Summary
Long-term potentiation (LTP) of mossy fiber EPSCs in the cerebellar nuclei is controlled by synaptic inhibition from Purkinje neurons. EPSCs are potentiated by a sequence of excitation, inhibition, and disinhibition, raising the question of how these stimuli interact to induce plasticity. Here, we find that synaptic excitation, inhibition, and disinhibition coupled to different calcium-dependent signaling pathways. In LTP induction protocols, constitutively active calcineurin can replace synaptic excitation, and constitutively active α-CaMKII can replace calcium influx associated with resumption of spiking upon disinhibition. Additionally, nimodipine can replace hyperpolarization, indicating that inhibition of firing decreases Ca influx through L-type Ca channels, providing a necessary signal for LTP. Together, these data suggest that potentiation develops after a calcineurin priming signal combines with an α-CaMKII triggering signal if and only if L-type Ca current is reduced. Thus, hyperpolarization induced by synaptic inhibition actively controls excitatory synaptic plasticity in the cerebellar nuclei.
Keywords: deep cerebellar nuclei, CaMKII, calcineurin, rebound, Purkinje, inhibition, LTP, eyelid conditioning
Introduction
Coincidence detection is a central component of cellular plasticity rules that are thought to underlie associative learning. In many quiescent neurons, coincidence detection depends on NMDA receptors that open only when glutamate binding coincides with membrane depolarization, permitting a calcium influx that drives long-term potentiation (LTP) (Kelso et al., 1986; Sastry et al., 1986; Bliss and Collingridge, 1993; Malenka et al., 1988; Lisman, 1989; McGlade-McCulloh et al., 1993). By contrast, in neurons of the cerebellar nuclei, which fire spontaneously, alternative plasticity mechanisms have emerged. In these cells, which receive strong inhibitory input from Purkinje cells as well as excitatory input from mossy fibers, pairing NMDA-receptor activation with postsynaptic spiking fails to potentiate EPSCs (Aizenman and Linden, 1999; Pugh and Raman, 2006). Instead, a synapse-specific LTP is induced by a repeated sequence of high-frequency excitation, hyperpolarization, and the relief of inhibition (Pugh and Raman, 2006; 2008). This induction protocol resembles stimuli that occur during delay eyelid conditioning, suggesting that this form of potentiation may be relevant to associative motor learning (McCormick and Thompson, 1984; Medina and Mauk, 1999; Hesslow et al., 1999; Jirenhed et al., 2007). Moreover, the requirement for both excitation and inhibition supports models of cerebellar learning predicting that potentiation of EPSCs in targets of Purkinje neurons is controlled by synaptic inhibition (Miles and Lisberger, 1981; Medina and Mauk, 1999).
This type of LTP raises the mechanistic question of how inhibition might interact with excitation to generate plasticity. Both excitation and disinhibition elicit calcium influx, via NMDA receptors and voltage-gated calcium channels, respectively, consistent with the calcium-dependence of potentiation (Pugh and Raman, 2006). Additionally, inhibiting either calcineurin or CaMKII prevents LTP induction, consistent with the idea that plasticity requires activity of multiple calcium-dependent enzymes. Moreover, the relative timing of the stimuli determines the efficacy of induction protocols: to generate LTP, excitation must precede disinhibition by less than 400 ms. These data suggest that excitation first primes activated synapses and a well-timed disinhibition then triggers potentiation (Pugh and Raman, 2008). Nevertheless, a central question is whether hyperpolarization serves only to facilitate calcium influx upon disinhibition (Llinás and Mühlethaler, 1988; Aizenman et al., 1998), or whether it also provides an independent signal, possibly by reducing tonic calcium levels generated by spontaneous firing (Muri and Knöpfel, 1994; Nelson et al., 2003; 2005; Pugh and Raman, 2008; Zheng and Raman, 2009). Distinguishing between these possibilities, however, is complicated by the fact that inhibition and disinhibition are electrophysiologically coupled.
In the present study, we reasoned that if different components of the induction protocol activate different signaling pathways, then inhibition and disinhibition might be dissociated biochemically. Therefore, to address the question of how NMDA receptors, voltage-gated calcium channels, and inhibition activate signaling pathways that induce mossy-fiber LTP, we applied modified induction protocols to cerebellar nuclear neurons infused with activated enzymes. We found that during LTP induction, activated calcineurin could replace excitatory synaptic stimulation and activated CaMKII could replace disinhibition. When excitation and disinhibition were both mimicked by activated enzymes, however, EPSCs were potentiated only when intracellular calcium was reduced by buffering, L-type calcium channel blockade, or hyperpolarization. Thus, synaptic inhibition plays the active signaling role of reducing calcium influx through L-type calcium channels, which is necessary to permit specific calcium-dependent enzymes to induce LTP.
Results
In the cerebellar nuclei, mossy fiber-mediated EPSCs are potentiated by a repeated sequence of synaptic excitation, inhibition (either by synaptic stimulation or by hyperpolarizing current injection), and disinhibition that evokes voltage-gated calcium influx (Pugh and Raman, 2006; 2008). In the present experiments, LTP was elicited in spontaneously firing cerebellar nuclear cells with a 100-Hz, 150-ms train of EPSPs, followed by a ~20-mV hyperpolarizing current injection that silenced firing for 250 ms before disinhibition and the resumption of spiking. This stimulus was applied 30 times at 0.2 Hz. This protocol elicits LTP in tissue from P13-16 mice (Figure 1A, right), as well as in older animals, P28–32 (Figure S1; EPSC change, 29.9 ± 11.4 %, n = 6; p < 0.05).
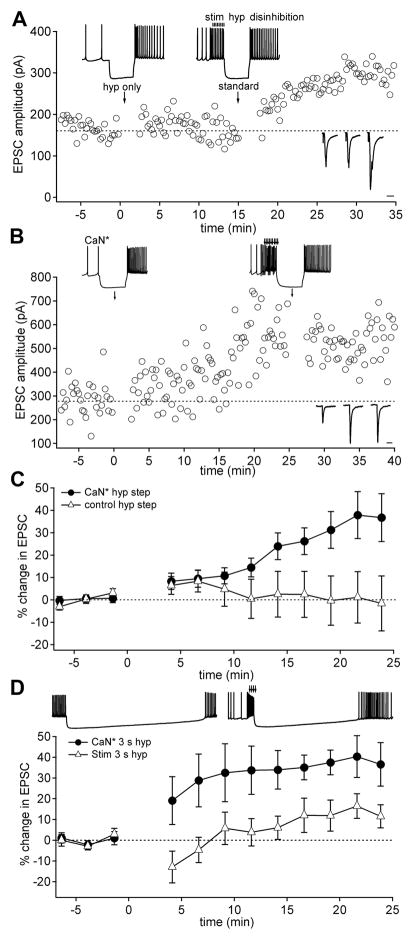
Figure 1.
