Abstract
We have analyzed at high resolution the neuroanatomical connections of the juxtaparaventricular region of the lateral hypothalamic area (LHAjp); as a control and comparison to this we also performed a preliminary analysis of a nearby LHA region that is dorsal to the fornix, namely the LHA suprafornical region (LHAs). The connections of these LHA regions were revealed with a coinjection tract-tracing technique involving a retrograde (cholera toxin B subunit) and anterograde (Phaseolus vulgaris leucoagglutinin) tracer. The LHAjp and LHAs together connect with almost every major division of the cerebrum and cerebrospinal trunk, but their connection profiles are markedly different and distinct. In simple terms the connections of the LHAjp indicate a possible primary role in the modulation of defensive behavior; for the LHAs a role in the modulation of ingestive behavior is suggested. However, the relation of the LHAjp and LHAs to potential modulation of these behaviors, as indicated by their neuroanatomical connections, appears to be highly integrative as it includes each of the major functional divisions of the nervous system that together determine behavior, i.e., cognitive, state, sensory, and motor. Furthermore, although a primary role is indicated for each region with respect to a particular mode of behavior, inter-mode modulation of behavior is also indicated. In summary, the extrinsic connections of the LHAjp and LHAs (so far as we have described them) suggest that these regions have a profoundly integrative role in which they may participate in the orchestrated modulation of elaborate behavioral repertoires.
Keywords: Lateral hypothalamic area, Ingestive behavior, Defensive behavior
INTRODUCTION
The periventricular region and adjacent medial zone of the hypothalamus have a fairly well defined cytoarchitecture characterized by circumscribed nuclei whose function is to some extent understood. In comparison, the cytoarchitectural differentiations of the larger lateral zone are more subtle: they form mostly regions rather than nuclei and the connections and functions of these regions are understood with considerably less clarity. Nevertheless, novel findings in recent years have contributed to a rise of interest especially in the lateral hypothalamic area (LHA) segment of the lateral zone. Before reviewing these developments, it is useful to consider first why a clear understanding of the LHA has long remained so elusive.
One of the more apparent features of the LHA is its intricate relationship with the most differentiated fiber system of the central nervous system, the medial forebrain bundle (Nieuwenhuys et al., 1982). In fact, one of the earliest allusions to the LHA as a specific region was made in one of the earliest studies of the medial forebrain bundle, in the mole (Ganser, 1882), where the LHA was referred to as a bed (or interstitial) nucleus of the medial forebrain bundle. Following this pioneering reference to the LHA, the first specific description of an identified lateral hypothalamic area appears to be that given by Franz Nissl for the rabbit (Nissl, 1913). During this early period, a region of the hypothalamus that included the LHA was also referred to as the substantia reticularis hypothalami (Malone, 1910), that is, the rostral extension or end of the reticular formation. The wide variety of LHA neuron morphology that Malone described may have informed his description of the LHA as reticular in nature; in this respect it does appear quite fitting, although (perhaps unfortunately) this nomenclature was not widely adopted. The LHA was subsequently described by Elisha Gurdjian in the rat using classical neuronal staining methods (Golgi for neuron morphology, Nissl for cytoarchitecture, and Weigert for myelinated fibers) (Gurdjian, 1927), and later (also in the rat) with similar methods by Wendell Krieg (Krieg, 1932).
According to Gurdjian, the rat LHA extends caudally from the lateral preoptic area (with which it is continuous) to the rostral aspect of the mammillary body (practically the full rostral-to-caudal extent of the hypothalamus) and consists of a group of characteristic medium- and large-sized neurons, distributed along the course of the medial forebrain bundle, intimately associated with the medial hypothalamic nuclei, and showing no “peculiar arrangement” (Gurdjian, 1927). Gurdjian’s description was supplemented by Krieg who clearly thought of the LHA as an interstitial nucleus that would act as a relay station from which information travelling through the medial forebrain bundle might reach the medial zone nuclei of the hypothalamus (Krieg, 1932). This general understanding of the LHA has been more or less affirmed by a cluster of complementary studies ranging across a wide variety of mammalian species, including the following: human (Le Gros Clark, 1938; Nauta and Haymaker, 1969), monkey (Crouch, 1934; Papez and Aronson, 1934), dog (Rioch, 1929), cat (Rioch, 1929; Ingram et al., 1932; Valverde, 1965), rat (Szentagothai et al., 1968), mouse (Millhouse, 1969), opossum (Warner, 1929), armadillo (Papez, 1932), and wild boar (Solnitzky, 1939) (for further references see (Fulton et al., 1940; Geeraedts et al., 1990).
The existence of a general consensus on the extent of the LHA and its relation to the medial forebrain bundle contrasts with little consensus on how this largest hypothalamic component should be differentiated and subdivided. Historically, a principal reason for this lack of agreement relates to the cytoarchitecture of the LHA: it has a subtle heterogeneity in which regional borders are not easily discernable in common Nissl preparations. Nevertheless, at least one differentiation of the LHA has consistently been identified, and this is a “perifornical region” that, in general terms, is considered to surround the column of the fornix. In this view the perifornical region is defined primarily by the column of the fornix as a fiducial marker, rather than by its cytoarchitecture. Nevertheless, species in which a perifornical region of the LHA has been variously indentified include the following: human (Le Gros Clark, 1938), monkey (Crouch, 1934; Papez and Aronson, 1934), dog (Rioch, 1929), cat (Rioch, 1929; Ingram et al., 1932), rat (Gurdjian, 1927) and wild boar (Solnitzky, 1939); for further references see (Fulton et al., 1940; Geeraedts et al., 1990).
A novel schema for subdividing the LHA in the rat based primarily on a fine-grained reanalysis of the cytoarchitecture was proposed recently (Swanson, 2004; Swanson et al., 2005). At least two developments are noteworthy for providing the impetus for this renewed interest: 1) LHA chemoarchitecture as revealed by the expression of melanin-concentrating hormone and the orexin/hypocretin peptides (Swanson et al., 2005; Hahn, 2009; see also de Lecea et al., 1998; Bittencourt et al., 1992), and 2) The identification of a topographically organized and highly restricted input to the subfornical region of the LHA (LHAsf) originating in the pontine nucleus incertus (Goto et al., 2001). Prior to the developments that led to the present schema for parceling the LHA, a comparably detailed account was suggested (Geeraedts et al., 1990), based on cytoarchitecture and its correspondence with the various components of the medial forebrain bundle (Nieuwenhuys et al., 1982; Veening et al., 1982).
Before the present study, the only systematic analyses of LHA projections with regard to current parcellations (Swanson, 2004) concerned the tuberal nucleus (Canteras et al., 1994), parasubthalamic nucleus (Goto and Swanson, 2004), and LHAsf (Goto et al., 2005), and no study has examined such connections with a co-injection methodology at high spatial resolution (i.e mapped to numerous consecutive atlas levels). Nevertheless, several earlier studies of LHA connections are noteworthy for providing evidence that the LHA is regionally differentiated rather than relatively homogenous (Conrad and Pfaff, 1976b; Saper et al., 1979; Hosoya and Matsushita, 1980; Hosoya and Matsushita, 1981b; Veening et al., 1982; Luiten et al., 1987; Allen and Cechetto, 1992; Roeling et al., 1993a; Deller et al., 1994; Abrahamson and Moore, 2001; Yoshida et al., 2006). In the present study, two nearby regions of the LHA middle group are subjected to a high spatial resolution analysis of their macro connection pattern; as delineated by the new parcellation they are the LHA juxtaparaventricular (LHAjp) region of the medial tier, and LHA suprafornical (LHAs) region of the perifornical tier. The LHAjp was the primary target for analysis, while the nearby LHAs served as a comparison site and control.
Our decision to make the LHAjp the primary focus of this study was prompted largely by its close proximity to a critically important neuroendocrine, autonomic, and behavioral part of the hypothalamus, the paraventricular nucleus (PVH) -- physiologic control of PVH neurons by local populations of neurons lying laterally adjacent to the PVH (within the LHA) has been suggested (Boudaba et al., 1997). In addition, a previous PHAL study provided a rudimentary description of projections from a region of the LHA corresponding approximately to the LHAjp (Roeling et al., 1993b). Determining the connections of the LHAjp and LHAs also allows for comparison with the already published connections of the LHAsf (Goto et al., 2005) -- establishing collectively the connections of three nearby LHA regions that are respectively ventral (LHAsf), medial (LHAjp), and dorsal (LHAs) to the fornix. More generally, a better knowledge of the neural connections of the LHA is a highly desirable goal given the extensive evidence implicating it in control of several fundamental behaviors including: eating and drinking (Mogenson and Stevenson, 1967; Watts et al., 1999), foraging and exploration (Lammers et al., 1988b; Goto et al., 2005), sleep/wake states (Alam et al., 2002), defense and aggression (Lammers et al., 1988a; Lammers et al., 1988b; Hrabovszky et al., 2005), and social behaviors (Lammers et al., 1987; Roeling et al., 1990).
MATERIALS AND METHODS
2.1. Animals and surgical procedures
Combined PHAL + CTB injections were made in 81 adult male Sprague-Dawley rats (supplier Harlan; 300 – 350g). The animals were kept under conditions of controlled temperature (22°C) and illumination (12-hour light cycle) and had access to food and water ad libitum. Animal housing conditions and all experimental procedures were in accordance with current NIH Guidelines for the Care and Use of Laboratory Animals; in addition, all protocols were approved by the University of Southern California Institutional Animal Care and Use Committee. Our method for the central co-injection of PHAL and CTB is the first reported use of this method in the rat, and follows its first application for central tract-tracing studies in the hamster (Coolen and Wood, 1998). The present co-injection method combines earlier separate methods for the central iontophoretic injection of PHAL (Gerfen and Sawchenko, 1984) and CTB (Luppi et al., 1990) in the rat.
For the surgical procedure, animals were anesthetized with an equal mixture (1ml/kg body weight) of ketamine (50mg/ml) and xylazine (10mg/ml), delivered intramuscularly. Under anesthesia they received a single stereotaxically placed iontophoretic injection of a mixture of 2.5% PHAL (Vector Laboratories, Burlingame, CA) and 0.25% CTB (List Biological Laboratories, Campbell, CA) prepared in 0.1M sodium phosphate-buffered saline, pH 7.4. The tracers were ejected from a glass micropipette (12–20 µm tip diameter) by applying a positive current (5 µA, 7 sec on/off intervals) for 5–10 min; the pipette was then left in place for 10 minutes before retraction. From 12 – 20 days later, the animals were anesthetized deeply with sodium pentobarbital (40 mg/kg body weight, intraperitoneal) and perfused transcardially with 150 ml ice cold 0.9% NaCl, followed by 300 ml ice cold 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5).
2.2. Tissue processing and immunocytochemistry
The fixed brains were postfixed for 20 hours at 4°C in 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5) containing 10% sucrose. Serial 30 µm thick transverse-plane sections (one-in-five series) were cut on a sliding microtome. One complete series of sections was processed for immunocytochemical detection of PHAL; another series was processed similarly for detection of CTB; an intervening series was thionin-stained. Except for the series allocated to thionin staining, the sections were collected in an anti-freeze solution and stored at −20°C until further processing. The anti-freeze solution consisted of 30% ethylene glycol and 20% glycerol in 0.02M potassium phosphate buffered saline (KPBS).
Immunocytochemical method for detection of tracers
From anti-freeze solution sections were washed in 0.02M KPBS (6 changes over 2 hours). The series of sections that were allocated for detection of CTB were then treated to suppress endogenous peroxidase activity: they were washed in a solution of 70% methanol containing 0.3% hydrogen peroxide for 15 minutes, and then washed twice with 2 changes of 0.02M KPBS. Sections were treated to block non-specific antibody binding: for PHAL detection, the sections were washed in 0.02 M KPBS containing 0.3% Triton X-100 and 10% non-fat milk (10 minutes); sections for CTB detection were washed in 0.02 M KPBS containing 0.3% Triton X-100 and 2% donkey serum. Following these steps, the sections were transferred to 0.02M KPBS containing primary antibodies raised against either CTB (species: goat, dilution: 1:10,000, supplier: List Biological Laboratories) or PHAL (species: rabbit, dilution: 1:3,000, supplier: Dako, Carpinteria, CA); these solutions also contained either 10% non-fat milk and 0.3% Triton X-100 (for PHAL) or 2% donkey serum and 0.1% Triton X-100 (for CTB). Sections were incubated in primary antibody solution for approximately 60 hours (3 nights); they were placed on a rotating platform and maintained at 4°C.
Following the primary antibody, the sections were washed in 0.02M KPBS (8 changes over 2 hours), and then washed in either 2% goat serum with 0.3% Triton X-100 and (for PHAL) or 2% donkey serum with 0.1% Triton X-100 and (for CTB). The sections were then exposed to a biotinylated secondary antibody (90 min, diluted 1:1000): donkey anti-goat for CTB (Jackson Immunoresearch, West Grove, PA), goat anti-rabbit for PHAL (Vector). The secondary antibody solutions also contained either 2% goat serum and 0.3% Triton X-100 (PHAL sections) or 2% donkey serum and 0.1% Triton X-100 (CTB sections).
Following the secondary antibody (the solution was retained), the sections were washed in 0.02M KPBS (6 changes over 30 minutes) and exposed to an avidin-biotin-horse radish peroxidase complex (ABC reagent, Vector, dilution: 1 drop reagent A and B in 30ml for CTB; 3 drops reagent A and B in 30ml for PHAL) in 0.02M KPBS for 2 hours. The sections were then washed in 0.02M KPBS (5 changes over 20 minutes) and placed again in their respective recycled secondary antibody solutions for about 16 hours (overnight) under the same conditions as for the primary antibody. Following the second secondary antibody exposure, the sections were washed in 0.02M KPBS (6 changes over 30 minutes), and then exposed to freshly prepared ABC reagent for 90 minutes. They were then washed in 0.02M KPBS (6 changes over 30 minutes). In order to visualize CTB and PHAL, the sections were exposed to 0.05% 3, 3’-diaminobenzidine (DAB) with 0.005% hydrogen peroxide in 0.02M KPBS; in order to enhance the visualization of the CTB label, 0.1 % ammonium nickel (II) sulphate was also included in the reaction mixture. The reaction was monitored and reaction times were typically between 10–15 minutes for PHAL and between 4–8 minutes for CTB. The reaction was stopped by successive washes in 0.02M KPBS (8 changes).
The sections were then mounted on to glass slides, air-dried, dehydrated through an ascending series of alcohols, and cleared in xylene. Some sets of sections processed for PHAL were then rehydrated and treated with osmium tetroxide (0.008%, for 60 minutes) in order to enhance visualization of the DAB reaction product under dark field illumination. Following this treatment, the PHAL sections were washed for 2 hours in tap water, again dehydrated and cleared in xylene and then (as was the case for all other mounted sections) treated with DePeX mountant and cover-slipped.
Antibody specificity
In order to provide a measure of specificity for the polyclonal primary antibodies that we used to detect either CTB or PHAL, we applied these antibodies to brain tissue sections collected from animals in which no tracer was injected. Following this procedure, no specific labeling was seen. Similarly, specificity of the polyclonal secondary antibodies was indicated by the observed absence of specific labeling in tissue sections collected from animals that had received a tracer injection, to which the immunocytochemical method was applied with omission of the primary antibody step.
2.3. Analysis
Injection site maps
Sites of tracer deposition within the LHA were plotted with the camera lucida method and with reference to adjacent thionin stained sections. The plotted data was then transposed on to a series of computerized standard drawings of the rat brain (Swanson, 2004); this was done with the use of illustration software (Adobe Illustrator CS3).
Tracer maps
PHAL-labeled fibers and CTB-labeled neurons were plotted either with the aid of the camera lucida method and then transposed to a computerized standard series of rat brain atlas maps (Swanson, 2004) using illustration software (Adobe Illustrator CS3), or they were plotted directly on to the latter. As was the case for mapping the sites of the tracer deposits, an adjacent series of thionin stained tissue sections was used as an aid in determining the cytoarchitectural boundaries between brain regions. Parceling of the rat brain, terminology for describing morphological features of tracer-labeled axons and cell bodies, and mapping strategies and procedures follow Swanson (2004), unless indicated otherwise.
Photomicrography
Individual photomicrographs were taken with a microscope-mounted digital camera (Diagnostics Instruments Spot/Leica DMRE, or Hamamatsu Orca ER/Zeiss Axio Imager.Z1) at a resolution of 1 megapixel, and saved as 8-bit TIFF files. The acquired image files were opened in photo-editing software (Adobe Photoshop CS3) where they were composed (cropped, rotated) and adjusted (brightness, contrast and color balance).
RESULTS
3.1. Normal anatomy
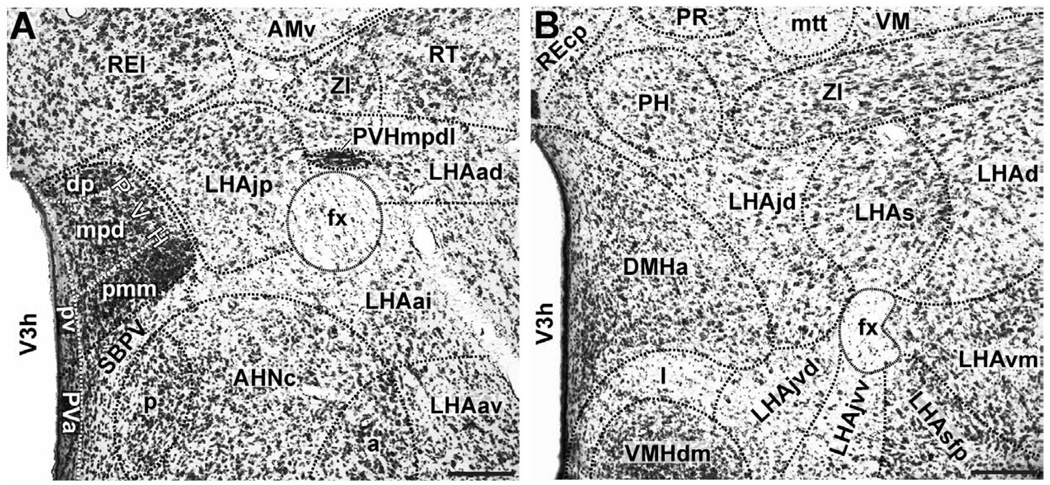
In Nissl-stained material the LHAjp and LHAs are spatially separate and visually distinguishable regions with less distinct borders than generally associated with neuron populations called nuclei; they are readily identifiable in sections cut roughly through the center of their spatial extent (Fig. 1), but gradations rather than clear borders between adjacent regions are typical. The borders and features of the LHAjp and LHAs have been mentioned elsewhere (Swanson, 2004; Swanson et al., 2005; Hahn, 2009) but a more careful account is useful here for describing our experimental results.
Figure 1.
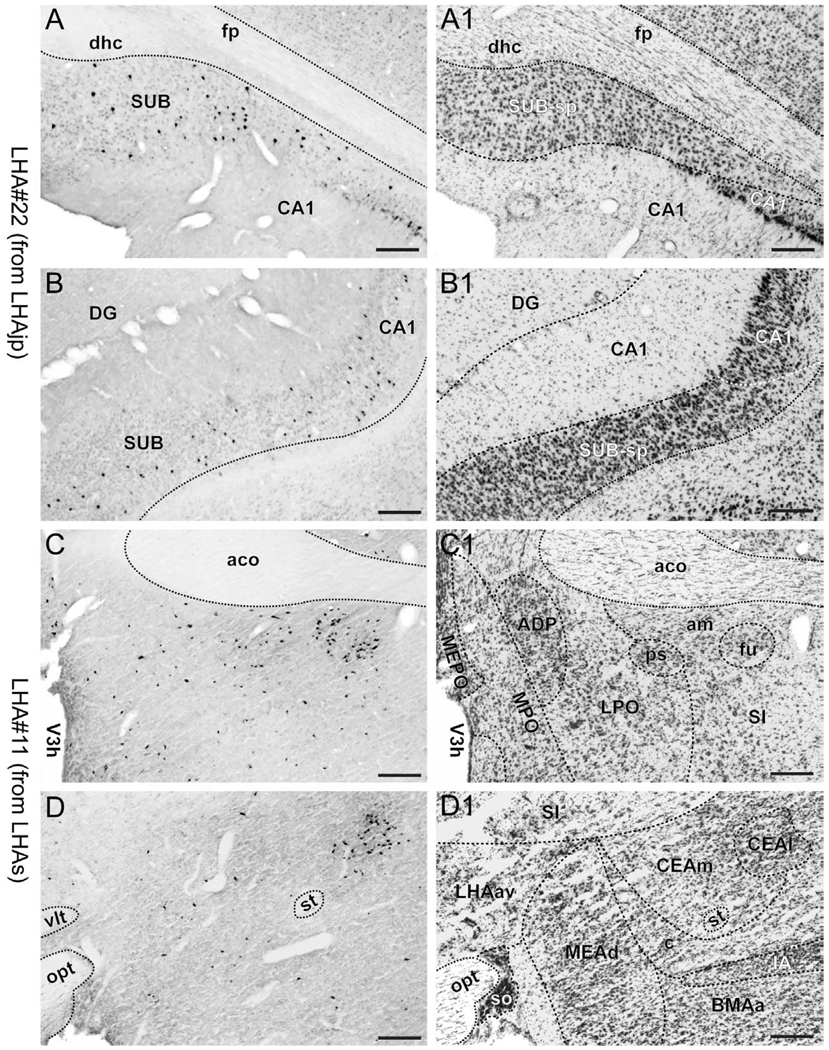
Representative photomicrographs of LHAs and LHAjp cytoarchitecture. (A and B) Brightfield photomicrographs of thionin stained transverse sections showing the cytoarchitecture and boundaries of the lateral hypothalamic area juxtaparaventricular region (LHAjp) at a caudal level (A) and the LHA suprafornical region (LHAs) at a midrostrocaudal level (B). Boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond approximately to those delineated in an atlas of the rat brain (Swanson, 2004). A list of abbreviations used in this figure (and throughout this paper) is present from the second page of this paper. Scale bars = 100 µm.
3.1.1. Borders and cytoarchitecture of the LHAjp (Fig. 1A; see also Fig. 3)
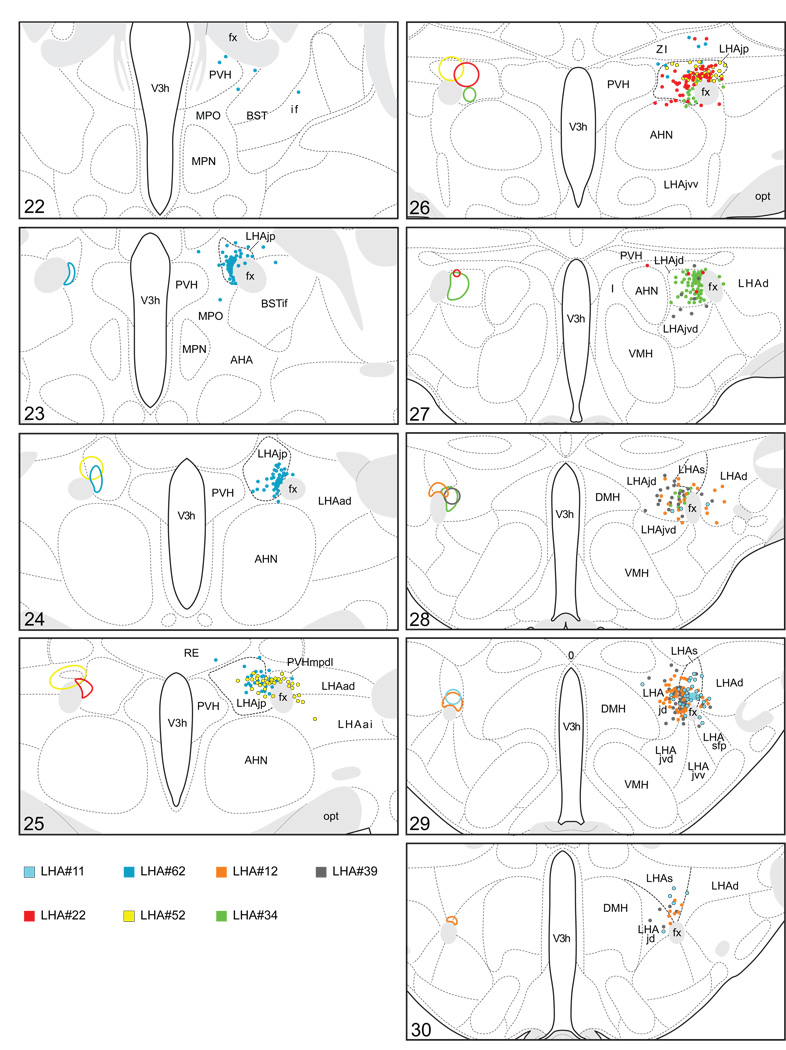
Figure 3.
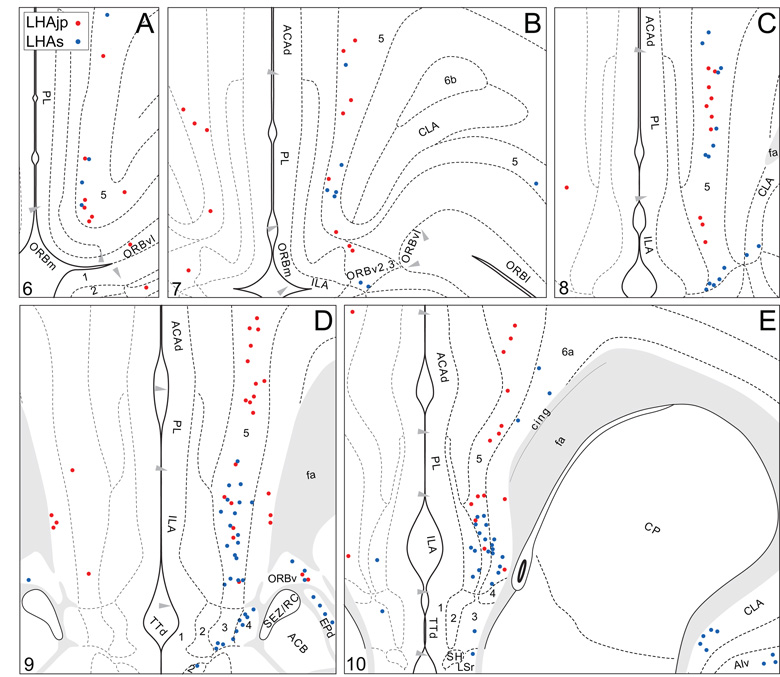
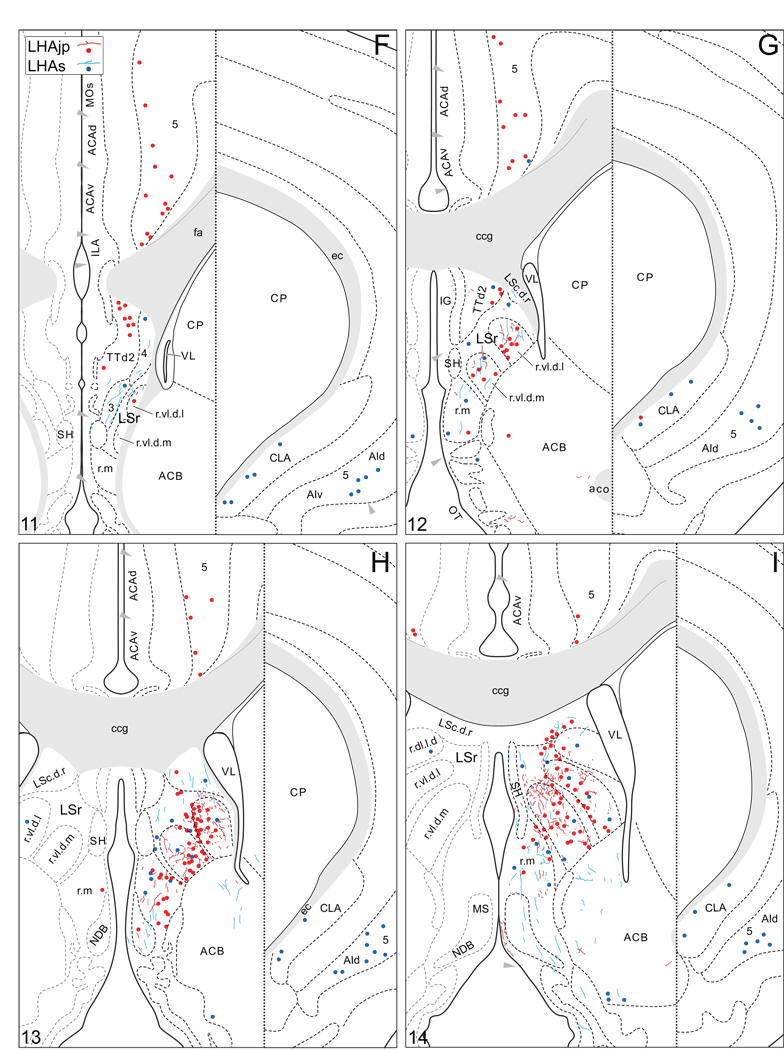
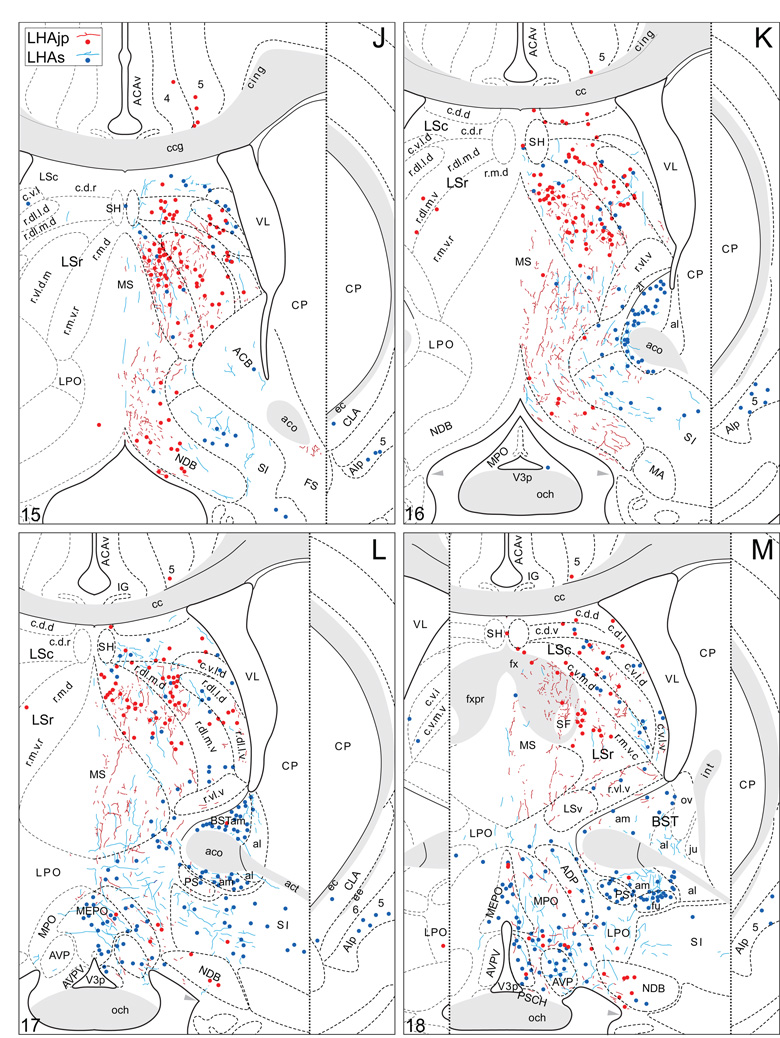
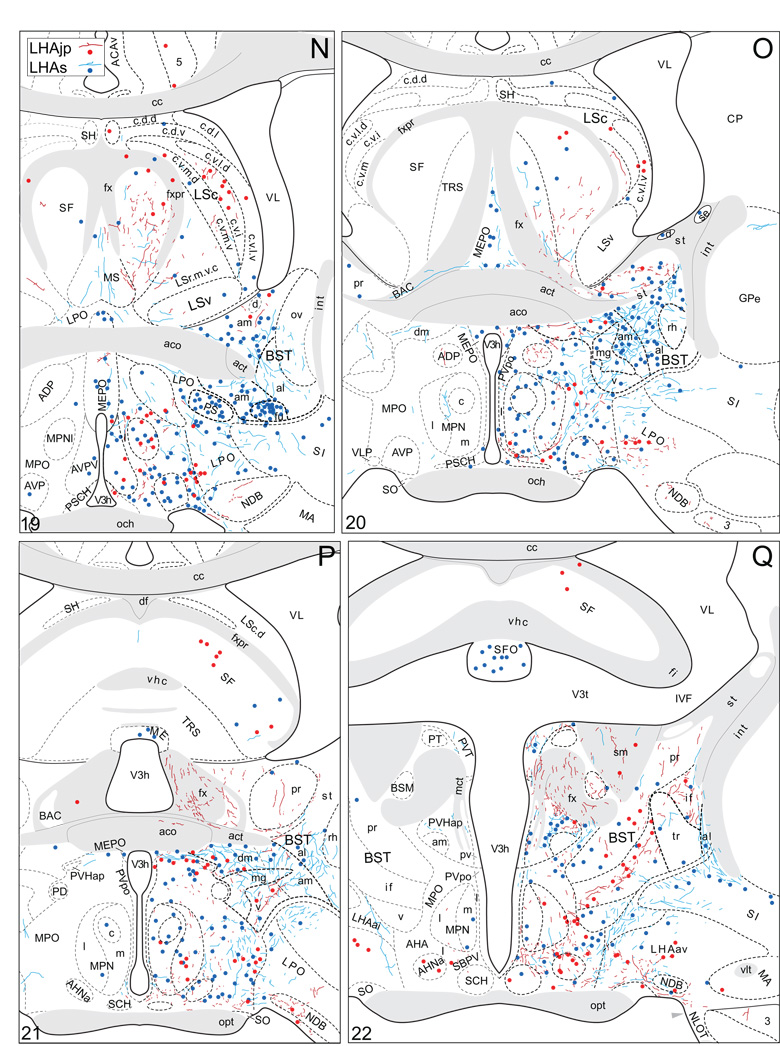
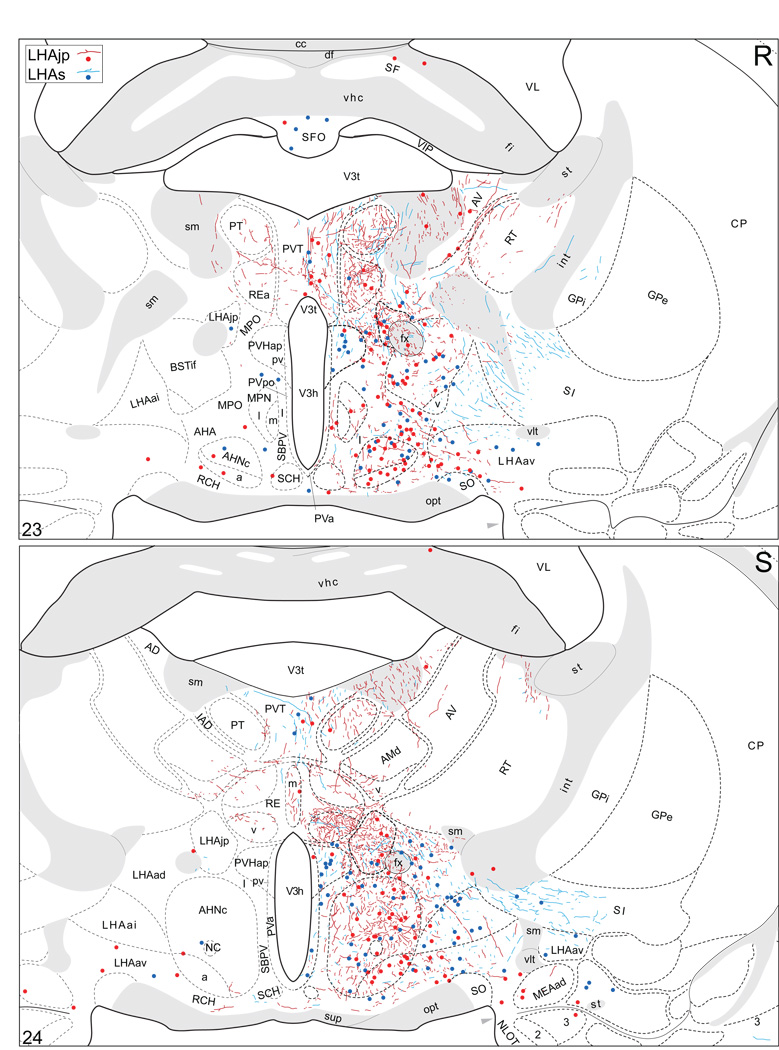
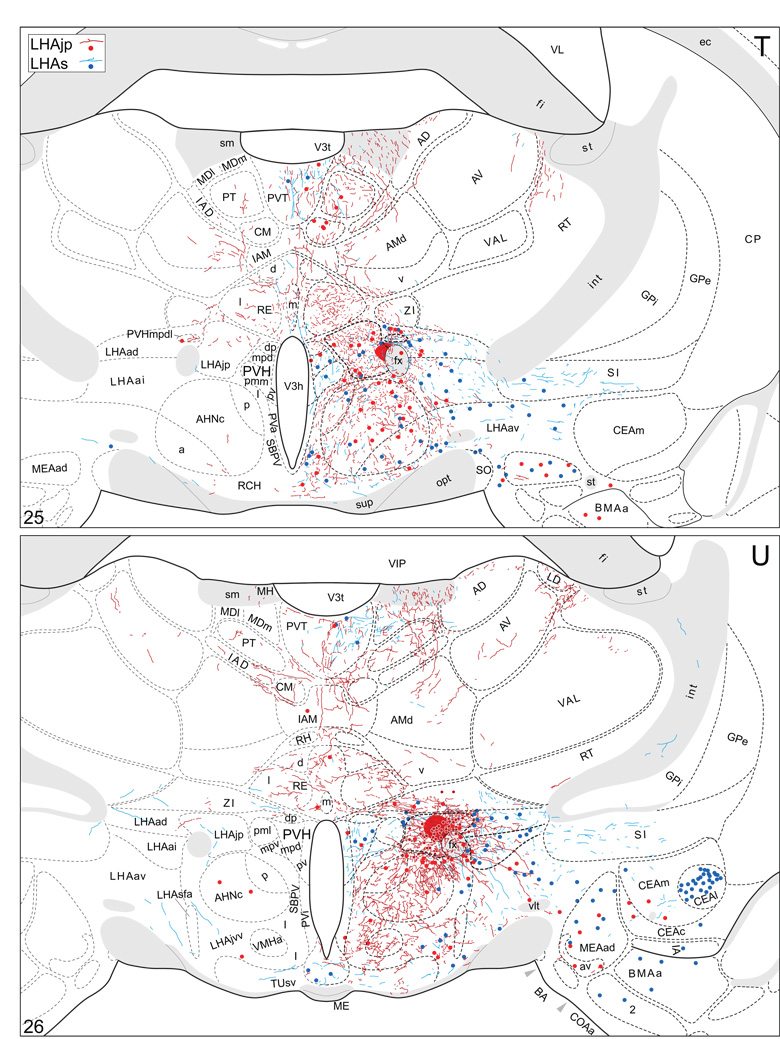
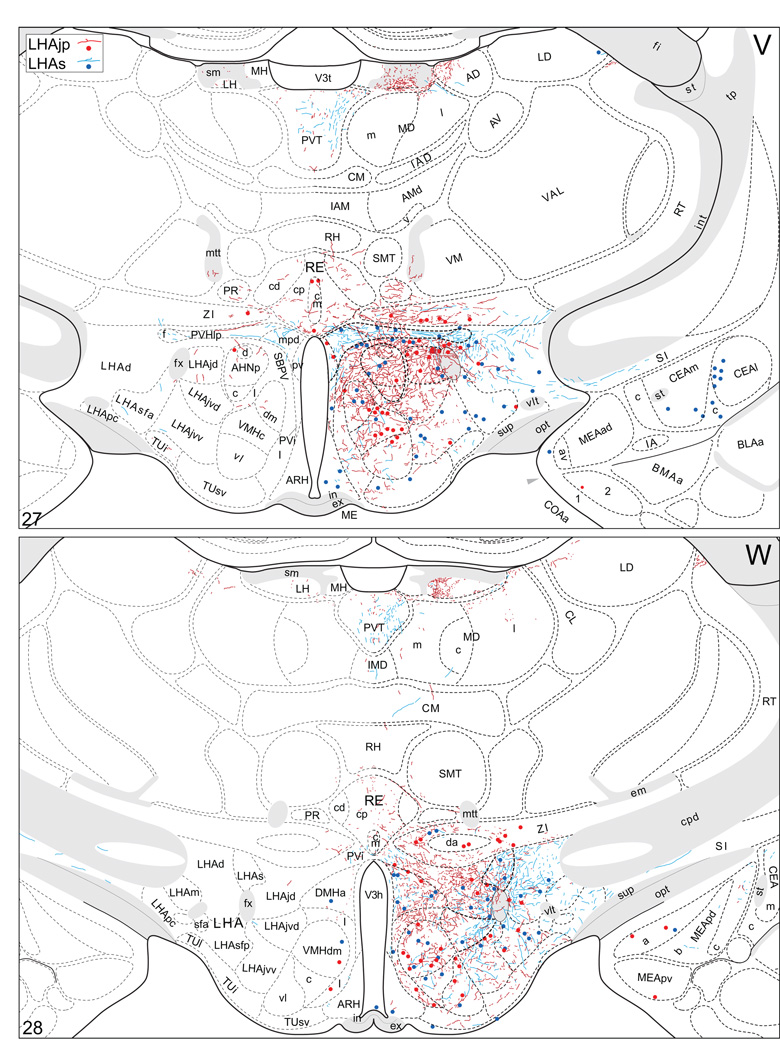
Plots of injection sites for representative experiments. The approximate extent of deposition sites for seven different tract-tracing experiments resulting from a combined injection of cholera toxin B subunit (CTB) and Phaseolus vulgaris leucoagglutinin (PHAL). The experiments presented involved either the lateral hypothalamic area juxtaparaventricular (LHAjp) or suprafornical (LHAs) regions, or immediately adjacent regions, to a greater or lesser extent as shown. Each experiment is represented by a different color, with PHAL-labeled cells shown as colored dots and the approximate extent of each CTB deposit shown in colored outline. All injections were unilateral, but for clarity the CTB site outlines are transposed to the contralateral side of the atlas plates. These data were plotted with the aid of the camera lucida method from tissue sections containing immunocytochemically detected PHAL and CTB, and with reference to an adjacent set of thionin stained sections; the plots were then transposed to corresponding atlas plates. Approximate boundaries of brain regions (dashed lines) and the shown fiber tracts (grayed areas) correspond to those delineated in an atlas of the rat brain (Swanson, 2004).
The LHAjp is a hypothalamic region of moderate size situated at a rostral dorsomedial site within the hypothalamus; in relation to other parts of the LHA, the LHAjp forms a rostrodorsal specialization of the medial tier (Swanson et al., 2005). In thionin-stained sections, the perikarya of LHAjp neurons stain with moderate intensity; cell bodies are medium-sized and have a somewhat regular round to oval shape. Overall the LHAjp has a rather uniform, if not homogenous, appearance.
Rostrally, the LHAjp appears at approximately the same level as the anterior parvicellular part of the PVH (PVHap), and from there it extends caudally to the level of the posterior magnocellular part of the PVH (PVHpm). Along the entire length of its rostral-caudal axis, the LHAjp apposes closely the column of the fornix; this apposition occurs with the medial, dorsomedial, and dorsal borders of the fornix. At its most rostral and most caudal, the LHAjp apposes the medial, dorsomedial, and dorsal aspects of the fornix, whereas at midrostrocaudal levels of the LHAjp, the apposition occurs predominantly with the medial border of the fornix.
Caudal to the level of its rostral end, the LHAjp extends medially until it comes to appose the distinct lateral border of the PVH, first at the level of the PVHap and then its caudal half. Ventrally, except for its rostral tip, the LHAjp apposes the rather distinct dorsal border of the anterior hypothalamic nucleus (AHN); at its rostral tip, the non-forniceal borders of the LHAjp are not easily discerned, but are nevertheless formed by neurons within a dorsal region of the medial preoptic nucleus (medial border), a ventromedial region of the rostral tip of the reticular nucleus of the thalamus (RT; dorsal and dorsolateral border), and a dorsomedial region of the interfascicular nucleus of the bed nuclei of the stria terminalis (BSTif; ventral border).
At a level just caudal to its rostral pole, the LHAjp forms an indistinct ventromedial border with the internuclear region situated between the PVH and AHN; also at this level the LHAjp is bounded distinctly by the thalamus: dorsomedially by the ventral part of the rostral division of the nucleus reuniens (REv), and dorsolaterally by the RT. At a midrostrocaudal level, the LHAjp continues to be bounded dorsomedially by the REv, while its dorsolateral border is formed by the medial tip of the rostral end of the zona incerta. The caudal end of the LHAjp is bounded dorsally by the zona incerta, and dorsolaterally by the dorsal zone of the anterior region of the LHA (LHAad); the border with the latter appears to occur slightly lateral to the fornix although it is not distinct.
Finally, an additional feature of the LHAjp, and one that provides a useful fiducial marker for this region, is the presence of a peculiar and neglected lateral wing of the dorsal zone of the medial parvicellular part of the hypothalamic paraventricular nucleus (PVHmpdl) (Simmons and Swanson, 2008) that penetrates the rostral half of the LHAjp, and in transverse section appears to bisect it. This lateral wing protrudes laterally from the PVH and then forms a rather narrow columnar arch that describes a lateral path through the LHAjp to the dorsal border of the fornix. At the dorsal border of the fornix, the PVHmpdl bulges slightly and extends rostrally to the level of the PVHap. The perikarya of the PVHmpdl stain intensely, making this structure appear quite distinct in relation to the medially adjacent LHAjp with its lighter stained perikarya (Fig. 1A). In addition, the cell bodies in the PVHmpdl have a distribution that is particularly dense in relation to the cell bodies of LHAjp neurons, further distinguishing this structure from the LHAjp.
3.1.2. Borders and cytoarchitecture of the LHAs (Fig. 1B; see also Fig. 3)
The perikarya of the LHAs show variety in their type: some cell bodies stain intensely with thionin, and these are intermingled with a greater abundance of smaller more lightly stained neurons (Fig. 1B). As its name suggests the LHAs sits atop the dorsal aspect of the fornix. Beginning rostrally at a level immediately caudal to the PVH, it extends caudally, to the level of the caudal-dorsal border of the dorsomedial nucleus. Along its rostral-caudal axis, the LHAs extends laterally slightly beyond the width of the fornix to merge with the dorsal region of the lateral tier of the LHA (LHAd); medially it merges principally with the juxtadorsomedial region of the medial tier of the LHA (LHAjd), and at its most caudal extent it merges with a ventrolateral region of the posterior hypothalamic nucleus. The LHAs forms a distinct dorsal boundary with the ventral border of the zona incerta.
