Abstract
Due to similar routes of viral transmission, many individuals infected with the human immunodeficiency virus (HIV) are also infected with the hepatitis C virus (HCV). Each virus can cause cognitive compromise among mono-infected individuals; evidence is accumulating that HIV/HCV co-infection may have a particularly deleterious impact on cognition. We present neuropsychological data obtained from 118 HIV+ adults with advanced HIV disease, 35 of whom were co-infected with HCV, who completed a comprehensive neurocognitive evaluation. Rates of global cognitive impairment were higher among co-infected patients than among those with HIV alone (63% vs. 43%). Within the specific domains of learning and memory, co-infected individuals were significantly more likely to be impaired than were the HIV mono-infected participants. Finally, we discuss implications of these findings and potential future directions for research in this area.
Keywords: HIV infection, HCV infection, HIV/HCV co-infection, neurocognition
Just as infection with the human immunodeficiency virus (HIV) has been shown to be a risk factor for neuropsychological impairment, it is becoming increasingly clear that infection with the hepatitis C virus (HCV) may be associated with some degree of neurocognitive decline.1 To date, however, few studies have examined whether co-infection with both HIV and HCV is associated with cognitive impairment above and beyond that seen in mono-infected individuals. Below we briefly review what is known regarding the neuropsychological effects of HIV and HCV mono-infection, as well as HIV/HCV co-infection, and then present data examining cognitive performance in HIV/HCV co-infected adults compared to that of a cohort of HIV mono-infected adults.
NEUROPSYCHOLOGICAL SEQUELAE OF HIV MONO-INFECTION
The deleterious effects of HIV-infection on neuropsychological function are well established.2 HIV-related neurocognitive dysfunction can range from subtle deficits in information-processing speed and efficiency to a pronounced dementia syndrome. Memory impairment, characterized especially by forgetfulness, motor and psychomotor slowing, attentional disruption, and executive systems dysfunction have all been repeatedly observed among HIV-infected individuals. This pattern of neurocognitive deficits is suggestive of disruption to the circuits that connect the prefrontal cortex with subcortical structures and is consistent with data from brain imaging and autopsy studies, which implicate preferential subcortical involvement.3-5 While studies have found that cognitive deficits are more likely to be found in patients with more advanced immune suppression,6 a small subset of patients who are otherwise asymptomatic and without significant immuno-compromise, nonetheless show cognitive deficits.6,7 In addition, and more importantly, some patients with what appears to be adequately treated HIV infection have developed new onset neurocognitive impairment, implying that HAART is not always fully neuroprotective.8 How HCV co-infection affects response to HAART has yet to be explored.
In addition to the neurocognitive consequences of HIV/AIDS, neuropsychiatric features such as depression, apathy, and irritability are frequently present among infected individuals.9-11 Clearly, psychiatric symptoms (e.g., depression, anxiety, apathy, irritability) among HIV-infected individuals are not always a consequence of central nervous system (CNS) disturbance. Social stigmatization and marginalization, increased medical and financial stressors, bereavement, and compromised social support may all contribute to elevated rates of mood disturbance. However, there is evidence suggesting that subcortical neuropathology may lead to psychiatric disturbance among a subset of HIV+ individuals. Work from our group has linked apathy to neurocognitive disruption (i.e., executive dysfunction) among HIV-infected adults and posited a common CNS etiology for both.10
NEUROPSYCHOLOGICAL FEATURES OF HCV MONO-INFECTION
In a subset of patients with advanced chronic HCV infection and severe liver disease, decompensated cirrhosis and elevated ammonia levels can lead to a syndrome known as hepatic encephalopathy.12 However, a substantial minority of HCV+ patients who do not have cirrhosis also report significant cognitive complaints,13 typically in the domains of attention/concentration, learning and memory, and slowed information processing speed. A subset of these patients demonstrate significantly lower than expected performance on neurocognitive testing.13,14 The prevalence of neurocognitive dysfunction in persons with HCV was explored by Hilsabeck and colleagues, who found that approximately one-third of HCV-infected patients seen in a tertiary care liver clinic showed impairment on two or more neuropsychological measures relative to normative data.15,16 While impaired performance was most prevalent on measures of information-processing/psychomotor speed (observed in 19-38% of the sample, depending on the measure), nearly 15% of this HCV+ sample showed impairment on some aspect of learning and memory as well.16