Calcineurin substitutes for synaptic excitation in the mossy fiber LTP protocol. (A) EPSC amplitudes before and after hyperpolarizing steps (“hyp only”; t = 0; upper inset, left) and the standard induction protocol (“standard”; t = 15 min; upper inset, right). Right lower insets: example EPSCs before and after the two induction protocols. Scale bar, 10 ms. (B) EPSC amplitudes in the presence of active calcineurin (CaN*) before and after the hyperpolarizing step conditioning protocol (applied at t = 0; upper inset, left) and the standard induction protocol (applied at t = 25 min; upper inset, right). Right lower insets show example EPSCs before and after the two protocols. (C) Mean EPSC amplitudes before and after hyperpolarizing steps in neurons infused with CaN* (closed circles; n = 11) or in control conditions (open triangles; n = 8). Error bars represent s.e.m. (D) Mean EPSC amplitudes before and after 3-sec hyperpolarizing steps applied either with synaptic stimulation (open triangles; n = 6) or in the presence of CaN* (closed circles; n=6).
Previous work indicates that the LTP induced by this standard protocol requires calcium influx, likely through NMDA receptors and voltage-gated calcium channels, and it is prevented by blockers of the calcium-dependent enzymes calcineurin and CaMKII (Pugh and Raman, 2006; 2008). To test whether synaptic excitation and disinhibition couple differentially to these enzymes, we infused cerebellar nuclear neurons with activated enzymes and applied modified induction protocols that restricted the sources of calcium influx to either voltage-gated channels or synaptic receptors. Because NMDA receptor activation stimulates calcineurin at other synapses (Mulkey et al., 1994), we first tested whether constitutively active calcineurin (CaN*) could replace synaptic excitation in inducing LTP. In these experiments, the induction protocol consisted of only a hyperpolarizing current step that interrupted spontaneous spiking, with no synaptic excitation. With control intracellular solutions, this protocol did not potentiate EPSCs (Figure 1A, Figure 1C; EPSC change, 1.25 ± 8.5%; n = 8; p > 0.05, baseline vs. postconditioning), although EPSCs could still be potentiated by the standard protocol (Figure 1A). With intracellular CaN* (100 U/ml), however, EPSCs potentiated by 20.4 ± 4.8% (Figure 1B, Figure 1C; n = 13; p < 0.01), reaching 36.7 ± 10.6% at 24 min after the induction protocol. After CaN*-mediated LTP, EPSC amplitudes did not further increase in response to the standard protocol (Figure 1B; EPSC change 5 min pre vs. post standard protocol: −16.0 ± 7.0%; n = 6; p > 0.05), demonstrating that enzyme-induced LTP occluded synaptically driven LTP. These results suggest that synaptic excitation activates calcineurin during LTP induction, implicating calcineurin in priming synapses for subsequent potentiation.
For protocols to be effective at inducing LTP, excitation must precede the offset of inhibition by no more than 400 ms (Pugh and Raman, 2008), suggesting that the putative priming signal has a limited lifetime. To test whether the temporal constraints on the inhibition-disinhibition sequence might be relieved by tonic activation of calcineurin, we assessed the efficacy of induction by synaptic excitation or CaN* when the period of hyperpolarization was prolonged. When the hyperpolarizing current injection after the train of synaptic stimuli was prolonged more than 10-fold (3 sec), EPSCs were not significantly potentiated (EPSC change, 10.5 ± 6.4%, n = 6; p > 0.1). Conversely, when CaN* replaced synaptic excitation, EPSCs increased by 35.6 ± 8.3% (Figure 1D, n = 6; p < 0.05), reaching 36.5 ± 10.5% at 24 min after the induction protocol. Thus, in the presence of CaN*, LTP induction indeed became insensitive to the duration of hyperpolarization, suggesting that a decay in calcineurin activity after synaptic stimulation defines the temporal window in which combined excitation and disinhibition can elicit LTP.
Because the hyperpolarizing step protocol includes spontaneous firing, inhibition, and resumption of firing, we next examined which of these features interact with CaN* to generate LTP. We began by testing whether spontaneous firing in the presence of CaN* was sufficient to induce LTP. Although spontaneous action potentials alone generate substantial voltage-gated calcium influx (Zheng and Raman, 2009), the combination of CaN* and spiking did not potentiate EPSCs (Figure 2A; EPSC change, −8.1± 4.2%, n = 7; p > 0.05). Tonically hyperpolarizing neurons to −70 mV in the presence of CaN*, however, did not lead to potentiation either (Figure 2A; EPSC change, −9.8 ± 6.3%, n = 6; p > 0.05). Finally, CaN* infusion along with synaptic excitation also failed to induce LTP (Figure 2A; −14.1 ± 9.6%, n = 8; p > 0.05). Together, these data suggest that signals generated by both action potential firing and inhibition are necessary to induce LTP in the presence of CaN*.
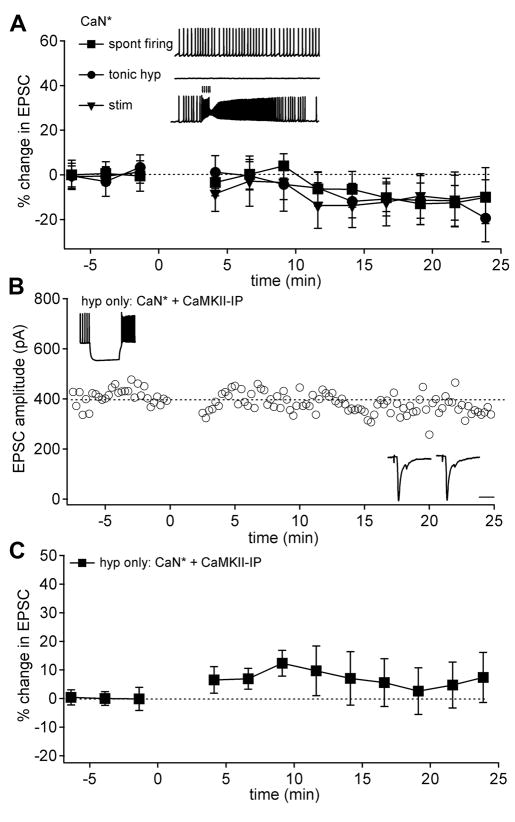
Figure 2.
