Abstract
The etiology of type 1 diabetes (T1D) remains unknown, but a growing body of evidence points to infectious agents and/or components of early childhood diet. The National Institutes of Health has established the TEDDY Study consortium of six clinical centers in the United States and Europe and a data coordinating center to identify environmental factors predisposing to, or protective against, islet autoimmunity and T1D. From 2004–2009, TEDDY will screen more than 360,000 newborns from both the general population and families already affected by T1D to identify an estimated 17,804 children with high-risk HLA-DR, DQ genotypes. Of those, 7,801 (788 first-degree relatives and 7,013 newborns with no family history of T1D) will be enrolled in prospective follow-up beginning before the age of 4.5 months. As of May 2008, TEDDY has screened more than 250,000 newborns and enrolled nearly 5,000 infants—approximately 70% of the final cohort. Participants are seen every 3 months up to 4 years of age, with subsequent visits every 6 months until the subject is 15 years of age. Blood samples are collected at each visit for detection of candidate infectious agents and nutritional biomarkers; monthly stool samples are collected for infectious agents. These samples are saved in a central repository. Primary endpoints include (1) appearance of one or more islet autoantibodies (to insulin, GAD65 or IA-2) confirmed at two consecutive visits; (2) development of T1D. By age 15, an estimated 800 children will develop islet autoimmunity and 400 will progress to T1D; 67 and 27 children have already reached these endpoints.
Keywords: type 1 diabetes, islet autoimmunity, environmental triggers, epidemiologic study, HLA, infectious agents, dietary factors, psychosocial factors
Background
Over the past 60 years, the incidence of type 1 diabetes (T1D) worldwide has been increasing by 3–5% per year,1–3 (Fig. 1) doubling approximately every 20 years.4 While several T1D susceptibility genes are known, such a rapid increase can only be explained by a powerful influence in the environment interacting with a relatively common genetic background. In some populations, the incidence has increased most markedly in the very youngest children,3 suggesting a role for very early exposures. The disease also appears to spread to children who carry lower-risk HLA-DR, DQ genotypes,5,6 consistent with an increase in the penetrance of the environmental exposure(s). Population-based cohort studies that preceded TEDDY7–9 as well as rapid advances in immunology and genetics have provided new insights into the pathogenesis of T1D. On the other hand, none of the candidate environmental exposures has been shown beyond reasonable doubt to cause a significant number of the cases. The role of the TEDDY study is to accelerate progress towards preventing T1D prevention through a large-scale sustained international effort to clearly define the causes of T1D.
Figure 1.
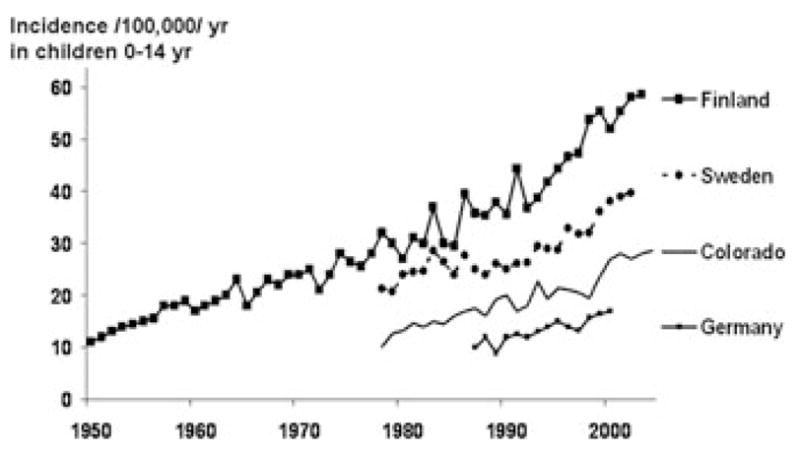
T1D incidence has doubled every 20 years. Data for Finland are from the Finnish National Public Health Institute (V. Harjutsalo and J. Tuomilehto); data for Sweden are from the Swedish Childhood Diabetes Registry;66 data for Germany are a compilation of two reports;67,68 data for Colorado are from the Colorado IDDM Registry, the Barbara Davis Center for Childhood Diabetes, and SEARCH for Diabetes in Youth.4
Islet autoimmunity, marked by the presence of autoantibodies to pancreatic β cell antigens such as GAD65, insulin, or IA-2, precedes clinical T1D in most cases by a few years (Fig. 2). This preclinical period provides a theoretical opportunity for prevention. However, two large randomized trials in relatives of T1D patients—the European Nicotinamide Diabetes Intervention Trial10 and the Diabetes Prevention Trial-1 (using parenteral11 and oral insulin12)—failed to prevent or delay progression from autoimmunity to diabetes. Significant β cell damage present at trial entry could also play a role. In contrast, TRIGR (the Trial to Reduce IDDM in the Genetically at Risk)13 is attempting T1D prevention by eliminating cow’s milk in infant nutrition before the onset of islet autoimmunity. Pilot studies using omega-3 fatty acids (NIP) or human oral insulin (Pre-Point) are under way in genetically susceptible young children to prevent islet autoimmunity and T1D. While these approaches may be effective, we lack convincing evidence concerning the initiators of islet autoimmunity to design optimal primary prevention trials. Of importance, our current understanding of T1D etiology originates predominantly from studies of first-degree relatives (FDRs) of T1D patients. These data may not be directly applicable to the causes and prevention of T1D in the general population, in which 90% of the cases occur. TEDDY is filling important gaps in our understanding of the natural history of T1D by studying from birth high-risk general population children and relatives followed systematically for environmental determinants of T1D.
Figure 2.
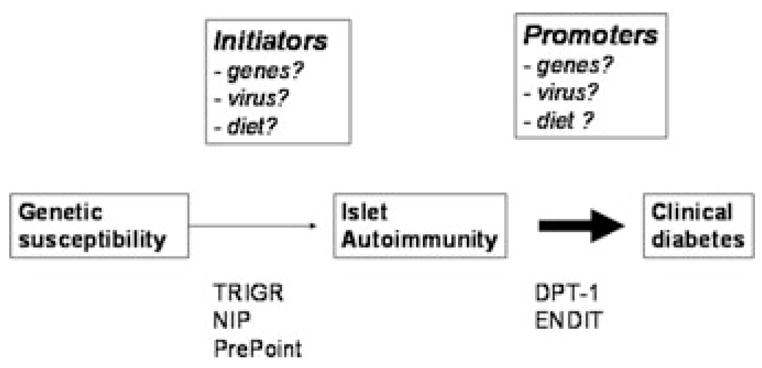
Natural history of T1D and prevention opportunities.