3.2. Neuroanatomical connections of the LHAjp and LHAs
From 81 combined PHAL and CTB tracer experiments we obtained 42 instances where the injection site included and was largely restricted to regions of the LHA medial and perifornical tiers; 22 of these included the region of the LHAjp, and 11 included the region of the LHAs. In describing these results, first we give an account of the experiments that we selected for analysis, and for those mapped out in detail, a description of the morphology of PHAL-labeled axons; we also give an account of the distribution of the PHAL-labeled axons and CTB-labeled perikarya with respect to their lateralization. We follow this with a brief description of the regions of most prominent anterograde and retrograde labeling obtained in these experiments from either the LHAjp or LHAs. This overview is followed by a complete description of the anterogradely-labeled projections, and then the sites of retrograde labeling, first for the LHAjp and then for the LHAs. In order to provide logical structure to our descriptions, the way in which we have organized our description of the efferent and afferent projections differs: our description of the efferent projections begins at the locus of the injection site, and is guided by the projections themselves; in contrast, our description of the retrograde labeling is organized according to a systematic hierarchy of cell groups and regions in the rat central nervous system (Swanson, 2004). To allow us greater focus on possible functional implications of these voluminous data in the Discussion, we make reference in the Results to previous tract-tracing studies that have a direct bearing on the present findings.
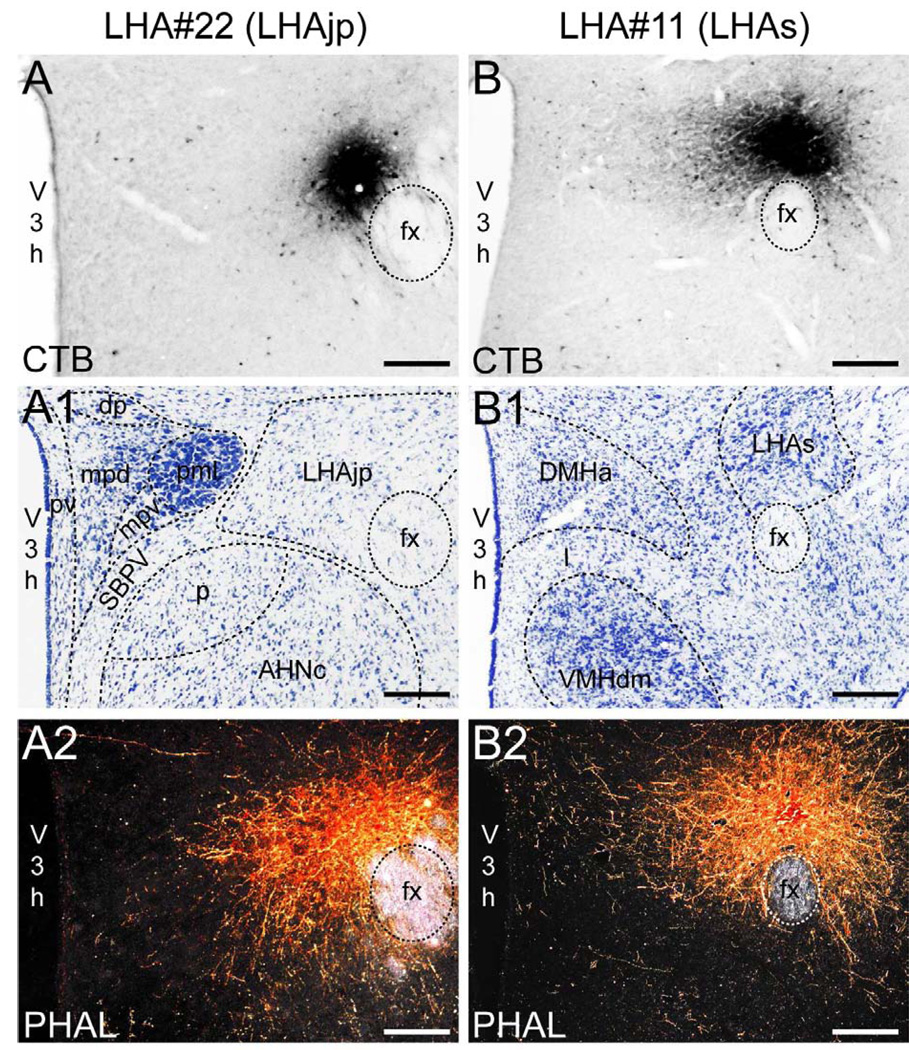
We reserved our most detailed analysis for two experiments where the site of tracer deposition was most restricted to the caudal end of the LHAjp (the level at which it presents at its fullest) and a midrostrocaudal portion of the LHAs: these were experiments LHA#22 (LHAjp) and LHA#11 (LHAs) (Figs. 2 and 3). Additional analysis was performed on five experiments out of the available set of 33 (22 including LHAjp and 11 including LHAs) where the injection site included and was most restricted to either the LHAjp or LHAs (Fig. 3). One of these experiments (LHA#62) was particularly useful because it was concentrated in the rostral half of the LHAjp, thus providing together with experiment LHA#22 (caudal end of the LHAjp) an overview of the total connections of the LHAjp (these findings are summarized at the end of the Results, and also represented graphically in Figs. 12 and 13 of our Discussion section). Plots of the anterograde and retrograde labeling obtained in experiments LHA#22 (LHAjp) and LHA#11 (LHAs) are shown in Figure 4; these data are also represented in summary form on a 'flat map' of the rat brain (Fig. 5). We also carried out a semi-quantitative analysis of the labeling obtained in experiments LHA #22, LHA #11 and LHA #62; the results of this analysis are given in tabular form (Table 1).
Figure 2.
Representative photomicrographs of PHAL and CTB injections sites. (A and B) Brightfield photomicrographs showing immunocytochemically identified deposits of cholera toxin B subunit (CTB) tracer for experiment LHA#22 within caudal levels of the lateral hypothalamic area juxtaparaventricular region (LHAjp) (A) and experiment LHA#11 within midrostrocaudal levels of the LHA suprafornical region (LHAs) (B). (A1 and B1) Thionin stained sections adjacent to the sections shown in (A, A2) and (B, B2). (A2 and B2) Darkfield photomicrographs showing immunocytochemically identified deposits of Phaseolus vulgaris leucoagglutinin (PHAL) tracer for experiment LHA#22 within caudal levels of the LHAjp (A2) and experiment LHA#11 within the midrostrocaudal levels of the LHAs (B2). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 100 µm.
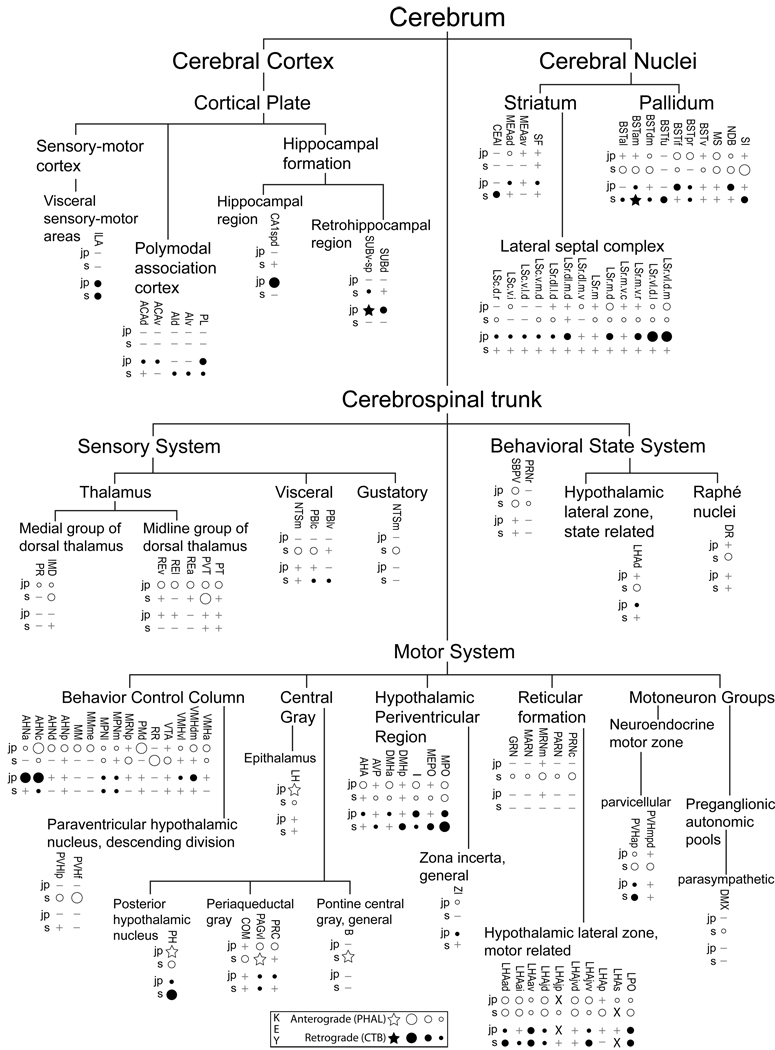
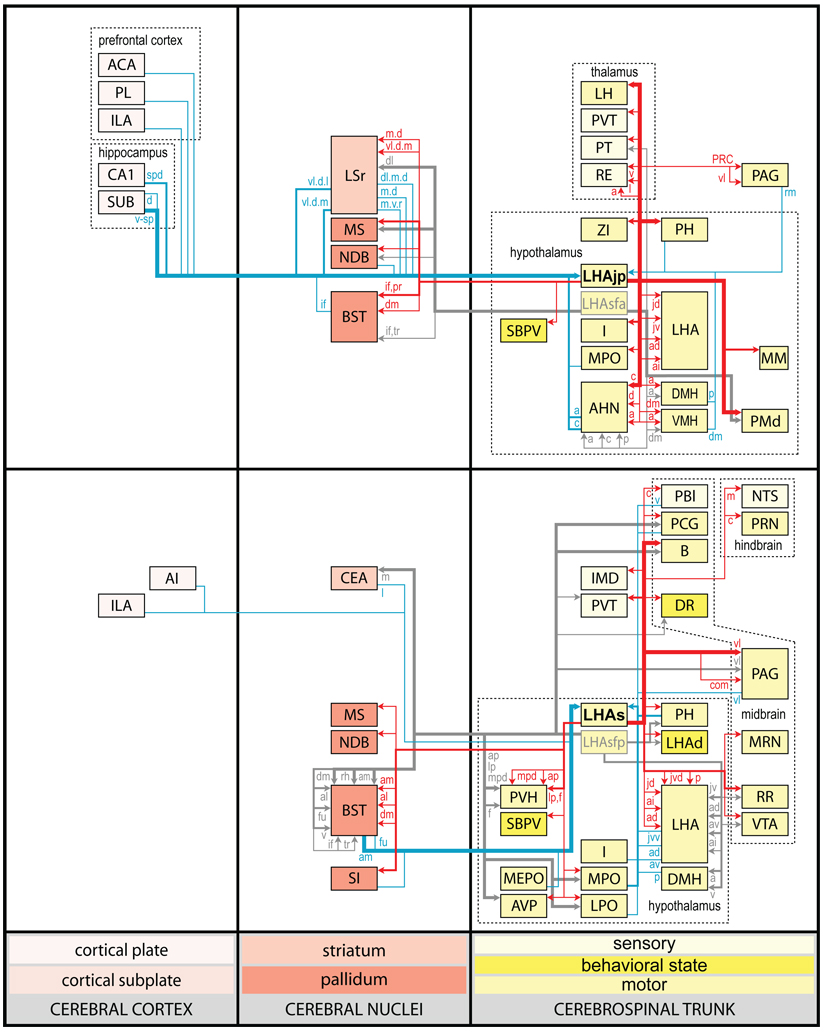
Figure 12.
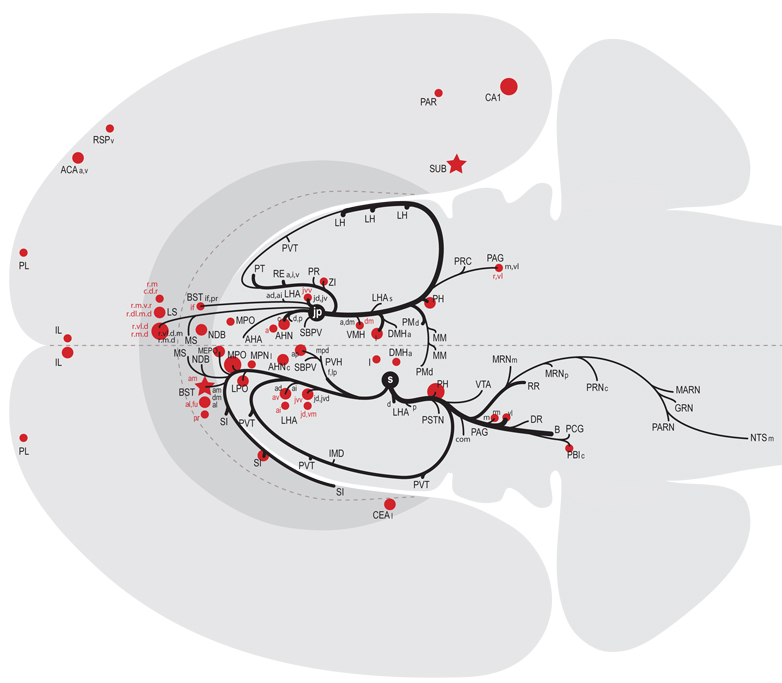
All regions of approximately moderate or higher anterograde (white-filled dots) and retrograde (black dots) labeling resulting from deposits of Phaseolus vulgaris leucoagglutinin (PHAL) and cholera toxin B sububit (CTB) within the lateral hypothalamic area juxtaparaventricular region (LHAjp; combined analysis of caudal LHAjp experiment LHA#22 and rostral LHAjp experiment LHA#62) or LHA suprafornical region (LHAs; midrostrocaudal level, experiment LHA#11) represented within a cerebral hierarchy. Regions in which the overall level of labeling amounted to approximately moderate (or above) are represented even if the constituent subregions contained a lower level of labeling (e.g., the MPN). For a more detailed comparison of the connections of caudal and rostral levels of the LHAjp (for experiment LHA#22 and LHA#62, respectively) and midrostrocaudal levels of the LHAs (for experiment LHA#11) see Fig. 4 and Table 1. The diameter of the round symbols corresponds to the relative abundance of labeling, 3 levels are represented: low, moderate and high. Stars indicate sites of highest abundance of labeling; “+” signs indicate the presence of labeling at a very low level; “−” signs indicate absence of labeling. “X” indicates the site of tracer injections.
Figure 13.
Summary diagram illustrating the major axonal inputs and outputs of the LHAjp (combined experiment LHA #22 and LHA #62) and midrostrocaudal levels of the LHAs (experiment LHA #11) represented within a cerebral hierarchy (see Swanson, 2004). Red lines indicate outputs and blue lines indicate inputs; directionality is also indicated by arrowheads. The thickness of each line corresponds to the relative magnitude of each projection. Small colored lettering indicates subdivisions of larger regions that are specifically targeted or that specifically provide an indicated input. For comparison, also shown (gray lines with gray arrow heads and gray lettering) are the previously published outputs of the LHA subfornical region (LHAsf) (Goto et al., 2005); note the similarity between the outputs or projections of the posterior zone of the LHAsf (LHAsfp) and midrostrocaudal levels of the LHAs, and the anterior zone of the LHAsf (LHAsfa) (Goto et al., 2005) and the LHAjp.
Figure 4.
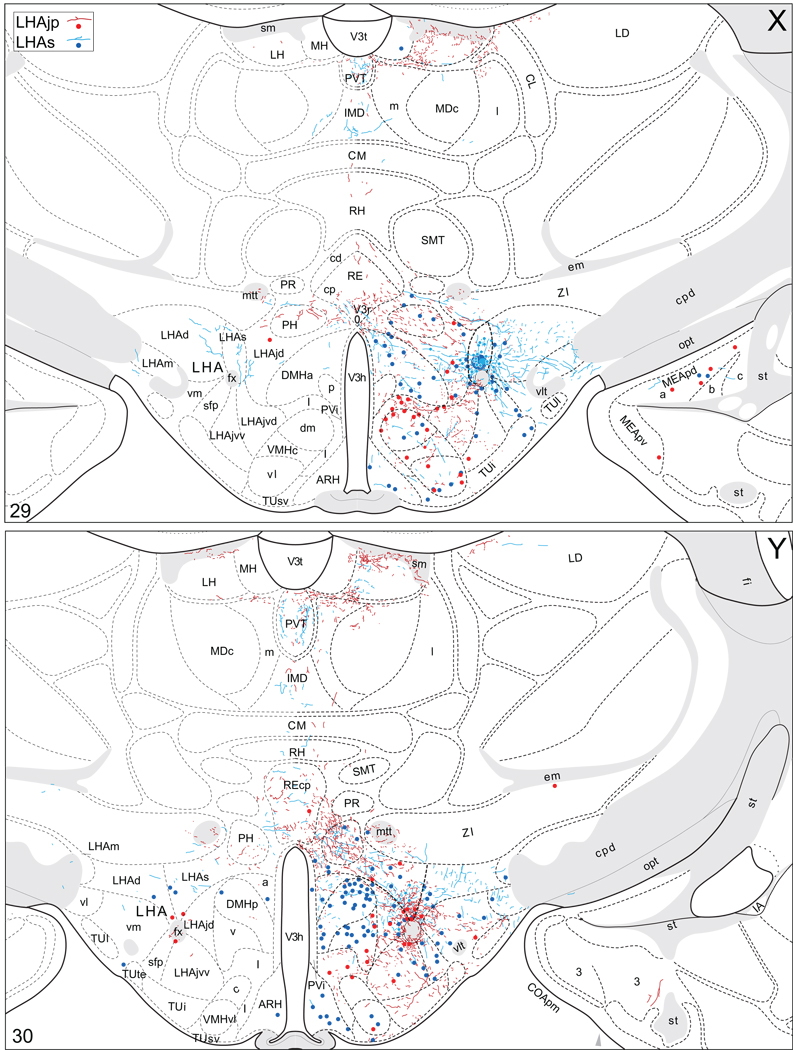
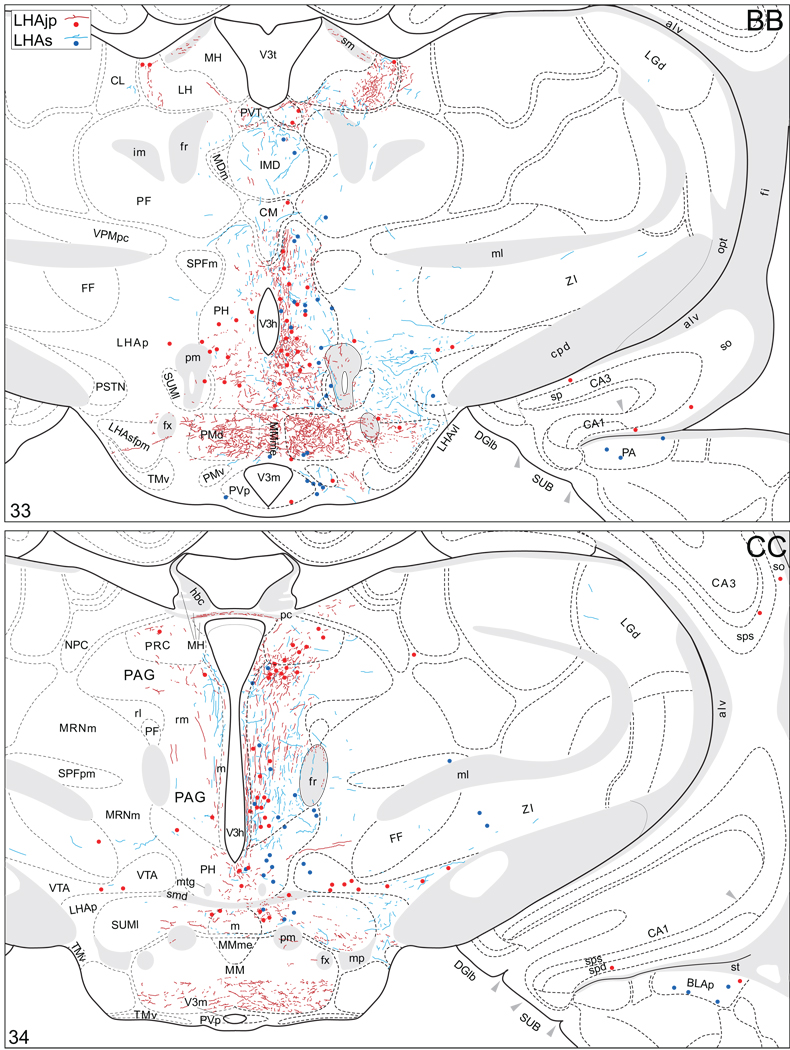
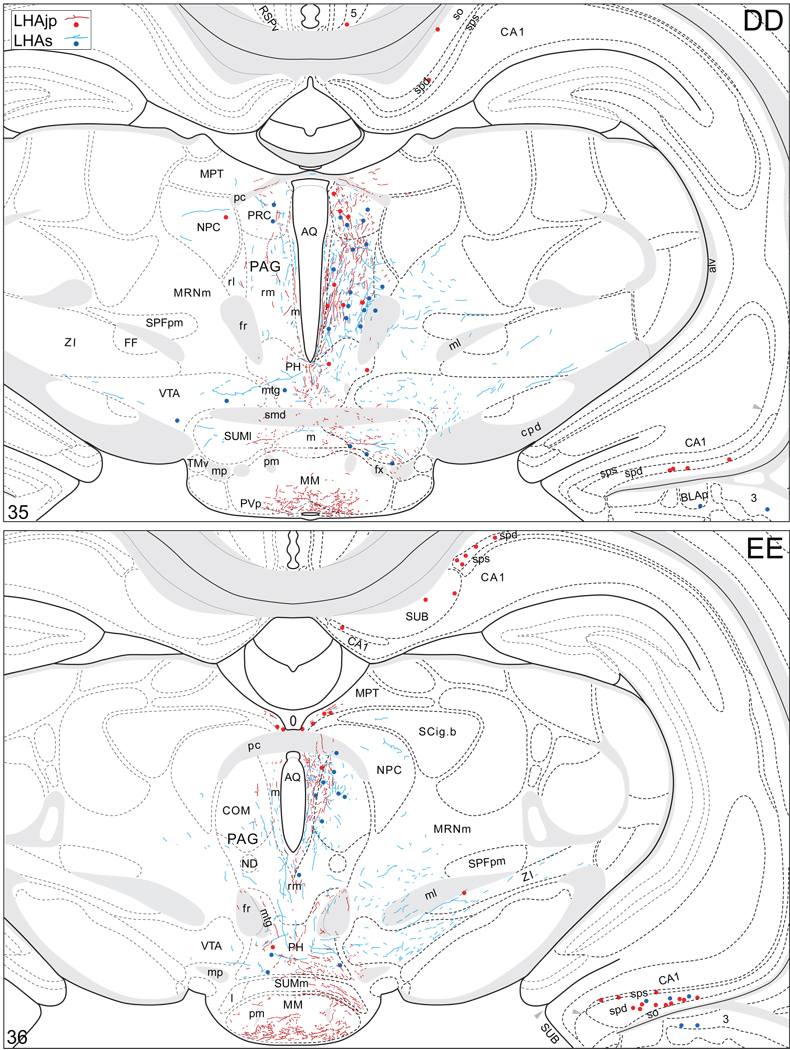
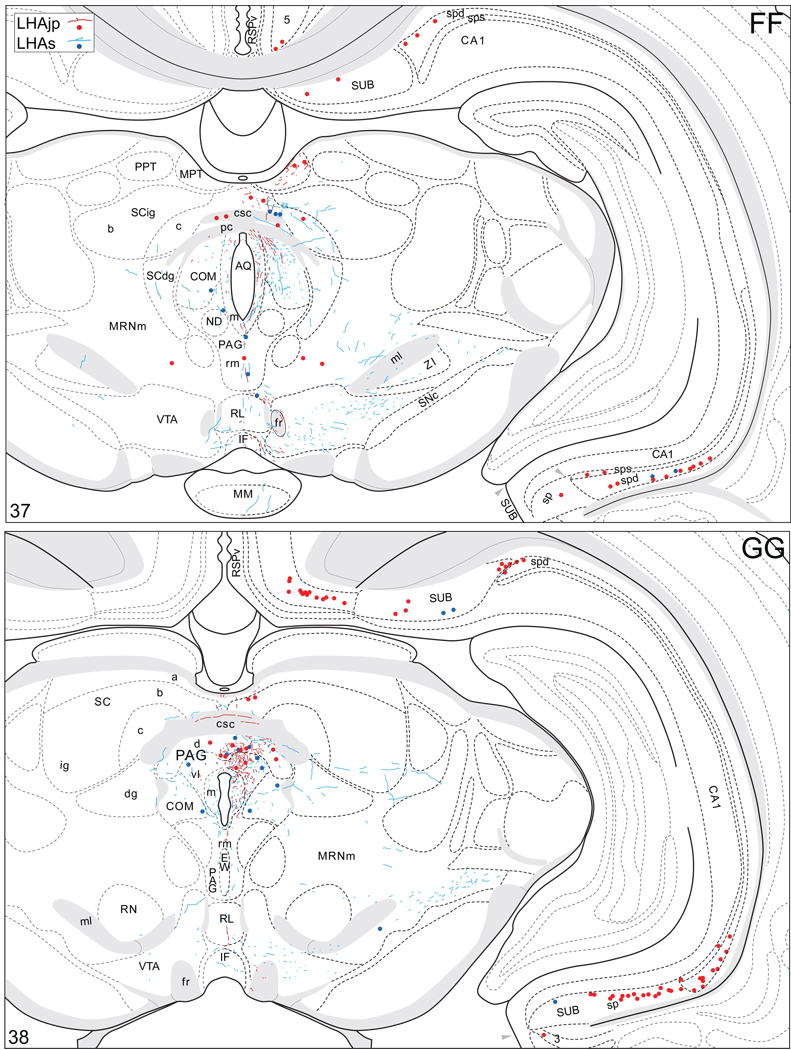
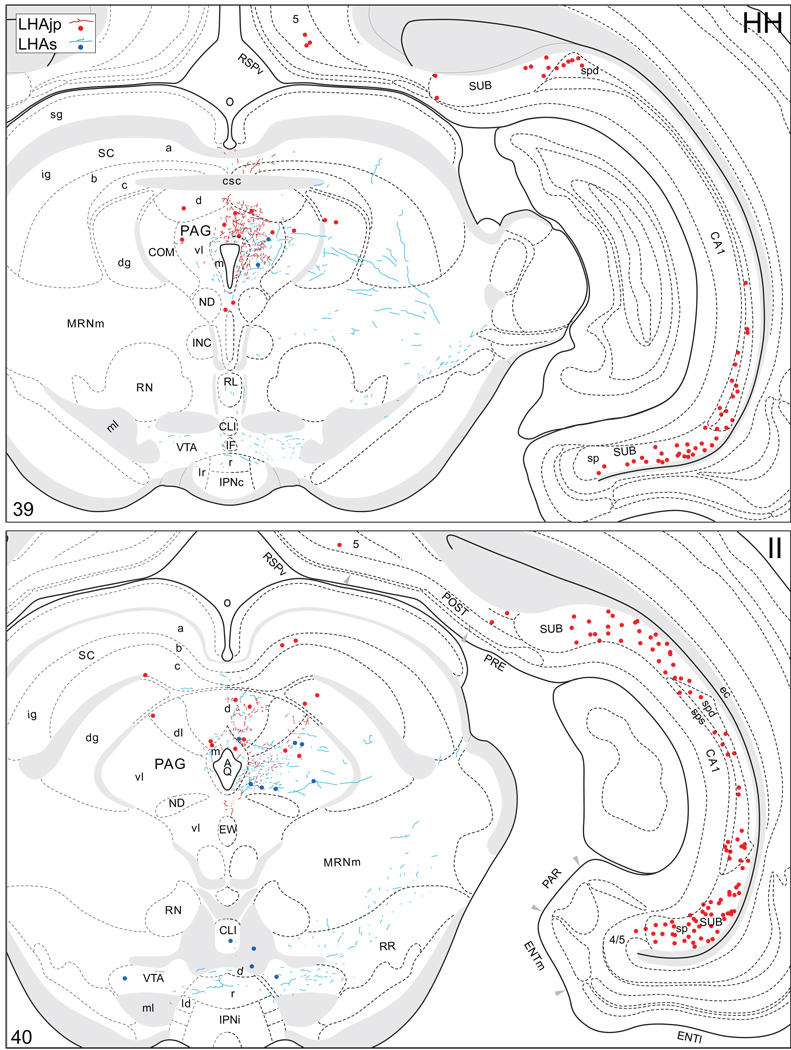
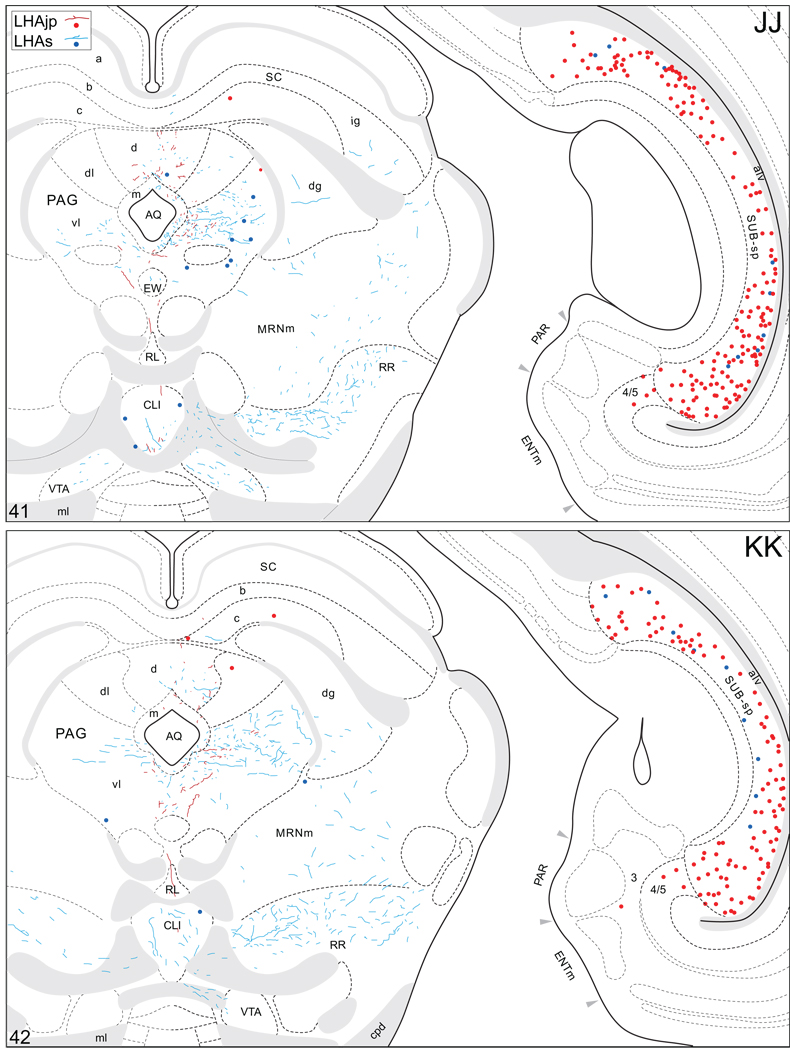
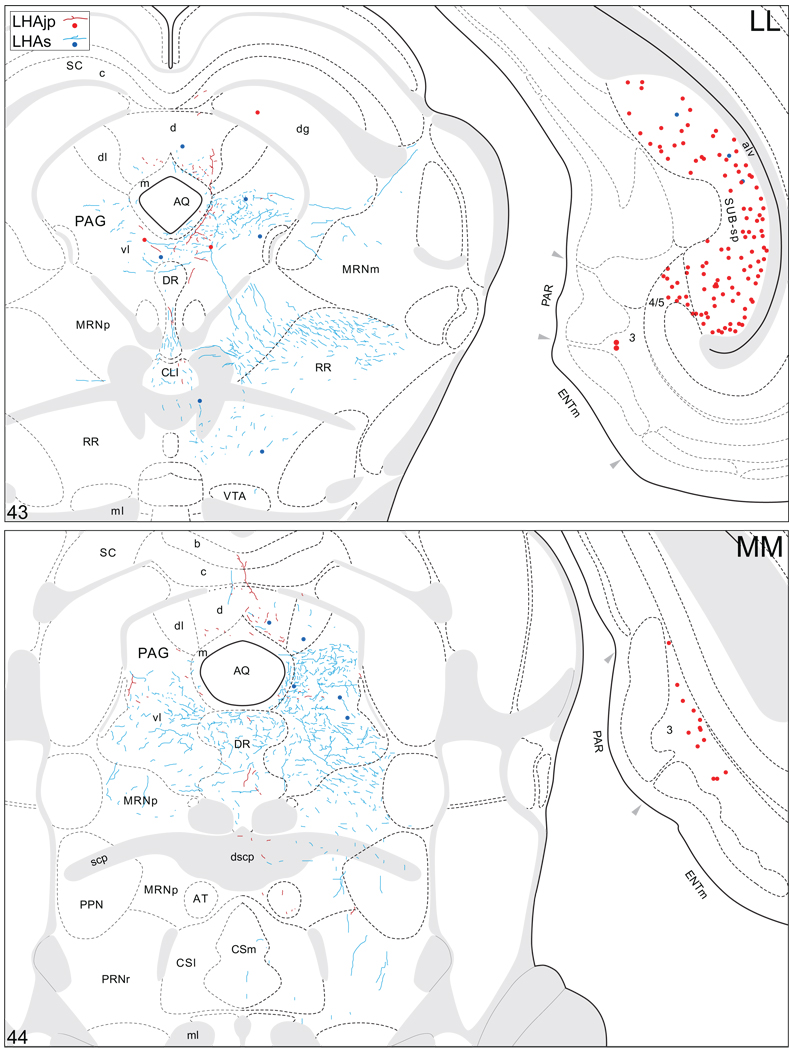
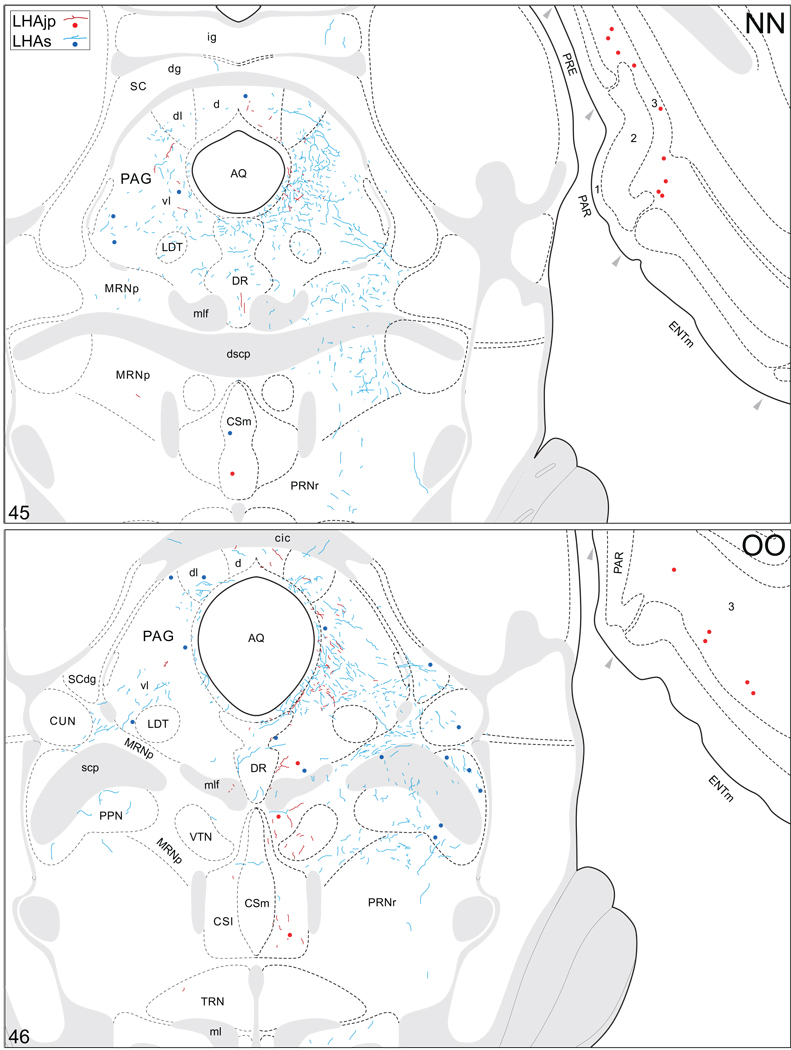
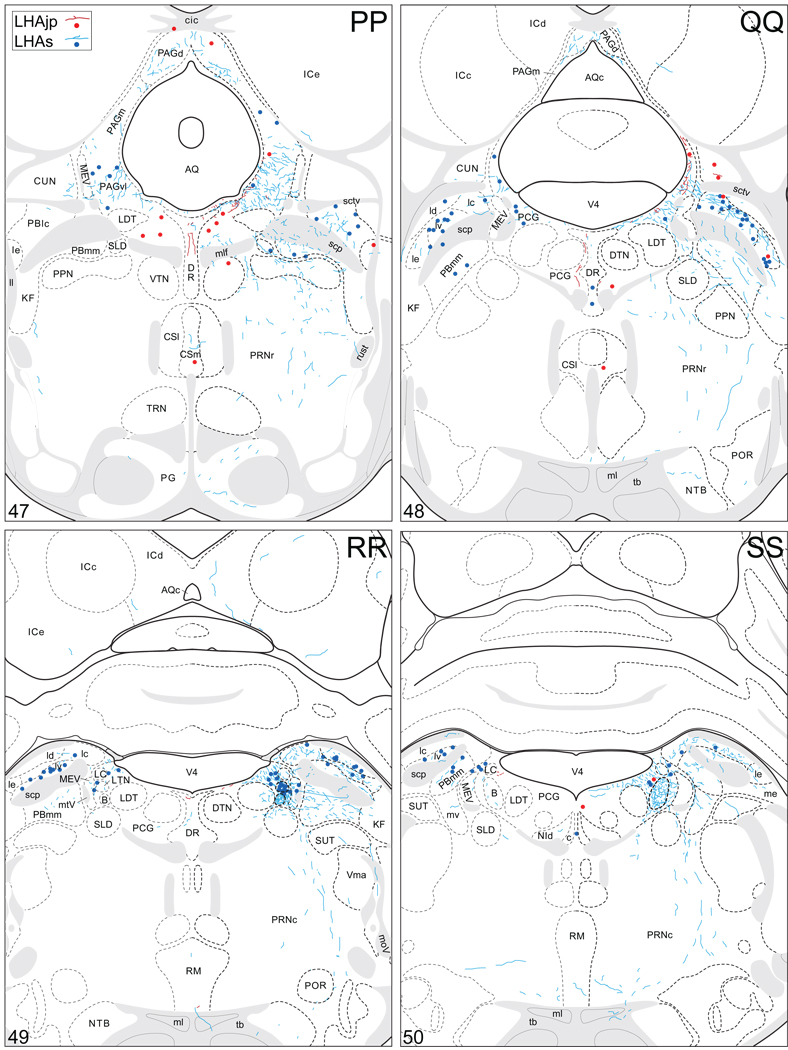
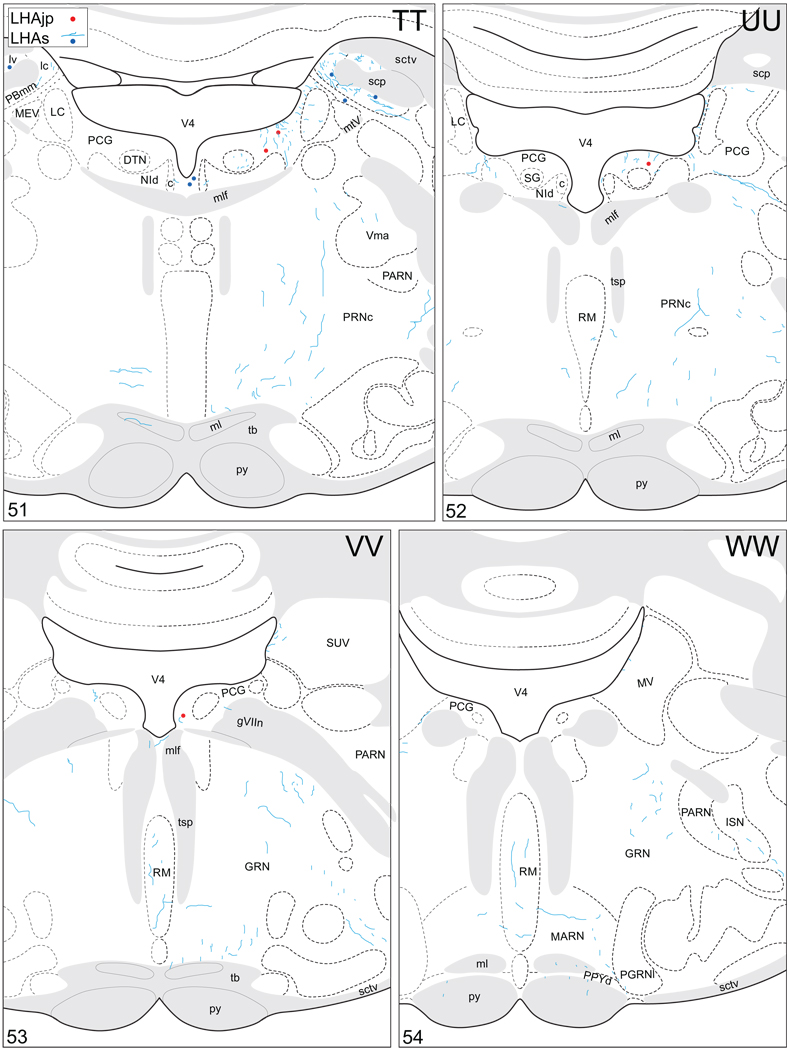
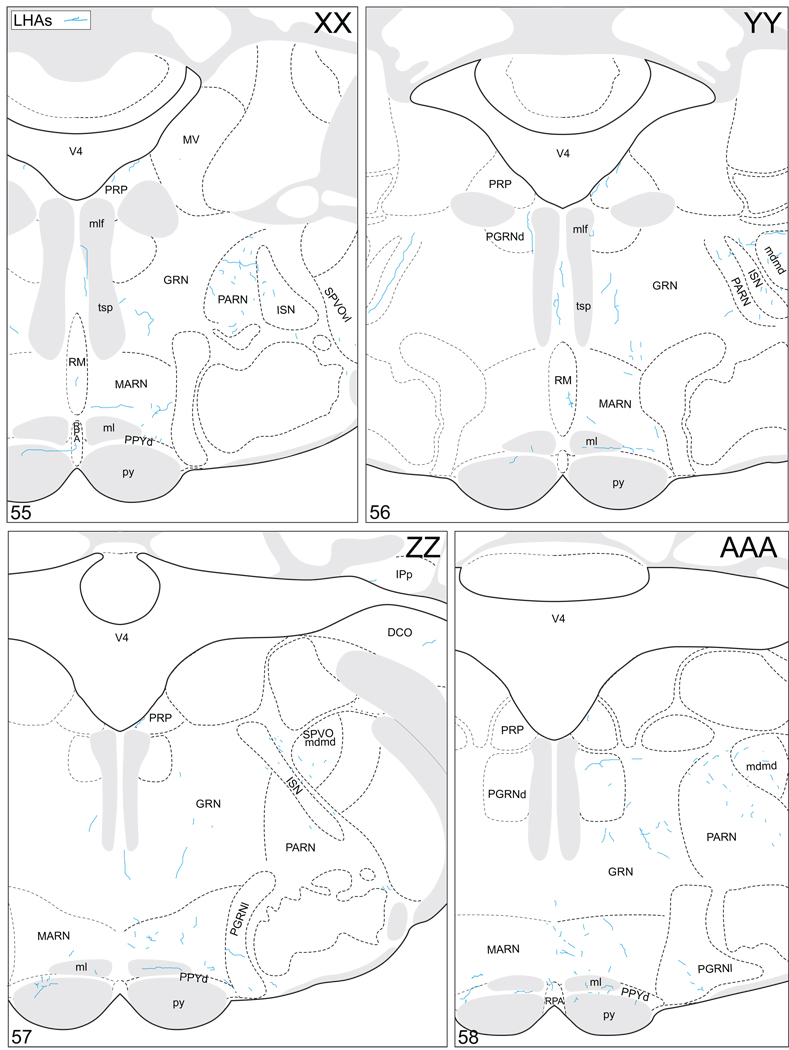
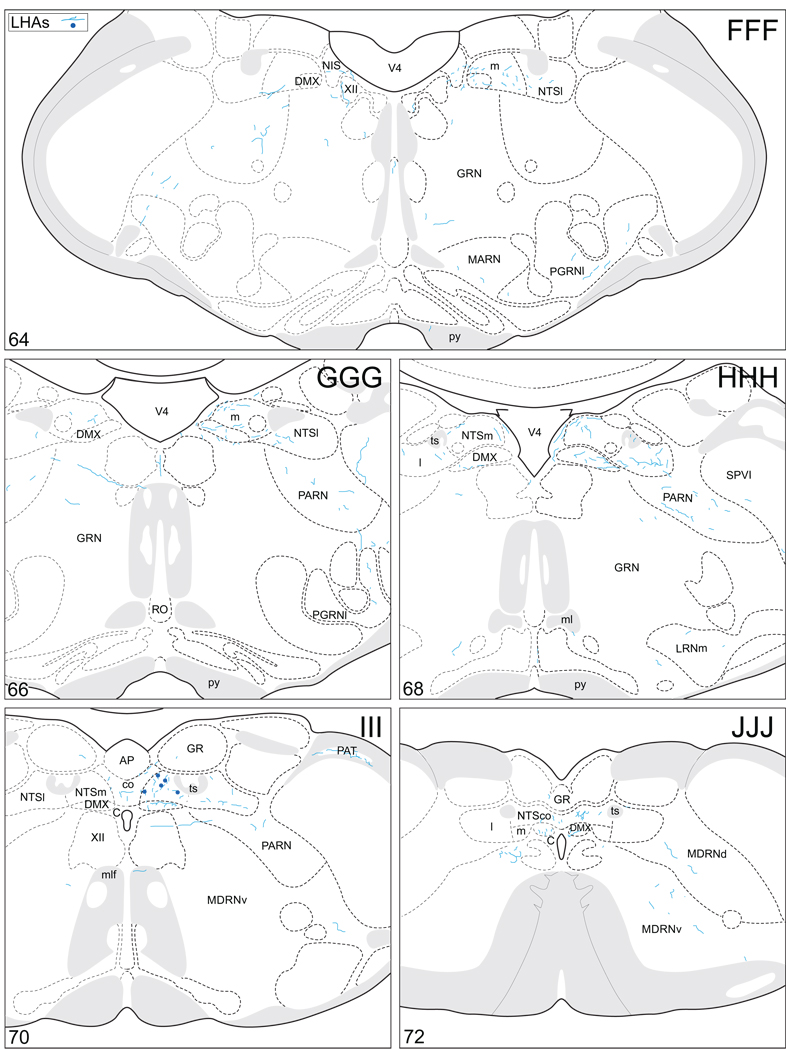
Projections from and to the LHAjp and the LHAs. The distribution of Phaseolus vulgaris leucoagglutinin (PHAL)-labeled axons is represented by the colored lines (red = LHAjp experiment #22; blue = LHAs experiment #11). Cholera toxin B subunit (CTB) retrogradely labeled neurons are represented as colored dots with black outlines (red = LHAjp experiment #22; blue = LHAs experiment #11); one dot = one labeled neuron. The data is plotted onto a series of rat brain reference templates derived from Swanson (2004) and arranged from rostral to caudal (A-JJJ); atlas levels are indicated by a numeral in the lower left corner of each figure. PHAL-filled cells at the site of the injection sites are indicated by colored dots with a white outline (red = LHAjp experiment #22; blue = LHAs experiment #11); the approximate extent of the visualized CTB injection site is indicated by a colored area with a black outline (red = LHAjp experiment #22; blue = LHAs experiment #11). It should be noted that in any given region where neurons retrogradely labeled from the LHAs and LHAjp are intermingled it is possible that a portion of them may project to both sites.