CaN* combines specifically with disinhibition and requires CaMKII to induce LTP. (A) Upper panel: Examples of alternative induction protocols combined with CaN* infusion. Lower panel: Mean EPSC amplitudes before and after the protocols shown above; tonic hyperpolarization (circles; n = 6), spontaneous firing (squares; n = 7), synaptic excitation (down triangle; n = 8). (B) EPSC amplitudes in neuron infused with CaN* and CaMKII inhibitory peptide (fragment 290–309, 25 μM) before and after a hyperpolarizing step conditioning protocol at t = 0. Inset conventions as in Figure 1. (C) Mean EPSC amplitudes before and after hyperpolarizing step only protocol in neurons infused with CaN* and CaMKII inhibitors (inhibitory peptide, n=9; KN-62, n=1).
Because blockade of CaMKII activity interferes with LTP induction (Pugh and Raman 2008), we next investigated whether the pattern of inhibition and disinhibition might activate CaMKII within the protocol that included only the hyperpolarizing step. Including a CaMKII inhibitor with CaN* indeed prevented EPSCs from potentiating in response to hyperpolarizing steps that interrupted firing (Figure 2B, Figure 2C; EPSC change 5.8 ± 7.2%, n =10; p > 0.1), supporting the hypothesis that activation of CaMKII provides the signal that triggers potentiation.
We next tested whether inhibition acted as an independent signal in the LTP protocol. If hyperpolarization were simply required to activate CaMKII upon disinhibition, then a modified induction protocol consisting only of synaptic excitation in the absence of inhibition should induce LTP with constitutively active α-CaMKII (CaMKII*) added to the intracellular solution. These experiments required the addition of α-CaMKII as well as Ca and CaM to activate the α-CaMKII. We therefore verified that the addition of free Ca did not significantly affect LTP by testing the efficacy of the standard protocol with 50 nM free Ca in the intracellular solution. Indeed, under these conditions, LTP developed normally in all cells (EPSC change 45.0 ± 15.2%, n = 4). With either control intracellular solutions or infusion of heat-inactivated α-CaMKII, synaptic excitation alone of spontaneously firing neurons induced a significant depression of EPSCs (Figure 3A, Figure 3D; control: EPSC change, −18.6 ± 6.3%, n = 6; p < 0.05; heat-inactivated CaMKII: EPSC change −25.2 ± 8.5%, n = 6; p < 0.05; p > 0.5 vs. control). These data are consistent with previous work demonstrating LTD induction by repeated, high-frequency trains of excitation applied to depolarized cells (Zhang and Linden, 2006; Pugh and Raman, 2008). When CaMKII* (200 nM) was included in the pipette, however, EPSC amplitudes did not depress, but instead remained stable (Figure 3B, Figure 3D; change in EPSC 4.4 ± 9.0%, n=10; p > 0.5). Similar results were obtained when synaptic excitation was prolonged to 250 ms (EPSC change, −9.5 ± 10.6, n = 13; p > 0.1). EPSCs also remained stable in the presence of CaMKII* when the induction protocol included only spontaneous firing in the absence of synaptic excitation, with or without a hyperpolarizing current injection (EPSC change, −7.0 ± 10.0%, n = 6; p>0.5; and −6.7 ± 6.8%; n = 6; p>0.1). Thus, although CaMKII* interfered with the development of LTD following EPSP trains, in none of these protocols did it trigger LTP.
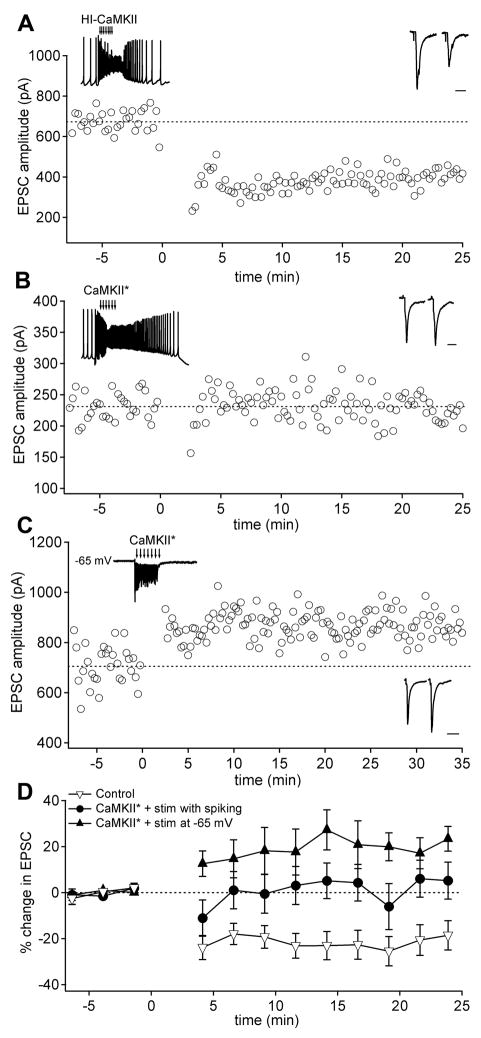
Figure 3.
Constitutively active CaMKII* combines with synaptic excitation and hyperpolarization to trigger LTP. (A) EPSC amplitudes in the presence of heat inactivated CaMKII (HI-CaMKII) before and after trains of EPSPs. (B) EPSC amplitudes in the presence of active CaMKII (CaMKII*) before and after trains of EPSPs delivered to a spontaneously firing neuron. (C) EPSC amplitudes before and after excitatory synaptic trains delivered to a neuron held at −65 mV (inset). (D) Mean EPSC amplitudes before and protocols illustrated in A–C: neurons infused with either HI-CaMKII or no enzyme (open triangles; n = 6 in each condition; pooled); CaMKII* with spontaneous firing (circles; n = 10); or CaMKII* in voltage clamped neurons (closed triangles; n = 6).