A number of environmental exposures have been proposed to contribute to T1D risk. These include exposures taking place during pregnancy, infancy, childhood, and beyond. Not all islet autoantibody–positive subjects progress to diabetes,11 and hence the importance of distinguishing whether an environmental agent triggers development of islet autoimmunity or promotes disease progression. This can only be determined by prospective follow-up of large numbers of genetically at-risk children from a very young age.
Exposure to rubella during pregnancy has resulted in diabetes in about 20% of children.14 Similarly, the risk for T1D in childhood is reported to be increased in children born to mothers with enterovirus infections during pregnancy.15,16 Other potential risk factors include ABO incompatibility and hyperbiliru-binemia,17,18 preeclampsia,19 mother’s age,20 and high birth weight for gestational age.20,21 Further evidence of fetal programming of T1D risk comes from the still unexplained decreased T1D risk in children of mothers with T1D as compared to children of fathers with T1D.22,23 Finally, the HLA type of the child appears to affect fetal growth, suggesting potential genetic programming that goes beyond the immune repertoire.24,25 There is a gap in understanding to what extent gestational factors, including genetic interactions, may trigger islet autoimmunity or merely increase T1D susceptibility in the offspring. It cannot be excluded that gestational infection may induce immunologic tolerance to the virus.26,27 An ability of the offspring immune system to regard a virus as self may have consequences for latency and reinfection.
Seroconversion to positivity for islet autoantibodies, the earliest measure of islet autoimmunity which may lead to clinical T1D, may occur already 3–6 months after birth.20,28 Candidate autoimmunity and T1D risk factors operating in infancy include those related to exposure to infectious agents, improved hygiene,29 mucosal exposure to dietary constituents,30,31 and requirement for increased beta cell functioning.32,33
Previous virus studies have sought to provide a direct evidence for virus-induced T1D. However, in some of these patients developing T1D, it was found that either the insulitis was chronic or that the patients already had islet cell autoantibodies. It could therefore not be excluded that the virus infection accelerated an already ongoing process of islet autoimmunity.34 Further studies of these phenomena as well as of other microbial agents are therefore warranted to take into account that subjects with an increased T1D risk may show responses that lead to islet autoimmunity or affect ongoing islet autoimmunity.
Enteroviruses, and in particular coxsackie B viruses, remain the prime candidate by nature of their tropism for beta cells,34,35 possible molecular mimicry,36 and early and more recent reports of their presence in beta cells of patients with T1D.37,38 Data from Finland showing a relationship between enterovirus infection and the appearance of islet autoantibodies as well as a seasonal fluctuation in the appearance of islet autoantibodies supports a role early in the disease.39 However, substantially more evidence is required to establish a causal role for enterovirus in T1D, especially as a trigger of the islet autoimmunity, because the association could not be demonstrated in children outside Scandinavia, including those in Colorado40 and Germany.41 Rotavirus has also been shown to infect beta cells and to have a link to islet autoimmunity by way of molecular mimicry,42,43 but evidence for a causal role is lacking.44
Seemingly in contrast to the infectious hypotheses is the notion that improved hygiene is responsible for upward trends in T1D incidence as well as incidences of other hyperimmune response diseases such as allergy.29 There are epidemiologic studies indicating that crowding and exposure to others in day care are associated with reduced T1D risk, supporting the hygiene hypothesis. Few studies have examined the relationship of hygiene to the development of islet autoimmunity. Related to hygiene is a potential role of vaccinations in the development of islet autoantibodies or progression to T1D. Some have suggested that vaccination increases T1D risk, but well-designed studies have found no evidence for this.45,46 The temporal relationship of vaccinations to the development of islet autoantibodies or T1D has never been examined. Prospective analyses of children from 3 months of age through the entire period of mandatory or voluntary vaccination are needed to establish effects of vaccinations on islet autoimmunity and progression to T1D.
Substantial data have been generated on the role of breast-feeding and early exposure to cow’s milk47 or cereals.30,31 In addition to these there are reports of associations of T1D development with low intakes of vitamin D,48 tocopherols,49 ascorbic acid,50 vitamin E,51 and omega-3 fatty acids.52 Other suspected exposures include drinking water, with an increased risk if water is from a local well compared to water-plant drinking water, possibly related to the amount of zinc.53 Moreover, N-nitroso compounds54 and mycotoxins55 have been associated with an increased risk of T1D.
The TEDDY study is uniquely positioned to elucidate the association between T1D and celiac disease because the study eligibility HLA genotypes confer susceptibility to both diseases.56–58 TEDDY is measuring autoantibodies against tissue transglutaminase (tTG), which is a very sensitive and specific marker of celiac disease.
Psychosocial factors may also contribute to appearance of T1D. Stress has long been considered a potential trigger for TID.59 Screening for high-risk genes associated with T1D could induce anxiety and distress in family members.60 Prospective studies utilizing detailed psychosocial evaluation of participating parents as well as children as they grow older will be necessary to effectively determine whether life events or stress may increase the risk for islet autoimmunity. Experiences in studies of children at genetic risk for T1D who have gone on to develop T1D have identified benefits such as absence of severe ketoacidosis and a reduction in hospitalization.61
While there are preliminary data and intriguing hypotheses as to the etiology of T1D, the data are often confounded by imprecise assessment of exposure, recall bias, failure to account for genetic susceptibility, failure to assess exposures at very early ages, or the inability to follow a sufficient sample of children long-term with high intensity. Most of the few studies that have attempted to look at exposure from an early age and in relation to the development of islet autoantibodies were underpowered. TEDDY will fill important gaps in our understanding of the events leading to T1D. In addition, samples collected by TEDDY will create a valuable resource for investigators proposing innovative hypotheses concerning candidate environmental and genetic factors.
