Abstract
Individualized medicine is the healthcare strategy that rebukes the idiomatic dogma of ‘losing sight of the forest for the trees’. We are entering a new era of healthcare where it is no longer acceptable to develop and market a drug that is effective for only 80% of the patient population. The emergence of “-omic” technologies (e.g. genomics, transcriptomics, proteomics, metabolomics) and advances in systems biology are magnifying the deficiencies of standardized therapy, which often provide little treatment latitude for accommodating patient physiologic idiosyncrasies. A personalized approach to medicine is not a novel concept. Ever since the scientific community began unraveling the mysteries of the genome, the promise of discarding generic treatment regimens in favor of patient-specific therapies became more feasible and realistic. One of the major scientific impediments of this movement towards personalized medicine has been the need for technological enablement. Nanotechnology is projected to play a critical role in patient-specific therapy; however, this transition will depend heavily upon the evolutionary development of a systems biology approach to clinical medicine based upon “-omic” technology analysis and integration. This manuscript provides a forward looking assessment of the promise of nanomedicine as it pertains to individualized medicine and establishes a technology “snapshot” of the current state of nano-based products over a vast array of clinical indications and range of patient specificity. Other issues such as market driven hurdles and regulatory compliance reform are anticipated to “self-correct” in accordance to scientific advancement and healthcare demand. These peripheral, non-scientific concerns are not addressed at length in this manuscript; however they do exist, and their impact to the paradigm shifting healthcare transformation towards individualized medicine will be critical for its success.
Keywords: Nanotechnology, Individualized therapy, Personalized medicine, Nanoparticle, -Omic technologies, Systems biology, Implantable device, Tissue engineering, Nanomedicine, Nanovector, Regulatory process
1. Introduction
Individualized therapy is the subsequent evolutionary expansion of the conventional clinical examination routine that cascades from patient evaluation, differential diagnosis, to the treatment of disease. Traditional medicine has historically been grounded upon evidence-based methods predicated upon observation, symptomatic analysis and pathologic expression/presentation. At one time, this individualized attention and tailored clinical course of action was broadly accepted as “personalized medicine”, however contemporary emerging technologies are providing scientists and clinicians with extraordinary access to a wealth of information with tremendous clinical potential. Unfortunately, the utilization of this expansive collection of raw patient data has been mildly successful in providing information with substantiated clinical value—but the promise still remains. Equipped with these new modern insights, a fresh conceptual approach to personalized therapy is now gaining momentum and the state of standard healthcare is at the brink of significant improvement. Soon standard treatment will no longer be associated with standardized “one-size-fits-all” therapy. There is more than sufficient evidence that physicians are adopting this philosophy as new resources of clinically relevant information are being employed. No longer are treatments prescribed solely based upon mammogram images and/or histological pathology; clinicians are now inclined to investigate the molecular profile or genetic map, of a patient to augment their final treatment decision. This utilization of genetic information is a medical leap forward in elevating the level of patient care; however genomic information represents only the “tip of the iceberg” as it relates to the total resource of information whose clinical relevance still predominately remains in the shadows of the unknown and undiscovered.
So what promise lies beneath the water’s depths that constitutes the bulk of the proverbial “iceberg”—the “-omic” technologies! Traditionally “-omic” technologies have included genomics, transcriptomics, proteomics, and metabolomics; however advances in the field of systems biology have driven the creation or classification of new “-omic” fields such as pepitidomics, glycomics, phosphoproteomics, and lipidomics. The technical definitions and clinical significances of the most established “-omic” technologies are summarized in Table 1. In short, systems biology begins at an extremely granular level in attempts to resolve the arrangements and interactions of cellular networks that drive the intricacies of cell function. The fundamental components of the “-omic” technologies (e.g. genome, transcriptome, proteome, metabolome) and their promiscuous non-linear and dynamic interactions all contribute to the overall complexity of defining their specific role(s) and function(s) as it pertains to the ultimate pathophysiology. Computational methods and mathematical models are then employed to facilitate the data processing and comparative analysis of “-omic” data subsets for disease signature validation and biomarker identification.
Table 1.
“-Omic” technologies.
| Technology | Definition | Clinical significance |
|---|---|---|
| Genomics | The study of the function and interactions of all of the genes in the genome. | Genomics provides information regarding the molecular mechanisms and the relationship between genetic and environmental factors of disease [1]. |
| Transcriptomics | The study of the complete set of RNA transcripts, or transcriptome, produced by the genome at any given moment. | Transcriptomics provides information about the global mRNA expression of particular tissue yielding information about the transcriptional differences between two or more disease states [2]. |
| Proteomics | The study of all proteins in a cell, tissue, or organism—including their identity, their biochemical properties and functional roles, and how their quantities, modifications, and structures change in response to the needs of the body or in disease [3]. | Proteomics provides genomic and post-translational information that yields functional signatures of biological events associated with pathophysiology. |
| Metabolomics | The study of the complex time-related concentration, activity, and flux of endogenous metabolites in cells, tissues, and other biosamples: blood, urine, and saliva. Metabolites include small molecules that are the products and intermediates of metabolism, as well as carbohydrates, peptides, and lipids [4]. | The metabolomic profile provides a “snapshot” of the cumulatively reflects the states of gene expression, protein expression, and the cellular environment as well as multidirectional interactions among these elements [5]. The metabolomic information can provide important insights into physiological and disease states and facilitate in depth understanding of underlying biochemical pathways [5]. |
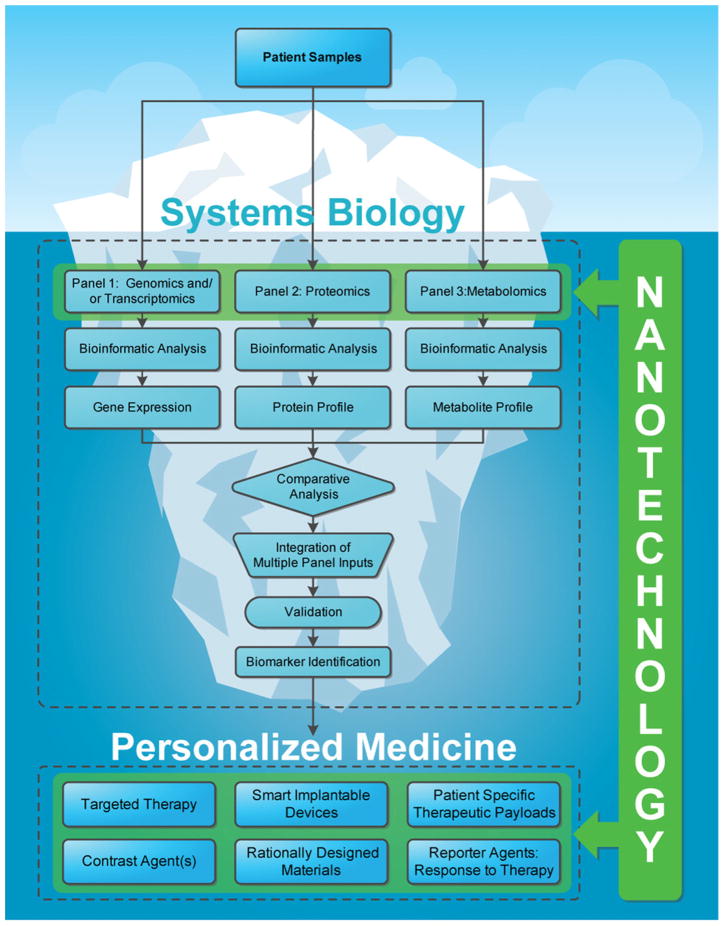
The envisioned role of nanotechnology is twofold: (1) provide access to previously inaccessible data as related to “-omic” technology components with unparalleled efficiency and resolution and (2) enable innovative therapeutic modalities that leverage the validated systems biology outputs for exquisitely specific individualized therapy. Systems biology has the potential of utilizing subtle biological clues (i.e. “-omic” technology components) for early detection of disease, predicting patient response to therapy, and identifying biomarkers to enable effective targeting of drug-delivery modalities to the disease site. The field of systems biology is still evolving; however there is strong evidence in scientific literature that supports the promise of nanotechnology as an enabling contributor to extracting the elusive “-omic” data for clinical analysis. For example, investigators have recently shown the ability to reproducibly enhance the presence of low molecular weight proteome from serum and plasma samples to differentiate the stages of disease as well as predict a patient’s response to therapy [6]. As the utility of nanotechnology expands to other “-omic” technologies, the ability to compare and integrate multiple panels of data subsets will tremendously strengthen the validation process for biomarker identification. Furthermore, nanotechnology has already demonstrated a clinical impact upon drug-delivery strategies for a variety of ailments, especially cancer indications. The inherent scale of nanotechnology enables a combinatory library of surface modifications (e.g. targeting moieties, charge modifications, stealth) of nanoparticulates, as well as control over size, shape, and other particle characteristics pending on particle material. This variety of options allows the rational design of personalized therapies that are predicated upon established biomarker evidence through systems biology discovery, imaging analysis, mathematical modeling and access to effective chemotherapeutics and other agents (Fig. 1).
Fig. 1.
The iceberg: the promise of “-omic” technologies. Nanotechnology will play a critical role in the discovery and validation of future biomarkers by providing access to a wealth of information provided by “-omic” technologies. Furthermore, nanotechnology offers a mechanism to utilize this patient-specific information to create novel individualized therapies and treatment strategies for patients in form of implantable devices, diagnostics, contrast agents, and innovative drug-delivery vectors.
Nanotechnology is not new to clinical medicine; PEGylated liposomal doxorubicin entered the market in 1995. Despite having nano-based therapeutics being commercially available for almost 15 years with an associated $5.4 billion in total sales [7], the strategic utilization of nano-products rationally designed through the comprehensive analysis of “-omic” technologies has yet to be realized. The field of nanomedicine is evolving with emphasis upon patient-specific treatment and soon should mature into the grandiose “tip-of-the-spear” therapeutic solution as the direct result of systems biology analysis and its integration with imaging technologies and available therapeutic agent options. This manuscript will provide a “technology snapshot” of contemporary nano-based products that inherently offer different levels of patient specificity as it pertains to individualized therapy, that are under development or are currently clinically available.
2. Nanotechnology overview
The advent and potential of materials and devices manufactured at the nano-scale level, the burgeoning field of nanotechnology, has raised both great promise and concern since its inception and introduction to the public. Nanotechnology, in the simplest form of the word, typically encompasses components with at least one feature smaller than a few hundred nanometers [8]. However, a more formal definition would be all objects that are man-made and contain nano-scale dimensions, all the while possessing distinctive properties that arise specifically due to their nano-dimension [8,9]. The small sizes that approach that of the atomic level, more often than not, invalidate the governing rules at the macroscopic level of these materials. At this scale, quantum mechanical effects begin to emerge leading to varied and unexpected physico-chemical properties [10–13]. Hence, modeling and indirect methods are frequently employed to accurately investigate the intricate interactions and properties of materials at the nano-scale. It is important to note, however, that it is these novel and unique properties that enable nanotechnology, specifically nanomedicine, to provide powerful solutions to a diverse field of problems.
With the introduction of the scanning tunneling microscopy (STM) in the early 1980s, the manipulation of individual atoms became possible [14,15], greatly assisting in the discovery and development of fullerenes (carbon-60 molecules) [16], carbon nanotubes [17] and nanocrystalline quantum dots [18–20]. These discoveries have not only yielded significant fruits for basic research, but also resulted in the awarding of Nobel Prizes to Richard Smalley, Robert Curl and Harold Kroto in Chemistry for fullerenes, and to Ernst Ruska, Heinrich Rohrer and Gerd Binning in Physics for STM [21]. Moreover, their seminal contributions served to successfully elevate nanotechnology into the mainstream and to increase public awareness regarding this field and its potential. Nano-scale materials had already been well established within the contexts of semiconductor [22] and optoelectronics [23], but after the scientific breakthroughs that occurred in 1980s, its potential to impact other fields, such as the chemical and energy industry, aerospace, and consumer products, was beginning to be realized. As an example, consider the following potential contributions: (1) increased efficiency and power in solar cells and batteries [24], (2) reduction of the amount of mercury that is released from fluorescent lamps, commonly found in households and industrial buildings [25], and (3) applications in the agriculture and food sectors that rapidly determine the presence of contamination [26]. Moreover, composite nanomaterials are capable of adding strength and reducing weight to produce such items as tennis rackets, baseball bats, and bicycles. In the case of optical lenses, nanocoatings have improved their surfaces and strength, thus reinforcing their structure and creating lenses less vulnerable to scratches. Nanoceramics have been applied to dental and bone implant arenas, with fillings now able to be tuned to match the mechanical and chemical properties of the surrounding tissue [27,28]. Last, but certainly not least, spherical nanoparticles such as liposomes (tiny vesicles composed of phospholipids) have achieved success in consumer products, with their widespread use in sunscreens, offering strong ultraviolet protection while remaining colorless, and in cosmetics with their ability to deliver moisturizers and other vital ingredients to the skin [29,30]. The success of nanotechnology in these products has paved the road for the future application and increased public awareness of nanotechnology, which is fundamentally important for its acceptance in fields such as medicine.
While the full potential of nanotechnology has yet to be realized in most industry sectors, medicine has benefited and been influenced by this field for several years, particularly in oncology. The premier drug-delivery nanoparticle currently in clinical use is the liposome [31]. Doxorubicin, a powerful and toxic chemotherapeutic, was encapsulated into liposomes and was initially approved for treatment of Kaposi’s sarcoma in the USA in 1995. This formulation, whose trade name is Doxil™, has since been approved for the treatment of metastatic breast cancer and recurrent ovarian cancer [32]. Another approved nano-therapeutic agent is an albumin nanoparticle comprising paclitaxel, otherwise known as Abraxane™. Approved in January of 2005, Abraxane™ allows for the administration of greater doses of paclitaxel and takes advantage of the natural properties of albumin to increase its extravasation into the tissue [33]. The success of these platforms has led the FDA to approve several investigational new drug (IND) applications for the treatment of several types of cancers. Currently, there are more than 400 ongoing clinical trials involving nanotechnology; the majority of which are for cancer treatment [34].
Since the introduction of liposomes as a viable delivery vehicle for chemotherapeutics, several nano-based platforms have been developed and proposed to examine their potential for cancer treatment, which include nanovectors and nanomaterials. Nanovectors are particles with nano-scale dimensions that can be used for the delivery of therapeutic or diagnostic agents through either encapsulation or physical attachment of the desired moiety to the nanoparticle [35]. Typically these systems are composed of lipids (e.g. liposomes [36]), nano-/microfabricated materials (e.g. fullerenes [16], carbon nanotubes [17], silicon [37,38], silica [39]), metals (gold [40], silver [41], iron [42], platinum [43], quantum dots [44]) and polymers [35] (micelles [45], dendrimers [46–48]). Moreover, these nanoparticles can adopt several different shapes, such as spherical, rods, wires, discs, hemispherical and ellipsoidal [49–52]. In the field of nanomedicine, specifically in the case of oncology, nanovectors can be divided into those that either provide treatment or disease diagnosis. The ability to concentrate and localize agents at the tumor site is the ultimate goal of these platforms, with the benefits including enhanced tumor treatment and/or improved contrast for imaging. Early studies with liposomes demonstrated that particles below one micron were rapidly cleared by the reticuloendothelial system (RES) [53]. Though, if the particles are coated with a molecule to increase their hydrophilicity, the clearance could be hindered, and hence, “stealth” liposomes were introduced [54]. These liposomes are able to increase their circulation time by decorating their surface with polyethylene glycol (PEG) [55]. These “stealth” liposomes are the prototypical example of first-generation nanovectors, where a nano-based delivery system enhances the delivery of a cytotoxic agent for improved therapeutic outcome. Recently, a second-generation of nanovector has been introduced that integrates additional functionality, such as the attachment of a bio-recognition molecule to the surface of the vector, to target a specific marker that is overexpressed on a tumor. Thanks to phage screening libraries and insights into the underlying biology of tumors, several antibodies, aptamers, peptides and ligands have been identified that can facilitate molecular targeting [56,57]. At present, the FDA has not approved any second-generation vectors, but several are being investigated in clinical trials. Lastly, novel, multifunctional systems are being proposed that offer new degrees of particle sophistication which improves the probability of localizing therapeutic payloads at the disease site. These systems are examples of third-generation nanovectors, which are adept at performing several functions, such as RES avoidance, molecular targeting, and localized therapeutic delivery. For example, there is active research being performed that features exogenously activated gold nanoparticles enclosed by bacteriophages that are molecularly targeted and capable of producing sufficient energy to thermally ablate tumor cells upon exposure to specific wavelengths of radiation [58].
As previously mentioned, nanoparticle material properties can be exploited to elicit clinical advantage for many applications, such as for medical imaging and diagnostic procedures. Iron oxide constructs and colloidal gold nanoparticles can provide enhanced contrast for magnetic resonance imaging (MRI) and computed tomography (CT) imaging, respectively [59,60]. Optical imaging has been plagued by the inability to provide effective solutions to in vivo imaging due to photobleaching and the ability of agents to be highly active in the near-infrared region, where light can easily penetrate through the body without harm. Quantum dots provide a plausible solution due to their tunable emission spectra and inherent ability to resist bleaching [44]. For ultrasound imaging, contrast relies on impedance mismatch presented by materials that are more rigid or flexible than the surrounding tissue, such as metals, ceramics or microbubbles [61]. Continued advancements of these nano-based contrast agents will allow clinicians to image the tumor environment with unprecedented resolution for enhanced understanding of disease progression and tumor location.
Additional nanotechnological-based detection and therapeutic devices were made possible using photolithography and nucleic acid chemistry [62,63]. The same technology that enabled integrated circuitry, produced micro-electro-mechanical systems (MEMS) for selective molecular sensing, sieving, and controlled drug release [64]. Microfluidic systems, also known as “lab-on-chip”, are fabricated by soft lithography of inexpensive polymers [65]. Micro- and nano-arrays, have experienced success for molecular diagnostic, genotyping and biomarker-guided therapeutic targeting [64,66,67]. Moreover, advances in proteomics have been made possible due to the technical refinement of lithographic resolution [68]. Recent interest in nanowires [69,70] and cantilevers arrays [71–73] for biomarker detection has shown promise. The former are biologically gated transistors able to detect multiple, real-time, simultaneous molecular binding events. The latter are miniature beams that deflect when molecules of interest bind and transmit a quantitatively measurable electrical signal. These innovative nanodevices equal or exceed the sensitivity of commercially available approaches [74] and are anticipated to be clinically available in the near future.
The commitment of federal resources to fund nanotechnology-based research has greatly aided its advancement thus far and will continue to play a critical role for its future success. In 2005, the National Cancer Institute (NCI) launched a $144 million Alliance for Nanotechnology in Cancer to support novel and continued research for nanotechnological-based approaches for oncology. At this time, the field of nanomedicine has arrived at a critical juncture where academic research efforts are now transitioning the technical and financial responsibilities of clinical translation to commercial ventures. An example of this academic handoff to a corporate partner is Rice University’s commercialization of its nanoshell technology, developed by Drs. Halas and West. Nanospectra Biosciences, Inc. has recently entered a Phase I clinical trial featuring the proprietary nanoshell technology for patients diagnosed with refractory head and neck cancer under an open Investigational Device Exemption (IDE) [75]. It can be anticipated from the number of ongoing clinical trials (400+) that corporate participation will be a growing trend in nanomedicine.
The following review will provide a comprehensive portrayal of the current status of nanotechnology. Importantly, we will address the potential of nanomedicine for individualized therapy. The review begins with a discussion focusing upon the utility of rational design when creating materials for personalized medicine applications, followed by the role nanotechnology has played in the early detection of disease, and an overview of nanotechnological implantable devices for controlled drug delivery. The review then progresses with a broad description of “injectable” nanovectors, highlighting both contrast agents and therapeutics. We then segue to the role of nanotechnology in tissue engineering, and conclude with a powerful commentary from a cancer survivor who articulates her experiences with chemotherapy and thoughts on the promise of individualized medicine.
3. The rational design of nanotechnologies for individualized therapy
The mathematical modeling of biophysical phenomena is crucial for identifying the main parameters governing the spatio-temporal evolution of the system under investigation, for elucidating their role and quantifying their effects and, most importantly, for predicting the evolution of the system without running extensive and expensive experiments. Mathematical models can indeed be used to design ‘rational’ experiments and ‘inspire’ experimentalists. Given the complexity of biology and the huge biological diversity among apparently similar individuals, mathematical models are clearly of fundamental importance for the effective development of tools to be used in personalized medicine. Two examples are briefly discussed in sequence, namely (1) the rational design of intravascularly injected nanoparticles (NPs) for biomedical imaging and drug delivery and (2) the development of orthopedic implants (OI) for post-traumatic osteo-regeneration of long bones with critical size defects.
NPs are man-made small objects, with a nanometer characteristic size, that are injected at the systemic level (intravascularly) to execute specific diagnostic and/or therapeutic missions at the biological target site. This could be a solid tumor mass, an inflamed portion of the vasculature, or any district within the human body where abnormal cells are proliferating. Before reaching the target site, the blood-borne NPs must make their way into the circulatory system passing a multitude of barriers that simply tend to sequester, digest and/or expel any foreign object, as the NPs. In the case of tumor targeting, such physiological barriers are presented as: (1) the spatially and temporally heterogenous blood flow in tumors [76] due to hyper-permeable blood vessels with fenestration [77] and lack of a functional lymphatic system; (2) the increased interstitial fluid pressure that may reduce transvascular and interstitial transport of free molecules within the extracellular matrix [78]; and (3) the highly intricate extracellular matrix (ECM) constituting an additional barrier to the delivery and transport of drugs [79]. Additional impediments are of the biological barrier type, which include: (1) the reticular endothelial system (RES), constituted by phagocytes, specialized cells lining the liver, spleen, bone marrow, and lymphatic tissue, which recognizes external molecules and remove them from the circulation [80] and (2) the insufficient expression of receptors on the membrane of the target cells, making more unlikely the specific recognition of the target cell by the imaging tracers or the therapeutic molecules [80]. It is here important to emphasize that the type and severity of the barrier is disease and patient specific.
Many laboratories are developing NPs which differ in (1) size: ranging from few tens of nanometers, as dendrimers, gold and iron oxide nanoparticles [81], to few hundred of nanometers, as liposomes and gold nanoshells [82], and up to the micron scale (1–2 μm) [38]; (2) shape: from the classical spherical nanoparticle to conical and discoidal, spheroidal, cylindrical [38,83,84]; and (3) surface physico-chemical properties: with some particles just coated with functional groups imparting a specific positive (amine groups) or negative electrostatic charge (carboxyl groups) and other particles decorated with polymeric linkers, as PEG, and biologically active molecules, as antibodies, peptides, aptamers and ligands [58,84,85].
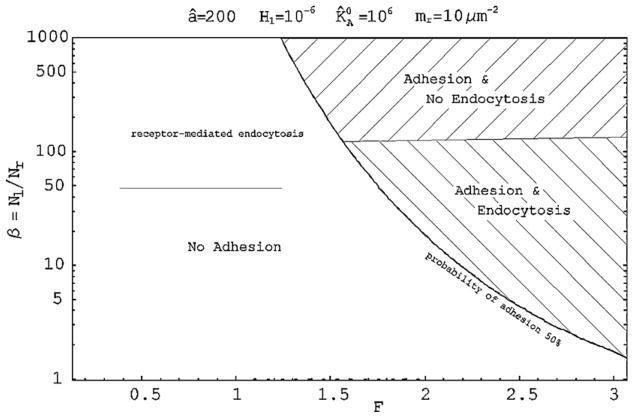
With such a complex biological scenario, with the multitude of NP combination available, accurate predictive mathematical models are fundamental in identifying those properties that can maximize NP accumulation at the target site. In the last years, mathematical modeling has been quite extensively used to predict and optimize the performances of intravascularly injected NPs for biomedical applications. In particular, the journey of blood-borne NPs has been divided into three sub-problems, transport and margination dynamics [49,86], adhesion dynamics [87,88], and internalization dynamics [89,90], showing how the size, shape and surface properties of the NPs dramatically affect their behavior [91]. In such applications, the final recognition of the diseased tissue and the response of the abnormal cells to the administered therapeutic molecule are highly patient specific depending on the vascular architecture, local hemodynamic conditions, level of expression of specific vascular and extravascular receptors, type of disease (cancer, hemorrhagic, cardiovascular, etc.), localization of the diseased tissue within the body and so on. The mathematical models do take into account disease and patient-specific features and can effectively be used to study the behavior of different NP combinations in the patient-specific vasculature, under the patient-specific biological and biophysical conditions. In particular, “Design Maps” can be generated for predicting the adhesive and internalization propensity of NPs as a function of non-specific interactions (van der Waals, double layer electrostatic, steric and acid–base) through the factor F, and specific interactions (ligand–receptor binding) through the ratio β. A representative map is shown in Fig. 2 for fixed hydrodynamic conditions and in the case of spherical beads: different NPs formulations can be ‘tested’ by changing F and β.
Fig. 2.
Design maps for spherical beads as a function of the patient-specific parameter β and F[90].
The “Design Maps” allow for identifying a subset of NPs that exhibit optimal performances in silico (mathematical modeling) and from which the optimal NP formulation can be selected through few and cost-effective in vivo experiments.
Another application where mathematical modeling has shown to be fundamental involves the design of a novel orthopedic implant (OI) for the post-traumatic osteo-regeneration of long bones with critical defects. This “fracture putty” is a complex biomaterial made up of individual components with characteristic dimensions that span across a variety of length scales (from nano to macroscales). These components are (1) a biodegradable porous polymeric matrix reinforced with (2) stiffer inclusions of a different material, as sketched in Fig. 3. The optimal “fracture putty” has to accommodate two main functions: support the normal loads acting on the bone (mechanical stability) and facilitate the growth of new bone that eventually would fully replace the artificial bone implant (osteo-regeneration).
Fig. 3.
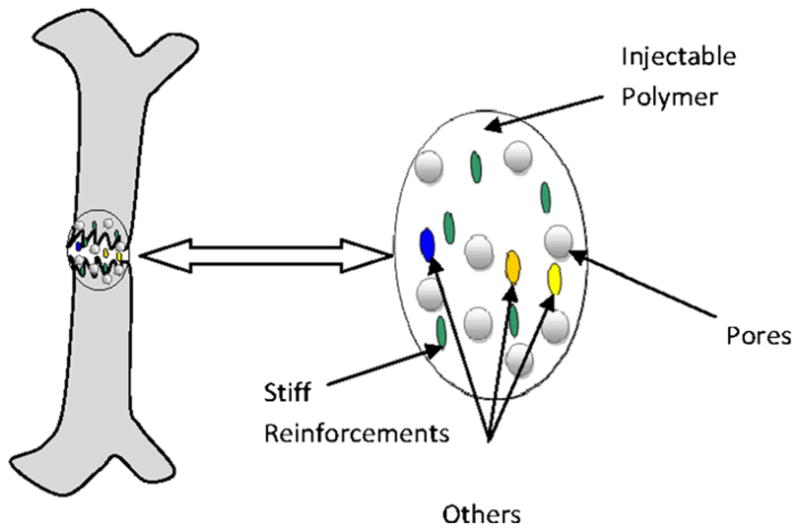
Schematic of fracture putty.