These observations raised the possibility that hyperpolarization as well as CaMKII* might be necessary for LTP induction. We therefore applied trains of synaptic excitation to neurons voltage-clamped to −65 mV. Under these conditions, mossy fiber stimulation still elicits synaptic calcium influx owing to weak Mg2+ block of NMDA receptors (Audinat et al., 1990; Anchisi et al., 2001; Pugh and Raman, 2006; 2008). With intracellular CaMKII*, this protocol potentiated EPSCs (Figure 3C, Figure 3D; EPSC change, 20.7 ± 6.7%, n = 6; p < 0.05; 23.4 ± 5.4% after 24 min), while with heat-inactivated CaMKII, EPSCs depressed (EPSC change, −32.0 ± 12.8, n = 8; p < 0.05; p < 0.01 vs. CaMKII*). These data indicate that CaMKII* can indeed replace disinhibition. Importantly, however, they also suggest that the hyperpolarizing step during the LTP induction protocol plays a signaling role distinct from setting up post-inhibitory firing that activates CaMKII.
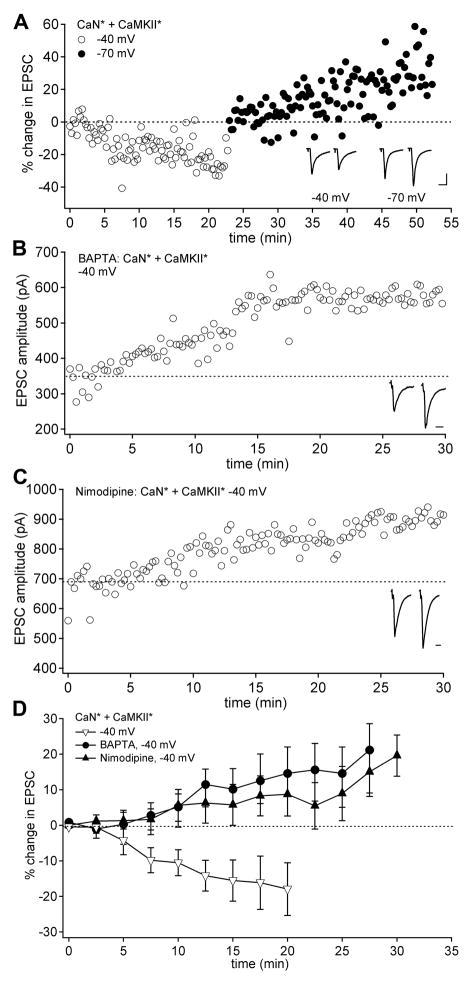
To explore further the relationship between membrane potential and the Ca-dependent pathways, we tested whether the combination of CaMKII* and CaN* was sufficient to induce plasticity by monitoring EPSCs in neurons held at −70 mV that were dialyzed with one or both enzymes. With both CaN* and CaMKII* present, EPSC amplitudes significantly increased over 20 minutes (Figure 4A, Figure 4C; EPSC change, 18.6 ± 4.8%, n = 15; p < 0.01, reaching 35.8 ± 13.5% after 25 min), and this run-up occluded LTP induction by the standard protocol (Figure 4A; EPSC change −14.7 ± 14.5%, n = 3). EPSC amplitudes remained stable when only one of the enzymes was included, however (Figure 4B, Figure 4C; EPSC change, 2.98 ± 7.0%, n = 11; p > 0.5). These results indicate that the activation of both enzymes generates an LTP-like run-up of EPSC amplitude at −70 mV. Because hyperpolarization was a necessary component of effective induction protocols, we reasoned that a tonic calcium load produced by activation of voltage-gated channels during firing (Zheng and Raman, 2009) might interfere with LTP induction. To test this possibility, we repeated the co-infusion experiment in cells held at −40 mV, a potential that activates voltage-gated calcium channels in nuclear cells (Zheng and Raman, 2009). In contrast to responses at −70 mV, EPSCs ran down in amplitude at −40 mV (Figure 5A, Figure 5D, EPSC change, −13.2 ± 2.3%, n = 9; p < 0.005). In a subset of these neurons (7/9), the voltage was switched to −70 mV, which reversed the downward trend, instead permitting EPSCs to increase in amplitude (Figure 5A, EPSC change from the point of switch, 9.1 ± 4.0%; n = 7; p < 0.005 vs. changes at −40 mV). Thus, the CaN*/CaMKII*-dependent EPSC run-up was voltage-sensitive, raising the possibility that the standing calcium influx that is predicted to occur at −40 mV interferes with run-up.
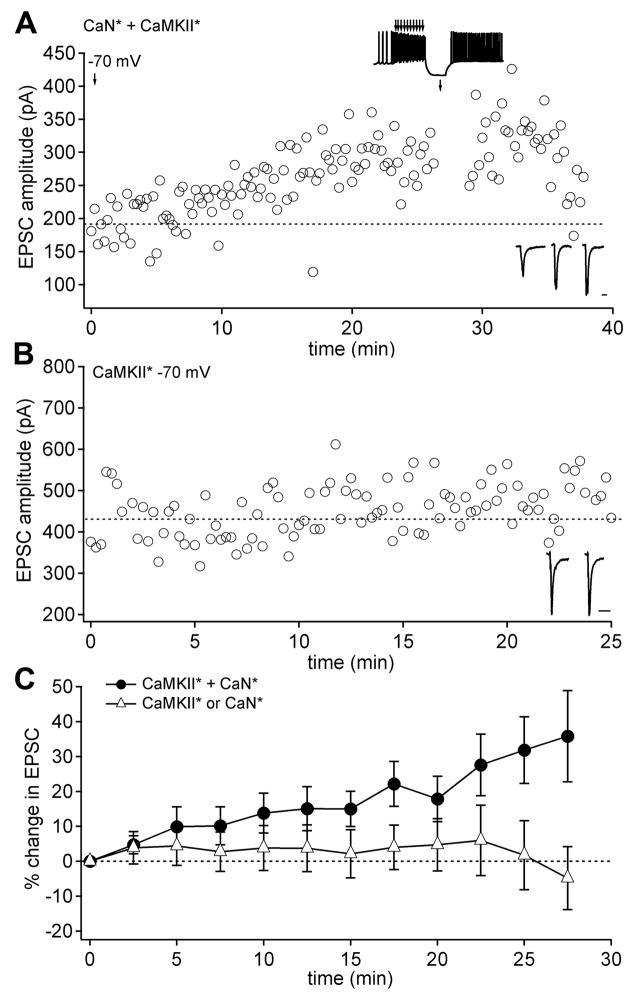
Figure 4.