TEDDY Study: Organization and Goals
The Environmental Determinants of Diabetes in the Young (TEDDY), a multicenter prospective cohort study, was initiated in 2003 to identify environmental factors that trigger or protect against the development of islet autoimmunity and T1D. The details of the TEDDY study’s organization and protocol have been previously published.62 In brief: the consortium comprises 6 clinical centers located in Denver (Colorado), Augusta (Georgia)/Gainesville (Florida), and Seattle (Washington) in the United States, and in Finland (Turku), Sweden (Malmo), and Germany (Munich). The Data Coordinating Center is in Tampa, Florida. Autoantibody Reference Laboratories are located in Denver (serving the U.S. Clinical Centers) and Bristol (serving the European Clinical Centers). The Central Genetics Reference Laboratory is in Oakland, California, and the Central mRNA Laboratory is in Augusta, Georgia. The NIDDK Bio-sample and Genetics Repositories store samples. For more details, see the Appendix.
The primary objectives of this study are:
-
To identify environmental factors that trigger or protect against the development of islet autoantibodies or T1D.
-
Infectious agents:
Blood and stool samples as well as other bodily fluids are collected and analyzed for infectious agents to test the hypothesis that specific virus(es) may trigger islet autoimmunity or promote progression to T1D. In addition, the hypothesis that certain infections may reduce the risk of islet autoimmunity or T1D will also be tested.
-
Dietary factors:
Primary caretakers provide 3-day food diaries and 24-hour-recall dietary records, and blood samples are used to analyze vitamin D, alpha-tocopherol, gamma-tocopherol, cartenoids, ascorbic acid, and red blood cell membrane fatty acids to test the hypothesis that dietary factors may trigger/accelerate/reduce islet autoimmunity or promote progression to T1D.
-
Psychosocial factors:
Stressful life events and other indicators of stress in the child and family are monitored to assess their contribution, if any, to the development of islet autoimmunity or T1D. In addition, participating families provide structured information that may help to identify factors affecting retention and study participation.
-
Other:
TEDDY will evaluate other factors such as toxins, immunizations, pets, and allergies in the triggering of and/or protection against islet autoimmunity or T1D.
-
Genes both within and outside the HLA region are typed to identify gene–environment interactions. It is expected that the joint analyses of genetic and environmental data will improve the identification of both genetic and environmental factors influencing development of islet autoimmunity and T1D, and may explain mechanisms of these interactive effects.
The prospectively collected specimens from TEDDY subjects (DNA, RNA, serum and plasma, cells and other samples) provide a unique opportunity for scientists within and outside the TEDDY consortium to test novel hypotheses.
Study Progress
Consent for genetic screening is obtained from parents of babies born in area hospitals or identified after birth. This blood sample is analyzed at approved and monitored laboratories for the HLA haplotypes that qualify a child for TEDDY. Those results are returned to the DCC and local clinical centers, who notify all subjects of the genetic screening outcome. Among those who are HLA-eligible, trained local staff (e.g., study nurses) contact the subject’s parent to explain genetic risk and introduce in some detail the follow-up phase of TEDDY for which their child is now eligible to join. Enrollment, informed consent, and completion of the first visit must occur before the child reaches the age of 4.5 months. Once this visit has been completed the subject is then on the follow-up visit schedule for data collection described below. A portion of the 9-month blood sample is used to confirm HLA eligibility at a central reference laboratory, where additional high-resolution typing is done to confirm eligibility. Table 1 summarizes current TEDDY accruals by center and cohort (FDRs versus infants without a history of T1D in a FDR). Table 2 compares current accruals with the overall study goals for the number of infants to complete screening, found eligible, and enrolled into follow-up. As of May 2008, TEDDY has screened more than 250,000 newborns and enrolled nearly 5,000 infants—approximately 70% of the study goals. As shown in Figure 3, TEDDY is ahead of the enrollment goals by several months and on track to complete the screening and enrollment by the end of 2009.
TABLE 1.
Current TEDDY Accruals (as of May 22, 2008)
| Clinical Center | Infants Screened |
Infants HLA-Eligible |
Infants Enrolled |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FDRs | Gen Pop | Total | FDRs | Gen Pop | Total | FDRs | Gen Pop | Total | |
| Colorado | 575 | 41,900 | 42,475 | 123 | 2212 | 2335 | 79 | 649 | 728 |
| Finland | 571 | 39,813 | 40,384 | 191 | 2221 | 2412 | 103 | 1039 | 1142 |
| Georgia/Florida | 621 | 55,903 | 56,524 | 113 | 1814 | 1927 | 50 | 480 | 530 |
| Germany | 1011 | 18,277 | 19,288 | 187 | 687 | 874 | 140 | 166 | 306 |
| Sweden | 674 | 31,433 | 32,107 | 128 | 2303 | 2431 | 91 | 1526 | 1617 |
| Washington | 526 | 63,733 | 64,259 | 116 | 2437 | 2553 | 62 | 584 | 646 |
| Total | 4011a | 251,059 | 255,070a | 866 | 11,674 | 12,540 | 531a | 4444 | 4975a |
FDRs = first-degree relatives newborn of a person with T1D; Gen Pop = newborn without a first-degree relative with T1D.
Including 43 FDRs screened and 6 enrolled by the Pittsburgh and New York TRIGR sites.
TABLE 2.
Expected Number of Infants to Complete Screening, Eligible, Enrolled and Endpoints Ascertained
| General population | % of goal attained as of May 22, 2008 | FDRs | % of goal attained as of May 22, 2008 | Total | |
|---|---|---|---|---|---|
| Screened | 355,992 | 71% | 5,596 | 72% | 361,588 |
| Follow-up eligible | 16,588 | 70% | 1,216 | 71% | 17,804 |
| Enrolled | 7,013 | 63%a | 788 | 67%a | 7,801 |
| With persistent islet autoantibodies by 6 years of age | 281 | 53b | 105 | 16b | 386 |
| With type 1 diabetes by 15 years of age | 281 | 21b | 105 | 6b | 386 |
Enrollment is completed on average 3–4 months after screening, hence lower % of goal attained than for screening;
As of March 31, 2008.
