Abstract
A series of laboratory experiments was conducted with a multiple-component immiscible liquid, collected from the Picillo Farm Superfund Site in Rhode Island, to examine liquid-vapor mass-transfer behavior. The immiscible liquid, which comprises solvents, oils, pesticides, PCBs, paint sludges, explosives, and other compounds, was characterized using gas chromatography and gas chromatography/mass spectrometry to determine mole fractions of selected constituents. Batch experiments were conducted to evaluate equilibrium phase-partitioning behavior. Two sets of air-stripping column studies were conducted to examine the mass-transfer dynamics of five selected target compounds present in the immiscible-liquid mixture. One set of column experiments was designed to represent a system with free-phase immiscible liquid present; the other was designed to represent a system with a residual phase of immiscible liquid. Initial elution behavior of all target components generally appeared to be ideal for both systems, as the initial vapor-phase concentrations were similar to vapor-phase concentrations measured for the batch experiment and those estimated using Raoult’s law (incorporating the immiscible-liquid composition data). Later-stage removal of 1,2-dichlorobenzene appeared to be rate limited for the columns containing free-phase immiscible liquid and no porous medium. Conversely, evaporative mass transfer appeared to be ideal throughout the experiment conducted with immiscible liquid distributed relatively uniformly as a residual phase within a sandy porous medium.
Keywords: Raoult’s law, multiple-component NAPL, SVE, rate-limited mass transfer
1. INTRODUCTION
Soil vapor extraction (SVE) has been shown to be an effective method for removing volatile and semi-volatile organic compounds from the unsaturated zone, and has become a standard protocol for remediation of sites contaminated with such compounds. However, SVE systems often exhibit reduced efficiency at some point, characterized by reduced mass-removal rates and extended lower-concentration tailing. This behavior may be caused by a number of physical, chemical, and biological factors influencing contaminant transport, including but not limited to changes in immiscible-liquid composition, rate-limited evaporation, diffusion-controlled transport, rate-limited sorption/desorption, and constraints associated with subsurface heterogeneity (e.g., Rathfelder et al., 1991; Ho and Udell, 1992; Armstrong et al., 1994; Hayden et al., 1994; Wilkins et al., 1995; Lingineni and Dhir, 1996; Nadim et al., 1997; Popovicova and Brusseau, 1997; Popovicova and Brusseau, 1998; Schaefer et al., 1998; Schaefer et al., 1999a; Schaefer et al. 1999b; Rathfelder et al., 2000; Yoon et al., 2002; Abriola et al., 2004). The partitioning and mass-transfer behavior of multiple-component immiscible liquids is one of several factors important for understanding contaminant transport in the vadose zone and for designing and implementing effective SVE systems.
Raoult’s law is often used to characterize partitioning behavior between a multiple-component immiscible liquid and the vapor or aqueous phases. Several studies have been reported in which Raoult’s law was used successfully to estimate component concentrations for aqueous solutions in equilibrium with multiple-component immiscible liquids, including gasoline (Cline et al., 1991), diesel fuel (Lee et al., 1992a), and coal tar (Lee et al., 1992b). In addition, several researchers have used Raoult’s law to successfully predict effluent vapor concentrations of volatile organic compounds during air-stripping of gasoline (Baehr et al., 1989), synthetic multiple-component mixtures (Rathfelder et al., 1991; Ho et al., 1994; Farhan et al., 2002), and jet fuel (El-Beshry et al., 2001). However, non-ideal contaminant removal behavior has also been reported, with measured effluent vapor-phase concentrations significantly lower than those predicted based on equilibrium partitioning (e.g., Ho and Udell, 1992; Hayden et al., 1994; Harper et al., 2003). For example, Hayden et al. (1994) found the applicability of the local equilibrium assumption and predictions based on Raoult’s law to be limited during late venting times (i.e, low mole fractions of constituents in the immiscible liquid). Deviation from ideal behavior predicted by Raoult’s law may be caused by mass-transfer limitations, by the non-ideality of the complex chemical mixture, or some combination thereof.
Understanding the mass-transfer behavior of multiple-component immiscible liquids is important for accurate prediction of contaminant transport and for the design of successful remediation systems. However, the mass-transfer dynamics of immiscible liquids comprised of very complex mixtures of chemicals, such as found in the Picillo Farm site immiscible liquid, has not been investigated to a significant extent. The objectives of this study were to investigate the mass-transfer dynamics associated with air-stripping of the complex Picillo Farm immiscible liquid, and to evaluate the ideality of evaporation with respect to the applicability of Raoult’s law.
2. MATERIALS AND METHODS
2.1 Site Background
The Picillo Farm Superfund Site, located in Coventry, Rhode Island, was placed on the National Priorities List (NPL) in September of 1983. The site covers approximately 7.5 acres of a 100-acre former pig farm where it was discovered that more than 10,000 drums of hazardous waste and an unknown volume of liquid chemicals, including solvents, oils, pesticides, PCBs, paint sludges, and explosives, were illegally disposed of in several unlined trenches. It was determined that contamination of soil, groundwater, and surface water existed on the site. In 1999, while the Environmental Protection Agency (EPA) was completing an investigation of the groundwater, surface water, and residual soil contamination, free-phase immiscible liquid was discovered in a well. The present study was implemented to help develop a more comprehensive understanding of the immiscible liquid behavior at the field site.
