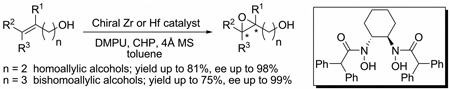
Abstract
In this report, zirconium(IV) and hafnium(IV)-bishydroxamic acid complexes were utilized in the highly enantioselective epoxidation of homoallylic alcohols and bishomoallylic alcohols, which used to be quite difficult substrates for other types of asymmetric epoxidation reactions. The performance of the catalyst was improved by adding polar additive and molecular sieves. For homoallylic alcohols, the reaction could provide epoxy alcohols in up to 81% yield and up to 98% ee, while for bishomoallylic alcohols, up to 75% yield and 99% ee of epoxy alcohols rather than cyclize compounds could be obtained in most cases.
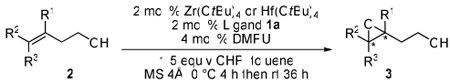
Despite the tremendous success of asymmetric epoxidations,1 more efficient epoxidation reactions are still of great importance. For example, there are only sporadic reports of catalytic enantioselective epoxidation of challenging substrates such as homoallylic alcohols and bishomoallylic alcohols to date.2 In recent years, our group has developed highly efficient vanadium-hydroxamic acid and bishydroxamic acid (BHA) catalyst systems for the asymmetric epoxidation of allylic alcohols and homoallylic alcohols.3 However, asymmetric epoxidation of bishomoallylic alcohols is an even more challenging problem because the longer carbon chain is much more flexible in the transition state.4 Although syntheses of epoxides, tetrahydrofuran (THF) and tetrahydropyran (THP) derivatives via epoxidation of bishomoallylic alcohols are well established using either achiral metal catalyst5 or Shi’s chiral-ketone-catalyzed epoxidation procedures,2f,6 a highly efficient metal-catalyzed enantioselective direct epoxidation is still missing from the list. Herein, we would like to report a zirconium(IV) and hafnium(IV)-catalyzed highly enantioselective epoxidation of homoallylic alcohols and bishomoallylic alcohols (Scheme 1).
