was some tissue autofluorescence in the fish gut, skin, eyes and sensory cells, no significant difference in red-channel intensity was detected between embryos injected with tumor cells or control beads or non-injected embryos. It should be noted that approximately 30 minutes after injection of Dextran-Texas-Red, Dextran started to leak uniformly from vessels containing tumor cells or controls, possibly because of a non-specific transendothelial exclusion process. Uniform Dextran leakage was also detected outside lymphatic vessels 15-30 minutes after injection. At least six animals were analyzed for each MDA cell mutant. By contrast, rapid Dextran leakage was detected within 3 minutes of injection in embryos injected with VE-cadherin morpholino (Fig. 4A, right panel).
Quantification of tumor-cell-induced vessel remodeling
Embryos that contain ISV arrested tumor cells or control beads were embedded in small 0.8% agarose drops 24 hours after injection of tumor cells or control beads, as described above. z-stacks (10-20 steps) of ISV-arrested tumor cells or control beads were acquired using 60× objective with a step of 0.5 μm (Fig. 4C). Vessel-wall thickness was measured based on a single optical section that contained maximum diameter of vessel-arrested tumor cells or control beads. Measurements were done randomly in the vessel-wall areas that directly contacted tumor cells or control Sepharose beads. Twenty-five random vessel wall measurements were done for each MDA tumor cell mutant, 12 animals were used for each condition.
Quantification of tumor-cell-induced endothelial cell nuclei movement
For time-lapse analysis and quantification of tumor-cell-induced endothelial cell nuclei movement (Fig. 4D,E) tumor-cell-injected embryos were anesthetized and embedded in 0.8 or 1.5% agarose, as described above. Imaging field (512×512, 60×) was selected to include ISV-arrested tumor cell and entire (or most of) ISV. z-stacks (10-40 steps) were taken every 5 minutes for a total time of up to 5 hours with a step of 0.5-1 μm. If necessary, the imaging field was adjusted manually during the imaging procedure to correct for embryo movement. Acquired 4D datasets were analyzed using Imaris Track function to measure track speed and duration. Specifically, endothelial cell nuclei positions were first assigned automatically using Imaris spot detection function and if necessary, nuclei positions were adjusted manually for each time point. Based on the nuclei positions individual nuclei tracks were created and track speed was measured using the Imaris built-in tracking function. Track speeds of control (empty), bead or tumor cell containing ISV endothelial cell nuclei were measured and used for analysis. Individual endothelial cell nuclei were included in analysis if they were within 30 μm of ISV-arrested tumor cell surface. Since significant variability between average background endothelial nuclei movement speeds in individual embryos was detected (up to five times) data was displayed as increase or decrease (percentage) above the average endothelial nuclei movement speed for a particular embryo. Ten endothelial cell nuclei from at least five independent animals were tracked for each MDA cell mutant or controls.
Quantification of tumor-cell-induced nuclei clustering
For quantification of tumor-cell-induced nuclei clustering (Fig. 4G), fluorescently labeled (CFP or DsRed) MDAwt cells or control 10-15 fluorescent Sepharose beads were injected into Tg(fli1:nEGFP) embryos (www.zebrafish.org). This transgenic zebrafish line specifically expresses EGFP in the endothelial cell nuclei. 24 hours after injection, embryos were fixed using 4% paraformaldehyde. z-stacks (20-30 steps) of ISV-arrested tumor cells or control beads were acquired using 20× objective with a step of 1 μm. The number of ISV nuclei within 20 μm of tumor cells or control beads was counted. ‘Empty vessel’ shows average number of endothelial cell nuclei per 20 μm of ISV length. A total of 26 (6-10 animals) measurements for each MDA cell mutant or bead were made.
Statistical analysis
All data were analyzed using unpaired Student's t-test built into the GraphPad Prizm software (www.graphpad.com) for statistical significance. Data plots show mean values ± s.e.m., except Fig. 3B and Fig. 4C, which depict distributions of single measurements (Fig. 4C shows single measurements and mean values).