Similar to HIV, the pattern of neurocognitive deficits in HCV-infected individuals is suggestive of frontal-subcortical dysfunction, with complex attention, information processing, and psychomotor speed preferentially impaired.14,16,17 Neuroimaging studies using 1H-MRS also indicate frontal-subcortical changes in persons with HCV.1 For example, a group of HCV-infected individuals had significantly higher Choline/Creatine ratios in the basal ganglia and frontal white matter than did persons infected with the hepatitis B virus (HBV) and healthy volunteers, and also had higher rates of neuropsychological impairment. Of note, HCV+ subjects who were cognitively impaired had significantly higher Cho/Cr ratios in the basal ganglia as compared to unimpaired HCV-infected persons and healthy volunteers.1 Within a sample of methamphetamine users, Taylor and colleagues reported that those subjects with HCV showed lower N-acetylaspartate levels (a neuronal and axonal marker) in frontal white matter than those without HCV, and a larger percentage of HCV-infected individuals showed cognitive compromise on psychometric testing.17
NEUROPSYCHOLOGICAL FEATURES OF HIV/HCV CO-INFECTION
Recently, studies have begun to explore the impact of HIV/HCV co-infection on cognition. Co-infected adults have been shown to evidence impairment on measures of executive function,18 psychomotor speed,19,20 and global cognitive function.21 Our group also has previously demonstrated that HIV/HCV co-infection is associated with a higher rate of impairment on measures of cognitive speed as well as an increased rate of drug and alcohol abuse.22 Studies employing 1H-MRS have found that both HIV and HCV infection are associated with elevated choline/creatine ratios in frontostriatal structures, suggesting the presence of glial proliferation. Increased levels of proinflammatory cytokines such as IL1, IL6, and TNF-α are also common to both diseases and may represent a common mechanism leading to neuropsychological compromise.
In the present study we sought to replicate our previous investigation with a different cohort of HIV infected individuals, a subset of whom were HIV/HCV co-infected. We hypothesized that co-infected subjects would demonstrate greater neuropsychological impairment than mono-infected participants; particularly on cognitive tasks sensitive to frontostriatal dysfunction.
METHOD
Research Participants
A total of 118 HIV-seropositive adults were enrolled in the current study, 35 who were co-infected with the Hepatitis C virus. Data for this investigation were collected at the National Neurological AIDS Bank (NNAB) in Los Angeles, California. The NNAB is a member site of the National NeuroAIDS Tissue Consortium, a multi-site, longitudinal investigation of the neurocognitive and neuropathological consequences of HIV infection.23 Due to the possible cognitive sequelae associated with current substance abuse as well as opportunistic infections affecting the central nervous system (CNS-OI), such as toxoplasmosis, cryptococcal meningitis, or progressive multifocal leukoencephalopathy, subjects receiving such diagnoses were excluded from data analysis in the present study.
At the time of participation, 27% of subjects were diagnosed as neurocognitively normal and 52% impaired due to HIV-associated Minor Cognitive Motor Disorder (MCMD) or HIV Associated Dementia (HAD) or based on criteria described below. Average absolute CD4+-cell count for the group as a whole was 226 cells/mm3 (sd = 275). Thirty-eight percent of participants were Caucasian, 29% were African American, 29% were Hispanic, and 4% were Asian/Pacific Islander. Women comprised 18% of the sample. Mean age of the participants was 43 years (8.3) with a range of 26-62. The majority of subjects had completed high school and had a mean of 13.5 (3.1) years of education.
Procedure
Study procedures were approved by the institutional review boards (IRB) of the University of California, Los Angeles, the Olive View-UCLA Medical Center, and the Charles R. Drew University of Medicine and Science. After providing written informed consent, participants completed self-report questionnaires, a structured psychiatric interview, neurological examination, and neurocognitive testing as described below. Subjects also provided blood, urine, and in some cases, cerebrospinal fluid (CSF) samples for analysis. All subjects agreed to donate their tissues and organs in the event of death.