Figure 5.
General organization of the principal projections from and to the caudal end of the LHAjp (experiment LHA#22) and midrostrocaudal levels of the LHAs (experiment LHA#11). Anterograde projections are indicated by black lines, retrograde labeling by red discs (or stars). The relative strength of each projection is indicated by the thickness of the black line/diameter of the red disc. Three levels are shown, and for the retrograde labeling a red star is used to indicate the site of most abundant retrograde labeling from the LHAjp or the LHAs. For regions that contained retrogradely labelled cells as well as antereogradely labeled fibers, red type is used to indicate particular subdivisions that were retrogradely labeled; black type indicates regions where anterogade-labelled projecting fibers were present. The flatmap is adapted from Swanson (2004).
Table 1.
Relative abundance of retrograde and anterograde labeling resulting from three separate experiments of combined cholera toxin B subunit (CTB) and Phaseolus vulgaris leucoagglutinin (PHAL) injections within the lateral hypothalamic area (LHA) of male rats; two of these were centered within the LHA juxtaparaventricular region (LHAjp, caudal = experiment LHA#22; rostral = experiment LHA#62), the other (experiment LHA#11) was centered within midrostrocaudal levels of the LHA suprafornical region (LHAs) -- listed as “central LHAs” in column title for brevity. The relative abundance of labeling (retrogradely – CTB – labeled neurons or anterogradely – PHAL – labeled fibers) is represented by the following semi-quantitative grading schema: − = absence of labeling; + = very low; ++ = low; +++ = moderate; ++++ = high; +++++ = very high; +++++* = region of highest amount of retrograde labeling for each experiment. Regions that contained PHAL-labeled axons with the appearance of fibers of passage, or regions that contained only a single CTB-labeled neuron, or PHAL-labeled fiber, are not included in the table (for further details see Fig. 4). The brain region hierarchy follows Swanson (2004).
| ANTEROGRADE (PHAL) | RETROGRADE (CTB) | |||||
|---|---|---|---|---|---|---|
| rostral LHAjp (LHA#62) |
caudal LHAjp (LHA#22) |
central LHAs (LHA#11) |
rostral LHAjp LHA#62) |
caudal LHAjp (LHA#22) |
central LHAs (LHA#11) |
|
| CELL GROUP OR REGION | ||||||
| 1. Cerebrum | ||||||
| 1.1. Cerebral Cortex | ||||||
| 1.1.1. Cortical Plate | ||||||
| Sensory-motor cortex | ||||||
| Somatomotor areas | ||||||
| secondary somatomotor area (MOs) | − | − | − | − | + | − |
| Visceral sensory-motor areas | ||||||
| infralimbic area (ILA) | − | − | − | ++++ | ++ | ++++ |
| Olfactory areas | ||||||
| anterior olfactory nucleus | ||||||
| posteroventral part (AONpv) | − | − | − | + | − | − |
| tenia tecta | ||||||
| dorsal part (TTd) | − | − | − | +++ | ++ | ++ |
| ventral part (TTv) | − | − | − | + | − | − |
| piriform area (PIR) | − | − | − | + | + | − |
| postpiriform transition area (TR) | − | − | − | − | − | + |
| nucleus of the lateral olfactory tract (NLOT) | − | − | − | + | − | − |
| cortical amygdalar area | ||||||
| anterior part (COAa) | − | − | − | ++ | + | + |
| posterior part | ||||||
| lateral zone (COApl) | − | + | − | + | − | − |
| medial zone (COApm) | − | − | − | + | − | − |
| Polymodal association cortex | ||||||
| anterior cingulate area | ||||||
| dorsal part (ACAd) | − | − | − | ++ | ++ | + |
| ventral part (ACAv) | − | − | − | + | ++ | + |
| prelimbic area (PL) | − | − | − | ++++ | ++ | ++ |
| orbital area | ||||||
| lateral part (ORBl) | − | − | − | − | − | + |
| medial part (ORBm) | − | − | − | − | + | − |
| ventral part (ORBv) | − | − | − | + | − | + |
| ventrolateral part (ORBvl) | − | − | − | − | + | + |
| agranular insular area | ||||||
| dorsal part (Ald) | − | − | − | − | − | +++ |
| ventral part (Alv) | − | − | − | − | − | + |
| posterior part (Alp) | − | − | − | − | − | ++ |
| retrosplenial area | ||||||
| ventral part (RSPv) | − | − | − | − | ++ | − |
| hippocampal formation | ||||||
| retrohippocampal region | ||||||
| entorhinal area | ||||||
| lateral part (ENTl) | − | − | − | + | − | − |
| medial part, dorsal zone (ENTm) | − | − | − | ++ | + | − |
| presubiculum (PRE) | − | − | − | + | + | − |
| parasubiculum (PAR) | − | − | − | − | ++ | − |
| subiculum | ||||||
| pyramidal layer (SUB-sp) | − | − | − | +++++* | +++++* | ++ |
| hippocampal region | ||||||
| Ammon's horn | ||||||
| field CA1 | ||||||
| pyramidal layer | ||||||
| deep (CA1spd) | − | − | − | +++++* | ++++ | + |
| superficial (CA1sps) | − | − | − | ++++ | ++ | − |
| stratum oriens (CA1so) | − | − | − | + | − | − |
| field CA2 | − | − | − | + | − | − |
| pyramidal layer (CA2sp) | ||||||
| field CA3 | ||||||
| pyramidal layer (CA3sp) | − | − | − | ++ | − | − |
| stratum oriens (CA3so) | − | − | − | + | + | − |
| 1.1.2. Cortical Subplate | ||||||
| Claustrum (CLA) | − | − | − | + | − | ++ |
| Endopiriform nucleus | ||||||
| dorsal part (EPd) | − | − | − | + | − | + |
| Basolateral amygdalar nucleus | ||||||
| posterior part (BLAp) | − | − | − | + | + | + |
| anterior part (BMAa) | − | − | − | + | + | + |
| Posterior amygdalar nucleus (PA) | − | − | − | + | − | − |
| 1.2. Cerebral Nuclei | ||||||
| 1.2.1. Striatum | ||||||
| Nucleus accumbens (ACB) ("shell" region) | − | − | + | + | + | + |
| Lateral septal complex | ||||||
| lateral septal nucleus | ||||||
| caudal (caudodorsal) part | ||||||
| dorsal zone | ||||||
| rostral region (LSc.d.r) | − | − | ++ | ++ | ++ | ++ |
| dorsal region (LSc.d.d) | − | − | − | − | + | − |
| lateral region (LSc.d.l) | − | − | − | + | − | − |
| ventral region (LSc.d.v) | − | − | − | + | + | + |
| ventral zone | ||||||
| medial region | ||||||
| dorsal domain (LSc.v.m.d) | − | − | + | ++ | + | + |
| ventral domain (LSc.v.m.v) | − | + | + | + | − | + |
| intermediate region (LSc.v.i) | − | + | − | +++ | ++ | + |
| lateral region | ||||||
| dorsal domain (LSc.v.l.d) | − | − | − | ++ | − | + |
| ventral domain (LSc.v.l.v) | − | + | + | ++ | + | + |
| rostral (rostroventral) part | ||||||
| medial zone | ||||||
| dorsal region (LSr.m.d) | +++ | +++ | + | +++ | ++++ | + |
| ventral region | ||||||
| rostral domain (LSr.m.v.r) | − | + | + | +++ | +++ | + |
| caudal domain (LSr.m.v.c) | − | + | + | ++ | ++ | + |
| ventrolateral zone | ||||||
| dorsal region (LSr.vl.d) | ||||||
| medial domain (LSr.vl.d.m) | +++ | +++ | + | +++++ | ++++ | + |
| lateral domain (LSr.vl.d.l) | ++ | + | + | +++++ | ++++ | + |
| ventral region (LSr.vl.v) | − | + | + | ++ | − | + |
| dorsolateral zone | ||||||
| medial region | ||||||
| dorsal domain (LSr.dl.m.d) | − | + | + | ++++ | ++++ | + |
| ventral domain (LSr.dl.m.v) | +++ | − | − | ++ | − | + |
| lateral region | ||||||
| dorsal domain (LSr.dl.l.d) | − | + | + | +++ | ++ | ++ |
| ventral domain (LSr.dl.l.v) | − | + | + | − | + | − |
| ventral part (LSv) | + | + | − | + | − | − |
| septofimbral nucleus (SF) | + | + | + | +++ | ++ | + |
| septohippocampal nucleus (SH) | − | − | + | + | − | ++ |
| Anterior amygdalar area (AAA) | − | − | − | − | − | + |
| Central amygdalar nucleus | ||||||
| medial part (CEAm) | − | − | + | − | + | + |
| lateral part (CEAl) | − | − | − | − | − | +++ |
| capsular part (CEAc) | − | − | + | − | + | + |
| Medial amygdalar nucleus | ||||||
| anterodorsal part (MEAad) | ++ | + | − | ++ | ++ | ++ |
| anteroventral part (MEAav) | − | + | − | − | + | − |
| posterodorsal part | ||||||
| sublayer a (MEApd-a) | − | − | − | + | + | + |
| sublayer b (MEApd-b) | − | − | + | + | − | − |
| sublayer c (MEApd-c) | − | + | − | + | − | − |
| posteroventral part (MEApv) | − | − | − | + | + | − |
| 1.2.2. Pallidum | ||||||
| Substantia innominata (SI) | − | − | ++++ | + | − | ++++ |
| Medial septal complex | ||||||
| Medial septal nucleus (MS) | ++++ | +++ | +++ | ++ | + | + |
| Diagonal band nucleus (NDB) | ++ | + | +++ | +++ | +++ | + |
| Triangular nucleus septum (TRS) | − | + | + | ++ | − | + |
| Bed nuclei of the stria terminalis | ||||||
| Anterior division | ||||||
| anterolateral area (BSTal) | − | + | +++ | + | − | +++ |
| anteromedial area (BSTam) | − | ++ | ++++ | +++ | + | +++++* |
| dorsomedial nucleus (BSTdm) | ++ | + | +++ | + | + | ++ |
| fusiform nucleus (BSTfu) | − | − | − | − | − | +++ |
| ventral nucleus (BSTv) | − | + | ++ | ++ | + | + |
| magnocellular nucleus (BSTmg) | − | − | ++ | + | − | + |
| Posterior division | ||||||
| principal nucleus (BSTpr) | +++ | ++ | − | +++ | ++ | ++ |
| interfascicular nucleus (BSTif) | +++ | ++ | + | +++ | ++ | ++ |
| transverse nucleus (BSTtr) | − | − | − | + | − | + |
| dorsal nucleus (BSTd) | − | − | − | − | + | − |
| 2. Cerebellum | − | − | − | − | − | − |
| 3. Cerebrospinal Trunk | ||||||
| 3.1 Sensory System | ||||||
| 3.1.1. Thalamus | ||||||
| Sensory-motor cortex related | ||||||
| Ventral group of the dorsal thalamus | ||||||
| subparafascicular nucleus thalamus | ||||||
| magnocellular part (SPFm) | − | + | − | + | − | − |
| parvicellular part | ||||||
| lateral division (SPFpl) | − | − | − | + | − | − |
| Polymodal association cortex related | ||||||
| Lateral group of the dorsal thalamus | ||||||
| lateral posterior nucleus thalamus (LP) | − | + | − | − | − | − |
| Anterior group of the dorsal thalamus | ||||||
| anteroventral nucleus thalamus (AV) | − | ++ | − | + | + | − |
| anteromedial nucleus thalamus | ||||||
| dorsal part (AMd) | − | + | − | − | − | − |
| ventral part (AMv) | − | ++ | − | − | − | − |
| anterodorsal nucleus thalamus (AD) | − | + | − | − | − | − |
| interanteromedial nucleus thalamus (IAM) | − | + | − | − | − | − |
| interanterodorsal nucleus thalamus (IAD) | − | ++ | − | − | − | − |
| lateral dorsal nucleus thalamus (LD) | − | + | − | − | − | − |
| Medial group of the dorsal thalamus | ||||||
| mediodorsal nucleus thalamus | ||||||
| medial part (MDm) | − | ++ | + | − | − | − |
| lateral part (MDl) | − | + | − | − | − | − |
| intermediodorsal nucleus thalamus (IMD) | − | − | ++ | − | − | + |
| perireuniens nucleus (PR) | − | ++ | − | − | − | − |
| Midline group of the dorsal thalamus | ||||||
| paraventricular nucleus thalamus (PVT) | +++ | +++ | ++++ | +++ | ++ | ++ |
| paratenial nucleus (PT) | ++ | ++++ | ++ | + | + | − |
| nucleus reuniens | ||||||
| rostral division | ||||||
| anterior part (REa) | ++ | ++++ | + | − | + | − |
| dorsal part (REd) | − | ++ | − | + | + | − |
| ventral part (REv) | − | +++++ | − | + | − | − |
| lateral part (REl) | ++ | ++++ | − | + | − | − |
| median part (REm) | − | + | + | − | − | − |
| caudal division | ||||||
| caudal part (REc) | − | + | + | + | + | − |
| dorsal part (REcd) | − | + | − | − | − | − |
| median part (REcm) | − | + | − | − | − | − |
| Intralaminar group of the dorsal thalamus | ||||||
| rhomboid nucleus (RH) | − | + | + | − | − | − |
| central medial nucleus thalamus (CM) | + | ++ | ++ | − | + | − |
| central lateral nucleus thalamus (CL) | − | + | + | − | − | − |
| parafascicular nucleus (PF) | − | − | − | + | − | − |
| Reticular nucleus thalamus (RT) | − | + | + | + | − | − |
| 3.1.2. Visual | − | − | − | − | − | − |
| 3.1.3. Somatosensory | − | − | − | − | − | − |
| 3.1.4. Auditory | − | − | − | − | − | − |
| 3.1.5. Gustatory | ||||||
| Nucleus of the solitary tract, medial part, rostral zone (NTSmr) | − | − | +++ | − | − | − |
| 3.1.6. Visceral | ||||||
| Nucleus of the solitary tract | ||||||
| commissural part (NTSco) | − | − | + | − | − | − |
| lateral part (NTSl) | − | − | + | − | − | − |
| medial part, caudal zone (NTSmc) | − | − | +++ | − | + | + |
| Parabrachial nucleus | ||||||
| lateral division | ||||||
| central lateral part (PBlc) | − | − | +++ | + | + | ++ |
| dorsal lateral part (PBld) | − | − | − | − | − | + |
| external lateral part (PBle) | − | − | + | − | − | + |
| ventral part (PBlv) | − | − | + | − | − | +++ |
| medial division | ||||||
| medial medial part (PBmm) | − | − | ++ | − | − | + |
| 3.1.7. Humerosensory | ||||||
| Subfornical organ (SFO) | − | − | − | − | − | ++ |
| 3.2. Behavioral state system | ||||||
| Suprachiasmatic nucleus (SCH) | − | − | − | + | − | − |
| Subparaventricular zone (SBPV) | +++ | +++ | +++ | ++ | − | + |
| Hypothalamic lateral zone, dorsal region (LHAd) | − | ++ | ++++ | + | ++ | ++ |
| Supramammillary nucleus | ||||||
| medial part (SUMm) | − | + | + | + | − | − |
| lateral part (SUMl) | − | ++ | + | + | + | + |
| Pedunculopontine nucleus (PPN) | − | − | ++ | + | − | + |
| Pontine reticular nucleus, rostral part (PRNr) | − | − | ++ | − | − | − |
| Raphé nuclei | ||||||
| interfascicular nucleus raphé (IF) | − | + | − | − | − | − |
| interpeduncular nucleus | ||||||
| lateral subnucleus | ||||||
| intermediate part (IPNli) | − | − | + | − | − | − |
| rostral linear nucleus raphé (RL) | − | + | + | − | − | − |
| central linear nucleus raphé (CLI) | − | − | + | − | − | + |
| superior central nucleus raphé | ||||||
| lateral part (CSl) | − | − | − | − | + | − |
| dorsal nucleus raphé (DR) | − | ++ | +++ | + | − | + |
| nucleus incertus | ||||||
| diffuse part (NId) | − | − | − | + | − | − |
| nucleus raphé magnus (RM) | − | − | + | − | − | − |
| Locus ceruleus (LC) | − | − | + | − | − | − |
| 3.3. Motor System | ||||||
| 3.3.1. Behavior Control Column | ||||||
| Medial preoptic nucleus | ||||||
| lateral part (MPNl) | ++ | + | − | ++ | ++ | ++ |
| medial part (MPNm) | − | + | + | ++ | + | ++ |
| central part (MPNc) | − | − | − | + | − | + |
| Anterior hypothalamic nucleus | ||||||
| anterior part (AHNa) | ++ | + | − | +++++ | +++ | + |
| central part (AHNc) | +++++ | +++++ | + | +++++* | ++++ | +++ |
| posterior part (AHNp) | +++ | +++ | + | ++ | + | + |
| dorsal part (AHNd) | ++ | ++ | − | + | − | − |
| Paraventricular nucleus hypothalamus, descending division | ||||||
| dorsal parvicellular part (PVHdp) | − | + | − | − | − | − |
| lateral parvicellular part (PVHlp) | − | + | ++++ | − | − | + |
| forniceal part (PVHf) | − | − | ++++ | − | − | − |
| Ventromedial hypothalamic nucleus | ||||||
| anterior part (VMHa) | + | ++ | + | + | − | − |
| dorsomedial part (VMHdm) | ++ | +++ | + | ++++ | ++ | ++ |
| central part (VMHc) | ++ | ++ | + | ++ | ++ | + |
| ventrolateral part (VMHvl) | + | ++ | + | +++ | ++ | + |
| Ventral premammillary nucleus (PMv) | − | − | + | + | + | + |
| Dorsal premammillary nucleus (PMd) | ++ | +++++ | − | + | − | + |
| Mammillary body | ||||||
| medial mammillary nucleus | ||||||
| body (MM) | − | ++++ | − | − | − | − |
| median part (MMme) | − | +++ | − | − | − | − |
| Ventral tegmental area (VTA) | − | + | +++ | ++ | ++ | + |
| Midbrain reticular nucleus, retrorubral area (RR) | − | − | ++++ | + | − | − |
| Midbrain reticular nucleus, parvicellular part (MRNp) | − | + | +++ | + | − | − |
| 3.3.2. Superior Colliculus, motor related | ||||||
| Intermediate gray layer | ||||||
| sublayer a (SCig-a) | − | + | − | − | − | − |
| sublayer b (SCig-b) | − | + | ++ | − | + | − |
| sublayer c (SCig-c) | − | + | + | + | + | + |
| Deep gray layer (SCdg) | − | − | + | − | + | − |
| 3.3.3. Postcerebellar and Precerebellar Nuclei | − | − | − | − | − | − |
| 3.3.4. Vestibulomotor regions | − | − | − | − | − | − |
| 3.3.5. Central Gray | ||||||
| Epithalamus | ||||||
| lateral habenula (LH) | +++++ | +++++ | ++ | − | − | − |
| Posterior hypothalamic nucleus (PH) | +++++ | +++++ | +++ | +++++ | +++ | +++++ |
| Periaqueductal gray | ||||||
| precommissural nucleus (PRC) | +++ | +++ | + | +++ | ++ | + |
| commissural nucleus (COM) | − | + | +++ | + | − | ++ |
| rostromedial division (PAGrm) | − | + | ++++ | − | +++ | ++ |
| rostrolateral division (PAGrl) | − | − | + | − | − | − |
| medial division (PAGm) | ++ | +++ | +++ | + | + | − |
| dorsal division (PAGd) | + | + | ++ | ++ | ++ | + |
| dorsolateral division (PAGdl) | − | − | + | ++ | − | − |
| ventrolateral division (PAGvl) | +++ | +++ | +++++ | +++ | ++ | +++ |
| nucleus of Darkschewitsch (ND) | − | − | + | + | − | − |
| Pontine central gray, general | ||||||
| pontine central gray (PCG) | − | + | +++ | + | ++ | +++ |
| lateral tegmental nucleus (LTN) | − | − | + | + | − | ++ |
| Barrington's nucleus (B) | − | − | +++++ | − | − | − |
| 3.3.6. Hypothalamic Periventricular Region | ||||||
| Median preoptic nucleus (MEPO) | − | + | ++ | ++ | + | ++++ |
| Suprachiasmatic preoptic nucleus (PSCH) | − | − | − | − | − | + |
| Anteroventral periventricular nucleus (AVPV) | − | − | − | + | + | ++ |
| Anterodorsal preoptic nucleus (ADP) | − | + | − | + | + | + |
| Anteroventral preoptic nucleus (AVP) | − | − | + | + | − | +++ |
| Parastrial nucleus (PS) | − | + | − | − | − | ++ |
| Medial preoptic area (MPO) | +++ | + | +++ | +++++ | +++ | +++++ |
| Anterior hypothalamic area (AHA) | +++ | ++ | + | ++ | ++ | ++ |
| Dorsomedial hypothalamic nucleus | ||||||
| anterior part (DMHa) | +++ | ++ | ++ | ++ | + | ++ |
| posterior part (DMHp) | − | − | + | + | − | +++ |
| ventral part (DMHv) | − | − | − | ++ | + | ++ |
| Periventricular hypothalamic nucleus, posterior part (PVp) | − | + | − | + | + | ++ |
| Internuclear area, hypothalamic periventricular region (I) | +++ | ++++ | + | +++ | ++ | +++ |
| 3.3.7 Reticular Formation | ||||||
| Hypothalamic lateral zone, motor related | ||||||
| lateral preoptic area (LPO) | ++ | + | +++ | ++++ | ++ | +++ |
| lateral hypothalamic area, motor related (LHAmo) | ||||||
| juxtaparaventricular region (LHAjp) | ++++ | ++++ | ++ | +++ | + | ++ |
| juxtadorsomedial region (LHAjd) | ++ | ++++ | +++ | +++ | ++ | ++ |
| juxtaventromedial region | ||||||
| dorsal zone (LHAjvd) | +++ | +++ | ++ | + | − | + |
| ventral zone (LHAjvv) | +++ | +++ | ++ | +++ | ++ | +++ |
| anterior region | ||||||
| dorsal zone (LHAad) | ++ | +++ | ++ | ++ | ++ | +++ |
| intermediate zone (LHAai) | +++ | ++ | ++ | + | ++ | +++ |
| ventral zone (LHAav) | +++ | ++ | ++ | ++++ | ++ | +++ |
| retrochiasmatic area (RCH) | ++ | ++ | + | ++ | + | ++ |
| tuberal nucleus (TU) | ||||||
| subventromedial part (TUsv) | − | + | + | + | − | + |
| intermediate part (TUi) | − | + | − | + | − | + |
| lateral part (TUl) | − | − | − | + | − | − |
| suprafornical region (LHAs) | +++ | +++ | ++ | + | + | ++ |
| subfornical region | ||||||
| anterior zone (LHAsfa) | − | − | − | + | − | + |
| posterior zone (LHAsfp) | − | + | ++ | ++ | + | ++ |
| premammillary zone (LHAsfpm) | − | + | − | + | + | − |
| magnocellular nucleus (LHAm) | − | − | + | − | − | − |
| parvicellular region (LHApc) | − | − | + | − | − | − |
| ventral region | ||||||
| medial zone (LHAvm) | − | + | + | + | − | ++ |
| lateral zone (LHAvl) | − | − | + | − | − | − |
| posterior region (LHAp) | − | + | +++ | − | + | − |
| preparasubthalamic nucleus (PST) | − | − | + | − | − | − |
| parasubthalamic nucleus (PSTN) | − | − | + | − | − | − |
| Zona incerta, general | ||||||
| zona incerta (ZI) | ++ | +++ | + | +++ | ++ | + |
| pretectal region | ||||||
| posterior pretectal nucleus (PPT) | − | − | − | + | − | − |
| medial pretectal area (MPT) | − | + | − | + | + | − |
| midbrain reticular nucleus, magnocellular part, general | ||||||
| midbrain reticular nucleus, magnocellular part (MRNm) | − | + | +++ | + | + | + |
| ventral tegmental nucleus (VTN) | − | + | − | + | − | − |
| cuneiform nucleus (CUN) | − | − | − | − | + | − |
| pontine reticular nucleus, caudal part (PRNc) | − | − | +++ | − | − | − |
| gigantocellular reticular nucleus (GRN) | − | − | +++ | − | − | − |
| paragigantocellular reticular nucleus (PGRN) | ||||||
| dorsal part (PGRNd) | − | − | + | − | − | − |
| lateral part (PGRNl) | − | − | ++ | − | − | − |
| parapyramidal nucleus (PPY) | ||||||
| deep part (PPYd) | − | − | + | − | − | − |
| magnocellular reticular nucleus (MARN) | − | − | +++ | − | − | − |
| parvicellular reticular nucleus (PARN) | − | − | ++ | − | − | − |
| medullary reticular nucleus (MDRN) | ||||||
| ventral part (MDRNv) | − | − | + | − | − | − |
| 3.3.8. Motoneuron Groups | ||||||
| Neuroendocrine motor zone | ||||||
| magnocellular | ||||||
| paraventricular nucleus hypothalamus, magnocellular division | ||||||
| posterior magnocellular part | ||||||
| medial zone (PVHpmm) | − | − | + | − | − | − |
| lateral zone (PVHpml) | − | ++ | + | − | − | − |
| parvicellular | ||||||
| paraventricular nucleus hypothalamus, parvicellular division | ||||||
| anterior parvicellular part (PVHap) | + | + | ++ | +++ | ++ | +++ |
| medial parvicellular part, dorsal zone (PVHmpd) | − | + | +++ | + | + | + |
| periventricular part (PVHpv) | − | + | + | + | + | + |
| periventricular hypothalamic nucleus, anterior part (PVa) | − | − | − | + | − | + |
| periventricular hypothalamic nucleus, intermediate part (PVi) | − | + | − | + | − | + |
| arcuate hypothalamic nucleus (ARH) | − | + | − | + | − | ++ |
| Preganglionic autonomic pools | ||||||
| parasympathetic | ||||||
| inferior salivatory nucleus (ISN) | − | − | + | − | − | − |
| dorsal motor nucleus of the vagus nerve (DMX) | − | − | ++ | − | − | − |
Labeled fibers, whether projecting from the LHAjp or LHAs, rarely had the sole appearance of fibers of passage; the latter were predominantly confined to clearly identifiable fiber tracts. This is an important observation in so far as it suggests that neurons of the LHAjp and LHAs distribute broadly the signals they convey. Typically, labeled axons displayed numerous varicosities and formed numerous boutons of passage; in addition, their distribution was characterized by much convolution, often giving the appearance of a loose or more tightly woven mesh. Identified terminal fields, in addition to the presence of terminal boutons, also typically contained boutons of passage; furthermore, the terminal field in any given region showed much variation in the density of fiber labeling.
3.2.1. Summary of prominent neuroanatomical connections of the LHAjp and LHAs
3.2.1.1. Anterograde tracing summary
Our analysis of PHAL-labeled axons traced anterogradely from neurons located predominantly within the caudal end of the LHAjp (experiment LHA#22, Figs. 2A, 3) revealed projections to a diverse array of brain regions; most of them are within the diencephalon and prominent among them (in descending order) are the lateral habenula, dorsal premammillary nucleus, posterior and anterior hypothalamic nuclei, nucleus reuniens, and paratenial nucleus. In contrast, brain regions receiving prominent projections from neurons located predominantly within the LHAs (experiment LHA#11, Figs. 2B, 3) include those in the midbrain and hindbrain as well as the diencephalon; these are (in descending order) Barrington’s nucleus, ventrolateral division of the periaqueductal gray (PAG), lateral parvicellular and forniceal parts of the hypothalamic paraventricular nucleus (PVHlp/f), substantia innominata, and midbrain reticular nucleus (principally the retrorubral area).
3.2.1.2. Retrograde tracing summary
Perikarya retrogradely labeled from the LHAjp were abundant (in descending order) in the subiculum and adjacent field CA1 of the hippocampal formation, lateral septal nucleus, anterior hypothalamic nucleus, and posterior hypothalamic nucleus. These findings contrast with retrograde labeling from the LHAs that was prominent (in descending order) in the anteromedial and fusiform nuclei of the bed nuclei of the stria terminalis, medial preoptic area, and posterior hypothalamic nucleus. Labeling from the LHAs within the hippocampal formation was very sparse, in marked contrast to abundant retrograde labeling from the LHAjp.
3.2.2. Detailed description of the neuroanatomical connections of the LHAjp and the LHAs
3.2.2.1. Projections from the LHAjp (PHAL anterograde tracing)
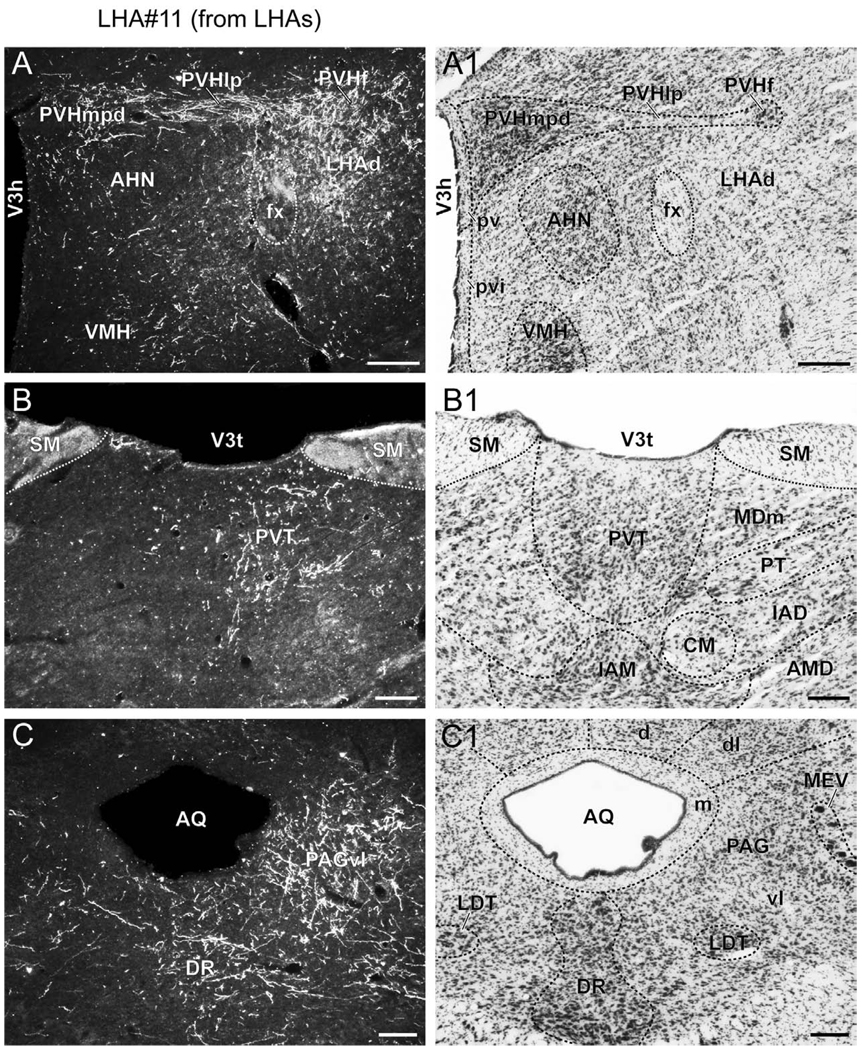
Axons arising from neurons located predominantly within the LHAjp reach their projection targets via several different paths (Fig. 5). The principal routes of egress for fibers as they emerge from the LHAjp are ventral, rostral, dorsal, and caudal; a relatively low abundance emerges laterally, and exceedingly few emerge medially to project into the adjacent PVH (Fig. 4T–W). In addition, there is a moderate contribution of fibers to the nearby column of the fornix, most of which appear to project rostrally (Fig. 4T–U). With regard to possible sources of LHA input to the PVH, it is noteworthy that a projection to the PVH from the LHA does have previous description (Watts et al., 1999; Csaki et al., 2000); however, the source of the projection identified in the earlier work was a region of the LHA caudal and lateral to the LHAjp, corresponding approximately to the LHAd (Watts et al., 1999; Csaki et al., 2000).
Ventrally adjacent to the LHAjp, a plexus of terminal boutons, boutons of passage and fibers of passage is present within the central part of the AHN (Fig. 4U): the posterior part of the AHN receives a lesser input (Fig. 4T–U), and there is very little input to the AHN’s dorsal (Fig. 4V) or anterior (Fig. Q–T) parts. Fibers enter the subparaventricular zone and internuclear regions dorsal and medial to the AHN (Fig. 4Q–V), but very few of these fibers continue on to enter the PVH or the periventricular nucleus; however, they do continue ventrally, to reach the anterior part of the VMH (Fig. 4U). In the vicinity of the anterior part of the VMH, fibers are distributed sparingly in the ventral zone of the juxtaventromedial LHA (LHAjvv; Fig. 4U), in the retrochiasmatic area (Fig. 4T), and rarely in the anterior zone of the subfornical region of the LHA, or the subventromedial part of the tuberal nucleus (principally as fibers of passage; Fig. 4U). Farther rostral, scattered fibers continue on through the medial preoptic and anterior hypothalamic areas, and rostral internuclear regions (Fig. 4M–R); a very light grouping of terminal fibers is present in caudal levels of the anterodorsal preoptic nucleus (Fig. 4O).
The rostral projection from the LHAjp via the column of the fornix appears to generate a moderate terminal field in the lateral and (to a lesser extent) medial septal nuclei (Fig. 4F–O); fibers also extend to the septofimbral (Fig. 4N–O) and diagonal band nuclei (Fig. 4H–M), although these are more sparsely populated with boutons. It is principally the rostral part of the lateral septal nucleus that is the major septal region recipient of projections from the LHAjp (Fig. 4F–N), and most prominent among the receiving rostral subdivisions are dorsal regions of the medial (LSr.m.d; Fig. 4J–L) and in particular the ventrolateral (LSr.vl.d; Fig. 4F–J) zones. In addition, a lower density of terminal fibers is distributed widely across the lateral septal nucleus, mostly within its rostral part. In all, a total of fourteen identified subdivisions of the lateral septal nucleus receive an input to a moderate or lesser degree. In general agreement with our findings, a moderate projection to lateral and a lesser projection to medial septal nuclei from a region of the LHA showing correspondence to the caudal end of the LHAjp has been described previously (Roeling et al., 1993b). In more specific agreement with our findings, a later study reported numerous back-labeled neurons in a perifornical region adjacent to the AHN (i.e., the LHAjp) following a deposit of the tracer fluorogold centered within the LSr.v (Risold and Swanson, 1997).
The axons that emerge laterally from the LHAjp are sparsely populated with boutons of passage; these fibers give rise to a comparatively minor projection whose fibers course rostral-caudal through LHA regions lateral to the fornix. This lateral projection field remains mostly within about the medial two-thirds of the lateral tier of the LHA; it extends caudally into the posterior group of the LHA. Its rostral course leads through the anterior group of the LHA, in the intermediate (LHAai) and ventral (LHAav) zones, to a light terminal field in the BSTif (Fig. 4Q–R), and a smattering of terminal fibers in the adjacent principal nucleus (BSTpr); in small amounts fibers also reach the lateral preoptic area (Fig. 4O–P). In addition to its rostrocaudal extension, a very few fibers from the lateral path appear to continue laterally before entering anterior parts of the medial amygdalar nucleus (Fig. 4S–T). At least one previous retrograde tracing study has described an output from the LHA to the BST (Weller and Smith, 1982); however, in that study (using horseradish peroxidase as the tracer) the particular LHA region that was the source of the output was not made clear, neither was it stated in which BST cell groups the injections were made. Similarly, in a previous PHAL study, a strong projection to the BST from the LHA was described (Roeling et al., 1993b), but the receiving BST cell groups were not identified, nor was the full extent of the PHAL injection sites in the LHA shown.
A moderate portion of the fibers that emerge dorsally from the LHAjp enter medial regions of the zona incerta and travel caudally within it, to the level of the posterior hypothalamic nucleus (Fig. 4 U–Y); most of these fibers are distributed rostrally within the zona incerta (Fig. 4 U–W). To reach the zona incerta, some of the fibers emanating from the LHAjp appear to traverse the PVHlp as fibers of passage, passing perpendicular to it without giving rise to terminal boutons (Fig. 4V). Directly adjacent to rostromedial regions of the zona incerta, and dorsomedial to the LHAjp, a dense terminal field is present within the rostral division of the thalamic nucleus reuniens in its ventral (REv), anterior (REa), and lateral (REl) parts (Fig. 4 R–U). This finding is consistent with a report showing very abundant retrograde labeling in what appears to correspond to dorsocaudal regions of the LHAjp following injection of the retrograde tracer fluorogold into the rostral division of the nucleus reuniens (McKenna and Vertes, 2004); earlier studies reported a similar finding (Risold and Swanson, 1995; Risold et al., 1997; McKenna and Vertes, 2004). Dorsal to the hypothalamic part of the third ventricle, some labeled fibers cross the midline and a light terminal field is present in the contralateral nucleus reuniens, approximately mirroring the fiber distribution on the tracer-injected side (Fig. 4R–U). Dorsal to the REa, a moderate terminal field is present in the paratenial nucleus (Fig. 4R–U); medial to the REa, numerous fibers extend caudally through rostral regions of the thalamic paraventricular nucleus (Fig. 4R).
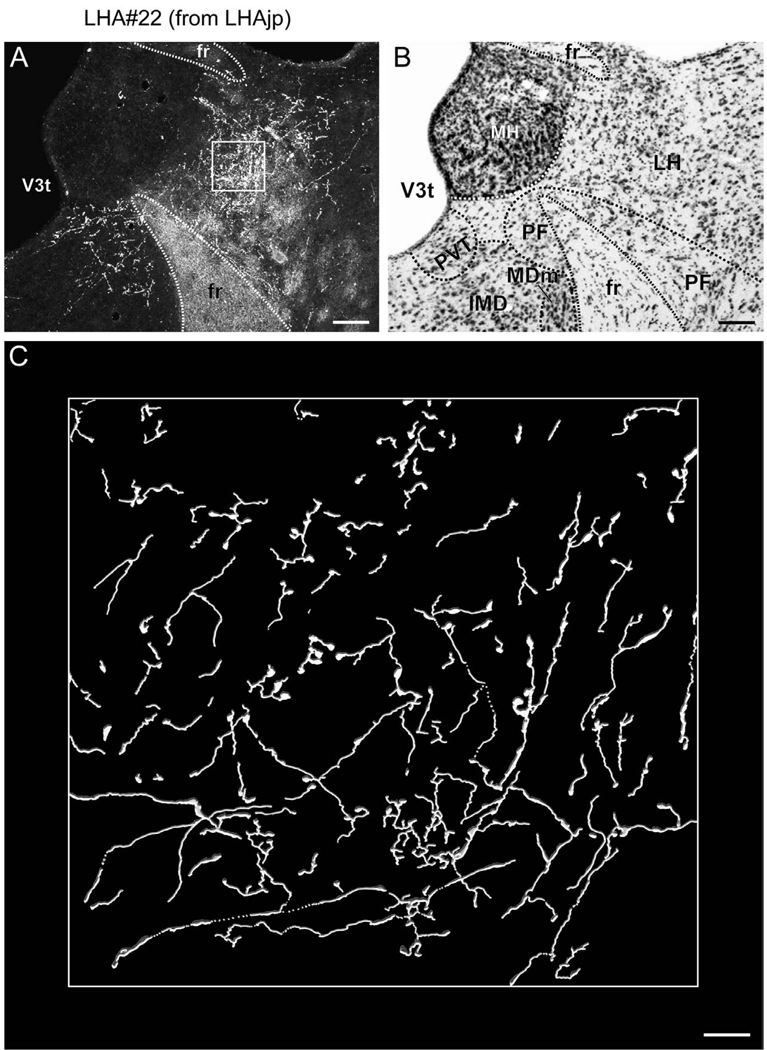
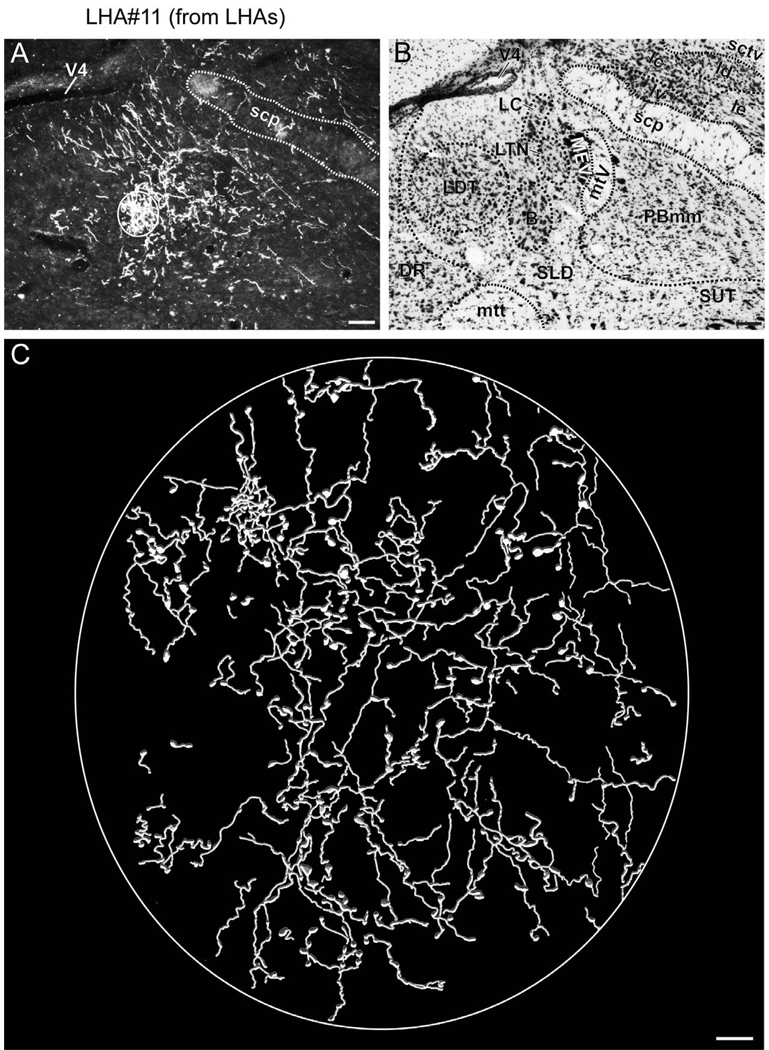
At the level of the rostral division of the nucleus reuniens, numerous labeled fibers are apparent within the stria medullaris; these give rise to an extremely dense terminal field within the lateral habenula (Figs. 4V–BB, 6A, 7). Within the lateral habenula the terminal field is for the most part differentiated into a dorsal and a ventral portion, and except for the most caudal level most of the fibers are present within the medial half. Several previous tracing studies confirm an input to the lateral habenula from LHA regions including one corresponding to the LHAjp, in the rat (Conrad and Pfaff, 1976b; Herkenham and Nauta, 1977; Parent et al., 1981; Shinoda and Tohyama, 1987; Ray et al., 1992), cat (Parent et al., 1981), and monkey (Parent et al., 1981); in addition, in the rat, this projection may include an enkephalinergic component (Shinoda and Tohyama, 1987). Further consistent with our results, previous tracing studies have also revealed in the rat a projection to the paraventricular thalamic nucleus (PVT) (Conrad and Pfaff, 1976b; Chen and Su, 1990; Kirouac et al., 2006), paratenial nucleus (Conrad and Pfaff, 1976b; Chen and Su, 1990), and rostral division of the nucleus reuniens from the LHA region including the LHAjp (Conrad and Pfaff, 1976b).
Figure 6.
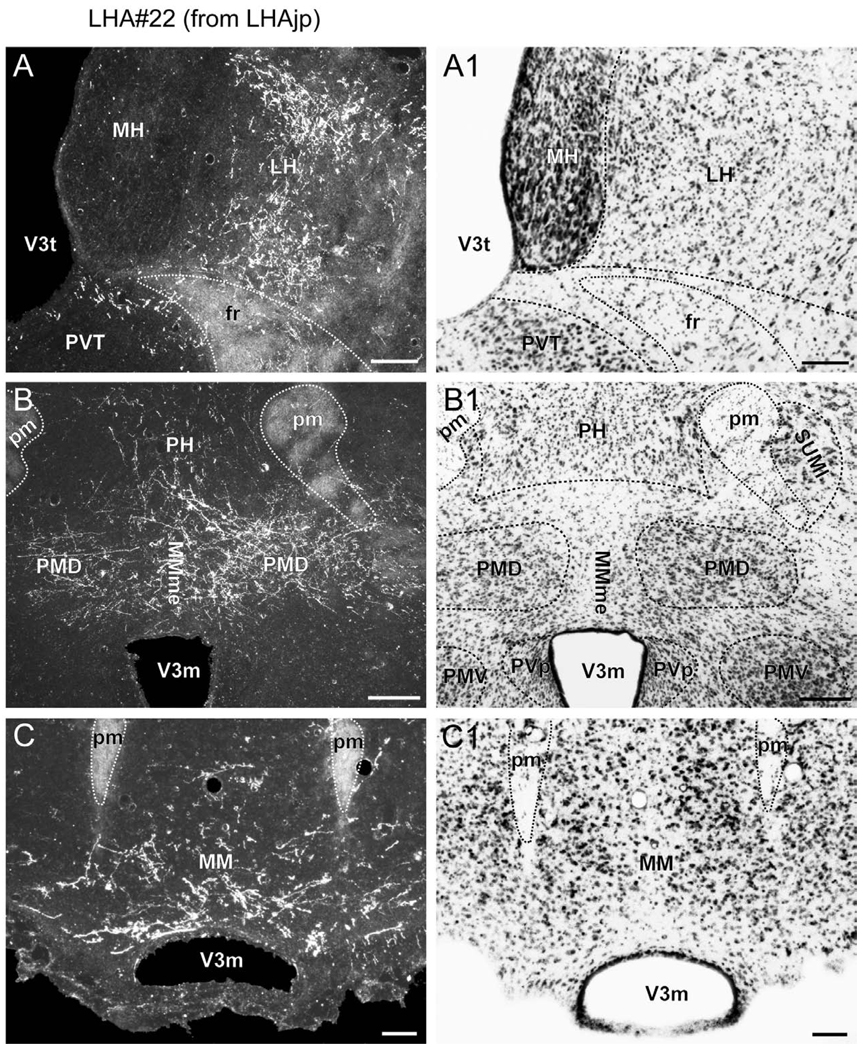
(A to C) Representative darkfield photomicrographs showing axons immunoreactive for Phaseolus vulgaris leucoagglutinin (PHAL) tracer in several brain regions following a deposit of PHAL within caudal levels of the lateral hypothalamic area juxtaparaventricular region (LHAjp) (experiment LHA#22). (A1 to C1) Brightfield photomicrographs of adjacent thionin stained sections. Note the presence of substantial labeling in: (A) lateral habenula (LH), (B) dorsal premammillary nucleus (PMd), and (C) medial mammillary nucleus (MM). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 100 µm in (A) and (A1), 200 µm in (B) and (B1), and 50 µm in (C) and (C1).
Figure 7.
(A) Representative darkfield photomicrograph showing axons immunoreactive for Phaseolus vulgaris leucoagglutinin (PHAL) tracer in the region of the lateral habenula (LH) following a deposit of PHAL within caudal levels of the lateral hypothalamic area juxtaparaventricular region (LHAjp) (experiment LHA#22). (B) Brightfield photomicrograph of adjacent thionin stained section. (C) Digitally rendered camera lucida drawing of the area delineated by the white box in (A). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 100 µm in (A) and (B), 10 µm in (C).