Both the mechanical stability and the osteo-regeneration are patient specific: the loads exerted over the critical size defects depend on the location of the defects as well as on the overall weight of the individual; whereas the osteo-regeneration rates depend on the age, gender and genetic features of the patient. By using mathematical modeling, the stress and strain fields at the site of the critical defects and the formation of new bone can be predicted in terms of the parameters listed above, which are patient-specific. Thus, the composition of the “fracture putty”, in terms of polymeric matrix porosity (geometry, size, and mechanical properties) and volume concentration of the inclusions (type and concentration of biomolecules) can be chosen to maximize osteo-regeneration while still offering sufficient mechanical—all for a specific individual.
These two examples emphasize the role played and the potentials of mathematical modeling in the pre- and post-development of devices for biomedical applications. Accurate and reliable mathematical modeling can be performed more easily than experiments. In silico evaluation can take into account the patient specificity of the problem and dramatically reduce the time and cost required to formulate a new device and therapeutic intervention, and eventually translate it into the clinical settings. In nanomedicine, the need for accurate mathematical models is even more pressing. Despite its rapid growth and extraordinary potentials, the field is still in its infancy, is highly interdisciplinary, and aims to solve problems of extraordinary and unprecedented complexity. With such a scenario, mathematical modeling could dictate the success of nanomedicine and make the difference between several years of unfruitful research, and the development of new revolutionary therapeutic strategies readily available to the public.
4. Early detection
Development of molecular diagnostics represents the first step to attain a real individually tailored medicine. However, current diagnostic and prognostic classifications which rely essentially on the anatomo-clinical methods do not reflect the vast heterogeneity of complex diseases such as cancer, and cannot predict clinical outcomes and response to therapy. Therefore, there is an urgent need for new molecular biomarkers to improve diagnosis, assess response to treatment, and evaluate disease progression [92,93]. Such biomarkers could be altered genes, RNAs, proteins or metabolites associated with a specific pathological stage or clinical outcome. Owing the complexity and heterogeneity of most diseases, it has been recognized that a single marker cannot reach sufficient specificity and sensitivity. Current strategies raise exciting opportunities of using multi-parametric analysis of “-omic” technology constituents (e.g. genome, transcriptome, proteome, and metabalome) for a diagnosis based on the molecular profiles of individual patients.
Human genome sequencing and advances in genomics provided a better understanding of the pathological mechanisms of diseases. Furthermore, the identification of specific molecular signatures as diagnostic and prognostic tools has opened the way for a more efficient and personalized medicine. Golub et al. reported the feasibility of gene expression profiling approach to discover and predict cancer classification [94]. Another study on diffuse large B-cell lymphoma demonstrated the correlation between specific gene expression patterns and clinically distinct subtypes of cancer [95]. In the post-genomic era, proteomics has demonstrated an increasing interest in biomarker research. Proteins are the products of the genes and represent the functional picture of the pathological state of patients [96–98]. Thousands of studies have shown the potential use of proteins as a promising source of biomarkers [99,100]. Developments in mass spectrometry technology have allowed the analysis of complex proteomes from minimally or non-invasive methods such as serum, plasma and other body fluids, offering opportunities for reliable early detection approaches [101,102]. In spite of the optimism brought by proteomics, the lack of sensitivity of those techniques remains a major limitation for the identification of clinically relevant protein biomarkers [103–105].
Nanotechnology has emerged as a new interdisciplinary field combining biology, chemistry, engineering, and will likely provide major progress in individualized medicine. In the field of biomarkers discovery and detection, nanotechnology will bring significant advances in molecular detection by improving the sensitivity and specificity of current technologies, or by providing novel approaches. The detection of traces of molecules will revolutionize diagnosis and allow for a real early detection of diseases.
4.1. Biomarker discovery
The major challenge yet to be addressed is the sensitive and selective detection of circulating biomarkers to improve diagnosis, assess treatment efficacy, and design personalized therapies with limited invasiveness. The low molecular weight (LMW) region of the blood proteome provides an unprecedented opportunity for clinical diagnosis or prognosis, and for monitoring response to therapy [92,99]. Proteins and peptide are degraded by proteases in the tumor stromal environment and shed into the circulation from leaky vessels, therefore, LMW peptidome presents an attractive opportunity to capture pathological changes occurring in the tumor [106]. However, despite such promise, successful translation of this technology to routine clinical application is limited due to: (1) the large dynamic range of blood proteins limiting the detection of low abundance biomarkers and (2) the rapid enzymatic degradation by endogenous and exogenous proteases [103].
To overcome the vast complexity and the relative instability of serum samples, a high throughput and reproducible fractionation system based on nanoporous silica chips (NSC) is currently being developed. The NSC effectively deplete most of the abundant high molecular weight (HMW) proteins and allow the enrichment and stabilization of LMW species present in the human circulating proteome [9,107]. The NSC are designed and engineered with defined nano-pore size and physico-chemical properties allowing substantial control over the molecular cut-off and the specific harvesting and stabilization of proteins and peptides [108]. This NSC technology in combination with mass spectrometry will provide a fast, efficient, and reliable fractionation system for high throughput enrichment, stabilization and detection of LMW biomarkers present in the human circulating proteome. Another approach presented by Luchini et al. demonstrated the use of smart hydrogel particles for the harvesting and protection of circulating LMW biomarkers [109]. The hydrogel particles are fabricated with a defined porosity and contained an affinity bait for a rapid onestep sequestration and concentration of the LMW fraction of serum molecules. The captured peptides and proteins are then protected from further enzymatic degradation. The ability to structurally design the nanoporous sieve and the chemical functionalization increases the selectivity of peptides enrichment. The combination of these enrichment methods with current proteomics technologies such as mass spectrometry profiling, can provide enormous enhancement of low abundant disease marker discovery.
4.2. Nano-biosensors
Highly sensitive biosensors that recognize genetic alterations or detect molecular biomarkers at extremely low concentration levels are crucial for the early detection of diseases and for early stage prognosis and therapy response. The use of nanowires to implement field effect transistor (FET) semiconductors presents an ultrasensitive and label-free strategy for the quantitative detection of biomolecules. The target binding events occurring on the nanowire result in conductance changes that can be monitored to detect specific molecules. The reduced diameter and the high surface to volume ratio of the nanowires offer an extremely high sensitivity due to the accumulation/depletion of carriers throughout a much larger wire cross-section [70,110]. This approach has been used to detect several biomolecular targets such as DNA and proteins. The identification of DNA alterations is crucial to better understand the mechanism of a disease such as cancer and to detect potential genomic markers for diagnosis and prognosis [111]. An innovative approach for the detection of gene mutations using nanowire FET has been developed by Wu et al. [112]. They demonstrated the ability of the nanodevice to detect BRAF gene mutations, an alteration that occurs in approximately 8% of human tumors [113]. Another study reported the development of a three-dimensional gold nanowire platform for the detection of mRNA with 100 fM sensitivity from cellular and clinical samples. This highly sensitive electrochemical sensing system uses peptide nucleic acid probes to directly detect specific mRNA molecules with no PCR amplification.
The development of immuno and aptamer-based nanowire biosensors to detect cancer biomarkers such as VEGF [114] and CA125 [115], or SARS virus N-protein [116] has shown a great sensitivity, providing the potential use of these nanodevices for point-of-care diagnostic applications. To improve the diagnostic efficacy of the biosensors, a multiplexed approach is needed to accurately identify heterogeneous diseases such as cancer. Zheng et al. have described nanowire arrays for the multiplexed detection at pg/mL level of several proteomic biomarkers including prostate-specific antigen (PSA), PSA-alpha1-antichymotrypsin, carcinoembryonic antigen and mucin-1 [117].
Micro- and nanocantilever systems are another category of biosensor devices that have been developed to realize specific and highly sensitive molecular detection. Silicon cantilevers can be micro- or nanofabricated with multiplexed capability for a label-free and cost-effective biomolecular detection technology. Modification of the surface stress due to specific binding events can be measurable and translated to molecular recognition [118]. McK-endry et al. have developed a label-free DNA microarray approach based on cantilever technology and demonstrated that the cantilever arrays can detect simultaneously multiple molecules at nanomolar concentrations [119]. Another study demonstrated the use of cantilever approach to specifically detect picomolar levels of mRNA biomarkers in total cellular RNA extract [120].
Cantilever nanosensors have also been used to detect minute amount of protein biomarkers. Label-free resonant microcantilever systems have been developed to detect ng/mL level of alpha-fetoprotein, a potential marker of hepatocarcinoma, providing an opportunity for early disease diagnosis and prognosis [121]. A bioassay described by Wu et al. demonstrated the capability of detecting two forms of prostate-specific antigen (PSA) over a large range of concentrations (from 0.2 ng/mL to 60 μg/mL) using microcantilevers with different geometries [72]. Recently, another group has enhanced the detection limit of this microcantilever approach using PSA polyclonal antibody as an additional surface stress inducer and PSA polyclonal antibody-conjugated silica nanoparticles (pAb-SiNPs) as a mass inducer [122]. This strategy increased the sensitivity of PSA detection to 1 pg/mL. Nanofabricated and functionalized devices such as nanowires and nanocantilevers are fast, multiplexed and label-free methods that provide extraordinary potential for the future of personalized medicine.
The development of nanomaterials and nanodevices offers new opportunities to improve molecular diagnosis, increasing our ability to discover and identify minute alterations in DNA, RNA, proteins or other biomolecules. Higher sensitivity and selectivity of nanotechnology-based detection methods will permit the recognition of trace amounts of biomarkers which will open extraordinary opportunities for systems biology analysis and integration to elicit effective early detection of diseases and improved therapeutic outcomes; hence paving the way to achieving individualized medicine.
5. Implantable drug-delivery devices
Targeted and controlled drug delivery play fundamental roles towards the goal of individualized therapies. While targeted delivery relates to the administration of drugs to the “right” place, controlled delivery relates to administering a drug at the “right” time. Drug targeting and controlled administration are being widely investigated through the opportunities brought forth through the utilization of nanotechnology. As a result, new strategies and novel nanotechnological embodiments are being developed for the individualized treatment of diseases, especially in the field of implantable systems, where the inherent dimensions of nanotechnology affords the miniaturization of scale that enables the integration of multiple functional components on a single device: telemetric control, on-board sensor systems, and innovative mechanisms enabling unparalleled control over therapeutic release profiles. Significant resources are being focused on the development of implantable nanotechnologies due to their potential benefit in for systemic or local treatment of a large number of pathologies. The ability to control the drug administration over the duration of weeks to months in accordance to the therapeutic needs of an individual patient is the justification for the development of implantable drug-delivery devices. Long-term therapies can potentially benefit of devices capable of sustaining the drug delivery and overcoming the need for multiple periodic administrations associated with conventional practice (generally oral or intravenous) which effectively improves patient compliance. This provides tremendous patient incentive since implantable devices can relieve people of their responsibilities of self-medication and/or frequent visits to the clinic.
5.1. Controlled therapeutic release
A vast majority of therapies are based upon the systemic administration of drug. The systemic delivery can be achieved orally, intravenously, arterially, dermally, transdermally, rectally, ocularly, through inhalation, subcutaneously, intramuscularly, or sublingually. All delivery strategies present advantages and side effects that are weighed to best address the therapeutic needs while minimizing discomfort to the patient. While oral and intravenous administration can deliver large doses of drugs, transdermal and ocular delivery can deploy smaller amount of drugs systemically or to specific areas of body. Despite their differences, most delivery strategies are associated with the rapid release of drug resulting in the subsequent increase of plasma drug concentration. Many drugs used in the clinic have a narrow therapeutic window, making the efficient administration of drug challenging due to possible toxicities associated with bordering doses. In order to maximize the therapeutic efficiency and minimize the side effects of the agents, a concentration of drug within the therapeutic range, is beneficial. This result can be obtained by employing implantable drug-delivery devices able to sustain the drug release over long periods of time, ranging from hours to years. While a large number of therapies require sustained constant drug administration, other diseases would benefit from variable drug administration over time.
Constant release
The constant sustained release of drugs has been largely investigated for the treatment of several diseases including hepatitis and various forms of cancer. A constant drug concentration in the plasma over a long period of time can be achievable through zero-order release kinetics. Zero-order release is reached when the gradient of drug molecule concentration throughout a delivery device reaches equilibrium. Commonly, continuum-based diffusive processes are concentration dependent: the diffusion of molecules out of a delivery device decreases with decreasing concentration in the reservoir. However, numerous technologies are now available for the control of molecular deployment and the achievement of concentration-independent release. A common strategy to attain zero-order release of drugs is by employing convective driving mechanisms such as osmotic pressure, mechanical pumping, and through electrokinetic transport. A constant drug release can also be achieved by tuning the properties of nanofluidic devices. It has been shown that under nano-scale molecular constraint, surface effects and charge interactions play a major role over the transport of molecules [123–126]—charge exclusion, concentration polarization, and streaming current phenomena have been observed [127]. Silicon nano-channel technology has provided a platform for the study of cell transplantation in immuno-isolated devices [128,129], biomolecular separation [130,131] and controlled concentration-driven release of drug molecules when integrated into implantable device strategies [132,133].
Modulable release
As opposed to a constant drug administration, multiple therapies would greatly benefit from the ability to tune drug release according to circadian cycles. It has been well documented that the presence of biological rhythms, such as the circadian cycles, affect body metabolism in living organisms over a 24-h cycle [134]. Organs such as the kidney, liver, and gastrointestinal tract, which are very critical to drug metabolism, are highly coupled with circadian rhythms [135]. The pharmacodynamics and efficacy of treatments have been demonstrated to be related to the time of administration during the circadian cycle [136]. Therefore, robust drug-delivery strategies need to consider the most ideal times for drug administration in order to reduce the toxicity in addition to the optimization of treatment efficacy [137,138]. In this regard, cancer medicine is adopting chronotherapy as a more effective strategy to treat cancer [139,140]. The clinical utilization of implantable devices has tremendous potential attributed to their ability to modulate the release of drugs to the physiological rhythms of an individual. The synchronization of drug delivery to bio-cycles represents an additional step towards personalized therapy. Numerous approaches have been investigated for the modulation of the drug release by means of pre-programed, remote control, and even self-regulating release of therapy.
Implantable device strategies for the remote controlled release of drugs have been largely explored to enable the “on-demand” administration of therapeutic agents [141–146]. Radiofrequency (RF), ultrasonic energy, and magnetic fields could potentially be employed to activate, tune, or arrest drug administration. Significant advances in the field of wireless communication technologies have opened new avenues for the employment of RF technologies for implantable drug-delivery devices. Micro- and nano-electronic components are commercially available and can be readily integrated into nanofluidic chip configurations. When combined with complex algorithms for data logging, manipulating and transmitting, sophisticated implantable devices can be created—RF remote activation of nanoliter-scale chemical release has been achieved utilizing novel microfabricated designs [147]. This study provided an attractive platform for the potential development of frequency-selective remote control of drug-delivery systems. A different remote control approach was demonstrated by Kohane, Langer and colleagues, which employed an applied magnetic field [148]. Nanocomposite membranes based on thermosensitive nanogels and magnetite nanoparticles were designed for the “on-demand” release of drug through a remotely applied magnetic field. On/off release of sodium fluorescein was shown over a period of 45 days of subcutaneous implantation. Other opportunities for remote control of drug release implants come from “switchable” surfaces and membranes which can be controlled by light exposure [149,150] and temperature variation [151,152]. In this context, magnetic temperature-sensitive nanocomposite hydrogels have been developed [153] which incorporate superparamagnetic Fe3O4 particles in negative temperature-sensitive hydrogels. The nanocomposite hydrogel was shown to swell under a temperature variation caused by an exogenous magnetic field source. Preliminary studies demonstrated a reduction in the molecular release rate in the presence of an alternating magnetic field.
The ability to remotely control drug administration broadens the limit of applicability of implantable devices. However, numerous studies are developing self-controlled implants able to trigger, calibrate, and discontinue the administration of drug compounds at the required time. An autonomously controlled device works in principle as an artificial gland. Such devices would be equipped to sense physical or biological variations in the surrounding environment, and provide a prompt response to the physiological stimulus by controlling the administration of a therapeutic agent. Attempts have been made to integrate sensor technology into implantable delivery devices to enable autonomous control. One such embodiment was designed to serve as an insulin delivery system which combined electronic components with microneedle arrays, micropumps and microsensors to sense the glucose concentration in the blood and provide insulin administration as needed [154]. Despite the large number of studies focusing upon the development of self-regulating devices and their enabling components, efforts have yet to be successful in achieving a reliable autonomous drug-delivery device. In order to attain the desired administration profile for a specific application, a variety of devices are currently being developed that feature multiple control strategies. In the following text, several families of implantable devices will be discussed according to their enabling technology. Many of the described technologies are microtechnology-based—they will remain in this discussion because of the novelty of their approach and potential ability to be advanced through nanotechnology.
5.2. Implantable drug-delivery nanotechnologies
Osmotic pumps
Osmotic pumps enabled by nanoporous membranes, represent one of the most mature approaches used for implantable drug-delivery devices. These pumps have been integrated into implantable drug-delivery devices for several decades [155,156]. Each embodiment employs the osmotic pressure of a solution with high concentration of electrolytes or sugars, to exert a pumping force capable of actively eluting a drug solution from a reservoir. The device is composed of two chambers which are separated by a movable piston. One reservoir contains a liquid solution of drug and the other compartment, sealed through a semipermeable nanomembrane, contains an osmotic solution. Fluids enter the osmotic compartment causing an increase in the fluid pressure thereby exerting a force which pushes the piston into the drug reservoir. As a result, a volume of drug solution is ejected from the device—ideally the motion of the piston is constant, forcing the drug release in a continuum fashion.
The DUROS® system, developed by the ALZA Corporation, was one of the first osmotic pumps brought to market [157]. The DUROS® implant is made of a titanium alloy cylinder with dimensions 4 mm × 45 mm, and features a capacity to hold ~150 μL of leuprolide DMSO-based solution. In vitro and in vivo studies in canines and humans demonstrated zero-order release rate for a year [157]. Another example of an implantable osmotic pump, the Chronogesic™, was developed for the subcutaneous delivery of sufentanil—the matchstick size, 4 mm × 44 mm, implant was designed for the sustained treatment of chronic pain and was used in clinical trials [158]. The titanium device held ~155 μL of concentrated solution of sufentanil in a benzyl alcohol solution. The mentioned clinical study showed a zero-order release profile comparable to the release achieved during in vitro test over a period of three months with 5 μg/h rate. Another commercially available osmotic pump, ALZET®, was developed for small animal research. Once implanted subcutaneously or intraperitoneally, these pumps were demonstrated to continuously deliver drug molecules at controlled release rates that ranged from one day to six weeks [159,160].
Other osmotic pumps have been designed to biodegrade after completion of therapy. In this context micro-electro-mechanical systems (MEMS) biodegradable osmotic pumps were microfabricated with polymeric structures [161]. This device was designed to deliver basic fibroblast growth factor (bFGF), which induces neovascularization, modulates osteoblastic proliferation, and promotes the differentiation in bone tissue. The pumping device was micro-molded on a layer of synthetic biodegradable polymer. The bottom layer contains the drug reservoir and houses an array of microchannels that facilitate drug release. An osmotic potential drives water into the reservoir through a semipermeable membrane. The water permeation inflates the reservoir and the increasing pressure promotes the ejection of therapeutics through the array of microchannels. It was shown that by varying the length of the microchannels, the design had a direct, predictable effect on the release rates [161]. This represents one of the few attempts at employing biodegradable polymers for the design of implantable osmotic pumps since difficulties in controlling the material properties and subsequent degradation processes, limited the success of this approach. Although osmotic pumps made their way to the clinics, their employment for clinical use is strictly limited to a near-continuous drug administration—not allowing for any modulation of the release rate.
Degradable polymers
Polymeric materials have shown great potential for their application to drug delivery. Specific properties such as biodegradability and biocompatibility make them convenient to serve as drug-delivery matrices. Fabrication techniques, (e.g. soft lithography, direct deposition, three-dimensional printing, laser stereolithography, and nanosphere lithograph) which are common to the semiconductor industry enable the fabrication of polymeric structures with features ranging from 50 μm to 50 nm in size [162]. Nanostructured degradable polymers allow the achievement of near zero-order releases of drug. Modifying the chemistry of polymers at the nano-scale allows for the inclusion of drug nanoparticles in the polymeric matrix. For these reasons, different nanostructured polymers are being studied for drug-delivery applications [163]. However, difficulties present when attempting to control the polymeric degradation kinetics—an initial “burst effect” is typical prior to reaching steady-state release regimes [164].
MEMS/NEMS
MEMS and NEMS (nano-electro-mechanical systems) represent two of the most advanced technologies for the development of multifunctional fluidic systems. Ferrari and colleagues focus their research on the fabrication and characterization of controlled diffusive transport in silicon nano-channel membranes [165,166]. The constrained motion of the molecules in nano-channels was demonstrated to affect the subsequent concentration-driven transport kinetics. By judiciously tailoring the surface chemistry and nano-channel dimensions, a constant release of biological molecules, such as bovine serum albumin, interferon-α and lysozyme, have been achieved in channel sizes ranging from 13 to 20 nm over a period of weeks [123–126]. This approach enabled the attainment of concentration-independent control over the molecular transport of drug by taking advantage of the surface–molecule interactions at the nano-scale.
MEMS and NEMS devices can also accommodate valves, pumps and mixers which allow for the precise transport of fluids and analytes in small quantities. As direct result of these capabilities, MEMS and NEMS have been developed for a variety of applications [167,168], including DNA analysis and sequencing [169,170], proteomics [171], metabolomics [172], and the detection of biological molecules and chemicals. MEMS and NEMS technologies can be applied to enable the development of innovative new drug-delivery devices that address the therapeutic needs pertaining to modulating drug release profiles, such as programable, cyclic, pulsatile, and/or continuous drug administration [133,173]. Several devices have been developed and characterized for in vitro and in vivo drug-delivery applications that utilize different approaches for modulating and triggering the release of the drug. For example, silicon-based piezoelectric micropumps were micro-fabricated by photolithographic techniques to achieve controlled drug release [174–176], while another embodiment incorporated sensors into a piezoelectric micropump device that featured microneedles to control the release of insulin for diabetic applications [177]. This sophisticated system was designed to autonomously adjust the insulin dose according to a patient’s physiologic status. Additional studies enabled fluid micropumping by employing a micro-fabricated thin film comprised of a nickel–titanium (Ni–Ti) shape-memory alloy [178]. Despite the potential applications of micropumping systems for drug delivery, moving components such as valves and pumps are difficult to successfully integrate within a microfluidic system due to failure as the result of mechanical stress and fabrication defects.
Other notable MEMS and NEMS approaches include those that are predicated upon the “burst release” of drug payloads. Among these are a controlled release device that features an array of micro-fabricated drug reservoirs capped by a gold membrane [179–182]. The devices allowed for the selective opening of reservoirs by applying an electrical potential across the desired number of gold membranes. As a result, the ensuing electrochemical reaction created soluble gold complexes and a complete dissolution of the membrane allowing for the drug release. By employing this device, the pulsatile release of therapeutic drugs was demonstrated in vitro and in vivo [181,183,184]. The studies demonstrated a comparable inhibition of tumor growth in rats to subcutaneous chemotherapy administration [183]. A similar technology was developed for the release of leuprolide by MicroCHIPS. The device presents an array of 100 reservoirs which can be individually activated. Release is achieved by removing the capping platinum and titanium layer by an electrothermal method mediated by an applied current [185]. The device design integrated wireless communication hardware, a power supply, and electrical components into a sealed shell, and was capable of the pulsatile release of leuprolide in a canine model for approximately six months [186]. Pulsatile release affords the administration of discrete amounts of therapeutic agents at any predetermined time. However, this system fails to achieve true continuous drug delivery and more closely resembles a multiple injection routine.
Additional devices employing burst release were developed for emergency care applications. The IRD3 device is a three layer MEMS [187] approach that employs a thick layer that comprises the drug reservoir, a sealing layer, and an actuation layer. The device is controlled through an applied current to the resistors fabricated within the actuation layer. The resistors heat the solution generating bubbles. The increase in the pressure bursts the sealing membrane allowing for the release of 20 μL of the drug solution in 45 s. The device was tested in vitro with of arginine vasopressin. The device demonstrated promise for further development [185], however, the study revealed that ~9% of the drug was degraded with respect to the original state due to the heating employed for the drug release.
Additional MEMS and NEMS technologies have been developed for the electrokinetic transport of molecules from a drug reservoir. The electrokinetic fluid transport has been investigated for a variety of fluidic applications. At the micro-/nano-scale, the electrokinetic phenomena enable the motion of ions or fluids by means of an applied electrical field; thus, mechanical moving parts are no longer required to achieve the motion of molecules. Electrokinetic transport has showed potential in applications such as drug delivery [188]. Electrokinetic membranes can be easily implemented by integrating electrodes into the device design; avoiding the need of moving components which are often prone to failure [189]. Electrophoretic and electroosmotic transport mechanisms have been investigated ranging from the macro- to nano-scale. Although both phenomena have been proven to be applicable to molecular transport, nano-scale electroosmosis will be highlighted as an efficient mechanism for the motion of molecules and fluids in channels [190]. Micro-/nano-channeled electroosmotic pumps have been fabricated and characterized in view of their potential application in implantable drug-delivery devices. Most studies, however, have developed electroosmotic pumps, which employ high applied voltages to elicit liquid flow [191]. The need for high applied voltages represents a limiting factor for the development of safe self-powered drug-delivery implants. For this reason additional studies have analyzed low-voltage driven electroosmotic devices. One of these studies employed nanomembranes that afford operation at low voltages and are capable of producing a large range of pumping rates [190,192]. In this context, parallel to the development of electroosmotic membranes, efforts have been spent in integrating electronics and sensors for the next generation of sophisticated drug-delivery devices. In this regard, Ferrari et al., are aiming to leverage their silicon nanomembrane technology for the development of a nano-channeled artificial gland that is capable of sensing environmental changes and equipped to appropriately respond with the controlled release of drug from an electroosmotic silicon membrane. Such an advanced integrated technology remains futuristic; however the promise of nanotechnology offers the technical potential to facilitate the realization of such clinical endeavors.