Scheme 1.
Zr(IV) and Hf(IV)-BHA Catalyzed Asymmetric Epoxidation of Homoallylic and Bishomoallylic Alcohols
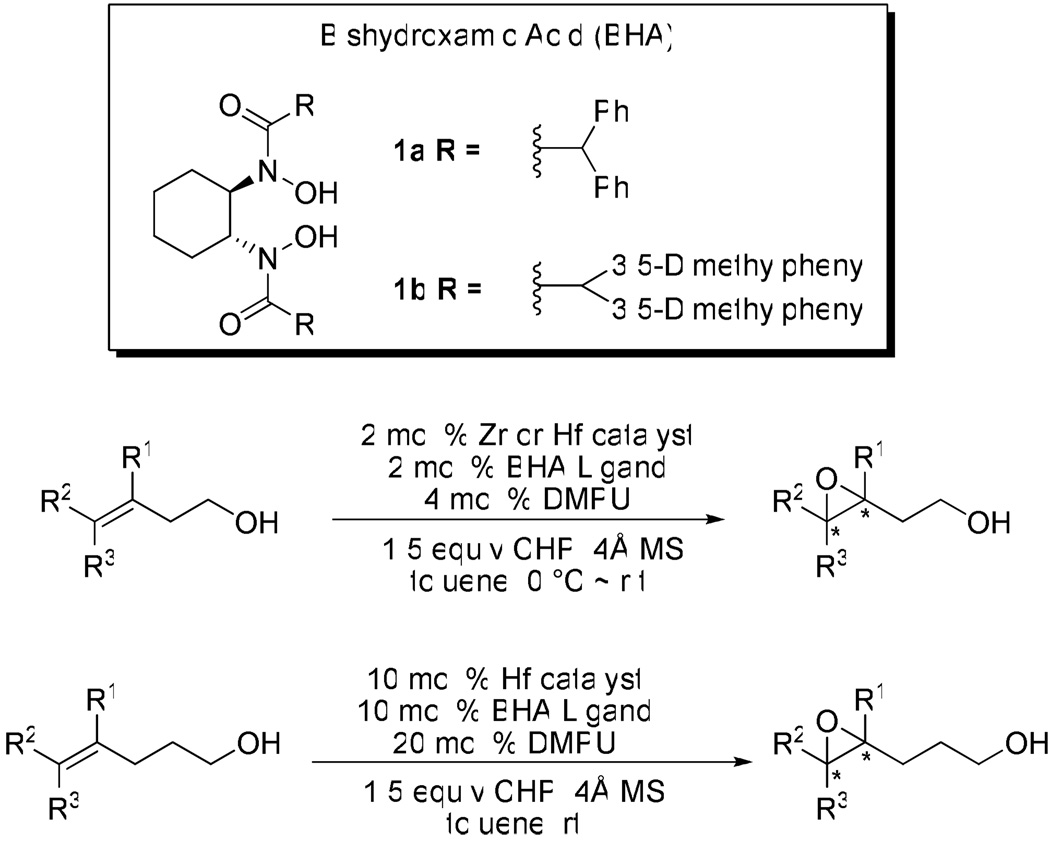
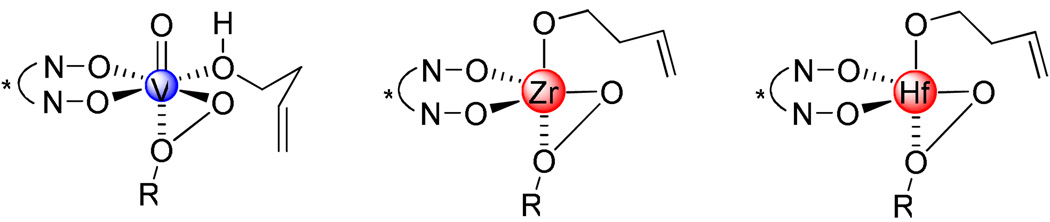
Since we depleted the catalytic ability of vanadium-BHA system with a very bulky BHA ligand,3d,3e we began to consider solving the problem using another approach. For example, we postulated that using a larger center ion would provide more space around it so that even a smaller ligand could arrange a proper cavity for stereoselective recognition of the substrate and oxidant molecules. There are many transition metals reported to efficiently catalyze epoxidation of alkenyl alcohols, especially group 4 and 5 metals such as Ti, Zr and V.7 Additionally, as a strong Lewis acid,8 Zr(IV) has been applied in asymmetric epoxidation of homoallylic alcohols.2b,2e Enlightened by these pioneering work, we therefore chose Ti, Zr, Hf, V and Nb as candidate metal species for our initial experiments. As we examined the candidate metal sources, Zr(OtBu)4 and Hf(OtBu)4 proved to be superior compared to other metals such as Ti(OiPr)4, Nb(OEt)5 and VO(acac)2 in terms of both reactivity and enantioselectivity. An explanation is presented in Scheme 2. In vanadium-catalyzed reactions, the transition state is believed to involve hexa-coordinate vanadium species;3d while in zirconium and hafnium-catalyzed reactions, the transition state should be a mono-nuclear penta-coordinate complex7,9 because 1) the ligand binds with the metal using two hydroxamate oxygens and the size of the ligand prevents catalyst oligomerization;10 2) the substrate binds with the metal using one alkoxide; 3) the alkylhydroperoxide binds with the metal as a bidentate ligand using both oxygens of the peroxy group as ligand donors.9 Non-linearity experiments showed linear relationship between enantiomer excess of ligand and product, which supported our hypothesis.
Scheme 2.