As we have described, by far the major portion of thalamic input arising from the LHAjp is present within the lateral habenula, PVT, PT, and RE. Nevertheless, a minor portion of thalamic input, in the form of sparsely distributed fibers, is also present in the anteroventral (AV), anteromedial (AM), anterodorsal (AD), interanteromedial (IAM), interanterodorsal (IAD), central medial (CM), mediodorsal (MD) (rostrally), lateral dorsal (LD), and RT (dorsal part) thalamic nuclei (Fig. 4S–BB). Together, these findings of thalamic (including zona incerta) input from the LHAjp are in general agreement with an earlier less comprehensive PHAL study in the rat (Roeling et al., 1993b).
Several components of the LHA caudal to the LHAjp receive an input from its neurons. In particular, the LHAjd (immediately caudal to the LHAjp) receives an extensive input (Fig. 4V–W); a less extensive fiber field is present in the LHAjvd, LHAjvv, and ventral levels of the LHAs (Fig. 4 U–Z). In the vicinity of the LHAs, there is a group of labeled fibers with terminals that forms a tight cluster adjacent to the fornix, within the LHAjvv, LHAjvd, LHAs, and posterior hypothalamic nucleus (Fig. 4Y–Z). Medial to the LHA, the dorsomedial and ventromedial nuclei of the hypothalamus receive a light input from the LHAjp; for the dorsomedial nucleus this is mostly within its anterior part (Fig. 4W), and for the ventromedial nucleus this is within its anterior and dorsomedial parts (Fig. 4U–W). A previous study of inputs to the dorsomedial nucleus using fluorogold found a low density of retrograde labeling in the region of the LHAjp (Thompson and Swanson, 1998). A very large projection reaches the posterior hypothalamic nucleus, and this extends dorsally and caudally mostly as fibers of passage, predominantly within medial regions (Fig. 4X–BB). A previous retrograde tracing study examining the afferent connections of the posterior hypothalamic nucleus showed a notable absence of retrograde labeling in a region of the LHA corresponding to the LHAjp (Abrahamson and Moore, 2001); this result is consistent with our present finding of numerous fibers of passage within the posterior hypothalamic area, arising from the LHAjp.
At the level of the posterior hypothalamic nucleus a very dense terminal field is present in the dorsal premammillary nucleus (PMd) (Figs. 4BB, 6B), apparently arriving there via a caudal route within the fornix. This projection was of much higher magnitude from the caudal end of the LHAjp (experiment LHA#22) than from the rostral half of the LHAjp (experiment LHA#62). Furthermore, the projection to the PMd is strikingly bilateral, with fibers appearing to reach the contralateral side via a route across the midline dorsal to the third ventricle at the level of the PMd (Figs. 4BB, 6B); the terminal field within the PMd is denser medially than laterally. In addition to targeting medial regions of the PMd, a moderate density of labeled fibers continue on to form a bilateral terminal field within the body of the medial mammillary nucleus, noticeably limited to its ventral half (Figs. 4CC, 6C); some of these fibers appear to enter the principal mammillary tract and then continue rostrally through the mammillothalamic tract, contributing to the light anterior thalamic projection already described. Consistent with our results, a previous retrograde tracing study of the afferent connections of the PMd reported cells in a region corresponding to the caudal end of the LHAjp, and they also reported a significant amount of contralateral labeling (Comoli et al., 2000). However, the level of retrograde labeling they reported in the caudal end of the LHAjp does appear to be lower than might be predicted by the density of the terminal field we found in the PMd. This could be accounted for by the extent of the injection site that formed the basis of their primary analysis because it appeared to barely include the medial PMd region where we observed much PHAL labeling from the LHAjp. Another factor may be the difficulty in determining the boundary between the LHAjp and immediately adjacent LHAjd because a higher abundance of retrograde labeling was observed rostrally in the LHAjd from the PMd (Comoli et al., 2000). It is also possible that relatively few cells in the caudal end of the LHAjp send axons to the PMd that ramify there greatly.
Numerous ribbons of fibers ascend dorsally within the posterior hypothalamic nucleus and rostromedial division of the periaqueductal gray (PAGrm; Fig. 4BB–DD). Within the PAG, some of these fibers pass through the precommissural nucleus (Fig. 4CC–DD), giving off boutons of passage en passant; at these levels a very light projection extends dorsally into the medial pretectal area (Fig. 4DD–FF), and caudally into medial regions of the superior colliculus (Fig. 4DD–HH). Farther caudal, a light terminal field is present within predominantly rostral levels of the ventrolateral division of the PAG and (to a much lesser extent) its medial and dorsal subdivisions (Fig. 4GG–LL). Consistent with these results, a previous CTB study of the connections of the precommissural nucleus found a moderate amount of retrograde labeling in an LHA region corresponding to the caudal end of the LHAjp (Canteras and Goto, 1999a). An earlier study using horseradish peroxidase as the retrograde tracer reported a large input to the ventrolateral and (to a lesser extent) dorsolateral divisions of the PAG from the LHA as a whole (Beitz, 1982); however, further direct comparison with the earlier study and our present data is not possible because regional LHA parcellation was not specified (Beitz, 1982). No PHAL-labeled axons arising from neurons within the LHAjp were found to extend caudal to the PAG in the pontine central gray.
3.2.2.2. Sources of projections to the LHAjp (CTB retrograde tracing)
3.2.2.2.1. Cerebral cortex
A striking difference between the regions targeted by projections from the LHAjp just described, and the sources of its inputs that we now describe, is the existence of a massive direct input to this moderately sized medial tier LHA region from the cerebral cortex. The major source of this cortical input is hippocampal; a more moderate input comes from the medial prefrontal cortex. Retrograde labeling in the hippocampal formation was localized mostly to the pyramidal layer of its intermediate region, and foremost among the sources of hippocampal input to the LHAjp was the subiculum. A moderate abundance was also present in field CA1 of the intermediate region. Labeling also reached to the dorsal or septal pole of the subiculum and field CA1, but not quite the ventral or temporal pole. In transverse Atlas Levels this translates to the presence of very abundant labeling caudally, with progressively less observed more rostrally (Fig. 4FF–LL; see also Fig. 8A, B). In addition, a small amount was present in the parasubiculum, and a very few labeled neurons were present in the presubiculum, medial entorhinal area, and hippocampal field CA3 (Fig. 4 JJ–OO). It is noteworthy that labeling in intermediate regions of the subiculum was by far the most abundant of any brain region.
Figure 8.
(A to D) Representative brightfield photomicrographs showing perikarya immunoreactive for cholera toxin B subunit (CTB) tracer in several brain regions. (A1 to D1) Thionin stained sections adjacent to the sections shown in (A to D). Images (A) and (B) show retrograde labeling in the dorsal (A) and ventral (B) halves of the intermediate third of the subiculum resulting from a CTB deposit within caudal levels of the lateral hypothalamic area, juxtaparaventricular region (LHAjp) (experiment LHA#22). Images (C) and (D) show retrograde labeling in the region of the bed nuclei stria of the terminalis anteromedial area (BSTam) and fusiform nucleus (BSTfu) (C), and central amygdalar nucleus lateral part (CEAl) (D) resulting from a CTB deposit within midrostrocaudal levels of the lateral hypothalamic area, suprafornical region (LHAs) (experiment LHA#11). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 100 µm.
The moderate amount of retrograde labeling present within the medial prefrontal cortex was the sum of a lower incidence of retrogradely labeled neurons in infralimbic (layers 5 and 6a), prelimbic (layer 5), and anterior cingulate (layer 5) areas (Fig. 4A–N); a small-moderate amount of cortical labeling was also present at an intermediate level in the ventral part of the retrosplenial area (layer 5) (Fig. 4FF–II). In addition to these cortical areas, a very low incidence of labeled neurons was also present in rostral levels of the secondary somatosensory area (layer 5; Fig. 4F) and the orbital area (medial, ventral, and ventrolateral parts; Fig. 4A–B); furthermore, a low incidence of retrograde labeling was found ventrally adjacent to the infralimbic area, in layer 2 of the dorsal part of the tenia tecta (Fig. 4F–G). Finally, within the cortical subplate, a very few cells were also present within the basolateral and basomedial amygdalar nuclei (Fig. 4Z, CC).
In comparing our data with the fruit of earlier work, we find that a fairly recent study of the hypothalamic projections of the subiculum showed essentially the same magnitude and distribution of retrograde labeling in the subiculum (and field CA1) as we observed following a deposit of CTB shown to include a region corresponding to the caudal end of the LHAjp (Kishi et al., 2000); in addition, in the same study, following an injection of PHAL at a site within the intermediate third of the subiculum, the authors describe a “dense plexus of labeled axons diverging from the postcommisural fornix…in an area between the PVH and fornix” (Kishi et al., 2000) -- i.e., caudally within the LHAjp. A dense terminal field in the region between the PVH and fornix arising from the intermediate third of the subiculum is also described in previous PHAL studies (Witter et al., 1990; Cullinan et al., 1993); an early study of the projections of the fornix in the rat using the degeneration method also indicated a projection to the region of the LHAjp (Nauta, 1956). Conversely, and also consistent with our results, several previous studies have shown that the ventral (temporal) and dorsal (septal) ends of Ammon’s horn and the subiculum project comparatively little to caudal regions of the LHAjp (Swanson and Cowan, 1975; Swanson and Cowan, 1977; Witter and Groenewegen, 1990; Canteras and Swanson, 1992a; Cenquizca and Swanson, 2006).
With regard to the moderate amount of retrograde labeling we found in the medial prefrontal cortex from the LHAjp, several previous tracing studies in the rat together provide a robust confirmation of the topography and magnitude of this projection, for example: (Hosoya and Matsushita, 1980; Luiten et al., 1987; Brittain, 1988; Sesack et al., 1989; Hurley et al., 1991; Floyd et al., 2001; Pajolla et al., 2001; Vertes, 2004; Gabbott et al., 2005). Projections from prefrontal cortex to the LHA have also been reported in species other than rat: thus, one previous study with PHAL in the rabbit described such projections, although these were reported to target an LHA region just lateral to the fornix (Buchanan et al., 1994); however, an earlier study in the cat (with autoradiographic tracing) described a projection from infralimbic and prelimbic areas to LHA regions including medial levels of the LHA (Room et al., 1985). Very dense projections to medial levels of the LHA from the medial prefrontal cortex have also been described with anterograde tracing methods in Old World (macaque) (Ongur et al., 1998; Rempel-Clower and Barbas, 1998) and more recently New World (marmoset) (Roberts et al., 2007) monkeys.
The low incidence of labeling we found in the basomedial amygdalar nucleus is corroborated by a previous PHAL study of this region (Petrovich et al., 1996); however, we could not find a comparable previous study for the basolateral amygdalar nucleus. Finally, we found only one previous study in which the efferent connections of the rat ventral part of the retrosplenial area had been studied, and there no mention was made of a projection to the hypothalamus (van Groen and Wyss, 1990); furthermore, to our knowledge, no previous tracing study has examined possible hypothalamic projections of the tenia tecta. Our finding of a small amount of retrograde labeling in the ventral part of the retrosplenial area and dorsal part of the tenia tecta from the LHAjp therefore appears to be novel.
3.2.2.2.2. Cerebral nuclei
3.2.2.2.2.1. Striatum
Aside from the extensive cerebral cortical input, the brain region showing the next most abundant retrograde labeling from the LHAjp was the lateral septal nucleus, where retrograde labeling was mostly present in the rostral part (Fig. 4F–N), with comparatively little in the caudal part (Fig.4 H–O). The subdivision of the rostral part of the lateral septal nucleus showing most abundant retrograde labeling from the LHAjp was the dorsal region (and lateral and medial domains) of the ventrolateral zone (LSr.vl.d.l/m), this was followed by the dorsal region of the medial zone (LSr.m.d) and then the medial region (and dorsal domain) of the dorsolateral zone (LSr.dl.m.d). Two more subdivisions of the lateral septal nucleus showed a small amount of labeling; these were the medial zone (central region) of the rostral part (LSr.m), and the dorsal zone (rostral region) of the caudal part (LSc.d.r). A lower amount of retrograde labeling, ranging from a few to about 10 retrogradely labeled neurons was also present in the following lateral septal subdivisions (see table of abbreviations for explanation): LSc.d.d, LSc.d.v, LSc.v.i, LSc.v.l.d, LSc.v.l.v, LSc.v.m.d, LSr.dl.l.d, LSr.dl.l.v, LSr.dl.m.v, LSr.m.v.c. It is noteworthy that the two lateral septal regions showing most abundant retrograde labeling from the LHAjp were the same two regions with the most extensive terminal field arising from the LHAjp (principally from its rostral half), namely: LSr.vl.d.m/l (Fig. 4 F–J) and LSr.m.d (Fig. 4J–L).
Among the most prominent of studies that have examined the projections of the lateral septal nucleus, an extensive tract-tracing study in the rat stands out in which they were examined comprehensively (Risold and Swanson, 1997). It was shown in the previous study that a PHAL injection into either the region of the LSr.vl.d or LSr.m.d would give rise to a moderate terminal field in the LHA region situated between the fornix and PVH (Risold and Swanson, 1997). Similarly, injections of the retrograde tracer fluorogold into regions of the AHN that included a portion of the LHAjp at rostral (their experiment AH3) or caudal (their experiment AH1) levels, resulted in numerous back-labeled cells in the LSr.vl.d.l/m (their experiment AH3) (Risold and Swanson, 1997); these results are consistent with our present findings. A projection from the lateral septal nucleus to the LHA has also been described in several earlier tract-tracing studies in species including (for example) rat (Meibach and Siegel, 1977; Kita and Oomura, 1982; Deller et al., 1994), cat (Krayniak et al., 1980), and pigeon (Krayniak and Siegel, 1978); however, the opportunity for direct comparison of these earlier studies with our present findings is limited because none describe the detail of this projection with respect to the precise delineations we present here.
Apart from the substantial amount of retrograde labeling in the lateral septal nucleus, we found very little retrograde labeling from the LHAjp in any other striatal cerebral nuclei. Nevertheless, a small amount of retrograde labeling was present in the septofimbral nucleus (Fig. 4N–R), and farther caudal a few cells were retrogradely labeled in the medial part of the central amygdalar nucleus (Fig. 4U); slightly more were back-labeled in anterior and (to a lesser extent) posterior parts of the medial amygdalar nucleus (Fig. 4S–X). In agreement with the latter finding, a previous PHAL study of the projections from the medial amygdalar nucleus revealed a predominantly light projection to the vicinity of the LHAjp from its anterior (and to a lesser extent) posterior parts (Canteras et al., 1995).
3.2.2.2.2.2. Pallidum
Two pallidal regions showed some degree of retrograde labeling from the LHAjp, although this was not particularly abundant. A moderate amount of retrograde labeling was present in the diagonal band nucleus (Fig. 4J–M); a similarly moderate amount was divided between several of the bed nuclei of the stria terminalis (BST). Within the BST, retrograde labeling (although modest overall) was most abundant in its posterior division, in the interfascicular nucleus (BSTif; Fig. 4Q–R), followed by the principal nucleus (BSTpr; Fig. 4Q). A very few cells were also back-labeled in the anterior division of the BST, in its dorsomedial nucleus (BSTdm) and anteromedial (BSTam) areas (Fig. 4M–P). Few studies appear to have examined the projections of the diagonal band nucleus with respect to the LHA; however, in general agreement with our present findings, at least two previous retrograde tracing studies in the rat have described (at relatively low spatial resolution) an output from the diagonal band nucleus to the LHA (Shiosaka et al., 1980; Kita and Oomura, 1982). Conversely, at higher spatial resolution, and also in agreement with our findings, a moderate projection from the BSTif and (to a lesser extent) the BSTpr to the region of the LHAjp is confirmed in a previous PHAL study (Dong and Swanson, 2004b); an earlier autoradiographic study also reported a similar finding (Swanson, 1976). The very low incidence of back-labeling we found in anterior levels of the BSTam is also confirmed by a previous PHAL study of the BST that indicated a very light projection from the BSTam to the LHAjp (Dong and Swanson, 2006a). However, our finding of very few cells in the BSTdm back-filled from caudal levels of the LHAjp is somewhat at odds with the findings of a previous PHAL study that indicated a light to moderate projection from the BSTdm to the LHAjp (Dong and Swanson, 2006b).
3.2.2.2.3. Cerebrospinal trunk
3.2.2.2.3.1. Sensory System
A small amount of retrograde labeling was present in regions classed as being part of the sensory system of the cerebrospinal trunk (although it should be noted that our analysis did not extend to the spinal cord), and most of this was within the thalamic paraventricular nucleus (PVT) at rostral levels (Fig. 4R–U). A very low incidence of labeling was also present at rostral levels within the anterior thalamic nuclei, the paratenial nucleus, and the central medial nucleus (Fig. 4 R–T). The only other regions of the sensory system we found to contain any retrograde labeling from the LHAjp were the viscerosensory-related caudal zone of the medial part of the nucleus of the solitary tract, and the lateral division of the parabrachial nucleus; however, the retrograde labeling in the latter two regions consisted of single isolated cells. Consistent with our findings, a very recent PHAL study of projections from the PVT shows a light input to the LHA region situated between the PVH and fornix (Vertes and Hoover, 2008); in contrast, a previous PHAL study of PVT output in the rat showed the region of the LHAjp to be almost devoid of labeled fibers (Moga et al., 1995), whereas labeling was reported to be more pronounced in more caudal LHA regions, ventral and lateral to the fornix (Moga et al., 1995). Nevertheless, an even earlier PHAL study did report a light terminal field in the region surrounding the magnocellular part of the PVH (which would include the LHAjp) (Puigbonet and Ciriello, 1991); furthermore, a later anterograde tracing study with DiI, also in the rat, identified broadly the LHA, and in particular its perifornical region, as one of the main efferent targets of the PVT (Arluison and Derer, 1993). In addition, a previous HRP retrograde tracing study in the cat identified the PVT as a region that provides a light output to a region of the LHA that appears to include the LHAjp (Smith and Flynn, 1980).
3.2.2.2.3.2. Behavioral State System
There was very little retrograding labeling in regions that form part of the behavioral state system of the cerebrospinal trunk; most of this was present in the dorsal region of the LHA (LHAd; Fig. 4V–W); isolated cells were also present in the hypothalamic subparaventricular zone, the lateral part of the supramammillary nucleus, and the superior central nucleus of the raphé. One recent PHAL study that examined projections from the subfornical region of the LHA (Goto et al., 2005), also described the results of an injection that included (but was not restricted to) rostral levels of the LHAd; a moderate projection to the LHAjp was noted (Goto et al., 2005).
3.2.2.2.3.3. Motor System
The bulk of cerebrospinal trunk retrograde labeling from the LHAjp was present within components of the motor system (Swanson, 2004), yet overall this was moderate (at most) in comparison to the combined labeling in the cerebral cortex and cerebral nuclei. Much of the labeling was present within regions classed as part of a behavior control column, and foremost among these regions was the anterior hypothalamic nucleus (AHN), which contained a moderate to high abundance of retrogradely labeled perikarya; these were distributed between the central (AHNc; Fig. 4R–U) and anterior (AHNa; Fig. 4Q–T) parts of the nucleus. For our experiment LHA-22, most of the labeled cells were present in the AHNc; however, CTB injections that were concentrated in the rostral half of the LHAjp (e.g., experiment #LHA62, Fig. 3) produced a far greater abundance of labeling in the AHNa and AHNc overall, and proportionally most of this was in the AHNa (Table 1). Rostral to the AHN, a very small amount of labeling was widely distributed in the lateral part of the medial preoptic nucleus (MPNl). Consistent with the AHN retrograde labeling, a previous PHAL study of projections from the AHN in the rat described a dense input to a region corresponding to the LHAjp from the AHNc and AHNa (Risold et al., 1994); a similar finding was described in a more recent PHAL study in the golden hamster (Delville et al., 2000). A previous PHAL study of the projections from the MPN (Simerly and Swanson, 1988) showed in one experiment involving the MPNl the presence of a light fiber plexus in the vicinity of the LHAjp (Simerly and Swanson, 1988).
Caudal to the PVH, a moderate amount of labeling was present in the hypothalamic ventromedial nucleus (VMH; Fig. 4U–Y), and this was mostly within the dorsomedial (VMHdm) part; however, a smaller amount was also present in the ventrolateral (VMHvl) and central (VMHc) parts. Caudal to the VMH, a very small group of cells was back-labeled in the rostral tip of the ventral tegmental area (Fig. 4CC), and a few cells were back-labeled in the ventral premammillary nucleus (PMv; Fig. 4AA–BB). Our finding of retrograde labeling in the VMH from the LHAjp is largely in agreement with a previous PHAL study of projections from the VMH that reported a terminal field in this LHA region from the VMHc, VMHvl, and VMHdm, with the latter part providing the most robust input (Canteras et al., 1994). In addition, a previous study of projections from the PMv revealed a light terminal field through the vicinity of the LHAjp (Canteras et al., 1992); similar findings for the VTA do not appear to have previous report.
The superior colliculus contained a small amount of retrograde labeling from the LHAjp (Fig. 4II–LL); more specifically this was within its intermediate (most in sublayer c) and deep gray layers. This observation does not appear to have previous wide confirmation; however, in a brief report of a previous anterograde tracing study of the superior colliculus in the rat and rabbit using the autoradiographic method, a projection to the LHA is mentioned, although its detail is not described (Fallon and Moore, 1979). Several central gray regions contained a moderate amount of retrograde labeling. This was most abundant in rostral levels of the posterior hypothalamic nucleus (PH) and PAG (Fig. 4Z–HH). Within the PAG, most of the back-labeled cells were present within the rostromedial division (PAGrm); a small group of cells was also present in the adjacent precommissural nucleus of the PAG. By comparison to the PAGrm, a lesser amount of labeling was present within mostly rostral levels of the ventrolateral division (PAGvl); a lower incidence was present in the dorsal (PAGd) division, and farther caudal within the pontine central gray (Fig. 4PP).
In agreement with our finding of moderate retrograde labeling in the PH from the LHAjp, a projection from rostral levels of the PH was shown in an earlier PHAL study to end partly in the vicinity of the LHAjp (Vertes et al., 1995). A previous PHAL study of the projections from selected PAG divisions described a high density of fibers within the LHA arising from rostral levels of the PAGvl (Cameron et al., 1995); these were shown mostly in an LHA region lateral to the fornix, however also shown, and consistent with our present results, was a lighter input to a region corresponding to the LHAjp (Cameron et al., 1995). Our finding of retrograde labeling in the precommissural nucleus (Fig. 4 CC–DD) is confirmed by a previous PHAL study that described an output from that region to the vicinity of the LHAjp (Canteras and Goto, 1999a). However, the density of the terminal field in the vicinity of the LHAjp shown in the previous study is higher than might be predicted by the relatively small amount of retrograde labeling we found in the precommissural nucleus; this may be accounted for by inclusion of PHAL-labeled perikarya in the subjacent PAGrm (Canteras and Goto, 1999a) where we also found cells retrogradely labeled from the LHAjp. In another previous PHAL study of projections from the PAG, a dense terminal field in the vicinity of the LHAjp was shown to arise from both the commissural nucleus and the PAGrm (Krout and Loewy, 2000).
A few parts of the hypothalamic periventricular region contained a moderate amount of retrograde labeling from the LHAjp; these were the medial preoptic area (MPO; Fig. 4N–R), and to a lesser extent the caudally continuous anterior hypothalamic area (AHA; Fig. 4Q–R). Besides the latter two regions, very little retrograde labeling was present elsewhere within the periventricular region -- a very small amount was present in the median preoptic nucleus, and a similarly small amount was present in the anterior and ventral parts of the dorsomedial nucleus. Few previous tracing studies have looked specifically at the connections of either the AHA or the MPO; however, a couple of early studies did examine the outputs of these regions in the rat with the autoradiographic tracing method (Conrad and Pfaff, 1976a; Conrad and Pfaff, 1976b). In agreement with our present results, the previous autoradiographic studies do show that the MPO (Conrad and Pfaff, 1976a) and AHA (Conrad and Pfaff, 1976b) both appear to generate a light projection to the hypothalamic region situated between the PVH and fornix; for the AHA, in addition to its efferent connections in the rat, similar findings with the autoradiographic method in another early study were reported for the cat and squirrel monkey (Saper et al., 1978). An extensive PHAL study of projections from the DMH showed few fibers within the region between the PVH and fornix, and these were described mostly as fibers of passage (Thompson et al., 1996); this is consistent with the very small amount of retrograde labeling we found within the DMH.
Several regions within the reticular formation (mostly in the rostral end, the LHA) were together retrogradely labeled to a moderately high extent. The highest abundance of this intra-LHA labeling was present within the LHAjvv (Fig. 4U–Y). In addition, a low-moderate amount of retrograde labeling was present in the LHAjd and LHAav; low amounts were also present in the LHAad, LHAai, subfornical region of the LHA, and more rostral levels of the LHAjp and LHAs. Elsewhere in the reticular formation there was relatively little retrograde labeling; nevertheless, a low incidence of labeled cells was present in the zona incerta (mostly in medial regions; Fig. 4 U–W) and in the lateral preoptic area (at rostral levels; Fig. 4 J–O). Isolated cells were also labeled in the retrochiasmatic area, midbrain reticular nucleus, and medial pretectal area. Finally, very little retrograde labeling was present in the neuroendocrine motor zone of the hypothalamic paraventricular nucleus (PVH; Fig. 4P–V), and the little that was present was mostly within its anterior parvicellular part (PVHa); a very few cells were also labeled in the dorsal zone of the medial parvicellular (PVHmpd), and periventricular (PVHpv) parts.
Very few studies have examined the connections of the zona incerta; however, in agreement with our results, one previous PHAL study of medial levels of the zona incerta in the rat did report a light projection in and around the LHAjp (Wagner et al., 1995). Similarly few studies have examined local projections from the PVH; nevertheless, one previous intracellular filling study showed boutons of passage on neuroendocrine CRH neuron axons in the region of the LHAjp (Rho and Swanson, 1987), and a PHAL study showed a few axons projecting from the PVHmpd to the LHA region just lateral to the PVH, that is, caudally in the LHAjp (Roeling et al., 1993b). At present, there exists no systematic study of intra-LHA connections. However, an already referred to recent study of the connections of the subfornical region of the LHA (Goto et al., 2005) did also include data from control injections within the LHAa/d and LHAjv regions, from which projections to the LHAjp were reported. With reference to the previous study (Goto et al., 2005), in further general agreement with our results, the projection to the LHAjp from a PHAL injection centered in the LHAjvv was reported to be dense, whereas that from a site including the LHAa/d was reported to be moderate; a light to moderately dense projection was reported to arise from PHAL injections centered in the subfornical region of the LHA.
3.2.2.3. Projections from the LHAs (PHAL anterograde tracing)
A deposit of PHAL (experiment LHA#11) centered and mostly contained within midrostrocaudal levels of the LHAs (Atlas Level 29 in Swanson, 2004) labeled perikarya that were mostly restricted to this region (Figs. 2B, 3). The principal route of fiber egress from this LHA site was apparent from numerous fibers that fanned out laterally, heavily innervating the adjacent LHAd and reaching across its rostrocaudal extent, predominantly in the medial half (Figs. 4W–Y, 9A). The laterally emerging fibers also extended to several other LHA regions: a moderately strong projection coursed caudally into the LHAp (Fig. 4AA–BB), while a lighter projection coursed rostrally to innervate the LHAad and LHAai (Fig. 4 Q–U), and a light-very light projection reached the LHAav, LHAsfp, LHApc, LHAm, LHAvm, LHAvl, and preparasubthalamic nucleus (Fig. 4Q–BB). In addition to the laterally emerging fibers, a lesser projection emerged medially from the LHAs to innervate moderately the LHAjd and LHAjvd, and lightly the LHAjvv, LHAsfp, and LHAjp (Fig. 4W–Y); a light projection also extended throughout the rest of the LHAs. Furthermore, a sparse distribution of fibers with terminals was present at the level of the injection site in the contralateral LHAs, LHAd, and LHAjd, appearing to reach these regions via a route above the roof of the hypothalamic part of the third ventricle.
Figure 9.
(A to C) Representative darkfield photomicrographs showing axons immunoreactive for Phaseolus vulgaris leucoagglutinin (PHAL) tracer in several brain regions following a deposit of PHAL within midrostrocaudal levels of the lateral hypothalamic area suprafornical region (LHAs) (experiment LHA#11). (A1 to C1) Brightfield photomicrographs of adjacent thionin stained sections. Note the presence of PHAL-labeled fibers in: (A) LHA dorsal part (LHAd) and paraventricular hypothalamic nucleus forniceal (PVHf) and lateral parvicellular (PVHlp) parts, (B) paraventricular thalamic nucleus (PVT), (C) periaqueductal gray ventrolateral division (PAGvl) and dorsal raphé nucleus (DR). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 200 µm in (A) and (A1), 100 µm in (B), (B1), (C) and (C1).
In addition to intra-LHA connections, certain medial and periventricular hypothalamic nuclei also received a moderate input from the LHAs. At the level of the injection site, a light innervation throughout the anterior part of the DMH was apparent (Fig. 4W–X); a very few fibers were also present within the posterior and ventral parts of the DMH caudal to the level of the injection site. A previous study of inputs to the DMH using fluorogold reported retrograde labeling in the LHA that was mostly medial and most abundant between the levels of the DMH and the caudal half of the PVH (Thompson and Swanson, 1998) that include the LHAs; retrograde labeling was shown caudally in the LHAs, although midrostrocaudal levels of the LHAs (Atlas Level 29) were not represented (Thompson and Swanson, 1998).
A smattering of fibers with few boutons was present in the VMH, and these were mostly rostral within the ventrolateral, central, and dorsomedial parts of the nucleus. Except for a very few fibers, most apparent as fibers of passage, there was no input to the arcuate nucleus. Perhaps most striking within the hypothalamus was a dense projection from the LHAs to the forniceal (f) and lateral parvicellular (lp) parts of the PVH (Figs. 4V, 9A); this projection continued medially and rostrally within the PVH to innervate moderately the PVHmpd and PVHap (Figs. 4Q–V, 9A); much lighter innervation, consisting of few fibers, was also present in the PVHpml, PVHpmm, and PVHpv. It is noteworthy that the projection to caudal levels of the PVHmpd and PVHlp/f included a light contralateral component that was not apparent in other parts of the PVH. The projection from the LHAs to the PVH contrasts with the very minor projection to the PVH from the LHAjp, and this was restricted principally to the PVHap. A projection to the PVH from the LHAs has not been described previously; however, an earlier PHAL study of a region of the LHA lateral and rostral to the LHAs (corresponding approximately to rostromedial levels of the LHAd) did show a pattern of input to the PVH similar to that described here (Watts et al., 1999).
Dorsal to the LHAs (and LHAd) a relatively few fibers, with the appearance of fibers of passage, were present in the zona incerta (ZI); some of these reached medially into the adjacent LHAjd and caudal end of the LHAs where they intermingled with the projection that emerged medially from the LHAs. This rather spare group of fibers continued medially, passing through the rostral third (or thereabout) of the posterior hypothalamic nucleus (PH), reaching the nucleus reuniens, and extending dorsally through midline nuclei of the thalamus, including more rostral levels of the central medial (CM) and intermediodorsal (IMD) thalamic nuclei (Fig. 4X–Z). In contrast to this light projection, a rather more substantial projection ascended through the PH and this consisted of many fibers of passage apparently on route to the PAG, as well as smaller terminal fibers with numerous boutons; some of the fibers ascending within the caudal half of the PH appeared to support a light projection to the more caudal levels of the CM and IMD (Fig. 4Z–BB).
Dorsal within the midline thalamus, numerous fibers coursed along the entire rostral-caudal extent of the PVT (Fig. 4Q–BB); this was a major projection target of the LHAs and the fibers appeared to enter the PVT via a caudal and a rostral route. Within the PVT the heaviest labeling was apparent as a terminal field in the caudal half (or thereabout) (Figs. 4Z–AA, 9B). A very light projection also reached rostral levels of the mediodorsal thalamic nucleus (medial part) and paratenial nucleus. There was also a very light projection via the stria medullaris to predominantly medial levels of the lateral habenula; this is in contrast to the projection to the lateral habenula from the LHAjp that is extremely dense and more broadly distributed (Figs. 4V–BB, 6A, 7).
The lateral projection from the LHAs continued laterally through the LHA and gave rise to a robust plexus of fibers within the substantia innominata (SI), mostly in medial regions (Fig. 4J–W). This projection continued across much of the SI's rostral-caudal extent (except for the most rostral levels), amounting to a sizeable projection overall. The fibers of this projection appeared to include fibers of passage intermingled with smaller branched axons possessing numerous boutons; the terminal field was densest at the level of the anterior parvicellular part of the PVH (Fig. 4R–T), it thinned out more rostrally, and was thinnest more caudally. Dorsal to the SI, a few fibers entered and descended within the internal capsule. Consistent with our present findings, a previous retrograde tracing study of the afferent connections of the SI showed retrograde labeling in the LHA, including a region corresponding approximately to midrostrocaudal levels of the LHAs, following a deposit of the tracer WGA-HRP in medial regions of the SI at about the level of the anterior parvicellular part of the PVH (Grove, 1988b); it is also noteworthy that retrograde labeling was shown to be restricted lateral and dorsal to the fornix in the LHA and did not extend medial to the fornix in the LHA (Grove, 1988b), a result that would be predicted by our observed absence of a projection to the SI from the LHAjp.
Adjacent to the pallidal cerebral nucleus of the SI there was a comparatively miniscule projection to the striatal cerebral central (CEA) and medial (MEA) amygdalar nuclei; this consisted of few fibers within the medial part of the CEA, and very few within the posterodorsal part of the MEA (Fig. 4U–X). However, despite the small size of this projection, it is in part confirmed by a previous study that showed a low incidence of retrograde labeling within a region corresponding to the LHAs following injection of HRP into the medial part of the CEA of the rat and cat (Ottersen, 1980). Farther rostral, within the ventral striatum, a low incidence of labeled fibers was distributed sparsely at mid-caudal levels of the nucleus accumbens, within the medial or “shell” region, medial and proximal to the ventral tip of the lateral ventricle (Fig. 4H–J). This light projection is in accord with the results of an earlier study of nucleus accumbens inputs using the tracer fluorogold, in which a low incidence of retrograde labeling was observed in the LHAs following a tracer deposit in the “septal pole” of the accumbens shell (Brog et al., 1993).
Turning our attention to rostral hypothalamic projections from the LHAs, few fibers were present within the AHN, predominantly in its central part -- in marked contrast to the very dense projection to the AHN from the LHAjp. Ventral to the AHN, a low density of fibers was present within the subventromedial part of the tuberal nucleus and the retrochiasmatic area. At very rostral levels of the PVH, a low density of fibers was also present in a region of the medial preoptic area dorsal to the fornix; at this level fibers were also clearly present within the stria medullaris, and in addition a few fibers were visible within the column of the fornix. Ventral to the fornix there were few fibers within the anterior hypothalamic area and median preoptic nucleus, and very few within the medial-, anterodorsal-, or anteroventral preoptic nuclei; however, a moderate terminal field extended across the medial preoptic area and a light projection extended to the lateral preoptic area (Fig. 4 K–R).
Returning to the pallidum, several bed nuclei of the stria terminalis received an input from the LHAs. A very few fibers were present in the BSTif; similarly few were present in the BSTov, BSTrh, and BSTfu. A light projection was apparent in the BSTmg; a moderate terminal field was present in the BSTdm (Fig. 4 O–P), and a moderate to dense terminal field was present in the BSTal and BSTam (Fig. 4 L–P). In terms of overall density, the projections to the BST from the LHAs exceeded those from the LHAjp; more striking are the differences in target region -- the prime targets for the LHAjp being the BSTif and BSTpr. Also within the pallidum, a low density of predominantly fibers of passage was apparent within the diagonal band nucleus, and these fibers extended into the medial septal nucleus, giving rise to a fairly light terminal field.
Returning to the striatum, the projection to the lateral septal nucleus was sparse overall; this is in contrast to the lateral septal input from the LHAjp, which was more substantial overall, and preferentially targeted the LSr.m.d and LSr.vl.d.m. No single subdivision of the lateral septal nucleus received more than a light input from the LHAs, and this was present in the form of scattered fibers with boutons and fibers of passage rather than a well defined terminal field; nevertheless, these fibers did pass through at least fifteen different lateral septal subdivisions (Table 1). Finally, for the rostral projections of the LHAs, a very few fibers appeared to extend rostral from the lateral septal nucleus into the dorsal part of the tenia tecta, and could be followed no farther.
Shifting focus to the caudal hypothalamus, except for the already described labeling in the LHAp, and PH, there was no appreciable additional input from the LHAs; this contrasts with a massive input to the dorsal premammillary nucleus and a moderate input to the mammillary body from the LHAjp. However, at the level of the mammillary body, a moderate projection composed of fibers with boutons and fibers of passage was present in the rostral end of the midbrain ventral tegmental area (VTA; Fig. 4 CC–FF); this result is consistent with a recent study of VTA inputs using fluorogold that showed rather abundant retrograde labeling in a region corresponding to the LHAs (Geisler and Zahm, 2005). The projection from the LHAs via the LHAp to the PH and VTA, continued caudally along two broadly distinguishable routes: one route went principally via the PH to the PAG, other midline and dorsal regions of the midbrain, and dorsal regions of the rostral hindbrain; the second route, caudal to the VTA, described a mainly ventrolateral path through reticular nuclei of the midbrain and hindbrain, but also extended to dorsal and medial regions of the hindbrain (Fig. 5). This dual descending route from the LHA has been described in similar terms previously (Saper et al., 1979; Hosoya and Matsushita, 1981b).
Considering first the ventrolateral descending route in more detail, this involved a robust projection to the midbrain reticular nucleus (MRN). This was most apparent as a dense net of axons with terminals in the retrorubral area (Fig. 4JJ–LL). Terminals and boutons of passage were also evident in moderate abundance in the parvicellular division (MRNp; Fig. 4LL–OO); fibers within the MRNm appeared to include fibers of passage. A small proportion of the MRN-projecting axons appeared to enter (and descend within) the medial lemniscus. Caudal to the MRN, a projection of moderate density extended caudally into the hindbrain through the pontine reticular nucleus; this then continued more thinly into the gigantocellular-, magnocellular-, and parvicellular reticular nuclei (Figs. 4PP–III, 5). A sparse lateral continuation of this projection extended to the inferior salivatory, oral part of the spinal trigeminal, and paratrigeminal nuclei; a medial branch extended very lightly to the nucleus raphé magnus and nucleus raphé obscurus (very few axons).
In addition to these projections, a dorsal offshoot of the descending reticular projection reached the nucleus of the solitary tract (NTS; Fig. 4BBB–JJJ). Labeling within the NTS included both rostral (“gustatory”) and caudal zones of the NTS; it was moderate in its medial part and of low density in its lateral and commissural parts; furthermore, a few labeled axons extended to the dorsal motor nucleus of the vagus nerve (DMX; Fig. 4FFF–JJJ). The most caudal extension of this descending path that we traced reached with very few fibers into the ventral and dorsal parts of the medullary reticular nucleus; at this caudal level a few fibers were also present within the fiber tracts of the medial lemniscus and the medullary pyramids. Given that several previous reports have identified projections from the LHA (including the region of the LHAs) to the spinal cord, it is likely that some of the fibers we identified in the caudal medulla descended farther (Hancock, 1976; Saper et al., 1976a; Hosoya, 1980; Swanson and Kuypers, 1980).
The second major descending route from the LHAs into the midbrain was apparent as a projection of numerous labeled fibers within the PH that entered and coursed dorsally within the rostromedial division of the PAG (Fig. 4CC–DD); a small proportion of these fibers reached the precommissural nucleus of the PAG, a more moderate proportion reached the commissural nucleus of the PAG (Fig. 4EE–HH), and a very few extended to the nucleus of the posterior commissure. A moderate density of fibers was also present in the medial division of the PAG. Most notable within the PAG, an extensive terminal field was present within its ventrolateral division (Figs. 4GG–PP, 9C); rostrally this was fairly sparse, but farther caudal it became extremely dense. A sparse distribution of labeled fibers with terminals was also present within the dorsal and dorsolateral divisions of the PAG (mostly caudally; Fig. 4OO–QQ). In addition, at the level of the commissural nucleus of the PAG, a spare projection of fibers was apparent within the superior colliculus; this extended caudally and included the intermediate layer but was largely within the deep layer, mostly in its deeper regions (Fig. 4EE–OO). At the caudalmost level of the PAG, a very few fibers were also apparent within the inferior colliculus (Fig. 4QQ–RR).
Certain more ventral midline regions of the midbrain also received an input. A light projection of mostly fibers of passage was present within the rostral linear and interfascicular nuclei of the raphé as well as the rostral subnucleus of the interpeduncular nucleus. In association with the latter site, it is noteworthy that a few fibers could be traced caudally from more rostral levels within the fasciculus retroflexus. Farther caudal a light terminal field was present within the raphé’s caudal linear nucleus (Fig. 4KK–LL), and a moderate terminal field was present in the dorsal nucleus of the raphé (Figs. 4LL–NN, 9C). A very few fibers were also apparent in the superior central nucleus of the raphé (medial part). A moderate density of fibers could be traced within the superior cerebellar peduncle, although their possible terminations within the cerebellum were not apparent. A few fibers were also observed within the medial longitudinal fascicle.
At caudal levels of the PAG, a moderate projection to a rather broad extent of the parabrachial nucleus (PB) became obvious (Fig. 4PP–TT); this appeared to involve a contribution of fibers from each of the two main descending routes. Across the PB, the central lateral part in particular appeared to be the major recipient, with a much lesser projection to the external lateral and ventral lateral parts. Also at this level, a moderate density of fibers was present in the pontine central gray, appearing to be mostly a continuation from the ventrolateral division of the PAG. The most striking feature in this dorsal region adjacent to the midbrain-hindbrain border was the existence of a highly targeted and extremely dense terminal field in Barrington's nucleus (Figs. 4RR–SS, 10).
Figure 10.
(A) Representative darkfield photomicrograph showing axons immunoreactive for Phaseolus vulgaris leucoagglutinin (PHAL) tracer in the region of Barrington's nucleus (B) following a deposit of PHAL within midrostrocaudal levels of the lateral hypothalamic area suprafornical region (LHAs) (experiment LHA#11, see Fig. 2). (B) Brightfield photomicrograph of adjacent thionin stained section. (C) Digitally rendered camera lucida drawing of the area delineated by the white oval in (A). Approximate boundaries of brain regions (dashed lines) and fiber tracts (finer dashed lines) correspond to those delineated in an atlas of the rat brain (Swanson, 2004). Scale bars = 100 µm in (A) and (B), 10 µm in (C).
There is a paucity of earlier reports at a high spatial resolution (i.e. mapped to numerous consecutive atlas levels) for projections to the midbrain and hindbrain from the LHA, let alone the LHAs. Nevertheless, the earlier studies deserve some mention, in so far as they provide a measure of corroboration for our findings. For example, with regard to the labeling we observed in the region of the NTS and the DMX, a previous anterograde tracing study described labeling in those regions following a deposit of tritiated amino acids into a region encompassing the column of the fornix at a caudal level of the LHA (Hosoya and Matsushita, 1981b). In addition, a more recent retrograde tracing study (Grove, 1988b) showed a clearly demarcated cluster of cells in the vicinity of the LHAs following a large deposit of the tracer WGA-HRP into a region that included the NTS and DMX (Grove, 1988a); retrograde labeling in the vicinity of the LHAs was also noted in an earlier HRP study of the DMX (ter Horst et al., 1984). Furthermore, an earlier study identified cells in the vicinity of the LHAs that projected to the region of the NTS or to the spinal cord, or (in a very few instances) to both (Swanson and Kuypers, 1980).
Also previously noted are clear projections from the caudal “perifornical LHA” (which includes the LHAs) to the region of the VTA (Saper et al., 1976b; Saper et al., 1979; Hosoya and Matsushita, 1981b), PAG (Saper et al., 1976b; Saper et al., 1979; Hosoya and Matsushita, 1981b; Beitz, 1982), dorsal nucleus of the raphé (Hosoya and Matsushita, 1981a; Hosoya and Matsushita, 1981b; Peyron et al., 1998), PB (Hosoya and Matsushita, 1981a; Hosoya and Matsushita, 1981b), and Barrington's nucleus in the rat (Allen and Cechetto, 1992; Semba and Fibiger, 1992; Valentino et al., 1994) and cat (Kuipers et al., 2006); projections reaching farther caudal have also been described to regions including the nucleus raphé magnus (Hosoya and Matsushita, 1981b; Hermann et al., 1997), as well as the pontine central gray, reticular nuclei of the pons and medulla, salivatory nuclei, and spinal cord (Hosoya and Matsushita, 1981a; Hosoya and Matsushita, 1981b). Taken together, our present data are in rather good agreement the findings of the earlier work.
3.2.2.4. Sources of projections to the LHAs (CTB retrograde tracing)
3.2.2.4.1. Cerebral cortex
In marked contrast to the abundant retrograde labeling in the cerebral cortex from the LHAjp, what we see from the LHAs is of substantially reduced abundance, and often quite different in distribution. The difference in abundance is most apparent in the hippocampal formation where a very low incidence of back-labeled cells was present in essentially the same overall distribution pattern. This finding appears to be consistent with the results of a recent PHAL study of the hypothalamic targets of subicular projections in the rat (Kishi et al., 2000); specifically, a PHAL deposit within the intermediate third of the subiculum labeled very few fibers in the vicinity of the LHAs, although numerous fibers and terminals were present within the LHA region immediately medial to the LHAs (Kishi et al., 2000). In addition, no input to the LHAs was reported from PHAL injections in the ventral end of the subiculum (Canteras and Swanson, 1992a).
Within the medial prefrontal cortex very few cells were back-labeled from the LHAs in the anterior cingulate area (layer 5 and 6a); this compares to a moderate amount of retrograde labeling in the anterior cingulate area from the LHAjp. A small amount of labeling was present in the prelimbic area (layer 5, occasionally layer 6a and possibly occasionally layer 3), rather similar in amount, but somewhat more rostral in distribution, than that from the LHAjp. A moderate amount of retrograde labeling was present in the infralimbic area (layer 5 and occasionally layer 6a) from the LHAs (Fig. 4D–E); this was about double the abundance of labeling seen in this region from the LHAjp. Also different from the input to the LHAjp, we did not find any cells back-labeled from the LHAs in the retrosplenial area, but we did find a moderate incidence of labeling in the agranular insular area (layer 5), divided mostly between its dorsal and posterior parts (Fig. 4E–I); a low incidence of labeling was also present in the ventral part of the orbital area and dorsal part of the tenia tecta (layers 3 and 4). Finally, within the cortical subplate, a very low incidence of labeling was present in the claustrum, dorsal part of the endopiriform nucleus, and (as was the case for the LHAjp), the basolateral and basomedial amygdalar nuclei.
Previous reports corroborate our data for the labeling we found in the prelimbic (Sesack et al., 1989; Vertes, 2004; Yoshida et al., 2006) and infralimbic (Brittain, 1988; Hurley et al., 1991; Vertes, 2004; Yoshida et al., 2006) areas. Past studies that speak to projections from the insular region have not examined them at a high spatial resolution with respect to the LHA. Nevertheless, at least three previous studies in the rat indicate a projection from the agranular insular area to the vicinity of the LHAs (Hurley et al., 1991; Floyd et al., 2001; Yoshida et al., 2006). In further agreement with one of the previous studies (Hurley et al., 1991), the labeled neurons we found in the agranular insular area were restricted to layer 5. This is at odds with a more recent report indicating that cells retrogradely labeled from the LHA (including the LHAs) are present in layer 3 of the agranular insular area, at a level of occurrence described as “moderate to heavy” (Yoshida et al., 2006); in the same study the authors reported very heavy labeling in the claustrum from the LHA (including the LHAs) (Yoshida et al., 2006), whereas we noted very few cells. Finally, the retrograde labeling we found in the basolateral and basomedial amygdalar nuclei has some confirmation from a previous PHAL study in which projections from the basomedial amygdalar nucleus were described (Petrovich et al., 1996); these were found to include a low density of labeled fibers in the vicinity of the LHAs.