EPSC amplitudes run up with CaN* and CaMKII* infused together in neurons held at −70 mV. (A) EPSC amplitudes at −70 mV in a neuron infused with both CaN* and CaMKII*. The standard conditioning protocol was delivered at t = 27 min (upper inset). (B) EPSC amplitudes in a neuron infused only with CaMKII*. (C) Mean EPSC amplitudes in neurons held at −70 mV and infused with both CaN* and CaMKII* (circles; n = 17) or either enzyme alone (triangles; n = 11).
Figure 5.
Calcium blocks CaN*/CaMKII*-induced EPSC run up. (A) EPSC amplitudes in neurons infused with both CaN* and CaMKII* and held at −40 mV (open circles) and at −70 mV (closed circles). EPSC amplitudes were normalized to the first 10 measurements at each voltage. Scale: 200 pA, 10 ms. (B) EPSCs recorded at −40 mV in neurons infused with 10 mM BAPTA, CaN* and CaMKII*. (C) EPSCs at −40 mV in neurons infused with CaN* and CaMKII* during bath application of 10 μM nimodipine. (D) Mean EPSC amplitudes at −40 mV for neurons infused with both CaN* and CaMKII* in control solutions (open triangles; n = 9), with BAPTA (circles; n = 7) or in nimodipine (closed triangles; n = 6).
To test more directly whether intracellular calcium inhibited the LTP-like run-up of EPSC amplitude, neurons held at −40 mV were infused with the fast calcium chelator BAPTA (10 mM), along with CaN* and CaMKII*. Under these conditions, rather than running down, EPSC amplitudes tended to increase (Figure 5B, Figure 5D; EPSC change, 14.9 ± 9.8%, n=7; p < 0.05 vs. −40 mV EGTA control), reaching 21.2 ± 15.9% after 25 min. Moreover, this run-up was indistinguishable from that measured at −70 mV (p > 0.5). Finally, because sustained calcium influx at −40 mV is likely to include a large contribution of L-type calcium current in these neurons (Zheng and Raman, 2009), we tested whether blocking L-type calcium channels might permit run-up at −40 mV even with normal buffering (1 mM EGTA). Indeed, in the presence of nimodipine (10 μM), EPSCs significantly increased (Figure 5C, Figure 5D, EPSC change, 10.3 ± 7.4%, n = 6; p < 0.05 vs. −40 mV; p > 0.1 vs. −70 mV), reaching 15.1± 5.9% after 25 min. Thus, calcium influx, at least in part through L-type calcium channels, prevents CaN*/CaMKII*-induced potentiation, suggesting that the drop in calcium induced by hyperpolarization provides a distinct and necessary signal in generating LTP.
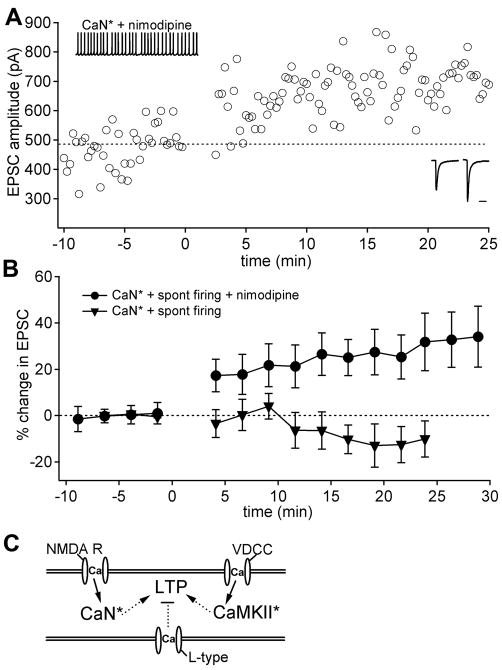
If the primary role of inhibition is to create a permissive condition for LTP by deactivating L-type calcium channels, then nimodipine should be able to replace the hyperpolarizing step in the induction protocol. If, however, hyperpolarization also recruits T-type or other low-voltage-activated channels that must activate upon disinhibition, then LTP will not proceed without a hyperpolarizing step, even when L-type channels are blocked. To distinguish between these possibilities, we tested whether the presence of nimodipine could convert the ineffective protocol of spontaneous firing with activated CaN* without hyperpolarization (Figure 2A) into one that generated LTP. Under these conditions, EPSCs potentiated by 25.9 ± 9.1% (Figure 6A, Figure 6B; n = 5; p < 0.05), reaching 32.7 ± 9.1% after 25 min, in contrast to EPSCs recorded with the same stimuli without nimodipine, which did not potentiate. These results rule out the idea that disinhibition itself provides a specific calcium signal for LTP induction. Instead, they support the idea that spontaneous firing maintains a basal activation of α-CaMKII (Nelson et al., 2003) as a result of calcium influx through high-voltage-activated (HVA) Ca channels, but that L-type current alone prevents LTP induction. Inhibition therefore plays the direct role of suppressing L-type current, thereby allowing LTP to proceed.
Figure 6.
CaN* and spontaneous firing induce LTP without hyperpolarization in the presence of the L-type Ca channel blocker nimodipine. (A) EPSCs before and after conditioning protocol of CaN* and spontaneous firing in the presence of nimodipine (10 μM). (B) Mean EPSC amplitudes before and after conditioning protocols with nimodipine present (open circles; n = 5) or absent (triangles; data from Figure 2). (C) Diagram summarizing the interaction of Ca-dependent pathways that regulate LTP. Ca influx through NMDA receptors activates calcineurin. Activation of α-CaMKII by Ca influx through voltage-dependent Ca channels (VDCC) triggers LTP if the suppressive effect of L-type Ca current is reduced by hyperpolarization-driven closure of these channels.