2.2 Immiscible-Liquid Composition
The composition of the multiple-component immiscible liquid collected from the Picillo Farm site was characterized both before and after air-stripping experiments were conducted. Immiscible-liquid composition was characterized by gas chromatography/mass spectrometry (GC/MS) using EPA Test Methods 8260, and 8270, and by gas chromatograph/flame ionization detection (GC/FID) using EPA Test Method 8015 (Transwest Geochem; Phoenix, AZ). EPA Test Method 8260 is used to quantify volatile organic compound concentrations, and EPA Test Method 8270 is used to quantify semi-volatile organic compound concentrations. EPA Test Method 8015 is used to quantify organic compound concentrations within the C6–C32 carbon range. More specifically, a summation method, based on analysis times for gasoline, diesel, and oil, is used to report carbon-profile group concentrations as C6–C10, C10–C22, and C22–C32, respectively. Any mass not accounted for by EPA Test Method 8015 is either an organic compound outside of the C6–C32 range or an inorganic compound.
2.3 Batch Studies
A batch experiment was conducted to investigate equilibrium phase-partitioning behavior. The batch reactors were set up by adding 0.90 g of Picillo Farm site immiscible liquid to 40-ml glass vials with an open-top screw cap and Teflon-coated rubber septa (Kimble-Kontes, Vineland, New Jersey). The vials were then placed on a shaker table set at 200 rpm and allowed to equilibrate at room temperature (21 ± 3 °C) for more than one week. Following equilibration, 2-ml vapor samples were collected over a period of time using a 2.5 ml gas-tight syringe (Hamilton Company, Reno, Nevada) and injected into 20-ml glass headspace vials, with open-top aluminum crimp-seal caps (Kimble-Kontes, Vineland, New Jersey) and Teflon-coated rubber septa (National Scientific Company, Duluth, Georgia). The vapor samples were analyzed by headspace gas chromatography (Tekmar-Dohrmann 7050 coupled with a Shimadzu GC-17A) flame ionization detector (FID). Chromatographic analysis was done using a glass capillary column (SPB-624, 30-m length, 5-µm film thickness) with oven temperature programming. Specifically, analysis began with the oven temperature set for 2 minutes at 40 °C, ramped to 150 °C at a rate of 10 °C per minute, and held for 2 minutes. The GC’s injection port temperature was set at 180 °C with the detector at 180 °C. Aqueous-phase standards were analyzed every 48 hours with check standards and blanks analyzed for quality assurance every 10 to 15 samples. Quantifiable detection limits for GC/FID analysis are presented in Table 1.
Table 1.
Target compound chemical properties.
| Chemical | Molecular Weight+ |
Density+ (g/cm3) |
Vapor Pressure+ (atm) |
Saturated Vapor-phase Concentration# (mg/L) |
QDL* (mg/L) |
|---|---|---|---|---|---|
| Trichloroethene | 131.39 | 1.464 | 0.0978 | 532 | 0.027 |
| Toluene | 92.14 | 0.867 | 0.0374 | 143 | 0.009 |
| Tetrachloroethene | 165.83 | 1.623 | 0.0239 | 164 | 0.016 |
| Ethylbenzene | 106.17 | 0.867 | 0.0126 | 55.4 | 0.009 |
| 1,2-Dichlorobenzene | 147.00 | 1.306 | 0.0018 | 11 | 0.018 |
Lide, 1995
Vapor-phase concentration in equilibrium with pure-phase immiscible liquid.
Quantifiable detection limit for GC/FID vapor-phase analysis.
2.4 Column Studies
Column experiments were performed at room temperature (21 ± 3 °C) to examine the effect of air stripping on the Picillo Farm site immiscible liquid. These experiments were conducted using 15-cm long, 2.1-cm inner diameter, stainless steel columns (Alltech, Deerfield, Illinois). All tubing (Ohio Valley Specialty Chemical, Marietta, Ohio), fittings, and connectors (Arizona Valve and Fitting Company, Phoenix, Arizona) were stainless steel. Dynamic flow experiments were performed under two types of column conditions: 1) column containing immiscible liquid and air, and 2) column containing porous media, water, immiscible liquid, and air. The columns containing only immiscible liquid and air were designed to represent a system with free-phase immiscible liquid present. Such systems would be found at a site with floating free product above a water table or pooled immiscible liquid above a water-saturated low permeability zone. For the purpose of this research, the system was simplified to contain only immiscible liquid and air. The column containing porous media, water, immiscible liquid, and air was designed to represent a vadose-zone system with a residual phase of immiscible-liquid contamination.
Gas flow for each column experiment was generated using a high-pressure gas cylinder containing high-purity (99.95%) nitrogen. Flow was monitored using a digital flow meter (Model ADM 2000, JandW Scientific, Folsom, California). Discrete vapor samples were collected from the column effluent throughout the experiments using a 2.5-ml gas-tight syringe. For each sample, 2 ml of vapor were injected into a 20-ml glass vial, with open-top aluminum crimp-seal cap and Teflon-coated rubber septa, and analyzed by GC/FID.
As previously stated, the first series of dynamic flow experiments were conducted in a column containing only immiscible liquid and air. For these experiments, an empty column was clamped in a horizontal position on a ring stand with the end caps and plugs secured. A sub-sample of immiscible liquid was collected using a 5-ml luerlock glass syringe (Micro-mate Interchangable, Popper and Sons, Inc., New Hyde Park, New York) fitted with a 25 gage, 3.8-cm Precision Guide needle (Becton Dickinson and Company, Franklin Lakes, New Jersey). One of the plugs on the column was removed, the needle was inserted, and the immiscible liquid was injected through the end cap into the column. The column was then connected to the apparatus and allowed to equilibrate for more than 24 hours. The experiment was then initiated by flushing nitrogen through the column at a flow rate of 35 ml/min (linear velocity = 11.2 cm/min, residence time ≈ 1.3 min). The flow rate chosen for the column experiments was in the range of values reported for prior gas-phase column studies (e.g., Hayden et al., 1994; Wilkins et al., 1995; Popovicova and Brusseau, 1997, Popovicova and Brusseau, 1998), and is generally representative of velocities associated with forced-gradient (e.g., SVE) systems.