Figure 3.
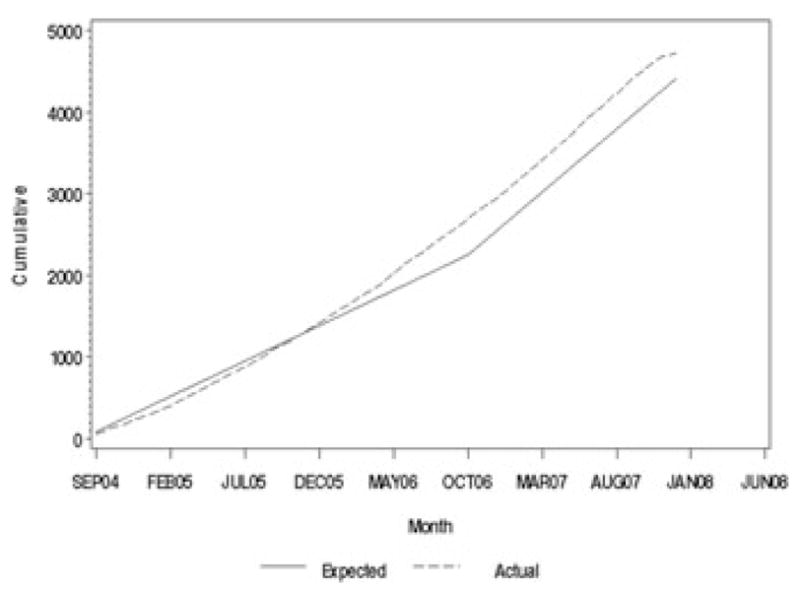
Cumulative number of enrolled subjects (data as of 3/31/08).
Eligible children are enrolled into intensive prospective follow-up before the age of 4.5 months.
The childrens’ exposure to dietary and other environmental factors is recorded at clinic visits every three months for the first 4 years of life and then biannually until age 15. Stool samples are collected to assess viral exposures at monthly intervals for the first 4 years and then biannually until age 15. The prospective data collection protocol is summarized in Table 3. Compliance rates to specific data collection components of the protocol are at high levels (Table 4).
TABLE 3.
Follow-up Protocol
| Age (months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | >24 q3 mo | >48 q6 mo | |
| Cord blood (mL) | 0.3–7.0 | ||||||||||
| Venous blood (mL) | |||||||||||
| Serum | 2.0 | 2.0 | 3.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | ||
| Plasma | 2.0 | 4.0 | 5.0 | 8.0 | 8.0 | 8.0 | 12.0 | 12.0 | 12.0 | ||
| mRNA (ABI) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | ||
| DNA | 1.0 | ||||||||||
| Total blood volume (mL) | 6.5 | 8.5 | 11.5 | 16.5 | 16.5 | 16.5 | 20.5 | 20.5 | 20.5 | TBD | |
Note: Follow-up included: stool samples (monthly); TEDDY Book (parental records of nutrition, infections, medications, and immunization); maternal pregnancy diet, child’s 24-hour recall, and 3-day food record; heights/weights (q 3 mo.); negative life events, parental distress, anxiety, and depression; family history; tap water, toenail clippings.
TABLE 4.
Percentage of Forms and Samples That Have Been Collected of TEDDY Study Visits with a Physical Exama
| (Forms/labs) | 3 mos. (N = 4418/4252) | 6 mos. (N = 3934/3763) | 9 mos. (N = 3432/3288) | 12 mos. (N = 2974/2853) | 15 mos. (N = 2555/2445) | 18 mos. (N = 2173/2031) | 21 mos. (N = 1768/1649) | 24 mos. (N = 1415/1300) | 27 mos. (N = 1103/991) | 30 mos. (N = 795/689) | 33 mos. (N = 500/413) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical exam | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Diet recall | 97 | 96 | 94 | 94 | 89 | 86 | 86 | ||||
| Serum | 99 | 99 | 99 | 100 | 99 | 99 | 98 | 95 | 97 | 99 | 99 |
| Plasma | 74 | 80 | 81 | 84 | 86 | 85 | 87 | 85 | 83 | 86 | 85 |
| mRNA | 62 | 71 | 73 | 76 | 79 | 79 | 81 | 79 | 79 | 83 | 82 |
| TEDDY book | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Percentages are based upon expected numbers. Data as of January 31, 2008.
Figure 4 indicates that there is a small early loss to follow-up after the initial couple of visits that is consistent with observations from previous similar cohort studies (DAISY in Colorado and DIPP in Finland). The loss seems to plateau after the subjects reach 1 year of age and is noticeably lower among the FDRs. The overall rate of disenrollment is 9% over 2 years, and 12% by the age of 30 months, well within the planning parameters of the study. Single missed visits range from 2% (month 27) to 7% (month 18). Data beyond 30 months are considered too recent to provide reliable estimates.
Figure 4.
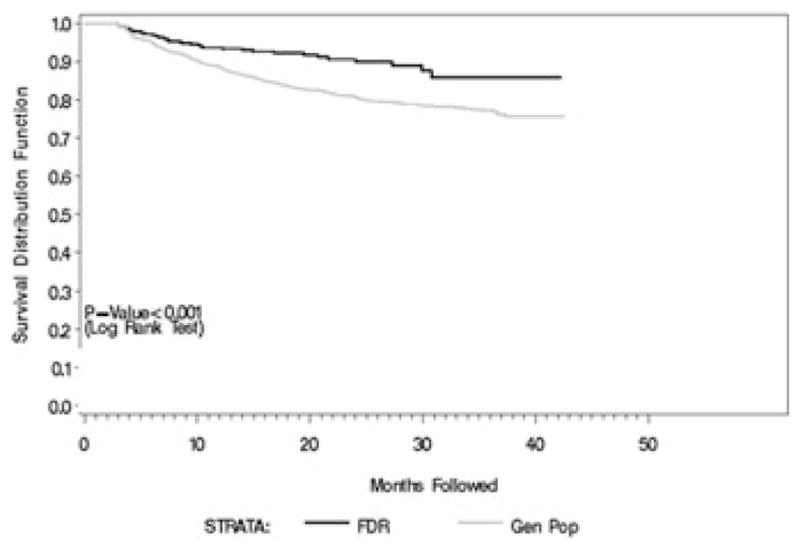
Early indicators of future retention (data as of 3/31/08).