3.2.2.4.2. Cerebral nuclei
3.2.2.4.2.1. Striatum
Overall, there was a moderate abundance of retrograde labeling in the striatum from the LHAs; most of this was distributed widely within the lateral septal nucleus (Fig. 4G–P). However, no single region of the lateral septal nucleus contained more than a low incidence of retrograde labeling from the LHAs, in marked contrast to retrograde labeling from the LHAjp, which was abundant in several components of the lateral septal nucleus. Almost every recognized differentiation of the lateral septal nucleus contained some retrograde labeling from the LHAs, although the majority of labeled cells were in the rostral part. In general agreement with these findings, a comprehensive previous study of the connections of the lateral septal complex in the rat revealed relatively few projections to the vicinity of the LHAs from either rostral or caudal parts (Risold and Swanson, 1997).
After the lateral septal nucleus, the second most abundantly labeled striatal component was the lateral part of the central amygdalar nucleus (CEAl; Figs. 4U–V, 8D); however, although in terms of overall amount of labeling the CEAl ranked second to the lateral septal nucleus, as a single striatal nucleus it contained the highest density and abundance of retrograde labeling from the LHAs. Aside from the moderate amount of labeling in the lateral septal nucleus and CEAl, there was comparatively little labeling in other striatal cerebral nuclei; this was present at a low incidence in the anterodorsal part of the MEA, septofimbral nucleus, and septohippocampal nucleus; and at a very low incidence in the anterior amygdalar area, CEAm, CEAc, and posterodorsal part of the MEA (sublayer a). Our finding of densely grouped neurons back-labeled in the CEAl is consistent with a previous report of this following an injection of CTB into a region of the LHA that included (but was not restricted to) the LHAs (Yoshida et al., 2006) -- labeling was also reported in the CEAm and CEAc, albeit at a much lower amount (Yoshida et al., 2006). An earlier PHAL study of output from the CEAl reported “no significant projections” to the LHA (Petrovich and Swanson, 1997); however, the PHAL study described principally the connections of a posterior region of the CEAl, whereas the retrograde labeling we report in the CEAl from the LHAs is highly restricted to its rostral tip.
3.2.2.4.2.2. Pallidum
The most abundant retrograde labeling in the entire brain from the LHAs was present within pallidal cerebral nuclei. Foremost among the pallidal regions back-labeled from the LHAs was the bed nuclei of the stria terminalis (BST), where labeled cells were very numerous; in addition to the BST, a moderate amount of labeling was present within the substantia innominata (SI) -- the latter is consistent with previous studies of projections from the SI (Grove, 1988a). Labeling in the BST was most prominent in its anterior division, more specifically within the anteromedial area (BSTam; Figs. 4K–P, 8C), which contained the highest abundance of labeling of any single delineated brain region. Next to the BSTam, the BST fusiform nucleus (BSTfu) contained the second most abundant BST labeling (a more moderate amount but a much higher density) (Figs. 4M–N, 8C), followed by the caudal half or so of the anterolateral area (BSTal), which contained a moderate amount (Fig. 4O–P). Within the anterior division of the BST, a low incidence of labeling was also present in dorsomedial (BSTdm) and interfascicular (BSTif) nuclei; a very few cells were also present in magnocellular (BSTmg) and ventral (BSTv) nuclei. One component of the posterior division of the BST contained a low incidence of labeling, and this was the principal nucleus (BSTpr; Fig. 4Q); also within the posterior division of the BST, a very few cells were retrogradely labeled within the transverse nucleus (BSTtr). By comparison to the BST, labeling within the SI was of a lower density; nevertheless, this extended over much of the rostral-caudal extent of the SI and summated to a somewhat higher than moderate amount in total (Fig. 4K–T). Outside of the BST and SI, there was no other site within the pallidum that contained more than a very few isolated back-labeled cells; these sites were in essence the medial septal nucleus and its ventrolateral extension, the diagonal band nucleus.
Several comprehensive PHAL studies of projections from the BST have been published in recent years; these studies by Hong-Wei Dong and colleagues include the BST divisions where we report retrograde labeling from the LHAs, and thus they provide a very useful set of data with which to make comparisons to our present findings (Dong et al., 2001b; Dong and Swanson, 2004a; Dong and Swanson, 2004b; Dong and Swanson, 2006a; Dong and Swanson, 2006b; Dong and Swanson, 2006c). Beginning with the BSTam, where we found such a very high abundance of retrograde labeling from the LHAs, following PHAL deposits in either dorsal or ventral regions of the BSTam, Dong and Swanson reported a “dense terminal plexus” in the LHAs (Dong and Swanson, 2006a). In a separate PHAL study, a projection from the BSTfu to the LHAs is reported (Dong et al., 2001b); this is shown to be dense within rostral levels of the LHAs, and of much lower density caudally (Dong et al., 2001b). Also in their study of BSTfu projections, Dong et al. described the projections of the BST oval nucleus (BSTov) (Dong et al., 2001b); this is a useful comparison here because the BSTov is adjacent to both the BSTam and BSTfu and yet provides essentially no projection to the LHAs (Dong et al., 2001b), which finding is consistent with our noted absence of retrograde labeling in the BSTov from the LHAs. Finally among the sites we have identified as providing a moderate or higher input to the LHAs from the BST, the retrograde labeling we found in caudal levels of the BSTal is confirmed by a shown moderate projection from this BST region to the vicinity of midrostrocaudal levels of the LHAs (Dong and Swanson, 2004a); in further agreement with our results, it is also noteworthy that a projection to the vicinity of midrostrocaudal levels of the LHAs from rostral levels of the BSTal appears to be of considerably lower magnitude (Dong and Swanson, 2004a).
We now compare our results with respect to the BST studies of Dong and colleagues for those BST regions where we have reported a low degree of retrograde labeling from the LHAs. To begin, the low incidence of labeling we saw in the BSTdm at first glance appears to contradict the report of an extremely dense terminal plexus in the LHAs following a PHAL deposit centered within the BSTdm (Dong and Swanson, 2006b). However, on closer examination, it is apparent that the PHAL deposit in the experiment used for the most detailed analysis included adjacent regions; in particular it included a region of the BSTam that, as we have already discussed, provides a major input to the LHAs -- this may account to some extent for the seeming discrepancy. A similar interpretive situation is presented by the results of a PHAL study of the BSTmg and BSTv, both of which regions are reported to generate a dense terminal field in the LHAs (Dong and Swanson, 2006c) -- recall we found very few back-labeled neurons in either region from the LHAs. In this instance, inclusion of the BSTam is also a distinct possibility given the combination of its direct proximity to the BSTv and BSTmg, and the difficulties inherent in distinguishing boundaries between these differentiations and the BSTam. Following this theme, the BSTtr is also reported to generate a rich terminal field in the LHAs (Dong and Swanson, 2004b) -- much more than one might expect given the very few cells we found in the BSTtr back-labeled from the LHAs. In this case, the unpredicted result may to some extent be explained by the inclusion of some dorsomedial regions of the SI directly adjacent to the BSTtr in the PHAL deposit used for the analysis (Dong and Swanson, 2004b) because we did overall find a moderate amount of retrograde labeling in this region of the SI. Another possibility in all these cases is that a relatively few neurons in the BSTdm, BSTtr, BSTv, or BSTmg give rise to a relatively dense terminal field in the LHAs. Finally, the results of a study of projections from the BSTif and BSTpr (Dong and Swanson, 2004b) indicate very little input to the LHAs; however, an earlier autoradiographic study of the preoptic region that included analysis of a control injection within the BSTif/pr is more in agreement with our findings in that it indicated a moderate projection to the vicinity of the LHAs (Swanson, 1976). Clearly, further experimentation will be required to clarify these discrepancies.
3.2.2.4.3. Cerebrospinal Trunk
3.2.2.4.3.1. Sensory System
We found a relatively low abundance of retrograde labeling from the LHAs in brain regions classed generally as part of the sensory system; nevertheless, this was more abundant than the retrograde labeling we found in these brain regions from the LHAjp, and its distribution was considerably more extensive. Essentially, the labeling was divided between three components of the sensory system: (1) Polymodal sensory -- paraventricular nucleus of the thalamus (PVT; Fig. 4Q–U), (2) Viscerosensory – lateral division of the parabrachial nucleus (PBl; Fig. 4PP–SS) and a caudal (gustatory related) region of the medial part of the nucleus of the solitary tract (NTSm; Fig. 4III), and (3) Humorosensory -- the subfornical organ (SFO; Fig. 4Q–R). In and of these regions, in descending order, labeling was most abundant in the PBl, followed by the SFO, PVT and then the NTSm. Within the PBl, most of the labeled cells were located in the ventral lateral part (PBlv); a lower incidence was present in the central- (PBlc), dorsal- (PBld) and external- (PBle) lateral parts -- a few cells were also noted in the medial part of the medial parabrachial nucleus (PBmm). However, most of the labeled cells in the PBlv were present contralateral to the injection site; this is noteworthy not only by comparison with the other PBl labeling (which was almost exclusively ipsilateral) but also for being the site of most numerous contralateral retrograde labeling from the LHAs. The low incidence of labeling within the PVT was confined to its more rostral levels; outside of the PVT, a few isolated cells were also noted in the lateral dorsal, intermediodorsal, anterior reuniens, and parafascicular nuclei of the dorsal thalamus.
Two previous studies (one very recent) have examined in detail the output of the PVT (Moga et al., 1995; Vertes and Hoover, 2008); in neither of these is a projection to the vicinity of the LHAs described. Nevertheless, in the more recent study (Vertes and Hoover, 2008) a very few fibers were shown in the vicinity of the LHAs in the figures depicting the distribution of the PHAL labeling. Furthermore, in the earlier study (Moga et al., 1995) a low incidence of retrograde labeling in rostral levels of the PVT is shown following a large injection of fluorogold into caudal levels of the LHA that may have included some of the LHAs. Consistent with the labeling we observed in the lateral division of the parabrachial nucleus, a previous PHAL study revealed a moderately dense fiber plexus in the vicinity of the LHAs labeled from a PHAL injection that appeared to include the PBlv, PBlc, and PBld (Bester et al., 1997); in addition, a plexus of fibers was shown within the vicinity of contralateral midrostrocaudal levels of the LHAs. Further consistent with our findings, a PHAL deposit in a region corresponding to the PBle resulted in very few labeled fibers in the vicinity of the LHAs (Bester et al., 1997). In an earlier and lower spatial resolution PHAL study of the PBl, a “light density” of labeling was reported in the LHA but its distribution was not specified (Krukoff et al., 1993).
In agreement with the retrograde labeling we observed from the LHAs in the SFO, a previous PHAL study of the SFO described a dense plexus of fibers in the LHA and showed clearly that this involves the dorsal perifornical region of the LHA at a level that includes most of the LHAs (Swanson and Lind, 1986). An earlier anterograde tracing study using the autoradiographic method also reported a dense terminal field in the dorsal perifornical area (Miselis, 1981), although this was mapped to an apparently more rostral site than was the case in the more recent PHAL study. Finally, for caudal levels of the NTSm, a recent PHAL study reported a small terminal field in the vicinity of the LHAs following a deposit of PHAL that labeled cells within the caudal half of the NTS (Geerling and Loewy, 2006). A previous PHAL study of the NTS reported a similar finding (ter Horst et al., 1989) and also showed that the caudal, but not the rostral, half of the NTS projects to the vicinity of the LHAs -- consistent with our findings. Furthermore, our present finding and the earlier PHAL studies are all consistent with an earlier autoradiographic study of NTS outputs in which sparse and diffuse labeling in the vicinity of the LHAs was reported (Ricardo and Koh, 1978).
3.2.2.4.3.2. Behavioral State System
Retrograde labeling in regions of the behavioral state system from the LHAs was similar to the labeling we obtained in these regions from the LHAjp, both in terms of its low incidence and regional distribution. Thus, the dorsal region of the LHA (LHAd) contained the most labeled cells (Fig. 4V–Z); a very low incidence of labeling was also present in the hypothalamic subparaventricular zone, lateral part of the supramammillary nucleus, and more caudally within the caudal linear nucleus and dorsal nucleus of the raphé, and the pedunculopontine nucleus. The labeling we found in the LHAd from the LHAs concurs with the results of a recent PHAL study (already mentioned in reference to the LHAjp) that included results from a PHAL injection involving (but was not restricted to) rostral levels of the LHAd (Goto et al., 2005); a moderately dense projection to the LHAs was noted (Goto et al., 2005).
3.2.2.4.3.3. Motor System
As with the LHAjp, the bulk of cerebrospinal trunk retrograde labeling from the LHAs was present within regions classed as part of the motor system (Swanson, 2004); however, this was more extensive than the labeling in these regions from the LHAjp and in fact constituted the greater part of all the retrograde labeling from the LHAs. The majority of the labeled neurons were distributed between four of the eight divisions of the motor system: (1) Behavior control column, (2) Central gray, (3) Hypothalamic periventricular region, and (4) Motor related hypothalamic lateral zone (LZm) of the reticular formation. Among these divisions, most of the labeled cells were present within the hypothalamic periventricular region (in contrast to the LHAjp from which most of the back-labeled cells were within the behavior control column), but numerous cells were also present within the behavior control column, central gray, and LZm. In addition to these, a relatively low incidence of labeling was present in the neuroendocrine motor zone; this is in stark contrast to the LHAjp from which labeling in the neuroendocrine motor zone was essentially absent.
Beginning with the comparatively little amount of retrograde labeling in the behavior control column from the LHAs, the regions that nevertheless contained the most back-labeled cells were the AHN (central part) (Fig. 4R–U) followed by the MPN (medial and lateral parts) (Fig. 4O–Q). A lower incidence of labeling was present in the dorsomedial part of the VMH (Fig. 4V–X) and a very low incidence was present in the ventrolateral part, and in the anterior and posterior parts of the AHN; in addition, a very few isolated cells were present in the central parts of the MPN and VMH, dorsal and ventral premammillary nuclei, and VTA. Although not part of the behavior control column, it is noteworthy that similarly few back-labeled cells were also present within the hierarchically-adjacent motor related intermediate gray layer of the superior colliculus (sublayer c). At least two previous PHAL studies in the rat showed a light to moderate projection from the central part of the AHN to the region of the LHAs (Risold et al., 1994; Delville et al., 2000), and one previous PHAL study of the MPN showed a moderate projection to the vicinity of the LHAs following a deposit of PHAL that included the medial, lateral, and central parts of the MPN (Simerly and Swanson, 1988).
Within the central gray numerous cells were back-labeled in rostral levels of the posterior hypothalamic nucleus (PH; Fig. 4X–BB), and a comparatively moderate number were present in the PAG (Fig. 4CC–PP). Labeling within the PAG was for the most part the sum of two small and diffuse groups of cells in the PAGvl and (to lesser extent) PAGrm; a very low incidence was also present in the PAGd and commissural nucleus. Farther caudal, a small group of cells was back-labeled (bilaterally) in the pontine central gray, and a small but densely clustered group of cells was present within the lateral tegmental nucleus (LTN). An earlier PHAL study of PH outputs described especially dense labeling in the “dorsal perifornical area” (including a region corresponding to the LHAs) (Vertes et al., 1995) resulting from a PHAL injection in rostral levels of the PH.
The third division of the motor system of the cerebrospinal trunk that contained abundant retrograde labeling was the hypothalamic periventricular region. About a third of all labeling in the hypothalamic periventricular region was within the medial preoptic area (MPO; Fig. 4M–R). Besides the MPO, the median preoptic nucleus (MEPO) ranked second and contained a moderately high amount of labeling (Fig. 4L–P). Farther caudal, the DMH also contained a moderately high amount of labeling (Fig. 4W–Z); most of this was present in the posterior and anterior parts, and a small amount was present in the ventral part. In addition to these cell groups, a low to moderate incidence of labeling was present in the anteroventral preoptic nucleus (AVP; Fig. 4L–O) and also distributed in the internuclear region of the hypothalamic periventricular region. Furthermore, a low incidence of labeling was present in anteroventral periventricular (AVPV) and parastrial (PS) nuclei, and a very low incidence was present in the anterior hypothalamic area (AHA), posterior part of the periventricular nucleus (PVp), and anterodorsal preoptic (ADP) nucleus.
Consistent with the very abundant labeling we found in the MPO, a previous study of MPO outputs using the autoradiographic tracing method revealed a dense terminal field in the region of the LHAs following a large deposit of 3H-leucine that was restricted mostly to the MPO (Conrad and Pfaff, 1976a). Much more recently, projections from the MEPO to the vicinity of the LHAs were shown using PHAL (Uschakov et al., 2007). With regard to the DMH, a previous PHAL study described and showed a dense plexus of terminal fibers and fibers with boutons of passage in the vicinity of the rostral half of the LHAs following a deposit of tracer that included both anterior and posterior parts of the DMH (Thompson et al., 1996). In an earlier PHAL study of the DMH a robust projection to the vicinity of the (principally) rostral half of the LHAs arising from the anterior and posterior parts of the DMH was also described (ter Horst and Luiten, 1986). A previous PHAL study of projections from several of the more rostral periventricular hypothalamic regions including the AVP, AVPV, PS, and MEPO showed relatively light inputs from these regions to caudal levels of the LHAs, although its mid and rostral levels were not shown (Thompson and Swanson, 2003). An earlier PHAL study of the AVPV in the female rat revealed a light projection to the vicinity of the LHAs that appeared to be more pronounced rostrally (Gu and Simerly, 1997).
The final division of the motor system of the cerebrospinal trunk that contained abundant labeling was the motor related hypothalamic lateral zone (LZm) of the reticular formation. Labeled cells in LZm were present almost exclusively within one of several regions of the LHA or the lateral preoptic area (LPO -- the rostral extension of the LHA). A moderate amount of retrograde labeling was present in the LPO (Fig. 4K–P); in the ventral (LHAav), dorsal (LHAad), and intermediate (LHAai) zones of the anterior region of the LHA (Fig. 4Q–V); and in the ventral zone (LHAjvv) of the juxtaventromedial region of the LHA (Fig. 4T–Z). A low incidence of labeling was present in the LHAjd and LHAvm, and a very low incidence was present in the LHAsfp, LHAjp, and retrochiasmatic area; few isolated cells were present in the zona incerta, tuberal nucleus, LHAjvd, and midbrain reticular nucleus (magnocellular part). In a previous retrograde tracing study of the LHA (Kishi et al., 2000), moderate labeling was shown in the LPO following an injection of CTB into a dorsal perifornical region that included the LHAs; labeling was also apparent in several other regions of the LHA, but this was not analyzed at a high resolution or with regard to the present LHA parcellation.
As was mentioned at the start of this final section of our results, one other division of the motor system, the neuroendocrine motor zone (NEM), contained a relatively low incidence of labeling. About half of the labeling within the NEM (a moderate amount) was present within the anterior parvicellular part of the paraventricular hypothalamic nucleus (PVHap; Fig. 4P–S); a low incidence was present in the arcuate nucleus (Fig. 4U–Y), a very low incidence in the PVH medial parvicellular part dorsal zone (PVHmpd), and a very few cells were present in adjacent periventricular regions: PVH periventricular part (PVHpv), and periventricular hypothalamic nucleus anterior (PVa) and intermediate (PVi) parts. Our finding of moderate retrograde labeling in the PVHap is consistent with the results of an earlier lower spatial resolution study in which labeling was described in the “parvocellular regions of the PVH” apparently including the PVHap and to a lesser extent the PVHmpd following a deposit of CTB into a dorsal perifornical region that included the LHAs (Yoshida et al., 2006).
3.3. Comparison with additional experiments (control injections)
It is not possible with current methodology to reproduce exactly the same placement and distribution of tracer injection across experiments, and even if it were possible it is likely that the resultant pattern of labeling obtained would in each experiment be somewhat different. The reasons for this variation may include normal variation between individual animals that manifests as differences that affect not only the tracer placement but also its spread and the resultant neuronal labeling. Among the most apparent sources of variation are differences in brain dimensions, differences in the position of Bregma relative to the brain, and differences in the particular distribution of neurons and their processes within the neuropil. Nevertheless, these limitations duly noted, a measure of control is provided by comparing the results of experiments that are optimal (i.e., most restricted to the target region of interest) with experiments that either overlap or are adjacent to the target region.
In this study the LHAjp is the primary focus, and experiment LHA#22 was selected as optimal for the most detailed analysis, being mostly restricted to the LHAjp and centered in the caudal end of this region where the LHAjp presents at its fullest, laterally adjacent to the fullest extent of the PVH (Figs. 1, 2A, 3); an additional experiment (LHA#62) that was also quite restricted to the LHAjp extended primarily over approximately the rostral half of this region (Fig. 3). As a nearby control region for experiment LHA#22, experiment LHA#11 was selected for being mostly restricted to a dorsal perifornical region within a topographically central zone (i.e., midrostrocaudal levels) of the LHAs (Figs. 1, 2B, 3). A related adjacent region control is provided by the already published connections of the subfornical region of the LHA (Goto et al., 2005) (see also Fig. 13). In addition to experiments LHA#22, LHA#62, and LHA#11, four additional experiments were selected for comparison that partially overlapped with either the LHAjp or LHAs (Fig. 3). For further comparison, the connections of the adjacent juxtadorsomedial region of the LHA (LHAjd) will be described in the next paper in this series.
In general terms, deposits of combined CTB and PHAL that included either the region of the LHAjp or LHAs as described for experiments LHA#22 (caudal end LHAjp) or LHA#11 (midrostrocaudal levels of LHAs) yielded a pattern of labeling that largely reproduced the pattern seen in those experiments as well as producing an additional data set presumably resulting from the inclusion of adjacent regions. It is not within the scope of this paper to present and compare the fully detailed results of our analysis for all of these experiments. However, as a prelude to further publications we summarize here some of the more striking observations. Much similarity was noted in the pattern of labeling obtained in experiment LHA#22 and that obtained in experiment LHA#34 (mostly restricted to the rostral half of the LHAjd), indicating that the caudal end of the LHAjp and directly apposed rostral end of the LHAjd connect similarly with other brain regions (we intend to present this data in a subquent paper). Furthermore, there do appear to be certain clear quantitative differences between the rostral and caudal halves of the LHAjp, as evidenced by comparing experiments LHA#22 (caudal end of LHAjp) and LHA#62 (rostral half of LHAjp). It is important to note, however, that these differences—to be described next—are generally quantitative rather than qualitative; that is, they generally represent differences in magnitude rather that the presence or absence of a pathway.
The caudal end of the LHAjp (experiment LHA#22) shows a substantially stronger projection to the following regions than the rostral half of the LHAjp (experiment LHA#62): medial mammillary and dorsal premammillary nuclei, rostral division of the nucleus reuniens and perireuniens nucleus, paratenial nucleus, anterior and dorsomedial parts of the VMH, and lateral and medial parts of the MPN. Conversely a substantially stronger projection from the rostral half of the LHAjp (experiment LHA#62) was noted in the following regions: medial preoptic area, medial septal nucleus, and LSr.dl.m.v. Retrograde labeling from the caudal end of the LHAjp was substantially more abundant than from the rostral half in the following areas: dorsal third of the subiculum, RSPv, ACA, LSr.m, and LSr.m.d. Conversely, many regions showed substantially more abundant retrograde labeling from the rostral half of the LHAjp than from the caudal end, as follows: Cerebral cortex -- ILA, PL, ventral third of the subiculum, TTd; Striatum -- LSc.v.l.d, LSr.dl.l.d, LSr.vl.d.l, LSr.vl.d.m; Pallidum -- BSTam/if/pr, SF, TRS, SI; Thalamus -- PVT; Hypothalamus -- AHNa/c/p, LHAav/jd/jvv, LPO, I, MEPO, MPNm, MPO, PH, PVHap, SBPV, VMHdm, VMHvl, ZI; Brainstem -- PAGvl, PRC. A fuller comparison of the inputs and the outputs of the rostral and caudal ends of the LHAjp is presented in Table 1.
DISCUSSION
The data from these tract-tracing experiments reveal much complexity in the neural connections of the LHA (Table 1; Fig. 4), and they affirm a view of the LHA as an heterogeneous region in terms of its neuronal structure -- a region in which discrete LHA parcels in close proximity to one another can have quite different patterns of connectivity. Here we have shown this to be the case for the regions we refer to as LHAjp and LHAs, and this is underscored further by the recent description of axonal projections from the subjacent subfornical region of the LHA (Goto et al., 2005). The very different patterns of connections for the LHAjp and LHAs have been indicated to some extent in previous studies as was presented in parallel with the description of the Results. In the following discussion we address additional extrinsic connections previously described and related to the present data; in relation to this, we also suggest some possible functional roles for the LHAs and LHAjp that are indicated by the very different patterns of their neural connections. Before this in-depth discussion of the data, we discuss briefly the technical limitations of the tract-tracing technique that was used in these experiments.
4.1. Methodological considerations
The goal of these experiments was to determine and compare systematically at high spatial resolution the source of neural inputs to, and the axonal pathways and associated terminal fields generated by, two nearby regions of the LHA. In this we aimed to provide a firmer and more substantive basis for further critical and hypothesis driven study of these LHA regions. To this end we employed a dual tract-tracing strategy that involved coinjecting from a single micropipette a mixture of two sensitive tracers -- PHAL and CTB; both tracers have had wide application in central anterograde (PHAL, and to a lesser extent CTB) and retrograde (CTB) tracing studies since their introduction about 25 years ago (Luppi et al., 1987; Gerfen and Sawchenko, 1984). When delivered by iontophoresis using the method described here, there is no unequivocal evidence that PHAL is taken up by fibers of passage (bearing in mind that long dendrites often extend into certain fiber tracts, for example, in the spinal cord). Instead, it is taken up by dendrites and cell bodies and is transported exclusively anterogradely to reveal the morphology of labeled axons with the clarity of a Golgi preparation (Gerfen and Sawchenko, 1984).
In contrast to PHAL, the tracer CTB is transported anterogradely and retrogradely following central injection; however the axonal labeling CTB produces is of lower clarity than that achieved with PHAL and it does not confound analysis of the retrograde labeling. Retrogradely transported CTB typically distributes rather uniformly in the perikaryon to reveal the morphology of the cell body and (to varying degree) its dendrites. However, despite wide application, questions pertaining to the efficacy of CTB persist (as is the case more generally for most if not all tracers); thus, one early report indicated that CTB is not taken up by undamaged fibers of passage in the olivocerebellar pathway following iontophoretic injection of CTB into a region of the cat ventrolateral medulla they assumed contained the olivocerebellar pathway (Luppi et al., 1990). In contrast, as evidence of uptake by fibers of passage, in a later study iontophoretic injection of CTB in the ventrolateral medulla of the rat was reported to result in retrograde labeling in the contralateral inferior olive, and anterograde labeling in the cerebellum (Chen and Aston-Jones, 1995); whether or not this was due to uptake by damaged or undamaged fibers was not established. Consistent with the former claim, in this study, for every region we reported retrogradely labeled with CTB we found no example of a previously published PHAL report to contradict this by indicating a given projection was not present (where the resolution of the former study was sufficient to allow such analysis); furthermore, for every instance where a previous PHAL report was available, the findings were consistent with our results. However, these are not definitive controls and future studies to resolve questions of tracer efficacy remain a critical priority. In any event, it can be concluded with certainty that retrograde labeling reported here was due to the uptake of CTB by axons generating terminals within and/or passing through the injection site.
Questions of efficacy aside, there are two intrinsic limitations of the coinjection method, one general and one (in this study) related to the LHAjp and LHAs. First, as considered in the Results, different neuronal pathway tracers have different diffusion patterns when injected into nervous tissue -- i.e. their injection sites have different characteristics, even when the same volume is injected, or they are injected together; second, it is difficult to establish with precision the effective spread of a tracer in solution from the tip of an injector -- that is, the actual spatial dimensions of the region of the injection site that produces detectible labeling of neuronal inputs or outputs -- this is particularly so when the neural connections of the injected region are poorly understood (as is the case with the LHAjp and LHAs). The specific relevance of this for the present coinjections is that the volume of neuropil exposed to effective concentrations of the two tracers cannot be assumed to be equal, although central regions of the injection site obviously contain effective concentrations of both tracers. Nevertheless, in this regard it is also important to point out an essential difference between PHAL and CTB (when the latter is employed as a retrograde tracer). In the case of PHAL, although the effective spread has not been measured, the labeled perikarya at the site of injection can be identified and quantified to some extent; this is not the case for CTB where the particular axons that take up the tracer for retrograde transport have not been similarly identified.
With regard to the limitations of the coinjection method used in the present experiments as they apply to the LHAjp and LHAs, firstly these are two relatively diminutive, irregularly shaped gray matter regions -- features that imposed limits on how confined to these regions our injections sites could be. Secondly, in each of our experiments, in addition to the possibility of undetected effective spread of tracer beyond the regions of interest, we always found at least a few PHAL labeled perikarya outside of the LHAjp or LHAs; the latter may also be due to dendrites extending from neighboring regions in to the target region. Thus, we cannot rule out the possibility that some of the lighter labeling we report relates not the LHAjp or the LHAs but to adjacent nuclei or regions. An effective and standard way to address this issue is to examine retrogradely labeled neuron populations with an anterograde tracer, and anterogradely labeled terminal fields with a retrograde tracer (whether through new experiments or those previously published). Another consideration is that with the present method it is not possible to determine whether or not neurons retrogradely labeled from two different sites that are intermingled in a given region project to both sites -- a double retrograde method in a single animal would be necessary to address this. As a final point here, neuronal gene expression patterns restricted to or selective for all of the projection neuron populations in the LHAjp and LHAs have not yet been established. This precludes the use of genetic dissection methods for determining the global neuronal input/output relationships of these two cytoarchitecturally differentiable gray matter regions (see Luo et al., 2008).
4.2. Connectional and functional overview
The present results indicate that the primary connections of the LHAjp and LHAs are differentiable but very complex, involving together almost 250 other definable gray matter differentiations of the brain (Table 1) -- this equates to approximately half the total number (about 550) currently recognized in the rat brain as a whole (Table B in Swanson, 2004). To place these results into a meaningful context necessitates a discussion of the functional implications. However, given the magnitude of these results we take a conservative approach and limit our discussion to what is known at a basic level about the structure-function attributes of a selection of the neuron populations that project directly to, or receive direct inputs from, these two nearby LHA differentiations. A theoretical framework for this discussion is provided by a four subsystem structure-function network model of the nervous system (Swanson, 2003b; Swanson, 2004) -- with motor, sensory, cognitive, and behavioral state subsystems. A single page summary of our results in the context of this four subsystem model is presented in the form of a tree diagram (Fig. 12); this is also referenced in a circuit diagram (Fig. 13), and these diagrams together with the summary ‘flatmap’ (Fig. 5) may be refered to as a guide throughout the following discussion.
One approach to the four subsystem model concerns cerebral hemisphere (cognitive) regulation of motivated behavior (Swanson, 2000) and argues for the existence of a triple descending projection from the cerebral hemispheres to the motor system, including a behavior control column located medially within the hypothalamus and midbrain. The projection comprises interconnected cortical excitatory, striatal inhibitory, and pallidal disinhibitory components. Generally, recipient regions of the behavior control column in turn project to midbrain regions where patterns of behavior are initiated; the latter project to motor pattern generator networks within the hindbrain and spinal cord, and it is the activity of these that are largely responsible for the activity of specific sets of motoneuron pools that effect the corresponding specific behaviors. Although the fine details and functional dynamics of the circuitry remain to be fully elucidated, this model, characterized by topographically based hierarchical, parallel, and network organizing principles, provides a useful conceptual frame of reference for the complex circuitry presented here.
4.3. LHAjp discussion: Connectional and functional considerations
With the wider vista in mind, if we now select as a starting point the source of the most abundant input to the LHAjp, this directs us to the cerebral cortex and in particular the hippocampal formation (HPF). Before proceeding, we address certain difficulties inherent in description of HPF topography and present a novel approach (Fig. 11). As is known, the longitudinal axis of the rat HPF is roughly “C”-shaped, with a dorsal or septal end and a ventral or temporal end (see Swanson and Cowan, 1977, their Figs. 1 and 2). However, beyond this basic distinction, several different qualitative and arbitrary descriptions exist. For example, the subiculum has been divided into dorsal and ventral halves (e.g., Swanson, 2004), or dorsal, intermediate, and ventral “parts” (e.g., Groenewegen et al., 1987; Kishi et al., 2000) or “zones” (Swanson and Cowan, 1977); Groenewegen (1977) further divided the intermediate part of the subiculum into dorsal and ventral “parts”.
Figure 11.
A lateral view of Ammon’s horn and the subiculum projected on the right cerebral hemisphere of the rat. The curved longitudinal axis of these parts of the hippocampal formation is indicated by a dashed line, with D at the dorsal or septal end and V at the ventral or temporal end. Dashed lines a and b are transverse to the longitudinal axis and divide qualitatively Ammon's horn and the subiculum into approximate dorsal, intermediate, and ventral thirds for descriptive purposes. The transverse solid line 1 divides qualitatively Ammon’s horn and the subiculum into dorsal and ventral halves for descriptive purposes. OB, olfactory bulb (rostral end of cerebral hemisphere). Adapted from Swanson and Cowan (1977). It is noteworthy that the site of the most abundant retrograde labeling in this study identified a projection to the LHAjp from the ventral half (predominantly) of the intermediate third of the subiculum.
Our current approach is to qualitatively divide the hippocampal region (Ammon's horn and dentate gyrus) along with the adjacent subiculum into approximate thirds to give dorsal, intermediate, and ventral volumes, with further specificity of description achieved by referring to dorsal and ventral halves (Fig. 11). Applying this model to our results we can say that retrograde labeling in the HPF from the LHAjp was most abundant within the intermediate third of field CA1 and the subiculum (caudal region of field CA1 and subiculum in transverse section); more specifically it predominated within the ventral half of the intermediate third of the subiculum (Fig. 4 HH–LL). Also noteworthy is an alternate collective parceling scheme for field CA1 and the subiculum proposed by Risold and Swanson (1996), who divided the longitudinal extent of these cortical areas into five sequential domains based on their predominant mode of topographically organized projection to the lateral septal nucleus, which in turn establishes topographically organized bidirectional connections with various parts of the hypothalamus. The topographic relation of these domains to LHA terminal fields will be dealt with in a subsequent paper.
4.3.1. LHAjp discussion: Hippocampal formation
A recent study examined in the rat the behavioral effects of bilateral excitotoxic (ibotenic acid) lesions of the HPF (Pentkowski et al., 2006). In that study it was reported that lesions of the ventral half of the intermediate third of the HPF (including at least dentate gyrus, Ammon’s horn, and subiculum) were effective at reducing an unconditioned (innate) defensive emotional response to the odor, but not the sight, of a predator (in this case a cat). Conversely, in the same study lesions restricted to the dorsal third of the HPF (including Ammon’s horn and subiculum) were without effect on these measures of behavior; the effect of lesion of the subiculum and Ammon’s horn in between was not assessed (Pentkowski et al., 2006). The authors went on to suggest an anxiolytic but not anti-fear effect of their effective lesions proposed to be consistent with a reduction in exploratory risk assessment behavior to the odor, but no attenuation of escape or freezing behavior to the presence of a clear visual threat (cat exposure). In an earlier study by a different group, bilateral excitotoxic combined lesion of the ventral half of the dentate gyrus, Ammon’s horn, and subiculum resulted in rats that spent more time in the open arms of an elevated plus maze -- an effect that was mimicked by the potent anxiolytic drug midazolam (a benzodiazepine derivative and potentiator of GABAergic neurotransmission) (Kjelstrup et al., 2002); furthermore, when confined for short periods in a brightly lit room these rats were also reported to defecate with much reduced frequency and to have lower plasma corticosterone in comparison to rats with combined lesion of the dorsal half of the dentate gyrus, Ammon’s horn, and subiculum (Kjelstrup et al., 2002).
Additional previous work has also implicated the ventral half of the intermediate third of the subiculum in defensive responses. For example, bilateral electrolytic lesion involving the ventral half of the intermediate third of the HPF (reported as 50 – 60% lesion of the “posteroventral hippocampus”, and shown to include the dentate gyrus, subiculum, and parts of parasubiculum and adjacent entorhinal area but not Ammon’s horn) in the rat has been reported to elicit a “rage syndrome” (McGowan et al., 1972) akin to a classic report of such behavior resulting from lesion of the lateral septal nucleus (Brady and Nauta, 1953). Furthermore, bilateral electrolytic lesion of the ventral half of the intermediate third of the HPF (shown to include at least the dentate gyrus, subiculum, and entorhinal area) (Blozovski and Harris, 1986) or combined lesion of the intermediate third of the HPF (shown to include at least subiculum and dentate gyrus) (Kimrua, 1958) in the rat results in a deficit in the acquisition of a passive avoidance response (Kimrua, 1958; Blozovski and Harris, 1986). Also noteworthy is a recent report indicating a requirement for roughly the ventral (but not dorsal) half of field CA1 in the temporal processing of olfactory information (Hunsaker et al., 2008); whereas the entire field CA1 appears to be involved in the temporal processing of visual information (Hunsaker et al., 2008).
The examples given above, indicative of an established role for the HPF in processing emotional stimuli, echoes a classical view of the HPF that has it positioned as a central regulator of emotion (Papez, 1937) -- a view that led to the proposal of a more specific role for the HPF in behavioral inhibition (Gray, 1976; Gray, 1982). However, these views have to some extent been overshadowed by studies emphasizing a role for the HPF in spatial navigation, for examples see: (O'Keefe and Nadel, 1978) and learning and memory, for examples see: (Eichenbaum, 2000a; Squire et al., 2004). Together these differing roles speak to a broader integrative function for the HPF -- one in which the seamless integration of spatial information and emotional salience with mnemonic processes facilitates appropriate modulation of an animal's behavior. An integrative role for the HPF is not a novel idea; several studies, many of them recent, grapple with this notion from differing perspectives, for examples see: (Hirsh, 1974; Eichenbaum, 2000b; Dolan, 2002; Holscher, 2003; Bannerman et al., 2004; Erdi et al., 2005; Kulvicius et al., 2008).
It is evident there exists regional division of function within the HF, and most apparent is a graded dorsal and dorsal intermediate to ventral intermediate and ventral differentiation in which the dorsal regions have a role in spatial navigation that is not necessarily (directly) influenced by anxiety-related information, but may be influenced by other emotionally salient stimuli (Bannerman et al., 2004; Pentkowski et al., 2006; O'Mara, 2006; Engin and Treit, 2008); whereas ventral regions (and in particular the ventral- and ventral intermediate subiculum) appears not to have a primary role in spatial navigation, but a central role in the processing of anxiety-related information (Herman et al., 1998; Kjelstrup et al., 2002; Bannerman et al., 2004; Pentkowski et al., 2006; O'Mara, 2006; Herman and Mueller, 2006; Engin and Treit, 2008), including involvement in an activation of the neuroendocrine response to stress (Herman et al., 1998; Kjelstrup et al., 2002). It is also of note that whereas glucocorticoid receptors are expressed throughout the HF, the ventral regions of Ammon’s horn and subiculum are reported to express approximately 2½ times the number of glucocorticoid receptors than the dorsal regions (Reul and de Kloet, 1985). To what extent differing views of the HPF can be reconciled remains to be determined and the details of the topography have yet to be fully elucidated. Nevertheless, a demonstrated involvement of the ventral intermediate region of the HPF in defensive responses is consistent with a strong projection from this region to the LHAjp, which as we shall now discuss, receives from and projects to several regions that are positively identified as components of a medial hypothalamic defensive behavior system.
4.3.2. LHAjp discussion: The medial hypothalamic defensive behavior system
The medial hypothalamic defensive behavior system was identified in the rat following studies in which rats were exposed to a cat (predator) in order to elicit innate defensive behavior. Brain regions showing increased neuronal activation associated with the defensive behavior (as determined by elevated numbers of Fos immunopositive neurons) were identified (Canteras et al., 1997; Canteras and Goto, 1999b). From this work three medial hypothalamic differentiations were identified for their especially elevated levels of Fos expression, these were the AHN, VMHdm, and PMd (Canteras et al., 1997); more recent work has confirmed these findings (Cezario et al., 2008). In another related study in which rats were exposed to the odor (but not the site) of a cat, regions corresponding to the AHN, VMHdm, and PMd also showed a marked increase in Fos expression (Dielenberg et al., 2001). In the present study we have shown for each of these three regions (AHN, VMHdm, and PMd) that they receive a projection from the LHAjp (major to the AHN and PMd and moderate to the VMHdm) (Fig. 13); in addition, the LHAjp also receives a major input from the AHN (Fig. 13). Furthermore, previous PHAL studies have revealed the AHN, VMHdm, and PMd to be highly interconnected (Canteras, 2002; Thompson and Swanson, 2003).
In the predator exposure defensive behavior model just described, bilateral chemical (excitotoxic: ibotenic- or N-methyl-D-aspartic acid) lesion of the PMd results in a severe reduction of escape and freezing responses (Canteras et al., 1997; Markham et al., 2004; Blanchard et al., 2005; Cezario et al., 2008), as well as chronic docility and attenuated vocalization when the animals are handled (Canteras et al., 1997). Bilateral electrolytic lesion of the PMd results in a similarly dramatic reduction in rat defensive behavior to the presence or odor of a cat (Blanchard et al., 2003; Blanchard et al., 2005). Most recently, blockade of glutamatergic neurotransmission (Canteras et al., 2008) or facilitation of GABAergic transmission (Cezario et al., 2008) within the PMd was shown to result in essentially the same behavioral deficit as that resulting from PMd lesion. Conversely, other studies have shown that PMd electrical stimulation (Yardley and Hilton, 1986) or microinjection of a GABAA receptor antagonist (bicuculline methiodide) into the vicinity of the PMd (Di Scala et al., 1984) will elicit the expression of behaviors resembling those presented by an animal exposed to a predator.
Interestingly, the AHN is implicated in different types of defensive response. Thus, electrical stimulation studies in the rat have identified a region encompassing much of the AHN from which escape (Lammers et al., 1988b) or attack (Kruk et al., 1983; Lammers et al., 1988a; Dielenberg et al., 2001; Hrabovszky et al., 2005) behavior can be evoked. It is noteworthy that a region corresponding to the anterior part of the AHN in particular was identified in these studies (Kruk et al., 1983; Lammers et al., 1988a; Lammers et al., 1988b); this is notable because in the present study we found the anterior part of the AHN to be a site of very abundant retrograde labeling from the LHAjp (in particular from the rostral half of the LHAjp). More apposite, a region corresponding to the LHAjp is identified as a site at which electrical stimulation will elicit bite attacks in male rats exposed to conspecifics (Kruk et al., 1983), or nonspecific jumps (Lammers et al., 1988b).
The hypothalamic PMd gives rise to very robust and topographically organized descending projections to the midbrain PAG; although our understanding of the topography is currently incomplete, in broad terms it is clear that the more ventral (or ventrolateral) PMd targets very heavily rostral levels of the PAGdl, whereas the more dorsal (or dorsomedial) PMd gives rise to a very light projection to rostral levels of the PAGdl, and a very dense projection to a dorsolateral region of the PAGvl (Goto et al., 2005; Motta et al., 2009). Recall that we found the LHAjp to also target the PAG lightly, principally to rostral levels of the PAGvl (Fig. 4 GG–II). A strong descending projection from the LHAjp to the PMd and then PMd to the PAG, and a more moderate projection from the LHAjp directly to the PAG, is in line with hierarchical control of defensive behavior suggested by early work in this field (Hunsperger, 1956; Fernandez de Molina and Hunsperger, 1962); it is also in line with a general model for the control of motivated behavior expressed more recently and emphasizing separate, hierarchically ordered regions for behavior control (hypothalamus) and behavior initiation (midbrain) (Swanson, 2000).
4.3.3. LHAjp discussion: Beyond the medial hypothalamic defensive behavior system
Thus far we have shown the LHAjp to be intimately connected to a proposed medial hypothalamic defensive behavior system, which itself is part of a behavior control column within the hypothalamus. According to our data several extra-hypothalamic regions are likely to influence this system via the LHAjp, and prominent among these regions are: in the cerebral cortex the HPF and prefrontal cortex, in the striatum the lateral septal nucleus, and (less prominently) in the pallidum the bed nuclei of the stria terminalis and diagonal band nucleus (Figs. 12, 13). In this way, with reference to the concept of a triple descending projection from the cerebral hemispheres to a behavior control column, we have shown for the LHAjp a direct descending input from the cerebral cortex (presumed excitatory), striatum (presumed inhibitory), and pallidum (presumed disinhibitory). Thus, in addition to medial hypothalamic nuclei, the LHAjp could also be considered an integral component of, or intimately related to, the behavior control column. In this context also worthy of note is a less prominent ascending projection within the cerebrospinal trunk from the rostromedial division, and (to a lesser extent) precommissural nucleus, of the PAG to the LHAjp (Fig. 4 CC–DD). Given the importance of the PAG for the expression of defensive behaviors (Bandler et al., 1985; Krieger and Graeff, 1985), and the strong descending projections from the PMd to the PAG (Goto et al., 2005), an ascending projection from the PAG to the LHAjp provides a direct route whereby activity of the midbrain behavior initiation region in the PAG could provide feedback to modulate activity of the LHAjp (and therefore the behavior control column).
The LHAjp may well be involved in the modulation of defensive behaviors. However, it is likely that the defensive responses in which it may participate are influenced quite differently by different sensory modalities. For example, shock-based fear conditioning to an otherwise innocuous odor will strongly activate the PMd (Canteras et al., 2008); conversely, PMd activation is not noted when the conditioned stimulus is auditory (Pezzone et al., 1992). In addition, PMd lesion, while effective at reducing defensive behavior to cat odor, appears to have little effect on freezing responses to electric shock or in the elevated plus maze (Blanchard et al., 2003). We cited an article at the start of this discussion indicating involvement of the ventral intermediate HPF in rat defensive responses to predator odor (Pentkowski et al., 2006), and we suggested that this may involve the LHAjp via the massive input it receives from the ventral half of the intermediate third of field CA1 and the subiculum. This in turn may influence activity of the medial hypothalamic defensive behavior system, which presumably would result in modulation of defensive behavior. Consistent with the latter, a recent study showed an approximately 2.5-fold increase in Fos expression specifically within the LHAjp following exposure to a live cat (Cezario et al., 2008). In the same study an approximately 8-fold increase in Fos expression in the PMd was noted -- it is suggested the PMd may act to amplify the signals it receives relating to defensive behavior (Cezario et al., 2008).
It is important to be clear that a possible role for the LHAjp in the modulation of defensive behavior in response to olfactory cues does not preclude its possible involvement in other behaviors that may or may not also be susceptible to influence by olfactory cues. For example, grooming behavior has been reported to occur following injection of various neurochemicals into a region corresponding to or including the LHAjp. These neurochemicals include adrenocorticotropic hormone (ACTH) (Van Erp et al., 1991) and the ionotropic glutamate receptor agonists kainic acid (Roeling et al., 1990) and N-methyl-D-aspartic acid (NMDA) (Roeling et al., 1991); grooming behavior is also reported following electrical stimulation in the vicinity of the LHAjp (Lammers et al., 1987). A further consideration is that grooming behavior may also be related to defensive behavior, in the form of displacement behavior -- the nature of which was so well described by Nikolaas Tinbergen is his classic studies of instinct (Tinbergen, 1951).