6. Nano-based injectable therapeutics
In the early 20th century, Paul Ehrlich, considered by many to be the father of pharmacology, envisioned the concept of a “magic bullet;” a notion where malignancies in the body could be treated by chemical substances equipped with a high affinity for that malignancy [193]. That time, the notion seemed too avant-garde and outlandish taking into consideration the fact that no agents and molecular disease targets were known. In the last decade, tremendous advances have been made in understanding the pathological processes and identifying molecular moieties specific for disease location. These incredible developments provide us with an opportunity to design specific and efficient drug-delivery carriers utilizing biology and physics of the diseased loci. As an example, the FDA has approved over 26 anticancer drugs for clinical use in the last decade alone [194], as well as a vast variety of other therapeutic agents for a wide range of conditions from cardiovascular disease to inflammation. Though the curative potential of these drugs on the molecular level is indisputable, there are several limitations which hinder clinical translation and success. Firstly, the physico-chemical properties of the agents prevent them from being efficiently administered in the molecular form. As an example, the polycyclic nature of the majority of drugs makes them practically insoluble in aqueous environments [195]. Drugs such as paclitaxel and dexamethasone have water solubility values of 0.0015 mg/mL [196] and 0.1 mg/mL [197], respectively, which makes them unacceptable for intravenous injection in aqueous media. Even more prominent obstacles lies in the presence of multiple biological barriers, preventing the administered drug or imaging agent from reaching its target tissue. When administered in a solution, the distribution of an agent is highly unspecific, with only 1 in 10,000 to 1 in 100,000 molecules reaching their intended site of action [198]. This lack of specificity, resulting in a much higher dose to be administered for obtaining the desired effect also largely affects the therapeutic window of the majority of drugs, making the range between efficiently and toxicity very narrow [199]. Doxorubicin, possessing prominent cardiotoxicity, represents an example of such an agent [200]. Taking these factors into account, it would be desirable to chemically modify the drug with features that would pharmacologically guarantee increased stability, solubility, and targeting to the site of action. However, these alterations are more than often not viable. This realization is the fundamental driving force behind the concept of nano-therapeutic drug delivery—to enable drug function regardless of poor intrinsic pharmacological properties.
Nanovectors are being developed and investigated as carriers for individualized therapeutic and imaging contrast agents based on the simultaneous, anticipated advantages of homing at the diseased site, such as cancer lesions and atherosclerotic plaque. This behavior encounters nanoparticles’ ability to cross the various obstacles, so-called “biobarriers”, located between the administration site and the target organ. Historically, oncology represents the field of medicine to which nanotechnology made the most prominent contributions. During the last 15 years, nanocarriers occupied an important niche in treatment of cancer patients, with liposomes being the first commercially available drug nanocarrier for injectable therapeutics [201–203]. Liposomal doxorubicin has been granted with FDA approval in the mid-1990s for use against Kaposi’s sarcoma. Later, it was also approved for metastatic breast cancer and recurrent ovarian cancer therapy. Starting from this point, a variety of nanocarrier-based drug-delivery systems have been in different stages of development, including particles with various compositions, physico-chemical characteristics, geometry and surface functionalizations [9,204]. The library, generated by all the possible combinations is gigantic, and clear considerations should be taken when developing carriers for specific drugs or conditions.
There is a general taxonomy that can be applied to nanovectors, which divides them into the three main subclasses or generations [9,201,205] as schematically shown in Fig. 4. The first-generation of nanovectors describes nanoparticles that home into the disease site by using passive mechanisms. The main subclass in this category comprise the liposomes [206], including those in clinical applications. In the case of cancer, liposomes utilize the enhanced permeability of the neovasculature as the mechanism to localize into the disease site through so-called the enhanced permeation and retention (EPR) mechanism [77,207]. The extravasation of nanovectors is favored due to the presence of the large (several hundred nanometers) vascular fenestrations on newly formed angiogenic vessels. The carriers in this subcategory can posses some surface modifications with, for example, a polyethylene glycol (PEG), making the nanovectors “stealth” and preventing their uptake by the RES. “Stealth” particles have substantially prolonged circulation time and increasing the likelihood of tumor homing [55,206,208,209]. Significant strides in the fields of chemistry and materials science have yielded several other nano- sized carriers with immense potential for drug delivery, including polymer–drug conjugates [210], polymer micelles [196], and dendrimers [211]. While first-generation of nanovector describes carriers with no active mechanisms of disease site location and therapy, the second-generation of nanovectors encompasses delivery systems with further functionality [212–216]. This functionality can be of two origins: (1) specific molecular recognition moieties on the nanovector to receptors overexpressed on the tumor cells or adjacent blood vessels (e.g. mAb-conjugated liposomes) or (2) a possibility for active/triggered release of the payload at the diseased location (e.g. magnetic liposomes). Superior to their precursors, employing additional complexities such as targeting moieties, remote activation, and environmentally sensitive components, enables second-generation of nanovectors to have additional emerging degrees of sophistication, though the second-generation predominantly represents simply a progressive evolution of the first-generation nanovectors. The fundamental problem of various obstacles on the way of therapeutics to reach their target, has given rise to a paradigm shift in the design of nanoparticles with the emergence third-generation particles. We strongly believe, that further developments of the nanovectors for personalized therapy will rely on the third-generation of the carriers, or logic embedded vectors (LEVs) [217]. LEVs are therapeutic multi-component constructs specifically engineered to avoid biological barriers, in which the functions of bio-recognition, cytotoxicity and biobarrier avoidance are decoupled, yet act in efficacious operational harmony. The ideal injected chemotherapeutic strategy is envisioned to be capable of navigating through the vasculature after intravenous administration, to reach the desired tumor site at full concentration, and to selectively kill cancer cells with a cocktail of agents with minimal harmful side effects.
Fig. 4.
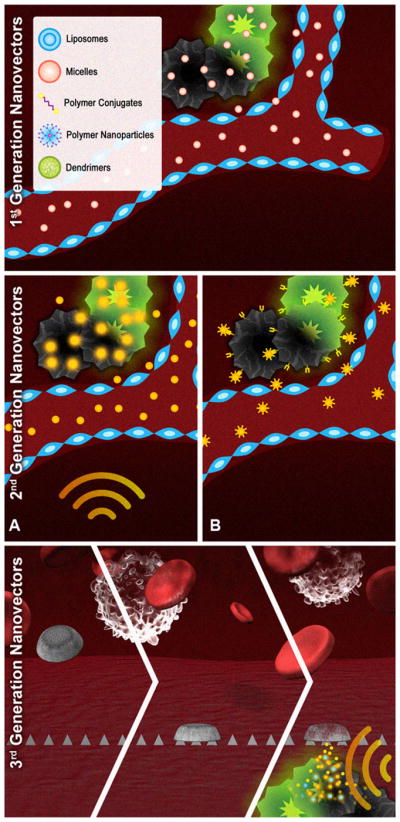
Schematic presentation of three generations of therapeutic nanovectors. First generation: nanoparticles localizing in tumor through the EPR passive mechanism; second generation: nanovectors possessing additional level of complexity such as (a) remote activation by means of radiofrequency (RF) or near-infrared (NIR) energy or (b) active targeting through specific ligands overexpressed on tumor cells; third generation: logic embedded vectors, LEV comprised of different nano-components which act through a time-sequence of synergistic and logic-driven events.
Further in this manuscript, we will provide examples of nanovectors belonging to the three above-mentioned generations for advanced therapy and imaging. These nanovectors demonstrate immense potential for enhanced drug delivery, which will undoubtedly have a high impact on the future of personalized medicine.
6.1. Drug delivery
6.1.1. The first- and second-generations of nanovectors
6.1.1.1. Liposomes
As it was mentioned above, the first generation of nanovectors encompasses a delivery system that localizes into the lesion through passive mechanisms. The homing to the disease site is driven only by the particles’ nano-dimensions, and is not related to any specific recognition of the target. Nonetheless, localization through EPR as in the case of tumor has been quite successful in particular in changing the pharmacokinetic behavior, bioavailability and toxicity of the delivered drug. Liposomes are vesicular nanostructures formed from phospholipid and cholesterol molecules—constituent components of cell membranes [206]. These microscopic phospholipid bubbles with bilayered membrane structures prove extremely advantageous for drug delivery given their inner hydrophilic compartment that can encapsulate water-soluble drugs, as well as therapeutic proteins, DNAs, and siRNAs. Non-PEGylated liposomes (Myocet™) and PEGylated liposomes (Doxil®) were among the first liposomal systems in clinical use [206]. For the liposomal encapsulated doxorubicin, the elimination half-life for the free drug is only 0.2 h, but this increases to 2.5 and 55 h, respectively, for non-PEGylated and PEGylated liposomal formulations. Moreover, the area under the time–plasma concentration profile (the AUC), which indicates the bioavailability of an agent following its administration, is increased 11- and 200-fold for Myocet™ and Doxil®, respectively, compared to the free drug [218]. Either through physical entrapment within the nanoparticle, or via conjugation to constituent components, drugs are effectively solubilized and habitually protected from enzymatic degradation and inactivation [218]. Encapsulation into the liposomal carrier also causes a significant reduction in the most significant adverse side effect of doxorubicin, namely cardiotoxicity, as demonstrated in clinical trials [203,207,208]. Other liposomal drugs which are either currently in use or are being evaluated in clinical trials include non-PEGylated liposomal daunorubicin (DaunoXome®) and vincristine (Onco-TCS), PEGylated liposomal cisplatin (SPI-77) and lurtotecan (OSI-211) [32].
Liposomes and other antibody-targeted nanoparticles, have also been the most investigated example of the second-generation nanovectors [9,49,201,203,206,212,214,220,221]. The additional functionality on the surface of the nanovectors enables specific recognition of the ligands on the disease site. One of the main questions considering any targeting moieties on the surface of nanovectors is the pro and contra of high or low binding affinity of the ligand for its antigen or receptor. Inside the disease mass, when the binding affinity is high, there is the ‘binding-site barrier’ which impairs the penetration of the carrier all the way through. The main reason for that is the strong binding of an agent to the forefront of the target tissue blocking further penetration in the deeper layers. On the other hand, for targets in which most of the cells are readily accessible to the delivery system—for example, tumor vasculature and certain hematological malignancies—a high binding affinity is desirable.
A variety of targeting moieties besides antibodies are under extensive investigation worldwide. These include ligands, aptamers, small peptides and phage-display peptide binding to specific target cell-surface markers or surface markers expressed in the disease microenvironment and will be further discussed in our review [222,223]. Examples of other nanocarriers in the first- and second-generations of nanovectors include metal nanoparticles for use in diagnostics (further described in this manuscript) [224,225], albumin paclitaxel nanoparticles approved for use in metastatic breast cancer [226], drug–polymer constructs dendrimers and polymeric micelles which are described below.
6.1.1.2. Drug–polymer constructs
Much like liposomes, polymer–drug conjugates have shown immense clinical potential early on as efficacious drug-delivery strategies. From a historical perspective, polymer–drug conjugates were among the earliest nano-therapeutic platforms explored for drug-delivery purposes and to achieve clinical translation [210]. At first glance, the underlying principle of the strategy appears facile, simply involving conjugation of drugs or proteins to water-soluble polymers. The polymers typically employed are polyethylene glycol (PEG), N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, and polysaccharides such as dextran [209]. The specific advantages afforded by polymer–drug conjugates for diseases such as cancer, for example, are similar to those of nanoparticles, chief among them being increased blood residence times, reduced immunogenicity, and passive targeting to tumors through the EPR effect. While attractive, a limiting factor includes the requirement for the presence of functionalizable chemical groups on the drug molecules [227]. Moreover, the small size of polymer–drug conjugates, typically <10 nm, means that they can easily cross basement membranes in the glomeruli of kidneys and be quickly cleared, leading to much shortened blood half-lives [32,228]. Nonetheless, these systems have found widespread clinical acceptance, especially in cancer therapeutics.
In the 1980s, Maeda and coworkers developed SMANCS, a conjugate of neocarzinostatin (NCS) and poly(styrene-co-maleic acid) (SMA), later approved for clinical use in the early 1990s [229]. As a result of this conjugation, the blood half-life of NCS was extended to 10 times that of the free drug, yielding enhanced accumulation at tumor sites [230]. In 1994, PEG-L-asparaginase (Oncaspar) was the first PEGylated enzyme approved for clinical use for treatment of acute lymphoblastic leukemia [231]. This formulation rapidly showed its advantages over free enzyme administration, mainly by reducing hypersensitivity reactions in patients and prolonging the half-life of the enzyme to 357 h (compared to 20 h for enzyme alone) [232]. Following the success of these polymer–drug conjugates, several HPMA–drug conjugates were explored in clinical trials, and these included conjugation with well-established anticancer drugs such as paclitaxel [233] and doxorubicin [234]. Presently, several polymer–drug conjugate platforms can be found in all stages of clinical trials, including dextran–doxorubicin, PEG–camptothecin, and polyglutamate–paclitaxel conjugates [210].
6.1.1.3. Polymer micelles
In the early 1980s, Ringsdorf and coworkers worked towards the development of polymer micelles as drug-delivery vehicles [235]. The result of their efforts is a promising platform with immense therapeutic potential presently on the cusp of clinical translation. Polymer micelles are spherical, supramolecular constructs, with a size ranging from 10 to 100 nm, formed from the self-assembly of biocompatible amphiphilic block copolymers in aqueous environments [236]. Briefly, the hydrophobic portion of the polymer forms a semi-solid core, wherein drug molecules can be entrapped. All the while, the hydrophilic portion forms a hydrating layer, protecting the carrier from opsonization and subsequent phagocytic clearance by the RES [237]. The hydrophobic polymer components can be varied, and include poly(D,L-lactic acid) (PDLLA), poly(ε-caprolactone) (PCL), and poly(propylene oxide) (PPO) to name a few, with sizes ranging from 2 to 15 kDa [45]. The hydrophilic portion is typically composed of PEG, although polymers such as poly(N-vinyl pyrrolidone) (PVP) and poly(N-isopropylacrylamide) (pNIPAM) have also been explored [196]. Polymer micelles prove attractive for drug-delivery purposes mainly because of their ability to solubilize hydrophobic drugs within the cores. The innate chemistry of micelles, which includes a PEG component, allows for the presence of a hydrophilic corona that prevents opsonization and RES uptake. It is important to note at this time that ligands can be added to the hydrophilic portion of the amphiphilic block copolymer for tumor targeting strategies [238]. Last but not least, the small size of micelles leads to their preferential accumulation in tumor tissue through the EPR effect.
Currently, polymer micelles are being explored in various phases of clinical trials. Kataoka and coworkers were able to formulate poly(ethylene glycol)–poly(L-aspartic acid) micelles containing doxorubicin, and showed impressive preclinical antitumor efficacy [220]. This formulation, known as NK911, displayed long blood circulation times, nearly tripling the half-life of the free drug, and showed much reduced drug clearance [239]. Another micellar formulation in clinical trials, Genexol-PM, consists of paclitaxel encapsulated within PEG–PLA micelles. Findings from these studies have shown that the micellar formulation of paclitaxel was much more tolerable than the clinically used formulation containing Cremephor® EL, an excipient shown to lead to hypersensitivity reactions [240]. As a result, Genexol-PM allowed for significant dose escalation of paclitaxel, which in turn translated into antitumor responses in at least two patients who were previously unresponsive to traditional paclitaxel administration [241].
6.1.1.4. Dendrimers
Dendrimers are polymeric vectors made of monomers that branch out radially from a central core [211]. The size of these globular structures is typically on the order of 10 nm, but this size can be fine-tuned simply by varying the dendrimer generation number. In addition to their size, their architecture and chemical constitution (e.g. end-groups) can be precisely controlled, a direct function of the step-by-step synthesis involved in dendrimer fabrication [242]. While dendrimers can be formed from a variety of polymers such as polyesters and polyamines, the polymer of choice remains polyamidoamine (PAMAM) given its stability, availability, and tolerability [243]. The resulting vehicle is an attractive platform for drug delivery, in light of the presence of a central cavity and channels between dendrons wherein drugs can be entrapped [244]. In addition to drug loading within these void spaces, drugs can be grafted onto tailorable functional groups [245]. This affords the possibility of incorporating not only multiple and different drug molecules within the same dendrimer, but also multiple targeting ligands as well [246]. Of paramount importance for in vivo applications is the ability of dendrimers to be functionalized with PEG, reducing their uptake by the RES. Last but not least, the unique chemistry of dendrimers allows for the controlled degradation, through depolymerization of dendrimers, which may in turn result in controlled drug release profiles at the site of action.
While they have yet to find their way into the clinical arena, dendrimers are showing promise as efficacious drug-delivery vehicles in several preclinical studies. Currently, dendrimers are being explored as vehicles for transdermal, ocular, and oral drug-delivery vectors [247]. For example, Jain and coworkers formulated artemether-containing dendritic micelles for treatment of multidrug-resistant strains of malaria, resulting in a 15-fold enhancement of drug solubility and increased stability [248]. For cancer chemotherapy, dendrimers have been explored as carriers for a variety of drugs, including doxorubicin [249], 5-fluorouracil [250], etoposide [251], and paclitaxel [252]. Recently, methotrexate-containing polyamidoamine dendrimers, fashioned with folate for targeting purposes, were shown to reduce growth of human KB tumors that overexpress the folic acid receptor in mice [221]. Hence, while still a technology in its infancy, the field of dendrimer drug delivery has immense potential for applications in a variety of diseases with a variety of administration routes. Although the representatives of the second-generation have not yet been approved by FDA, there are numerous ongoing clinical trials involving targeted nanovectors, especially in cancer applications.
6.1.2. The third-generation of nanocarriers: logic embedded nanovectors
Third generation nanovectors, such as multi-stage agents, are capable of more complex functions which enable sequential overcoming of multiple biobarriers following a certain time/site determined “logic” of events [217]. In these multitasking constructs each component is responsible for a different task among the following: bio-recognition, protection from degradation, avoidance of toxicity, overcoming biobarriers and efficient intracellular delivery. These vectors are able to act in a pre-programable sequential manner, encoded in the properties of the material. In these multi-stage carriers each stage performs part of the journey from the site of administration towards the target lesion, negotiating one or more biological barriers, and adding a degree of targeting selectivity in the process. To multiply the probability of homing into the disease location, expertise in molecular biology, physics, mathematics, chemistry and engineering are crosslinked. This novel generation of nano-therapeutics is exemplified through the employment of multiple nano-based products that synergistically provide distinct functionalities. These systems may incorporate imaging and therapeutic component in such a manner that enables an individualization of therapy built-in in the vector. The time dynamics of the evolution of the lesion do not necessarily require a change in cytotoxic payload—the response to the evolution of the lesion and its microenvironment may be built in the individualization of carrier.
Ferrari and colleagues have recently designed the multi-stage technology platform, which incorporates the fundamental components of the above-described LEVs [38]. These are comprised of nanoporous silicon microparticles that utilize their unique particle size, shape and other physical characteristics in concert with active biological targeting moieties to efficiently deliver payloads of nanoparticles to the disease loci, resolving sequential mission-critical issues. The multi-stage drug-delivery system is predicated upon a Stage I nanoporous silicon microparticle that is specifically designed (through mathematical modeling) to exhibit superior margination and adhesion properties during its negotiation through the systemic blood flow en route to the tumor site. As an example, the optimal mathematical design of first-stage vector particles with respect to margination [253,254], firm cellular adhesion [90,255], internalization [37,89] was shown and the initial biodistribution studies translating the rational design into the in vivo data were performed. Through using the photolithographic techniques and bioconjugation methods it is possible to yield an exponential amount of particle configurations by modifying the size and shape of “first-stage” particles and choosing specific surface characteristics, to meet the criteria chosen by the design maps (Fig. 2). These first-stage particles enable efficient margination of the vector in the blood vessels as well as the recognition of the diseased vasculature. The “mother-ship” first-stage vectors carries within its biodegradable nanoporous structure various payloads, or the second stage nanovectors, which can essentially be any of the above-mentioned first or second generation vectors [38,203]. It has also demonstrated that the release profiles of the second stage vector from the multi-stage particle can be finely tuned to take place at different times, and through different paths, particles can be intracellularly internalized [37,256] to deliver their payloads to different subcellular structures. The multi-stage drug-delivery system is emblematic of third-generation nanoparticle technology, since the strategy combines numerous nanocomponents to deliver multiple nanovectors to a tumor lesion. The versatility of this LEV platform allows for a vast variety of applications.
Other examples of the third-generation nanovectors include nanoshuttles [58,215] and ‘nanocell’ [257] vectors. Nanoshuttles are the biologically active molecular networks comprised of self-assemblies of gold nanoparticles within a bacteriophage matrix. These systems combine various functionalities encoded in their structure: the biological targeting capabilities phage-display peptides and gold nanoparticles with hyperthermic response to near-infrared radiation, CT imaging contrast and surface-enhanced Raman scattering detection. ‘Nanocell’ nanovectors are “disease inspired” systems comprised of a lipid-based nanoparticle enveloping a polymeric nanoparticle core, each of the components of the system encapsulates different therapeutic agents that are released in a sequential, time-sensitive manner. As an example, a conventional chemotherapeutic drug (e.g. doxorubicin) is conjugated to a polymer core and an anti-angiogenic agent (combretastatin) is entrapped within the lipid envelope. The carrier is localized to the tumor site through the above-mentioned EPR effect, and then the sequential time release of the anti-angiogenic agent, followed by the cytotoxic drug, provides an efficient time-sensitive combination therapy.
To summarize, the last century has witnessed the discovery of a vast arsenal of agents for diseases ranging from cancer to cardiovascular disease. And while efficacious in in vitro settings, their unique chemistry, narrow therapeutic windows and unfavorable tissue distribution, due to the multiplicity of obstacles, precludes their successful translation to the clinics. Presently, nano-scale drug-delivery vehicles continue to enable the use of preexisting drugs by providing longer circulation times, greater tolerability, and site specific delivery; factors that result in better patient outcomes. And while future drugs become more and more specific in their mechanisms of action, the future of nanotechnology in drug delivery will surely shift towards functionalization of these vehicles, moving towards LEV as the vector of choice to provide multiple therapeutic benefits with the hopes of affording the patient the most personalized mode of therapy possible.
6.2. Nanovectors for thermal ablation
6.2.1. Thermal ablation: the concepts
The standard of care for patients with unresectable malignancies is chemotherapy and external-beam radiation. When these fail, innovative treatments are sought. These currently include local thermal ablation under ultrasound or computed tomography guidance [258–260]. Thermal ablation is a cancer treatment modality that uses heat to destroy a tissue or to impair its function. It has been routinely used in the clinics for treatment of uterine bleeding, atrial fibrillation primary lung and liver cancers and liver metastasis [261,262].
Ablation is achieved by using various heating sources, like laser light, focused ultrasound, microwaves, radiofrequency field and magnetic resonance. Traditionally, the most common method used, radiofrequency ablation (RFA), is based on radiofrequency electric fields that utilized the natural differences in properties of the normal and carcinogenic tissues to achieve differential heat deposition. RFA has been used for the treatment of primary and metastatic liver tumors and is credited with a low occurrence of side effects. This treatment is most suited for small numbers of lesions since each must be targeted individually. The goal of RFA therapy is to destroy the entire tumor and at least a 0.5 cm margin by heating the tissue to 50–100 °C, causing “coagulation necrosis” [263]. Radio waves produce heat by ionic agitation (resistive forces) as they travel from the implanted electrode tip to the ground source placed outside the body [264]. Therapeutic response is typically monitored by CT or FDG-PET. Studies have shown that RF energy has low tissue specific absorption rates (SAR) and therefore, has excellent whole body tissue penetration with documented safety in humans exposed to an RF field for 10 min up to several hours [265,265].
However, the difference in sensitivity of normal and abnormal tissues is too small, thus normal tissue can also be damaged under irradiation, further the heterogeneity of electrical conductivity of tissues makes selective heating very difficult. To overcome this problem and increase the contrast between the two tissues, nanovectors are being studied for effective treatment. Gordon et al. suggested the use of submicron particles to enable cellular uptake and cause intracellular hyperthermia, thereby increasing the selectivity of the thermal destruction [266]. Introduction of nano-scale devices have prominent advantages of increased sensitivity to radiofrequency energy, lower required doses and exposure times, selective delivery, and improved homogeneity of heat induction.
The nanovectors, used for thermal ablation therapies include superparamagnetic iron oxide nanoparticles (SPIONs), paramagnetic copper–nickel alloy nanoparticles, magnetite cationic liposomes, carbon particles (single walled carbon nanotubes and fullerenes), gold nanoparticles and nanoshells. By definition, these nanoparticles include the second and the third-generation nanovectors that could be injected directly into the tumor or local (e.g. hepatic) vein and heated, or conjugated with ligands to enable active targeting, respectively. Below, a brief overview will be provided on different classes of nanovectors used for thermal ablation therapies.
6.2.2. Thermal ablation using magnetic nanoparticles
One option for thermal ablation includes seeding the tumor with magnetic nanoparticles for selective generation of heat in the tumor. Iron oxide nanoparticles have a very good magnetic properties that when placed in an alternating magnetic field gets heated up by hysteresis loss, induced eddy currents and Neel relaxation [267]. In a study by Hilger et al. [268], three sizes of Fe3O4 nanoparticles (i.e. SPIONs) were tested for heat induction in human breast adenocarcinoma xenografts established by subcutaneous administration in immunodeficient mice. Tumors were loaded with 7.7 ± 2.3 mg magnetite per 100 mg tissue. At a magnetic field amplitude of 6.5 kA/m and 400 kHz frequency the rate of energy per mass of SPIONs (i.e. specific absorption rate) was higher for smaller nanoparticles (10 vs 220 nm). Mice were exposed to an AC magnetic field for 4 min, amplitude 6.5 kA/m; frequency 400 kHz using a circular coil applicator. At sites containing magnetite agglomerates, temperatures were elevated between 18 and 55 °C. Limitations included nonhomogeneous particle distribution and migration of particles from the tumor tissue. Similar limitations were experienced in human Phase I studies for the treatment of prostate cancer, specifically suboptimal intratumoral distribution of magnetic nanoparticles [269]. The key to optimization of this technique is the future of less invasive targeted delivery of magnetic nanoparticles to the tumor.
In a recent study [270], rabbits with malignant kidney tumors were implanted with super paramagnetic iron oxide nanoparticles (SPIONs) using CT guided placement and exposed to an alternating electromagnetic field (0.32 kA/m) for 15 min. The resulting tumor necrosis was verified by CT perfusion imaging and histological evaluation. In an earlier study by the same group [271], a single injection of SPIONs (8–10 nm) was compared to continuous infusion during exposure to the magnetic field. Continuous infusion of SPIONs resulted in a larger zone of necrosis compared to the single injection, however, irregular ferrofluid distribution lead to highly variable coagulation necrosis volumes.
In a study by the German Research Foundation, cobalt–palladium thermoseeds were implanted in the prostate of 57 cancer patients [272]. In six weekly sessions hyperthermia was induced using a magnetic field along with 3D-conformal radiotherapy of 1.8 Gy. Temperature elevations between 42 and 46 °C were achieved, with no major side effects. Evaluations of efficacy are ongoing but early studies show a steep decrease in PSA levels, a marker for prostate cancer.
6.2.3. RF and NIR ablation with carbon nanoparticles, gold nanoparticles and nanoshells
Among the most interesting examples of the second-generation of the nanovectors are gold nanoparticles and nanoshells that can be remotely activated by near-infrared light (NIR, 650–950 nm) [273] as schematically presented in Fig. 4 (middle panel A). Generally, gold nanoparticles were reported to be biocompatible [274–276].