Comparison of V, Zr and Hf-Catalyzed Reactions
We then started conditions optimization with the Zr(IV)-BHA system (Table 1). Compared with the V(V)-BHA system, Zr-BHA gave much higher selectivity (entries 1 and 2). The simpler ligand 1a showed the best reactivity and selectivity (entries 2 and 3). A 1:1 ratio of metal to ligand was crucial, while two equivalents of ligand completely inactivated the catalyst (entry 4).11 and Zr(acac)4 did not work at all as the metal source (entry 5). Since using toluene and dichloromethane as solvent both gave similar level of enantioselectivity, we chose to use toluene because it is more environmental friendly. Interestingly, we also found that the results were significantly improved when a polar aprotic ligand such as 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) was added (entry 7). The additive may serve two functions in the system. First, as seen in Scheme 2, there are vacant coordination sites on Zr and Hf metal during the reaction. These vacant sites could possibly facilitate a slow but significant oligomerization of Zr and Hf species by bridging two or more metals with primary alkoxide anion generated from the substrate or product. Adding DMPU could help the catalyst stay in its monomeric form by occupying these vacant sites on the metals as well as regeneration of the monomeric catalytic species.8b,12 Second, the nucleophilic oxygen of DMPU may help release the coordinated product from the metal and complete the catalytic cycle. Final screening showed Hf(IV) gave more promising results than Zr(IV) as the center metal ion (entry 8). Furthermore, addition of molecular sieves (MS) also greatly improved the results, especially the 4Å powdered molecular sieves (entry 9).13
Table 1.
Screening of Reaction Conditions
 | ||||||
|---|---|---|---|---|---|---|
| entrya | metal | ligand | additive | solvent | %yieldb | %eec |
| 1 | VO(OiPr)3 | 1ad | - | toluene | 29 | 69 |
| 2 | Zr(OtBu)4 | 1a | - | toluene | 25 | 90 |
| 3 | Zr(OtBu)4 | 1b | - | toluene | 28 | 71 |
| 4 | Zr(OtBu)4 | 1ae | - | toluene | N.R. | - |
| 5 | Zr(acac)4 | 1a | - | toluene | N.R. | - |
| 6 | Zr(OtBu)4 | 1a | - | CH2Cl2 | 48 | 90 |
| 7 | Zr(OtBu)4 | 1a | DMPU | toluene | 56 | 90 |
| 8 | Hf(OtBu)4 | 1a | DMPU | toluene | 60 | 95 |
| 9f | Hf(OtBu)4 | 1a | DMPU MS 4Å |
toluene | 81 | 97 |
All reactions were performed in the presence of 1.0 equiv of cumene hydroperoxide (CHP) (1 mmol) unless otherwise noted.
Isolated yield after chromatographic purification.
Enantiomeric excess values were determined by chiral gas chromatography.
1.5 mol % ligand was used.
2.0 mol % ligand was used.
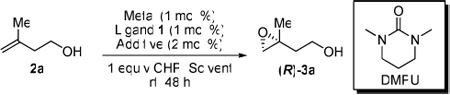
Conditions: 2 mol % metal, 2 mol % ligand, 4 mol % DMPU, 1.5 equiv of CHP, and 100 mg MS were used in a 0.5 mmol scale reaction. The reaction is initially cooled to 0 °C for 4 h then left at room temperature for the next 36 h.
A variety of homoallylic alcohols with different substituted patterns were subjected to the optimum epoxidation conditions of both Zr(IV) and Hf(IV). The results are summarized in Table 2. In general, Hf is better than Zr in terms of reactivity and selectivity. 1,1-disubstituted (entries 1, 3 and 5) and (Z)-substituted substrates (entries 7 and 8) provide higher reactivities and selectivities. It is interesting to note that these two substitute patterns lead to completely reversed enantioface selection as shown in Table 2. Entries 1 and 2 indicated that without (Z)-substituent, the epoxidation gave (3R)-product predominately; while entries 7 and 8 showed (Z)-substituted substrates mainly provided (3S,4R)-products.
Table 2.