Measures
Neurocognitive Status
Participants completed a comprehensive battery of neuropsychological tests at baseline to assess functioning in the domains of attention/working memory, speed of information processing, learning, memory, verbal fluency, abstract executive functioning, and motor/psychomotor speed. Two standardized sets of scores were derived from these measures. First, raw scores were converted to demographically corrected t-scores (with a mean of 50 and a standard deviation of 10) using test manuals and published normative data. Domain t-scores were obtained by calculating the mean t-score for all tests comprising a given domain. A global t-score was calculated by summing individual t-scores and dividing by the number of tests administered. Second, using an algorithm developed by Heaton and colleagues,24 deficit scores (ranging from 0 to 5) were assigned to each measure based on the following T scores: T > 39 = 0; T ≤ 39 and ≥ 36 = 1; T ≤ 35 and ≥ 30 = 2; T ≤ 29 and ≥ 25 = 3; T ≤ 24 and ≥ 20 = 4; T < 20 = 5. Such a technique gives more weight to low scores, thereby increasing sensitivity to detect deficit within a domain. The deficit scores for all neuropsychological measures that comprised a particular domain were then averaged to produce a domain deficit score. Subjects were classified as impaired in a domain if their average deficit score was greater than or equal to 1.0. In addition, subjects with an average deficit score greater than or equal to 1.0 across all tests were classified as having global neuropsychological impairment.
Neurological Diagnoses
Neurological examinations were performed by board certified neurologists with extensive experience in Neuro-AIDS. Neurological diagnoses (e.g., CNS-OIs, possible or probable MCMD/HAD) were arrived at via consensus agreement between the examining neurologist and neuropsychologist after consideration of the subject's lab results (blood and CSF), neuroimaging, and medical history. Subjects who received a consensus diagnosis were classified as neurocognitively normal, impaired but subsyndromic, or impaired due to MCMD or HAD, according to the same procedure. Subjects with a clinical diagnosis of overt hepatic encephalopathy were specifically excluded from this analysis.
Psychiatric Illness
The Psychiatric Research Interview for Substance and Mental Disorders, or PRISM,25 was administered to all participants at baseline. The substance use disorders and depressive disorders modules were administered.
Data Analysis
All data were analyzed using the Statistical Package for Social Sciences, version 11.0.26 Descriptive statistics were conducted on all variables to summarize the data. Comparisons on demographic variables between the two groups were performed using ANOVA for continuous variables (i.e., age and education) and chi-square frequency analysis for categorical variables (i.e., ethnicity and gender). HIV disease progression was assessed by CD4 + count subsets using ANOVA. Group differences in rates of neurocognitive disorders (i.e., sub-syndromic, MCMD/HAD) were compared using chi-square. Finally, participants were classified as “normal” or “impaired” in each neurocognitive domain based upon their average performance on tests within that domain. Those with an average deficit score of greater than or equal to 1 were classified as impaired. Differences between the groups in rate of impairment in each domain were compared with Pearson chi-square analyses.
RESULTS
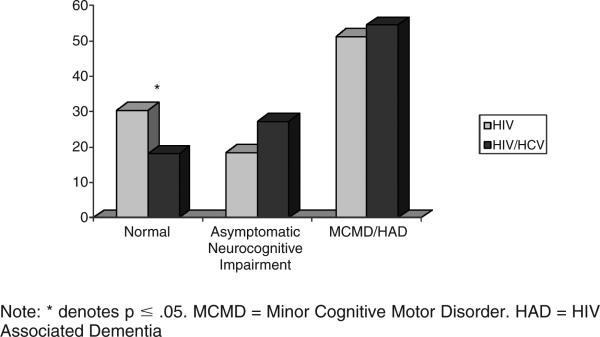
Results of demographic comparisons are presented in Table 1. Groups did not differ significantly with regards to age, education, ethnicity, or gender. HIV disease severity as measured by absolute CD4+ count was also statistically similar between the groups. Prevalence rates of HIV-related dementia and associated syndromes did not differ significantly between the groups (χ2 = 2.16, p = .34). However, as Figure 1 indicates, only 18% of the co-infected group members, compared to 30% of those with HIV alone, were considered “normal.” In addition, there appears to be slightly, though not significantly, higher rates of asymptomatic neurocognitive impairment among the co-infected group.