Treatment of mammalian and human cancer cells with gold or carbon nanoparticles in vitro and in vivo followed by brief treatments in a non-invasive shortwave RF field has been shown to produce thermal cytotoxicity in the malignant cells [277–281]. A treatment strategy based on molecular targeting of gold nanoparticles to cancer cells to create RF-induced hyperthermic cytotoxicity has several advantages: gold and carbon nanoparticles are simple and inexpensive to synthesize; they are easily characterized due to the signature optical absorptions; their surface chemistry readily permits manipulation of charge and shape; and attaching cancer cell targeting molecules, including antibodies, peptides, or pharmacologic agents, is easily achieved. Lastly, a major advantage to using 5–10 nm diameter gold nanoparticles is the ability of nanoparticles to penetrate effectively through pores and fenestrations in the neovasculature of solid tumors [282]. These physico-chemical and biologic properties allow targeted gold nanoparticles to gain access to the surface of cancer cells and bind to identified target surface ligands or receptors, followed by internalization into the cytoplasm of the cells [278]. These intracytoplasmic gold nanoparticles can then be activated by absorption of RF energy to release heat sufficient to produce thermal cytotoxicity in the cancer cells. Finally, an additional advantage of gold for therapeutic use is that it is already used clinically to treat some patients with severe rheumatoid arthritis and is known to have a low toxicity profile. Carbon fullerene nanoparticles (nano-C60) have the advantage of being even smaller (~2 nm) than traditionally used AuNPs, can be functionalized with a number of biologic molecules, and can be loaded into cancer cell targeting antibodies without altering the binding capacity of the antibody [283].
In a recent study DNA-encasement of multi-walled carbon nanotubes (MWNTs) was shown to enhance absorption of NIR energy and subsequent heat conversion. In this study thermal effect was proportional to irradiation time and laser power and DNA-encasement required threefold less concentration of the MWNTs to produce the same shift in the temperature of the bulk solution. Further, intratumoral injection of MWNTs followed by irradiation with NIR laser resulted in complete eradication of the prostate cancer xenograft tumors in mice [284].
Nanoshells are composed of an ultrathin metal shell surrounding a dielectric core [285]. Gold-coated nanoshells with silica core can be developed to prominently absorb light in the NIR wavelength region and since live tissues do not posses significant absorption in this region, it provides a pan for the selective ablation [286]. NIR-absorbing nanoshells have been used for cancer therapy [273,287]; showing up to 100% regression of tumors after photothermal treatment [288]. In these works nontargeted nanoshells localized passively in the tumor based on EPR mechanism. Further degree of selectivity is achievable through linking gold nanoparticles and nanoshells to antibodies that recognize target cells enabling active targeting of the abnormal cells prior to applying near-infrared light or radiofrequency energy source to heat them up, thus minimizing non-specific adverse reaction. The gold surface allows easy conjugation of biomolecules to the surface by the use of a poly(ethylene glycol) (PEG) linker with a sulfur moiety. The targeting moieties for gold nanoparticles and nanoshells that were reported in the literature include Eph, HER-2, aptamers, EGF, guanylyl cyclase C ligands [289–294]. As an example, targeting towards Eph receptors, which belong to a tyrosine kinase family of proteins overexpressed in many types of cancers, including prostate, lung, esophageal, melanoma, leukemia colorectal, cervical, ovarian, and breast cancers [295,296], have shown specific binding to PC-3 cells overexpressing Eph receptor. Subsequent photothermal therapy with NIR light selectively killed PC-3 cells, demonstrating the efficacy of targeted nanoshell therapy [293].
Tissue thermal ablation using nanovectors as specific thermal agents has a great potential of treating unresectable tumors with high specificity, which can be personalized based on the ligand attached to the particle surface. The ideal system in this case is envisioned to be capable of navigating through the vasculature after intravenous administration, to reach the desired tumor site at full concentration, and to selectively kill cancer cells with remotely applied energy. Some of the above-mentioned systems are now being tested in various clinical trials.
7. Nano-based contrast agents
Advances in the molecular characterization of disease have motivated the development of molecular-specific contrast agents. The purpose of these contrast agents is to facilitate the non-invasive detection and visualization of morphological and biochemical changes that influence disease and/or its response to therapy. Progress in this field has been driven largely by applications in oncology, from the identification of specific molecular pathways associated with tumorigenesis to the clinical monitoring of cancer biomarkers before and after treatment [297]. The integration of new molecular-specific contrast agents with more conventional diagnostic imaging techniques is expected to have a major impact on the detection, diagnosis, and decision-making for personalized molecular-based treatment.
Molecular-specific imaging is already in clinical practice today. Positron emission tomography (PET), single-photon-emission computed tomography (SPECT), and magnetic resonance imaging (MRI) are some of the first clinical imaging modalities capable of generating images with molecular specificity. These technologies monitor the localization of different exogenously administered contrast agents to collect information about tissue anatomy, physiology, and metabolism. New contrast agents for these and other imaging modalities are continually being introduced in order to enhance clinical care.
Nanoparticles have been proposed as a promising platform technology for the synthesis of molecular-specific contrast agents. Advantages of nanoparticles include high contrast, tunable size, shape, and surface properties, ease of integrating multiple functionalities, and long circulation times [298,299]. A variety of nanoparticle platforms are currently in development for a range of clinical indications, including superparamagnetic agents, metal nanoparticles, liposomes, and more. Each of these platforms differs in bioavailability, pharmacokinetics, toxicity, immunogenicity, and specificity. Thus it is likely that a variety of different and specialized nanoparticle platforms will be required for targeting different disease processes. Several nanoparticle-based contrast agents have entered the market and additional products are currently undergoing clinical testing or entering the pipeline.
7.1. Nanoparticle-based contrast agents approved for clinical use
The first nanoparticle-based contrast agents were developed to compete with gadolium(Gd)-based contrast agents for MRI. However nanovectors for imaging that are currently in clinical use are all based on SPIONs. To-date, two intravenous formulations have reached clinical use and one additional formulation has been approved for oral use (Table 2). SPIONs are the first nanoparticle system to be clinically approved for in vivo imaging. In 1996, Feridex I.V.® (ferumoxides injectable solution) was introduced as the world’s first organ-specific MR contrast agent. Later, Resovist® (ferucarbotran injectable solution) was approved in the European Union (EU), Australia, and Japan for the detection and evaluation liver lesions using MRI. Both Feridex I.V.® and Resovist® are considered “negative” contrast agents: their iron content produces strong local disruptions in the magnetic field of MRI scanners, leading to increased T2* relaxation and decreased signal intensity in areas of nanoparticle accumulation. These contrast agents rely on passive targeting strategies to detect alterations in the reticuloendothelial system (RES), making normal liver, spleen, bone marrow, and lymph nodes appear dark. Inflammation, scarring, and most focal lesions of the liver reduce the uptake of SPIONs, producing localized regions of signal [300].
Table 2.
Clinically approved nanoparticle-based contrast agents.
| Composition | Trade name | Company | Indication | Administration |
|---|---|---|---|---|
| Dextran-coated SPIO (ferumoxides) | Feridex I.V./Endorem | Bayer Healthcare Pharmaceuticals, Inc. | Detection and evaluation of liver lesions associated with an alteration in the RES | i.v. |
| Carboxydextran-coated SPIO (ferucarbotran) | Resovist/Cliavist(EU, AUS, JPN only) | Bayer Schering Pharma AG | Detection and evaluation of liver lesions associated with an alteration in the RES | i.v. |
| Silicon-coated SPIO (ferumoxsil) | GastroMARK | Covidien, Ltd. | Bowel marking | Oral |
A key advantage of SPIONs in comparison to other heavy-metal based MRI contrast agents is their ability to integrate physiologically. Iron and iron oxides are metabolized, stored in intracellular pools as ferritin, and incorporated into hemoglobin. Dose escalation studies in rodent models elicited no identifiable side effects at 100 mg iron/kg [301], a dose well above that used for MRI procedures (<5 mg/kg). Radiotracer and histological studies in rodents have demonstrated that nanoparticulate iron becomes part of the body iron pool, first accumulating in the RES and then slowly disappearing over the course of 14–28 days [302–304]. In humans, intravenous administration of iron oxide nanoparticles coated with semi-synthetic carbohydrates was found to safely increase mean blood hemoglobin concentrations by approximately 1.0 g/dL over a 35-day period [305]. As a result, in June 2009, Feraheme™ (ferumoxytol injectable solution) was approved for the treatment of iron-deficiency anemia in adult patients with chronic kidney disease. Ongoing clinical trials are evaluating the suitability of ferumoxytol as an MRI contrast agent for nervous system disease, brain neoplasms, and peripheral artery disease.
These first-generation SPIONs will likely play an important role in advancing personalized medicine. Feridex I.V.® is administered as a slow infusion in conjunction with delayed phase imaging and is therefore well suited for the detection of small focal lesions with high accuracy, particularly when images are collected before and after contrast agent injection [306,307]. Resovist® can be administered as a rapid bolus and can therefore be monitored using dynamic imaging to produce higher liver-to-tumor contrast [308]. New applications of these contrast agents are also being actively pursued, including the pre-operative staging of pancreatic cancer (currently in Stage IV clinical trials), monitoring of tissue margins following radiofrequency ablation to predict tumor recurrence [309], non-invasive differentiation of hepatocellular cancer grades [310], and the monitoring of macrophage infiltration into other pathologic tissues [311]. There is also interest in using SPIONs to track cell movement in vivo following transplantation (reviewed in [312]) for the long-term goal of developing and monitoring personalized cell-based therapies.
7.2. Nanoparticle-based contrast agents in clinical trials
Increasing numbers of nanoparticles are currently undergoing clinical trials (Table 3). The most advanced nanoparticles are still based on the SPIO platform, but vary in surface coating, size, and function. Also under investigation is the first molecular-specific, nanoparticle-based injectable contrast agent. These contrast agents are described in further detail below.
Table 3.
Nanoparticle-based contrast agents in clinical trials.
| Composition | Trade name | Developer | Indication | Administration | Status |
|---|---|---|---|---|---|
| Dextran-coated USPIO (ferumoxtran-10) | Combidex/Sinerem | AMAG Pharmaceuticals, Inc. | Differentiation of cancerous from noncancerous lymph nodes | i.v. | Phase III |
| Carboxy dextran-coated USPIO (ferucarbotran) | Supravist | Bayer Schering Pharm AG | Detection of blood pooling using MRA | i.v. | Phase III |
| Polyglucose sorbitol carboxymethyl ether-coated SPIO (ferumoxytol) | – | AMAG Pharmaceuticals, Inc. | Nervous system disease, brain neoplasms, peripheral artery disease | i.v. | Phase II |
| Citrate-coated very small SPIO | VSOP-C184 | Charité - Universitätsmedizin Berlin | Detection of blood pooling using MRA | i.v. | Phase I |
| Radiolabeled Her-2 affibody | ABY-025 | Affibody Holding AB | Breast cancer | i.v. | Phase I |
Combidex®(ferumoxtran-10) is an ultra-small (20 nm diameter) SPIO (USPIO) coated with low-molecular weight dextrans under development for lymph node imaging. Following intravenous administration, these nanoparticles are phagocytosed by macrophages and accumulate in benign lymph nodes. Disturbances in lymph flow and/or nodal architecture lead to abnormal patterns of nanoparticle accumulation that can be detected by MRI [313]. Although Combidex® has been approved for use in some EU countries, it has had difficulty gaining widespread regulatory approval due to a high false-positive rate. For example, a recent multi-center study evaluated the use of Combidex® and MRI to identify lymph node metastases occurring outside the normal area of pelvic lymph node dissection in 296 patients with prostate cancer [314]. There was a 24.1% false-positive rate in this study, leading to unnecessary surgical interventions. Based on advice from the FDA, Combidex® is currently undergoing additional clinical trials in attempt to better define the specific applications for which Combidex® is both safe and accurate. These potential applications include the screening and assessment of therapeutic response to “anti-inflammatory” interventions [315], the imaging of brain and pelvic neoplasms, lymph node staging in prostate cancer, and the prediction abdominal aortic aneurysm instability.
Two SPIOs are under clinical investigation as contrast agents for MR angiography (MRA). Supravist® (Ferucarbotran), a T1-weighted reformulation of Resovist®, has been developed for “positive” detection of blood pooling. Supravist® has shown promising results using both first-pass and steady-state angiography following bolus injection [316], comparable to those achieved using gadolinium(Gd)-based contrast agents [317]. Phase III clinical trials in patients with peripheral artery disease and renal vascular disease have been completed but not yet published [318]. VSOP-C184, a 7 nm citrate-coated SPIO formulation, has also generated first-pass images equivalent to those using Gd-based agents [319]. Phase I clinical trials have demonstrated favorable safety, tolerability, and efficacy data [320]. Such nanoparticle-based MRA agents are expected to advance angiography as imaging modality for personalized medicine, since their long plasma-half-life is well-suited for detection of small vessels with slow and/or complex flow [321]. Potential applications tested in humans and/or animals include perfusion imaging [322], functional imaging [323], dynamic detection of bleeding [324], and the characterization of tumor-related angiogenesis [325].
The first molecular-specific nanoparticle-based contrast agent, an engineered compact three-helix bundle (i.e. affibody®) that binds Her-2, is poised to enter Phase I clinical trials. In August 2009, Affibody AG announced that it has obtained the final approval and funding for a Phase I study of its ABY-025 compound. Her-2 is a growth factor whose over-expression is associated with more aggressive and malignant breast cancer phenotypes [326,327]. In clinical practice today, breast cancer treatments are selected based on the expression (or lack) of specific biomarkers including Her-2. And while treatments are personalized based on disease stratification, there is no available method for detecting or monitoring changes in Her-2 expression in a non-invasive manner. The 18F-labeled ABY-025 affibody® has the potential to revolutionize how and when Her-2 specific treatments are administered. In animal studies, ABY-025 allowed the direct assessment of Her-2 expression in vivo using PET and the monitoring of changes in Her-2 expression following therapeutic intervention [328]. In humans, this technique could be used to detect Her-2 expression without the need for biopsy, both before and after treatment. It is easy to envision that this approach could be used to perform regular non-invasive follow-up studies to evaluate treatment efficacy for on-demand therapy tailoring.
7.3. Nanoparticle-based contrast agents in preclinical development
The recent success of nanoparticle-based contrast agents has led to the development of more complex nanoparticle systems. A search of the NIH Molecular Imaging and Contrast Agent Database (MICAD) reveals over 40 nanoparticle-based systems in preclinical development. These systems utilize a variety of different chemical, physical, and biological properties for a range of clinical indications. The latest designs in nanoparticle-based contrast agents are comprised of one or more contrast generating materials (para/superparamagnetic, radioactive, electron dense, or fluorescent), bioactive targeting moieties (peptides, antibodies, growth factors, etc.), a biocompatibility coating (carbohydrates, polymers, etc.), and other promising surface functionalizations (Fig. 5). These nanoparticles are generally designed and assembled in a modular manner, allowing multiple properties to be integrated at desired ratios. Besides SPIOs, the most common nanoparticle platforms include non-magnetic metals, liposomes, synthetic carbon structures, polymeric nanoparticles, and emulsions (Table 4).
Fig. 5.
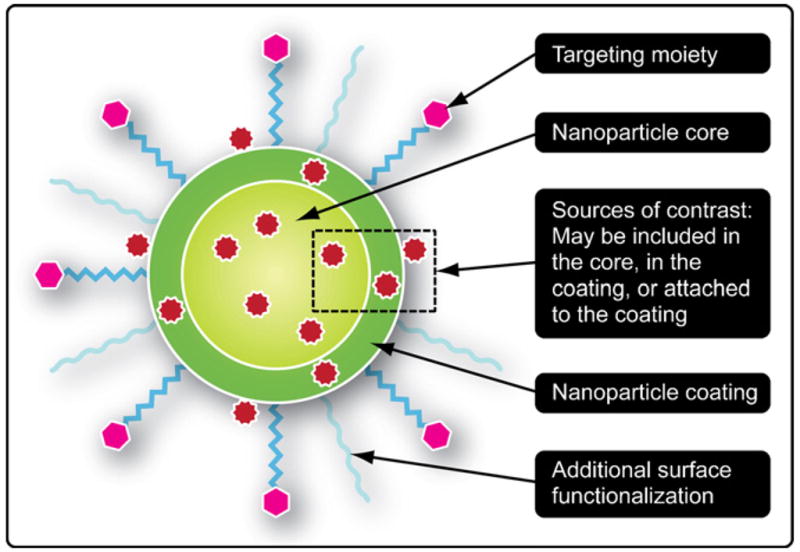
Generalized cross-section of a nanoparticle-based contrast agent, adapted from [329].
Table 4.
Nanoparticle-based contrast agents in preclinical development.
| Composition | Contrast source | Target | Indication | Ref. |
|---|---|---|---|---|
| Superparamagnetic metal nanoparticles | ||||
| Poly-L-lysine coated IO | IO | Mammalian cells | Tracking of transplanted cells | [330–333] |
| Antibody-targeted IO | IO | Her-2 | Breast cancer | [334] |
| Peptide/protein-targeted SPIO | IO | Clotted plasma proteins, MMP-2 | Various tumors | [335,336] |
| Radiolabeled antibody-targeted SPIO | 111In, IO, IRDye 800CW | Membrane glycoproteins, EGFR-2 | Various cancers | [337,338] |
| Aptamer-doxorubicin SPIO conjugate | IO, doxorubicin | PSMA | Prostate cancer | [339] |
| Peptide-targeted USPIO | IO | αvβ3, E-selectin | Various tumors, inflammation | [340,341] |
| Antibody-targeted USPIO | IO | CD20 antigen, E-selectin | Non-Hodkin’s lymphoma, inflammation | [342,343] |
| Baculovirus-targeted USPIO | IO, LacZ | Mammalian cells | Gene therapy | [344] |
| Micelle-encapsulated MnSPIO | IO | Macrophages | Liver lesions | [345] |
| Antibody-targeted MnMEIO | IO | Her-2 | Breast cancer | [346] |
| Radiolabeled passive-targeted MnMEIO | 124I, IO | Lymph nodes | Lymph node mapping | [347] |
| Fluorescent CLIO | IO, Cy5.5 | Macrophages | Macrophage infiltration | [348] |
| Radiolabeled fluorescent CLIO | 64Cu, IO, Cy5.5 | Macrophages | Macrophage infiltration | [349] |
| Fluorescent peptide-targeted CLIO | IO, Cy5.5/FITC | Proteases, bombesin receptor, plectin, uMUC-1, hepsin, αvβ3, H-2Kd, VCAM-1, phosphatidylserine | Various tumors, autoreactive T-cells, inflammation, apoptosis | [350–357] |
| Fluorescent siRNA-CLIO conjugate | IO, Cy5.5 | Birc5 gene | Various cancers | [358] |
| Other metal nanoparticles | ||||
| Polymer-coated gold nanoshells | Au | Tumor accumulation | Solid tumors | [287] |
| Fluorescent passive-targeted gold | Au, Hilyte 647 | Tumor accumulation | Solid tumors | [359] |
| Fluorescent antibody-targeted gold | Au, ICG | EGFR | Epithelial cancer | [360] |
| Antibody-targeted QD | QD | Her-2, PSMA, VEGFR | Various tumors | [361,362] |
| Growth factor-targeted QD | QD | EGFR | Epithelial cancers | [363] |
| Radiolabeled peptide-targeted QD | 64Cu, QD | αvβ3, VEGF | Various cancers | [364,365] |
| Protein-targeted paramagnetic QD | Gd, QD | Phosphatidylserine | Apoptosis | [366] |
| Liposome-based nanoparticles | ||||
| Radiolabeled peptide-targeted liposomes | 18F | Macrophages | Inflammation | [367] |
| Antibody-targeted paramagnetic liposomes | Gd, Texas red | ICAM-1 | Inflammation and neuroinflammatory disease | [368] |
| Radiolabeled, dye-filled liposomes | 99mTc, blue dye | Lymph nodes | Lymph node identification, inflammation | [369,370] |
| Fluorescent protein-targeted paramagnetic liposomes | Gd, AF680 | Transferrin receptor, E-selectin | Various cancers | [371,372] |
| Electron dense liposomes | Iodine | – | Blood pooling | [373] |
| Synthetic carbon-based nanoparticles | ||||
| Peptide-targeted SWNT | SWNT | Integrin αvβ3 | Various cancers | [374] |
| Gd-filled fullerenes, fullerenols, and SWNT | Gd | Macrophages | Macrophage infiltration, blood pooling | [375–377] |
| Radiolabeled antibody-targeted SWNT | 111In, SWNT | CD20 | Lymphoma | [378] |
| Radiolabeled peptide-targeted SWNT | 64Cu, 111In, SWNT | Integrin αvβ3,EGFR | Various cancers | [379] |
| Radiolabeled MWNT | 99mTc, 125I | – | TBD | [380] |
| Other platforms | ||||
| Bismuth sulfide polyvinylpyrrolidone nanoparticles | Bi | – | Blood pooling | [381] |
| Radiolabeled hormone-targeted bacteriophage | 111In | MC-1 receptor | Melanoma | [382] |
| Ioxilan carbonate particles | Iodine | Macrophages | Liver lesions | [383] |
| Antibody-targeted paramagnetic perfluorocarbon emulsions | Gd, 19F | Fibrin, Integrin αvβ3, collagen III | Atherosclerosis | [384–386] |
| Radiolabeled amphiphillic block copolymers | 64C | Folate receptor | Various cancers | [387] |
| Iodinated amphiphillic block copolymers | Iodine | Macrophages | Lymph lesions | [388] |
| Fluorescent paramagnetic dendrimers | Gd, Cy5.5 | – | Sentinal lymph node identification | [389] |
New classes of superparamagnetic nanoparticles, as well as nanoparticles similar to those in clinical trials, are being tested for use with MRI. USPIONs, which have demonstrated higher liver-to-tumor contrast than conventional gadolinium and SPION contrast agents [308], are under investigation for cancer and inflammation detection [340–344]. New classes of SPIONs include magnesium-doped iron oxide (MnSPION) and magnetism-engineered iron oxide nanoparticles (MnMEION) [346,347] for MRI contrast enhancement, and crosslinked iron oxide nanoparticles (CLION) for the controlled addition of nanoparticle surface modifications. MnMEIONs have been used to study in vivo cancer biomarker expression [346] and perform sentinel lymph node mapping in animals. CLIONs, comprised of a magnetite core caged by dextran and functionalized with amine groups, have been developed for a wide range of clinical indications [350–358].
Nanoparticles containing non-magnetic metals have fluorescent and scattering properties that make them attractive for optical imaging. Gold nanoparticles, for example, can be designed to absorb or reflect light at specific wavelengths [390]. Nanoshells and solid particles of various size, shape, and surface properties have been tested preclinically as optical contrast agents [287,359,360]; however, their optical properties have not yet been validated in humans. The electron density of gold has also made it a popular for X-ray computed tomography (CT) [391], although it is not yet clear whether gold is the best material for this use [392]. Quantum dots (QDs), another popular metal nanoparticle platform [361–364,366], provide excellent contrast for fluorescence imaging. These nanoparticles have a broad excitation window, a narrow emission window, high quantum yield, and minimal photobleaching. Interest in use of QDs has resurged with advent of heavy metal-free quantum dots, which are expected to have less toxicity than earlier designs [393].
Liposomes present a popular platform for contrast agent design. Comprised of natural or synthetic amphiphilic lipid molecules arranged in bilayer membrane structure, liposomes can encapsulate a range of hydrophilic and hydrophobic agents. Lipids functionalized with polymers, targeting moieties, radiolabels, and/or paramagnetic ions can be intercalated into the membrane structure to provide unique surface properties [394]. To date, several liposome formulations have been clinically approved for therapeutic applications, validating the clinical promise of this approach. Liposomes bearing contrast sources are under development for CT, PET, SPECT, MRI, MRA, and optical imaging [337,367–373]. For example, liposomes loaded with an electron-dense iodinated aqueous interior have been proposed for CT imaging, offering significant advantages over more traditional CT contrast agents [373].
Carbon nanotubes and fullerenes have shown promise as contrast agents for a variety of imaging modalities. These synthetic molecules respond to local dielectric changes, allowing them to absorb and emit light without photobleaching for near IR imaging [374,395]. More recent approaches have used these molecules as a structural backbone for the incorporation of alternative contrast sources, including confinement of gadolium ions within the carbon structure [377] and functionalization of the exterior with radio-and fluorescent labels [378–380]. A major advantage of single-walled (SWNT) and multi-walled (MWNT) nanotubes is that a single molecule provides many potential attachment sites, allowing different surface functionalizations and their replicates to co-exist [378]. As a caveat, synthetic carbon-based molecules have not yet been tested in humans.
Many other nanoparticle platforms are also under investigation. Polymers offer a flexible approach for the controlled assembly, functionalization, and degradation of contrast agents [396]. Dendrimers, like carbon nanotubes, provide a large number of functional sites for contrast agent and targeting moiety attachment [397]. Lipid-based emulsions and micelles, which are structurally distinct from liposomes, have also demonstrated promise as in vivo contrast agents [398,399]. Nature-derived nanoparticles including viruses and bacteriophages are also under development but few have been tested in animals.
7.4. Design trends for individualized medicine
Many of the new nanoparticle systems in development contain active targeting moieties. These moieties are used to enhance the specificity of contrast agents, resulting in the localized accumulation of contrast agents at the molecular target of interest. Targets include cancer biomarkers (e.g. Her-2, EGFR, integrin αvβ3, PSMA, CD20), inflammatory biomarkers (e.g. E-selectin, ICAM-1, VCAM-1), apoptosis markers, and many others. An early example of molecular-specific targeting for in vivo imaging was provided by Weissleder et al., who used monocrystalline iron oxide functionalized with antimyosin Fab fragments to detect myocardial infarcts in rats [400]. More recently, considerable effort has been directed towards the rational design of targeting moiety attachment [49]. It has been demonstrated, for example, that nanoparticles can accommodate multiple small ligands, enabling multivalent targeting to one or more biomarkers and increasing target affinity for individual probes [401]. Mathematical models that consider parameters such as ligand density, ligand accessibility, and receptor distribution have been used to successfully improve nanoparticle specificity in vivo [402,403].
Molecular-specific nanoparticle-based contrast agents have the ability to provide information that is not readily available using conventional techniques. In the simplest case, intravenously injected contrast agent could be used to non-invasively detect the expression of biomarkers important for disease diagnosis and treatment selection, without the need for biopsy. Many nanoparticle-based contrast agents in preclinical testing today are designed around this principal. The design of contrast agents with long circulation times, or the repeat administration of contrast agents, would facilitate dynamic monitoring of how biomarker expression changes with time, which is important for determining disease progression and response to therapy. More complex contrast agents, also known as “smart” bioprobes, could be used to collect functional information from specific molecular targets. In cancer, for example, elevated telomerase activity is associated with poor prognosis and increased risk of recurrence [404–407]. Measurement of telomerase activity and other prognostic proteins could be used for the smarter selection of personalized therapy.
Another emerging trend in the field of nanoparticle-based contrast agents is the synthesis of multi-modal particles, i.e. particles that can be detected using two or more imaging modalities. An elegant example is the protease-activatable CLION developed by the Weissleder group [350]. Multiple Cy5.5 molecules are bound in close proximity along a polymer backbone encapsulating the iron oxide particle. The fluorescent emission remains quenched until cleavage of the lysine–lysine bonds by specific enzymes such as cathepsin-B. When injected into atherosclerotic-prone mice, nanoparticle fluorescence was found to co-localize with histologically confirmed atherosclerotic regions [408]. The uptake of this nanoparticle by macrophages was subsequently studied in the infarcted myocardium of mice. In vivo fluorescence tomography images showed co-registration with MR images, demonstrating the possibility of simultaneously monitoring macrophage localization and activity with a single contrast agent [409]. Besides CLIOs, other multi-modal nanoparticles tested in animals include radiolabeled QDs [364,365], gadolium-coated QDs [366], radio-labeled dye-filled liposomes [369,370], and fluorescently labeled gadolium-encapsulating liposomes [371].