Standardization of Assays for Islet Autoantibodies
TEDDY is unique among the NIDDK-sponsored studies in having two core autoantibody laboratories with confirmation of positive samples in both laboratories before samples are classified as positive. This strategy was agreed on (i) to have a high degree of certainty in positive results, an important consideration in view of the numerous samples tested for three autoantibodies from each subject, and (ii) to provide an internal mechanism for checking and improving assay performance. We believe that the strategy has proved highly successful in both of these areas. Having two laboratories has posed additional requirements with respect to establishing concordance and the TEDDY study has performed several tasks addressing this point.
Prior to inception of the study protocol, and in order to determine and improve concordance of measurement during TEDDY, both laboratories measured autoantibodies to insulin, GAD65, and IA-2 in serum samples from 496 nondiabetic high-risk children (from the DAISY study) and from 60 patients with new-onset disease. Analysis of these data showed that there were differences between calling sample positives caused by different relative thresholds in the two laboratories. The data were used to simulate performance (ROC analysis) and concordance at different thresholds and to determine the thresholds from each laboratory that were expected to be equivalent on a common sample set. These thresholds were selected for the TEDDY protocol.
Because of the number of studies using islet autoantibodies as an outcome or selection criteria and the observations made with respect to two laboratories in TEDDY, the NIDDK decided to endorse this proposal and to embark on developing a common method of measurement (Islet Autoantibody Measurement Harmonization Project).
Children are born with maternal IgG, including maternal islet autoantibodies, especially if the child has a mother with diabetes. It is necessary to exclude positive results that are due to this maternal IgG transmission when defining subject outcome. An algorithm was developed that considered islet autoantibody status of the mother (measured when the child was aged 6 or 9 months), whether a child had a negative sample prior to their first positive sample, and whether the islet autoantibody titer increased or decreased in subsequent samples.
TEDDY participants are considered persistently islet autoantibody–positive (major study end point) if they had at least two confirmed positive samples that were not due to maternal islet autoantibody transfer or if they had one confirmed positive sample and developed diabetes prior to the next sample collection. The study protocol requires that all positive samples and 5% of negative samples be tested in the second central laboratory. As of March 31, 2008, 53 children from the general population and 16 FDRs have developed persistent islet autoantibody. The cumulative incidence of this endpoint by the age of 40 months was 4.6% in FDRs and 2.8% in the general population children. However, some young FDRs may later be reclassified as having had maternal autoantibodies as data mature.
Development of Type 1 Diabetes
As of March 31, 2008, 21 children from the general population and 6 FDRs have developed T1D. The cumulative incidence of this endpoint by the age of 40 months was 2.5% in FDRs and 1.8% in the general population children. Preliminary observations suggest that the participation in TEDDY significantly reduced the severity of clinical presentation and has eliminated most of the expected hospitalizations and DKA in these very young patients.
Other Accomplishments
TEDDY has mapped the frequencies of T1D susceptibility genotypes in diverse populations including African Americans, Asian Americans, and Hispanics in the United States. Preliminary results have been presented elsewhere.63
The study developed novel comparisons and standardization between the four national TEDDY food databases (U.S., German, Swedish, and Finnish). A comprehensive nutrient data dictionary has been compiled of different nutrients that are calculated in TEDDY: units of measurement, methods of analysis, and derivations and descriptions of each nutrient in each TEDDY food database. Most nutrients are comparable between the databases, whereas some need to be recalculated (protein, energy) and some are not comparable between all of the four databases (fiber, folate), and all the databases do not contain all TEDDY nutrients (e.g., some of the fatty acids). The Finnish and Swedish national food databases (which both are used in TEDDY) take part in an EU program (Eurofir), which aims at harmonization of algorithms that will result in more uniform procedures (especially at the food level) and will, in the long run, benefit TEDDY. TEDDY has already discovered significant differences in infant feeding practices between the United States and Europe64 and is exploring variability in infant nutrition within the U.S. population.
The TEDDY protocol includes studies focused on identification of psychosocial factors that predispose to or protect from β cell autoimmunity and T1D. A secondary objective is to explore the psychosocial corollaries of the ascertainment of risk status for autoimmunity and T1D in newborns. In addition, the TEDDY Psychosocial Committee assists with developing appropriate procedures and identifying resources to assure adequate informed consent, minimize study burden, maximize procedure convenience/comfort, thank and support participants, and provide psychosocial support, as needed. Parent anxiety in response to the infants’ increased risk is assessed using a 6-item short form of Spielberger’s State Anxiety Inventory (SAI) administered when the child was 3–4.5 months old and again when the child is 6-months old. Postpartum depression is measured when the child is 6 months of age by the Edinburgh Post-natal Depression scale. Families that failed to enroll in TEDDY are asked to provide reasons for their decision. We also examined those who dropped out of TEDDY within 1 year of recruitment and found several important predictors. TEDDY has also uncovered significant differences between the levels of parental stress and postpartum depression between the U.S. and European societies.65
In conclusion, the TEDDY Consortium is nearing the final stages of the initial phase of the project, with more than 70% of the screening and 63% of the enrollment goals already achieved. The retention of study participants and compliance remain high despite the very demanding protocol.
Preliminary findings point to major inter-population differences in genetic susceptibility and candidate environmental risk factors for T1D.
Acknowledgments
This work was funded by Grants DK 63829, 63861, 63821, 63865, 63863, 63836, and 63790 and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and the Centers for Disease Control and Prevention (CDC).
Appendix
TEDDY Study Group
[Note: Superscript numbers refer to the Committees listed below.]