Supplementary Material
Acknowledgments
We are grateful to Yinchun Wang, Sharif Rumjahn and Theresa Reno for critical reading of the manuscript and to Emerald Butko for help with image analysis. This work was supported by California Breast Cancer Research Program Postdoctoral Fellowship 11FB-0088 (to K.S.) and National Institutes of Health Grants CA129231 and CA097022 (to R.K.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/13/2332/DC1
References
- Al-Mehdi A. B., Tozawa K., Fisher A. B., Shientag L., Lee A., Muschel R. J. (2000). Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 6, 100-102 [DOI] [PubMed] [Google Scholar]
- Blum Y., Belting H. G., Ellertsdottir E., Herwig L., Lüders F., Affolter M. (2008). Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312-322 [DOI] [PubMed] [Google Scholar]
- Bos P. D., Zhang X. H., Nadal C., Shu W., Gomis R. R., Nguyen D. X., Minn A. J., van de Vijver M. J., Gerald W. L., Foeken J. A., et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmone A., Amorim M., Andrea L., Pontier A. L., Wang S., Jablonski E., Sipkins D. A. (2008). Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322, 1861-1865 [DOI] [PubMed] [Google Scholar]
- Crawford Y., Ferrara N. (2008). VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 335, 261-269 [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Wolburg H. (2004). Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 34, 2955-2963 [DOI] [PubMed] [Google Scholar]
- Fischer R. S., Gardel M., Ma X., Adelstein R. S., Waterman C. M. (2009). Local cortical tension by myosin II guides 3D endothelial cell branching. Curr. Biol. 19, 260-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P. (2004). Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14-23 [DOI] [PubMed] [Google Scholar]
- Gupta G. P., Nguyen D. X., Chiang A. C., Bos P. D., Kim J. Y., Nadal C., Gomis R. R., Manova-Todorova K., Massagué J. (2007). Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765-771 [DOI] [PubMed] [Google Scholar]
- Haddad O., Chotard-Ghodsnia R., Verdier C., Duperray A. (2010). Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFkappaB: differential role of the shear stress. Exp. Cell. Res. 316, 615-626 [DOI] [PubMed] [Google Scholar]
- Haldi M., Ton C., Seng W. L., McGrath P. (2005). Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9, 139-151 [DOI] [PubMed] [Google Scholar]
- Hewitt R. E., McMarlin A., Kleiner D., Wersto R., Martin P., Tsokos M., Stamp G. W., Stetler-Stevenson W. G. (2000). Validation of a model of colon cancer progression. J. Pathol. 192, 446-454 [DOI] [PubMed] [Google Scholar]
- Heyder C., Gloria-Maercker E., Hatzmann W., Zaenker K. S., Dittmar T. (2006). Visualization of tumor cell extravasation. Contrib. Microbiol. 13, 200-208 [DOI] [PubMed] [Google Scholar]
- Isogai S., Lawson N. D., Torrealday S., Horiguchi M., Weinstein B. M. (2003). Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281-5290 [DOI] [PubMed] [Google Scholar]
- Kienast Y., von Baumgarten L., Fuhrmann M., Klinkert W. E., Goldbrunner R., Herms J., Winkler F. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116-122 [DOI] [PubMed] [Google Scholar]
- Koop S., Schmidt E. E., MacDonald I. C., Morris V. L., Khokha R., Grattan M., Leone J., Chambers A. F., Groom A. C. (1996). Independence of metastatic ability and extravasation: metastatic ras-transformed and control fibroblasts extravasate equally well. Proc. Natl. Acad. Sci. USA 93, 11080-11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubens B. S., Zänker K. S. (1998). Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Cancer Lett. 131, 55-64 [DOI] [PubMed] [Google Scholar]
- Lee S. L., Rouhi P., Dahl Jensen L., Zhang D., Ji H., Hauptmann G., Ingham P., Cao Y. (2009). Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc. Natl. Acad. Sci. USA 106, 19485-19490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi K. J., MacDonald I. C., Schmidt E. E., Kerkvliet N., Morris V. L., Chambers A. F., Groom A. C. (1998). Multistep nature of metastatic inefficiency. Am. J. Pathol. 153, 865-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Teruya-Feldstein J., Weinberg R. A. (2007). Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682-688 [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Claesson-Welsh L. (2001). VEGF receptor signal transduction. Sci. STKE 112, 1-17 [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M., Swirsding K., Orsenigo F., Cotelli F., Mione M., Dejana E. (2009). Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. PLoS ONE 4, 1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozevich G., Kozlova N., Cheglakov I., Ushakova N., Berman A. (2009). Integrin alpha5beta1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity. Cell Cycle 8, 2219-2225 [DOI] [PubMed] [Google Scholar]