Another set of experiments was conducted using a column containing 20/30-mesh quartz sand, water, immiscible liquid, and air. The sand (Accusand, Unimin Corporation, Le Sueur, MN) has a mean particle diameter of 0.71 ± 0.02 mm and an organic carbon content of 0.4 g/kg (Schroth et al., 1996). For the porous-media filled columns, stainless steel diffusion disks with a pore size of 20 um (Alltech, Deerfield, IL) were placed at each end of the column. Before packing the column, the sand was mixed in a beaker with sufficient water to attain 5% water saturation when packed. The column was packed in incremental steps to establish a uniform bulk density (~1.6 g/cm3) and porosity (~0.4).
Once the column had been packed, immiscible liquid was injected into the column to establish a targeted residual immiscible liquid saturation of approximately 10%. A sample of immiscible liquid was collected from the immiscible liquid reservoir using a 5-ml luerlock glass syringe fitted with a 25 gage, 3.8-cm Precision Guide needle. The column was placed in a vertical position and immiscible liquid was injected at various points across the cross sectional area at the top of the column. The column was then sealed and the immiscible liquid was allowed to redistribute for approximately 48 hours. After this period, the column was rotated 180° and additional immiscible liquid was injected as before and allowed to redistribute for another 48 hours. The column was then connected to the apparatus, humidified nitrogen was injected at a flow rate of 10.5 ml/min (linear velocity = 11.2 cm/min, residence time ≈ 1.3 min), and discrete vapor samples were collected. Following the air-stripping of the porous-medium packed column, the contents were pushed through the top of the column in 0.5 cm increments and photographs were taken to record the distribution of immiscible liquid within the column. These data confirmed a uniform distribution of immiscible liquid within the column.
2.5 Data Analysis
Raoult’s law was used to determine ideal vapor-phase concentrations of selected target compounds present in the Picillo Farm site immiscible liquid. To use Raoult’s law, molecular weights of each compound in the immiscible liquid are needed. Because the immiscible liquid is such a complex mixture, not all compounds in the immiscible liquid were identified through the compositional analysis. However, using the carbon profile data obtained for the immiscible liquid, representative molecular weights were selected to represent each carbon profile group (see Table 2). Once molecular weights had been assigned to each fraction of the immiscible-liquid mixture, the mole fraction of each target compound could be calculated using equation 1:
| (1) |
where Xi is the mole fraction of component i; Ci is the concentration of component i in the immiscible liquid mixture (mg/kg); and, MWi is the molecular weight of component i. For the above equation, the subscript i will represent not only the individual compounds but also the carbon profile groups.
Table 2.
Carbon profile data for immiscible liquid.
| Carbon Profile | Representative Compound -- Molecular Weight |
Pre-stripping Concentration (mg/kg) |
Post-stripping Concentration (mg/kg) |
|---|---|---|---|
| C6–C10 | C8H10 – 114.23 | 340,000 | < 7,400* |
| C10–C22 | C16H34 – 226.45 | 240,000 | 290,000 |
| C22–C32 | C27H56 – 380.75 | 160,000 | 420,000 |
| Other than C6–C32 | C32H66 – 452.56 | 260,000 | 290,000 |
Value less than detection limit for EPA Test Method 8015. Reported value based on composition data reported in Table 3.
Once the mole fractions had been estimated for each target compound, equilibrium vapor-phase concentrations were determined according to Raoult’s law. To predict partitioning between a multi-component immiscible liquid and the gas phase, a combination of the ideal gas law and Raoult’s law has typically been used:
| (2) |
where Cg,i is the equilibrium concentration in the gas phase of compound i; MWi is the molecular weight of compound i; Pi is the vapor pressure of compound i as a pure component; R is the universal gas constant; T is temperature; and, Xi is the mole fraction of compound i in the immiscible liquid mixture.
Equilibrium vapor-phase concentrations of our specific target components were determined using the above equations. First, the saturated vapor-phase concentration of the selected compound was calculated using the Ideal Gas Law. Raoult’s law was then used to predict the equilibrium vapor-phase concentration of the target component given the mole fraction of the compound in the immiscible liquid. The resultant concentrations calculated in this manner have an estimated error of approximately 15%, which encompasses the error associated with chemical analysis as well as uncertainty in the estimates of compound molecular weights. Final predicted vapor-phase concentration values were compared to equilibrium and steady-state vapor-phase concentrations determined from the batch and column experiments, respectively.
3. RESULTS AND DISCUSSION
3.1 Chemical Analyses and Batch Experiments
Compositional analysis of the Picillo Farm site immiscible liquid was performed both before and after the first air-stripping experiment, which was conducted for approximately 10 days. Results of the chemical analysis using carbon profile test method 8015 are reported in Table 2. Results of the chemical analysis using EPA Test Method 8260 are reported in Table 3. The results of the carbon profile test show that the immiscible liquid was composed primarily of organic compounds within the C6–C32 carbon range, with this group comprising approximately 74% before air-stripping and approximately 72% after air-stripping. The remaining portion of the immiscible liquid is assumed to consist of either organic compounds outside of the C6–C32 range or inorganic compounds.
Table 3.