Colorado Clinical Center
Marian Rewers, M.D., Ph.D., PI,1,4,6,10,11,13 Katherine Barriga,12 Judith Baxter,9,12 George Eisenbarth, M.D., Ph.D., Patricia Gesualdo,2,12,14 Michelle Hoffman,12,13,14 Lisa Ide, Jill Norris, Ph.D.,2,12 and Kathleen Waugh.7,12 University of Colorado at Denver and Health Sciences Center, Barbara Davis Center for Childhood Diabetes.
Georgia/Florida Clinical Center
Jin-Xiong She, Ph.D., PI, 1,3,4,11 Andy Muir, M.D.,^7,13 Desmond Schatz, M.D.,*4,5,7,8 Diane Hopkins,12 Leigh Steed,12 Angela Choate,*12 Katherine Silvis,2 Meena Shankar,*2 Yi-Hua Huang, Ph.D., Ping Yang, Hong-Jie Wang, Jessica Leggett, Kim English, and Richard McIndoe, Ph.D., Angela Wilcox*, Michael Haller, M.D.*14 Medical College of Georgia, *University of Florida, and ^Emory University.
German Clinical Center
Anette G. Ziegler, M.D., PI,1,3,4,11 Julia Bollwein,2,12 Ezio Bonifacio, Ph.D.,*5 Sandra Hummel, Ph.D.,2 Mathilde Kersting,¥2 Stephanie Koenig,2 Annette Knopff,7 Lydia Rank,2,12 Roswith Roth, Ph.D.,^9 Stefanie Schoen,¥2 Petra Schwaiger,7 Wolfgang Sichert-Hellert, Ph.D.,¥2 and Christiane Winkler.2,12 Diabetes Research Institute and *Center for Regenerative Therapies, TU Dresden, ^Institute of Psychology, University of Graz, Austria, ¥Research Institute for Child Nutrition, Dortmund.
Finland Clinical Center
Olli G. Simell, M.D., Ph.D., PI,¥ ^,1,4,11,13 Kirsti Nanto-Salonen, M.D., Ph.D.,¥ ^,12 Jorma Ilonen, M.D., Ph.D.,¥¶,3 Mikael Knip, M.D., Ph.D.,*± Riitta Veijola, M.D., Ph.D., μ¤ Tuula Simell, Ph.D.,¥ ^,9,12 Heikki Hyöty, M.D., Ph.D.,*±,6 Suvi M. Virtanen, M.D., Ph.D.,*§,2 Carina Kronberg-Kippilä, §,2 Maija Torma,¥ ^,12,14 Barbara Simmell,¥ ^ Eeva Ruohonen,¥ ^ Minna Romo,¥ ^ Elina Mantymaki,¥ ^ Tiina Niininen,*± Mia Nyblom,*± and Aino Stenius.μ¤ ¥University of Turku, *University of Tampere, μUniversity of Oulu, ^Turku University Hospital, ±Tampere University Hospital, ¤Oulu University Hospital, and §National Public Health Institute, Finland, ¶University of Kuopio.
Sweden Clinical Center
Åke Lernmark, Ph.D., PI,1,3,4,5,8,10,11 Daniel Agardh, M.D.,13 Ph.D., Peter Almgren, Marie Andersson-Turpeinen, Carin Andrén-Aronsson,2,13 Maria Ask, Ulla-Marie Carlsson, Corrado Cilio, M.D., Ph.D., Jenny Bremer, Emilie Ericson-Hallström, Joanna Gerardsson, Barbro Gustavsson, Gertie Hansson,12,14 Ida Hansson, Monica Hansen, Susanne Hyberg, Rasmus Håkansson, Sten Ivarsson, M.D., Ph.D.,6 Jesper Johansoon, Helena Larsson M.D., Ph.D.,14 Barbro Lernmark, Ph.D.,9,12 Maria Markan, Jessica Melin, Maria Månsson-Martinez, Anita Nilsson, Kobra Rahmati, Monica Sedig Järvirova, Birgitta Sjöberg, Carina Törn, Ph.D., Anne Wallin, Ingrid Wigheden, and Åsa Wimar Lund University.
Washington Clinical Center
William A. Hagopian, M.D., Ph.D., PI,1,3,4,7,11,13 Peng Hui, M.D., Ph.D., Michael Brantley,7,12 Claire Cowen Crouch, Kristen M. Hay,2 Stephen Ayres, Carla Hammar, Viktoria Steptikova, Jenn Skidmore, Bonnie Bang, Denise Mulenga, Nicholas Vanneman,12 Judy Ewing, Isaac Whitaker, and Emily Wion. Pacific Northwest Research Institute.
New York Satellite Center
Robin S. Goland, M.D., Barney Softness, M.D., Ellen Greenberg, Diana Arnold,12 and Erica Arrecis2, and Lee Trope12 Columbia University, Naomi Berrie Diabetes Center.
Pennsylvania Satellite Center
Dorothy Becker, M.D., Margaret Franciscus,12 MaryEllen Dalmagro-Elias,2 and Ashi Daftary, M.D. Children’s Hospital of Pittsburgh of UPMC.
Data Coordinating Center
Jeffrey P. Krischer, Ph.D., PI,1,4,5,10,11 Michael Abbondondolo, Lori Ballard, London Bounmananh, Rasheedah Brown,12 David Cuthbertson, Christina Foster, Veena Gowda, Hye-Seung Lee, Ph.D., Shu Liu, Jamie Malloy, Cristina McCarthy,12 Wendy McLeod,2,9 Lavanya Nallamshetty, Susan Smith,12 Ulla Uusitalo, Ph.D.,2 Kendra Vehik, Ph.D., and Jimin Yang, Ph.D.2 University of South Florida.
Project officer
Beena Akolkar, Ph.D.,1,3,4,5,7,10,11 National Institutes of Diabetes and Digestive and Kidney Diseases.
Other contributors
Thomas Briese,6 Columbia University; Henry Erlich,3 Children’s Hospital Oakland Research Institute; Suzanne Bennett Johnson,9,12 Florida State University; and Steve Oberste,6 Centers for Disease Control and Prevention.
