Abstract
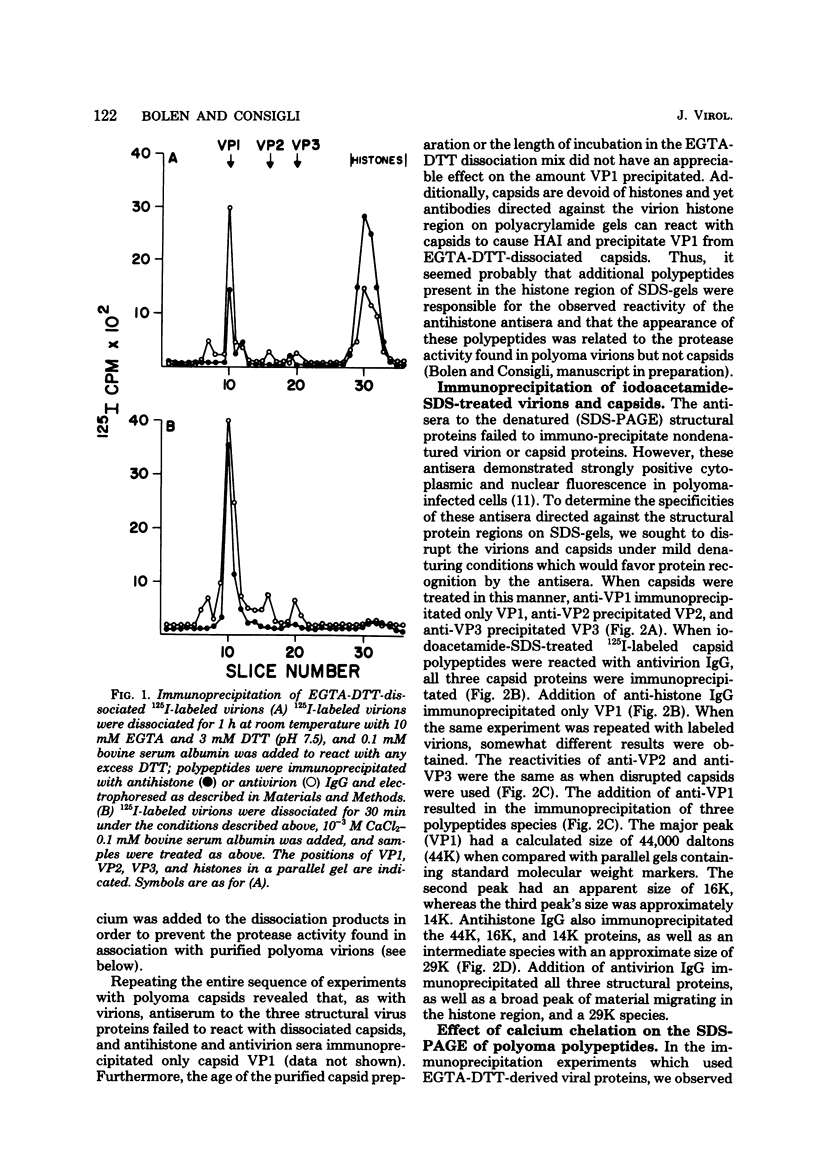
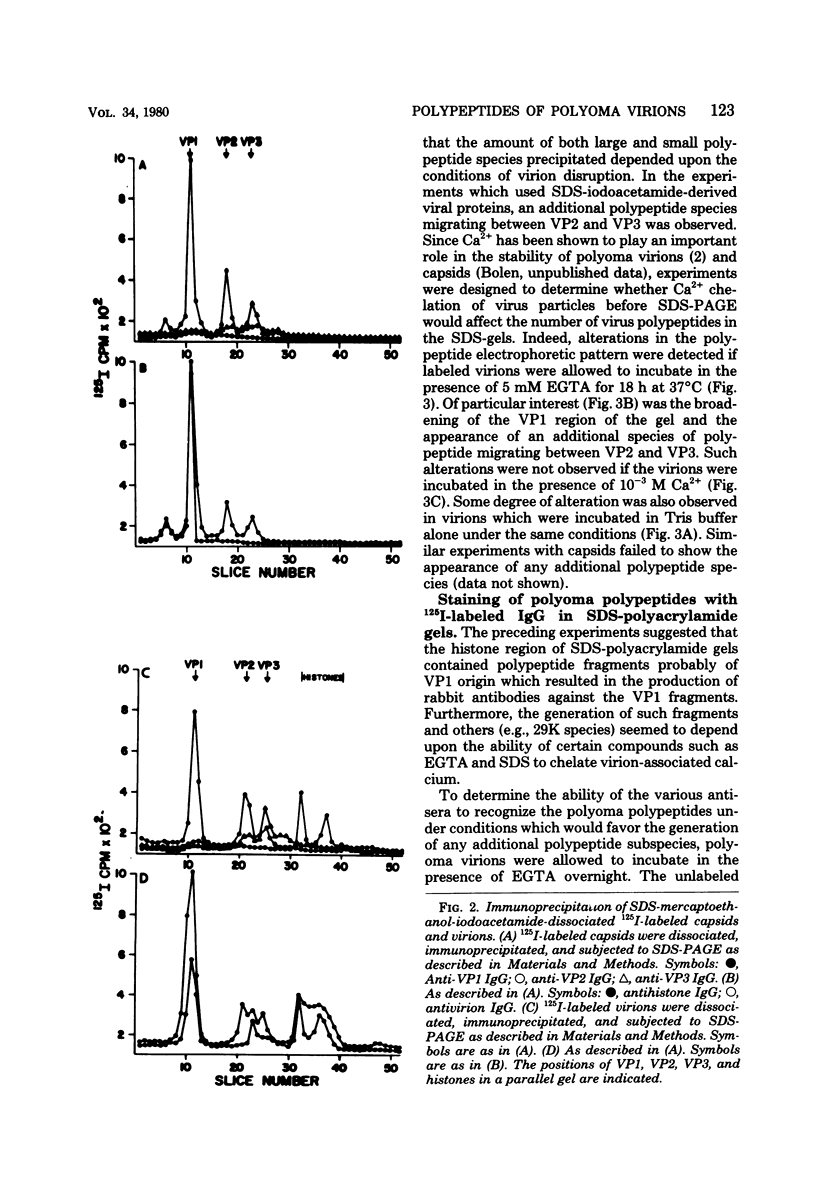
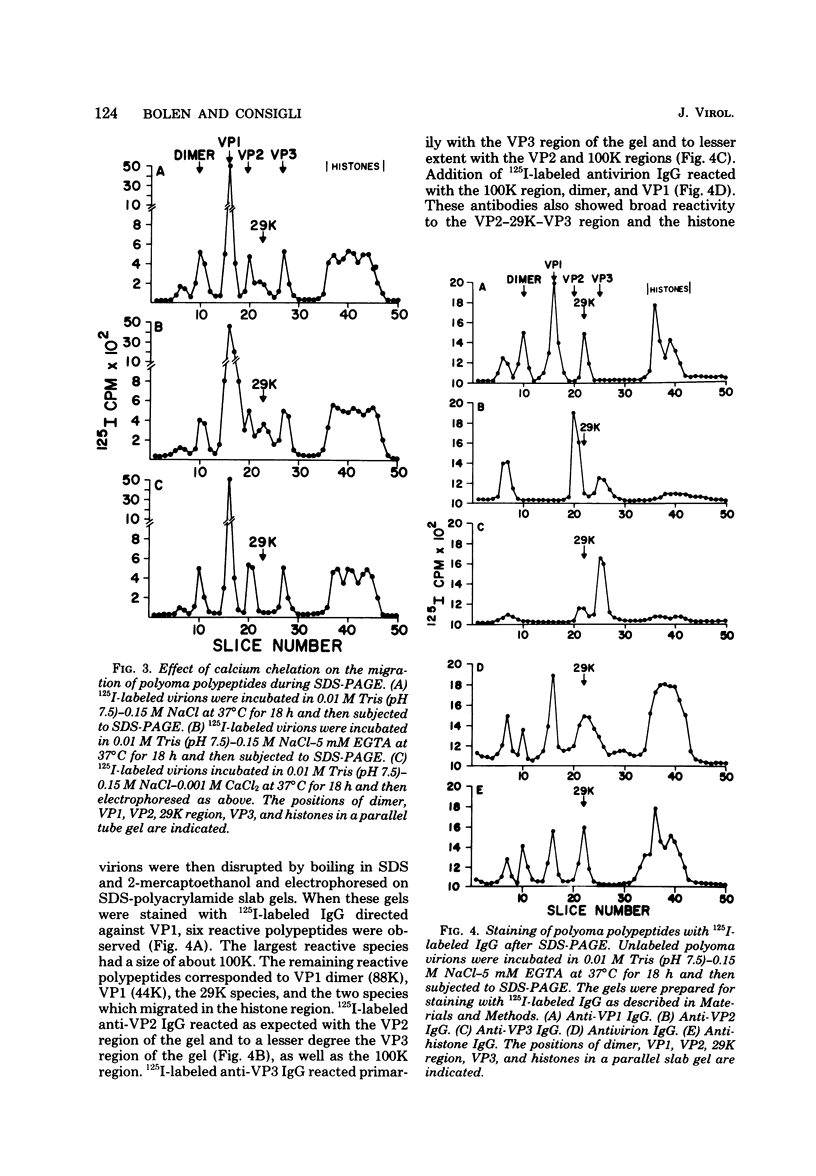
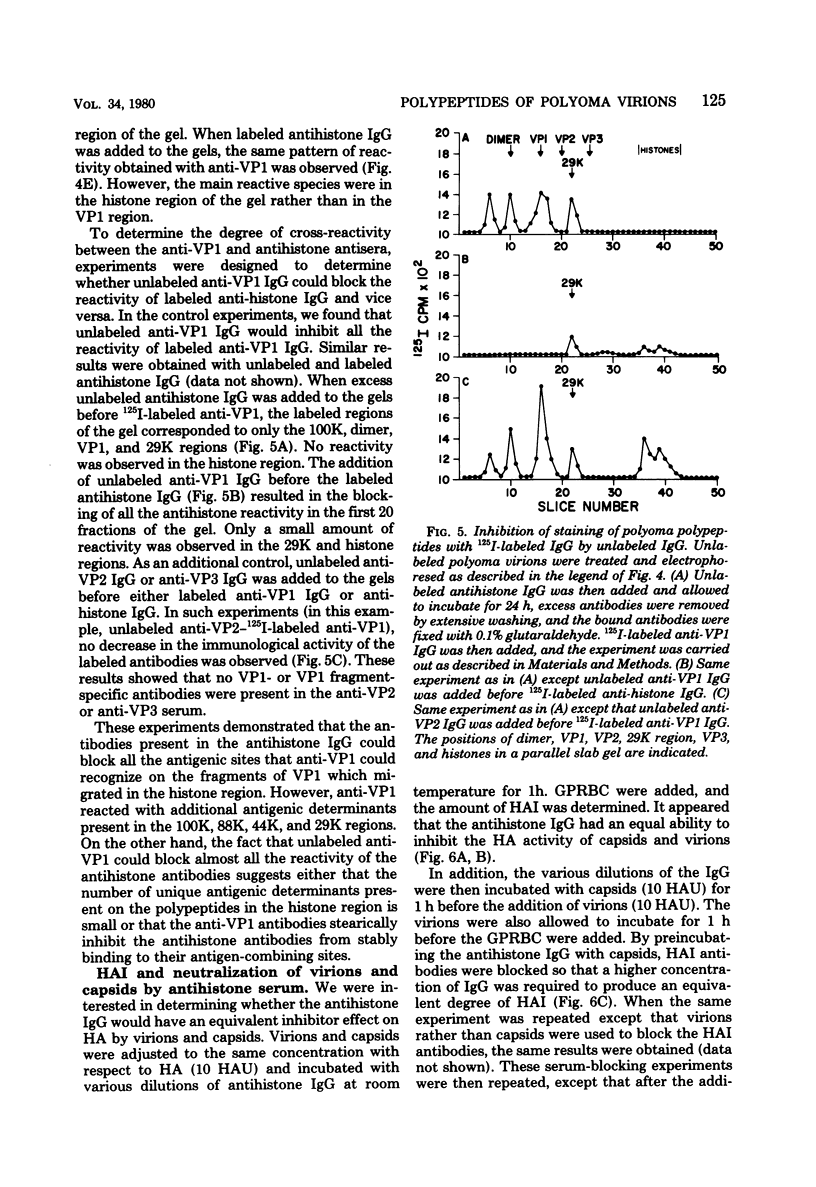
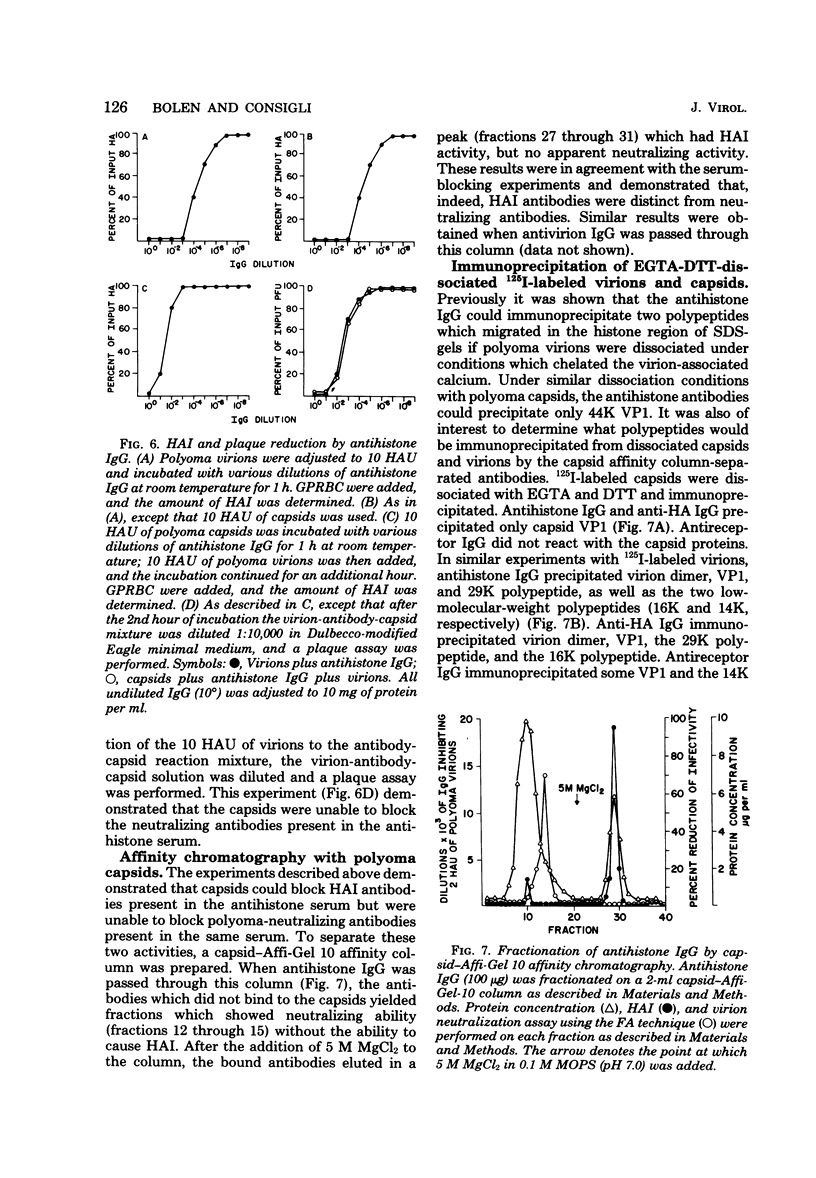
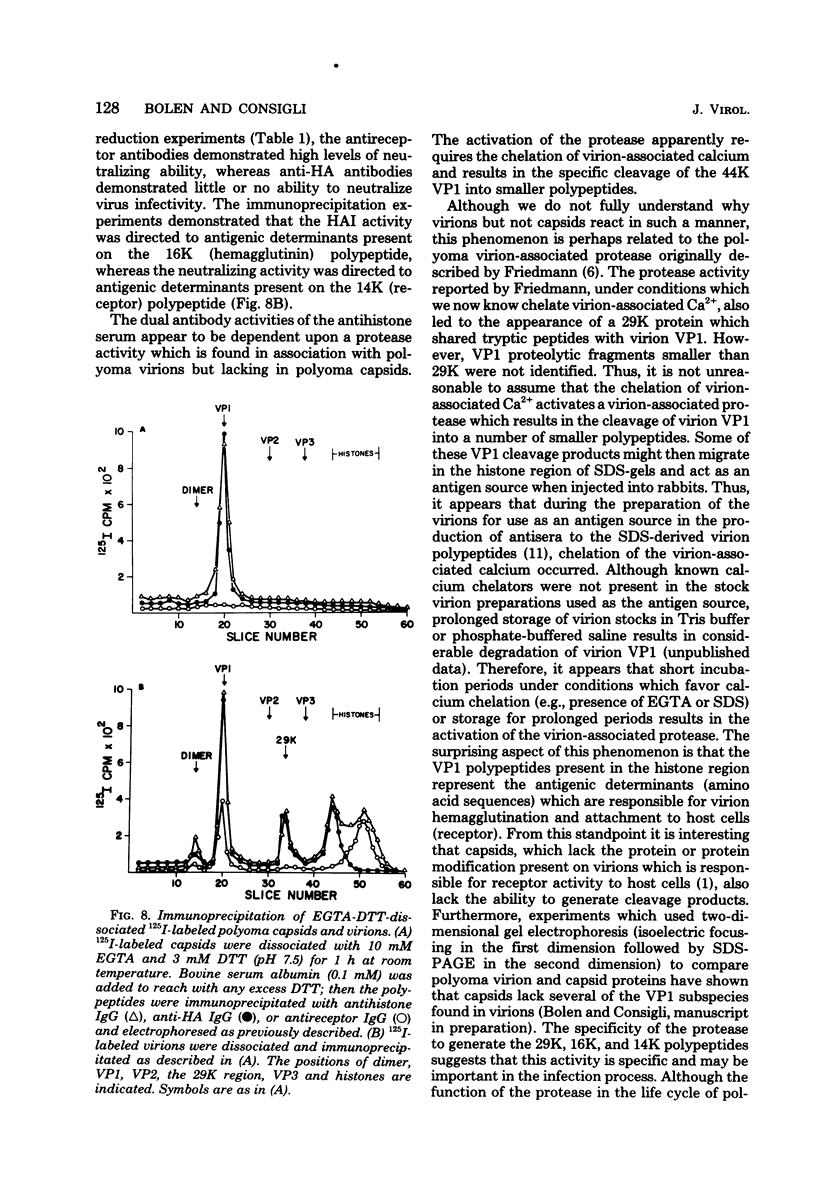
Antisera to the sodium dodecyl sulfate (SDS)-polyacrylamide gel-derived polyoma virion polypeptides were used in immunoprecipitation experiments with ethylene glycol-bis-N,N'-tetraacetic acid (EGTA)-dissociated polyoma virions and capsids to determine the specificity of the antipolyoma polypeptide sera. Additionally, a