An understanding of any given brain region merits a consideration of the relations of its input-providing regions and recipient sites. For the LHAjp we have thus far done this for regions that together constitute elements of a medial hypothalamic defensive behavior system. We now consider with respect to the LHAjp, the relations and possible functional roles of additional hypothalamic and extra-hypothalamic sites that project to or receive from the region of the LHAjp. Within the hypothalamus, in addition to the aforementioned regions, ventral levels of the medial mammillary nucleus (MM) receive a dense and strikingly targeted projection from the caudal end of the LHAjp (Fig. 6C). The MM, as is the case for the LHAjp, also receives a strong projection from the subiculum (Swanson and Cowan, 1975; Swanson and Cowan, 1977; Kishi et al., 2000) as does the PMd (Comoli et al., 2000). Moreover, the topography of this projection is noteworthy in that the dorsal half of the subiculum projects predominantly to the dorsal MM (Swanson and Cowan, 1975; Swanson and Cowan, 1977; Shibata, 1989; Kishi et al., 2000), whereas the ventral half of the subiculum projects predominantly to the ventral MM (Shibata, 1989; Kishi et al., 2000); a similar topography is also reported in the macaque monkey (Aggleton et al., 2005).
Thus the ventral half of the subiculum targets heavily ventral levels of the MM in addition to the LHAjp. To what extent these projections arise from the same neurons remains to be determined. Nevertheless, this projection, together with a projection from the caudal end of the LHAjp to ventral levels of the MM, places the LHAjp as an intermediary within the classic loop alluded to earlier and originally described by James Papez (Papez, 1937). This loop (in essence: hippocampal formation > mammillary body > anterior thalamic group > medial isocortex > hippocampal formation) he proposed as an anatomical substrate for the processing of emotion. Although the basic anatomical loop described by Papez has essentially stood the test of time, it has undergone substantial refinement (Swanson and Cowan, 1975; Swanson and Cowan, 1977; Seki and Zyo, 1984; Shibata, 1989; Shibata, 1992; van Groen and Wyss, 1995; Dalgleish, 2004). Furthermore, the functional role attributed to “the Papez circuit” has undergone revision, with a more current emphasis placed on mnemonic processes relating to spatial navigation rather than emotion per se (Vertes et al., 2001; Vann et al., 2003; Vann and Aggleton, 2003; Vann and Aggleton, 2004). Nevertheless, our results together with recent work on hippocampal function (Herman et al., 1998; Pentkowski et al., 2006; O'Mara, 2006; Engin and Treit, 2008) suggest the “circuit for emotion” as conceived by Papez continues to provide a useful heuristic.
4.3.4. LHAjp discussion: Thalamus
Given our proposed involvement of the LHAjp in the modulation of defensive behaviors, a direct link between the LHAjp and the MM is interesting in so far as it provides a link between a region implicated in behavior (LHAjp) and one implicated in learning and memory (MM, via its projection to the anterior thalamic group). More specifically the MM via the mammillothalamic tract (mtt) is implicated in visuo-spatial learning -- this is disrupted by lesions of either the MM or the mtt (Vann et al., 2003; Vann and Aggleton, 2003). Recent work suggests a similar role for PMd (Cezario et al., 2008; Carvalho-Netto et al., 2008) that also projects massively to the anterior thalamic group (specifically to the AMv) (Canteras and Swanson, 1992b). In short, what we have identified is a direct input from the hippocampal formation to the LHAjp and an indirect route by which the neurons of the LHAjp may (via the MM/PMd as a component of the Papez circuit) influence activity of the hippocampal formation. In terms of possible function, in essence what is described thus far is a basic circuit including the LHAjp that may be involved not only in the emotive expression of a behavior but also in experience-dependent changes to that behavior, i.e. learning and memory.
If we now consider the connections of ventral regions of the MM to the thalamus and beyond in more detail, we see that it is principally a dorsorostral subregion of the anteroventral thalamic nucleus (AV) that is the thalamic recipient region from the ventral MM (Seki and Zyo, 1984; Shibata, 1992). The dorsorostral subregion of the AV in turn has bidirectional connections with intermediate to caudal levels of the dorsal part of the retrosplenial area (RSPd) (van Groen and Wyss, 1995; Shibata, 1998; van Groen and Wyss, 2003); it also projects prominently to the pre- and (to a lesser extent) postsubiculum (van Groen and Wyss, 1995). Recall that we found a low amount of retrograde labeling at an intermediate rostrocaudal level of the ventral part of the retrosplenial area (RSPv) from the LHAjp (Fig.4 FF–II). The RSPv has major bidirectional connections with the RSPd (in addition to the contralateral RSPv) (van Groen and Wyss, 1990; van Groen and Wyss, 2003). The RSPv also projects strongly to dorsal regions of the AV and more lightly to a dorsal region of the lateral dorsal (LD) and reticular (RT) nuclei of the thalamus (van Groen and Wyss, 1990; Shibata, 1998) (the latter two regions also receive a very light input from the LHAjp); the RSPv also receives a strong input from the AD (van Groen and Wyss, 1990; van Groen and Wyss, 1995).
As is the case with lesion of the MM or mtt (Vann et al., 2003; Vann and Aggleton, 2003), lesion of the AD or AV impairs spatial learning (Byatt and Dalrymple-Alford, 1996; van Groen et al., 2002a; Mitchell and Dalrymple-Alford, 2005), as does lesion of the LD (van Groen et al., 2002b). Lesion of the RSPd and RSPv impairs spatial learning also, and this is of much greater severity if the RSPd and RSPv are lesioned together rather than independently (Vann and Aggleton, 2002; van Groen et al., 2004). In addition to learning impairment, recent work indicates that retrosplenial area lesion also impairs spatial navigation that is reliant on directional cues (Pothuizen et al., 2008). Recent work also suggests related involvement of two additional thalamic regions that share a connection with the RSP, these are the rostral division of the nucleus reuniens together with ventral levels of the anteromedial thalamic nucleus (AMv) (Carvalho-Netto et al., 2008) -- recall that the AMv receives a massive input from the PMd (Canteras and Swanson, 1992b).
Combined lesion of the rostral division of the nucleus reuniens and AMv is reported to result in a “drastic reduction of contextual conditioned defensive responses” (Carvalho-Netto et al., 2008). It is salient to recall that the rostral division of the nucleus reuniens receives a dense projection from the LHAjp (Fig.4 R–U): the rostral division of the nucleus reuniens also has robust bidirectional connections with field CA1 and the subiculum (Wouterlood et al., 1990; McKenna and Vertes, 2004; Vertes et al., 2006) that, as we have shown, project strongly to the LHAjp. Recall too that the subiculum and field CA1 also target heavily ventral regions of the MM (Shibata, 1989; Kishi et al., 2000), RSPv (van Groen and Wyss, 1990), and PMd (Comoli et al., 2000), and that lesion of the ventral half of the intermediate third of the HPF (including at least dentate gyrus, Ammon’s horn, and subiculum) reduces a defensive response to a threat signified by an odor (Pentkowski et al., 2006). Given these functional data together with the connectional data, one could predict that lesion of the LHAjp may have an effect on spatial (contextual) learning and navigation, and especially in relation to defensive behavior that has an olfactory component.
4.3.5. LHAjp discussion: Theta rhythms
An additional functional perspective and higher order of integration is suggested by previous work indicating that the Ammonic theta rhythm (characteristic of locomotor and attentive behavior in rats and associated with mnemonic processes) resonates throughout structures of the Papez circuit (Vertes et al., 2004); this raises the intriguing possibility that neurons within the LHAjp (as a putative accessory component of the Papez circuit) might also show theta oscillations. In further support of this proposition, several hypothalamic sites have been identified for their responsiveness to stimulation of the rostral (oral) part of the pontine reticular nucleus (Vertes and Kocsis, 1997; Woodnorth et al., 2003), a brainstem site capable of eliciting the synchrony of hippocampal activity that is a necessary prerequisite for theta rhythm generation (Vertes, 1981; Vertes, 1982). Moreover, in addition to its classic association with locomotion in the rat (Vanderwolf, 1969), the hippocampal theta rhythm is also associated with other processes that may be predicted to have a bearing on defensive behavior. Thus, hippocampal mnemonic processes are affected by the theta rhythm (Staubli and Lynch, 1987; Huerta and Lisman, 1996; Vertes and Kocsis, 1997); environmental novelty appears to reduce its frequency (Jeewajee et al., 2008; Lever et al., 2009); while an increase in theta rhythm frequency (also associated with a heightened motivational-emotional state) is reported to correlate with an increase in the speed of locomotion (Slawinska and Kasicki, 1998; Jeewajee et al., 2008).
The possibility of a theta rhythm influence on defensive behavior involving neurons within the LHAjp is also likely to involve certain neuron types that have particular roles in spatial cognition; these are the so-called “head-direction” (Taube et al., 1990a), hippocampal “place” (O'Keefe and Dostrovsky, 1971), and (most recently identified) entorhinal area “grid” (Hafting et al., 2005) neurons. Head-direction neurons as a group include subtypes that respond to the present or anticipated facing direction subject to the influence of a present or previous environment (Taube et al., 1990a; Taube et al., 1990b; Chen et al., 1994a; Chen et al., 1994b; Muller et al., 1996; Taube, 1998). Complimentary to head-direction neurons, place and grid neurons respond essentially to a specific locus (place) and locus progression (grid). Together with head-direction neurons, these three neuron types collectively comprise central elements of a system for spatial cognition -- a system essential for most behaviors, not least defensive behavior.
Of further relevance, head-direction neurons are present and prominent in several regions associated with the classic Papez circuit, including some that have light direct connections with the LHAjp, these regions are: lateral mammillary nucleus, anterolateral and lateral dorsal thalamic nuclei, and postsubiculum and retrosplenial area (Taube and Bassett, 2003). Head-direction neurons are therefore in a position to be influenced by the hippocampal region theta rhythm (Vertes et al., 2004), as are place neurons (being intrinsic to Ammon’s horn); whereas grid neurons in the entorhinal area are in a strong position to influence the Ammonic theta rhythm via the perforant and direct temporoalvear paths. Therefore, the neurons of the LHAjp are in a position to integrate and contribute to a temporally coded stream of information relating to spatial cognition, locomotion, and emotional salience. Furthermore, a link is indicated between the rhythmic processes (associated and coordinated with respiration) that shape the flow of information into the olfactory system and modulate other central rhythms, not least the hippocampal region theta rhythm, as means to facilitate perceptive behavior (Uchida et al., 2006; Kepecs et al., 2006; Grosmaitre et al., 2007).
Returning to and extending further our discussion of the connections of the retrosplenial area with regard to connections of the LHAjp, it is noteworthy that the RSPv receives a major input from the ventral intermediate subiculum (van Groen and Wyss, 1990). Dorsal to the RSPv, and adjacent to the RSPd, the agranular part of the retrosplenial area (RSPagl) also has relevance to this discussion given its strong bidirectional connections with the RSPv (van Groen and Wyss, 1990; van Groen and Wyss, 1992; Risold et al., 1997). In addition, the RSPagl has bidirectional connections with the adjacent anteromedial visual area (VISam), as well as more rostral parts of the medial isocortex including the anterior cingulate area (van Groen and Wyss, 1992; Risold and Swanson, 1995). As is the case for other parts of the retrosplenial area, the RSPagl has major connections with the thalamus. Prominent among them are bidirectional connections with anterior and lateral groups, and the rostral division of the nucleus reuniens (van Groen and Wyss, 1992; Risold and Swanson, 1995).
The RSPagl also projects to the superior colliculus (van Groen and Wyss, 1992), and this projection (in addition to its connections with the lateral group of the thalamus) identify it and the RSPv and RSPd with which it is intimately connected, as being in a position to influence eye movements and so have a role in visual attention. Strong indirect connections of the LHAjp with the RSP (in addition to a minor direct input from both the RSPv and superior colliculus) are therefore indicative of a possible role in visual attention. This certainly fits with our proposed involvement of the LHAjp in the modulation of defensive behavior; in the context of the discussion so far, it also refines our understanding of a previously proposed hypothalamus-thalamus-cortical circuit for mediating pheromonal influences on eye and head movements (Risold and Swanson, 1995).
We have now identified and highlighted certain medial hypothalamic, thalamic (anterior and lateral groups, and nucleus reuniens), cortical (retrosplenial area and subiculum), and midbrain (periaqueductal gray) regions that by their interconnections and connections with the LHAjp, together with some elucidation of their functional roles, provide the basis of a theoretical framework for understanding the position of the LHAjp within a broader structural and functional network. However, several additional components of this network remain to be addressed in order to provide a more coherent framework. These components include four regions that we mentioned earlier in this discussion, namely: the medial prefrontal cortex, lateral septal nucleus (striatum), diagonal band nucleus and bed nuclei of the stria terminalis (pallidum). In addition to these, a projection to the lateral habenula is noteworthy. Before entering into this final phase of our discussion for the LHAjp, it is apposite first to review briefly the principal neural pathways likely to be involved in the transmission of pheromonal information to the LHAjp because this forms a basic starting point for the possible behavioral role we are emphasizing.
4.3.6. LHAjp discussion: Olfactory system
Recent studies of the mouse olfactory system indicate that the odor sensing capabilities of the main and accessory olfactory epithelia overlap more than was previously thought (Trinh and Storm, 2004; Lin et al., 2004; Xu et al., 2005; Wang et al., 2006). Furthermore, recent studies also reveal more extensive overlap in the central projection fields of the main and accessory olfactory systems in rats and mice (Halpern and Martinez-Marcos, 2003; Pro-Sistiaga et al., 2007; Pro-Sistiaga et al., 2008) than was indicated in a previous classic work (Scalia and Winans, 1975). Nevertheless, the vomeronasal organ persists as a site that is specialized for the detection of pheromones (Halpern and Martinez-Marcos, 2003). In addition there exists a rostrocaudal differentiation within the accessory olfactory system, characterized by molecular and cytoarchitectural differences in the accessory olfactory epithelium (Jia and Halpern, 1996; Herrada and Dulac, 1997; Jia et al., 1997a) and accessory olfactory bulb (Jia and Halpern, 1996; Jia and Halpern, 1997; Jia et al., 1997a; Larriva-Sahd, 2008) and by differences in a subset of the central projections of rostral and caudal levels of the accessory olfactory bulb (Mohedano-Moriano et al., 2007).
Specifically, rostral (but not caudal) levels of the accessory olfactory bulb (AOB) appear to project to the bed nuclei of the stria terminalis, principal nucleus (BSTpr); whereas caudal (but not rostral) levels of the AOB project to dorsal levels of the anterior amygdalar area (AAA), deep levels of the bed nucleus of the accessory olfactory tract (BA), and the anteroventral part of the medial amygdalar nucleus (MEAav) (Mohedano-Moriano et al., 2007). In addition to segregated regions of termination, most of the amygdalar differentiations targeted by the AOB receive jointly from its rostral and caudal region; these differentiations are the MEAad, MEApv, MEApd, and cortical amygdalar nucleus (COA) (Scalia and Winans, 1975; Mohedano-Moriano et al., 2007).
To recap: we found the LHAjp to have a light bidirectional connection with the BSTpr and BSTif (Fig. 4Q–R), and a very light bidirectional connection with the MEAad (Fig. 4S–U). Thus, these two cell groups provide a possible direct link between the accessory olfactory system and the LHAjp. Further possible links, more substantial yet indirect, are provided by the connections of additional AOB-recipient parts of the medial and cortical amygdalar nuclei. Before describing these it is noteworthy that with the exception of the MEApd, subdivisions of the medial and cortical amygdala are highly interconnected (Canteras et al., 1995). The MEApd projects heavily to the BSTpr, while the MEApv and MEAad (and possibly MEAav) project heavily to the interfascicular nucleus of the BST (BSTif) (Dong et al., 2001a), which itself has a moderate bidirectional connection with the LHAjp (Dong and Swanson, 2004b) (and our results) and also projects to all components of the medial hypothalamic defensive behavior system (i.e., the AHN, VMHdm, and PMd) (Dong and Swanson, 2004b); the posterior part of the COA also sends a moderate projection to the BSTif (Dong et al., 2001a).
In addition to possible connections of the AOB with the LHAjp via the projections of AOB-recipient parts of the amygdalar region that project to the BSTif and BSTpr, there are also projections from the medial amygdalar nucleus to several other sites that strongly project to the LHAjp; these sites are principally within the cerebral cortex and hypothalamus. Specifically, the MEAad/v, MEApv, and MEApd all send a moderate projection to the ventral halves of the subiculum and field CA1 and the infralimbic area (Canteras et al., 1995). The MEAad/v and MEApv also project moderately to the prelimbic area (Canteras et al., 1995). All parts of the medial amygdalar nucleus also project to the entorhinal area (Canteras et al., 1995) that itself projects via the perforant and temporoalvear paths to the subiculum and CA1 (Wyss, 1981; Witter et al., 2000). Hypothalamic sites receiving a robust projection from the medial amygdalar nucleus and projecting to the LHAjp include, from the MEAad/v: AHNc, MPO, MPNl, PH; from the MEApv: AHNa/c, MPO, MPN, VMHdm, PH; from the MEApd: PH. The medial amygdalar nucleus also projects to the lateral septal nucleus; however, the principal projection is from the MEAad/v to the LSv (Canteras et al., 1995), which projects little to the LHAjp. Especially noteworthy is that the MEApv is specifically implicated in the processing of pheromonal odor related to defensive behavior (McGregor et al., 2004; Choi et al., 2005).
4.3.7. LHAjp discussion: Lateral septal nucleus
In reviewing centripetal pathways from the AOB to the LHAjp, we have identified certain regions and routes by which pheromonal information may influence the activity of neurons within the LHAjp; we have also touched on two of the principal regions projecting to the LHAjp that remain to be more fully discussed in terms of the functional implications. These regions are the lateral septal nucleus and the prefrontal cortex. The lateral septal nucleus appears to have a central role in defensive behavior. Classic studies describe a hyperdefensive behavioral response resulting from lesion to the lateral septal nucleus (Brady and Nauta, 1953; Brady and Nauta, 1955; Albert and Chew, 1980); this is illustrated well by a description of the hyperdefensive response in the rat following lesion of the lateral septal nucleus: “Immediately following recovery from the anesthetic, an explosive wildness and fierceness developed in these animals which made them virtually impossible to handle without special precautions and which appeared to leave them hyper-reactive to all sensory stimulation, particularly tactual.” (Brady and Nauta, 1955). Of particular relevance to our present results, the region of the lateral septal nucleus that was identified as being mainly involved in producing the hyperdefensiveness was the “rostrolateral septal nucleus” (Albert and Chew, 1980); this would have included the region of the lateral septal nucleus that we have identified as having strong bidirectional connections with the LHAjp (LSr.vl.d.l/m).
One interpretation of the classic septal lesion studies is that the lateral septal nucleus provides a tonic behavioral inhibition. This interpretation is broadly consistent with an inhibitory role for the striatum, of which the lateral septal nucleus is a component (Swanson, 2000). Given a massive cortical excitatory (presumed glutamatergic) projection from Ammon’s horn and the subiculum to the lateral septal nucleus (Risold and Swanson, 1997), it is also consistent with a previously suggested role for the hippocampal formation in behavioral inhibition (Gray, 1976; Gray, 1982) and may account for the early report of “rage syndrome” following bilateral electrolytic lesion of the ventral half of the hippocampal formation (that appeared to include at least the subiculum and dentate gyrus as well as parts of the parasubiculum and adjacent entorhinal area) (McGowan et al., 1972). However, various studies of lateral septal function have produced equivocal results, some suggesting behavioral inhibition, others activation, for examples see: (Gray and McNaughton, 1983). Nevertheless, strong bidirectional connections of the rostral part of the lateral septal nucleus with the LHAjp are consistent with our proposed involvement of the LHAjp in defensive behaviors. Furthermore, this raises the possibility that the previous equivocal results on the effects of septal lesions may have resulted from differential disruption of either a descending inhibitory projection or a (possibly inhibitory) ascending projection involving the LHAjp, as this might be predicted to produce differential effects on defensive behavior.
In this context it is also noteworthy that the rostral part of the lateral septal nucleus projects massively to the AHN (Risold and Swanson, 1997), which in turn has very strong bidirectional connections with the LHAjp. In addition, the part of the lateral septal nucleus that provides the strongest projection to the AHN (LSr.vl.d) (Risold and Swanson, 1997) is the same part that has the strongest bidirectional connections with the LHAjp. This speaks to the topography of the projections, and our findings confirm and extend previous work that has elucidated a highly organized topography in the projections of hippocampal domains via the fimbria to the lateral septal nucleus and between the latter and the hypothalamus (Risold and Swanson, 1996; Risold and Swanson, 1997). Specifically, the region of the hippocampal formation that projects most strongly to the LHAjp (intermediate regions of field CA1 and subiculum) also projects most strongly to the part of the lateral septal nucleus (LSr.vl.d) that has the strongest bidirectional connections with the AHN (Risold and Swanson, 1997) and LHAjp. These findings are also well viewed in light of recent work on hippocampal theta oscillations in the freely moving rat where these oscillations (recorded as field potentials from CA1) are revealed as travelling waves that propagate roughly along the dorsal to ventral (septotemporal) axis of the hippocampal formation (Lubenov and Siapas, 2009); one would predict such a functional topography to be determined by the underlying structural topography within the HPF, and to extend to its inputs and outputs.
Integration of the function of the LHAjp and lateral septal nucleus and further evidence of a highly organized topography is indicated by much commonality in the connections of the LHAjp and LSr.vl.d (Risold and Swanson, 1997). These common connections differ in magnitude, nevertheless efferent targets of the LHAjp and LSr.vl.d include (in addition to the AHN): anterior part of DMH, dorsal premammillary nucleus and ventral regions of medial mammillary nucleus, paraventricular thalamic nucleus, paratenial nucleus and rostral division of nucleus reuniens, lateral habenula, superior colliculus, and ventrolateral division of PAG; whereas projections to the LHAjp and LSr.vl.d come from (in addition to AHN, field CA1, and subiculum): dorsomedial part of VMH and the posterior hypothalamic nucleus (Risold and Swanson, 1997).
4.3.8. LHAjp discussion: Lateral habenula
The lateral habenula merits special attention for the dense terminal projection it receives from the LHAjp. Interest in the role of the lateral habenula has burgeoned as a result of recent evidence implicating this epithalamic structure in the processing of information relating to negative reward and motivation (Matsumoto and Hikosaka, 2007; Hikosaka et al., 2008; Matsumoto and Hikosaka, 2009). To elaborate: an increase in lateral habenula excitation correlates with an increase in perceived negative reward value, whereas an increase in perceived positive reward value correlates with an increase in lateral habenula inhibition (Matsumoto and Hikosaka, 2007; Hikosaka et al., 2008; Matsumoto and Hikosaka, 2009). Furthermore, excitation of lateral habenula neurons temporally precedes and correlates with inhibition of midbrain dopamine neurons (in the ventral tegmental area and compact part of the substantia nigra) (Christoph et al., 1986; Matsumoto and Hikosaka, 2007) and serotonergic neurons (in the dorsal nucleus of the raphé) (Wang and Aghajanian, 1977; Park, 1987). The dorsal (and median, that is superior central) nuclei of the raphé and ventral tegmental area receive a direct projection from the lateral habenula (Herkenham and Nauta, 1979; Araki et al., 1988; Kim, 2009), as does the cholinergic neuron-rich laterodorsal tegmental nucleus (Herkenham and Nauta, 1979; Cornwall et al., 1990; Semba and Fibiger, 1992; Kim, 2009) -- also recall we found the ventral tegmental area projects directly and lightly to the LHAjp. Thus the lateral habenula is in a position to possibly effect a broad modulation of behavioral state, consistent with a possible role for the LHAjp in defensive behavior via its projection to the lateral habenula.
With further regard to an association between the lateral habenula and our proposed involvement of the LHAjp in the modulation of defensive behavior, a recent study is noteworthy in which it was reported that chemical stimulation of the lateral habenula in the rat with kainic acid would facilitate inhibitory avoidance in an elevated T-maze paradigm (purported to be a measure of anxiety), while bilateral electrolytic lesion of the lateral habenula produced an opposite effect (Pobbe and Zangrossi, Jr., 2008); an effect of electrolytic lateral habenula lesion to reduce active avoidance also has previous report (Wilcox et al., 1986). Given a projection from the intermediate subiculum and field CA1 to the LHAjp and from the LHAjp to the lateral habenula, the effect of lateral habenula lesion has an intriguing parallel to the effect of ventral intermediate hippocampal formation lesions that include Ammon’s horn and the subiculum because these also attenuate a defensive response (risk assessment) considered to be a measure of anxiety (Pentkowski et al., 2006). Clearly a neural mechanism for associating a defensive (or other) behavior with the negative reward value of that behavior in a particular context would have utility for reining in behavior that if left unchecked could lead to undesired outcomes.
A novel understanding of lateral habenula function shows its connections in a new light. In addition to the already mentioned ventral tegmental area and nuclei of the raphé, one of the more well described and major targets of medial regions of the lateral habenula (the principle habenula region targeted by the LHAjp) is the nucleus incertus (Herkenham and Nauta, 1979; Goto et al., 2001; Olucha-Bordonau et al., 2003). The nucleus incertus is a distinct cell group situated adjacent to the dorsal tegmental nucleus in caudoventral regions of the pontine central gray (Goto et al., 2001); its diverse connections are well and lengthily described (Goto et al., 2001; Olucha-Bordonau et al., 2003); suffice to say here that the connections of the nucleus incertus have been taken to indicate a general role for it in behavioral planning, attention, execution, and learning (Goto et al., 2001; Olucha-Bordonau et al., 2003). A more specific role for the nucleus incertus is suggested to be control of the hippocampal theta rhythm (Olucha-Bordonau et al., 2003); this realted to our earlier proposition that the LHAjp may be influenced by theta oscillations (Vertes et al., 2004). Indeed electrical stimulation of the nucleus incertus in the rat is reported to evoke a theta rhythm in the hippocampal formation (recorded as a field potential centered in CA1) (Nunez et al., 2006), whereas lesion of the nucleus incertus can disrupt this rhythm (Nunez et al., 2006). Also noteworthy is a prominent projection from the nucleus incertus to the supramammillary nucleus, and bidirectional connections between the nucleus incertus and the posterior hypothalamic nucleus, and medial septal complex (Goto et al., 2001) -- each of these regions are understood to have a prominent role in either the generation or modulation of the hippocampal theta rhythm (Bland, 1986; Kocsis and Vertes, 1994; Vertes and Kocsis, 1997; Kirk, 1998; Pan and McNaughton, 2004).
Another structure of importance in relation to the lateral habenula and nucleus incertus is the interpeduncular nucleus (IPN). The IPN receives a topographically distinct input from the habenula (predominantly medial but also lateral) (Kim, 2009) and it has massive bidirectional connections with the nucleus incertus (which also connects massively with the superior central or median nucleus of the raphé) (Goto et al., 2001). Lesion of the IPN was shown recently to disrupt the activity of head-direction neurons in the anterodorsal nucleus of the thalamus (Clark et al., 2009); the latter is significant given the parity, discussed earlier, between certain regions showing theta oscillations (Vertes et al., 2004) and containing head direction neurons (Taube and Bassett, 2003), and connecting with the LHAjp. Continuing this theme, it is noteworthy that lesion of the dorsal tegmental nucleus or lateral mammillary nucleus also disrupts the activity of head-direction neurons in the anterodorsal nucleus of the thalamus (Bassett et al., 2007). The dorsal tegmental nucleus receives an input to its capsule from the nucleus incertus, and a direct input from the lateral mammillary and interpeduncular nuclei (Goto et al., 2001). In addition, lesion (ibotenic acid) of the laterodorsal tegmental nucleus -- a site of cholinergic neurons that receives a direct input from the nucleus incertus (Goto et al., 2001) as well as from the lateral habenula (Herkenham and Nauta, 1979; Araki et al., 1988; Cornwall et al., 1990; Semba and Fibiger, 1992; Kim, 2009) -- attenuates locomotion induced by scopolamine (a muscarinic acetylcholine receptor antagonist); this attenuation of locomotion is likely to be due in part to disruption of a cholinergic projection from the laterodorsal tegmental nucleus to dopamine neurons in the ventral tegmental area (Brudzynski et al., 1988; Oakman et al., 1995; Kocsis et al., 2001) -- the ventral tegmental area is also implicated in theta rhythm modulation (Kocsis et al., 2001; Bassant and Poindessous-Jazat, 2001).
As a final consideration to round out this section of the discussion, it is noteworthy that the nucleus incertus targets heavily an LHA site, namely the subfornical region of the LHA (and in particular its anterior zone) (Goto et al., 2001), which itself shares many of the projection targets of the LHAjp (Goto et al., 2005; see also Fig. 13). A further level of integration is suggested by the very abundant expression of corticotropin-releasing hormone (CRH) type 1 receptor expression in the nucleus incertus (Chalmers et al., 1995), indicating that the nucleus incertus (and thus perhaps the theta rhythm) may be modulated by the stress-related neuropeptide CRH, as has been suggested (Goto et al., 2001; Olucha-Bordonau et al., 2003) and indicated (Chan et al., 2000; Bittencourt and Sawchenko, 2000; Tanaka et al., 2005). In summary, a connection between the LHAjp and nucleus incertus via lateral habenula may provide a route whereby negative reward (error correction) information associated with defensive behavior could influence simultaneously the activity of head direction cells, theta oscillations, and locomotion, which in turn would influence any activity of the LHAjp modulated by these other activities. Relating the above considerations to the flow of the discussion overall, the findings argue a possible role for the LHAjp in the contextual, behavioral state-dependent and experience-dependent modulation and modification of behavior.
4.3.9. LHAjp discussion: Medial prefrontal cortex
Now we turn to consider the interconnections of the medial prefrontal cortex (rostromedial isocortex) with respect to the LHAjp. Overall, the medial prefrontal cortex (including here the anterior cingulate, prelimbic, and infralimbic areas) projects strongly to the LHAjp. Infralimbic and prelimbic areas project strongly to the rostral half of the LHAjp and lightly to the caudal end of the LHAjp; whereas the anterior cingulate area projects more strongly to the caudal end of the LHAjp and more lightly to the rostral half. The medial prefrontal cortex has a central role in diverse aspects of cognition and in that role is associated with several interrelated processes, broadly including: attention (especially visual) (Desimone and Duncan, 1995; Sarter et al., 2006), working memory (Repovs and Baddeley, 2006), decision making and goal directed behavior (Bechara et al., 1997; de Bruin et al., 2000), and visceromotor control (Neafsey, 1990) -- for further review see: (Bechara et al., 2000; Miller and Cohen, 2001; Dalley et al., 2004; Vertes, 2006; Robbins, 2007).
The distinct yet interrelated nature of these cognitive processes is reflected in the topographic organization of the medial prefrontal cortex. Possibly most evident is a graduated dorsal to ventral division (not unlike that for the hippocampal formation). In very broad functional terms, in the rat dorsal levels of the medial prefrontal cortex (including the anterior cingulate area and dorsal levels of the prelimbic area) is indicated to have primary role in cognitive processes associated with exteroception; whereas ventral levels of the medial prefrontal cortex (including ventral levels of the prelimbic area and the infralimbic area) is indicated to have a primary role in cognitive processes associated with interoception (Gisquet-Verrier et al., 2000; Heidbreder and Groenewegen, 2003; Vertes, 2006; Hoover and Vertes, 2007). These appellations are tentative and come with the caveat that there persists some lack of consensus regarding the organization, function, and nomenclature of the medial prefrontal cortex across species (Uylings et al., 2003). Nevertheless, given a stronger projection to the caudal end of the LHAjp from dorsal levels of the prefrontal cortex and a stronger projection to the rostral half of the LHAjp from ventral levels of the prefrontal cortex, this suggests that cognitive influences on any defensive behavior in which the LHAjp may participate is likely to be differentially modulated by different cognitive processes.
Most previous studies of prefrontal cortex connections have detailed the efferent projections, for example: (Room et al., 1985; Sesack et al., 1989; Hurley et al., 1991; Takagishi and Chiba, 1991; Buchanan et al., 1994; Ongur et al., 1998; Floyd et al., 2001; Chiba et al., 2001; Vertes, 2002; Gabbott et al., 2003; Vertes, 2004; Gabbott et al., 2005; Vertes et al., 2007); however, extensive studies of the afferent connections are also available (Audinat et al., 1988; Conde et al., 1990; Conde et al., 1995; Hoover and Vertes, 2007). From these studies it is clear that the anterior cingulate, prelimbic, and infralimbic areas are highly interconnected with one another, and with diverse regions of the cerebral cortex and cerebrospinal trunk. Of particular relevance to this discussion, beginning with the cerebral cortex, is a major input to the prefrontal cortex from field CA1 and the subiculum (Swanson, 1981). The graduated dorsal-to-ventral division of function within the medial prefrontal cortex referred to above is reflected in a dorsal-to-ventral gradation of this projection: retrograde tracing studies have revealed relatively moderate back-labeling in field CA1 and the subiculum from the anterior cingulate area that becomes progressively more abundant in transition through the prelimbic to the infralimbic area (Hoover and Vertes, 2007). Thalamic nuclei also project strongly to the prefrontal cortex, including three that receive a notable input from the LHAjp, i.e. anteromedial, paratenial, and reuniens nuclei (Vertes et al., 2006; Hoover and Vertes, 2007; Vertes et al., 2007; Vertes and Hoover, 2008); projections to the medial prefrontal cortex from the midbrain (ventral tegmental area and dorsal nucleus of the raphé) and pons (laterodorsal tegmental nucleus) are also prominent (Hoover and Vertes, 2007). In these connections, the areas of prefrontal cortex that project to the LHAjp receive major inputs from several regions that are, as we have discussed, either connected first order to the LHAjp or connected as second order regions that are likely to be instrumental participants in the elaborated functions of the LHAjp.
Major projections from the medial prefrontal cortex that relate directly to the interconnections of the LHAjp (in addition to the direct LHAjp projection already described) include pathways from the anterior cingulate area to the retrosplenial area (van Groen and Wyss, 1992; Risold and Swanson, 1995; Shibata and Naito, 2008) and anteromedial and lateral dorsal thalamic nuclei (Shibata and Naito, 2005) -- relating strongly, as discussed, to aspects of attention (especially visual), learning, and memory. Similarly, major projections from the prelimbic and infralimbic areas include those to the thalamic paratenial and reuniens nuclei -- the paratenial nucleus and rostral division of the nucleus reuniens receive a substantial projection from the LHAjp. The paratenial and reuniens nuclei in turn project massively (predominantly rostral division of reuniens) to the hippocampal formation (as well as back to the medial prefrontal cortex), thereby forming a nodal point for the flow of information between the LHAjp (our results), medial prefrontal cortex, thalamus, and hippocampal formation (Ohtake and Yamada, 1989; Wouterlood et al., 1990; Risold et al., 1997; Vertes, 2002; Vertes, 2004; Vertes et al., 2004; Vertes et al., 2006; Vertes, 2006; Hoover and Vertes, 2007; Vertes et al., 2007; Vertes and Hoover, 2008). Hippocampal formation projections from the nucleus reuniens appear to target all of field CA1 and the subiculum (Wouterlood et al., 1990; Risold et al., 1997; Vertes et al., 2006), whereas those from the paratenial nucleus are predominantly restricted to the ventral half of the subiculum (Vertes and Hoover, 2008).
The infralimbic (but not prelimbic) area also targets heavily the lateral septal nucleus, in particular its rostral part (Vertes, 2004) -- the same region of the lateral septal nucleus that has a strong bidirectional connection with the LHAjp. The lateral septal projection of the infralimbic area has particular resonance with our discussion given a general function of 'cognitive affect' associated with this region of the medial prefrontal cortex, an association of the lateral septal nucleus with behavioral inhibition, and a suggested role for the LHAjp in defensive behavior. Furthermore, with our emphasis on the potential contribution of an olfactory component to modulation of LHAjp neurons, it is noteworthy that several olfactory-related structures receive differentiated projections from the pre- and (predominantly) infralimbic areas, these include: anterior olfactory nucleus, tenia tecta, and olfactory recipient parts of the amygdalar region and BST (Vertes, 2004). More generally the described interconnections of the LHAjp, thalamus, and hippocampal formation with the prefrontal cortex, highlight certain routes that are likely to be central to the influence of cognitive processes on the activity of the LHAjp.
Further noteworthy projections from the prefrontal cortex include a robust midbrain projection to differentiations of the PAG that receive (and to a lesser extent project to) the LHAjp, namely the precommissural nucleus and ventrolateral division (Floyd et al., 2001; Vertes, 2004). In addition, prefrontal cortex projections to the PAG may also reflect a graduated dorsal to ventral division of the prefrontal cortex in that the anterior cingulate area and dorsal levels of the prelimbic area are reported to project preferentially to the dorsolateral division of the PAG, whereas ventral levels of the prelimbic area and infralimbic area may project preferentially to the ventrolateral division of the PAG (Floyd et al., 2001). With regard to our discussion of how cerebral oscillations (and in particular the hippocampal theta rhythm) may involve the LHAjp, it is relevant to mention a robust projection to the supramammillary nucleus from the infralimbic area (Vertes, 2004). Also relevant is a strong projection from the infralimbic area to the diagonal band nucleus (NDB) -- the NBD projects moderately to, and receives lightly from, the LHAjp; the NDB also has bidirectional connections with the nucleus incertus and both regions, along with the supramammillary nucleus, are implicated in modulation of the hippocampal theta rhythm (Bland, 1986; Kocsis and Vertes, 1994; Vertes and Kocsis, 1997; Kirk, 1998; Pan and McNaughton, 2004; Nunez et al., 2006).
We have to this point discussed principal connections of the LHAjp with other brain regions, and certain of the interconnections of those regions as they relate to the place of the LHAjp within a structural network, and as they may relate in terms of some possible functions. There remain several further aspects of this for future discussion. One of the more prescient topics is the relation of the connections of the LHAjp to the connections of the nucleus accumbens -- we found essentially no direct connections of the LHAjp with the nucleus accumbens. A further future consideration, given our emphasis on defensive behavior, would be to consider how our findings relate to systems involved in other behaviors.
4.4. LHAs discussion: Connectional and functional considerations
In this study we set out primarily to present and discuss the connections of the LHAjp and we have included an analysis of the connections of the LHAs as a control for, and comparison, to the connections of the LHAjp, and to the already published connections of the subfornical region of the LHA (Goto et al., 2005). Our analysis is partial in so far as the experiment we selected for most detailed analysis (experiment LHA#11) is one in which the tracer deposit is sited within a central part of the LHAs -- it remains to be determined how the connections of these midrostrocaudal levels of the LHAs compare to its more peripheral levels. Nevertheless, the connections of the LHAs in so far as we have described them are quite different to those of the LHAjp, indicative of a very different functional role for the LHAs and underscoring the heterogeneity of the middle group of the LHA.
4.4.1. LHAs discussion: Cerebral cortex
The most obvious difference between the LHAjp and LHAs in terms of neural input is the (almost) absence of a projection to the LHAs from the hippocampal formation. In addition to this major difference, projections to the LHAs from other cortical areas are very different overall from those to the LHAjp: both LHA regions receive a moderate input from the infralimbic area, but input to the LHAs from the prelimbic area is considerably less than to the LHAjp; there is essentially no projection to the LHAs from anterior cingulate- or retrosplenial areas, and (unique to the LHAs) a moderate input from the agranular insular area. These differences immediately suggest a different functional perspective: they indicate the LHAs is not directly modulated by hippocampal mnemonic processes relating to either spatial navigation or stress (anxiety), and that prefrontal cortex influence on the LHAs is weighted more to cognitive processes associated with interoception. Also noteworthy is the absence of of a projection from a mid rostrocaudal level of the LHAs (and also from the LHAjp) to regions of the cerebral cortex that have been shown in earlier retrograde tracing studies to receive considerable input from the LHA (Allen et al., 1991; Saper, 1985; Saper et al., 1986). Nevertheless, our data appears to be consistent with the earlier work in that cerebral cortical projections were not shown to arise from regions of the LHA corresponding to the LHAjp, or a midrostralcaudal level of the LHAs. In fact, the region corresponding to a mid rostrocaudal level of the LHAs was shown specifically to not be retrogradely labeled following injections of the retrograde tracers fast blue or diamadino yellow in (respectively) the infralimbic or insular areas (Saper et al., 1986).
4.4.2. LHAs discussion: Cerebral nuclei
4.4.2.1. Striatum
Connections of the LHAs with cerebral nuclei are also quite different from those of the LHAjp. Beginning with the striatum, a tightly circumscribed group of cells back-labeled in rostral levels of the CEAl is striking -- no such retrograde labeling was found from the LHAjp. Pallidal connections with the LHAs are overall considerably more pronounced than with the LHAjp, and the involved regions are mostly different. Foremost are strong bidirectional connections of the LHAs with the BST and the substantia innominata. The LHAs also projects moderately to the medial septal nucleus and diagonal band nucleus.
The projection to the LHAs from the rostral tip of the CEAl provides further evidence of interoceptive (viscerosensory) and also gustatory influence on the LHAs because the CEAl is highly connected with regions involved in these processes. These connections include extremely dense projections to the rostral end of the CEAl from the gustatory/viscerosensory region of the insular area (McDonald et al., 1999) and projections to the CEA from the infralimbic area (Vertes, 2004). In addition, the CEA has predominantly bidirectional connections with (mostly) the anterior division of the BST (Dong et al., 2000; Dong et al., 2001a; Dong et al., 2001b; Dong and Swanson, 2003; Dong and Swanson, 2004a; Dong and Swanson, 2004b; Dong and Swanson, 2006a; Dong and Swanson, 2006b; Dong and Swanson, 2006c), parabrachial nucleus (prominently PBlc and PBle) (Veening et al., 1984; Bernard et al., 1993; Petrovich and Swanson, 1997), PAGvl (Rizvi et al., 1991), dorsal nucleus of the raphé (Rizvi et al., 1991; Peyron et al., 1998; Lee et al., 2007), ventrolateral medulla (Jia et al., 1992; Jia et al., 1997b; Viltart et al., 2006), and dorsal vagal complex (NTS and DMX) (Veening et al., 1984; Jia et al., 1997b; Viltart et al., 2006).
An identified role for rostral levels of the CEAl in the transmission of viscerosensory and gustatory information to the LHAs relates more generally to involvement of the CEA in autonomic control (Swanson and Petrovich, 1998); yet a more commonly ascribed (although not necessarily unrelated) role for the amygdalar region as a whole is in the expression of fear responses (Ledoux, 2000; Antoniadis and McDonald, 2001). In this, a clear and unequivocal understanding of the role of the amygdalar region remains somewhat elusive, not least because of poor consensus of definition of its parts (Swanson and Petrovich, 1998; Swanson, 2003a). Connected to this, a further confound relates to the different ways in which fear may manifest, with different somatic and autonomic effects possibly involving different parts of the amygdalar region. With regard to the CEAl, its connections are consistent with its involvement in the expression of conditioned fear (Petrovich and Swanson, 1997); however, excitotoxic lesion studies indicate that the CEA (all parts) is not involved in a commonly used model of fear conditioning in which an unconditioned noxious stimulus (footshock) is paired with a conditioned benign stimulus (tone) to produce a conditioned reflex motor response (freezing) (Koo et al., 2004). Nevertheless, the CEA appears to have a role in other somatic (e.g., exploratory behavior) (Moreira et al., 2007) and autonomic (e.g., cardiovascular) (Roozendaal et al., 1990) responses associated with conditioned fear.
In fact, the projection from the CEA to the LHA (including the region of the LHAs but not the LHAjp) is implicated specifically with changes in arterial pressure associated with conditioned fear (Ledoux et al., 1988). In addition, an inhibition of the CEAl (as evidenced by a decrease in cFos mRNA expression) occurs in models of unconditioned “fear” (environmental novelty, loud noise, and restraint) (Day et al., 2001; Day et al., 2005) and conditioned “fear” (footshock paired with light) (Day et al., 2008). Given that most, if not all, CEAl neurons express GABA (Day et al., 1999) it is likely that the neurons inhibited under these conditions are GABAergic; however, the projection targets of these neurons remain to be determined. Evidently, the full extent of responses (somatic or autonomic) associated with expressions of presumed fear and involving the CEA requires further clarification. Any role of the LHAs in these responses will have to take into account not only the direct projection from the CEAl to the LHAs but also the extensive intra- and inter-connections of amygdalar and BST nuclei, and the direct connections of the BST -- also a site of abundant GABAergic neurons (Stefanova et al., 1997; Day et al., 1999) -- with the LHAs.
4.4.2.2. LHAs discussion: Pallidum
With regard to the BST, the LHAs receives major input from the BSTam and BSTfu (the BSTam also receives a moderate input from the LHAs). Together, the BSTam and BSTfu are thought to be involved in a broad coordination of responses (autonomic, neuroendocrine, and somatic or behavioral) to homeostatic challenges -- in particular those relating to stress and energy balance (Dong et al., 2001b; Dong and Swanson, 2006a). Furthermore, previous work has described a projection to the BSTam (previously subdivided into anterodorsal and anteroventral parts) and BSTfu from the CEAl (Dong et al., 2001a). In the previous work a PHAL injection restricted principally to a mid rostrocaudal level of the CEAl (experiment #12) labeled moderately the BSTam and densely the BSTfu (see their Fig. 7) (Dong et al., 2001a); however, projections from the rostral end of the CEAl (where retrograde labeling from the LHAs in our experiment LHA#11 was observed) were not examined in the previous study (Dong et al., 2001a). Nevertheless, in an earlier study a PHAL injection centered within rostral levels of the CEAl was reported to label varicose fibers within the BST (Grove, 1988b); the distribution of these fibers within the BST was not described in the text, but they were shown to be present within a region corresponding to the anterior division of the BST (see her Fig. 9) (Grove, 1988b).
In addition to strong inputs to the LHAs from the BSTam and BSTfu, a moderate combined input arises from the BSTal, BSTdm, BSTif, and BSTpr; the BSTal and BSTdm also receive a moderate projection from the LHAs, and the BSTif a light projection. The BSTal and BSTdm have extremely diverse neural output profiles indicative of equally diverse roles in coordinating responses to homeostatic challenges (Dong and Swanson, 2004a; Dong and Swanson, 2006b). For the BSTdm a role in responding to thirst and salt appetite is suggested to be foremost among its possible functions (Dong and Swanson, 2006b); the BSTam is suggested to have a central role in the coordination of visceral and somatic motor responses relating to ingestive (eating and drinking) behavior (particularly associated with noxious stimuli) (Dong and Swanson, 2004a). In contrast to the BSTam, BSTfu, BSTdm, and BSTal, the projections of the BSTif and BSTpr are consistent with a central role for these regions in reproductive and defensive behaviors (Dong and Swanson, 2004b).
Strikingly, combined projection profiles of the BSTfu, BSTam, BSTal, and BSTdm show extensive overlap with regions that are either a target of (or are targeted by) the LHAs (Dong et al., 2001b; Dong and Swanson, 2004a; Dong and Swanson, 2006a; Dong and Swanson, 2006b). This correlation is strengthened by including the projections of additional BST regions (notably the BSTov, BSTju, BSTrh, BSTmg, BSTad) that have little or no direct connection with the LHAs, but are nevertheless highly interconnected with other BST regions. The high degree of overlap in the terminal fields of these BST regions and the connections of the LHAs suggests a high degree of functional interrelation. Conversely, the main projection targets of the BSTif and BSTpr are to regions that connect with the LHAs relatively little, whereas the BSTif projects to regions with which the LHAjp has robust connections (Dong and Swanson, 2004b). Given a central role for the BSTif and BSTpr in defensive (BSTif) and sexual/reproductive (BSTpr) behaviors this suggests that the LHAs may not be directly involved in the control of these behaviors, but is nevertheless a recipient of information relating to them -- such integration has clear utility for the appropriate modulation of one behavior (such as ingestive) in response to changes in another (such as defensive or sexual/reproductive). It also alludes to a hierarchy of behavioral control.