Zr(IV) and Hf(IV)-Catalyzed Asymmetric Epoxidation of Homoallylic Alcohols
 | |||||
|---|---|---|---|---|---|
| entrya | product | metal | %yieldb | %ee c | |
| 1 | 3a | Zr | 61 | 91 | |
| Hf | 81 | 97 | |||
| 2 | 3b | Hf | 37 | 63 | |
| 3 | 3c | Zr | 67 | 92 | |
| Hf | 69 | 98 | |||
| 4 | 3d | Zr | 67 | 63 | |
| Hf | 70 | 71 | |||
| 5 | 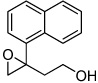 |
3e | Hf | 31d | 91 |
| 6 | 3f | Hf | 47 | 73 | |
| 7 | 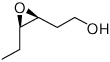 |
3g | Zr | 45 | 93 |
| Hf | 82 | 94 | |||
| 8 | 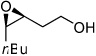 |
3h | Zr | 80 | 92 |
| Hf | 83 | 96 | |||
| 9 | 3i | Hf | 41 | 71 | |
| 10 | 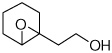 |
3j | Zr | 72 | 76 |
| Hf | 81 | 89 | |||
All reactions were performed in the presence of 1.5 equiv of CHP.
Isolated yield after chromatographic purification.
Enantiomeric excess values were determined by chiral GC and chiral HPLC.
61% of 2e was recovered.
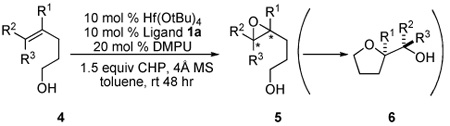
The reactivity of epoxidation of bishomoallylic alcohols was lower as the length of carbon chain was increased, requiring 10 mol % of catalyst in most cases. The best substrates for this reaction are 1,1-disubstituted olefins bearing an aromatic group as shown in Table 3. We anticipated that making the δ-carbon a benzylic position would accelerate the THF forming process; however, in most cases, the δ-aromatic group actually suppressed the THF formation in that we were able to isolate a high yield of corresponding epoxides. Several bishomoallylic alcohols bearing δ-aromatic group were subjected to the optimized conditions and they underwent smooth reactions. Electron-deficient substrates such as 4b, 4c and 4d provide corresponding epoxides efficiently (entries 2, 3 and 4). Electron-rich but sterically hindered substrate 4e gave epoxide in high ee but low reactivity, presumably due to the size of the ortho-methoxy group which is very close to the double bond (entry 5). Substrate 4g which contains a para-methoxyphenyl substituent was converted to THF products with lower ee presumably because the para-methoxy effect stabilizes the benzilic cation therefore facilitates the SN1 type racemizing cyclization. (Z)-substituted substrates such as 4h also exhibited high enantioselectivity although the reactivity was much lower. THF formation was also exclusively observed in this reaction.
Table 3.
Zr(IV) and Hf(IV)-Catalyzed Asymmetric Epoxidation of Bishomoallylic Alcohols
 | ||||
|---|---|---|---|---|
| entrya | product | %yieldb | %eec | |
| 1 | 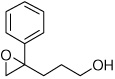 |
5a | 75 | 99 |
| 2 | 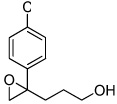 |
5b | 57 | 98 |
| 3 | 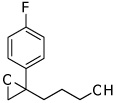 |
5c | 73 | 97 |
| 4 | 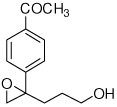 |
5d | 79 | 97 |
| 5 | 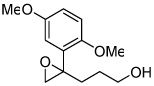 |
5e | 25d | 97 |
| 6 | 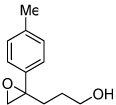 |
5f | 53 | 99 |
| 7 | 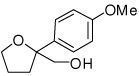 |
6g | 47 | 59 |
| 8 | 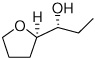 |
6h | 41 | 95e |
All reactions were performed in the presence of 1.5 equiv of CHP.
Isolated yield after chromatography purification.
Enantiomeric excess values were determined by chiral HPLC.
42% of 4e was recovered.