technique for applying 125I-labeled immunoglobulins to SDS-polyacrylamide gels was used to explore the antigenic specificities of the antisera. The results demonstrated that antisera directed against the SDS-gel-derived VP1, VP2, and VP3 did not react with native polyoma proteins, but would react with the appropriate antigens on denatured polyoma proteins. Antisera against the histone region of such gels reacted with native and denatured polyoma VP1. Separation of neutralizing antibodies from hemagglutination inhibition (HAI) antibodies to polyoma in antisera directed against the histone region of polyacrylamide gels was done by using a polyoma capsid affinity column. The antibodies eluted from this column which did not react with capsids possessed only neutralizing activity, whereas antibodies which bound to capsids possessed only HAI activity. These isolated immunoglobulin G fractions were then used in immunoprecipitation experiments to demonstrate that the antigenic determinants responsible for the HAI activity of the serum were contained on a 16,000-dalton polypeptide, whereas those antigenic determinants responsible for neutralizing activity were contained on a 14,000-dalton polypeptide. Both of these polypeptides present in the histone region of the SDS-gels appeared to be derived from the major virion protein VP1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T. Structural proteins of polyoma virus: proteolytic degradation of virion proteins by exogenous and by virion-associated proteases. J Virol. 1976 Nov;20(2):520–526. doi: 10.1128/jvi.20.2.520-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Decapsidation of polyoma virus: identification of subviral species. Virology. 1975 Nov;68(1):245–255. doi: 10.1016/0042-6822(75)90165-8. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Fried M., Waterfield M. D. Nonhistone virion proteins of polyoma: characterisation of the particle proteins by tryptic peptide analysis by use of ion-exchange columns. Virology. 1975 Aug;66(2):408–419. doi: 10.1016/0042-6822(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Flory P. J., Jr Synthesis of the SV40 viral polypeptide Vp1 during infection. Virology. 1978 May 15;86(2):344–353. doi: 10.1016/0042-6822(78)90075-2. [DOI] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