Pre- and post-air stripping immiscible-liquid composition data.
| Chemical | Pre-stripping Concentration (mg/kg) |
Post-stripping Concentration (mg/kg) |
|---|---|---|
| 1, 1, 1-Trichloroethane | 1,600 | <5+ |
| 1, 1-Dichloroethene | 160 | <10+ |
| 1,2,4-Trimethylbenzene | 4,900 | 130 |
| 1,3,5-Trimethylbenzene | 1600 | 33 |
| 1,2-Dichlorobenzene | 120,000 | 6,900 |
| 4-Isopropyltoluene | 670 | 39 |
| Ethylbenzene | 11,000 | 27 |
| n-Butylbenzene | 360 | 50 |
| n-Propylbenzene | 940 | <25+ |
| sec-Butylbenzene | 190 | <25+ |
| Styrene | 12,000 | 42 |
| Tetrachloroethene | 4,500 | 7.3 |
| Toluene | 8,200 | <10+ |
| Trichloroethene | 580 | <5+ |
| Xylenes, Total | 34,000 | 110 |
Value less than detection limit.
As would be expected, the mass of the volatile compounds present in the immiscible liquid was significantly lower after the stripping event. This resulted in an increase in the density of the immiscible liquid (ρpre = 0.939 g/cm3 vs. ρpost = 0.983 g/cm3), as well as a change in the immiscible-liquid composition. As shown in Table 2, almost all of the mass comprising the C6–C10 carbon profile group was removed. Concomitantly, the post-stripping concentrations for the C10–C22 and C22–C32 carbon profile groups increased. As the most volatile compounds were removed, the larger carbon profile groups accounted for a larger portion of the immiscible-liquid mass. These results are as expected and are consistent with what has been observed in prior work (e.g., Kayano and Wilson, 1992).
It is interesting to point out that, although volume limitations prevented quantitative viscosity measurements, a noticeable change in the immiscible-liquid viscosity was observed upon visual inspection of the Picillo Farm site immiscible liquid following the air-stripping events. Specifically, it was observed that not only had the viscosity of the immiscible liquid significantly increased but the immiscible liquid appeared to exist as two distinct phases, a liquid phase as well as a semi-solid phase. Many of the constituents of the Picillo Farm immiscible liquid are solid compounds in their pure states. As compounds are removed from an immiscible liquid containing such compounds, phase transformations may occur and eventual solidification of the immiscible liquid may result (Peters et al., 1997; Peters et al., 2000).
Target components present in the Picillo Farm site immiscible liquid were selected to be monitored as a function of gas venting for the dynamic flow experiments. Based on batch experiment results, the target compounds selected were trichloroethene (TCE), tetrachloroethene (PCE), toluene, ethylbenzene (EB), and 1,2-dichlorobenzene (1,2-DCB). The selected compounds represent three primary chemical classes (i.e., chlorinated aliphatics, chlorinated aromatics, alkyl aromatics) and major contaminants of concern (i.e., chlorinated solvents, petroleum hydrocarbons). Values for molecular weight, density, and vapor pressure as well as calculated saturated vapor-phase concentrations for each target compound are reported in Table 1.
The calculated mole fractions and predicted vapor-phase concentrations for the target compounds, estimated using Raoult’s law, are reported in Table 4. Mole fractions of the target compounds were calculated using compositional data determined by GC/FID and GC/MS analysis of the immiscible liquid prior to air stripping (reported in Tables 2 and 3, respectively), including the estimated molecular weights selected to represent the carbon profile groups. These results yielded an average molecular weight for the Picillo Farm immiscible liquid equal to 211 ± 10 g/mol. The calculated mole fractions were then used to predict the equilibrium vapor-phase concentration of each target compound using Raoult’s law. As shown in Table 4, the vapor-phase concentration values determined from the batch experiment are similar to the predicted vapor-phase concentration values. These results indicate that Raoult’s law provided representative equilibrium vapor-phase concentrations for the selected target compounds. This suggests that the Picillo Farm site immiscible liquid behaved ideally with respect to immiscible-liquid/vapor partitioning.
Table 4.
Target compound mole fractions, predicted vapor-phased concentrations, and measured vapor-phase concentrations (mg/L).
| Chemical | Mole Fraction |
Predicteda | Batch | Immiscible Liquid- Free-phase Columnsb |
Immiscible Liquid- Residual Column |
|---|---|---|---|---|---|
| Trichloroethene | 0.001 | 0.53 ± 0.08 | 0.43 ± 0.06 | 0.48 ± 0.09 | 0.45 ± 0.09 |
| Toluene | 0.018 | 2.57 ± 0.39 | 2.50 ± 1.05 | 2.26 ± 0.44 | 2.89 ± 0.30 |
| Tetrachloroethene | 0.006 | 0.99 ± 0.15 | 0.97 ± 0.32 | 0.81 ± 0.16 | 1.03 ± 0.25 |
| Ethylbenzene | 0.021 | 1.16 ± 0.17 | 1.44 ± 0.43 | 1.17 ± 0.21 | 1.50 ± 0.21 |
| 1,2-Dichlorobenzene | 0.169 | 1.85 ± 0.28 | 1.43 ± 0.45 | 0.77 ± 0.28 | 1.22 ± 0.39 |
Based on Raoult’s law. Values were determined using estimated mole fractions.
The concentration values represent the mean of the steady-state initial elution concentration values of all three column experiments.