The combination of data from multiple imaging techniques offers many advantages over data collected from a single modality. Potential advantages include: improved sensitivity and specificity of disease detection and monitoring, smarter therapy selection based on larger data sets, and faster assessment of treatment efficacy. The successful combination of imaging modalities, however, will be difficult to achieve with multiple contrast agents. Multimodal contrast agents stand to fill this niche by providing spatial, temporal, and/or functional information that corresponds with anatomic features of interest.
There is also great interest in the design of multi-functional nanoparticles, such as those that combine contrast and therapeutic agents. The integration of diagnostics and therapeutics, known as “theranostics”, is attractive because it allows the imaging of therapeutic delivery, as well as follow-up studies to assess treatment efficacy. The Wickline group has extensively studied perfluorocarbon-based emulsions targeted to various atherosclerotic plaque lesions components including the Integrin αvβ3 integrin [384], fibrin [385], and collagen type III [386]. Their innovative use of gadolium-containing lipids around a perfluorocarbon core allows both 19F and conventional MR imaging. Animal studies were performed in which Integrin αvβ3-targeted nanoparticles containing the anti-angiogenesis drug fumagillin were repeatedly administered to atherosclerotic rabbits [384]. In the first round, the theranostic showed significant accumulation at the atherosclerotic lesions. A week later, a second round of theranostic injection showed little accumulation in the same regions, which the authors attributed to the successful anti-angiogenic effect of the first injection. This study supports the idea of detecting disease, targeting therapies, and assessing therapy response with a single nanoparticle agent. Other theranostic nanoparticles currently under development include siRNA CLIONs [358], siRNA molecular beacons [410], polymer-coated SPIOs [411,412], and liposome-encapsulated quantum dots [413].
8. Nanotechnology in tissue engineering
8.1. Benefits of nanotechnology and of nanomaterials in tissue engineering
To achieve optimal tissue growth, the natural extracellular environment must be mimicked for the necessary cell adhesion, mobility, and differentiation to occur [414]. Synthetic polymers meet most demands for tissue engineering (TE) scaffolds; they are capable of serving as bulk mechanical and structural platforms as well as enable the molecular interactions with the cells that are necessary to induce tissue healing. Most of the synthetic polymers used in TE are non-toxic, consistently available, inexpensive to create, and easy to alter [415]. However, they often lack the ability to create biological cues as natural polymers do in order to induce a desired cell response [416]. For this to happen, the cells rely on several topographical and physiochemical signals. These signals can be provided either by the proteins contained in the extra cellular matrix (ECM) or by the growth factors that bind to the receptors present on the cell surface. As the cells move over a natural matrix or an artificial scaffold, they sense the presence of grooves and ridges through the extension and retraction of filopodia [415]. Through this interaction, the cells determine their behavior, adjust their response to the environment, and regulate their terminal differentiation [414]. In response to these phenomena, the relevance of chemical modifications and physical features at the nano-scale proves crucial in the development of the ideal scaffold for the repair and growth of tissue. The emergence of nanotechnology offered a new toolset for the discovery, engineering and manufacturing of nanopatterned surfaces, and nanostructured scaffolds for implantable devices. Moreover, nanotechnology offers novel and improved solutions for the localized release of the biomolecules and growth factors that are needed in any TE approach. Nanotechnology in TE overcomes many downfalls that micron structured implants face, such as infection, chronic inflammation, and poor binding with the surrounding tissue. To improve these issues, nano-scale features have been implemented, providing enhanced biointegration [417]. Natural tissues contain various nanometer features because of the presence of collagen fibrils and other proteins that are less than 100 nm in one dimension [417]. The nanometer-scaled surface structures enhance cellular response through mimicking natural tissue. Due to the tunability and adaptability of the manufacturing processes, several different scaffold types can be obtained and ideally optimized for the particular needs and requirements of the individual patient or application. Currently, nanomaterials have been proven to assist in the restoration of several tissues and organs as seen in Fig. 6 [417].
Fig. 6.
Various applications of nanotechnology in regenerative medicine.
8.2. Nanostructured scaffolds
The primary approach to achieve optimal tissue repair is to implement a scaffold capable of mimicking the structure of the tissue [418]. In order to ensure the host cell colonization the scaffold, besides being biodegradable and biocompatible, has to be capable of replacing the function of the extracellular matrix [414]. Nanofibrous scaffolds physically resemble the extracellular environment needed for tissue repair and growth and have been proven to be advantageous over other materials for several reasons. Their high surface area and porous structure promote the colonization of the host cells as well as the necessary exchange of nutrients and metabolic waste between the scaffold and the surrounding tissues [414]. They are also suitable for high-density functionalization and can be modified in order to provide the right environment for the recruitment, growth, and differentiation of cells. Nanofibers can be made out of synthetic and natural materials and can be formed through electrospinning, phase separation, template synthesis, melt-blowing, drawing and self-assembly of peptides and block copolymers [414,415]. However, only electrospinning and self-assembly techniques have been used for the production of scaffolds for regenerative medicine [419–421] for applications as diverse as cartilage, bone, nerve, skeletal muscle, skin and blood vessels replacement [422–424].
Nano-fibrous scaffolds can be created through a bottom-up self-assembly approach through which the material is assembled molecule by molecule. This assembly takes place through non-covalent bonds such as hydrophobic, van der Waals, and ionic interactions. In order to achieve an efficient assembly, not only do the components need to possess a certain level of complementary chemical, but they also have to maintain a conformational complementary relationship as well. Recently in the field of TE, amphiphilic peptides have gained popularity due to their ability to form into strong and fast recovering hydrogels through self-assembly. The resulting 3D nanofiber scaffolds show biomimetic properties, can resemble the structure of the ECM, and naturally support cell proliferation and differentiation [425].
The creation of nano-fibrous scaffolds through electrospinning is a relatively more complex and cost-effective process that results in the production of either aligned or randomly dispersed fibers [414]. The use of an electric field is at the basis of the manufacturing process and it leads to fibers with diameters around 100 nm or less. Also these fibers closely resemble the ECM arrangement and are able to mimic the proteins found in the structural make up of the ECM and mimic the dimensions of the collagen fibrils within the ECM. Electrospinning is capable of producing nanofibers that vary in size, shape and composition. For example, they can be solid, composite, hollow, decorated, helical and branched [426]. Materials used to create these scaffolds vary from natural polymers such as collagen, gelatin, elastin, silk fibroin, fibrinogen, chitin, chitosan and hyaluronan to synthetic polymers such as poly-(α-hydroxy ester), poly(ethylene terephthalate), poly(ethylene oxide) and polyurethane [427–430].
8.3. Nanopatterning of surfaces of devices
In order for an implantable device to be successfully grafted in the body, the interaction between the cells and its surface is critical. When strong biointegration of an implant to the surrounding tissue is achieved, there is a significant increase in the lifetime of the biomaterial [417]. The type of chemical modification, biomaterial used and coating process employed, must aim at promoting cell adhesion, mobility, and differentiation. The nanotopography and chemical composition of the surface greatly influences the cell behavior [431]. In several instances nanomaterials have been used to tailor the surface features of an implant or of a drug eluting device to reduce the instance of infection, inhibit chronic inflammation and accelerate the appropriate tissue growth [417]. As a general statement, implants presenting surfaces that mimicked those of natural tissues resulted in better and faster tissue growth compared to conventional implants that possessed flat or atomic smooth surfaces. The control of the chemical composition of a scaffold and the tailoring of its surface charge are closely linked to protein adsorption which is an essential step for the initial interaction of cells with the synthetic platform and for the subsequent cascade of biologic events that lead to tissue regeneration [417]. This is particularly true for orthopedic implants where surface features at the nano-scale have been shown to increase interactions with cell-adhesive proteins such as collagen and laminin which is key for promoting osteoblast functions [417].
Nanomaterials and nanofeatures can also be used to avoid or postpone the onset of harmful complications. As an example, orthopedic devices often fail due to a prolonged inflammatory response induced by an up regulation of macrophage activity, overproduction of cytokines, chemokines and matrix enzymes [417]. Implementation of nano-scale features onto the surface of implants has been shown to reduce the function of these overactive macrophages. The deposition of carbon nanotubes on polycarbonate urethane increased both roughness and surface energy thus down regulating macrophage adhesion [432]. Also alumina with surface texture at the nano-scale level showed decreased macrophage adhesion proliferation, and pro-inflammation cytokine release, compared to alumina with surface feature in the micron size [433]. Another major factor in the failure of orthopedic implants is the formation of biofilms. Having a chronic bacterial infection surrounding an implant can lead to osteomyelitis, acute sepsis, and even death [417]. The most common bacteria found to invade orthopedic implants is Staphylcoccus epidermidis which originates from the patient’s skin and can easily spread to the scaffold during implantation. Materials such as ZnO and AgO have shown anti-microbial properties that become even more pronounced when formulated at the nano-scale [434]. Notably, even materials that have not been generally referred to as anti-bacterial (like TiO2) acquire anti-bacterial properties at the nano-scale.
8.4. Nanoparticles
Nanoparticles have been profusely used in the realm of TE for the delivery of molecules, drugs, growth factors and DNA. They can be either embedded within the scaffold or adsorbed to its surface for the release of the necessary biomolecules. Nanoparticles provide for three main advantages: control over the dosage, over the release kinetics and over the spatial distribution. The controlled delivery of biomolecules is a crucial step in the growth of tissue. Within the realm of regenerative medicine it is common knowledge that colonizing cells need multiple factors in a sequential and well-ordered fashion to proliferate and differentiate as they do in natural tissue. Nanoparticles also allow for the targeted delivery to specific sub-populations of cells through standard receptor–ligand interactions or through more sophisticated forms of intracellular delivery. Several nanoparticles have been explored for their potential as delivery systems, each with specific release properties. Examples of these are spheres, capsules, liposomes, micelles and dendrimers. These different types of nanoparticles have been developed as solid, hollow, or porous [414]. Biodegradable polymers such as polylactic acid (PLA), poly-glycolic acid (PGA), polyethylene glycol (PEG) and its co-polymers have been used due to their different release properties and for their ability to be responsive to the environmental or external stimuli such as temperature, pH, and magnetic field.
Nanoparticles are not only used as delivery systems, but can also be exploited as enclosures for the mechanical reinforcements of polymeric scaffolds [431]. Nanoparticles appropriately dispersed in the polymeric matrix, have been shown to increase the compressive, tensile, torsional or flexural strength of synthetic polymers such as PLA, PGA, polycaprolactone (PCL) or crosslinked poly(propylene fumarate) [415]. If the nanoparticles are added directly to the polymer solution, poor interfacial interactions will lead to precipitate formation. However, a surfactant can be added to enhance dispersion or the surface of the nanoparticles can be chemically modified to covalently crosslink to the polymer network [435].
8.5. Nanotechnology in skin regeneration
Skin is considered to be the first successful TE endeavor. Several products for skin regeneration are already FDA approved for the treatment or replacement of skin damaged by severe burns or diabetic ulcers [436]. One example of a successfully developed product is Apligraf® produced by Organogenesis. This graft is primarily for treating venous leg ulcers and diabetic foot ulcers and has been used to treat over 200,000 patients thus far. The graft itself consists of a layer of human keratinocytes cultured on a matrix of bovine type I collagen and human dermal fibroblasts. As a drawback, products such as Apligraf® are usually very expensive mainly because of the time needed for the graft to mature in vitro before being clinically viable. The use of nanotechnology has the potential to reduce this culture time making the scaffold a more desirable and cell friendly environment.
The electrospun scaffolds aforementioned have been shown to promote a more rapid vascularization compared to the tightly woven proteins with sparse winding voids which are characteristic of decellularized human dermis scaffolds. Electrospinning methods have also been used to develop silk fibroin and collagen nanofibers [437,438]. In all cases, skin fibroblasts, keratinocytes, and endothelial cells (individually and in co-culture) integrated within the scaffold and displayed distinct regions of organization when cultured on an electrospun scaffold. The high porosity and surface area contributed to the promotion of keratinocytes/fibroblast adhesion and spreading, making these nanofibers ideal scaffolds for skin TE. Solvent spin-etching of PCL scaffolds grafted with nanostructured chitosan (CS) were recently described as relatively inexpensive and successful scaffolds for the growth of human dermal fibroblasts. Their significantly higher surface roughness compared to the traditional smooth CS/PCL surfaces proved to induce higher rates of fibroblast proliferation and viability compared to the smooth polymer surfaces [439]. Similarly, electrospun PCL fibers and gelatin about 300–600 nm in diameter seeded with human dermal fibroblasts exhibited a significant amount of proliferation and solid viability. This novel technique called autologous layered dermal reconstitution (ALDR) holds several advantages over the traditional methods since it allows for the rapid, layer-by-layer deposition of tissue in deep wounds. This is possible due to the electrospinning process that occurs on top of a commercially available polyurethane wound dressing. After the scaffold is implanted the wound dressing can be removed after a couple of days and be replaced with another scaffold construct and this process is repeated until the wound is fully healed. Using a layer-by-layer technique such as this eliminates the long problematic in vitro culture time that is usually needed in order to establish cellular infiltration and growth throughout an entire single-layer scaffold. The final result of the ALDR technique is a continuous layer of tissue with rapid cell proliferation and integration between the layers made possible by porous nanostructured scaffolds [440].
8.6. Nano approach to vascular tissue engineering
Tissue engineering is a desirable solution to the problems of intimal hyperplasia and thrombosis associated with synthetic vascular graft materials such as PTFE and Dacron because it yields autologous, healthy vascular tissue [416]. However, current methods for the bio-fabrication of vascular scaffolds rely on the use of bio-reactors which makes them expensive, time consuming and not automated [441]. This drastically limited the transition of these technologies into clinical use. In the future years, nanotechnology will play a crucial role for the survival of the field of vascular TE as nanotechnology-based methods such as nano-assembly of natural-like vascular ECMs, magnetic-force-driven TE and electro-spinning of vascular scaffolds and living cells introduce cheaper and more efficient alternatives to the current bio-reactor based methods [442].
As mentioned before, nanostructured scaffolds can mimic the organization of a natural vascular ECM and thus improve cell attachment. Vascular graft patterned with nanofilms can reduce athrombogeneity and increase adhesion of circulating endothelial progenitor cells [441]. Hydrogels have been modified using nanopatterned growth factors and ECM peptides to increase their functionalities and improved their capacity to direct cell and tissue differentiation [443,444]. By varying ligand identity, presentation and density, nanopatterning, material architecture, and mechanical properties it is even possible to control the vascular cell phenotype [443], cell behavior [445,446] and intracellular signaling in stem cells [447].
Thromboresistant luminal surfaces are critical in the design of vascular grafts. There are three different ways nanotechnology is used in creating a thromboresistant luminal surface. One way is through immobilization of athrombogenic molecules; the second way is the immobilization of molecules to enhance endothelialization; the third way is to increase endothelialization via iron oxide nanoparticles coating and magnetically labeled endothelial cells. It has been proven possible to magnetically label cells or cell sheets using functionalized iron oxide nanoparticles [441]. Once magnetically labeled, the cells or cell sheets can then be driven to the desired locations via magnetic forces. This technology has been named magnetic-force-driven TE [441]. The endothelialization of the internal lumen can then be achieved faster as the magnetic forces have been shown to significantly accelerate the cell seeding, adhesion and monolayer assembly [448–450].
An interesting one-step rapid fabrication of a vascular scaffold with integrated living cells has been developed combining methods for the encapsulation of living cells with the electrospinning of nanofibers thus eliminating the time consuming and expensive bioreactor-based cell seeding and scaffold cellularization which is a key component to the future success of vascular TE [451,452].
8.7. Nanostructured materials for neural tissue engineering
There are several barriers that must be overcome when approaching the repair of the nervous tissue. Formation of scar tissue after tissue injury, gaps in the tissue due to phagocytosis of dying cells, inhibition of axon growth in the mature central nervous system (CNS), and the failure of many adult neurons to initiate axonal extension are all likely events that one needs to consider when attempting to regenerate nerves [453–457]. Implantable nanometer-scale scaffolds such as nanotubes and nanofibers that mimic the ECM and tubular structures of axons and dendrites have been used for neural tissue regeneration applications. Achieving axonal regeneration after injury in the CNS is particularly challenging and until now the use of nanotechnology and molecular self-assembly to repair injured brain structures has not been fully explored. In particular, self-assembling peptide nanofiber scaffolds (SAPNS) have been used for the regeneration of central nervous system function. Injury of the optic tract in a hamster model of induced blindness resulted in the restoration of vision within a mere three weeks after the scaffold-forming nanomaterial was injected into the brain. The regenerated axons reconnected the target tissue and restored functional vision to the animal. This study is evidence that it is in fact possible to reconnect or in a sense “knit” two separate damaged areas of the brain through the use of a scaffold tissue-bridging structure. The nanofibers themselves provided a framework structure for axonal re-innervation by direct interaction between the scaffold, the ECM and the neural tissue on both sides of the inflicted lesion. SAPNS are nontoxic and the degradation products of L-amino acids have the potential to be uptaken by nearby cells that can use them in turn for their growth and repair. Having the ability to also facilitate neural tissue reconstruction within 24 h after injury peptide scaffolds are a promising alternative to autografts of peripheral nerve or other tissues currently used for recovery from CNS injury. Other studies have shown advantageous results using nanofibrous scaffolds to promote neuron growth and differentiation for regenerating damaged neural tissue [458,459].
Nanomedicine has also made strides in peripheral nervous system (PNS) regeneration by morphologically orienting Schwann cells and directing neurite growth from neurons [460,461]. Using biomimetic materials created via soft lithography, researchers have proven that through nanotopographical cues that replicate the cellular features of Schwann cells, they can enhance the functions of neurons [462] and without any biochemical signal differentiate mesenchymal stem cells into neuronal-like cells [463]. Another way to promote neuron growth is through electrical stimulation, which has become a key consideration in the design of neuronal implants. Carbon nanotubes are ideal candidates for applications such as TE [464], bionics [465], neural interfaces [466,467] and electrochemical biosensors [468,469] because of their unique electrical, chemical and physical properties. Nanomaterials have also demonstrated an ability to limit gliotic cell responses. Since device failure often occurs due to the fibrotic response which is mediated by the glial cells [470,471], limiting their activity will reduce the likelihood of failure. Novel carbon nanofiber based electrode arrays were developed for the CNS neuronal stimulation using compressed carbon nanofibers with reinforcing polycarbonate urethane. The strong interaction with neurons on the carbon nanofibers limited astrocyte functions and therefore decreased gliotic scar tissue formation [472].
8.8. Nanocomposite materials for bone regeneration
In 2008, the US Department of Defense’s special project division, the Defense Advanced Research Projects Agency (DARPA) sent out a request for proposals addressing the immediate need to develop innovative medical solutions to be applied on the battlefield that are capable of stabilizing and treating severely fractured bone as the result of roadside bombs or other explosive devices. This type of injury is called a non-union fracture and generally leads to the amputation of the wounded limb(s). The ideal solution to this common wartime injury would be an injectable material, or “fracture putty”, that is capable of being administered in the field of action and would allow load bearing use within 24 h post-injury. The success of the fracture putty will be measured by its ability to remodel the shattered bone, provide sufficient mechanical properties to facilitate the return of load bearing responsibilities to the injured limb, and the ability to regenerate natural bone in harmonious synchronicity with putty biodegradation over time; without loss of continuity of mechanical strength. It is obvious that such technology has tremendous translational potential for applications in civilian and veterinary medicine as well.
In 2009, Ferrari et al. elected to approach this complex regenerative bone problem by employing nanotechnology to develop a nanocomposite material. Mathematical models, as mentioned in Section 3 of this review, are being used to rationally design a biologically active construct capable of promoting rapid bone regeneration, while simultaneously restoring the essential biomechanical functions of the missing bone, fighting biofilm formation, controlling pain and promoting angiogenesis. Biodegradable nanoporous silicon enclosures (NSEs) are being embedded into a polymer matrix to provide immediate mechanical reinforcement at a level comparable to healthy bone. The putty is being engineered such that independent ambulation will be attainable as early as one week post-fracture. The degradation of the polymer matrix and its strengthening inclusions gradually transfer the mechanical load to the regenerating bone; aiding in its functional recovery. Due to the integration of multiple biodegradable and biocompatible nano-components, this fracture putty prototype will be tailored to match the exact mechanical and biological requirements for a specific individual to ensure optimal healing for any bone fracture. The envisioned formulation of this novel composite can be seen in Fig. 3.
8.9. Conclusions and future perspectives of nanotechnology and tissue engineering
Through the use of nanotechnology within TE, tissue regeneration will be impacted in ways that will change the face of medicine indefinitely. Through the use of nanostructures capable of mimicking natural tissues, nanopatterning of the surface of materials, and in combination with the use of nanoparticles capable of delivering multiple biomolecules in a time and space defined fashion, optimal constructs and scaffolds can be formed, leading to an ideal biointegration between regenerating and pre-existing tissues. One of the possible scenarios for the future is the generation of “cell-free” scaffolding materials “off the shelf”. Those constructs will have the perfect structure and function, they will be biodegradable and will be personalized by the addition of autologous stem cells freshly isolated from the patient. As soon as the necessary studies to assess the biocompatibility of nanomaterials and to rule out any toxicity for the body will be performed a new era for TE will begin.
9. A patient advocate’s perspective
To use the analogy included in the introduction to this chapter, one would equate the trees with patients. My tree has been pruned starting nearly 20 years ago. Since that time much has changed in cancer treatment. In 1989 tests were ordered to help the medical oncologist determine the course of my treatment, but basically the question was whether or not chemotherapy was indicated. There was at that time a typical “chemo cocktail” for my disease. With the awareness that medical advances are made initially through research, and eventually validated in clinical trials, and with the benefit of a nearby comprehensive cancer center, unique opportunities were available to me. Therefore when presented with the opportunity to participate in a clinical trial, I accepted, even though it involved four additional courses of an experimental regimen with notable side effects. Patients are an important ingredient in the formula for progress. Clearly, new approved treatments result from such trials and other trials lead to the elimination of treatments that are shown not to work in humans. Over the course of time, through the careful evaluation of the results of clinical protocols and the conduct of epidemiologic studies, critical knowledge is gained relevant to the treatment of specific diseases.
Much more is known today regarding different tumor cell types, cell-surface receptors, potential for metastasis and resistance to therapeutics to name a few new criteria. Consequently the numbers of tests have greatly increased resulting in the accrual of much more data on each individual patient. More often now, a formally defined disease is viewed as a category with numerous subtypes each requiring specialized treatments based on identifiable patient characteristics. Surveys and questionnaires evaluate life styles and this data coupled with assessment of family histories are scrutinized to categorize patients.
We are fast approaching the ability to use preventative, diagnostic and therapeutic interventions that are targeted to patients based on their specific risk as determined through genetic testing, clinical determinates and family histories. Additionally, physicians are better able to understand the molecular signatures of cancer cells enabling them to target abnormally activated pathways. The times of one shot for all are over. The medical professionals are learning to recognize patterns of biomarkers and subsequently use this knowledge to tailor strategies for detection, treatment, or prevention of life threatening diseases. If appropriate testing could accurately predict individual risk, there might be behavior modifications or early interventions to actually prevent the disease.
Ultimately, each patient’s data could be analyzed and a personalized plan of action formulated. There would be more information to determine the possibility of recurrence, and which courses of action would be critical for success. Would there be a necessity for surgery or radiation? Is immune therapy appropriate? Could hormone therapies be beneficial? These treatments would only have to be as invasive as necessary. For the patient the results could be immeasurable with respect to increases in quality of life. There are significant side effects and recovery time with surgery, radiation and chemotherapy. One would hope that the medical professionals would take the time to explain each of the tests, their outcomes and other clinical indications in order for the patient to make informed decisions. In my case, I felt very much a part of the team making treatment choices. The journey to fight the disease was 14 months long and included surgery, chemotherapy and radiation. Mind you, I am grateful, as I have had no recurrence in the time since. With personalized medicine and the advancements made in the last 20 years, it might be a shorter journey in the future. For those patients, my hope would be that each would be treated with the most optimal methods to cure their specific disease.
10. Concluding remarks
It is certain that nanotechnology has yet to impart an enabling contribution towards the overall movement to individualized medicine; however, the potential of nanomedicine remains undeniable. This manuscript has assembled a comprehensive review of the clinical indications that have been influenced by nanotechnology and a discovery roadmap which may lead to effective personalized therapy. In the foreseeable future, a single drop of blood may provide sufficient clinical information to accurately assess the current state of patient health; including critical prognostic insights on appropriate future treatment strategies and preventative care. Nanotechnology has revealed itself as an effective mechanism to extracting vast amounts of data from multiple clinically relevant biologic panel inputs and then leveraging this patient-specific information to elicit favorable therapeutic outcomes through the utilization of its inherent scale domain. The complexities of disease, such as found in cancer, has taught us several hard truths: (1) a one-size-fits-all therapy is not a clinically responsible course of action; (2) the problem is too overwhelmingly complex for a single discipline to resolve—a multidisciplinary, and integrated effort is required to successfully unravel the mysteries of disease processes; and (3) emerging technologies, such as nanomedicine, must be employed to address the biological complexities of disease at a scale relevant to the processes driving the pathologic condition.
From a technology perspective, it is realistic to anticipate that nanotechnology will continue to achieve incremental advances on numerous clinical fronts from early detection, tissue engineering, to drug delivery. Eventually, a critical mass of clinical innovations will be reached that will allow the patient population to received individualized treatment pending upon the detailed analysis and integration of patient data. In reality however, the most formidable barrier to individualized medicine is not a technical challenge; it is regulatory issue. At the time of publication, the FDA does not offer any official regulatory guidelines that address nano-based medical products—each biomedical nanotechnology is evaluated on a case-by-case basis. This represents a very responsible approach by the Agency to ensure the safety and efficacy of all nano-based products; however it presents a serious impediment for the translation of individualized therapy. The current practice for evaluating medical products is through a robust series of clinical trials that enroll patient populations to evaluate products. This obviously poses a fundamental problem for those advocating personalized treatment strategies, which by design, are not created to be safe and efficacious for a population; but for an individual. The current state of technology affords us the ability to choose the size, shape, surface modification, and therapeutic payload of nanoparticles, which offers an exponentially large combinatorial library of nanovector possibilities. It is inconceivable to attempt to “approve” the entire menu of possible nanoparticle configurations, especially when the average therapeutic has an associated development cost of 10 years and $1.0 billion [473].
Current efforts by the FDA have clearly indicated a proactive approach to resolve the issues pertaining to nanotechnology. In August 2007, the FDA formed the FDA Nanotechnology Task Force (FDA-NTF) to lead Agency efforts to define regulatory processes that encourage the continued development of innovative, safe, and effective FDA-regulated products that employ nanotechnology. Furthermore, the FDA created the NanoTechnology Interest Group (NTIG) to facilitate the regulation of nano-based products, which is comprised of representatives from all of the Centers of the FDA. In March 2009, the FDA published a news release announcing a new collaborative effort under their Nanotechnology Initiative, that involves a consortium of eight universities and hospitals of the Texas Medical Center in Houston, Texas, called the Alliance for NanoHealth (ANH) [474]. The FDA-ANH Nanotechnology Initiative (FANTI) is a program envisioned to help speed development of safe and effective medical products in the field of nanomedicine.