TABLE 1.
Group Demographic Characteristics
| Variable | HIV | HIV/HCV | p-value |
|---|---|---|---|
| Mean (sd) | |||
| Age (years) | 42.5 (8.3) | 44.7 (8.4) | .20 |
| Education (years) | 13.5 (3.2) | 13.2 (2.8) | .66 |
| Absolute CD4+ cell count | 226 (275) | 190 (230) | .54 |
| % of participants in each group | |||
|---|---|---|---|
| Gender (% female) | 20.5 | 11.4 | .24 |
| Ethnicity: | .41 | ||
| Caucasian | 39.8 | 34.3 | |
| Hispanic | 10.8 | 5.7 | |
| African American | 24.1 | 40 | |
| Asian, Alaskan | 3.6 | 5.7 | |
| Mixed Hispanic | 21.7 | 14.3 | |
FIGURE 1.
Rates of neurocognitive syndromes
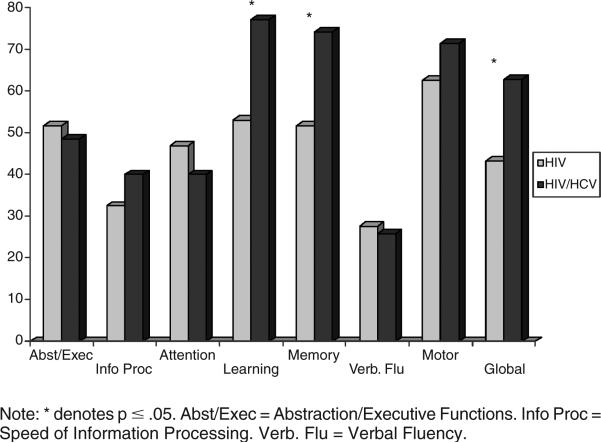
Rates of cognitive impairment for the two groups across neurocognitive domains differed. The HIV/HCV co-infected group was significantly more likely to be globally neuropsychologically impaired than was the HIV mono-infected group (χ2 = 3.74, p = .05). Among the HIV/HCV co-infected group, 63% were classified as globally impaired versus only 43% among the HIV only group. Examination of the component neurocognitive domains revealed that the HIV/HCV co-infected group had significantly higher rates of impairment in learning (χ2 = 5.98, p = .01) and memory (χ2 = 5.12, p = .02). As shown in Figure 2, 77% of co-infected subjects were classified as having learning impairment, compared to only 53% of mono-infected individuals. Similarly, 74% of co-infected participants had memory impairments, compared to only 52% of mono-infected subjects. Note also in the figure that co-infected individuals appear to have slightly higher rates of impairment, though not significantly so, in information processing speed and motor ability. Conversely, the mono-infected group appears to have higher rates of impairment in attention.
FIGURE 2.
Rates of impairment across neurocognitive domains
DISCUSSION
As hypothesized, HIV/HCV co-infected subjects were more likely to be classified as neuropsychologically impaired than were HIV mono-infected adults. Specifically, co-infected participants were almost three times more likely to be classified as impaired in the domains of learning as well as memory. Although not statistically significant due to power limitations, the co-infected cohort also had slightly higher rates of impairment on measures of psychomotor speed and upper extremity motor speed.
Consistent with our prior work,22 as well as that of others,14,18,19,21 co-infection with both HIV and HCV appears to be associated with an increase risk of cognitive impairment. Several possible explanations can be advanced to explain this finding. Given that the primary route of transmission for HCV is injection drug use, it may simply be that the incremental rate of neurocognitive impairment among the co-infected patients is due to the deleterious effects of drug abuse rather than HCV infection. While this explanation cannot be definitively ruled out, we did exclude all subjects with a DSM-IV diagnosis of current drug abuse in the present study. Since the adverse effects of drug use on cognition typically tend to dissipate over time, it is unlikely that our current findings are due to the effects of historical drug use alone.