3.2 Column Experiments
Three sets of column experiments, in which the column contained Picillo Farm site immiscible liquid and air, were conducted to examine the mass-transfer dynamics of the immiscible-liquid target components. The initial elution behavior of all target compounds was similar for the three experiments (results for Experiments 1 and 2 are presented in Figures 1 and 2, respectively, and results for Experiment 3 are not shown), demonstrating method reproducibility. Inspection of Figure 1 reveals that, as expected, the initial maximum effluent concentrations are significantly lower than the single-compound saturated vapor-phase concentrations. A comparison of the initial concentration values for each of the target compounds to the batch equilibrium concentrations and to the predicted values determined using Raoult’s law is presented in Table 4. It is observed that the initial steady-state elution concentrations, batch equilibrium concentrations, and predicted concentrations are generally equivalent within a 95% confidence interval. Given this equivalence, it appears that the initial elution behavior of these target components is ideal, and that Raoult’s Law provides a reasonable description of partitioning behavior.
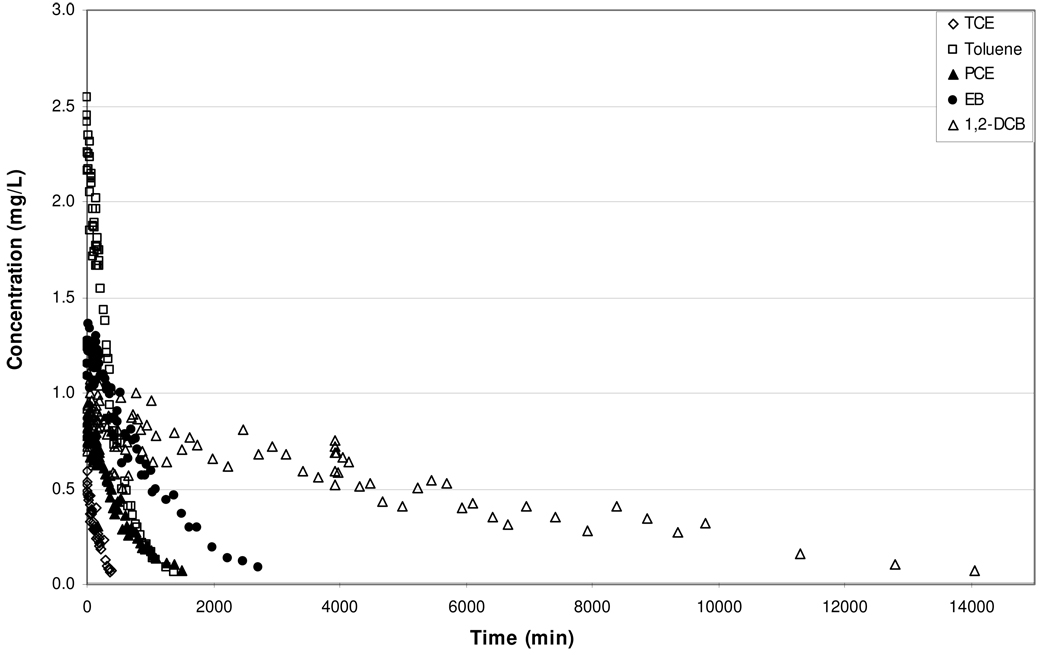
Figure 1.
Elution curves for trichloroethene, toluene, tetrachloroethene, ethylbenzene, and 1,2-dichlorobenzene obtained for a free-phase immiscible liquid system (Experiment 1).
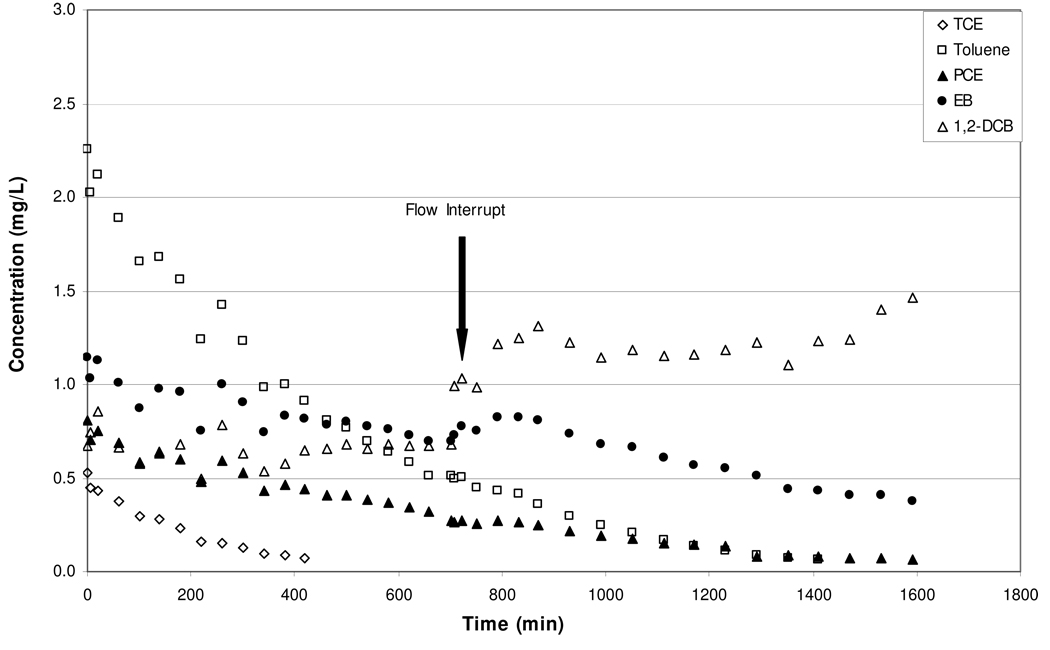
Figure 2.
Elution curves for trichloroethene, toluene, tetrachloroethene, ethylbenzene, and 1,2-dichlorobenzene obtained for a free-phase immiscible liquid system with a two-week flow interrupt at 700 minutes (Experiment 2).