The connection of the LHAs with the substantia innominata (SI) is bidirectional, with a strong projection to a mostly dorsal region at medial levels of the SI, and a relatively light input to the LHAs from a similar region. Relating this to what we have just discussed it is noteworthy that the dorsal/medial region of the SI appears to have rather similar projections to the CEAl and also to the BST regions associated with homeostatic processes relating to ingestive behaviors (Grove, 1988a). Furthermore, this region of the SI appears to receive abundant input from areas of the cerebral cortex involved in similar processes that connect directly or indirectly to the LHAs, notably the gustatory/viscerosensory part of the insular area and the infralimbic area (Grove, 1988b). It is also noteworthy that connections of the LHAs with the lateral septal nucleus are much reduced in comparison to the strong bidirectional connections of the lateral septal nucleus with the LHAjp; the LHAs sends a light projection to primarily the rostral part of the lateral septal nucleus and receives very little lateral septal input. Moderate projections from the LHAs to the medial septal complex provide a link to our earlier discussion with regard to possible theta rhythm influences on the LHAjp, in which the medial septal complex is known to be critically involved (Bland, 1986; Kocsis and Vertes, 1994; Vertes and Kocsis, 1997; Kirk, 1998; Pan and McNaughton, 2004). It is also noteworthy that in the rat the medial septal nucleus, and especially the diagonal band nucleus (recipient of a moderate input from the LHAs), is a principal site of gonadotropin-releasing hormone neurons (Witkin et al., 1982; Merchenthaler et al., 1984; Wray and Hoffman, 1986) -- whether or not this projection has any influence on the reproductive function of the GnRH neurons remains to be tested.
4.4.3. LHAs discussion: Cerebrospinal Trunk
4.4.3.1. Sensory System
Outside of the cerebral cortex and cerebral nuclei, a major target of the LHAs within the sensory system of the cerebrospinal trunk is the thalamus, specifically the PVT; a lighter projection also reaches from the LHAs to the IMD and PT, and the PVT and IMD also provide a light input to the LHAs. The dorsal midline (including PT and PVT) and medial (including IMD) nuclei of the thalamus are thought to have a general role in awareness, and four different groupings of these nuclei have been suggested to include different aspects of awareness (Van der Werf et al., 2002). Based primarily on their connectivity, the PVT, PT, and IMD have been assigned to the same group that is thought to be involved in viscerosensory awareness (Van der Werf et al., 2002). The appropriateness of this designation is better understood in light of the projection targets of the PVT, PT, and IMD. Among the most pronounced connections of these thalamic structures are strong projections to the nucleus accumbens (Van der Werf et al., 2002; Ligorio et al., 2009); robust connections also exist with parts of the amygdalar region (including CEA), BST (anterior division) and cerebral cortex (including insular area, infralimbic area, and ventral levels of the prelimbic area) that have major association with visceral sensorimotor processing (Van der Werf et al., 2002). In passing, it is noteworthy that the IMD receives a significant input from the supramammillary nucleus that itself has a role in control of the theta rhythm (Vertes, 1992); in this context also noteworthy is an already discussed projection from the PT (itself a major target of the LHAjp) to the ventral half of the subiculum (Vertes and Hoover, 2008).
Caudal to the thalamus and also within the sensory system, the LHAs has moderate bidirectional connections with the lateral division of the parabrachial nucleus, and projects to rostral (“gustatory”) and caudal (“visceral”) levels of the NTSm; the LHAs also sends a very few fibers to the NTSco and NTSl and it receives a light direct input from caudal levels of the NTSm. These connections indicate a role for LHAs in the modulation of ascending viscerosensory signals, in particular those relating to ingestive behavior -- this is also indicated by light projections from the LHAs to the rostral (oral) part of the pontine reticular nucleus (PRNr) and very light projections to the oral part of the spinal trigeminal nucleus and to the paratrigeminal nucleus; the projection to the PRNr is also noteworthy for the effect of electrical stimulation of this region (at the level of the locus ceruleus) to elicit synchronous hippocampal oscillations at theta frequency (Vertes, 1981; Vertes, 1982).
The projection from the LHAs to the parabrachial nucleus is moderate and extensive, targeting the medial and lateral divisions (most evidently the PBlc). The LHAs also receives a moderate input from the PB, primarily from the PBlc and PBlv. It is noteworthy that among the connecting regions of the PB are several that we have already discussed in relation to their connections with the LHAs, namely: the insular area, CEA, BST, dorsal regions of the SI, and midline group of the thalamus (Bernard et al., 1993; Alden et al., 1994; Petrovich and Swanson, 1997; Dong et al., 2001b; Dong and Swanson, 2004a). The CEA and BST in particular receive a massive projection from the PB; with regard to the CEA, the CEAm is targeted principally by the PBmm and PBlv, and the CEAl by the PBlc and PBle (Bernard et al., 1993). The major BST recipient site is the BSTov (Alden et al., 1994), which in turn targets densely the BSTfu; other regions targeted strongly by BSTov include the BSTam, dorsal regions of SI, CEAm, and PB (Dong et al., 2001b).
An additional part of the sensory system, the subfornical organ (SFO), is notable for providing an input to the LHAs. The SFO is a circumventricular organ extensively characterized for its humorosensory role in the homeostatic control of arterial pressure and plasma sodium ion concentration (Fitzsimons, 1998; Geerling and Loewy, 2008). More recent work indicates the SFO (and other circumventricular organs) may also have additional roles in energy and thermal homeostasis (Cairns et al., 2005; Fry et al., 2007; Smith et al., 2009). The means by which the SFO effects its homeostatic functions involves the widespread projections of its neurons to regions involved in cognitive, behavioral state, and motor-related processes (visceral, neuroendocrine, and somatic) (Miselis, 1981; Swanson and Lind, 1986). Our finding of retrograde labeling in the SFO from the LHAs is consistent with a putative role for the LHAs in processes relating to ingestive behavior -- this may relate in particular to the ingestion of water and salt, given an established role for the SFO in thirst and salt appetite (Fitzsimons, 1998; Geerling and Loewy, 2008); however, clearly other functions in which the SFO has a role may also influence the LHAs.
It is of particular note that one of the sources of direct neural (as opposed, say, to hormonal) input to the SFO is caudal levels of the NTSm (viscerosensory part) (Zardetto-Smith and Gray, 1987); because the NTSm (rostral and caudal levels) also receives a direct neural input from the LHAs, this identifies the LHAs as a site that is in a position not only to be directly influenced by the SFO but to potentially directly modulate that influence via a feedback projection to the NTS. The possibility of a modulatory feedback role for the LHAs is also indicated by the preceding discussion in this section; it would appear that this may well be far reaching, not only in terms of the topography, but also in terms of the functional processes that may be modulated.
4.4.3.2. LHAs discussion: Behavioral State System
The next major division of the cerebrospinal trunk to which the LHAs directly projects is the behavioral state system. Moderate projections reach from the LHAs to the SBPV and dorsal nucleus of the raphé, and moderate to strong projections extend across the LHAd; very light terminal projections also reach to the hindbrain regions of the nuclei raphé magnus and obscurus (very few fibers). The SBPV has very strong bidirectional connections with the suprachiasmatic nucleus (Watts et al., 1987; Moga and Moore, 1997; Krout et al., 2002b) and evidence from transneuronal tracing studies indicates that LHAs projections to the SBPV may include some that innervate neurons projecting to the suprachiasmatic nucleus (Krout et al., 2002b) -- this may therefore be a route whereby LHAs neurons could influence circadian rhythms. The region of the LHAd is implicated in control of ingestive behavior. For example, CRH gene expression is induced (and neurotensin gene expression is increased) in rostromedial levels of the LHAd concurrently with anorexia induced in rats that imbibe a supraphysiological saline solution (Watts, 1992; Watts et al., 1995; Kelly and Watts, 1998; Watts et al., 1999; Watts, 2001) or that are subject to dehydration resulting from water deprivation (Kelly and Watts, 1996). The anorexia occurring in rats that drink supraphysiological saline is also associated with changes to CRH or neurotensin gene expression in several other sites that either directly project to the LHAs (CEAl, BSTfu, PVH, RCH) (Watts, 1992; Watts et al., 1995; Kelly and Watts, 1998; Watts et al., 1999; Watts, 2001) or are in a position to influence strongly the LHAs (BSTov) (Watts et al., 1995).
Furthermore, three regions closely associated with ingestive behavior and fluid homeostasis that project to LHAs, namely the BSTfu, MEPO, and SFO, also project to rostromedial levels of the LHAd (Kelly and Watts, 1996). In addition, neurons retrogradely labeled with fluorogold in these regions from injections centered rostromedially in the LHAd show increased expression of the c-fos gene in rats that have received intraperitoneal injections of supraphysiological saline (Kelly and Watts, 1996). Intriguingly, no increase in cFos protein in the CEAl is apparent in the dehydrated condition, but a conspicuous increase is apparent in this region following the re-presentation of water (Alan G. Watts, unpublished observation). In the previous study, fluorogold injections were centered in the component of the LHA in which CRH gene expression occurs in association with dehydration anorexia; this LHA component is located dorsal and lateral to the fornix between Atlas Levels 27 and 28 of Swanson (2004) and appears to include rostral levels of the LHAd and the rostral tip of the LHAs (Kelly and Watts, 1996). Inclusion of rostral levels of the LHAs due to spread of fluorogold tracer makes it likely that at least some of the fluorogold labeled neurons were retrogradely labeled from the rostral end of the LHAs in addition to the rostral end of the LHAd (Kelly and Watts, 1996). Furthermore, there does appear to be considerable overlap in the projection profiles of the LHAs (our data) and LHAd (Kelly and Watts, 1996; Kelly and Watts, 1998); this is consistent with an established role for the LHAd (and a proposed role for the LHAs) in processes relating to ingestive behavior. Additional studies will be required to clarify the similarities and differences between these two apparently interrelated LHA regions.
The dorsal nucleus of the raphé is a principal site of brain serotonergic neurons (Steinbusch, 1981) and projections from this region extend widely throughout the central nervous system (Vertes, 1991; Vertes and Kocsis, 1994), reflecting the wide ranging functions of this nucleus as they relate to the modulation of behavioral state and the maintenance of homeostasis (Michelsen et al., 2008). Consistent with this, diverse neural inputs to the dorsal nucleus of the raphé indicate a high degree of functional integration, as evidenced in a very recent analysis that employed a conditional viral transneuronal tracing methodology to reveal the sources of projections to cell groups containing serotonergic neurons -- in either the dorsal nucleus of the raphé or the nucleus raphé magnus (Braz et al., 2009). Prominent among the functions in which the dorsal nucleus of the raphé is implicated are: sleep (Imeri and Opp, 2009), anti-nociception/analgesia (Wang and Nakai, 1994; Millan, 2002), and temperature regulation (Hillegaart, 1991; Tao and Auerbach, 1995); prominent among the dysfunctions it is implicated in are major depression (Stockmeier et al., 1998; Michelsen et al., 2008) and Alzheimer's disease (Michelsen et al., 2008).
Projections from the LHAs target almost exclusively rostral levels of the dorsal nucleus of the raphé (Swanson rat brain Atlas Levels 44/45). Notable differences in the ascending projections of rostral and caudal levels of the dorsal nucleus of the raphé are reported to include comparatively little projection to the hippocampal formation from rostral levels, yet denser projections to the rest of the cerebral cortex (Vertes, 1991); descending projections of rostral levels are reported to be heavier and to show wider distribution than those of caudal levels to several parts of the medulla (Vertes and Kocsis, 1994). Aside from these differences, targets common to both rostral and caudal levels of the dorsal nucleus of the raphé include widespread regions of the cerebrum and cerebrospinal trunk (Vertes, 1991; Vertes and Kocsis, 1994).
Among the notable sources of neural input to rostral levels of the dorsal nucleus of the raphé is a recently clarified projection from the prefrontal cortex, in particular from regions that also project to the LHAs, prominently the infralimbic area and adjacent ventral levels of the prelimbic area, and the dorsal agranular insular area (Goncalves et al., 2009). Additional areas of the prefrontal cortex projecting to the LHAs and rostral levels of the dorsal nucleus of the raphé include those dorsal to the rostral pole of the nucleus accumbens corresponding to the TTd, ORBv, EPdm, and also the claustrum (Goncalves et al., 2009). Finally, although we have focused here on the dorsal nucleus of the raphé, it should be noted that the additional (behavioral state) parts of the raphé to which the LHAs projects very lightly (i.e., the nuclei raphé magnus and obscurus), are, like the dorsal nucleus of the raphé, also implicated in a diverse range of homeostatic autonomic functions in which serotonergic neurons appear to have a central role (Krowicki and Hornby, 1993; Hermann et al., 1997).
4.4.3.3 LHAs discussion: Motor system
4.4.3.3.1. Behavior Control Column
The LHAs has robust connections with numerous regions that are classified as part of the motor system of the cerebrospinal trunk. In line with our discussion so far, these connections indicate a similar primary role for the LHAs: control of somatomotor, autonomic, and neuroendocrine processes relating to ingestive behavior and more broadly to the maintenance of homeostasis. Beginning with regions of the motor system's behavior control column, sites receiving prominent LHAs input include the midbrain reticular nucleus (principally the retrorubral area and more moderately the parvicellular part) and VTA. The descending division of the PVH also receives a robust input; lighter projections reach to the anterior part of the VMH, central part of the AHN, and lateral part of the MPN.
The retrorubral area has an identified role in orofacial motor control associated with eating and drinking in the rat (Uchida et al., 2005), cat (Joseph et al., 1985; Arts et al., 1998), and monkey (Mora et al., 1977; DeLong et al., 1983). This effect appears to be under tonic GABAergic inhibition: a GABAA receptor antagonist (bicuculline) will elicit repetitive jaw movements when applied directly to the retrorubral area; conversely, a GABAA receptor agonist (muscimol) when applied to the same area will counteract this effect (Uchida et al., 2005). In addition to the identified input from the LHAs, the retrorubral area receives massive innervation from the anterior division of the BST, most prominently from the BSTrh (Dong and Swanson, 2003), and also from the BSTdm, BSTal, BSTfu, and BSTju (Dong et al., 2000; Dong et al., 2001b; Dong and Swanson, 2004a; Dong and Swanson, 2006b). Prominent projections also reach the retrorubral area from the dorsal nucleus of the raphé (Vertes, 1991), CEA (including its rostral levels) (Gonzales and Chesselet, 1990), and dorsomedial levels of the SI (Swanson et al., 1984). These are all regions that have substantial direct connections with the LHAs. In this context, also noteworthy is recent work documenting extensive projections to the retrorubral area from hindbrain adrenergic and noradrenergic neurons (Mejias-Aponte et al., 2009).
A main projection target of the retrorubral area (along with the VTA and substantia nigra with which it is closely associated) is the caudoputamen; additional projections also reach to the SI, CEA (Prensa and Parent, 2001), and hippocampal formation (Gasbarri et al., 1996). The retrorubral area (and adjacent components of the substantia nigra) also projects to the parvicellular reticular nucleus (PARN) and adjacent regions that contain orofaciopharyngeal motor pattern generators, see: (Dong and Swanson, 2003). Another structure with notable direct projections to the retrorubral area is the parasubthalamic nucleus (PSTN) (Goto and Swanson, 2004). The PSTN (a caudal and far lateral part of the LHA adjacent to the subthalamic nucleus) has striking relevance here because its projection profile identifies it as a region whose functions are likely to be very closely allied to those of the LHAs (as also appears to be the case for, collectively, the anterior division of the BST). In particular, the PSTN has massive projections to hindbrain regions directly involved in ingestive behavior and autonomic (in particular cardiovascular) control: these include parasympathetic preganglionic neurons in the medulla (in particular salivatory nuclei and DMX), and in the PB and NTS (Goto and Swanson, 2004).
Furthermore, the PSTN has ascending projections to many differentiations that also connect to the LHAs: thalamus (PVT, MDm), CEA (mostly CEAm and CEAc, but not CEAl), anterior division of the BST (mostly BSTam, BSTal, BSTrh, and BSTfu, and also BSTov and BSTdm), SI (heavier than that from the LHAs and mostly more dorsal and lateral), infralimbic and agranular insular areas, PB, and NTS (Goto and Swanson, 2004). Other PSTN connections of note are to cortical areas associated with hippocampal formation and the main olfactory system (postpiriform transition area, lateral entorhinal area, and posterior part of the basolateral amygdalar nucleus). The PSTN also projects to the striatal fundus (implicated in somatomotor control), which in turn projects to the retrorubral area and substantia nigra (Shammah-Lagnado et al., 2001); for further discussion see (Goto and Swanson, 2004). Incidentally, we described a light projection to the rostral tip of the PSTN from the rostral half of the LHAs (it remains to be seen whether this topography extends caudally).
We have highlighted an indicated role for the retrorubral area of the MRN in rhythmic patterned orofacial movement; the parvicellular MRN (MRNp) is suggested to participate in a different rhythmic patterned movement, namely locomotion (Rye et al., 1988). The MRNp was identified previously as the midbrain extrapyramidal area (Rye et al., 1987); before that it had also been included as part of the (ventrally and laterally) adjacent pedunculopontine nucleus (PPN) (Swanson et al., 1984). The PPN as now defined is currently implicated in rhythmic activity pertaining to functions including paradoxical sleep, arousal, and cognition (Garcia-Rill, 1991; Steckler et al., 1994; Inglis and Winn, 1995; Winn et al., 1997). Nevertheless, participation of the MRNp in orofacial control (and possibly other functions attributed to the PPN) should not be ruled out; this assertion is supported by transneuronal (viral) tracing studies that have identified reticular formation components (including the region of the MRNp) that collectively provide input to trigeminal, facial, and hypoglossal motor nuclei that control jaw, facial, and lingual muscles (Fay and Norgren, 1997a; Fay and Norgren, 1997b; Fay and Norgren, 1997c) -- the integrated nature of the reticular formation cautions against overly reductionist interpretation.
Few studies have examined specifically the connections of the MRNp -- much attention has been focused on the PPN. Nevertheless, one study has described the afferent connections of the MRNp in comparison to those of the PPN, and this revealed the strongest source of neural input to the MRNp to be the lateral habenula (Steininger et al., 1992). Major inputs to the MRNp also arise from the vicinity of the LHAs (confirming our data), LHAd, dorsal nucleus of the raphé, PAGvl, lateral division of the PB, and several other brain parts that have connections with the LHAs (Steininger et al., 1992). Notably, a projection to the MRNp from a dorsomedial region of the SI that is bidirectionally connected with the LHAs has been independently described (Swanson et al., 1984; Groenewegen et al., 1993). The MRNp (but not the PPN) is also bidirectionally connected with the substantia nigra and internal segment of the globus pallidus (Rye et al., 1987). In essence, our data establish the presence of a projection to the MRNp from the LHAs and the broader connections of the LHAs. Together with the known connections of the MRNp, this suggests that it would be useful to revaluate the MRNp with particular regard to its role in autonomic and behavioral state control relating to ingestive behavior.
The projection from the LHAs to the VTA indicates a direct influence on processes relating to positive reward and motivation, in which function the VTA is prominently implicated (Ikemoto, 2007); however, a more complex picture is evident when this is viewed in light of our earlier discussion on the projection of the LHAjp to the lateral habenula (which also projects to the VTA), and the involvement of the lateral habenula in processes associated with negative reward (Matsumoto and Hikosaka, 2007; Hikosaka et al., 2008; Matsumoto and Hikosaka, 2009). Recent work has elucidated further the microcircuitry of the VTA, detailing intra-VTA connections and showing extensive and highly topographically organized connections with the adjacent retrorubral area and substantia nigra (Ferreira et al., 2008). The afferent connections of the VTA have also recently been reassessed and these confirm the VTA as a center where information relating to reward value (input from lateral habenula and nucleus accumbens) is integrated with information from a wide variety of brain parts whose overarching function relates most evidently to ingestive behavior and interoception (Geisler and Zahm, 2005; Jhou et al., 2009).
In line with these findings, and as is the case for the retrorubral area, extensive ascending catecholaminergic projections also reach the VTA (Mejias-Aponte et al., 2009). The VTA and retrorubral area (along with the substantia nigra) are further associated by their neurochemical architecture that includes the dopaminergic cell groups A8–A10 and by projections from all three regions to the subiculum and field CA1 (Gasbarri et al., 1994; Gasbarri et al., 1996; Gasbarri et al., 1997). The projections to the hippocampal formation are especially intriguing as they target regions (field CA1 and subiculum) that provide the strongest input to the LHAjp -- recall that the LHAjp targets strongly the lateral habenula that in turn targets the VTA. The retrorubral area, VTA, and substantia nigra could conceivably be a nodal axis for relating and integrating motivational or incentive value (positive or negative) associated with different modes or systems of behavior that involve different LHA components, for example, the LHAs (ingestive) and LHAjp (defensive).
The remaining part of the behavior control column to receive a strong input from the LHAs is the descending division of the PVH (lateral and forniceal parts); this clearly reveals the LHAs as being in a position to directly influence autonomic outflow (Sawchenko and Swanson, 1982). Conversely, lighter connections of the LHAs with the AHNc (bidirectional), MPNl (receiving), and VMHa (receiving) may indicate interoceptive and autonomic influence (via the LHAs) on processes that involve these regions. Given what is known about the roles and connections of the AHNc, MPNl, and VMHa, this suggests influence on processes relating to defensive (AHNc) (Risold et al., 1994; Canteras, 2002) and reproductive (VMHa and MPNl) (Saper et al., 1976b; Simerly and Swanson, 1986; Simerly and Swanson, 1988; Canteras et al., 1994) behaviors.
4.4.3.3.2 LHAs discussion: Central Gray
Several components of the central gray connect with the LHAs, although the pattern of interactions is quite different to those of the LHAs; in particular there is very little projection to the lateral habenula from the LHAs in comparison to the very dense terminal field in the lateral habenula from the LHAjp. Major LHAs targets in the central gray are the PAGvl (which also provides a light input to the LHAs) and Barrington's nucleus -- in fact these two regions receive the densest LHAs innervation in the entire brain. The LHAs also sends a substantial projection to the commissural nucleus of the PAG and to the posterior hypothalamic nucleus (which also provides a heavy input to the LHAs). We previously discussed the posterior hypothalamic nucleus in relation to possible influence of the LHAjp on the theta rhythm; the same could be true for the LHAs, and in addition the rather considerable input to the LHAs from the posterior hypothalamic nucleus suggests a more complex interaction with possible feedback. It is also noteworthy that the ascending and descending projections of the posterior hypothalamic nucleus target many of the regions that connect directly with the LHAs (Vertes et al., 1995; Vertes and Crane, 1996).
The PAG is implicated in the modulation of behaviors relating to defense and reproduction (Simerly and Swanson, 1986; Behbehani, 1995; Marson and Carson, 1999; Murphy and Hoffman, 2001; Marson and Murphy, 2006); in these functions it recruits (and is modulated by) components of the somatic and autonomic nervous systems -- in particular much attention has focused on its role in the modulation of nociception (Keay et al., 1994; Behbehani, 1995; Millan, 2002). A current understanding of the PAG describes its caudal half as being composed of topographically organized longitudinal columns or divisions (at least four have been identified), each of which participates in distinct (yet interrelated) aspects of behavioral control (Bandler and Shipley, 1994; Bandler and Keay, 1996; Floyd et al., 2000; Keay and Bandler, 2001). In addition to its established role in the modulation of defense- and reproduction-related behaviors, neural inputs to the PAG indicate that it is heavily influenced by processes relating to ingestive behavior; this is evident in the projection we describe to it from the LHAs, and more firmly by the input it receives from regions of the anterior division of the BST that are strongly implicated in ingestive behaviors (Dong et al., 2001b; Dong and Swanson, 2003; Dong and Swanson, 2004a). Less clear at present is the extent to which (and also how directly) the PAG modulates ingestive behaviors; nevertheless, in this regard massive connections of the PAG with the VMH and DMH are noteworthy (Saper et al., 1976b; Canteras et al., 1994; Thompson et al., 1996; Thompson and Swanson, 1998).
The projection from the LHAs to the commissural nucleus of the PAG compares to the projection from the LHAjp to the precommissural nucleus; while the connections of the latter have been studied specifically (Canteras and Goto, 1999a), the connections of the former PAG region have not. Nevertheless, on the basis of its connections and location, the precommissural nucleus is considered to be a rostral component of the PAG (Canteras and Goto, 1999a), and thus by definition a rostral extension of the caudally adjacent commissural nucleus. Functional correlates specifically for the commissural nucleus are not yet available; however, the precommissural nucleus has a similar pattern of connectivity to the PAGdl, and like the PAGdl it is suggested to have a positive role in defensive behavior (Canteras and Goto, 1999a; Schenberg et al., 2005).
In contrast to the commissural nucleus, much can be said about the connections and functional correlates of the PAGvl. To begin with, in light of what we discussed in the previous section on the behavior control column, of particular note are projections from the PAGvl to the PVH (its neuroendocrine and particularly its descending divisions) (Floyd et al., 1996), and GABAergic projections from the VTA and SN to the PAGvl (and dorsal nucleus of the raphé) (Kirouac et al., 2004); indeed it has been shown that stimulation of the VTA/SN will elicit cardiovascular depressor responses that appear to be mediated by this projection (Kirouac et al., 2004). With regard to the role of the PAGvl in defensive behavior, this is to some extent complimentary or opposite to that of more dorsal PAG divisions. Thus, dorsal levels of the PAGvl have a role in increasing sympathetic and somatomotor activation that manifests as (for example) hypertension, tachycardia, and the classic freeze, flight or fight response; whereas ventral levels of the PAGvl have a role in decreasing sympathetic and somatomotor activation that manifests as (for example) hypotension, bradycardia, quiescence, and anti-nociception (Carrive, 1993; Bandler and Keay, 1996; Brandao et al., 1999; Keay and Bandler, 2001). Further studies will be required in order to clarify how the LHAs projection to the PAGvl may influence these responses. Nevertheless, a putative role for the LHAs in processes relating to ingestive behavior is evidently more compatible with the spectrum of functions attributed to the PAGvl rather than to more dorsal divisions of the PAG.
Furthermore, the PAGvl is identified specifically as a site that receives convergent nociceptive afferent information from deep somatic and visceral sources (Keay et al., 1994) -- it is therefore in a position to respond by influencing somatic, autonomic (and neuroendocrine) responses accordingly. This is particularly interesting in light of the afferent autonomic-related projections to the LHAs and the fairly recent clarification of a common afferent pathway (to the insula area) for conveyance of interoceptive information associated with autonomic motor control (with interoceptive broadly redefined to include essentially all bodily sensations distinct from the exteroceptive system sensations of mechanorecption and proprioception) (Craig, 2002, 2003). The data suggest a possible involvement of the LHAs in feedback (or feed forward) modulation of somatic and autonomic efferent and afferent (interoceptive) information at the level of the PAG; for further thoughts on this see (Bandler et al., 2000a; Bandler et al., 2000b). As an additional point of interest, direct involvement of the PVH is also predicted given the robust projection to the PVH (in particular its descending division) from the LHAs (present data) and a strong projection to the (predominantly ventrolateral division of the) PAG from the PVH (Larry Swanson and Paul Sawchenko, unpublished observations).
In this last part of the discussion on the connections of the LHAs with the central gray, the remaining site of major LHAs connectivity is Barrington's nucleus -- recipient of very dense innervation from the LHAs (Fig. 10). In addition to a well established role in the control of micturition (Marson, 1997; Valentino et al., 2000), it is increasingly apparent that Barrington's nucleus is also implicated in far more diverse aspects of autonomic and behavioral control (Cano et al., 2000; Sved et al., 2002). Recent transneuronal tracing studies suggest the existence of multisynaptic projections from Barrington's nucleus to several organs and tissues besides the bladder, these include: the trachea (Haxhiu et al., 1993), kidney (Cano et al., 2000), spleen (Cano et al., 2000; Cano et al., 2001), pancreas (Loewy and Haxhiu, 1993), distal colon (Valentino et al., 2000), gonads (Marson et al., 1993; Marson, 1995; Marson and McKenna, 1996; Orr and Marson, 1998; Papka et al., 1998; Tang et al., 1999; Marson and Murphy, 2006), and brown adipose tissue (Cano et al., 2003). Furthermore, Barrington's nucleus is activated by a variety of stressors (Sved et al., 2002). Taken together, the current evidence indicates a novel possible influence of Barrington’s nucleus on physiological responses to stress.
There are some functional correlates consistent with this idea. For example, neurons retrogradely and transneuronally labeled with pseudorabies virus in Barrington's nucleus from intercapsular brown adipose tissue show a marked increase in Fos expression in rats placed in cold environment (Cano et al., 2003). Related to this, recent work also reaffirms a previously suggested role for the PVH in control of thermogenesis via its influence on sympathetic nerves innervating brown adipose tissue (Madden and Morrison, 2009); this is also suggested by the results of transneuronal tracing studies (Cano et al., 2003). Previous anterograde tracing studies using the autoradiographic method established the existence of a projection from Barrington's nucleus to the intermediolateral cell column at sacral levels of the spinal cord (Loewy et al., 1979); this has been confirmed with retrograde tracing (fluorogold) methods (Valentino et al., 1995). In addition, projections to Barrington's nucleus from the PVH have been demonstrated with anterograde (PHAL) and retrograde (CTB) methods (Valentino et al., 1994). Also noteworthy is a demonstrated ascending projection to Barrington's nucleus (PHAL method) from the central gray at lumbrosacral (but not thoracic) levels of the spinal cord (Wang et al., 1999).
This updated view of Barrington's nucleus indicates that the projection to it from the LHAs may be involved in the modulation of physiological responses to stress. This idea is reinforced by studies that have shown modulation in the expression of the stress-related peptide corticotropin-releasing hormone (CRH) in Barrington's nucleus under a variety of stressful conditions (Imaki et al., 1991; Imaki et al., 1992). It is also noteworthy that a population of CRH-expressing neurons in Barrington's nucleus projects to the PAGvl, DMX, and lumbosacral spinal cord (Valentino et al., 1995). Furthermore, because neural inputs to the LHAs indicate that it receives interoceptive afferent information, this affirms a possible function of the LHAs as a nodal point for interoceptive control (as we discussed in relation to its projection to the PAGvl). In addition, the realization of Barrington's nucleus as a link between autonomic innervation of the pelvic viscera and the prefrontal cortex has led to the suggestion that it may be involved in pelvic visceral dysfunctions (such as bowel disorders) that have comorbitiy with psychiatric disorders (Pavcovich et al., 1998; Valentino et al., 1999; Cairns et al., 2005); the connections of the LHAs indicate that it may have a central place within this circuitry. This may also be the case for dysfunctions that relate to the more classic understanding of Barrington's nucleus as the pontine micturition center, such as urge incontinence or other stress-related bladder dysfunctions (Kuipers et al., 2006; Wood et al., 2009).
4.4.3.3.3 LHAs discussion: Hypothalamic Periventricular Region
Several parts of the hypothalamic periventricular region connect with the LHAs, most notably the medial preoptic area (MPO), median preoptic nucleus (MEPO), and dorsomedial hypothalamic nucleus (DMH). Less substantial connections were noted between the LHAs and the anterior hypothalamic area, anteroventral preoptic nucleus, and anteroventral periventricular nucleus (AVP and AVPV, respectively), and periventricular internuclear area (I). Rather abundant retrograde labeling in the MPO, together with a moderate direct projection to this area from the LHAs, indicates interaction between the LHAs and an area that is thought to have a broad role in the initiation of motivated (goal-oriented) behavioral responses that involve the recruitment of autonomic, somatic, and endocrine components of the nervous system (Swanson and Mogenson, 1981). Motivated behaviors in which the MPO has been implicated include reproductive behavior (Balthazart and Ball, 2007), defensive behavior associated with male conspecific interactions (Motta et al., 2009), and behavioral responses associated with thirst and thermoregulation (Swanson and Mogenson, 1981). Intriguingly, one of the (anatomically and functionally) identified projections of the MPO is to the region of the MRNp that also receives an input from the LHAs (see earlier discussion); also, a proportion of the projecting neurons express galanin (Swanson et al., 1987). Furthermore, the same study indicated that some neurons projecting to the vicinity of the MRNp also send a collateral projection to a region centered caudally in the zona incerta -- it is apparent that this region may have included the LHAs (Swanson et al., 1987); the PAGvl is also heavily targeted by the MPO (Swanson et al., 1987).
The LHAs therefore connects with an area (the MPO) that appears to have broad scope of influence on behavior, from manifest locomotor behavior to endocrine and autonomic modulation; these compare to an indicated role for the LHAs in the control of a broad spectrum of ingestive-related processes. For the LHAjp we suggested that its projection to the BSTpr indicated an interaction between a system for defensive behavior (involving the LHAjp) and one for sexual/reproductive behavior (involving the BSTpr) -- an example of a possible neural substrate for inter-behavior modulation. In the case of LHAs the indicated interaction is between processes related to ingestive behavior, thermoregulation, and sexual behavior -- a role for the LHAs in control of reproductive behavior is also indicated by its projection to the AVPV. These very simple formulations draw attention to the fact that different modes of behavior necessarily modulate other modes of behavior -- one would predict the variety and scope of these interactions to be an emergent property of the underlying neurocircuitry. Drawing attention to the reality of interaction between different systems of behavior also reinforces the caution against assigning exclusive function to a given brain region.
The connection we found between the LHAs and the median preoptic nucleus (MEPO) -- moderate retrograde labeling and light projection -- is informed by our earlier discussion of an input to the LHAs from the SFO. The SFO and MEPO, and a second circumventricular organ -- the vascular organ of the lamina terminalis (OVLT), are highly interconnected (Miselis, 1981; Saper and Levisohn, 1983; Swanson and Lind, 1986; Kawano and Masuko, 2006); the functional significance of this has been demonstrated most clearly in relation to the MEPO as a downstream target of the SFO and OVLT and the actions of humoral angiotensin II on the SFO and OVLT to promote arterial pressure, plasma sodium ion homeostasis, and drinking (Fitzsimons, 1998). In addition to its role in cardiovascular and hydromineral homeostasis, the MEPO is also implicated in control of thermoregulation (Morrison et al., 2008) and sleep-wake states/arousal (Uschakov et al., 2007; Kumar et al., 2008). As we discussed for the MPO, these different functions may relate to inter-behavior modulation; for example, the influence of thermoregulatory or sleep/wake state on processes relating to ingestive behavior.
The remaining part of the hypothalamic periventricular region that has substantial connections with the LHAs is the DMH. Specifically, a light terminal field was apparent in the anterior part of the DMH, and a moderate abundance of retrograde labeling was present in the anterior, ventral, and posterior parts -- most of this was present as a cluster of neurons in a dorsolateral region of the posterior part of the DMH. The connections of the DMH and its parts have been studied comprehensively (ter Horst and Luiten, 1986; Thompson et al., 1996; Thompson and Swanson, 1998), although functional differences that may exist between its individual parts have not. Nevertheless, much evidence points to a role for the DMH in the control of energy homeostasis, and in particular a more recently established role for the DMH to integrate control of energy balance with the circadian rhythm (Bellinger and Bernardis, 2002; Chou et al., 2003; Gooley et al., 2006; Gao and Horvath, 2008).
With a view to future clarification of differential function between the different parts of the DMH, it is noteworthy that the pattern of parabrachial nucleus innervation of the DMH (Thompson and Swanson, 1998) appears to be quite similar to the distribution of retrograde labeling in the DMH seen from the LHAs; the DMH also receives very dense innervation from the parastrial nucleus (Thompson and Swanson, 1998). What also sets these two nuclei, parabrachial and parastrial, apart is that their projection to the DMH includes a robust innervation of the posterior part, unlike several other regions that project to the DMH (Thompson and Swanson, 1998). The projection to the DMH from the parabrachial nucleus indicates a further potentially important route for influence of the latter structure on the LHAs, in addition to the direct bidirectional connections the parabrachial nucleus has with the LHAs, as discussed.
We also found a low-moderate amount of retrograde labeling in the parastrial nucleus (PS) from the LHAs. The PS is indicated to participate in several functions and has connections with several regions that we have already discussed with regard to the LHAs. Thus the PS receives inputs from the subfornical organ (Swanson and Lind, 1986), BSTfu (Dong et al., 2001b), BSTmg (Dong and Swanson, 2006c), and CEA (Tsubouchi et al., 2007); PS projection targets include the PVH (Thompson and Swanson, 2003; Tsubouchi et al., 2007), AVPV (Hahn and Coen, 2006), MEPO, MPO, and AVP (Thompson and Swanson, 2003); the PS is also reported to be sexually dimorphic (Del Abril et al., 1990). As a final point to round out this section, it is quite interesting that six highly interconnected regions (AVP, ADP, AVPV, PS, MEPO, and DMH), put forward as a putative substrate for a hypothalamic visceromotor pattern generator network (Thompson and Swanson, 2003), provide to a greater or lesser degree an input to the LHAs -- the LHAs is therefore in a position to integrate and send on the activity of this network.
4.4.3.3.4. LHAs discussion: Reticular Formation
The LHAs, as is the case for the LHAjp, has substantial interconnections with other motor related parts of the hypothalamic lateral zone (Fig. 12); in addition, and unlike the LHAjp, the LHAs sends substantial projections to parts of the reticular formation located in the midbrain and hindbrain that are involved in the control of visceromotor processes, in particular those related to ingestive behavior (Fig. 12). With regard to the hypothalamic lateral zone, of note is a relatively light bidirectional connection between the LHAs and LHAjp. This connection and the connections of the LHAs with other regions of the hypothalamic lateral zone could provide a means for crosstalk between regions whose connections indicate a deeply integrative role -- this high-order crosstalk could be conceived as a form of meta-integration.
From midbrain to hindbrain, the first principal target of the LHAs within the reticular formation division of the motor system is the magnocellular part of the midbrain reticular nucleus (MRNm); the light to moderate projection of fibers is distributed diffusely and appears to include fibers of passage that continue on to form a more evident terminal field in the MRNp, as previously discussed. The sources of neural inputs to the MRNm include several that together strongly implicate the MRNm in the control of motor processes relating to ingestive behavior, these sources include a robust input from the midbrain dorsal nucleus of the raphé (Vertes, 1991), extrapyramidal area (corresponds to MRNp), and parabrachial nucleus (Rye et al., 1988), and substantial though lesser input from the retrorubral area, pedunculopontine nucleus, and subceruleal region (corresponds to sublaterodorsal nucleus) (Rye et al., 1988); it is noteworthy to recall that the LHAs projects to (or has bidirectional connections with) each of these regions.
Caudal to the MRN, the fibers that descend into and through the reticular parts of the hindbrain do so rather diffusely; although as a whole this amounts to a sizeable projection. These descending axons course with moderate abundance through pontine, gigantocellular, and paragigantocellular reticular nuclei; with moderate to light abundance through parvicellular and magnocellular reticular nuclei; and with light abundance through medullary reticular nuclei. A prominent subset of these fibers traverses the caudal (motor) part of the pontine reticular nucleus (PRNc), principally within its lateral half. The PRN, together with the other hindbrain parts of the reticular formation, sends massive and topographically organized projections to midline and intralaminar groups of thalamic nuclei (Krout et al., 2002a). These projections and the nuclei that generate them form a prominent part of the classically described reticular activating system that is capable of exerting a major influence on arousal (Gottesmann, 1999; Vincent, 2000). The LHAs is thus in a position to exert a modulatory low level influence on this system -- a possible gating mechanism.
To examine this possibility further we can take the PRNc as an example. The major thalamic targets of the PRNc include nuclei associated with motor responses to salient stimuli (parafascicular nucleus) and multimodal sensory processing (rhomboid nucleus) (Van der Werf et al., 2002; Krout et al., 2002a). Conversely, sources of substantial descending projections to the PRNc, in addition to the LHAs, include the precommissural nucleus of the PAG (Canteras and Goto, 1999a) and the dorsomedial and rhomboid nuclei of the anterior BST division (Dong and Swanson, 2003; Dong and Swanson, 2006b). Each of these cell groups (as appears to be the case for the LHAs) is considered to have very broad roles in the control of body homeostasis and behavior and, particularly in relation to ingestive behavior and energy balance (BSTrh and BSTdm) (Dong and Swanson, 2003; Dong and Swanson, 2006b), but also possibly in relation to defensive or reproductive behaviors (PRC) (Canteras and Goto, 1999a).
Although it is unclear what effect the LHAs may exert on the PRN (or on the other reticular formation nuclei it projects to), a clue to a possible role is indicated by studies that have identified the PRN for having a role in audiogenic stress (“startle”) responses (Koch, 1999). Given an indicated role for the LHAs in the modulation of autonomic responses to stress, it could be that its projection to the PRN (and other reticular nuclei) may be involved in modulating the responsiveness of the reticular activating system to external stressful stimuli (including auditory stimuli that generate a startle response) dependent on the current level of internal stress. Conversely, dysfunction of such a mechanism could contribute to an inappropriate increase or decrease in arousal.
4.4.3.3.5. LHAs discussion: Motoneuron Groups
The lowest level of the motor system hierarchy with which the LHAs has substantial connections, contains the motor neuron groups that collectively form the final common neuronal pathway for the expression of behavior and the control of vital functions. Two motoneuron groups are noteworthy for their connections with the LHAs: these are the parvicellular division of the neuroendocrine motor zone, and the parasympathetic division of the preganglionic motor neuron pools. More specifically, the LHAs sends a moderately strong projection directly to the anterior- and medial parvicellular (dorsal zone) parts of the hypothalamic paraventricular nucleus (PVHap and PVHmpd, respectively); it also receives a moderate input from the PVHap and a light input from the hypothalamic arcuate nucleus (ARH). A light projection also extends from the LHAs directly to the dorsal motor nucleus of the vagus nerve and to the inferior salivatory nucleus. These connections reinforce an indicated role for the LHAs as a broad modulator of processes relating to autonomic and neuroendocrine control, energy balance, and ingestive behavior.
5. Concluding remarks
In this paper we have described at length the first order connections of the LHAjp and LHAs, two differentiable, nearby regions of the lateral hypothalamic area -- a region traversed by the medial forebrain bundle, which is arguably the most complex fiber tract of the central nervous system. Given their location, it is perhaps not surprising that the connections of the LHAjp and the LHAs are revealed in their way to be similarly complex. However, this complexity is not without order; in fact a highly topographic organization is apparent in the connections of the LHAjp and the LHAs. This is also true for the previously described connections of the subfornical region of the LHA (LHAsf) (Goto et al., 2005). Furthermore, the regions targeted by the anterior zone of the LHAsf (LHAsfa) share much similarity with regions targeted by the LHAjp; whereas the projection pattern of the posterior zone of the LHAsf (LHAsfp) is similar in many respects to that of the LHAs (Goto et al., 2005; see also Fig 13). It remains to be seen how the differences and similarities in the topography of LHA projections for these LHA regions compare to other LHA differentiations. Nevertheless, the pattern of LHAjp and LHAs connections indicates a profoundly integrative role for these LHA regions in the coordination and appropriate modulation of behavioral expression and bodily homeostasis.
In this role, the connections of the LHAjp associate it primarily with defensive behavior, whereas the connections of the LHAs associate it with ingestive behavior. Furthermore, essentially all functional components of a basic four subsystem plan for the nervous system appear to be engaged: cognitive, state, sensory, and motor (Swanson, 2000). In addition to their indicated association with a primary mode of behavior, the connections of the LHAjp and LHAs also indicate a role in inter-mode behavioral modulation. Thus, for the LHAs a possible a role in generating visceral responses to cognitive stress is indicated; similarly, the LHAjp may have a role in modulating sexual behavior in response to changes in defensive behavior. While these hypotheses remain to be tested, in overtly simple terms analogies could be drawn between these processes and the inhibition of motoneurons for antagonistic muscle groups during limb movement, or the lateral inhibition of photoreceptor cells that occurs in the retina. However, much remains to be learned about the functional operation of the LHA regions, and the neurochemistry of their connections. For example, subsets of neurons within several LHA regions express the peptides melanin concentrating hormone or hypocretin/orexin (Swanson et al., 2005; Hahn, 2009). Therefore, as a guide to a better understanding of the contribution of individual neuron types, and in order to establish more general organizing principles, it will be necessary to determine in their entirety the first order connections of all LHA regions -- a goal we are working toward.
Acknowledgements
This work was funded by the National Institutes of Health (Grant NS016686).