There are at least two routes by which HCV could be postulated to cause neuropsychological impairment. First, subjects with decompensated liver disease might have hepatic encephalopathy, which is associated with elevated levels of ammonia.12 Subjects with clinical diagnoses of hepatic encephalopathy were excluded from the current study, but it is possible that some subjects might have had slight or sub-clinical hepatic encephalopathy that contributed to cognitive impairment.27 Alternatively, HCV has been found to invade the nervous system28 and can be recovered from CSF.29 HCV infects monocytes and macrophages, the same cells that are a target of HIV,28 leading us to suspect that similar neuropathogenic mechanisms might be at play.
Alternatively, it may be that the higher rates of neuropsychological impairment among the co-infected subjects are due to the additive, if not synergistic effects of HCV infection superimposed on HIV infection. Previous studies have demonstrated common neurobehavioral symptomatology and common abnormalities on neuroimaging, and have posited a common neurophysiologic mechanism underlying the shared cognitivedeficits. Whether this is due to the direct effects of these viruses on the brain, or an inflammatory cascade triggered by infection, remains to be determined.
Additional research on HIV/HCV co-infection is clearly needed. Such studies will have to give more careful consideration towards controlling for common confounding conditions such as comorbid drug and alcohol abuse. Similarly, different definitions of cognitive “impairment,” often determined by comparison to normative data, might overestimate the potential effects of HCV/HIV co-infection. Ideally, adequately powered studies that include groups of demographically matched healthy controls in addition to mono infected HIV+, mono-infected HCV+, as well as HIV/HCV co-infected subjects will better allow estimation of the potential effects of each virus alone and in combination. Future studies that employ multiple methodologies for assessing brain-behavior function, such as functional and structural neuroimaging in addition to neuropsychological assessment, are likely to be particularly informative.
There are several other practical implications of this study that bear mention. Given the elevated rate of cognitive symptomatology in HCV+ individuals, neuropsychological screening of HCV+ patients, particularly those with more pronounced drug use histories or those who are HIV co-infected, may prove useful in detecting patients with neurocognitive compromise. This may have particular relevance when structuring interventions to improve treatment compliance (such as medication adherence) or in risk-reduction efforts. In this context, risk-reduction should not only focus on drug use behaviors, but also on behavioral practices that may increase transmission of infection such as riskier sexual practices and needle sharing.
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (RO1 DA13799) to CHH and from the National Institute of Neurological Disorders and Stroke (NS38841) to EJS.
The authors thank Amanda Gooding for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Charles H. Hinkin, David Geffen School of Medicine at University of California at Los Angeles, VA Greater Los Angeles Health Care System, and the National Neurological AIDS Bank..
Steven A. Castellon, David Geffen School of Medicine at University of California at Los Angeles, VA Greater Los Angeles Health Care System, and the National Neurological AIDS Bank..
Andrew J. Levine, David Geffen School of Medicine at University of California at Los Angeles and the National Neurological AIDS Bank..
Terry R. Barclay, David Geffen School of Medicine at University of California at Los Angeles..
Elyse J. Singer, David Geffen School of Medicine at University of California at Los Angeles and the National Neurological AIDS Bank..