For Experiment 1, the vapor-phase elution concentrations for all five target compounds reached below detection during the course of the experiment (Figure 1). The mass of each target compound removed during the course of the experiment was determined by moment analysis. The masses remaining in the column at the end of Experiment 1 were then calculated by mass balance. The results showed that trichloroethene was completely removed, while approximately 85% (±10%) of the initial masses of toluene, tetrachloroethene, and ethylbenzene were removed. Although there is some uncertainty associated with the mass-removal analysis, the low elution concentrations observed for trichloroethene, toluene, tetrachloroethene, and ethylbenzene are consistent with the low mass/mole fractions remaining in the column at the end of the experiment. Conversely, the mass-removal analysis showed that approximately 65% of the 1,2-dichlorobenzene remained in the column at the end of Experiment 1, whereas the vapor-phase elution concentration of 1,2-dichlorobenzene was below detection. This suggests that as air-stripping of the immiscible liquid progressed, the evaporation of 1,2-dichlorobenzene became rate limited.
To further evaluate the possibility that evaporation of 1,2-dichlorobenzene was rate limited, the measured and expected final vapor-phase elution concentrations were examined. According to the moment analysis, 203 (±20) mg of 1,2-dichlorobenzene were removed during air-stripping of the immiscible liquid in Experiment 1, leaving 385 (±38) mg of 1,2-dichlorobenzene in the immiscible liquid at the completion of the experiment. Using this mass, the mole fraction of 1,2-dichlorobenzene at the end of the experiment was estimated to be 0.228. Assuming ideal, equilibrium conditions, the concentration of 1,2-dichlorobenzene in the vapor-phase should have been 2.4 (±0.24) mg/L at the end of the experiment. However, the concentration of 1,2-dichlorobenzene at the end of Experiment 1 was below detection (0.02 mg/L). This difference, approximately two orders-of-magnitude, supports the hypothesis that 1,2-dichlorobenzene mass transfer was rate limited.
As mentioned above, during Experiment 1, nitrogen was flushed continuously through the column until 1,2-dichlorobenzene vapor-phase concentrations were below detection. For Experiment 2, nitrogen flushing was stopped at approximately 700 minutes (approximately 3% mass removal for 1,2-dichlorobenzene), and no-flow conditions were maintained for approximately two weeks (see Figure 2). Following this no-flow period, nitrogen was again flushed through the system. The 1,2-dichlorobenzene vapor-phase concentrations increased after flow was resumed, from approximately 0.7 (±0.07) mg/L to 1.2 (±0.1) mg/L. The post-interrupt rebound concentrations are larger than those measured during the equivalent elution period of Experiment 1 (0.73 ±0.14 mg/L). These results are indicative of rate-limited mass transfer. In comparison to 1,2-dichlorobenzene, a much smaller rebound was observed for ethylbenzene, and no concentration perturbations were observed for the other target compounds. This suggests that the other target compounds were not influenced significantly by mass-transfer constraints at the point of flow interruption.
An analysis of the post flow-interruption vapor-phase concentrations of 1,2-dichlorobenzene was conducted for Experiment 2, similar to the analysis of final effluent concentrations conducted above for Experiment 1. The results show that the post-interrupt rebound concentrations were similar to the equilibrium value predicted using Raoult’s law. This suggests evaporation of 1,2-dichlorobenzene was under equilibrium conditions after the flow interrupt.
The results presented above support the hypothesis that evaporation of 1,2-dichlorobenzene from the immiscible liquid became rate limited during the course of the experiments. Given the absence of porous media, potential limiting factors such as air-flow constraints and sorption/desorption were not present for this system. Thus, the observed rate-limited mass transfer may be associated with the development of a non-uniform distribution of 1,2-dichlorobenzene within the immiscible liquid, and associated slow diffusion of 1,2-dichlorobenzene through the immiscible liquid to the immiscible-liquid/vapor interface. In addition, viscosity changes, solidification effects, and skin formation may have influenced the immiscible-liquid/vapor mass transfer behavior of 1,2-dichlorobenezene during the later phase of Experiment 1. As discussed previously, visual inspection of the immiscible liquid following the air-stripping event indicated a noticeable change in the immiscible-liquid viscosity, and the formation of a separate semirigid, nearly solid, phase. Similar behavior for aged immiscible-liquid mixtures have been reported in the literature (e.g., Southworth et al., 1983; Luthy et al., 1993; Peters et al., 1997; Peters et al., 2000). For example, Southworth et al. (1983) reported mass-transfer rate coefficients to be significantly dependent on immiscible-liquid viscosity. Luthy et al. (1993) observed mass-transfer limitations associated with the aging of coal tar as a semirigid film formed on the surface of the immiscible liquid.