- Narumiya S., Tanji M., Ishizaki T. (2009). Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 28, 65-76 [DOI] [PubMed] [Google Scholar]
- Nguyen D. X., Bos P. D., Massague J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274-284 [DOI] [PubMed] [Google Scholar]
- Nicoli S., Ribatti D., Cotelli F., Presta M. (2007). Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 67, 2927-2931 [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C., Dahm R. (2002). Zebrafish: A Practical Approach, 1st edn.Oxford: Oxford University Press; [Google Scholar]
- Pinner S., Sahai E. (2008). Imaging amoeboid cancer cell motility in vivo. J. Microsc. 231, 441-445 [DOI] [PubMed] [Google Scholar]
- Podsypanina K., Du Y. C., Jechlinger M., Beverly L. J., Hambardzumyan D., Varmus H. (2008). Seeding and propagation of untransformed mouse mammary cells in the lung. Science 321, 1841-1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. F., An D. S., Chen I. S., Baltimore D. (2003). Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc. Natl. Acad. Sci. USA 100, 183-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E. (2007). Illuminating the metastatic process. Nat. Rev. Cancer 7, 737-749 [DOI] [PubMed] [Google Scholar]
- Schluter K., Gassmann P., Enns A., Korb T., Hemping-Bovenkerk A., Holzen J., Haier J. (2006). Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am. J. Pathol. 169, 1064-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Weinberg R. A. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. USA 106, 10290-10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann A. F., Lawson N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781-784 [DOI] [PubMed] [Google Scholar]
- Sipkins D. A., Wei X., Wu J. W., Runnels J. M., Côté D., Means T. K., Luster A. D., Scadden D. T., Lin C. P. (2005). In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K., Klemke R. (2008). Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509-4520 [DOI] [PubMed] [Google Scholar]
- Stoletov K., Montel V., Lester R. D., Gonias S. L., Klemke R. (2007). High-resolution imaging of the dynamic tumor cell-vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 104, 17406-17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson J. L., Chambers A. F. (2006). Dormancy of solitary metastatic cells. Cell Cycle 5, 1744-1750 [DOI] [PubMed] [Google Scholar]
- Voura E. B., Jaiswal J. K., Mattoussi H., Simon S. M. (2004). Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med. 10, 993-998 [DOI] [PubMed] [Google Scholar]
- Wang H., Fu W., Im J. H., Zhou Z., Santoro S. A., Iyer V., DiPersio C. M., Yu Q. C., Quaranta V., Al-Mehdi A., et al. (2004). Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol. 164, 935-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Bodin L., Pandey M., Krause M., Coughlin S., Boussiotis V. A., Ginsberg M. H., Shattil S. J. (2008). Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin αIIbβ3. J. Cell Biol. 30, 1211-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S., Cui J., Barnes L., Cheresh D. (2004). Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 167, 223-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., von Andrian U. H., Deryugina E. I., Strongin A. Y., Bröcker E. B., Friedl P. (2003). Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J. B., Pinner S. E., Gschmeissner S., Condeelis J. S., Sahai E. (2006). ROCK-and myosin-dependent matrix deformation enables protease-independent tumor cell invasion in vivo. Curr. Biol. 16, 1515-1523 [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Yang M., Jiang P., Xu M., Yamamoto N., Tsuchiya H., Tomita K, Moossa A. R., Bouvet M., Hoffman R. M. (2006). Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 66, 4208-4214 [DOI] [PubMed] [Google Scholar]
- Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927-939 [DOI] [PubMed] [Google Scholar]
- Zijlstra A., Mellor R., Panzarella G., Aimes R. T., Hooper J. D., Marchenko N. D., Quigley J. P. (2002). A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 62, 7083-7092 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.