Committees
1Ancillary Studies, 2Diet, 3Genetics 4Human Subjects/Publicity/Publications, 5Immune Markers, 6Infectious Agents, 7Laboratory Implementation, 8Maternal Studies, 9Psychosocial, 10Quality Assurance, 11Steering, 12Study Coordinators, 13Celiac Disease, and 14Clinical Implementation.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Diabetes Epidemiology Research International Group. Secular trends in incidence of childhood IDDM in 10 countries. Diabetes. 1990;39:858–864. [PubMed] [Google Scholar]
- 2.Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl 3):B3–B8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 3.The DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 4.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 5.Kontiainen S, Scheinin T, Schlenzka A, et al. Differences in HLA types in children with insulin-dependent diabetes diagnosed in the 1960s, 1970s, and 1980s. Lancet. 1988;2:219. doi: 10.1016/s0140-6736(88)92320-3. [DOI] [PubMed] [Google Scholar]
- 6.Vehik K, Hamman RF, Lezotte D, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care. 2008 doi: 10.2337/dc07-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norris JM, Eisenbarth GS, Beaty B, et al. Dietary antibodies, infant diet and beta-cell autoimmunity: Diabetes Autoimmunity Study in the Young (DAISY) [abstract] Autoimmunity. 1995;21:22–A082. [Google Scholar]
- 8.Roll U, Christie MR, Füchtenbusch M, et al. Perinatal autoimmunity in offspring of diabetic parents. The German Multi-Center BABY-DIAB study: detection of humoral immune responses to islet antigens in early childhood. Diabetes. 1996;45:967–973. doi: 10.2337/diab.45.7.967. [DOI] [PubMed] [Google Scholar]
- 9.Kupila A, Erkkila S, Simell T, et al. The type 1 diabetes Prediction and Prevention Project in Finland: 4.5 years of population-based genetic screening, follow-up, and prevention. Diabetes. 2000;49:145. [Google Scholar]
- 10.Gale EA, Bingley PJ, Emmett CL, Collier T. European nicotinamide diabetes intervention trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 11.Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 12.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 13.Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Pediatr Diabetes. 2007;8:117–137. doi: 10.1111/j.1399-5448.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menser MA, Forrest JM, Bransby RD. Rubella infection and diabetes mellitus. Lancet. 1978;1:57–60. doi: 10.1016/s0140-6736(78)90001-6. [DOI] [PubMed] [Google Scholar]
- 15.Dahlquist G, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. Diabetes. 1995;44:408–413. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 16.Hyoty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM: Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 17.Dahlquist G, Kallen B. Maternal-child blood group incompatibility and other perinatal events increase the risk for early-onset type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:671–675. doi: 10.1007/BF00400261. [DOI] [PubMed] [Google Scholar]
- 18.Elfving AM, Lindberg BA, Landin-Olsson M, et al. Islet cell autoantibodies in cord blood from children with blood group incompatibility or hyperbilirubinemia. Autoimmunity. 2003;36:111–115. doi: 10.1080/0891693031000073109. [DOI] [PubMed] [Google Scholar]
- 19.Dahlquist GG, Patterson C, Soltesz G. Perinatal risk factors for childhood type 1 diabetes in Europe. The EURODIAB Substudy 2 Study Group. Diabetes Care. 1999;22:1698–1702. doi: 10.2337/diacare.22.10.1698. [DOI] [PubMed] [Google Scholar]
- 20.Stene LC, Barriga K, Norris JM, et al. Perinatal factors and development of islet autoimmunity in early childhood: the Diabetes Autoimmunity Study in the Young. Aje. 2004;160:3–10. doi: 10.1093/aje/kwh159. [DOI] [PubMed] [Google Scholar]
- 21.Blom L, Dahlquist G, Nystrom L, et al. The Swedish childhood diabetes study: social and perinatal determinants for diabetes in childhood. Diabetologia. 1989;32:7–13. doi: 10.1007/BF00265397. [DOI] [PubMed] [Google Scholar]
- 22.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–152. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 23.Harjutsalo V, Reunanen A, Tuomilehto J. Differential transmission of type 1 diabetes from diabetic fathers and mothers to their offspring. Diabetes. 2006;55:1517–1524. doi: 10.2337/db05-1296. [DOI] [PubMed] [Google Scholar]
- 24.Larsson HE, Lynch K, Lernmark B, et al. Diabetes-associated HLA genotypes affect birth-weight in the general population. Diabetologia. 2005;48:1484–1491. doi: 10.1007/s00125-005-1813-4. [DOI] [PubMed] [Google Scholar]
- 25.Hummel M, Marienfeld S, Huppmann M, et al. Fetal growth is increased by maternal type 1 diabetes and HLA DR4-related gene interactions. Diabetologia. 2007;50:850–858. doi: 10.1007/s00125-007-0607-2. [DOI] [PubMed] [Google Scholar]
- 26.Larsson HE, Lynch K, Lernmark B, et al. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50:1161–1169. doi: 10.1007/s00125-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 27.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 29.Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45:588–594. doi: 10.1007/s00125-002-0801-1. [DOI] [PubMed] [Google Scholar]
- 30.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler AG, Schmid S, Huber D, et al. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 32.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia. 2001;44:914–922. doi: 10.1007/s001250100548. [DOI] [PubMed] [Google Scholar]
- 33.Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia. 2006;49:20–24. doi: 10.1007/s00125-005-0076-4. [DOI] [PubMed] [Google Scholar]
- 34.Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45:1353–1361. doi: 10.1007/s00125-002-0852-3. [DOI] [PubMed] [Google Scholar]