“FDA’s Nanotechnology Initiative with the Alliance for NanoHealth is an effort to engage resources and technical expertise in this rapidly advancing field and is a clear example of leveraging science and scientists to advance the public good,” said the FDA’s Acting Commissioner, Frank M. Torti, M.D., M.P.H. “Nanotechnology holds great promise for the advancement of novel medical products.” [474]
The overarching goal of FANTI is to develop a framework of collaboration—that will include stakeholders from industry (pharmaceutical, biotech and devices), non-profit organizations, government and others—to work pre-competitively in identifying high priority scientific and translational gaps in moving nanoengineered medical products from preclinical stages of development through clinical stages and then to commercialization [204]. Further, under this first-of-a-kind partnership, it is anticipated that a series of projects will be implemented to address these gaps with immediate benefit to the partners and public health. These projects, which will be executed through a mechanism called a public–private partnership (PPP), which are intended to modernize the product development and regulatory sciences needed to reduce uncertainties about product performance throughout the product life cycle. Ultimately, the FANTI program was established to improve the safety and efficacy of nanoengineered biotechnologies and to optimize the regulatory process for industry members seeking approval for their nano-based products [475]. The activities and goals of the FANTI effort are aligned with the FDA’s Critical Path Initiative which cites nanomedicine as a priority in its 2006 report, “The Critical Path Opportunities List and Report”[204].
The resolution of the regulatory challenges that face nanotechnology will positively impact the research and development of nano-based products and facilitate their translation into clinical use. Regulatory reform will also significantly reduce the commercialization risks associated with the clinical translation of biomedical nanotechnologies and will certainly invoke the attention and interest from the corporate and investment sectors. Fortunately progress is being achieved on all fronts advocating that individualized therapy will be driven by the harmonious advancement of innovative science, progressive regulatory reform, and market demand. We are steadily approaching the horizon of a personalized healthcare system that is established upon saving the forest…one tree at a time.
Acknowledgments
The authors of this article are supported by the following funding sources: NIH–R33 CA122864, NASA NSPIRES–NNJ06HE06A, NIH-R01CA128797, DOD/TATRC–W81XWH-07-2-0101, DOD/MPA2–W81XWH-07-1-0596, NSF/ECCS—0725886, NASA—NNX08AW91G, DOE/DE—FG02-08ER64712, DOD/DARPA—W911NF-09-1-0044, DOD/BCRP—W81XWH-09-1-0212, NIH—U54CA143837, NIH—1RC2GM092599, State of Texas Governor’s Emerging Technology Fund, Nanomedical Systems, Inc., and the Alliance for NanoHealth.
References
- 1.Guttmacher AE, Collins FS. Genomic medicine—a primer. N Engl J Med. 2002;347(19):1512–20. doi: 10.1056/NEJMra012240. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. Bjog. 2006;113(Suppl 3):118–35. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health; National Cancer Institute. Proteomics and cancer. National Institutes of Health; Bethesda: 2009. [Google Scholar]
- 4.Office of Extramural Research. Thesaurus: computer retrieval of information on scientific projects, 2007. National Institute of Health; 2006. [Google Scholar]
- 5.National Institutes of Health; H.a.H. Services. Application of metabolomics for translational and biological research (R01) Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 6.Hu Y, Bouamrani A, Tasciotti E, Li L, Liu X, Ferrari M. ACS Nano. 2010. Tailoring of the Nanotexture of Mesoporous Silica Films and Their Functionalized Derivatives for Selectively Harvesting Low Molecular Weight Protein. (ACEEPTED) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 8.Theis T, Parr D, Binks P, Ying J, Drexler KE, Schepers E, et al. nan’o.tech.nol’o.gy n. Nat Nanotechnol. 2006;1(1):8–10. doi: 10.1038/nnano.2006.77. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Jenekhe SA, Perlstein J. Nanoscale Size Effects on Photoconductivity of Semiconducting Polymer Thin Films. Chemistry of Materials. 1996;8(8):1571–4. [Google Scholar]
- 11.Jenekhe SA, Zhang X, Chen XL, Choong V-E, Gao Y, Hsieh BR. Finite Size Effects on Electroluminescence of Nanoscale Semiconducting Polymer Heterojunctions. Chemistry of Materials. 1997;9(2):409–12. [Google Scholar]
- 12.Manandhar P, Jang J, Schatz GC, Ratner MA, Hong S. Anomalous Surface Diffusion in Nanoscale Direct Deposition Processes. Physical Review Letters. 2003;90(11):115505. doi: 10.1103/PhysRevLett.90.115505. [DOI] [PubMed] [Google Scholar]
- 13.Clark LA, Ye GT, Snurr RQ. Molecular Traffic Control in a Nanoscale System. Physical Review Letters. 2000;84(13):2893. doi: 10.1103/PhysRevLett.84.2893. [DOI] [PubMed] [Google Scholar]
- 14.Soler JM, Baro AM, Garcia N, Rohrer H. Interatomic forces in scanning tunneling microscopy: Giant corrugations of the graphite surface. Phys Rev Lett. 1986;57(4):444–7. doi: 10.1103/PhysRevLett.57.444. [DOI] [PubMed] [Google Scholar]
- 15.Binnig G, Garcia N, Rohrer H. Conductivity sensitivity of inelastic scanning tunneling microscopy. Phys Rev B Condens Matter. 1985;32(2):1336–8. doi: 10.1103/physrevb.32.1336. [DOI] [PubMed] [Google Scholar]
- 16.Curl RF, Smalley RE. Probing C60. Science. 1988;242(4881):1017–22. doi: 10.1126/science.242.4881.1017. [DOI] [PubMed] [Google Scholar]
- 17.Rao CNR, Satishkumar BC, Govindaraj A, Manashi N. Nanotubes. ChemPhysChem. 2001;2(2):78–105. doi: 10.1002/1439-7641(20010216)2:2<78::AID-CPHC78>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004;10(9):993–8. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 19.Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med. 2005;11(6):678–82. doi: 10.1038/nm1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alivisatos AP. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science. 1996;271(5251):933–7. [Google Scholar]
- 21.Foundation N. The Official Web Site of the Nobel Foundation. 2009 Available from: http://nobelprize.org/index.html.
- 22.Weaver JH, Waddill GD. Cluster Assembly of Interfaces: Nanoscale Engineering. Science. 1991;251(5000):1444–51. doi: 10.1126/science.251.5000.1444. [DOI] [PubMed] [Google Scholar]
- 23.Duan X, Huang Y, Cui Y, Wang J, Lieber CM. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature. 2001;409(6816):66–9. doi: 10.1038/35051047. [DOI] [PubMed] [Google Scholar]
- 24.Wan Y, Sha J, Chen B, Fang Y, Wang Z, Wang Y. Nanodevices based on silicon nanowires. Recent Pat Nanotechnol. 2009;3(1):1–9. doi: 10.2174/187221009787003348. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Sarin L, Johnson NC, Hurt RH. A nano-selenium reactive barrier approach for managing mercury over the life-cycle of compact fluorescent lamps. Environ Sci Technol. 2009;43(15):5915–20. doi: 10.1021/es9013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sozer N, Kokini JL. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27(2):82–9. doi: 10.1016/j.tibtech.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Ran S, Vleugels J, Huang S, Vanmeensel K, Blank DHA, Winnubst L. Manipulating microstructure and mechanical properties of cuo doped 3y-tzp nanoceramics using spark-plasma sintering. J Eur Ceram Soc. 2010;30:899–904. [Google Scholar]
- 28.Aronov D, Karlov A, Rosenman G. Hydroxyapatite nanoceramics: Basic physical properties and biointerface modification. J Eur Ceram Soc. 2007;27(13–15):4181–6. [Google Scholar]
- 29.Imanaka N, et al. Amorphous cerium–titanium solid solution phosphate as a novel family of band gap tunable sunscreen materials. Chem Mater. 2003;15(12):2289–91. [Google Scholar]
- 30.Wolf P, Cox P, Yarosh DB, Kripke ML. Sunscreens and T4N5 Liposomes Differ in Their Ability to Protect Against Ultraviolet-Induced Sunburn Cell Formation, Alterations of Dendritic Epidermal Cells, and Local Suppression of Contact Hypersensitivity. J Investig Dermatol. 1995;104(2):287–92. doi: 10.1111/1523-1747.ep12612828. [DOI] [PubMed] [Google Scholar]
- 31.Gabizon A. Tailoring liposomes for cancer drug delivery: from the bench to the clinic. Ann Biol Clin (Paris) 1993;51(9):811–3. [PubMed] [Google Scholar]
- 32.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–44. [PubMed] [Google Scholar]
- 34.Carmo VA, Ferrari CS, Reis EC, Ramaldes GA, Pereira MA, De Oliveira MC, et al. Biodistribution study and identification of inflammation sites using 99mTc-labelled stealth pH-sensitive liposomes. Nucl Med Commun. 2008;29(1):33–8. doi: 10.1097/MNM.0b013e3282f1bc0d. [DOI] [PubMed] [Google Scholar]
- 35.Jain RK. The next frontier of molecular medicine: delivery of therapeutics. Nature Med. 1998;4:655–7. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 36.Caplen NJ, Alton EWFW, Mddleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 37.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30(13):2440–8. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3(3):151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 39.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2(5):889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759–82. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 41.Bong-Hyun J, Mi Suk N, Jaeyun K, Gunsung K, Homan K, Min-Soo K, et al. Multifunctional silver-embedded magnetic nanoparticles as sers nanoprobes and their applications. Small. 2010;6:119–25. doi: 10.1002/smll.200901459. [DOI] [PubMed] [Google Scholar]
- 42.Winter PM, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with [alpha]v[beta]3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–4. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 43.Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287(5460):1989–92. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 44.Gao X, Chan WC, Nie S. Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding. J Biomed Opt. 2002;7(4):532–7. doi: 10.1117/1.1506706. [DOI] [PubMed] [Google Scholar]
- 45.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234(2):123–31. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomedicine. 2009;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Tomalia DA. A new class of polymers: starburst-dendritic macromolecules. Polymer J. 1985;17:117–32. [Google Scholar]
- 48.Patri AK, Kukowska-Latallo JF, Baker JJR. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliver Rev. 2005;57(15):2203–14. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–43. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 50.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev. 2006;35(11):1095–104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 52.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010 doi: 10.1016/j.jconrel.2009.10.014. in Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 53.Gabizon A, Peretz T, Sulkes A, Amselem S, Ben-Yosef R, Ben-Baruch N, et al. Systemic administration of doxorubicin-containing liposomes in cancer patients: a phase I study. Eur J Cancer Clin Oncol. 1989;25(12):1795–803. doi: 10.1016/0277-5379(89)90350-7. [DOI] [PubMed] [Google Scholar]
- 54.Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068(2):133–41. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- 55.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 56.Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–72. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- 57.Stupp SI, LeBonheur V, Walker K, Li LS, Huggins KE, Keser M, et al. Supramolecular Materials: Self-Organized Nanostructures. Science. 1997;276(5311):384–9. doi: 10.1126/science.276.5311.384. [DOI] [PubMed] [Google Scholar]
- 58.Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL, et al. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Natl Acad Sci USA. 2006;103(5):1215–20. doi: 10.1073/pnas.0509739103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11(11):2319–31. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 60.Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, et al. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Invest Radiol. 2007;42(12):797–806. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 61.Lindner JR. Contrast ultrasound molecular imaging: harnessing the power of bubbles. Cardiovasc Res. 2009;83(4):615–6. doi: 10.1093/cvr/cvp243. [DOI] [PubMed] [Google Scholar]
- 62.Fodor SE. Light-directed spatially addressable parallel chemical synthesis. Science. 1991;251:767–73. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 63.Nam JM, Mirkin CA. Bio-barcode-based DNA detection with PCR-like sensitivity. J Am Chem Soc. 2004;126:5932–3. doi: 10.1021/ja049384+. [DOI] [PubMed] [Google Scholar]
- 64.Santini JT, Richards AC, Scheidt R, Cima MJ, Langer R. Microchips as controlled drug-delivery devices. Angew Chem Int Edn. 2000;39:2396–407. [PubMed] [Google Scholar]
- 65.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Soft lithography in biology and biochemistry. Annual Review of Biomedical Engineering. 2001;3:335–73. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 66.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotech. 2003;21(10):1184–91. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 67.Orosco MM, Pacholski C, Sailor MJ. Real-time monitoring of enzyme activity in a mesoporous silicon double layer. Nat Nano. 2009;4(4):255–8. doi: 10.1038/nnano.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng MM, Cuda G, Bunimovich YL, Gaspari M, Heath JR, Hill HD, et al. Nanotechnologies for biomolecular detection and medical diagnostics. Current Opinion in Chemical Biology. 2006;10(1):11–9. doi: 10.1016/j.cbpa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Heath JR, Phelps ME, Hood L. NanoSystems biology. Molecular Imaging & Biology. 2003;5(5):312–25. doi: 10.1016/j.mibio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Cui Y, Wei Q, Park H, Lieber CM. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science. 2001;293(5533):1289–92. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 71.Hansen KM, Ji H-F, Wu G, Datar R, Cote R, Majumdar A, et al. Cantilever-Based Optical Deflection Assay for Discrimination of DNA Single-Nucleotide Mismatches. Analytical Chemistry. 2001;73(7):1567–71. doi: 10.1021/ac0012748. [DOI] [PubMed] [Google Scholar]
- 72.Wu G, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nat Biotech. 2001;19(9):856–60. doi: 10.1038/nbt0901-856. [DOI] [PubMed] [Google Scholar]
- 73.Hwang KS, Lee S-M, Kim SK, Lee JH, Kim TS. Micro- and Nanocantilever Devices and Systems for Biomolecule Detection. Annual Review of Analytical Chemistry. 2009;2(1):77–98. doi: 10.1146/annurev-anchem-060908-155232. [DOI] [PubMed] [Google Scholar]
- 74.Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R. Viral-Induced Self-Assembly of Magnetic Nanoparticles Allows the Detection of Viral Particles in Biological Media. Journal of the American Chemical Society. 2003;125(34):10192–3. doi: 10.1021/ja036409g. [DOI] [PubMed] [Google Scholar]
- 75.Biosciences, N. Tumor ablation using AuroLase therapy. [cited 2009 November 20]. Available from: http://www.nanospectra.com/index.html.
- 76.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1–3):149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 77.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34(4):633–41. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 79.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–503. [PubMed] [Google Scholar]
- 80.Alberts B. Molecular biology of the cell. 3. New York: Garland Pub; 1994. p. xliii.p. 1294. [66] [Google Scholar]
- 81.Majoros IJ, Williams CR, Baker JR., Jr Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem. 2008;8(14):1165–79. doi: 10.2174/156802608785849049. [DOI] [PubMed] [Google Scholar]
- 82.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100(23):13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 84.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci USA. 2007;104(29):11901–4. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA. 2008;105(7):2586–91. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee SY, Ferrari M, Decuzzi P. Design of bio-mimetic particles with enhanced vascular interaction. J Biomech. 2009;42(12):1885–90. doi: 10.1016/j.jbiomech.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33(2):179–90. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 88.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27(30):5307–14. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 89.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94(10):3790–7. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008;29(3):377–84. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 91.Decuzzi P, Ferrari M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials. 2007;28(18):2915–22. doi: 10.1016/j.biomaterials.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, et al. The case for early detection. Nat Rev Cancer. 2003;3(4):243–52. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 93.Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5(10):588–99. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- 94.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286(5439):531–7. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 95.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 96.Aebersold R, Anderson L, Caprioli R, Druker B, Hartwell L, Smith R. Perspective: a program to improve protein biomarker discovery for cancer. J Proteome Res. 2005;4(4):1104–9. doi: 10.1021/pr050027n. [DOI] [PubMed] [Google Scholar]
- 97.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1(11):845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 98.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452(7187):571–9. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 99.Liotta LA, Ferrari M, Petricoin E. Clinical proteomics: written in blood. Nature. 2003;425(6961):905. doi: 10.1038/425905a. [DOI] [PubMed] [Google Scholar]
- 100.Petricoin EF, Belluco C, Araujo RP, Liotta LA. The blood peptidome: a higher dimension of information content for cancer biomarker discovery. Nat Rev Cancer. 2006;6(12):961–7. doi: 10.1038/nrc2011. [DOI] [PubMed] [Google Scholar]
- 101.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 102.van der Merwe DE, Oikonomopoulou K, Marshall J, Diamandis EP. Mass spectrometry: uncovering the cancer proteome for diagnostics. Adv Cancer Res. 2007;96:23–50. doi: 10.1016/S0065-230X(06)96002-3. [DOI] [PubMed] [Google Scholar]
- 103.Diamandis EP. Mass spectrometry as a diagnostic and a cancer biomarker discovery tool: opportunities and potential limitations. Mol Cell Proteomics. 2004;3(4):367–78. doi: 10.1074/mcp.R400007-MCP200. [DOI] [PubMed] [Google Scholar]
- 104.Findeisen P, Sismanidis D, Riedl M, Costina V, Neumaier M. Preanalytical impact of sample handling on proteome profiling experiments with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chem. 2005;51(12):2409–11. doi: 10.1373/clinchem.2005.054585. [DOI] [PubMed] [Google Scholar]
- 105.Villanueva J, Philip J, Chaparro CA, Li Y, Toledo-Crow R, DeNoyer L, et al. Correcting common errors in identifying cancer-specific serum peptide signatures. J Proteome Res. 2005;4(4):1060–72. doi: 10.1021/pr050034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, et al. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Invest. 2006;116(1):271–84. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouamrani A, Hu Y, Tasciotti E, Li L, Chiappini C, Liu X, et al. Mesoporous silica chips for selective enrichment and stabilization of low molecular weight proteome. Proteomics. 2009 doi: 10.1002/pmic.200900346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaspari M, Cheng MMC, Terracciano R, Liu XW, Nijdam AJ, Vaccari L, et al. Nanoporous surfaces as harvesting agents for mass spectrometric analysis of peptides in human plasma. Journal of Proteome Research. 2006;5(5):1261–6. doi: 10.1021/pr050417+. [DOI] [PubMed] [Google Scholar]
- 109.Luchini A, Geho DH, Bishop B, Tran D, Xia C, Dufour RL, et al. Smart hydrogel particles: biomarker harvesting: one-step affinity purification, size exclusion, and protection against degradation. Nano Lett. 2008;8(1):350–61. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang WU, Chen C, Lin KH, Fang Y, Lieber CM. Label-free detection of small-molecule-protein interactions by using nanowire nanosensors. Proc Natl Acad Sci USA. 2005;102(9):3208–12. doi: 10.1073/pnas.0406368102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Degenhardt YY, Wooster R, McCombie RW, Lucito R, Powers S. High-content analysis of cancer genome DNA alterations. Curr Opin Genet Dev. 2008;18(1):68–72. doi: 10.1016/j.gde.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu CC, Ko FH, Yang YS, Hsia DL, Lee BS, Su TS. Label-free biosensing of a gene mutation using a silicon nanowire field-effect transistor. Biosens Bioelectron. 2009;25(4):820–5. doi: 10.1016/j.bios.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 113.Pratilas CA, Solit DB. Therapeutic strategies for targeting BRAF in human cancer. Rev Recent Clin Trials. 2007;2(2):121–34. doi: 10.2174/157488707780599393. [DOI] [PubMed] [Google Scholar]
- 114.Lee HS, Kim KS, Kim CJ, Hahn SK, Jo MH. Electrical detection of VEGFs for cancer diagnoses using anti-vascular endotherial growth factor aptamer-modified Si nanowire FETs. Biosens Bioelectron. 2009;24(6):1801–5. doi: 10.1016/j.bios.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 115.Bangar MA, Shirale DJ, Chen W, Myung NV, Mulchandani A. Single conducting polymer nanowire chemiresistive label-free immunosensor for cancer biomarker. Anal Chem. 2009;81(6):2168–75. doi: 10.1021/ac802319f. [DOI] [PubMed] [Google Scholar]
- 116.Ishikawa FN, Chang HK, Curreli M, Liao HI, Olson CA, Chen PC, et al. Label-free, electrical detection of the SARS virus N-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano. 2009;3(5):1219–24. doi: 10.1021/nn900086c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23(10):1294–301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 118.Fritz J, Baller MK, Lang HP, Rothuizen H, Vettiger P, Meyer E, et al. Translating biomolecular recognition into nanomechanics. Science. 2000;288(5464):316–8. doi: 10.1126/science.288.5464.316. [DOI] [PubMed] [Google Scholar]
- 119.McKendry R, Zhang J, Arntz Y, Strunz T, Hegner M, Lang HP, et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc Natl Acad Sci USA. 2002;99(15):9783–8. doi: 10.1073/pnas.152330199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J, Lang HP, Huber F, Bietsch A, Grange W, Certa U, et al. Rapid and label-free nanomechanical detection of biomarker transcripts in human RNA. Nat Nanotechnol. 2006;1(3):214–20. doi: 10.1038/nnano.2006.134. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Li X, Zhang Z, Zuo G, Cheng Z, Yu H. Nanogram per milliliter-level immunologic detection of alpha-fetoprotein with integrated rotating-resonance microcantilevers for early-stage diagnosis of heptocellular carcinoma. Biomed Microdevices. 2009;11(1):183–91. doi: 10.1007/s10544-008-9223-2. [DOI] [PubMed] [Google Scholar]
- 122.Lee SM, Hwang KS, Yoon HJ, Yoon DS, Kim SK, Lee YS, et al. Sensitivity enhancement of a dynamic mode microcantilever by stress inducer and mass inducer to detect PSA at low picogram levels. Lab Chip. 2009;9(18):2683–90. doi: 10.1039/b902922b. [DOI] [PubMed] [Google Scholar]
- 123.Cosentino C, Amato F, Walczak R, Boiarski A, Ferrari M. Dynamic Model of Biomolecular Diffusion through Two-Dimensional Nanochannels. J Phys Chem B. 2005;109(15):7358–64. doi: 10.1021/jp045478u. [DOI] [PubMed] [Google Scholar]
- 124.Lesinski G, Sharma S, Varker K, Sinha P, Ferrari M, Carson W. Release of biologically functional interferon-alpha from a nanochannel delivery system. Biomed Microdevices. 2005;7(1):71–9. doi: 10.1007/s10544-005-6174-8. [DOI] [PubMed] [Google Scholar]
- 125.Martin F, Walczak R, Boiarski A, Cohen M, West T, Cosentino C, et al. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. J Control Release. 2005;102(1):123–33. doi: 10.1016/j.jconrel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 126.Pricl S, Ferrone M, Fermeglia M, Amato F, Cosentino C, Cheng M, et al. Multiscale modeling of protein transport in silicon membrane nanochannels. Part 1. Derivation of molecular parameters from computer simulations. Biomed Microdevices. 2006;8(4):277–90. doi: 10.1007/s10544-006-0031-2. [DOI] [PubMed] [Google Scholar]
- 127.Eijkel JCT, Berg Avd. Nanofluidics: what is it and what can we expect from it? Microfluidics and Nanofluidics. 2005;1(3):249–67. [Google Scholar]
- 128.Desai T, Chu W, Tu J, Beattie G, Hayek A, Ferrari M. Microfabricated immunoisolating biocapsules. Biotechnol Bioeng. 1998;57(1):118–20. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 129.Desai TA, Chu WH, Rasi G, Sinibaldi-Vallebona P, Guarino E, Ferrari M. Microfabricated biocapsules provide short-term immunoisolation of insulinoma xenografts. Biomed Microdevices. 1999;1(2):131–8. doi: 10.1023/A:1009948524686. [DOI] [PubMed] [Google Scholar]
- 130.Chu W, et al. Silicon-micromachined direct-pore filters for ultrafiltration. 1997 [Google Scholar]
- 131.Chu W, Chin R, Huen T, Ferrari M. Silicon membrane nanofilters from sacrificial oxide removal. J Microelectromech Syst. 1999;8(1):34–42. [Google Scholar]
- 132.Lewis JR, Ferrari M Berg REOA. Lab-on-a-chip: miniaturized systems for (bio)chemical analysis and synthesis. New York: Elsevier Science; 2003. BioMEMS for drug delivery application; p. 402. [Google Scholar]
- 133.Sinha PM, Valco G, Sharma S, Liu X, Ferrari M. Nanoengineered device for drug delivery application. Nanotechnology. 2004;15(10):S585–9. [Google Scholar]
- 134.Schibler U. The daily rhythms of genes, cells and organs. EMBO reports. 2005;6(S1):S9. doi: 10.1038/sj.embor.7400424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Claudel T, Cretenet G, Saumet A, Gachon F. Crosstalk between xenobiotics metabolism and circadian clock. FEBS letters. 2007;581(19):3626–33. doi: 10.1016/j.febslet.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 136.Hrushesky W. Circadian timing of cancer chemotherapy. Science. 1985;228(4695):73. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- 137.Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev. 2007;59(9–10):828–51. doi: 10.1016/j.addr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Lemmer B. Chronobiology, drug-delivery, and chronotherapeutics. Adv Drug Deliv Rev. 2007;59(9–10):825–7. doi: 10.1016/j.addr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 139.Levi F. Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes Control. 2006;17(4):611–21. doi: 10.1007/s10552-005-9004-7. [DOI] [PubMed] [Google Scholar]
- 140.Eriguchi M, Levi F, Hisa T, Yanagie H, Nonaka Y, Takeda Y. Chronotherapy for cancer. Biomed Pharmacother. 2003;57(Suppl 1):92s–5s. doi: 10.1016/j.biopha.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 141.Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Deliv Rev. 2004;56(2):185–98. doi: 10.1016/j.addr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Nuxoll EE, Siegel RA. BioMEMS devices for drug delivery. IEEE Eng Med Biol Mag. 2009;28(1):31–9. doi: 10.1109/MEMB.2008.931014. [DOI] [PubMed] [Google Scholar]
- 143.Brewer M, Zhang T, Dong W, Rutherford M, Tian ZR. Future approaches of nanomedicine in clinical science. Med Clin North Am. 2007;91(5):963–1016. doi: 10.1016/j.mcna.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1(1):22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 145.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, et al. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials. 2003;24(11):1959–67. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 146.Ziaie B, Baldi A, Lei M, Gu Y, Siegel RA. Hard and soft micromachining for BioMEMS: review of techniques and examples of applications in microfluidics and drug delivery. Adv Drug Deliv Rev. 2004;56(2):145–72. doi: 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 147.Ye H, Randall C, Leong T, Slanac D, Call E, Gracias D. Remote radio-frequency controlled nanoliter chemistry and chemical delivery on substrates. Angew Chem-Int Ed Eng. 2007;46(26):4991. doi: 10.1002/anie.200604414. [DOI] [PubMed] [Google Scholar]
- 148.Hoare T, et al. A magnetically triggered composite membrane for on-demand drug delivery. 2009 doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abbott S, Ralston J, Reynolds G, Hayes R. Reversible wettability of photoresponsive pyrimidine-coated surfaces. Langmuir. 1999;15(26):8923–8. [Google Scholar]
- 150.Ichimura K, Oh S, Nakagawa M. Light-driven motion of liquids on a photoresponsive surface. Science. 2000;288(5471):1624. doi: 10.1126/science.288.5471.1624. [DOI] [PubMed] [Google Scholar]
- 151.Lin S, Lin Y, Chent K. A thermoswitchable membrane for drug delivery. Drug Delivery. 1995;2(2):123–7. [Google Scholar]
- 152.Chen K, Lin Y, Lin S. Thermally on-off switching nylon membrane for controlling drug penetration. Drug Deliv Syst-Osaka Tokyo. 1996;11:55–61. [Google Scholar]
- 153.Satarkar N, Zach Hilt J. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 2008;4(1):11–6. doi: 10.1016/j.actbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 154.Nagakura T, Ishihara K, Furukawa T, Masuda K, Tsuda T. Auto-regulated osmotic pump for insulin therapy by sensing glucose concentration without energy supply. Sensor Actuat: B Chem. 1996;34(1–3):229–33. [Google Scholar]