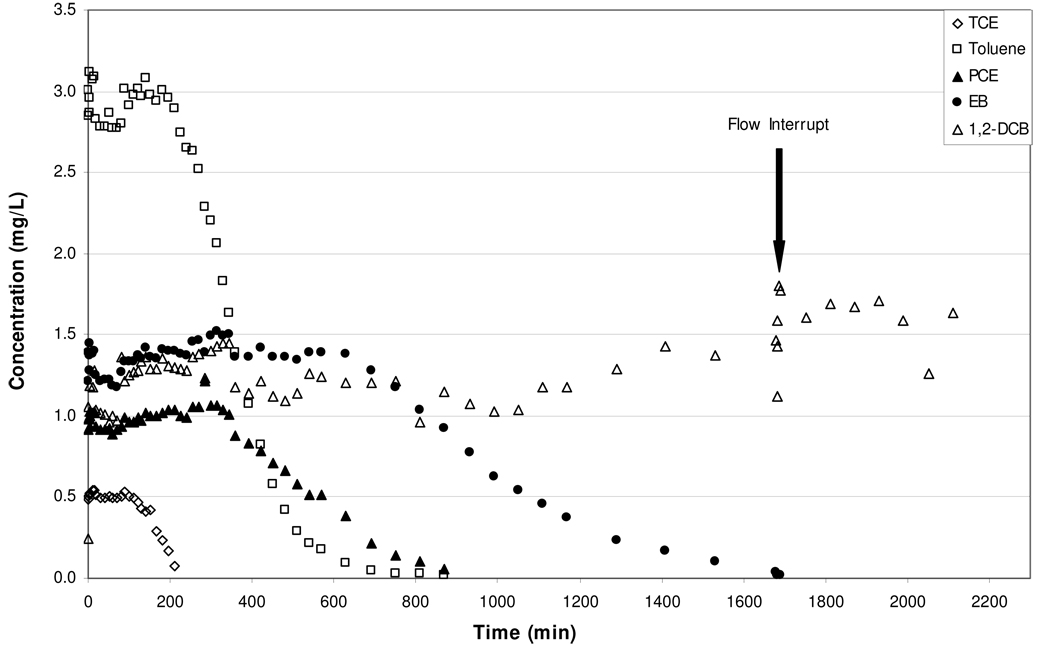
A column experiment was also conducted for a system with residual-phase (SN = 8.6%) immiscible-liquid contamination. For this experiment (#4), the column was flushed with nitrogen for 28 hours, flow was interrupted for approximately 3 days, and then flushing of the immiscible liquid continued for another 7 hours. Inspection of the porous medium following the experiment showed that the immiscible-liquid distribution was relatively uniform. The elution curves for trichloroethene, toluene, tetrachloroethene, ethylbenzene, and 1,2-dichlorobenzene for Experiment 4 are presented in Figure 3. As was previously demonstrated in Experiments 1, 2, and 3, the initial steady state elution concentrations for the residual immiscible liquid experiment are equivalent to the batch vapor-phase concentrations and to the predicted equilibrium concentrations determined using Raoult’s law (see Table 4). Minimal impact of the flow interruption was observed, indicating minimal constraints on mass transfer. The contrasting behavior observed between this experiment and the ones conducted with free-phase immiscible liquid with respect to 1,2-dichlorobenzene mass transfer may be related to the respective immiscible-liquid configurations employed. For the experiment conducted with a porous-medium packed column (experiment 4), the immiscible liquid was distributed relatively uniformly within the porous medium as ganglia at a relatively low saturation. This distribution would result in a large interfacial area and relatively uniform vapor flow, thereby allowing for significant mass transfer of components to the vapor phase. In contrast, the interfacial area for the free-phase experiments was significantly smaller. It should be noted that mass removal for 1,2-dichlorobenzene was approximately 16% for experiment 4, and thus mass-transfer behavior at later stages of mass removal was not evaluated for 1,2-dichlorobenzene.
Figure 3.
Elution curves for trichloroethene, toluene, tetrachloroethene, ethylbenzene, and 1,2-dichlorobenzene obtained for a residual-phase immiscible liquid system (Experiment 4).
4. SUMMARY
A series of laboratory experiments was conducted with a multiple-component immiscible liquid collected from the Picillo Farm Superfund Site, Coventry, Rhode Island. The Picillo Farm site immiscible liquid is a complex mixture of chemicals including solvents, oils, and pesticides. Few analyses of such complex mixtures of chemicals with respect to immiscible-liquid phase ideality and immiscible-liquid/vapor mass transfer have been reported. Column experiments were conducted to examine the elution behavior and mass-transfer dynamics of the target compounds. Initial elution behavior was shown to generally be ideal for all column experiments, as the initial vapor-phase concentrations were similar to batch-equilibrium concentrations and to predicted equilibrium vapor-phase concentrations calculated using Raoult’s Law. A system with free-phase immiscible liquid was represented for the first set of column experiments. For this system, 1,2-dichlorobenzene evaporation was shown to be rate limited. A system with residual¬phase immiscible liquid was also examined. For this system, elution behavior was shown to be ideal. Overall, Raoult’s law was shown to provide reasonable estimates of vapor concentrations for this complex immiscible-liquid mixture.
ACKNOWLEDGMENTS
We thank the U. S. Army Corps of Engineers and The National Institute of Environmental Health Sciences Superfund Basic Research Program (ES04940) for their financial support of this project. We also thank Dominic DiGuilio and Asami Murao for their assistance, and the reviewers for their helpful comments.