- 35.Yoon JW, Austin M, Onodera T, Notkins A. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson MA, Bowman MA, Campbell L, et al. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47:225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 38.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makela M, Vaarala O, Hermann R, et al. Enteral virus infections in early childhood and an enhanced type 1 diabetes-associated antibody response to dietary insulin. J Autoimmun. 2006;27:54–61. doi: 10.1016/j.jaut.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Graves PM, Rotbart HA, Nix WA, et al. Prospective study of enteroviral infections and development of beta-cell autoimmunity. Diabetes Uutoimmunity Study in the Young (DAISY) Diabetes Res Clin Pract. 2003;59:51–61. doi: 10.1016/s0168-8227(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 41.Fuchtenbusch M, Irnstetter A, Jager G, Ziegler AG. No evidence for an association of coxsackie virus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J Autoimmun. 2001;17:333–340. doi: 10.1006/jaut.2001.0550. [DOI] [PubMed] [Google Scholar]
- 42.Ebringer A, Wilson C. HLA molecules, bacteria and autoimmunity. J Med Microbiol. 2000;49:305–311. doi: 10.1099/0022-1317-49-4-305. [DOI] [PubMed] [Google Scholar]
- 43.Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319–1324. doi: 10.2337/diabetes.49.8.1319. [DOI] [PubMed] [Google Scholar]
- 44.Makela M, Oling V, Marttila J, et al. Rotavirus-specific T cell responses and cytokine mRNA expression in children with diabetes-associated autoantibodies and type 1 diabetes. Clin Exp Immunol. 2006;145:261–270. doi: 10.1111/j.1365-2249.2006.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graves PM, Barriga KJ, Norris JM, et al. Lack of association between early childhood immunizations and beta-cell autoimmunity. Diabetes Care. 1999;22:1694–1697. doi: 10.2337/diacare.22.10.1694. [DOI] [PubMed] [Google Scholar]
- 46.DeStefano F, Mullooly JP, Okoro CA, et al. Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus. Pediatrics. 2001;108:E112. doi: 10.1542/peds.108.6.e112. [DOI] [PubMed] [Google Scholar]
- 47.Borch-Johnsen K, Joner G, Mandrup-Poulsen T, et al. Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus: a hypothesis. Lancet. 1984;2:1083–1086. doi: 10.1016/s0140-6736(84)91517-4. [DOI] [PubMed] [Google Scholar]
- 48.Virtanen SM, Kenward MG, Erkkola M, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512–1521. doi: 10.1007/s00125-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 49.Uusitalo L, Knip M, Kenward MG, et al. Serum a-tocopherol concentrations and risk of type 1 diabetes mellitus: a cohort study in siblings of affected children. J Pediatr Endocrinol Metab. 2005;18:1409–1416. doi: 10.1515/jpem.2005.18.12.1409. [DOI] [PubMed] [Google Scholar]
- 50.Leinonen JS, Alho H, Harmoinen A, et al. Unaltered antioxidant activity of plasma in subjects at increased risk for IDDM. Free Radic Res. 1998;29:159–164. doi: 10.1080/10715769800300181. [DOI] [PubMed] [Google Scholar]
- 51.Knekt P, Reunanen A, Marniemi J, et al. Low vitamin E status is a potential risk factor for insulin-dependent diabetes mellitus. J Intern Med. 1999;245:99–102. doi: 10.1046/j.1365-2796.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- 52.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 53.Haglund B, Ryckenberg K, Selinus O, Dahlquist G. Evidence of a relationship between childhood-onset type I diabetes and low groundwater concentration of zinc. Diab Care. 1996;19:873–875. doi: 10.2337/diacare.19.8.873. [DOI] [PubMed] [Google Scholar]
- 54.Dahlquist GG, Blom LG, Persson LA, et al. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ. 1990;300:1302–1306. doi: 10.1136/bmj.300.6735.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamb MM, Myers MA, Barriga K, et al. Maternal diet during pregnancy and islet autoimmunity in offspring. Pediatr Diabetes. 2008;9:135–141. doi: 10.1111/j.1399-5448.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 56.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–148. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 57.Rewers M, Liu E, Simmons J, et al. Celiac disease associated with type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2004;33:197–214. doi: 10.1016/j.ecl.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Simell S, Kupila A, Hoppu S, et al. Natural history of transglutaminase autoantibodies and mucosal changes in children carrying HLA-conferred celiac disease susceptibility. Scand J Gastroenterol. 2005;40:1182–1191. doi: 10.1080/00365520510024034. [DOI] [PubMed] [Google Scholar]
- 59.Danowski TS. Emotional stress as a cause of diabetes mellitus. Diabetes. 1963;12:183–184. doi: 10.2337/diab.12.2.183. [DOI] [PubMed] [Google Scholar]
- 60.Johnson SB. Screening programs to identify children at risk for diabetes mellitus: psychological impact on children and parents. J Pediatr Endocrinol Metab. 2001;14(Suppl 1):653–659. doi: 10.1515/jpem.2001.14.s1.653. [DOI] [PubMed] [Google Scholar]
- 61.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27:1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 62.The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8:286–29. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 63.Hagopian W, Rewers M, Lernmark Å, et al. for the TEDDY Study Group. Major ethnic differences in HLA-DR-DQ contribution to the type 1 diabetes (T1D) risk pool in The Environmental Determinants of Diabetes in the Young (TEDDY) study [abstract] Diabetes. 2007;56(Suppl 1) [Google Scholar]
- 64.Norris JM, Virtanen SM, Moyers S, et al. for the TEDDY Study Group. Infant feeding practices in The Environmental Determinants of Diabetes in the Young (TEDDY) study [abstract] Diabetes. 2006;55(Suppl 1) [Google Scholar]
- 65.Johnson SB, Lernmark B, Baxter J, et al. for the TEDDY Study Group. At-risk for type 1 diabetes (T1D): Parent anxiety in response to newborn genetic screening results in The Environmental Determinants of Diabetes in the Young (TEDDY) study [abstract] Diabetes. 2007;56(Suppl 1) [Google Scholar]
- 66.Health Care and Medical Services – Status Report 2006. The National Board of Health and Welfare; Stockholm, Sweden: 2006. [Google Scholar]
- 67.Neu A, Willasch A, Ehehalt S, et al. Diabetes incidence in children of different nationalities: an epidemiological approach to the pathogenesis of diabetes. Diabetologia. 2001;44(Suppl 3):B21–B26. doi: 10.1007/pl00002948. [DOI] [PubMed] [Google Scholar]
- 68.Rosenbauer J, Icks A, Giani G. Incidence and prevalence of childhood type 1 diabetes mellitus in Germany: model-based national estimates. J Pediatr Endocrinol Metab. 2002;15:1497–1504. doi: 10.1515/jpem.2002.15.9.1497. [DOI] [PubMed] [Google Scholar]