Discussion
Together, the results demonstrate that excitation, inhibition, and firing each regulate calcium in distinct ways to potentiate mossy fiber EPSCs in the cerebellar nuclei. Synaptic excitation activates the calcium-dependent phosphatase calcineurin; inhibition prevents spiking, thereby decreasing calcium influx through L-type calcium channels; and the resumption of firing upon disinhibition restores the spike-dependent calcium influx that activates α-CaMKII. The data support a model in which calcineurin primes synapses for later potentiation, and the reduction in tonic calcium by inhibition creates a condition that is permissive for potentiation, which is then triggered by α-CaMKII. Further, they illustrate that the hyperpolarization associated with synaptic inhibition actively controls synaptic plasticity by regulating L-type calcium channel-dependent processes that otherwise prevent the induction of potentiation (Figure 6C).
Calcineurin and mossy-fiber LTP
Within the standard induction sequence of synaptic excitation, inhibition, and disinhibition, CaN* successfully substituted for synaptic excitation. Precedent for a role for phosphatases in inducing LTP comes from the postsynaptic LTP of parallel fibers onto Purkinje cells (Belmeguenai and Hansel, 2005). Since mossy fiber LTP in the cerebellar nuclei is prevented by antagonists of NMDA receptors (Pugh and Raman, 2006), it seems likely that trains of EPSPs normally activate endogenous calcineurin via calcium influx through NMDA receptors. Moreover, the synapse-specificity of LTP (Pugh and Raman, 2008) suggests that the action of calcineurin generally serves to prime activated synapses for potentiation. Here, we observe that synaptic excitation followed by long hyperpolarizations did not induce LTP, but that, in the presence of constitutively active calcineurin, long hyperpolarizations did not prevent potentiation. These data suggest that the priming signal decays within a few hundred milliseconds, during which time the resumption of firing, presumably restoring α-CaMKII activity, is required for triggering to occur.
Interestingly, CaN* was not only able to replace synaptic stimulation in effective induction protocols, but it also made EPSCs less susceptible to a net long-term depression. At least four stimulus protocols have been shown to induce long-term depression in cerebellar nuclear cells, all of which consist of brief, high-frequency trains of synaptic excitation that neither overlap with nor are closely followed by a hyperpolarization (Figure 3, Zhang and Linden, 2006, Pugh and Raman, 2008). When CaN* replaces synaptic stimulation, however, similar protocols fail to depress EPSCs (Figure 2), suggesting that CaN* biases EPSCs toward potentiation. Consistent with this idea, blocking calcineurin activation during the standard protocol elicits LTD (Pugh and Raman, 2008). Together, these data raise the possibility that high-frequency stimulation of glutamatergic afferents couples to multiple pathways in cerebellar nuclear neurons, some of which favor depression and others of which favor potentiation. Increasing calcineurin activity shifts the balance toward LTP, which may be accomplished either by preventing depression or by simply outweighing it to produce a net potentiation.
Disinhibition and α-CaMKII activation
Multiple experiments support the conclusion that activation of α-CaMKII is necessary to trigger potentiation of primed synapses. Because CaN* in spontaneously firing cells induces LTP as long as L-type Ca channels are blocked, it appears likely that Ca influx through HVA calcium channels that open during spontaneous action potentials is sufficient to activate α-CaMKII. Importantly, this observation rules out the possibility that CaMKII activation is coupled specifically to the activation of T-type or other LVA channels. In previous work, we found that LVA calcium channels that are activated after periods of strong hyperpolarization of voltage-clamped neurons were sufficient to trigger LTP (Pugh and Raman, 2006), consistent with the idea that it is possible to activated α-CaMKII by calcium influx associated specifically with disinhibition. Because of the high density of T-type calcium currents in the cerebellar nuclei (Llinás and Mühlethaler, 1988; Molineux et al., 2006), these data initially seemed to support a specific role for T-type channel activation. Subsequent studies, however, demonstrated that the relief of synaptic inhibition does not elicit sizable T-type currents because inhibition hyperpolarizes these neurons to voltages that permit only little T-channel recovery (Alviña et al., 2008; Zheng and Raman, 2009). Nevertheless, synaptically mediated inhibition is sufficient to induce LTP (Pugh and Raman, 2008), indicating that calcium influx through high voltage activated channels is sufficient to trigger LTP. Moreover, although spontaneous firing opens calcium channels that support a tonic calcium load, Ca levels do not rise above baseline upon synaptic disinhibition (Zheng and Raman, 2009). Thus, within the standard protocol, disinhibition evokes neither a special source of calcium, nor a particularly large calcium signal.
The present results provide more direct evidence that activation of α-CaMKII does not depend on calcium influx specifically associated with disinhibition, but that it is already activated in the basal state by the spikes that precede inhibition, as observed in the medial vestibular nuclei (Nelson et al., 2003; 2005). Nevertheless, with synaptic activation of calcineurin, the duration of hyperpolarization cannot be indefinitely prolonged, suggesting that α-CaMKII activity, too, falls off with inhibition. It therefore seems likely that the calcium influx associated with post-inhibitory firing restores α-CaMKII activity, providing a necessary signal for induction of LTP.
Inhibition and permissive conditions for LTP
Inhibition during the induction protocol does not, however, simply set up disinhibition; rather, it plays a distinct and necessary role in LTP induction by reducing L-type Ca current that interferes with the induction of plasticity. Tonic spiking therefore prevents potentiation, even in the face of synaptic excitation and basal activation of α-CaMKII, while synaptic inhibition actively regulates the strengthening of excitatory synapses by decreasing or interrupting ongoing action potentials and thereby reducing calcium influx through L-type channels. Thus, even in the absence of post-inhibitory (rebound) bursts of action potentials that evoke calcium influx, inhibition can couple to calcium-dependent intracellular signaling pathways in a manner that is relevant to plasticity. A similar situation exists in the medial vestibular nuclei, in which long-term increases in intrinsic excitability can be induced by repeated periods of inhibition. There, however, the reduction in basal firing and consequent drop in calcium levels decreases α-CaMKII activity, modulating ion channels that mediate excitability (Nelson et al., 2005), whereas, in the cerebellar nuclei, inhibition relieves a calcium-dependent repressor of potentiation that is coupled to L-type calcium channels.