REFERENCES
- 1.Forton DM, Allsop JM, Cox IJ, Hamiliton G, Wesnes K, Thomas HC, Taylor-Robinson SD. A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS. 2005;19(suppl. 3):S53–S63. doi: 10.1097/01.aids.0000192071.72948.77. [DOI] [PubMed] [Google Scholar]
- 2.Hinkin CH, Castellon SA, van Gorp WG, Satz P. Neuropsychological features of HIV infection. In: van Gorp WG, Buckingham SL, editors. A mental health practitioner's guide to the neruopsychiatric complications of HIV/AIDS. Oxford University Press; New York: 1997. pp. 1–41. [Google Scholar]
- 3.Hestad K, McArthur JH, Dal Pan GJ, et al. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- 4.Hinkin CH, van Gorp WG, Mandelkern MA, et al. Cerebral metabolic change in patients with AIDS: report of a six-month follow-up using positron emission tomography. J Neuropsychiatr Clin Neuro. 1995;7:180–187. doi: 10.1176/jnp.7.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Navia BA, Cho ES, Petito C, Price R. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 6.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500-neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 7.White DA, Heaton RK, Monsch AU, HNRC Group Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. J Int Neuropsychol Soc. 1995;1:304–315. doi: 10.1017/s1355617700000308. [DOI] [PubMed] [Google Scholar]
- 8.Cysique LA, Brew BJ, Halman M, et al. Undetectable cerebrospinal fluid HIV RNA and beta-2 micro-globulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr. 2005;39(4):426–429. doi: 10.1097/01.qai.0000165799.59322.f5. [DOI] [PubMed] [Google Scholar]
- 9.Castellon SA, Hinkin CH, Wood S, Yarema K. Apathy, depression and cognitive performance in HIV-1 infection. J Neuropsychiatr Clin Neuro. 1998;10:320–329. doi: 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- 10.Castellon SA, Hinkin CH, Myers H. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc. 2000;6:336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- 11.Rabkin JD, Fernando SJ, Jacobsberg LB, et al. Prevalence of axis I disorders in an AIDS cohort: a cross-sectional controlled study. Compr Psychiatry. 1997;38:146–154. doi: 10.1016/s0010-440x(97)90067-5. [DOI] [PubMed] [Google Scholar]
- 12.Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62(19-20):2295–2304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, et al. Hepatitis C virus infection affects the brain–evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41:845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatol. 2002;35:433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 15.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatol. 2002;35:440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- 16.Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MJ, Letendre SL, Schweinsburg BC, et al. Hepatitis C virus infection is associated with reduced white matter N-acetylasparatate in abstinent methamphetamine users. J Int Neuropsychol Soc. 2004;10:110–113. doi: 10.1017/S1355617704101161. [DOI] [PubMed] [Google Scholar]
- 18.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurol. 2004;62:957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin EM, Novak RM, Fendrich M, Vassileva JL, Gonzalez R, et al. Stroop performance in drug users classified by HIV and hepatic C virus serostatus. J Int Neuropsychol Soc. 2004;10:289–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- 20.von Giesen HJ, Heintges T, Abbasi-Boroudjeni N, Kucukkoylu S, Koller H, Haslinger BA, et al. Psychomotor slowing in hepatitis C and HIV infection. J AIDS. 2004;35:131–137. doi: 10.1097/00126334-200402010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Letendre S, Cherner M, Ellis RJ, et al. Individuals co-infected with hepatitis C and HIV are more cognitively impaired than those infected with either virus alone. J Neurovirol. 2002;8(Supplement):S14. [Google Scholar]
- 22.Hilsabeck RC, Castellon SA, Hinkin CH. Neuropsychological aspects of co-infection with HIV and hepatitis C virus. Clin Infect Dis. 2005;41:S38–S44. doi: 10.1086/429494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgello S, Gelman BB, Kozlowski PB, et al. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27(4):326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 24.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halsstead-Reitan battery: Demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, Florida: 1991. [Google Scholar]
- 25.Hasin D, Trautman K, Endicott J. Psychiatric research interview for substance and mental disorders: phenomenologically based diagnosis in patients who abuse alcohol or drugs. Psychopharmacol Bull. 1998;34(1):3–8. [PubMed] [Google Scholar]
- 26.Statistical Package for Social Sciences for Windows, Version 11.0. Chicago, Illinois: 2001. [Google Scholar]
- 27.Groeneweg M, Quero JC, De Bruijn I, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28(1):45–49. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- 28.Laskus T, Radkowski M, Adair DM, et al. Emerging evidence of hepatitis C virus neuroinvasion. Aids. 2005;19(Suppl 3):S140–144. doi: 10.1097/01.aids.0000192083.41561.00. [DOI] [PubMed] [Google Scholar]
- 29.Laskus T, Radkowski M, Bednarska A, et al. Detection and analysis of hepatitis C virus sequences in cerebrospinal fluid. J Virol. 2002;76(19):10064–10068. doi: 10.1128/JVI.76.19.10064-10068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]