REFERENCES
- Abriola LM, Bradford SA, Lang J, Gaither CL. Volatilization of binary nonaqueous phase liquid mixtures in unsaturated porous media. Vadose Zone J. 2004;3:645–655. [Google Scholar]
- Armstrong JE, Frind EO, McClellan RD. Nonequilibrium mass transfer between the vapor, aqueous, and solid phases in unsaturated soils during vapor extraction. Water Resour. Res. 1994;30:355–368. [Google Scholar]
- Baehr AL, Hoag GE, Marley MC. Removing volatile contaminants from the unsaturated zone by inducing advective air-phase transport. J. Contam. Hydrol. 1989;4:1–26. [Google Scholar]
- Cline PV, Delfino JJ, Rao PSC. Partitioning of aromatic constituents into water from gasoline and other complex solvent mixtures. Environ. Sci. Technol. 1991;25:914–920. [Google Scholar]
- El-Beshry MZ, Gierke JS, Bedient PB. Practical modeling of SVE performance at a jet-fuel spill site. J. Environ. Eng. 2001 July;:630–638. [Google Scholar]
- Farhan S, Budiman J, Holsen TM. Experimental investigation of the interaction of soil air permeability and soil vapor extraction. J. Environ. Eng. 2002 February;:120–130. [Google Scholar]
- Harper BM, Stiver WH, Zytner RG. Nonequilibrium nonaqeuous phase liquid mass transfer model for soil vapor extraction systems. J. Environ. Eng. 2003;129:745–754. [Google Scholar]
- Hayden NJ, Voice TC, Annable MD, Wallace RB. Change in gasoline constituent mass-transfer during soil venting. J. Environ. Eng. 1994;120:1598–1614. [Google Scholar]
- Ho CK, Udell KS. An experimental investigation of air venting of volatile liquid hydrocarbon mixtures from homogeneous and heterogeneous porous media. J. Contam. Hydrol. 1992;11:291–316. [Google Scholar]
- Ho CK, Liu SW, Udell KS. Propagation of evaporation and condensation fronts during multicomponent soil vapor extraction. J. Contam. Hydrol. 1994;16:381–401. [Google Scholar]
- Kayano S, Wilson DJ. Soil clean up by in-situ aeration. X. Vapor stripping of mixtures of volatile organics obeying Raoult’s law. Sep. Sci. Technol. 1992;27:1525–1554. [Google Scholar]
- Lee LS, Hagwall M, Delfino JJ, Rao PSC. Partitioning of polycyclic aromatic hydrocarbons from diesel fuel into water. Environ. Sci. Technol. 1992a;26:2104–2110. [Google Scholar]
- Lee LS, Rao PSC, Okuda I. Equilibrium partitioning of polycyclic aromatic hydrocarbons from coal tar into water. Environ. Sci. Technol. 1992b;26:2110–2115. [Google Scholar]
- Lingineni S, Dhir VK. Controlling transport processes during NAPL removal by soil venting. Adv. Water Resour. 1996;20:157–169. [Google Scholar]
- Luthy RG, Ramaswami A, Ghoshal S, Merkel W. Interfacial films in coal tar nonaqueous-phase liquid-water systems. Environ. Sci. Technol. 1993;27:2914–2918. doi: 10.1021/es00053a600. [DOI] [PubMed] [Google Scholar]
- Nadim F, Nadim A, Haog GE, Dahmani AM. Desorption rate limitation in the extraction of organic molecules from unsaturated soils during soil venting operations. J. Contam. Hydrol. 1997;25:21–37. [Google Scholar]
- Peters CA, Mukherji S, Knightes CD, Weber WJ., Jr Phase stability of multicomponent NAPLs containing PAHs. Environ. Sci. Technol. 1997;37:2540–2546. [Google Scholar]
- Peters CA, Wammer KH, Knightes CD. Multicomponent NAPL solidification thermodynamics. Transport Porous Media. 2000;38:57–77. [Google Scholar]
- Popovicova J, Brusseau ML. Dispersion and transport of gas-phase contaminants in dry porous media: effect of heterogeneity and gas velocity. J. Contam. Hydrol. 1997;28:157–169. [Google Scholar]
- Popovicova J, Brusseau ML. Contaminant mass-transfer during gas-phase transport in unsaturated porous media. Water Resour. Res. 1998;34:83–92. [Google Scholar]
- Rathfelder K, Yeh WWG, Mackay D. Mathematical simulation of soil vapor extraction systems: model development and numerical examples. J. Contam. Hydrol. 1991;8:263–297. [Google Scholar]
- Rathfelder KM, Lang JR, Abriola LM. A numerical model (MISER) for the simulation of coupled physical, chemical and biological processes in soil vapor extraction and bioventing systems. J. Contam. Hydrol. 2000;43:239–270. [Google Scholar]
- Schaefer CE, Unger DR, Kosson DS. Partitioning of hydrophobic contaminants in the vadose zone in the presence of a nonaqueous phase. Water Resour. Res. 1998;34:2529–2537. [Google Scholar]
- Schaefer CE, Arands RR, Kosson DS. Measurement of pore connectivity to describe diffusion through a non aqueous phase in unsaturated soils. J. Contam. Hydrol. 1999a;40:222–238. [Google Scholar]
- Schaefer CE, Roberts PV, Blunt MJ. Measurement and prediction of effective diffusivities through spreading and nonspreading oils in unsaturated porous media. Environ. Sci. Technol. 1999b;33:2879–2884. [Google Scholar]
- Schroth MH, Ahearn SJ, Selker JS, Istok JD. Characterization of Miller-similar silica sands for laboratory hydrologic studies. Soil Sci. Soc. Am. 1996;60:1331–1339. [Google Scholar]
- Southworth GR, Herbes SE, Allen CP. Evaluating a mass transfer model for the dissolution of organics from oil films into water. Water Res. 1983;17:1647–1651. [Google Scholar]
- Wilkins MD, Abriola LM, Pennell KD. An experimental investigation of rate-limited nonaqueous phase liquid volatilization in unsaturated porous media: steady state mass transfer. Water Resour. Res. 1995;31:2159–2172. [Google Scholar]
- Yoon H, Kim JH, Liljestrand HM, Khim J. Effect of water content on transient nonequilibrium NAPL-gas mass-transfer during soil vapor extraction. J. Contam. Hydrol. 2002;54:1–18. doi: 10.1016/s0169-7722(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Zaidel J, Zazovsky A. Theoretical study of multicomponent soil vapor extraction: propagation of evaporation-condensation fronts. J. Contam. Hydrol. 1999;37:225–268. [Google Scholar]