- 155.Santus G, Baker RW. Osmotic drug delivery: a review of the patent literature. J Control Release. 1995;35:21. [Google Scholar]
- 156.Verma RK, Aroa S, Garg S. Osmotic pumps in drug delivery. Crit Rev Ther Drug. 2004;21(6):44. doi: 10.1615/critrevtherdrugcarriersyst.v21.i6.20. [DOI] [PubMed] [Google Scholar]
- 157.Wright JC, Tao Leonard S, Stevenson CL, Beck JC, Chen G, Jao RM, et al. An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J Control Release. 2001;75(1–2):1–10. doi: 10.1016/s0168-3659(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 158.Fisher DM, Kellett N, Lenhardt R. Pharmacokinetics of an implanted osmotic pump delivering sufentanil for the treatment of chronic pain. Anesthesiology. 2003;99(4):929–37. doi: 10.1097/00000542-200310000-00028. [DOI] [PubMed] [Google Scholar]
- 159.Fish RE, Spitzer JA. Continuous infusion of endotoxin from an osmotic pump in the conscious, unrestrained rat: a unique model of chronic endotoxemia. Circ Shock. 1984;12(2):135–49. [PubMed] [Google Scholar]
- 160.Djalilian HR, Caicedo E, Lessan K, Grami V, Le CT, Spellman SR, et al. Efficacy of an osmotic pump delivered, GM-CSF-based tumor vaccine in the treatment of upper aerodigestive squamous cell carcinoma in rats. Cancer Immunol Immunother. 2007;56(8):1207–14. doi: 10.1007/s00262-006-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ryu W, Huang Z, Prinz FB, Goodman SB, Fasching R. Biodegradable microosmotic pump for long-term and controlled release of basic fibroblast growth factor. J Control Release. 2007;124(1–2):98–105. doi: 10.1016/j.jconrel.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 162.Lu Y, Chen SC. Micro and nano-fabrication of biodegradable polymers for drug delivery. Adv Drug Deliver Rev. 2004;56(11):1621–33. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 163.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99(11):3181–98. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 164.Narasimhan B, Langer R. Zero-order release of micro- and macromolecules from polymeric devices: the role of the burst effect. J Control Release. 1997;47(1):13–20. [Google Scholar]
- 165.Keller CG, Ferrari M. Milli-scale polysilicon structures. Technical Digest from the Solid State Sensor and Actuator Workshop; 1994; Hilton Head Island, South Carolina.. [Google Scholar]
- 166.Chu W, Ferrari M. Silicon nanofilter with absolute pore size and high mechanical strength. 1995 [Google Scholar]
- 167.Gardeniers JGE, van den AB, Rosenberg E. Micro- and nanofluidic devices for environmental and biomedical applications. Sixth Euroconference on environmental analytical chemistry; 2004. [Google Scholar]
- 168.Holtzel A, Tallarek U. Ionic conductance of nanopores in microscale analysis systems: Where microfluidics meets nanofluidics. J Separ Sci. 2007;30(10):1398–419. doi: 10.1002/jssc.200600427. [DOI] [PubMed] [Google Scholar]
- 169.Lien K-Y, Liu C-J, Lin Y-C, Kuo P-L, Lee G-B. Extraction of genomic DNA and detection of single nucleotide polymorphism genotyping utilizing an integrated magnetic bead-based microfluidic platform. Microfluidics Nanofluidics. 2009;6(4):539–55. [Google Scholar]
- 170.Chang H, Venkatesan B, Iqbal S, Andreadakis G, Kosari F, Vasmatzis G, et al. DNA counterion current and saturation examined by a MEMS-based solid state nanopore sensor. Biomed Microdevices. 2006;8(3):263–9. doi: 10.1007/s10544-006-9144-x. [DOI] [PubMed] [Google Scholar]
- 171.Li Y, Denny P, Ho C-M, Montemagno C, Shi W, Qi F, et al. The Oral Fluid MEMS/NEMS Chip (OFMNC): Diagnostic & Translational Applications. Adv Dent Res. 2005;18(1):3–5. doi: 10.1177/154407370501800102. [DOI] [PubMed] [Google Scholar]
- 172.Kraly JR, Holcomb RE, Guan Q, Henry CS. Review: Microfluidic applications in metabolomics and metabolic profiling. Anal Chim Acta. 2009;653(1):23–35. doi: 10.1016/j.aca.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van der Heyden FHJ, Stein D, Dekker C. Streaming Currents in a Single Nanofluidic Channel. Phys Rev Lett. 2005;95(11):116104. doi: 10.1103/PhysRevLett.95.116104. [DOI] [PubMed] [Google Scholar]
- 174.Junwu K, Zhigang Y, Taijiang P, Guangming C, Boda W. Design and test of a high-performance piezoelectric micropump for drug delivery. Sensor Actuat: A Phys. 2005;121(1):156–61. [Google Scholar]
- 175.Maillefer D, et al. A high-performance silicon micropump for an implantable drug delivery system. 12th IEEE international; 1999. [Google Scholar]
- 176.Teymoori M. Abbaspour-Sani 2005 Design and simulation of a novel electrostatic peristaltic micromachined pump for drug delivery applications. Sensor Actuat. 2005;117(2):7. [Google Scholar]
- 177.Cui Q, Liu C, Zha X. Study on a piezoelectric micropump for the controlled drug delivery system. Microfluidics and Nanofluidics. 2007;3(4):377–90. [Google Scholar]
- 178.Benard W, Kahn H, Heuer A, Huff M. Thin-film shape-memory alloy actuated micropumps. J Microelectromech Syst. 1998;7(2):245–51. [Google Scholar]
- 179.Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Yawen LI, Cima MJ, et al. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92(1):6–21. [Google Scholar]
- 180.Santini J, Jr, Richards A, Scheidt R, Cima M, Langer R. Microchips as controlled drug-delivery devices. Angew Chem Int Ed. 2000;39(14):2396–407. [PubMed] [Google Scholar]
- 181.Santini JT, Jr, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–8. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 182.Shawgo R, Richards Grayson A, Li Y, Cima M. BioMEMS for drug delivery. Curr Opin Solid St M. 2002;6(4):329–34. [Google Scholar]
- 183.Li Y, Ho Duc HL, Tyler B, Williams T, Tupper M, Langer R, et al. In vivo delivery of BCNU from a MEMS device to a tumor model. J Control Release. 2005;106(1–2):138–45. doi: 10.1016/j.jconrel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 184.Li Y, Shawgo R, Tyler B, Henderson P, Vogel J, Rosenberg A, et al. In vivo release from a drug delivery MEMS device. J Control Release. 2004;100(2):211–9. doi: 10.1016/j.jconrel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 185.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr, Pelta CM, Santini JT., Jr Electrothermally activated microchips for implantable drug delivery and biosensing. J Control Release. 2005;109(1–3):244–55. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 186.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr, Maloney JM, Coppeta J, et al. Chronic, programmed polypeptide delivery from an implanted, multireser-voir microchip device. Nat Biotechnol. 2006;24(4):437–8. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 187.Elman NM, Ho Duc HL, Cima MJ. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed Microdevices. 2009;11(3):625–31. doi: 10.1007/s10544-008-9272-6. [DOI] [PubMed] [Google Scholar]
- 188.Chen L, Conlisk AT. Electroosmotic flow and particle transport in micro/nano nozzles and diffusers. Biomed Microdevices. 2008;10(2):289–98. doi: 10.1007/s10544-007-9135-6. [DOI] [PubMed] [Google Scholar]
- 189.Laser D, Santiago J. A review of micropumps. J Micromech Microeng. 2004;14:35–64. [Google Scholar]
- 190.Miao J, Xu Z, Zhang X, Wang N, Yang Z, Sheng P. Micropumps based on the enhanced electroosmotic effect of aluminum oxide membranes. Adv Mater. 2007;19:4234–7. [Google Scholar]
- 191.Chen L, Choo J, Yan B. The microfabricated electrokinetic pump: a potential promising drug delivery technique. Expert Opin Drug Deliv. 2007;4(2):119–29. doi: 10.1517/17425247.4.2.119. [DOI] [PubMed] [Google Scholar]
- 192.Takamura Y, Onoda H, Inokuchi H, Adachi S, Oki A, Horiike Y. Low-voltage electroosmosis pump for stand-alone microfluidics devices. Electrophoresis. 2003;24(1–2):185–92. doi: 10.1002/elps.200390012. [DOI] [PubMed] [Google Scholar]
- 193.Winau F, Westphal O, Winau R. Paul Ehrlich—in search of the magic bullet. Microbes Infect. 2004;6(8):786–9. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 194.Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035–42. [PubMed] [Google Scholar]
- 195.Hatefi A, Amsden B. Camptothecin delivery methods. Pharm Res. 2002;19(10):1389–99. doi: 10.1023/a:1020427227285. [DOI] [PubMed] [Google Scholar]
- 196.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24(6):1029–46. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 197.Huuskonen J, Salo M, Taskinen J. Neural network modeling for estimation of the aqueous solubility of structurally related drugs. J Pharm Sci. 1997;86(4):450–4. doi: 10.1021/js960358m. [DOI] [PubMed] [Google Scholar]
- 198.Ferrari M. Nanovector therapeutics. Curr Opin Chem Biol. 2005;9(4):343–6. doi: 10.1016/j.cbpa.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 199.Canal P, Gamelin E, Vassal G, Robert J. Benefits of pharmacological knowledge in the design and monitoring of cancer chemotherapy. Pathol Oncol Res. 1998;4(3):171–8. doi: 10.1007/BF02905246. [DOI] [PubMed] [Google Scholar]
- 200.Tallaj JA, Franco V, Rayburn BK, Pinderski L, Benza RL, Pamboukian S, et al. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. J Heart Lung Transplant. 2005;24(12):2196–201. doi: 10.1016/j.healun.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 201.Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med. 2008;59:251–65. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang MD, Shin DM, Simons JW, Nie S. Nanotechnology for targeted cancer therapy. Expert Rev Anticancer Ther. 2007;7(6):833–7. doi: 10.1586/14737140.7.6.833. [DOI] [PubMed] [Google Scholar]
- 203.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine—challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–7. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 205.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3(5):242–4. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 206.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 207.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 208.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–19. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 209.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 210.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 211.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23(12):1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 212.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 213.Kale AA, Torchilin VP. “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J Liposome Res. 2007;17(3–4):197–203. doi: 10.1080/08982100701525035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 215.Souza GR, Staquicini FI, Christianson DR, Ozawa MG, Miller JH, Pasqualini R, et al. Combinatorial targeting and nanotechnology applications. Biomed Microdevices. 2009 doi: 10.1007/s10544-009-9340-6. [DOI] [PubMed] [Google Scholar]
- 216.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53. [PubMed] [Google Scholar]
- 217.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends in Biotechnol (TiBTec) 2010;786:1–8. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16(7):691–707. doi: 10.1097/01.cad.0000167902.53039.5a. [DOI] [PubMed] [Google Scholar]
- 220.Parveen S, Sahoo SK. Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs. Clin Pharmacokinet. 2006;45(10):965–88. doi: 10.2165/00003088-200645100-00002. [DOI] [PubMed] [Google Scholar]
- 221.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, et al. Development of the polymer micelle carrier system for doxorubicin. J Control Release. 2001;74(1–3):295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 222.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–24. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 223.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010 doi: 10.1016/j.jconrel.2009.10.032. in press [2009 Nov 4 -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 224.Hajitou A, Pasqualini R, Arap W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16(3):80–8. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–78. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 226.Latorre M, Rinaldi C. Applications of magnetic nanoparticles in medicine: magnetic fluid hyperthermia. P R Health Sci J. 2009;28(3):227–38. [PubMed] [Google Scholar]
- 227.Chuang VT, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm Res. 2002;19(5):569–77. doi: 10.1023/a:1015396825274. [DOI] [PubMed] [Google Scholar]
- 228.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10(1):35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 229.Greenwald RB, Choe YH, McGuire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev. 2003;55(2):217–50. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 230.Maeda H, et al. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostat: pronounced improvements in pharmacological properties. J Med Chem. 1985;28(4):455–61. doi: 10.1021/jm00382a012. [DOI] [PubMed] [Google Scholar]
- 231.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet. 2003;42(13):1089–105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 232.Graham ML. Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev. 2003;55(10):1293–302. doi: 10.1016/s0169-409x(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 233.Ho DH, Brown NS, Yen A, Holmes R, Keating M, Abuchowski A, et al. Clinical pharmacology of polyethylene glycol-L-asparaginase. Drug Metab Dispos. 1986;14(3):349–52. [PubMed] [Google Scholar]
- 234.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl) methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5(1):83–94. [PubMed] [Google Scholar]
- 235.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, et al. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12(4):315–23. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 236.Gros L, Ringsdorf H, Schupp H. Polymeric Anti-Tumor Agents on a Molecular and on a Cellular-Level. Angew Chem Int Edit. 1981;20(4):305–25. [Google Scholar]
- 237.Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300(5619):615–8. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- 238.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–72. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 239.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6(11):2427–30. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 240.Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer. 2004;91(10):1775–81. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47(9):2486–93. [PubMed] [Google Scholar]
- 242.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10(11):3708–16. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 243.Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharm. 2003;259(1–2):143–60. doi: 10.1016/s0378-5173(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 244.Wiwattanapatapee R, Carreno-Gomez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm Res. 2000;17(8):991–8. doi: 10.1023/a:1007587523543. [DOI] [PubMed] [Google Scholar]
- 245.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. Faseb J. 2005;19(3):311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 246.Svenson S. Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm. 2009;71(3):445–62. doi: 10.1016/j.ejpb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 247.Liu M, Frechet JM. Designing dendrimers for drug delivery. Pharm Sci Technolo Today. 1999;2(10):393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 248.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38(3):185–96. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 249.Bhadra D, Bhadra S, Jain NK. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J Pharm Pharm Sci. 2005;8(3):467–82. [PubMed] [Google Scholar]
- 250.Gillies ER, Frechet JMJ. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjugate Chemistry. 2005;16(2):361–8. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 251.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257(1–2):111–24. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 252.Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J. Synthesis and evaluation of a star amphiphilic block copolymer from poly(epsilon-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjug Chem. 2005;16(2):397–405. doi: 10.1021/bc049784m. [DOI] [PubMed] [Google Scholar]
- 253.Ooya T, Lee J, Park K. Hydrotropic dendrimers of generations 4 and 5: synthesis, characterization, and hydrotropic solubilization of paclitaxel. Bioconjug Chem. 2004;15(6):1221–9. doi: 10.1021/bc049814l. [DOI] [PubMed] [Google Scholar]
- 254.Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnology. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41(10):2312–8. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 256.Decuzzi P, Ferrari M. Modulating cellular adhesion through nanotopography. Biomaterials. 2010;31(1):173–9. doi: 10.1016/j.biomaterials.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 257.Serda RE, Gu J, Burks JK, Ferrari K, Ferrari C, Ferrari M. Quantitative mechanics of endothelial phagocytosis of silicon microparticles. Cytometry A. 2009;75(9):752–60. doi: 10.1002/cyto.a.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436(7050):568–72. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 259.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010 doi: 10.1245/s10434-009-0686-z. in press [2009 Aug 26. -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 260.Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin NE. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations—review article. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2009.07.034. in press [2009 Aug 21 -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 261.Mohnike K, Wieners G, Pech M, Seidensticker M, Ruhl R, Lopez-Haenninen E, et al. Image-guided interstitial high-dose-rate brachytherapy in hepatocellular carcinoma. Dig Dis. 2009;27(2):170–4. doi: 10.1159/000218350. [DOI] [PubMed] [Google Scholar]
- 262.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, 3rd, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.23.4450. in press [2009 Oct 19 -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 263.Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2(4):349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 264.Thanos L, Mylona S, Ptohis N, Tsiouris S, Sotiropoulou E, Pomoni A, et al. Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol. 2009;15(4):290–6. doi: 10.4261/1305-3825.DIR.1828-08.2. [DOI] [PubMed] [Google Scholar]
- 265.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–31. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 266.Erdreich LS, Klauenberg BJ. Radio frequency radiation exposure standards: considerations for harmonization. Health Phys. 2001;80(5):430–9. doi: 10.1097/00004032-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 267.Adair ER, Blick DW, Allen SJ, Mylacraine KS, Ziriax JM, Scholl DM. Thermo-physiological responses of human volunteers to whole body RF exposure at 220 MHz. Bioelectromagnetics. 2005;26(6):448–61. doi: 10.1002/bem.20105. [DOI] [PubMed] [Google Scholar]
- 268.Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia. A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses. 1979;5(1):83–102. doi: 10.1016/0306-9877(79)90063-x. [DOI] [PubMed] [Google Scholar]
- 269.Han SK, Kim RS, Lee JH, Tae G, Cho SH, Yuk SH. Core-Shell Nanoparticles for drug delivery and molecular imaging. In: Kumar C, editor. Nanomaterials for medical diagnosis and therapy. Darmstadt, Germany: Wiley VCH; 2009. pp. 143–89. [Google Scholar]
- 270.Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest Radiol. 2002;37(10):580–6. doi: 10.1097/00004424-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 271.Johannsen M, Gneveckow U, Taymoorian K, Cho CH, Thiesen B, Scholz R, et al. Thermal therapy of prostate cancer using magnetic nanoparticles. Actas Urol Esp. 2007;31(6):660–7. doi: 10.1016/s0210-4806(07)73703-8. [DOI] [PubMed] [Google Scholar]
- 272.Bruners P, Braunschweig T, Hodenius M, Pietsch H, Penzkofer T, Baumann M, et al. Thermoablation of malignant kidney tumors using magnetic nanoparticles: an in vivo feasibility study in a rabbit model. Cardiovasc Intervent Radiol. 2010 doi: 10.1007/s00270-009-9583-x. in press [2009 May 9 -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 273.Bruners P, Hodenius M, Baumann M, Oversohl J, Gunther RW, Schmitz-Rode T, et al. Magnetic thermal ablation using ferrofluids: influence of administration mode on biological effect in different porcine tissues. Cardiovasc Intervent Radiol. 2008;31(6):1193–9. doi: 10.1007/s00270-008-9387-4. [DOI] [PubMed] [Google Scholar]
- 274.Deger S, Bohmer D, Roigas J, Turk I, Budach V, Loening SA. Interstitial hyperthermia using thermoseeds in combination with conformal radiotherapy for localized prostate cancer. Front Radiat Ther Oncol. 2002;36:171–6. doi: 10.1159/000061342. [DOI] [PubMed] [Google Scholar]
- 275.Hirsch LR, Jackson JB, Lee A, Halas NJ, West JL. A whole blood immunoassay using gold nanoshells. Anal Chem. 2003;75(10):2377–81. doi: 10.1021/ac0262210. [DOI] [PubMed] [Google Scholar]
- 276.Zhang XD, Guo ML, Wu HY, Sun YM, Ding YQ, Feng X, et al. Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int J Nanomedicine. 2009;4:165–73. doi: 10.2147/ijn.s6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 278.Loo C, Hirsch L, Lee MH, Chang E, West J, Halas N, et al. Gold nanoshell bioconjugates for molecular imaging in living cells. Opt Lett. 2005;30(9):1012–4. doi: 10.1364/ol.30.001012. [DOI] [PubMed] [Google Scholar]
- 279.Marches R, Chakravarty P, Musselman IH, Bajaj P, Azad RN, Pantano P, et al. Specific thermal ablation of tumor cells using single-walled carbon nanotubes targeted by covalently-coupled monoclonal antibodies. Int J Cancer. 2009;125(12):2970–7. doi: 10.1002/ijc.24659. [DOI] [PubMed] [Google Scholar]
- 280.Curley SA, Cherukuri P, Briggs K, Patra CR, Upton M, Dolson E, et al. Non-invasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Exp Ther Oncol. 2008;7(4):313–26. [PubMed] [Google Scholar]
- 281.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110(12):2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 282.Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnology. 2008;6:2. doi: 10.1186/1477-3155-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chakravarty P, Marches R, Zimmerman NS, Swafford AD, Bajaj P, Musselman IH, et al. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc Natl Acad Sci USA. 2008;105(25):8697–702. doi: 10.1073/pnas.0803557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11(9):3530–4. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 285.Hartman KB, Wilson LJ. Carbon nanostructures as a new high-performance platform for MR molecular imaging. Adv Exp Med Biol. 2007;620:74–84. doi: 10.1007/978-0-387-76713-0_6. [DOI] [PubMed] [Google Scholar]
- 286.Ghosh S, Dutta S, Gomes E, Carroll D, D’Agostino R, Jr, Olson J, et al. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano. 2009;3(9):2667–73. doi: 10.1021/nn900368b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 288.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–7. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 289.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007;7(7):1929–34. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 290.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209(2):171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 291.Waldman SA, Fortina P, Surrey S, Hyslop T, Kricka LJ, Graves DJ. Opportunities for near-infrared thermal ablation of colorectal metastases by guanylyl cyclase C-targeted gold nanoshells. Future Oncol. 2006;2(6):705–16. doi: 10.2217/14796694.2.6.705. [DOI] [PubMed] [Google Scholar]
- 292.Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1(2):149–54. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2008;86(2):165–72. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- 294.Bickford LR, Agollah G, Drezek R, Yu TK. Silica-gold nanoshells as potential intraoperativemolecular probes for HER2-overexpression in ex vivo breast tissue using near-infrared reflectance confocal microscopy. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-009-0408-z. in press [2009 May 6 -Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 295.Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3(3):351–8. [PMC free article] [PubMed] [Google Scholar]
- 296.Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir. 2008;24(20):11860–5. doi: 10.1021/la801969c. [DOI] [PubMed] [Google Scholar]
- 297.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15(6):419–33. doi: 10.1016/j.cytogfr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 298.Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des. 2004;10(27):3431–42. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 299.Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer. 2008;8(2):94–107. doi: 10.1038/nrc2289. [DOI] [PubMed] [Google Scholar]
- 300.Emerich DF, Thanos CG. Targeted nanoparticle-based drug delivery and diagnosis. J Drug Target. 2007;15(3):163–83. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- 301.Groneberg DA, Giersig M, Welte T, Pison U. Nanoparticle-based diagnosis and therapy. Curr Drug Targets. 2006;7(6):643–8. doi: 10.2174/138945006777435245. [DOI] [PubMed] [Google Scholar]
- 302.Weissleder R, Stark D, Engelstad B, Bacon B, Compton C, White D, et al. Super-paramagnetic iron oxide: pharmacokinetics and toxicity. Am J Roentgenol. 1989;152(1):167–73. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 303.Simonsen CZ, Ostergaard L, Vestergaard-Poulsen P, Rohl L, Bjornerud A, Gyldensted C. CBF and CBV measurements by USPIO bolus tracking: Reproducibility and comparison with Gd-based values. J Magn Reson Im. 1999;9(2):342–7. doi: 10.1002/(sici)1522-2586(199902)9:2<342::aid-jmri29>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 304.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–27. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 305.Okon E, Pouliquen D, Okon P, Kovaleva ZV, Stepanova TP, Lavit SG, et al. Biodegradation of magnetite dextran nanoparticles in the rat. A histologic and biophysical study Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Lab Invest. 1994;71(6):895–903. [PubMed] [Google Scholar]
- 306.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity Biodegradation of magnetite dextran nanoparticles in the rat. A histologic and biophysical study Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. AJR Am J Roentgenol. 1989;152(1):167–73. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 307.Coyne DW. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin Pharmacother. 2009;10(15):2563–8. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- 308.Kim YK, Lee JM, Kim CS, Chung GH, Kim CY, Kim IH. Detection of liver metastases: gadobenate dimeglumine-enhanced three-dimensional dynamic phases and one-hour delayed phase MR imaging versus superparamagnetic iron oxide-enhanced MR imaging. Eur Radiol. 2005;15(2):220–8. doi: 10.1007/s00330-004-2570-3. [DOI] [PubMed] [Google Scholar]
- 309.Seneterre E, Taourel P, Bouvier Y, Pradel J, Van Beers B, Daures JP, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200(3):785–92. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- 310.Schnorr J, Wagner S, Abramjuk C, Drees R, Schink T, Schellenberger EA, et al. Focal liver lesions: SPIO-, gadolinium-, and ferucarbotran-enhanced dynamic T1-weighted and delayed T2-weighted MR imaging in rabbits. Radiology. 2006;240(1):90–100. doi: 10.1148/radiol.2393040884. [DOI] [PubMed] [Google Scholar]
- 311.Mori K, Fukuda K, Asaoka H, Ueda T, Kunimatsu A, Okamoto Y, et al. Radiofrequency ablation of the liver: determination of ablative margin at MR imaging with impaired clearance of ferucarbotran—feasibility study. Radiology. 2009;251(2):557–65. doi: 10.1148/radiol.2512081161. [DOI] [PubMed] [Google Scholar]
- 312.Chen RC, Lii JM, Chou CT, Chang TA, Chen WT, Li CS, et al. T2-weighted and T1-weighted dynamic superparamagnetic iron oxide (ferucarbotran) enhanced MRI of hepatocellular carcinoma and hyperplastic nodules. J Formos Med Assoc. 2008;107(10):798–805. doi: 10.1016/S0929-6646(08)60193-X. [DOI] [PubMed] [Google Scholar]
- 313.Kim HJ, Kim KW, Byun JH, Won HJ, Shin YM, Kim PN, et al. Comparison of mangafodipir trisodium- and ferucarbotran-enhanced MRI for detection and characterization of hepatic metastases in colorectal cancer patients. AJR Am J Roentgenol. 2006;186(4):1059–66. doi: 10.2214/AJR.04.1941. [DOI] [PubMed] [Google Scholar]
- 314.Rogers WJ, Meyer CH, Kramer CM. Technology insight: in vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006;3(10):554–62. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- 315.Islam T, Harisinghani MG. Overview of nanoparticle use in cancer imaging. Cancer Biomark. 2009;5(2):61–7. doi: 10.3233/CBM-2009-0578. [DOI] [PubMed] [Google Scholar]
- 316.Heesakkers RA, Jager GJ, Hovels AM, de Hoop B, van den Bosch HC, Raat F, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251(2):408–14. doi: 10.1148/radiol.2512071018. [DOI] [PubMed] [Google Scholar]
- 317.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, U-King-Im J-M, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53(22):2039–50. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 318.Reimer P, Bremer C, Allkemper T, Engelhardt M, Mahler M, Ebert W, et al. Myocardial perfusion and MR angiography of chest with SH U 555 C: results of placebo-controlled clinical phase i study. Radiology. 2004;231(2):474–81. doi: 10.1148/radiol.2312021251. [DOI] [PubMed] [Google Scholar]
- 319.Wyttenbach R, Gianella S, Alerci M, Braghetti A, Cozzi L, Gallino A. Prospective blinded evaluation of Gd-DOTA- versus Gd-BOPTA-enhanced peripheral MR angiography, as compared with digital subtraction angiography. Radiology. 2003;227(1):261–9. doi: 10.1148/radiol.2271011989. [DOI] [PubMed] [Google Scholar]