30 mol % catalyst loading was used and the reaction was stirred for 72 hr. 59% starting material 4h was recovered. Absolute configuration is determined as (R, R). See footnote 14.
In conclusion, we discovered that in combination with BHA ligands, Zr(IV) and Hf(IV) are able to catalyze highly enantioselective epoxidation of homoallylic alcohols and bishomoallylic alcohols in a very efficient manner. Further efforts are being employed to investigate the reaction mechanism and potential application in asymmetric synthesis.
Supplementary Material
Acknowledgment
National Institutes of Health (NIH) is greatly appreciated for providing financial support (GM068433-05).
Footnotes
Supporting Information Available: Representative experimental procedures and necessary characterization data for all compounds are provided. Results of non-linear effect experiments are provided. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.For recent reviews, see: Katsuki T, Martin VS. Org. React. 1996;48:1. Katsuki T. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Berlin: Springer; 1999. p. 621. Keith JM, Larrow JF, Jacobsen EN. Adv. Synth. Catal. 2001;343:5. Chatterjee D. Coord. Chem. Rev. 2008;252 Díez D, Núñez MG, Antón AB, García P, Moro RF, Garrido NM, Marcos IS, Basabe P, Urones JG. Curr. Org. Synth. 2008;5:186. Matsumoto K, Sawada Y, Katsuki T. Pure Appl. Chem. 2008;80:1071. Wong OA, Shi Y. Chem. Rev. 2008;108:3958. doi: 10.1021/cr068367v.
- 2.Ti- and Zr-catalyzed: Rossiter BE, Sharpless KB. J. Org. Chem. 1984;49:3707. Ikegami S, Katsuki T, Yamaguchi M. Chem. Lett. 1987:83. Hosokawa T, Kono T, Shinohara T, Murahashi S-I. J. Organometal. Chem. 1989;370:C13. Karjalainen JK, Hormi OEO, Sherrington DC. Tetrahedron: Asymmetry. 1998;9:3895. Okachi T, Murai N, Onaka M. Org. Lett. 2003;5:85. doi: 10.1021/ol027261t. Organocatalytic: Wang Z-X, Shi Y. J. Org. Chem. 1998;63:3099. doi: 10.1021/jo980604+. Shi Y. Acc. Chem. Res. 2004;37:488. doi: 10.1021/ar030063x. Wang B, Wong OA, Zhao M-X, Shi Y. J. Org. Chem. 2009;73:9539. doi: 10.1021/jo801576k.
- 3. Makita N, Hoshino Y, Yamamoto H. Angew. Chem. Int. Ed. 2003;42:941. doi: 10.1002/anie.200390250. Angew. Chem.2003, 115, 971; Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Angew. Chem. Int. Ed. 2005;44:4389. doi: 10.1002/anie.200500938. Angew. Chem.2005, 117, 4463; Barlan AU, Zhang W, Yamamoto H. Tetrahedron. 2007;63:6075. doi: 10.1016/j.tet.2007.03.071. Zhang W, Yamamoto H. J. Am. Chem. Soc. 2007;129:286. doi: 10.1021/ja067495y. Li Z, Zhang W, Yamamoto H. Angew. Chem. Int. Ed. 2008;47:7520. doi: 10.1002/anie.200802523. Angew. Chem.2008, 120, 7630. Some chiral BHA ligands such as 1a, 1b and those described in reference 3d have been commercialized and readily available from Sigma-Aldrich Co. and Tokyo Chemical Industry.