Molecular mechanisms of LTP expression
An interesting feature of mossy fiber LTP in the cerebellar nuclei is its dependence on a kinase and phosphatase that antagonize one another in other cell types. For example, in hippocampal CA1 pyramidal neurons, CaMKII and calcineurin are mutually inhibitory and trigger LTP and LTD, respectively (McGlade-McCulloh et al., 1993; Mulkey et al., 1994; Lledo et al., 1995; Lisman, 1989; Lisman et al., 2001). The present data nevertheless suggest a straightforward model in which calcineurin and α-CaMKII can work together to induce LTP. Since the paired pulse ratio and the variance of EPSC amplitudes remain unchanged after potentiation cerebellar mossy fiber LTP is likely expressed postsynaptically (Pugh and Raman, 2008). At calcineurin-primed synapses, α-CaMKII may (directly or indirectly) facilitate the insertion of AMPA receptors, while a distinct calcium-dependent process facilitates internalization of AMPA receptors. With high tonic calcium levels attained by spontaneous and driven firing of action potentials, synaptic strength may be actively maintained by balanced receptor turnover. With tonic low calcium, synaptic strength may be passively maintained by little or no turnover. When inhibition is followed by disinhibition, however, the changes in tonic calcium may affect the calcium-dependent signaling pathways differentially, yielding a net increase in the action of α-CaMKII and/or down-regulation of the processes driving internalization. In this way, insertion may be favored over internalization, inducing LTP.
Cellular plasticity in the cerebellar nuclei and learning
The original mossy fiber LTP protocol was designed to approximate predicted cerebellar activity during delay eyelid conditioning (Pugh and Raman, 2006; McCormick and Thompson, 1984). Although in subsequent studies (Pugh and Raman, 2008; the present work), we have reduced the protocol to identify the minimal stimuli necessary to induce plasticity, this form of LTP, which persists to ages at which delay eyelid conditioning is robust (Stanton et al., 1992), remains of interest because of its potential relevance to learning. In delay eyelid conditioning, animals are trained to associate conditioned stimuli, carried by mossy fibers, with unconditioned stimuli, carried by inferior olivary fibers (McCormick and Thompson, 1984, Mauk et al., 1986; Steinmetz et al., 1989; Hesslow et al., 1999). Because mossy fibers and inferior olivary fibers excite, directly or indirectly, both cerebellar nuclear cells and Purkinje cells, excitation and inhibition are predicted to coincide in cerebellar nuclear cells. Disinhibition is expected to occur as Purkinje cells transiently pause after the complex spike evoked by unconditioned stimulus, or, after training, at the end of the conditioned stimulus (Jirenhed et al., 2007). The present data suggest that the patterns of activity predicted to occur during learning should be sufficient to activate calcineurin through synaptic excitation, to lower calcium influx through L-type Ca channels via inhibition from concerted Purkinje cell activity, and to activate α-CaMKII upon disinhibition and resumption of firing, as Purkinje cell firing pauses. In this way, it is possible that synaptic excitation in the cerebellar nuclei is potentiated in vivo during conditioning. Such plasticity has the potential to work in parallel with plasticity in the cerebellar cortex that occurs during cerebellar learning (Koekkoek et al. 2003; Koekkoek et al. 2005; Hansel et al. 2006; Jirenhed et al. 2007), by increasing the responsiveness of cerebellar nuclear cells to conditioned stimuli.
In addition to mossy fiber LTP, cerebellar nuclear neurons express several other forms of cellular plasticity (Zheng and Raman, 2010), some of which appear well suited to work in concert with mossy fiber LTP, and others of which seem likely to oppose it. For instance, low calcium influx through NMDA receptors or L-type calcium channels leads to an LTD of inhibitory synaptic responses, whereas high calcium influx through the same channels potentiates synaptic inhibition (Morishita and Sastry, 1993; Morishita and Sastry, 1996; Aizenman et al., 1998; Ouardouz and Sastry, 2000). Thus, the conditions favoring depression of inhibition are similar to those that favor potentiation of excitation, namely, when excitatory synapses are activated but inhibition is nevertheless effective at suppressing action potential firing. Conversely, strong excitation without inhibition not only increases inhibitory strength but also promotes depression of EPSCs (Zhang and Linden, 2006; the present data). In addition, bursts of spikes, evoked either by EPSPs or after IPSPs, trigger increases in intrinsic excitability (Aizenman and Linden, 2000; Zhang et al., 2004); these changes, too, might be elicited by stimulus patterns associated with mossy fiber LTP. Thus, strengthening excitation, weakening inhibition, and increasing intrinsic excitability may work together to increase cerebellar output and thereby contribute to conditioned responses as well as other forms of cerebellar learning.
Experimental Procedures
Preparation of tissue
Parasagittal cerebellar slices were prepared from 13–16 day old C57BL/6 mice (Charles River Laboratories) in accordance with institutional guidelines for animal care and use. Mice were deeply anesthetized with isofluorane and transcardially perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 123 NaCl, 3.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1.5 CaCl2, 1 MgCl2, 10 glucose and equilibrated with 95/5% O2/CO2. Mice were then rapidly decapitated and the brains removed into ice-cold ACSF. Slices (300 μm thick) were cut on a Vibratome (Leica VT 100S) and incubated in warmed (35ºC), oxygenated ACSF for at least 1 hour before recording. In young adult (P28–32) mice, slices were cut in ACSF in which 210 mM sucrose replaced NaCl (Kirov et al., 2004).
Electrophysiological recording
Cerebellar slices were transferred to a recording chamber perfused continuously with warmed (33–37ºC), oxygenated ACSF containing the GABAA receptor antagonist SR95531 (10 μM) at a flow rate of 2–4 ml/min. Slices were visualized with IR-DIC microscopy and recordings were made from large neurons primarily within the interpositus and lateral cerebellar nuclei (soma diameters > 15 μm). Voltage- and current-clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices) and pClamp 10.0 data acquisition software (Molecular Devices). Series resistance was compensated 25–55%. Data were filtered at 2 kHz and sampled at 10 kHz.
For experiments involving both voltage- and current-clamp recordings, borosilicate patch pipettes were pulled to tip resistances of 2–4 MΩ and filled with an internal solution containing (in mM): 130 K-gluconate, 2 Na-gluconate, 6 NaCl, 10 HEPES, 2 MgCl2, 1 EGTA, 14 Tris-creatine phosphate, 4 MgATP, 0.3 Tris-GTP, and 10 sucrose. In experiments in which cells were voltage-clamped at −40 mV, the internal solution was supplemented with 600 μM QX-314 and K-gluconate was replaced with Cs-methanesulfonate. Where indicated in the text, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 10 mM) replaced EGTA in the internal solution.