- 320.Bremerich J, Bilecen D, Reimer P. MR angiography with blood pool contrast agents. Eur Radiol. 2007;17(12):3017–24. doi: 10.1007/s00330-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 321.Schnorr J, Wagner S, Abramjuk C, Wojner I, Schink T, Kroencke TJ, et al. Comparison of the iron oxide-based blood-pool contrast medium VSOP-C184 with gadopentetate dimeglumine for first-pass magnetic resonance angiography of the aorta and renal arteries in pigs. Invest Radiol. 2004;39(9):546–53. doi: 10.1097/01.rli.0000133944.30119.cc. [DOI] [PubMed] [Google Scholar]
- 322.Taupitz M, Wagner S, Schnorr J, Kravec I, Pilgrimm H, Bergmann-Fritsch H, et al. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Invest Radiol. 2004;39(7):394–405. doi: 10.1097/01.rli.0000129472.45832.b0. [DOI] [PubMed] [Google Scholar]
- 323.Allkemper T, Bremer C, Matuszewski L, Ebert W, Reimer P. Contrast-enhanced blood-pool MR angiography with optimized iron oxides: effect of size and dose on vascular contrast enhancement in rabbits. Radiology. 2002;223(2):432–8. doi: 10.1148/radiol.2232010241. [DOI] [PubMed] [Google Scholar]
- 324.Schoenberg SO, Aumann S, Just A, Bock M, Knopp MV, Johansson LO, et al. Quantification of renal perfusion abnormalities using an intravascular contrast agent (part 2): results in animals and humans with renal artery stenosis. Magn Reson Med. 2003;49(2):288–98. doi: 10.1002/mrm.10383. [DOI] [PubMed] [Google Scholar]
- 325.Karczmar GS, Fan X, Al-Hallaq H, River JN, Tarlo K, Kellar KE, et al. Functional and anatomic imaging of tumor vasculature: high-resolution MR spectroscopic imaging combined with a superparamagnetic contrast agent. Acad Radiol. 2002;9(Suppl 1):S115–8. doi: 10.1016/s1076-6332(03)80414-2. [DOI] [PubMed] [Google Scholar]
- 326.Weishaupt D, Hetzer FH, Ruehm SG, Patak MA, Schmidt M, Debatin JF. Three-dimensional contrast-enhanced MRI using an intravascular contrast agent for detection of traumatic intra-abdominal hemorrhage and abdominal parenchymal injuries: an experimental study. Eur Radiol. 2000;10(12):1958–64. doi: 10.1007/s003300000519. [DOI] [PubMed] [Google Scholar]
- 327.Gambarota G, Leenders W, Maass C, Wesseling P, van der Kogel B, van Tellingen O, et al. Characterisation of tumour vasculature in mouse brain by USPIO contrast-enhanced MRI. Br J Cancer. 2008;98(11):1784–9. doi: 10.1038/sj.bjc.6604389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 329.Varley JM, Swallow JE, Brammar WJ, Whittaker JL, Walker RA. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1(4):423–30. [PubMed] [Google Scholar]
- 330.Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 Expression in Breast Cancer Xenografts After Therapy Can Be Quantified Using PET and 18F-Labeled Affibody Molecules. J Nucl Med. 2009;50(7):1131–9. doi: 10.2967/jnumed.108.057695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Cormode DP, Skajaa T, Fayad ZA, Mulder WJM. Nanotechnology in Medical Imaging: Probe Design and Applications. Arterioscler Thromb Vasc Biol. 2009;29(7):992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116(11 Suppl):I38–45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 333.Ben-Hur T, van Heeswijk RB, Einstein O, Aharonowiz M, Xue R, Frost EE, et al. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. 2007;57(1):164–71. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 334.Ju S, Teng GJ, Lu H, Zhang Y, Zhang A, Chen F, et al. In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology. 2007;245(1):206–15. doi: 10.1148/radiol.2443061290. [DOI] [PubMed] [Google Scholar]
- 335.Yocum GT, Wilson LB, Ashari P, Jordan EK, Frank JA, Arbab AS. Effect of human stem cells labeled with ferumoxides-poly-L-lysine on hematologic and biochemical measurements in rats. Radiology. 2005;235(2):547–52. doi: 10.1148/radiol.2352040383. [DOI] [PubMed] [Google Scholar]
- 336.Chen TJ, Cheng TH, Chen CY, Hsu SC, Cheng TL, Liu GC, et al. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14(2):253–60. doi: 10.1007/s00775-008-0445-9. [DOI] [PubMed] [Google Scholar]
- 337.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci USA. 2007;104(3):932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, et al. In Vivo MRI Detection of Gliomas by Chlorotoxin-Conjugated Superparamagnetic Nanoprobes. Small. 2008;4(3):372–9. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Natarajan A, Gruettner C, Ivkov R, DeNardo GL, Mirick G, Yuan A, et al. Nano-Ferrite particle based radioimmunonanoparticles: binding affinity and in vivo pharmacokinetics. Bioconjug Chem. 2008;19(6):1211–8. doi: 10.1021/bc800015n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, et al. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007;48(9):1501–10. doi: 10.2967/jnumed.107.042234. [DOI] [PubMed] [Google Scholar]
- 341.Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, et al. Superparamagnetic Iron Oxide Nanoparticle-Aptamer Bioconjugates for Combined Prostate Cancer Imaging and Therapy. ChemMedChem. 2008;3(9):1311–5. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67(4):1555–62. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 343.Boutry S, Laurent S, Elst LV, Muller RN. Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media Mol Imaging. 2006;1(1):15–22. doi: 10.1002/cmmi.87. [DOI] [PubMed] [Google Scholar]
- 344.Baio G, Fabbi M, de Totero D, Ferrini S, Cilli M, Derchi LE, et al. Magnetic resonance imaging at 1.5 T with immunospecific contrast agent in vitro and in vivo in a xenotransplant model. MAGMA. 2006;19(6):313–20. doi: 10.1007/s10334-006-0059-6. [DOI] [PubMed] [Google Scholar]
- 345.Kang HW, Torres D, Wald L, Weissleder R, Bogdanov AA., Jr Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86(6):599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- 346.Raty JK, Liimatainen T, Wirth T, Airenne KJ, Ihalainen TO, Huhtala T, et al. Magnetic resonance imaging of viral particle biodistribution in vivo. Gene Ther. 2006;13(20):1440–6. doi: 10.1038/sj.gt.3302828. [DOI] [PubMed] [Google Scholar]
- 347.Lu J, Ma S, Sun J, Xia C, Liu C, Wang Z, et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials. 2009;30(15):2919–28. doi: 10.1016/j.biomaterials.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 348.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–9. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 349.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, et al. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl. 2008;47(33):6259–62. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 350.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63(23):8122–5. [PubMed] [Google Scholar]
- 351.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17(4):375–8. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 353.Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjug Chem. 2006;17(4):905–11. doi: 10.1021/bc060035+. [DOI] [PubMed] [Google Scholar]
- 354.Medarova Z, Pham W, Kim Y, Dai G, Moore A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int J Cancer. 2006;118(11):2796–802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 355.Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR, et al. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem. 2005;16(3):576–81. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 356.Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5(4):e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kelly KA, Setlur SR, Ross R, Anbazhagan R, Waterman P, Rubin MA, et al. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 2008;68(7):2286–91. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Moore A, Grimm J, Han B, Santamaria P. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes. 2004;53(6):1459–66. doi: 10.2337/diabetes.53.6.1459. [DOI] [PubMed] [Google Scholar]
- 359.Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54(3):718–24. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 360.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13(3):372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 361.Lee H, Lee K, Kim IK, Park TG. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials. 2008;29(35):4709–18. doi: 10.1016/j.biomaterials.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 362.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26(1):83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 363.Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007;67(3):1138–44. doi: 10.1158/0008-5472.CAN-06-1185. [DOI] [PubMed] [Google Scholar]
- 364.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 365.Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, Deorukhkar A, Shentu S, Kuno N, et al. Imaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin Cancer Res. 2008;14(3):731–41. doi: 10.1158/1078-0432.CCR-07-1958. [DOI] [PubMed] [Google Scholar]
- 366.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35(12):2235–44. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 367.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48(11):1862–70. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 368.Prinzen L, Miserus RJ, Dirksen A, Hackeng TM, Deckers N, Bitsch NJ, et al. Optical and magnetic resonance imaging of cell death and platelet activation using annexin a5-functionalized quantum dots. Nano Lett. 2007;7(1):93–100. doi: 10.1021/nl062226r. [DOI] [PubMed] [Google Scholar]
- 369.Marik J, Tartis MS, Zhang H, Fung JY, Kheirolomoom A, Sutcliffe JL, et al. Long-circulating liposomes radiolabeled with [18F]fluorodipalmitin ([18F]FDP) Nucl Med Biol. 2007;34(2):165–71. doi: 10.1016/j.nucmedbio.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sipkins DA, Gijbels K, Tropper FD, Bednarski M, Li KC, Steinman L. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol. 2000;104(1):1–9. doi: 10.1016/s0165-5728(99)00248-9. [DOI] [PubMed] [Google Scholar]
- 371.Phillips WT, Klipper R, Goins B. Use of (99m)Tc-labeled liposomes encapsulating blue dye for identification of the sentinel lymph node. J Nucl Med. 2001;42(3):446–51. [PubMed] [Google Scholar]
- 372.Shan L, Wang S, Sridhar R, Bhujwalla ZM, Wang PC. Dual probe with fluorescent and magnetic properties for imaging solid tumor xenografts. Mol Imaging. 2007;6(2):85–95. [PubMed] [Google Scholar]
- 373.Hirai M, Minematsu H, Kondo N, Oie K, Igarashi K, Yamazaki N. Accumulation of liposome with Sialyl Lewis X to inflammation and tumor region: application to in vivo bio-imaging. Biochem Biophys Res Commun. 2007;353(3):553–8. doi: 10.1016/j.bbrc.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 374.Mukundan S, Jr, Ghaghada KB, Badea CT, Kao CY, Hedlund LW, Provenzale JM, et al. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am J Roentgenol. 2006;186(2):300–7. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- 375.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 376.Fatouros PP, Corwin FD, Chen ZJ, Broaddus WC, Tatum JL, Kettenmann B, et al. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology. 2006;240(3):756–64. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 377.Mikawa M, Kato H, Okumura M, Narazaki M, Kanazawa Y, Miwa N, et al. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjug Chem. 2001;12(4):510–4. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 378.Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem Commun (Camb) 2005;(31):3915–7. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 379.McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48(7):1180–9. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 380.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nano. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 381.Guo J, Zhang X, Li Q, Li W. Biodistribution of functionalized multiwall carbon nanotubes in mice. Nucl Med Biol. 2007;34(5):579–83. doi: 10.1016/j.nucmedbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 382.Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5(2):118–22. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 383.Newton JR, Miao Y, Deutscher SL, Quinn TP. Melanoma imaging with pretargeted bivalent bacteriophage. J Nucl Med. 2007;48(3):429–36. [PubMed] [Google Scholar]
- 384.Li C, Kan Z, Yang DJ, Kuang LR, Liu CW, Wright KC, et al. Preparation, characterization, and evaluation of ioxilan carbonate particles for computed tomography contrast enhancement of liver. Invest Radiol. 1994;29(11):1006–13. doi: 10.1097/00004424-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 385.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, et al. Endothelial {alpha}{nu}{beta}3 Integrin-Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–9. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 386.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, et al. Novel MRI Contrast Agent for Molecular Imaging of Fibrin: Implications for Detecting Vulnerable Plaques. Circulation. 2001;104(11):1280–5. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 387.Cyrus T, Abendschein DR, Caruthers SD, Harris TD, Glattauer V, Werkmeister JA, et al. MR three-dimensional molecular imaging of intramural biomarkers with targeted nanoparticles. J Cardiovasc Magn Reson. 2006;8(3):535–41. doi: 10.1080/10976640600580296. [DOI] [PubMed] [Google Scholar]
- 388.Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, et al. 64Cu-Labeled Folate-Conjugated Shell Cross-Linked Nanoparticles for Tumor Imaging and Radiotherapy: Synthesis, Radiolabeling, and Biologic Evaluation. J Nucl Med. 2005;46(7):1210–8. [PubMed] [Google Scholar]
- 389.Kong WH, Lee WJ, Cui ZY, Bae KH, Park TG, Kim JH, et al. Nanoparticulate carrier containing water-insoluble iodinated oil as a multifunctional contrast agent for computed tomography imaging. Biomaterials. 2007;28(36):5555–61. doi: 10.1016/j.biomaterials.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 390.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, et al. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25(4):866–71. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 391.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2(5):681–93. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 392.Kim D, Park S, Lee JH, Yeon JY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo x-ray computed tomography imaging. Nanomed: Official J Am Acad Nanomed. 2007;3(4):352. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 393.Lumbroso P, Dick CE. X-ray attenuation properties of radiographic contrast media. Medical Physics. 1987;14(5):752–8. doi: 10.1118/1.595999. [DOI] [PubMed] [Google Scholar]
- 394.Manzoor K, Johny S, Thomas D, Setua S, Menon D, Nair S. Bio-conjugated luminescent quantum dots of doped ZnS: a cyto-friendly system for targeted cancer imaging. Nanotechnology. 2009;20(6):65102. doi: 10.1088/0957-4484/20/6/065102. [DOI] [PubMed] [Google Scholar]
- 395.Nahar M, Dutta T, Murugesan S, Asthana A, Mishra D, Rajkumar V, et al. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Crit Rev Ther Drug Carrier Syst. 2006;23(4):259–318. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 396.Cherukuri P, Gannon CJ, Leeuw TK, Schmidt HK, Smalley RE, Curley SA, et al. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci USA. 2006;103(50):18882–6. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Levine DH, Ghoroghchian PP, Freudenberg J, Zhang G, Therien MJ, Greene MI, et al. Polymersomes: a new multi-functional tool for cancer diagnosis and therapy. Methods. 2008;46(1):25–32. doi: 10.1016/j.ymeth.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Longmire M, Choyke PL, Kobayashi H. Dendrimer-based contrast agents for molecular imaging. Curr Top Med Chem. 2008;8(14):1180–6. doi: 10.2174/156802608785849021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19(1):142–64. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 400.Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res. 2009;42(7):904–14. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Weissleder R, Lee AS, Khaw BA, Shen T, Brady TJ. Antimyosin-labeled monocrystalline iron oxide allows detection of myocardial infarct: MR antibody imaging. Radiology. 1992;182(2):381–5. doi: 10.1148/radiology.182.2.1732953. [DOI] [PubMed] [Google Scholar]
- 402.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23(11):1418–23. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 403.Ghaghada KB, Saul J, Natarajan JV, Bellamkonda RV, Annapragada AV. Folate targeting of drug carriers: a mathematical model. J Control Release. 2005;104(1):113–28. doi: 10.1016/j.jconrel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 404.Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114(3):277–87. doi: 10.1016/j.jconrel.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 405.Bautista CV, Felis CP, Espinet JM, Garcia JB, Salas JV. Telomerase activity is a prognostic factor for recurrence and survival in rectal cancer. Dis Colon Rectum. 2007;50(5):611–20. doi: 10.1007/s10350-006-0820-y. [DOI] [PubMed] [Google Scholar]
- 406.El Samny T, El Halaby H, El Fiky L, Nassar M, El Makhzangy A, Eissa S. Prognostic value of telomerase and DNA ploidy in laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2005;262(10):799–803. doi: 10.1007/s00405-004-0904-z. [DOI] [PubMed] [Google Scholar]
- 407.Sanz-Casla MT, Vidaurreta M, Sanchez-Rueda D, Maestro ML, Arroyo M, Cerdan FJ. Telomerase activity as a prognostic factor in colorectal cancer. Onkologie. 2005;28(11):553–7. doi: 10.1159/000088525. [DOI] [PubMed] [Google Scholar]
- 408.Chen J, Tung C-H, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, et al. In Vivo Imaging of Proteolytic Activity in Atherosclerosis. Circulation. 2002;105(23):2766–71. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 409.Sosnovik DE, Nahrendorf M, Deliolanis N, Novikov M, Aikawa E, Josephson L, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115(11):1384–91. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 410.Bryson JM, Fichter KM, Chu WJ, Lee JH, Li J, Madsen LA, et al. Polymer beacons for luminescence and magnetic resonance imaging of DNA delivery. Proc Natl Acad Sci USA. 2009;106(40):16913–8. doi: 10.1073/pnas.0904860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Santra S, Kaittanis C, Grimm J, Perez JM. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small. 2009;5(16):1862–8. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Jain TK, Foy SP, Erokwu B, Dimitrijevic S, Flask CA, Labhasetwar V. Magnetic resonance imaging of multifunctional pluronic stabilized iron-oxide nanoparticles in tumor-bearing mice. Biomaterials. 2009;30(35):6748–56. doi: 10.1016/j.biomaterials.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Al-Jamal WT, Al-Jamal KT, Tian B, Cakebread A, Halket JM, Kostarelos K. Tumor targeting of functionalized quantum dot-liposome hybrids by intravenous administration. Mol Pharm. 2009;6(2):520–30. doi: 10.1021/mp800187d. [DOI] [PubMed] [Google Scholar]
- 414.Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 2008;26(1):39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 415.Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38(4):1139–51. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- 416.Harrington DA, Sharma AK, Erickson BA, Cheng EY. Bladder tissue engineering through nanotechnology. World J Urol. 2008;26(4):315–22. doi: 10.1007/s00345-008-0273-0. [DOI] [PubMed] [Google Scholar]
- 417.Chun YW, Webster TJ. The role of nanomedicine in growing tissues. Ann Biomed Eng. 2009;37(10):2034–47. doi: 10.1007/s10439-009-9722-1. [DOI] [PubMed] [Google Scholar]
- 418.Lanza RP, Langer RS, Vacanti J. Principles of tissue engineering. 2. San Diego, CA: Academic Press; 2000. pp. 143–156. [Google Scholar]
- 419.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, et al. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhang Y, Lim CT, Ramakrishna S, Huang ZM. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med. 2005;16(10):933–46. doi: 10.1007/s10856-005-4428-x. [DOI] [PubMed] [Google Scholar]
- 421.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Riboldi SA, Sadr N, Pigini L, Neuenschwander P, Simonet M, Mognol P, et al. Skeletal myogenesis on highly orientated microfibrous polyesterurethane scaffolds. J Biomed Mater Res A. 2008;84(4):1094–101. doi: 10.1002/jbm.a.31534. [DOI] [PubMed] [Google Scholar]
- 423.Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, et al. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26(26):5330–8. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 424.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–82. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 425.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29(19):2899–906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 426.Fujita K. The use of resin-sprayed thin paper for urinary bladder regeneration. Invest Urol. 1978;15:355. [PubMed] [Google Scholar]
- 427.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3(6):1233–9. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 428.Nagapudi K, Brinkman WT, Thomas BS, Park JO, Srinivasarao M, Wright E, et al. Viscoelastic and mechanical behavior of recombinant protein elastomers. Biomaterials. 2005;26(23):4695–706. doi: 10.1016/j.biomaterials.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 429.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27(5):724–34. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 430.Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27(7):1088–94. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 431.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 432.Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials. 2008;29(8):970–83. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 433.Khang D, Liu-Snyder P, Pareta R, Lu J, Webster TJ. Reduced responses of macrophages on nanometer surface features of altered alumina crystalline phases. Acta Biomater. 2009;5(5):1425–32. doi: 10.1016/j.actbio.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 434.Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res Part A. 2006;78A(3):595–604. doi: 10.1002/jbm.a.30789. [DOI] [PubMed] [Google Scholar]
- 435.Haque S, Rehman I, Darr JA. Synthesis and characterization of grafted nanohydroxyapatites using functionalized surface agents. Langmuir. 2007;23(12):6671–6. doi: 10.1021/la063517i. [DOI] [PubMed] [Google Scholar]
- 436.Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2008 doi: 10.1007/s10544-008-9264-6. [DOI] [PubMed] [Google Scholar]
- 437.Yeo IS, Oh JE, Jeong L, Lee TS, Lee SJ, Park WH, et al. Collagen-based biomimetic nanofibrous scaffolds: preparation and characterization of collagen/silk fibroin bicomponent nanofibrous structures. Biomacromolecules. 2008;9(4):1106–16. doi: 10.1021/bm700875a. [DOI] [PubMed] [Google Scholar]
- 438.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25(7–8):1289–97. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 439.Chung T-W, Wang Y-Z, Huang Y-Y, Pan C-I, Wang S-S. Poly (e-caprolactone) grafted with nano-structured chitosan enhances growth of human dermal fibroblasts. Artif Organ. 2006;30(1):35–41. doi: 10.1111/j.1525-1594.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 440.Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3(3):321–30. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 441.Mironov V, Kasyanov V, Markwald RR. Nanotechnology in vascular tissue engineering: from nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008;26(6):338–44. doi: 10.1016/j.tibtech.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 442.Carpenter J, Khang D, Webster TJ. Nanometer Polymer surface features: the influence on surface energy, protein adsorption and endothelial adhesion. Nanotechnology. 2008;19(18) doi: 10.1088/0957-4484/19/50/505103. [DOI] [PubMed] [Google Scholar]
- 443.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11(4):381–7. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15(3):467–80. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA. 2005;102(12):4300–5. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28(30):4409–17. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 447.Dobson J, Cartmell SH, Keramane A, El Haj AJ. Principles and design of a novel magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning, and dynamic in vitro screening. IEEE Trans Nanobioscience. 2006;5(3):173–7. doi: 10.1109/tnb.2006.880823. [DOI] [PubMed] [Google Scholar]
- 448.Perea H, Aigner J, Heverhagen JT, Hopfner U, Wintermantel E. Vascular tissue engineering with magnetic nanoparticles: seeing deeper. J Tissue Eng Regen Med. 2007;1(4):318–21. doi: 10.1002/term.32. [DOI] [PubMed] [Google Scholar]
- 449.Perea H, Aigner J, Hopfner U, Wintermantel E. Direct magnetic tubular cell seeding: a novel approach for vascular tissue engineering. Cells Tissues Organs. 2006;183(3):156–65. doi: 10.1159/000095989. [DOI] [PubMed] [Google Scholar]
- 450.Pislaru SV, Harbuzariu A, Agarwal G, Witt T, Gulati R, Sandhu NP, et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 2006;114(1 Suppl):I314–8. doi: 10.1161/CIRCULATIONAHA.105.001446. [DOI] [PubMed] [Google Scholar]
- 451.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28(17):2738–46. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kramer S, Xie H, Gaff J, Williamson JR, Tkachenko AG, Nouri N, et al. Preparation of protein gradients through the controlled deposition of protein-nanoparticle conjugates onto functionalized surfaces. J Am Chem Soc. 2004;126(17):5388–95. doi: 10.1021/ja031674n. [DOI] [PubMed] [Google Scholar]
- 454.Kim DH, Abidian M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res A. 2004;71(4):577–85. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 455.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res A. 2006;78(1):157–67. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 456.Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, Lee IS. Culture of neural cells on silicon wafers with nano-scale surface topograph. J Neurosci Methods. 2002;120(1):17–23. doi: 10.1016/s0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 457.Ai H, Meng H, Ichinose I, Jones SA, Mills DK, Lvov YM, et al. Biocompatibility of layer-by-layer self-assembled nanofilm on silicone rubber for neurons. J Neurosci Methods. 2003;128(1–2):1–8. doi: 10.1016/s0165-0270(03)00191-2. [DOI] [PubMed] [Google Scholar]
- 458.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 459.Nisbet DR, Pattanawong S, Ritchie NE, Shen W, Finkelstein DI, Horne MK, et al. Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. J Neural Eng. 2007;4(2):35–41. doi: 10.1088/1741-2560/4/2/004. [DOI] [PubMed] [Google Scholar]
- 460.Thompson DM, Buettner HM. Neurite outgrowth is directed by schwann cell alignment in the absence of other guidance cues. Ann Biomed Eng. 2006;34(1):161–8. doi: 10.1007/s10439-005-9013-4. [DOI] [PubMed] [Google Scholar]
- 461.Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syn-geneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12(9):3310–20. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Bruder JM, Lee AP, Hoffman-Kim D. Biomimetic materials replicating Schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. J Biomater Sci Polym Ed. 2007;18(8):967–82. doi: 10.1163/156856207781494412. [DOI] [PubMed] [Google Scholar]
- 463.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313(9):1820–9. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Chen X, Tam UC, Czlapinski JL, Lee GS, Rabuka D, Zettl A, et al. Interfacing carbon nanotubes with living cells. J Am Chem Soc. 2006;128(19):6292–3. doi: 10.1021/ja060276s. [DOI] [PubMed] [Google Scholar]
- 465.Baughman RH. Materials science. Playing nature’s game with artificial muscles. Science. 2005;308(5718):63–5. doi: 10.1126/science.1099010. [DOI] [PubMed] [Google Scholar]
- 466.Liopo AV, Stewart MP, Hudson J, Tour JM, Pappas TC. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface. J Nanosci Nanotechnol. 2006;6(5):1365–74. doi: 10.1166/jnn.2006.155. [DOI] [PubMed] [Google Scholar]
- 467.Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127(16):6021–6. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 468.Withey GD, Lazareck AD, Tzolov MB, Yin A, Aich P, Yeh JI, et al. Ultra-high redox enzyme signal transduction using highly ordered carbon nanotube array electrodes. Biosens Bioelectron. 2006;21(8):1560–5. doi: 10.1016/j.bios.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 469.Vamvakaki V, Tsagaraki K, Chaniotakis N. Carbon nanofiber-based glucose biosensor. Anal Chem. 2006;78(15):5538–42. doi: 10.1021/ac060551t. [DOI] [PubMed] [Google Scholar]
- 470.Mojarradi M, Binkley D, Blalock B, Andersen R, Ulshoefer N, Johnson T, et al. A miniaturized neuroprosthesis suitable for implantation into the brain. IEEE Trans Neural Syst Rehabil Eng. 2003;11(1):38–42. doi: 10.1109/TNSRE.2003.810431. [DOI] [PubMed] [Google Scholar]
- 471.Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans Neural Syst Rehabil Eng. 2003;11(2):151–5. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 472.McKenzie JL, Waid MC, Shi R, Webster TJ. Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials. 2004;25(7–8):1309–17. doi: 10.1016/j.biomaterials.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 473.Pharmaceutical Research and Manufacturers of America (PhRMA) Pharmaceutical industry profile 2009. 2009. pp. 36–39. [Google Scholar]
- 474.United States Food and Drug Administration (FDA) PRESS RELEASE. 2009 March 10;:1. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149541.htm.
- 475.United States Food and Drug Administration (FDA) Memorandum of understanding (MOU 225-07-8006) 2009. p. 1. [Google Scholar]