- 4.Mihelich ED, Daniels K, Eickhoff DJ. J. Am. Chem. Soc. 1981;103:7690. [Google Scholar]
- 5.(a) Masamune H, Hayase Y, Schilling W, Chan WK, Bates GS. J. Am. Chem. Soc. 1977;99:6756. doi: 10.1021/ja00462a049. [DOI] [PubMed] [Google Scholar]; (b) Fukuyama T, Vranesic B, Negri DP, Kishi Y. Tetrahedron Lett. 1978;19:2741. [Google Scholar]; (c) Nakata T, Schmidt G, Vranesic B, Okigawa M, Smith-Palmer T, Kishi Y. J. Am. Chem. Soc. 1978;100:2933. [Google Scholar]; (d) Harmange J-C, Figadère B. Tetrahedron: Asymmetry. 1993;4:1711. [Google Scholar]; (e) Hartung J, Drees S, Geiss B, Schmidt P. Synlett. 2003:223. [Google Scholar]; (f) Hartung J, Drees S, Greb M, Schmidt P, Svoboda I, Fuess H, Murso A, Stalke D. Eur. J. Org. Chem. 2003:2388. [Google Scholar]
- 6.(a) Morimoto Y, Iwai T, Kinoshita T. Tetrahedron Lett. 2001;42:6307. [Google Scholar]; (b) Elliott MC. J. Chem. Soc., Perkin Trans. 1. 2002:2301. [Google Scholar]; (c) Vilotijevic I, Jamison TF. Science. 2007;317:1189. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wan S, Gunaydin H, Houk KN, Floreancig PE. J. Am. Chem. Soc. 2007;129:7915. doi: 10.1021/ja0709674. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Byers JA, Jamison TF. J. Am. Chem. Soc. 2009;131:6383. doi: 10.1021/ja9004909. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Morten CJ, Byers JA, Van Dyke AR, Vilotijevic I, Jamison TF. Chem. Soc. Rev. 2009;38:3175. doi: 10.1039/b816697h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Morten CJ, Jamison TF. J. Am. Chem. Soc. 2009;131:6678. doi: 10.1021/ja9025243. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Van Dyke AR, Jamison TF. Angew. Chem. Int. Ed. 2009;48:4430. doi: 10.1002/anie.200900924. Angew. Chem.2009, 121, 4494. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Vilotijevic I, Jamison TF. Angew. Chem. Int. Ed. 2009;48:5250. doi: 10.1002/anie.200900600. Angew. Chem.2009, 121, 5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jøergensen KA. Chem. Rev. 1989;89:431. [Google Scholar]
- 8.(a) Sakurada I, Yamasaki S, Gottlich R, Iida T, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2000;122:1245. [Google Scholar]; (b) Yamasaki S, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2001;123:1256. doi: 10.1021/ja005794w. [DOI] [PubMed] [Google Scholar]
- 9.Lubben TV, Wolczanski PT. J. Am. Chem. Soc. 1987;109:424. [Google Scholar]
- 10.Ti-polymeric aggregates: Martin RL, Winter G. Nature. 1963;197:687. Russo WR, Nelson WH. J. Am. Chem. Soc. 1970;92:1521. evidence for Zr-polymeric species: Bradley DC, Holloway CE. J. Chem. Soc. A. 1968:1316.
- 11.It is probably due to a stable complex Zr(BHA)2 was formed. See: Fouché KF, le Roux HJ, Phillips F. J. Inorg. Nucl. Chem. 1970;32:1949.
- 12.Vogl EM, Gröger H, Shibasaki M. Angew. Chem. Int. Ed. 1999;38:1570. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1570::AID-ANIE1570>3.0.CO;2-Y. Angew. Chem.1999, 111, 1672; [DOI] [PubMed] [Google Scholar]
-
13.
Hanson RM, Sharpless KB. J. Org. Chem. 1986;51:1922.
Gao Y, Klunder JM, Hanson RM, Masamune H, Ko SY, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765. We also examined 3Å and 5Å MS as additive. No significant rate or ee improvement was observed with 3Å MS, while 5Å MS favored the formation of THF compounds.
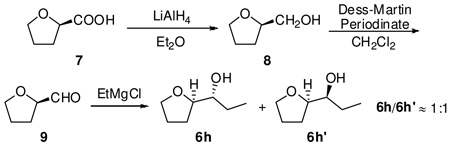
- 14.The absolute configuration of substituted THF was established by the synthesis of diastereomers of products from known chirality compound and comparison of chiral gas chromatography retention times.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.