For experiments with added free intracellular Ca, 0.1 mM CaCl2 was added to the intracellular solution, which is calculated to produce a free calcium concentration of 50 nM under our buffering conditions (CaEGTA calculator TS v1.3; Patton et al., 2004).
Excitatory synaptic currents and potentials were evoked by stimulating the white matter surrounding the cerebellar nuclei with 0.1 ms current pulses (<1 mA; Isoflex Stimulus Isolation Unit; AMPI) delivered through a concentric bipolar electrode (FHC). Mossy fibers form the bulk of this input, although a contribution from climbing fiber collaterals entering the nuclei cannot be excluded (Chan-Palay, 1977). Except as noted, baseline EPSCs (paired pulses, 50-ms interval) were recorded at −65 mV every 15 s for 7–10 minutes. The recording configuration was then switched to current clamp mode and current injections and/or EPSP trains were delivered 30 times, every 5 s. EPSCs were then monitored in current clamp for at least 25 minutes. Inhibition was mimicked with current injections (−200 to −600 pA) that interrupted firing and hyperpolarized the membrane by about −20 mV. In experiments including long hyperpolarizing steps, 500-ms square current injections were ramped back to 0 pA over 2.5 s, resulting in a mean interruption of firing of 2.7 s. In all recordings of EPSCs, a 100-ms, −10-mV test step was delivered on each sweep to monitor recording stability and access resistance. Recordings were aborted if neurons were unstable or if an increase in access resistance indicated resealing.
Enzymes, drugs, and other chemicals
Chemicals were obtained from Sigma-Aldrich, with the exception of SR95531 and KN-62 (Tocris). For CaN*, recombinant human myristolated calcineurin (PP2B) was obtained at 100 U/μl (Biomol International) and diluted 1:500 in internal solution before use. Fresh dilutions were made hourly. For CaMKII*, a truncated monomer of the α-CaMKII (residues 1–325; New England BioLabs) was activated in vitro by incubating CaMKII in a reaction buffer containing 400 μM ATPγS, 1.2 μM calmodulin, 0.5 mM CaCl2, 10 mM NaCl, 5 mM HEPES, and 2 mM MgCl2 in water for 10 minutes (McGlade-McCulloh 1993; Lledo et al., 1995). This solution was diluted ten-fold in the internal solution during recordings, to a final concentration of 200 nM. In interleaved control experiments, α-CaMKII was denatured by heating at 70ºC for 25–35 minutes before incubation in reaction buffer.
Data analysis
Peak amplitudes of synaptic currents were measured with Neuromatic (ThinkRandom.com) in IgorPro (Wavemetrics). Data are reported as mean ± s.e.m. EPSC amplitudes in each cell were normalized to the mean baseline (pre-conditioning) EPSC amplitude. To generate plots of population data, every ten sequentially evoked EPSCs (first EPSC only of paired EPSCs) were averaged for each cell. For statistical analysis, EPSC amplitudes from 5 minutes post-conditioning to the end of the recording were averaged and compared to the mean baseline amplitude. To facilitate comparisons between conditions, we also report the average percent change in EPSC amplitude measured 24 to 25 minutes after application of the induction protocol. In run-up/rundown experiments, the baseline was taken as the average of the first 10 EPSCs, which were compared to the mean of the EPSC amplitudes measured during the last 5 minutes of recording. Statistical significance was assessed with either paired (where possible) or unpaired, two-tailed Student’s t-tests, with significance taken to be p < 0.05. In some traces, stimulus artifacts have been digitally truncated.
Supplementary Material
Acknowledgments
Supported by NIH NS39395 (IMR). ALP was supported by T32 NS041234 and F32 NS067831. We thank Jason Pugh for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1687–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Alviña K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–1258. doi: 10.1038/nn.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchisi D, Scelfo B, Tempia F. Postsynaptic currents in deep cerebellar nuclei. J Neurophysiol. 2001;85:323–331. doi: 10.1152/jn.2001.85.1.323. [DOI] [PubMed] [Google Scholar]
- Audinat E, Knöpfel T, Gahwiler BH. Responses to excitatory amino acids of Purkinje cells’ and neurons of the deep nuclei in cerebellar slice cultures. J Physiol. 1990;430:297–313. doi: 10.1113/jphysiol.1990.sp018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmegunai A, Hansel C. A role for protein phosphatases 1, 2A and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Cerebellar Dentate Nucleus: Organization, Cytology, and Transmitters. Berlin: Springer-Verlag; 1977. [Google Scholar]
- Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, De Zeeuw CI, Elgersma Y. alphaCaMKII is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso SR, Ganong AH, Brown TH. Hebbian synapses in hippocampus. PNAS. 1986;14:5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. PNAS. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Mühlenthaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci. 1999;19:7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Plasticity in the vestibuloocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Morishita W, Sastry BR. Long-term depression of IPSPs in rat deep cerebellar nuclei. Neuroreport. 1993;4:719–722. doi: 10.1097/00001756-199306000-00030. [DOI] [PubMed] [Google Scholar]
- Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA. 2006;103:5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Nat Acad Sci USA. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Morishita W, Sastry BR. Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J Neurophysiol. 1996;76:59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Muri R, Knöpfel T. Activity induced elevations of intracellular calcium concentration in neurons of the deep cerebellar nuclei. J Neurophysiol. 1994;71:420–428. doi: 10.1152/jn.1994.71.1.420. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28:10549–10560. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry BR, Goh JW, Auyeung A. Associative induction of posttetanic and long-term potentiation in CA1 neurons of rat hippocampus. Science. 1986;232:988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shin JH, Linden DJ. Persistent changes in the intrinsic excitability of rat deep cerebellar nuclear neurones induced by EPSP or IPSP bursts. J Physiol. 2004;561:703–719. doi: 10.1113/jphysiol.2004.071696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26:6935–6944. doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman IM. Ca currents activated by spontaneous firing and synaptic disinhibition in neurons of the cerebellar nuclei. J Neurosci. 2009;29:9826–9838. doi: 10.1523/JNEUROSCI.2069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.