Abbreviations
- AAA
anterior amygdalar area
- ACAd
anterior cingulate area, dorsal part
- ACAv
anterior cingulate area, ventral part
- ACB
nucleus accumbens (“shell” region)
- aco
anterior commissure, olfactory limb
- act
anterior commissure, temporal limb
- AD
anterodorsal nucleus thalamus
- ADP
anterodorsal preoptic nucleus
- AHA
anterior hypothalamic area
- AHNa
anterior hypothalamic nucleus, anterior part
- AHNc
anterior hypothalamic nucleus, central part
- AHNd
anterior hypothalamic nucleus, dorsal part
- AHNp
anterior hypothalamic nucleus, posterior part
- Ald
agranular insular area, dorsal part
- Alp
agranular insular area, posterior part
- Alv
agranular insular area, ventral part
- alv
alveus of fornix
- AMd
anteromedial nucleus thalamus, dorsal part
- AMv
anteromedial nucleus thalamus, ventral part
- AONpv
anterior olfactory nucleus, posteroventral part
- AP
area postrema
- AQ
cerebral aqueduct
- AQc
cerebral aqueduct collicular recess
- ARH
arcuate hypothalamic nucleus
- AT
anterior tegmental nucleus
- AV
anteroventral nucleus thalamus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral periventricular nucleus
- B
Barrington's nucleus
- BA
bed nucleus of the accessory olfactory part
- BAC
bed nucleus anterior commissure
- BLAp
basolateral amygdalar nucleus, posterior part
- BMAa
basomedial amygdalar nucleus, anterior part
- BSM
bed nucleus of the stria medullaris
- BSTal
bed nuclei stria terminalis, anterior division, anterolateral area
- BSTam
bed nuclei stria terminalis, anterior division, anteromedial area
- BSTd
bed nuclei stria terminalis, posterior division, dorsal nucleus
- BSTdm
bed nuclei stria terminalis, anterior division, dorsomedial nucleus
- BSTfu
bed nuclei stria terminalis, anterior division, fusiform nucleus
- BSTif
bed nuclei stria terminalis, posterior division, interfascicular nucleus
- BSTmg
bed nuclei stria terminalis, anterior division, magnocellular nucleus
- BSTpr
bed nuclei stria terminalis, posterior division, principal nucleus
- BSTse
bed nuclei stria terminalis, anterior division, rhomboid nucleus
- BSTtr
bed nuclei stria terminalis, posterior division, transverse nucleus
- BSTv
bed nuclei stria terminalis, anterior division, ventral nucleus
- C
central canal
- CA1
field CA1, Ammon's horn
- CA1so
field CA1, stratum oriens
- CA1spd
field CA1, pyramidal layer, deep
- CA1sps
field CA1, pyramidal layer, superficial
- CA2sp
field CA2, pyramidal layer
- CA3so
field CA3, stratum oriens
- CA3sp
field CA3, pyramidal layer
- cc
corpus callosum
- ccg
genu of corpus callosum
- CEAc
central amygdalar nucleus, capsular part
- CEAl
central amygdalar nucleus, lateral part
- CEAm
central amygdalar nucleus, medial part
- cic
commissure of the inferior colliculus
- cing
cingulum bundle
- CL
central lateral nucleus thalamus
- CLA
claustrum
- CLI
central linear nucleus raphé
- CM
central medial nucleus thalamus
- COAa
cortical amygdalar area, anterior part
- COApl
cortical amygdalar area, posterior part, lateral zone
- COApm
cortical amygdalar area, posterior part, medial zone
- COM
periaqueductal gray, commissural nucleus
- CP
caudoputamen
- cpd
cerebral peduncle
- csc
commissure of the superior colliculus
- CSl
superior central nucleus raphé, lateral part
- CSm
superior central nucleus raphé, medial part
- CUN
cuneiform nucleus
- da
dopamine (cell group within zona incerta)
- DCO
dorsal cochlear nucleus
- df
dorsal fornix
- DGlb
dentate gyrus lateral blade
- DMHa
dorsomedial hypothalamic nucleus, anterior part
- DMHp
dorsomedial hypothalamic nucleus, posterior part
- DMHv
dorsomedial hypothalamic nucleus, ventral part
- DMX
dorsal motor nucleus vagus nerve
- DR
dorsal nucleus raphé
- dscp
decussation of the superior cerebellar peduncle
- DTN
dorsal tegmental nucleus
- ec
corpus callosum external capsule
- ee
corpus callosum extreme capsule
- em
external medullary lamina thalamus
- ENTl
entorhinal area, lateral part
- ENTm
entorhinal area, medial part, dorsal zone
- EPd
endopiriform nucleus, dorsal part
- EW
Edinger-Westphal nucleus
- fa
corpus callosum anterior forceps
- FF
fields of Forel
- fi
fimbria
- fr
fasciculus retroflexus
- FS
fundus of the striatum
- fx
columns of the fornix
- fxpr
precommissural fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- GR
gracile nucleus
- GRN
gigantocellular reticular nucleus
- gVIIn
genu of the facial nerve
- hbc
habenular commissure
- HPF
hippocampal formation
- I
internuclear area, hypothalamic periventricular region
- IA
intercolated nuclei amygdala
- IAD
interanterodorsal nucleus thalamus
- IAM
interanteromedial nucleus thalamus
- ICc
inferior colliculus central nucleus
- ICd
inferior colliculus dorsal nucleus
- ICe
inferior colliculus external nucleus
- IF
interfascicular nucleus raphé
- IG
induseum griseum
- ILA
infralimbic area
- im
internal medullary lamina thalamus
- IMD
intermediodorsal nucleus thalamus
- INC
interstitial nucleus of Cajal
- int
internal capsule
- IPNc
interpeduncular nucleus central subnucleus
- IPNd
interpeduncular nucleus dorsomedial subnucleus
- IPNi
interpeduncular nucleus intermediate subnucleus
- IPNld
interpeduncular nucleus lateral subnucleus dorsal part
- IPNli
interpeduncular nucleus, lateral subnucleus, intermediate part
- IPNlr
interpeduncular nucleus lateral subnucleus rostral part
- IPNr
interpeduncular nucleus rostral subnucleus
- ISN
inferior salivatory nucleus
- ivf
interventricular foramen
- KF
Kolliker-Fuse subnucleus
- LC
locus ceruleus
- LD
lateral dorsal nucleus thalamus
- LDT
laterodorsal tegmental nucleus
- LGd
dorsal part of the lateral geniculate complex
- LH
lateral habenula
- LHAad
lateral hypothalamic area, anterior region, dorsal zone
- LHAai
lateral hypothalamic area, anterior region, intermediate zone
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- LHAd
lateral hypothalamic area, dorsal region
- LHAjd
lateral hypothalamic area, juxtadorsomedial region
- LHAjp
lateral hypothalamic area, juxtaparaventricular region
- LHAjvd
lateral hypothalamic area, juxtaventromedial region, dorsal zone
- LHAjvv
lateral hypothalamic area, juxtaventromedial region, ventral zone
- LHAm
lateral hypothalamic area, magnocellular nucleus
- LHAmo
lateral hypothalamic area, motor related
- LHAp
lateral hypothalamic area, posterior region
- LHApc
lateral hypothalamic area, parvicellular region
- LHAs
lateral hypothalamic area, suprafornical region
- LHAsfa
lateral hypothalamic area, subfornical region, anterior zone
- LHAsfp
lateral hypothalamic area, subfornical region, posterior zone
- LHAsfpm
lateral hypothalamic area, subfornical region, premammillary zone
- LHAvl
lateral hypothalamic area, ventral region, lateral zone
- LHAvm
lateral hypothalamic area, ventral region, medial zone
- LP
lateral posterior nucleus thalamus
- LPO
lateral preoptic area
- LRNm
lateral reticular nucleus magnocellular part
- LSc.d.d
lateral septal nucleus, caudal (caudodorsal) part, dorsal zone, dorsal region
- LSc.d.l
lateral septal nucleus, caudal (caudodorsal) part, dorsal zone, lateral region
- LSc.d.r
lateral septal nucleus, caudal (caudodorsal) part, dorsal zone, rostral region
- LSc.d.v
lateral septal nucleus, caudal (caudodorsal) part, dorsal zone, ventral region
- LSc.v.i
lateral septal nucleus, caudal (caudodorsal) part, ventral zone, intermediate region
- LSc.v.l.d
lateral septal nucleus, caudal (caudodorsal) part, ventral zone, lateral region, dorsal domain
- LSc.v.l.v
lateral septal nucleus, caudal (caudodorsal) part, ventral zone, lateral region, ventral domain
- LSc.v.m.d
lateral septal nucleus, caudal (caudodorsal) part, ventral zone, medial region, dorsal domain
- LSc.v.m.v
lateral septal nucleus, caudal (caudodorsal) part, ventral zone, medial region, ventral domain
- LSr.dl.l.d
lateral septal nucleus, rostral (rostroventral) part, dorsolateral zone, lateral region, dorsal domain
- LSr.dl.l.v
lateral septal nucleus, rostral (rostroventral) part, dorsolateral zone, lateral region, ventral domain
- LSr.dl.m.d
lateral septal nucleus, rostral (rostroventral) part, dorsolateral zone, medial region, dorsal domain
- LSr.dl.m.v
lateral septal nucleus, rostral (rostroventral) part, dorsolateral zone, medial region, ventral domain
- LSr.m.d
lateral septal nucleus, rostral (rostroventral) part, medial zone, dorsal region
- LSr.m.v.c
lateral septal nucleus, rostral (rostroventral) part, medial zone, ventral region, caudal domain
- LSr.m.v.r
lateral septal nucleus, rostral (rostroventral) part, medial zone, ventral region, rostral domain
- LSr.vl.d
lateral septal nucleus, rostral (rostroventral) part, ventrolateral zone, dorsal region
- LSr.vl.d.l
lateral septal nucleus, rostral (rostroventral) part, ventrolateral zone, dorsal region, lateral domain
- LSr.vl.d.m
lateral septal nucleus, rostral (rostroventral) part, ventrolateral zone, dorsal region, medial domain
- LSr.vl.v
lateral septal nucleus, rostral (rostroventral) part, ventrolateral zone, ventral region
- LSv
lateral septal nucleus, ventral part
- LTN
lateral tegmental nucleus
- MA
magnocellular preoptic nucleus
- MARN
magnocellular reticular nucleus
- mct
medial corticohypothalamic tract
- MDc
mediodorsal nucleus thalamus, central part
- MDl
mediodorsal nucleus thalamus, lateral part
- MDm
mediodorsal nucleus thalamus, medial part
- MDRNd
medullary reticular nucleus, dorsal part
- MDRNv
medullary reticular nucleus, ventral part
- MEAad
medial amygdalar nucleus, anterodorsal part
- MEAav
medial amygdalar nucleus, anteroventral part
- MEApd-a
medial amygdalar nucleus, posterodorsal part, sublayer a
- MEApd-b
medial amygdalar nucleus, posterodorsal part, sublayer b
- MEApd-c
medial amygdalar nucleus, posterodorsal part, sublayer c
- MEApv
medial amygdalar nucleus, posteroventral part
- MEex
median eminence external
- MEin
median eminence internal
- MEPO
median preoptic nucleus
- MEV
mesencephalic nucleus of the trigeminal
- MH
medial habenula
- ml
medial lemniscus
- mlf
medial longitudinal fascicle
- MM
medial mammillary nucleus (body)
- MMme
medial mammillary nucleus, median part
- MO
secondary somatomotor area
- moV
motor root of the trigeminal nerve
- mp
mammillary peduncle
- MPNc
central part
- MPNl
medial preoptic nucleus, lateral part
- MPNm
medial part
- MPO
medial preoptic area
- MPT
medial pretectal area
- MRNm
midbrain reticular nucleus, magnocellular part
- MRNp
midbrain reticular nucleus, parvicellular part
- MS
medial septal nucleus
- mtg
mammillotegmental tract
- mtt
mammillothalamic tract
- mtV
mesencephalic tract of the trigeminal nerve
- MV
medial vestibular nucleus
- NC
supraoptic nucleus, accessory supraoptic group, nucleus circularis
- ND
nucleus of Darkschewitsch
- NDB
diagonal band nucleus
- NIc
nucleus incertus, compact part
- NId
nucleus incertus, diffuse part
- NIS
nucleus intercalatus
- NLOT
nucleus of the lateral olfactory tract
- NPC
nucleus of the posterior commissure
- NTB
nucleus of the trapezoid body
- NTSco
nucleus of the solitary tract, commissural part
- NTSl
nucleus of the solitary tract, lateral part
- NTSmc
nucleus of the solitary tract, medial part, caudal zone
- NTSmr
nucleus of the solitary tract, medial part, rostral zone
- och
optic chiasm
- opt
optic tract
- ORBl
orbital area, lateral part
- ORBm
orbital area, medial part
- ORBv
ventral part
- ORBvl
ventrolateral part
- OT
olfactory tubercle
- PA
posterior amygdalar nucleus
- PAGd
periaqueductal gray, dorsal division
- PAGdl
periaqueductal gray, dorsolateral division
- PAGm
periaqueductal gray, medial division
- PAGrl
periaqueductal gray, rostrolateral division
- PAGrm
periaqueductal gray, rostromedial division
- PAGvl
periaqueductal gray, ventrolateral division
- PAR
parasubiculum
- PARN
parvicellular reticular nucleus
- PAT
paratrigeminal nucleus
- PBlc
parabrachial nucleus, lateral division, central lateral part
- PBld
parabrachial nucleus, lateral division, dorsal lateral part
- PBle
parabrachial nucleus, lateral division, external lateral part
- PBlv
parabrachial nucleus, lateral division, ventral part
- PBmm
parabrachial nucleus, medial division, medial medial part
- pc
posterior commissure
- PCG
pontine central gray
- PF
parafascicular nucleus
- PG
pontine gray
- PGRNd
paragigantocellular reticular nucleus, dorsal part
- PGRNl
paragigantocellular reticular nucleus, lateral part
- PH
posterior hypothalamic nucleus
- PIR
piriform area
- PL
prelimbic area
- pm
principal mammillary tract
- PMd
dorsal premammillary nucleus
- PMv
ventral premammillary nucleus
- POR
superior olivary complex periolivary region
- POST
postsubiculum
- PPN
pedunculopontine nucleus
- PPT
posterior pretectal nucleus
- PPYd
parapyramidal nucleus, deep part
- PR
perireuniens nucleus
- PRC
periaqueductal gray, precommissural nucleus
- PRE
presubiculum
- PRNc
pontine reticular nucleus, caudal part
- PRNr
pontine reticular nucleus, rostral part
- PRP
nucleus prepositus
- PS
parastrial nucleus
- PSCH
suprachiasmatic preoptic nucleus
- PST
preparasubthalamic nucleus
- PSTN
parasubthalamic nucleus
- PT
paratenial nucleus
- PVa
periventricular hypothalamic nucleus, anterior part
- PVHam
paraventricular nucleus hypothalamus, magnocellular division, anterior magnocellular part
- PVHap
paraventricular nucleus hypothalamus, anterior parvicellular part
- PVHdp
paraventricular nucleus hypothalamus, dorsal parvicellular part
- PVHf
paraventricular nucleus hypothalamus, forniceal part
- PVHlp
paraventricular nucleus hypothalamus, lateral parvicellular part
- PVHmpd
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone
- PVHmpdl
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone, lateral wing
- PVHpml
paraventricular nucleus hypothalamus, posterior magnocellular part, lateral zone
- PVHpmm
paraventricular nucleus hypothalamus, posterior magnocellular part, medial zone
- PVHpv
paraventricular nucleus hypothalamus, periventricular part
- PVi
periventricular nucleus hypothalamus, intermediate part
- PVp
periventricular nucleus hypothalamus, posterior part
- PVpo
preoptic periventricular nucleus
- PVT
paraventricular nucleus thalamus
- py
pyramidal tract, crossed
- RCH
retrochiasmatic area
- REa
nucleus reuniens, rostral division, anterior part
- REc
nucleus reuniens, caudal division, caudal part
- REcd
nucleus reuniens, caudal division, dorsal part
- REcm
nucleus reuniens, caudal division, median part
- REcp
nucleus reuniens, caudal division, posterior part
- REd
nucleus reuniens, rostral division, dorsal part
- REl
nucleus reuniens, rostral division, lateral part
- REm
nucleus reuniens, rostral division, median part
- REv
nucleus reuniens, rostral division, ventral part
- RH
rhomboid nucleus
- RL
rostral linear nucleus raphé
- RM
nucleus raphé magnus
- RN
red nucleus
- RO
nucleus raphé obscurus
- RPA
nucleus raphé pallidus
- RR
midbrain reticular nucleus, retrorubral area
- RSPv
retrosplenial area, ventral part
- RT
reticular nucleus thalamus
- rust
rubrospinal tract
- SBPV
subparaventricular zone
- SCdg
superior colliculus, deep gray layer
- SCdg
superior colliculus, deep gray layer
- SCH
suprachiasmatic nucleus
- SCig-a
superior colliculus, intermediate gray layer, sublayer a
- SCig-b
superior colliculus, intermediate gray layer, sublayer b
- SCig-c
superior colliculus, intermediate gray layer, sublayer c
- scp
superior cerebellar peduncle
- SCsg
superior colliculus, superficial gray layer
- sctv
ventral spinocerebellar tract
- SEZ/RC
subependymal zone/rhinocele
- SF
septofimbral nucleus
- SFO
subfornical organ
- SH
septohippocampal nucleus
- SI
substantia innominata
- SLD
sublaterodorsal nucleus
- sm
stria medullaris
- smd
supramammillary decussation
- SMT
submedial nucleus of the thalamus
- SO
supraoptic nucleus
- SPFm
subparafascicular nucleus thalamus, magnocellular part
- SPFpl
subparafascicular nucleus thalamus, parvicellular part, lateral division
- SPVI
spinal nucleus of the trigeminal interpolar part
- SPVOmdmd
spinal nucleus of the trigeminal oral part middle dorsomedial part, dorsal zone
- SPVOvl
spinal nucleus of the trigeminal oral part ventrolateral part
- st
stria terminalis
- SUB-sp*
subiculum, pyramidal layer
- SUMl
supramammillary nucleus, lateral part
- SUMm
supramammillary nucleus, medial part
- sup
supraoptic commissures
- SUT
supratrigeminal nucleus
- SUV
superior vestibular nucleus
- tb
trapezoid body
- TMd
tuberomammillary nucleus dorsal part
- TMv
tuberomammillary nucleus ventral part
- tp
thalamic peduncles
- TR
postpiriform transition area
- TRN
tegmental reticular nucleus
- TRS
triangular nucleus septum
- ts
solitary tract
- tsp
tectospinal pathway
- TTd
tenia tecta, dorsal part
- TTv
tenia tecta, ventral part
- TUi
tuberal nucleus, intermediate part
- TUl
tuberal nucleus, lateral part
- TUsv
tuberal nucleus, subventromedial part
- TUte
tuberal nucleus, terete subnucleus
- V3h
third ventricle, hypothalamic part
- V3m
third ventricle mammillary recess
- V3p
third ventricle preoptic recess
- V3r
third ventricle periventricular recess
- V3t
third ventricle, thalamic part
- V4
fourth ventricle
- VAL
ventral anterior-lateral complex of the thalamus
- vhc
ventral hippocampal commissure
- VL
lateral ventricle
- VLP
ventrolateral preoptic nucleus
- vlt
ventrolateral hypothalamic tract
- VM
ventral medial nucleus of the thalamus
- Vma
motor nucleus of the trigeminal, magnocellular part
- VMHa
ventromedial nucleus hypothalamus, anterior part
- VMHc
ventromedial nucleus hypothalamus, central part
- VMHdm
ventromedial nucleus hypothalamus, dorsomedial part
- VMHvl
ventromedial nucleus hypothalamus, ventrolateral part
- VPMpc
ventral posteromedial nucleus of the thalamus parvicellular part
- VTA
ventral tegmental area
- VTN
ventral tegmental nucleus
- XII
hypoglossal nucleus
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In Swanson (2004) the subiculum (SUB) is further divided into dorsal and ventral halves; for clarity of description we have omitted those subdivisions in this article (see discussion section 4.3.).
Literature Cited
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DJ, Chew GL. The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav Neural Biol. 1980;30:357–388. doi: 10.1016/s0163-1047(80)91247-9. [DOI] [PubMed] [Google Scholar]
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J Comp Neurol. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ. Amygdala, hippocampus, and unconditioned fear. Exp Brain Res. 2001;138:200–209. doi: 10.1007/s002210000645. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Arluison M, Derer P. Forebrain connections of the rat paraventricular thalamic nucleus as demonstrated using the carbocyanide dye DiI. Neurobiology (Bp) 1993;1:337–350. [PubMed] [Google Scholar]
- Arts MP, Bemelmans FF, Cools AR. Role of the retrorubral nucleus in striatally elicited orofacial dyskinesia in cats: effects of muscimol and bicuculline. Psychopharmacology (Berl) 1998;140:150–156. doi: 10.1007/s002130050752. [DOI] [PubMed] [Google Scholar]
- Audinat E, Conde F, Crepel F. Cortico-cortical connections of the limbic cortex of the rat. Exp Brain Res. 1988;69:439–443. doi: 10.1007/BF00247590. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav Brain Res. 1985;15:107–119. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res. 1996;107:285–300. doi: 10.1016/s0079-6123(08)61871-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000a;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Price JL, Keay KA. Brain mediation of active and passive emotional coping. Prog Brain Res. 2000b;122:333–349. doi: 10.1016/s0079-6123(08)62149-4. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bassant MH, Poindessous-Jazat F. Ventral tegmental nucleus of Gudden: a pontine hippocampal theta generator? Hippocampus. 2001;11:809–813. doi: 10.1002/hipo.1096. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci Lett. 2003;345:145–148. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Harris P. Passive avoidance deficits following lesions of the posteroventral hippocampo-subiculo-entorhinal area in the developing rat. J Physiol (Paris) 1986;81:374–378. [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Brady JV, Nauta WJ. Subcortical mechanisms in emotional behavior: affective changes following septal forebrain lesions in the albino rat. J Comp Phsyiol Psychol. 1953;46:339–346. doi: 10.1037/h0059531. [DOI] [PubMed] [Google Scholar]
- Brady JV, Nauta WJ. Subcortical mechanisms in emotional behavior: the duration of affective changes following septal and habenular lesions in the albino rat. J Comp Phsyiol Psychol. 1955;48:412–420. doi: 10.1037/h0046406. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Anseloni VZ, Pandossio JE, de Araujo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J Comp Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain DA. PhD Thesis. 1988. The efferent connections of the infralimbic area in the rat; pp. 1–170. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Wu M, Mogenson GJ. Modulation of locomotor activity induced by injections of carbachol into the tegmental pedunculopontine nucleus and adjacent areas in the rat. Brain Res. 1988;451:119–125. doi: 10.1016/0006-8993(88)90755-x. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- Byatt G, Dalrymple-Alford JC. Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci. 1996;110:1335–1348. doi: 10.1037//0735-7044.110.6.1335. [DOI] [PubMed] [Google Scholar]
- Cairns MJ, Burns P, Di Nicolantonio R, McKinley MJ, Mathai ML. Influence of brain angiotensin on thermoregulation and hydromineral balance during pregnancy in rats. J Appl Physiol. 2005;98:1813–1819. doi: 10.1152/japplphysiol.00842.2004. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Rinaman L, Sved AF. Connections of Barrington's nucleus to the sympathetic nervous system in rats. J Auton Nerv Syst. 2000;79:117–128. doi: 10.1016/s0165-1838(99)00101-0. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Connections of the precommissural nucleus. J Comp Neurol. 1999a;408:23–45. doi: 10.1002/(sici)1096-9861(19990524)408:1<23::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999b;10:413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Kroon JA, Do-Monte FH, Pavesi E, Carobrez AP. Sensing danger through the olfactory system: The role of the hypothalamic dorsal premammillary nucleus. Neurosci Biobehav Rev. 2008;32:1228–1235. doi: 10.1016/j.neubiorev.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992a;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. The dorsal premammillary nucleus: an unusual component of the mammillary body. Proc Natl Acad Sci U S A. 1992b;89:10089–10093. doi: 10.1073/pnas.89.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Matinez RRC, Canteras NS. Evidence for a hypothalamo-thalamic circuit mediating emotional memory to predatory threats. Society for Neuroscience Abstracts. 2008;595:21. [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezario AF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Hypothalamic sites responding to predator threats--the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. Eur J Neurosci. 2008;28:1003–1015. doi: 10.1111/j.1460-9568.2008.06392.x. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. II. Contributions of visual and ideothetic information to the directional firing. Exp Brain Res. 1994a;101:24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994b;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Chen S, Aston-Jones G. Evidence that cholera toxin B subunit (CTb) can be avidly taken up and transported by fibers of passage. Brain Res. 1995;674:107–111. doi: 10.1016/0006-8993(95)00020-q. [DOI] [PubMed] [Google Scholar]
- Chen S, Su HS. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Sarma A, Taube JS. Head direction cell instability in the anterior dorsal thalamus after lesions of the interpeduncular nucleus. J Neurosci. 2009;29:493–507. doi: 10.1523/JNEUROSCI.2811-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Canteras NS. Afferent connections of the dorsal premammillary nucleus. J Comp Neurol. 2000;423:83–98. doi: 10.1002/1096-9861(20000717)423:1<83::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Conde F, Audinat E, Maire-Lepoivre E, Crepel F. Afferent connections of the medial frontal cortex of the rat. A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. I. An autoradiographic study of the medial preoptic area. J Comp Neurol. 1976a;169:185–219. doi: 10.1002/cne.901690205. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol. 1976b;169:221–261. doi: 10.1002/cne.901690206. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Crouch RL. The nuclear configuration of the hypothalamus and subthalamus of Macacus rhesus. J Comp Neurol. 1934;59:431–449. [Google Scholar]
- Csaki A, Kocsis K, Halasz B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 2000;101:637–655. doi: 10.1016/s0306-4522(00)00411-5. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Day HE, Badiani A, Uslaner JM, Oates MM, Vittoz NM, Robinson TE, Watson SJ, Jr, Akil H. Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala. J Neurosci. 2001;21:732–740. doi: 10.1523/JNEUROSCI.21-02-00732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- Day HE, Kryskow EM, Nyhuis TJ, Herlihy L, Campeau S. Conditioned fear inhibits c-fos mRNA expression in the central extended amygdala. Brain Res. 2008;1229:137–146. doi: 10.1016/j.brainres.2008.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci. 2005;21:441–454. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin JP, Feenstra MG, Broersen LM, Van Leeuwen M, Arens C, De Vries S, Joosten RN. Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Prog Brain Res. 2000;126:103–113. doi: 10.1016/S0079-6123(00)26010-X. [DOI] [PubMed] [Google Scholar]
- Del Abril A, Segovia S, Guillamon A. Sexual dimorphism in the parastrial nucleus of the rat preoptic area. Brain Res Dev Brain Res. 1990;52:11–15. doi: 10.1016/0165-3806(90)90216-l. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc.Natl.Acad.Sci.U.S.A. 95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Leranth C, Frotscher M. Reciprocal connections of lateral septal neurons and neurons in the lateral hypothalamus in the rat: a combined phaseolus vulgaris-leucoagglutinin and Fluoro-Gold immunocytochemical study. Neurosci Lett. 1994;168:119–122. doi: 10.1016/0304-3940(94)90430-8. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J Neurosci. 1983;3:1599–1606. doi: 10.1523/JNEUROSCI.03-08-01599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, de Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Di Scala G, Schmitt P, Karli P. Flight induced by infusion of bicuculline methiodide into periventricular structures. Brain Res. 1984;309:199–208. [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat“: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006a;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: Implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006b;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: Implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006c;494:108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000a;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: mapping or memory? Curr Biol. 2000b;10:R785–R787. doi: 10.1016/s0960-9822(00)00763-6. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. Dissociation of the anxiolytic-like effects of Avpr1a and Avpr1b receptor antagonists in the dorsal and ventral hippocampus. Neuropeptides. doi: 10.1016/j.npep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Erdi P, Huhn Z, Kiss T. Hippocampal theta rhythms from a computational perspective: code generation, mood regulation and navigation. Neural Netw. 2005;18:1202–1211. doi: 10.1016/j.neunet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Superior colliculus efferents to the hypothalamus. Neurosci Lett. 1979;14:265–270. doi: 10.1016/0304-3940(79)96159-7. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus. II. Facial muscle motor systems. Brain Res Brain Res Rev. 1997a;25:276–290. doi: 10.1016/s0165-0173(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. I. Masticatory muscle motor systems. Brain Res Brain Res Rev. 1997b;25:255–275. doi: 10.1016/s0165-0173(97)00026-x. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev. 1997c;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Fernandez de Molina A, Hunsperger RW. Organization of the subcortical system governing defence and flight reactions in the cat. J Physiol. 1962;160:200–213. doi: 10.1113/jphysiol.1962.sp006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JG, Del Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Keay KA, Arias CM, Sawchenko PE, Bandler R. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neurosci Lett. 1996;220:105–108. doi: 10.1016/s0304-3940(96)13240-7. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Fry M, Hoyda TD, Ferguson AV. Making sense of it: roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp Biol Med (Maywood) 2007;232:14–26. [PubMed] [Google Scholar]
- Fulton JF, Ranson SW, Friedman JM. The Hypothalamus and Central Levels of Autonomic Function. Baltimore: Williams and Wilkins; 1940. [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Ganser SJM. Vergleichende anatomische Studien über das Gehirn des Maulwurfs. Morph Jb. 1882;7:591–725. [Google Scholar]
- Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582:132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Sulli A, Pacitti C, Innocenzi R, Perciavalle V. The projections of the retrorubral field A8 to the hippocampal formation in the rat. Exp Brain Res. 1996;112:244–252. doi: 10.1007/BF00227643. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Geeraedts LM, Nieuwenhuys R, Veening JG. Medial forebrain bundle of the rat: IV. Cytoarchitecture of the caudal (lateral hypothalamic) part of the medial forebrain bundle bed nucleus. J Comp Neurol. 1990;294:537–568. doi: 10.1002/cne.902940404. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Wincour G, Delatour B. Functional dissociation between dorsal and ventral regions of the medial prefrontal cortex in rats. Psychobiology. 2000;28:248–260. [Google Scholar]
- Goncalves L, Nogueira MI, Shammah-Lagnado SJ, Metzger M. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull. 2009;78:240–247. doi: 10.1016/j.brainresbull.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Chesselet MF. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris leucoagglutinin (PHA-L) J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005;493:412–438. doi: 10.1002/cne.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J Comp Neurol. 2004;469:581–607. doi: 10.1002/cne.11036. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The neurophysiology of sleep and waking: intracerebral connections, functioning and ascending influences of the medulla oblongata. Prog Neurobiol. 1999;59:1–54. doi: 10.1016/s0301-0082(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioural inhibition system: a possible substrate for anxiety. In: Feldman MP, Broadhurst AM, editors. Theoretical and experimental bases of behaviour modification. London: Wiley; 1976. pp. 3–41. [Google Scholar]
- Gray JA. The neuropsychiatry of anxiety: an enquiry into the functions of the septohippocampal system. Oxford University Press; 1982. [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988a;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988b;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- Gurdjian ES. The diencephalon of the albino rat. Studies on the brain of the rat. No. 2. J Comp Neurol. 1927;43:1–114. [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. [DOI] [PubMed] [Google Scholar]
- Hahn JD. Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2009;468:12–17. doi: 10.1016/j.neulet.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Cells of origin of hypothalamo-spinal projections in the rat. Neurosci Lett. 1976;4:179–184. doi: 10.1016/0304-3940(76)90070-7. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Jansen AS, Cherniack NS, Loewy AD. CNS innervation of airway-related parasympathetic preganglionic neurons: a transneuronal labeling study using pseudorabies virus. Brain Res. 1993;618:115–134. doi: 10.1016/0006-8993(93)90435-p. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegaart V. Functional topography of brain serotonergic pathways in the rat. Acta Physiol Scand Suppl. 1991;598:1–54. [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Holscher C. Time, space and hippocampal functions. Rev Neurosci. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hosoya Y. The distribution of spinal projection neurons in the hypothalamus of the rat, studied with the HRP method. Exp Brain Res. 1980;40:79–87. doi: 10.1007/BF00236665. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. Cells of origin of the descending afferents to the lateral hypothalamic area in the rat, studied with the horseradish peroxidase method. Neurosci Lett. 1980;18:231–236. doi: 10.1016/0304-3940(80)90290-6. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. A direct projection from the hypothalamus to the area postrema in the rat, as demonstrated by the HRP and autoradiographic methods. Brain Res. 1981a;214:144–149. doi: 10.1016/0006-8993(81)90445-5. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neurosci Lett. 1981b;24:111–116. doi: 10.1016/0304-3940(81)90232-9. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Synaptic plasticity during the cholinergic theta-frequency oscillation in vitro. Hippocampus. 1996;6:58–61. doi: 10.1002/(SICI)1098-1063(1996)6:1<58::AID-HIPO10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal and ventral CA1 for the temporal processing of spatial locations, visual objects, and odors. Behav Neurosci. 2008;122:643–650. doi: 10.1037/0735-7044.122.3.643. [DOI] [PubMed] [Google Scholar]
- Hunsperger RW. Affektreaktionen auf elektrische Reizung im Hirnstamm der Katze. Helv physiol acta. 1956;14:70–92. [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Vale W, Sawchenko PE. Regulation of corticotropin-releasing factor mRNA in neuroendocrine and autonomic neurons by osmotic stimulation and volume loading. Neuroendocrinology. 1992;56:633–640. doi: 10.1159/000126286. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Ingram WR, Hannet FI, Ranson SW. The topography of the nuclei of the diencephalon of the cat. J Comp Neurol. 1932;55:333–394. [Google Scholar]
- Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2008;18:340–348. doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Goldman G, Halpern M. Development of vomeronasal receptor neuron subclasses and establishment of topographic projections to the accessory olfactory bulb. Brain Res Dev Brain Res. 1997a;102:209–216. doi: 10.1016/s0165-3806(97)00097-7. [DOI] [PubMed] [Google Scholar]
- Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G(o alpha)) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Jia C, Halpern M. Segregated populations of mitral/tufted cells in the accessory olfactory bulb. Neuroreport. 1997;8:1887–1890. doi: 10.1097/00001756-199705260-00019. [DOI] [PubMed] [Google Scholar]
- Jia HG, Rao ZR, Shi JW. Projection from the ventrolateral medullary neurons containing tyrosine hydroxylase to the central amygdaloid nucleus in the rat. Brain Res. 1992;589:167–170. doi: 10.1016/0006-8993(92)91180-m. [DOI] [PubMed] [Google Scholar]
- Jia HG, Rao ZR, Shi JW. Evidence of gamma-aminobutyric acidergic control over the catecholaminergic projection from the medulla oblongata to the central nucleus of the amygdala. J Comp Neurol. 1997b;381:262–281. doi: 10.1002/(sici)1096-9861(19970512)381:3<262::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Joseph JP, Boussaoud D, Biguer B. Activity of neurons in the cat substantia nigra pars reticulata during drinking. Exp Brain Res. 1985;60:375–379. doi: 10.1007/BF00235932. [DOI] [PubMed] [Google Scholar]
- Kawano H, Masuko S. Peptidergic and catecholaminergic synaptic contacts onto nucleus preopticus medianus neurons projecting to the subfornical organ in the rat. Neurosci Res. 2006;55:211–217. doi: 10.1016/j.neures.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Owler B, Depaulis A, Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61:727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Kelly AB, Watts AG. Mediation of dehydration-induced peptidergic gene expression in the rat lateral hypothalamic area by forebrain afferent projections. J Comp Neurol. 1996;370:231–246. doi: 10.1002/(SICI)1096-9861(19960624)370:2<231::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kelly AB, Watts AG. The region of the pontine parabrachial nucleus is a major target of dehydration-sensitive CRH neurons in the rat lateral hypothalamic area. J Comp Neurol. 1998;394:48–63. doi: 10.1002/(sici)1096-9861(19980427)394:1<48::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Kimrua D. Effects of selective hippocampal damage on avoidance behaviour in the rat. Can J Psychol. 1958;12:213–218. doi: 10.1037/h0083740. [DOI] [PubMed] [Google Scholar]
- Kirk IJ. Frequency modulation of hippocampal theta by the supramammillary nucleus, and other hypothalamo-hippocampal interactions: mechanisms and functional implications. Neurosci Biobehav Rev. 1998;22:291–302. doi: 10.1016/s0149-7634(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Li S, Mabrouk G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol. 2004;469:170–184. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol. 2006;497:155–165. doi: 10.1002/cne.20971. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kita H, Oomura Y. An HRP study of the afferent connections to rat medial hypothalamic region. Brain Res Bull. 1982;8:53–62. doi: 10.1016/0361-9230(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Di Prisco GV, Vertes RP. Theta synchronization in the limbic system: the role of Gudden's tegmental nuclei. Eur J Neurosci. 2001;13:381–388. [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayniak PF, Siegel A. Efferent connections of the septal area in the pigeon. Brain Behav Evol. 1978;15:389–404. doi: 10.1159/000123789. [DOI] [PubMed] [Google Scholar]
- Krayniak PF, Weiner S, Siegel A. An analysis of the efferent connections of the septal area in the cat. Brain Res. 1980;189:15–29. doi: 10.1016/0006-8993(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Krieg WJS. The hypothalamus of the albino rat. J Comp Neurol. 1932;55:19–89. [Google Scholar]
- Krieger JE, Graeff FG. Defensive behavior and hypertension induced by glutamate in the midbrain central gray of the rat. Braz J Med Biol Res. 1985;18:61–67. [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002a;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002b;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;424:111–141. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ. Serotonin microinjected into the nucleus raphe obscurus increases intragastric pressure in the rat via a vagally mediated pathway. J Pharmacol Exp Ther. 1993;265:468–476. [PubMed] [Google Scholar]
- Kruk MR, van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Kuipers R, Mouton LJ, Holstege G. Afferent projections to the pontine micturition center in the cat. J Comp Neurol. 2006;494:36–53. doi: 10.1002/cne.20775. [DOI] [PubMed] [Google Scholar]
- Kulvicius T, Tamosiunaite M, Ainge J, Dudchenko P, Worgotter F. Odor supported place cell model and goal navigation in rodents. J Comput Neurosci. 2008;25:481–500. doi: 10.1007/s10827-008-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Suntsova N, Rai S, McGinty D, Alam MN. Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat. Brain Res. 2008;1234:66–77. doi: 10.1016/j.brainres.2008.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988a;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced patterns of locomotion and escape jumps in the rat. Brain Res. 1988b;449:294–310. doi: 10.1016/0006-8993(88)91045-1. [DOI] [PubMed] [Google Scholar]
- Lammers JH, Meelis W, Kruk MR, van der Poel AM. Hypothalamic substrates for brain stimulation-induced grooming, digging and circling in the rat. Brain Res. 1987;418:1–19. doi: 10.1016/0006-8993(87)90956-5. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J Comp Neurol. 2008;510:309–350. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. Morphological Aspects of the Hypothalamus. In: Le Gros Clark WE, Beattie J, Riddoch G, Dott NM, editors. The Hypothalamus. Edinburgh: Oliver and Boyd; 1938. pp. 1–68. [Google Scholar]
- Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. 155-84. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Eum YJ, Jo SM, Waterhouse BD. Projection patterns from the amygdaloid nuclear complex to subdivisions of the dorsal raphe nucleus in the rat. Brain Res. 2007;1143:116–125. doi: 10.1016/j.brainres.2007.01.081. [DOI] [PubMed] [Google Scholar]
- Lever C, Jeewajee A, Burton S, O'Keefe J, Burgess N. Hippocampal theta frequency, novelty, and behavior. Hippocampus. 2009 doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligorio M, Descarries L, Warren RA. Cholinergic innervation and thalamic input in rat nucleus accumbens. J Chem Neuroanat. 2009;37:33–45. doi: 10.1016/j.jchemneu.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res. 1993;620:323–330. doi: 10.1016/0006-8993(93)90174-l. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–538. doi: 10.1016/0006-8993(79)90584-5. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–549. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K Neuron. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Sakai K, Salvert D, Fort P, Jouvet M. Peptidergic hypothalamic afferents to the cat nucleus raphe pallidus as revealed by a double immunostaining technique using unconjugated cholera toxin as a retrograde tracer. Brain Res. 1987;402:339–345. doi: 10.1016/0006-8993(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534:209–224. doi: 10.1016/0006-8993(90)90131-t. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. Uber die kerne des menschlichen diencephalon. Berlin: Konigl Press; 1910. [Google Scholar]
- Markham CM, Blanchard DC, Canteras NS, Cuyno CD, Blanchard RJ. Modulation of predatory odor processing following lesions to the dorsal premammillary nucleus. Neurosci Lett. 2004;372:22–26. doi: 10.1016/j.neulet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Marson L. Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett. 1995;190:41–44. doi: 10.1016/0304-3940(95)11495-i. [DOI] [PubMed] [Google Scholar]
- Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- Marson L, Carson C. Central Nervous System Innervation of the Penis, Prostate, and Perineal Muscles: A Transneuronal Tracing Study. Mol Urol. 1999;3:43–50. [PubMed] [Google Scholar]
- Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1996;374:161–179. doi: 10.1002/(SICI)1096-9861(19961014)374:2<161::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419–R428. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L, Platt KB, McKenna KE. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience. 1993;55:263–280. doi: 10.1016/0306-4522(93)90471-q. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McGowan BK, Hankins WG, Garcia J. Limbic lesions and control of the internal and external environment. Behav Biol. 1972;7:841–852. doi: 10.1016/s0091-6773(72)80176-7. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977;119:1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Gorcs T, Setalo G, Petrusz P, Flerko B. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res. 1984;237:15–29. doi: 10.1007/BF00229195. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. A Golgi study of the desending medial forebrain bundle. Brain Res. 1969;15:341–363. doi: 10.1016/0006-8993(69)90161-9. [DOI] [PubMed] [Google Scholar]
- Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Dissociable memory effects after medial thalamus lesions in the rat. Eur J Neurosci. 2005;22:973–985. doi: 10.1111/j.1460-9568.2005.04199.x. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Stevenson JA. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp Neurol. 1967;17:119–127. doi: 10.1016/0014-4886(67)90139-2. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- Mora F, Mogenson GJ, Rolls ET. Activity of neurons in the region of the substantia nigra during feeding in the monkey. Brain Res. 1977;133:267–276. doi: 10.1016/0006-8993(77)90763-6. [DOI] [PubMed] [Google Scholar]
- Moreira CM, Masson S, Carvalho MC, Brandao ML. Exploratory behaviour of rats in the elevated plus-maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Res Bull. 2007;71:466–474. doi: 10.1016/j.brainresbull.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain's fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc.Natl.Acad.Sci.U.S.A. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Ranck JB, Jr, Taube JS. Head direction cells: properties and functional significance. Curr Opin Neurobiol. 1996;6:196–206. doi: 10.1016/s0959-4388(96)80073-0. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. An experimental study of the fornix system in the rat. J Comp Neurol. 1956;104:247–271. doi: 10.1002/cne.901040205. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Haymaker W. Hypothalamic Nuclei and Fiber Connections. In: Haymaker W, Anderson E, Nauta WJH, editors. The Hypothalamus. Illinois: Charles C Thomas; 1969. pp. 136–209. [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nissl F. Die Grosshirnanteile des Kaninchens. Arch Psychiatr Nervenkr. 1913;52:867–953. [Google Scholar]
- Nunez A, Cervera-Ferri A, Olucha-Bordonau F, Ruiz-Torner A, Teruel V. Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci. 2006;23:2731–2738. doi: 10.1111/j.1460-9568.2006.04797.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- O'Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake T, Yamada H. Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci Res. 1989;6:556–568. doi: 10.1016/0168-0102(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Orr R, Marson L. Identification of CNS neurons innervating the rat prostate: a transneuronal tracing study using pseudorabies virus. J Auton Nerv Syst. 1998;72:4–15. doi: 10.1016/s0165-1838(98)00079-4. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Pajolla GP, Crippa GE, Correa SA, Moreira KB, Tavares RF, Correa FM. The lateral hypothalamus is involved in the pathway mediating the hypotensive response to cingulate cortex-cholinergic stimulation. Cell Mol Neurobiol. 2001;21:341–356. doi: 10.1023/a:1012650021137. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog Neurobiol. 2004;74:127–166. doi: 10.1016/j.pneurobio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Papez JW. The thalamic nuclei of the nine-banded armadillo (tatusia novemcincta) J Comp Neurol. 1932;56:49–103. [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;38:725–738. [Google Scholar]
- Papez JW, Aronson LR. Thalamic nuclei of Pithecus (Macacus) rhesus. 1. Ventral thalamus. Arch Nerol Psychiat. 1934;32:1–26. [Google Scholar]
- Papka RE, Williams S, Miller KE, Copelin T, Puri P. CNS location of uterine-related neurons revealed by trans-synaptic tracing with pseudorabies virus and their relation to estrogen receptor-immunoreactive neurons. Neuroscience. 1998;84:935–952. doi: 10.1016/s0306-4522(97)00563-0. [DOI] [PubMed] [Google Scholar]
- Parent A, Gravel S, Boucher R. The origin of forebrain afferents to the habenula in rat, cat and monkey. Brain Res Bull. 1981;6:23–38. doi: 10.1016/s0361-9230(81)80066-4. [DOI] [PubMed] [Google Scholar]
- Park MR. Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull. 1987;19:581–586. doi: 10.1016/0361-9230(87)90075-x. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington's nucleus: evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–361. doi: 10.1016/s0006-8993(97)01178-5. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res. 1992;597:41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Zangrossi H., Jr Involvement of the lateral habenula in the regulation of generalized anxiety- and panic-related defensive responses in rats. Life Sci. 2008;82:1256–1261. doi: 10.1016/j.lfs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Aggleton JP, Vann SD. Do rats with retrosplenial cortex lesions lack direction? Eur J Neurosci. 2008;28:2486–2498. doi: 10.1111/j.1460-9568.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Rosa-Prieto C, Saiz-Sanchez D, Martinez-Marcos A. Projections of olfactory bulbs to the olfactory and vomeronasal cortices. Neuroreport. 2008;19:1541–1544. doi: 10.1097/WNR.0b013e32831126ee. [DOI] [PubMed] [Google Scholar]
- Puigbonet ME, Ciriello J. Hypothalamic connections of the paraventricular thalamic nucleus. Society for Neuroscience Abstracts. 1991;12:707. [Google Scholar]
- Ray JP, Russchen FT, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the mediodorsal nucleus of the thalamus in the rat. J Comp Neurol. 1992;320:435–456. doi: 10.1002/cne.903200403. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rho JH, Swanson LW. Neuroendocrine CRF motoneurons: intrahypothalamic axon terminals shown with a new retrograde-Lucifer-immuno method. Brain Res. 1987;436:143–147. doi: 10.1016/0006-8993(87)91566-6. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rioch DM. Studies on the diencephalon of carnivora part I. The nuclear configuration of the thalamus, epithalamus, and hypothalamus of the dog and cat. J Comp Neurol. 1929;49:1–119. [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Evidence for a hypothalamothalamocortical circuit mediating pheromonal influences on eye and head movements. Proc Natl Acad Sci U S A. 1995;92:3898–3902. doi: 10.1073/pnas.92.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Roeling TA, Kruk MR, Schuurmans R, Veening JG. Behavioural responses of bicucculline methiodide injections into the ventral hypothalamus of freely moving, socially interacting rats. Brain Res. 1993a;615:121–127. doi: 10.1016/0006-8993(93)91122-9. [DOI] [PubMed] [Google Scholar]
- Roeling TA, Van Erp AM, Meelis W, Kruk MR, Veening JG. Behavioural effects of NMDA injected into the hypothalamic paraventricular nucleus of the rat. Brain Res. 1991;550:220–224. doi: 10.1016/0006-8993(91)91321-q. [DOI] [PubMed] [Google Scholar]
- Roeling TA, Veening JG, Peters JP, Vermelis ME, Nieuwenhuys R. Efferent connections of the hypothalamic “grooming area” in the rat. Neuroscience. 1993b;56:199–225. doi: 10.1016/0306-4522(93)90574-y. [DOI] [PubMed] [Google Scholar]
- Roeling TAP, Veening JG, Kruk MR, Nieuwnhuys R. Grooming behavior elicited by kainic acid evoked cell body stimulation in the hypothalamus of the rat. Neuroscience Research Communications. 1990;6:111–119. [Google Scholar]
- Room P, Russchen FT, Groenewegen HJ, Lohman AH. Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol. 1985;242:40–55. doi: 10.1002/cne.902420104. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Differential effect of lesioning of the central amygdala on the bradycardiac and behavioral response of the rat in relation to conditioned social and solitary stress. Behav Brain Res. 1990;41:39–48. doi: 10.1016/0166-4328(90)90052-g. [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- Saper CB, Akil H, Watson SJ. Lateral hypothalamic innervation of the cerebral cortex: immunoreactive staining for a peptide resembling but immunochemically distinct from pituitary/arcuate alpha-melanocyte stimulating hormone. Brain Res Bull. 1986;16:107–120. doi: 10.1016/0361-9230(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976a;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol. 1976b;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the anterior hypothalamic area of the rat, cat and monkey. J Comp Neurol. 1978;182:575–599. doi: 10.1002/cne.901820402. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Povoa RM, Costa AL, Caldellas AV, Tufik S, Bittencourt AS. Functional specializations within the tectum defense systems of the rat. Neurosci Biobehav Rev. 2005;29:1279–1298. doi: 10.1016/j.neubiorev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Seki M, Zyo K. Anterior thalamic afferents from the mamillary body and the limbic cortex in the rat. J Comp Neurol. 1984;229:242–256. doi: 10.1002/cne.902290209. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Shibata H. Descending projections to the mammillary nuclei in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1989;285:436–452. doi: 10.1002/cne.902850403. [DOI] [PubMed] [Google Scholar]
- Shibata H. Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. J Comp Neurol. 1992;323:117–127. doi: 10.1002/cne.903230110. [DOI] [PubMed] [Google Scholar]
- Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10:3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Shibata H, Naito J. Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Res. 2005;1059:93–103. doi: 10.1016/j.brainres.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Shibata H, Naito J. Organization of anterior cingulate and frontal cortical projections to the retrosplenial cortex in the rat. J Comp Neurol. 2008;506:30–45. doi: 10.1002/cne.21523. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Tohyama M. Analysis of the habenulopetal enkephalinergic system in the rat brain: an immunohistochemical study. J Comp Neurol. 1987;255:483–496. doi: 10.1002/cne.902550402. [DOI] [PubMed] [Google Scholar]
- Shiosaka S, Tohyama M, Takagi H, Takahashi Y, Saitoh Y, Sakumoto T, Nakagawa H, Shimizu N. Ascending and descending components of the medial forebrain bundle in the rat as demonstrated by the horseradish peroxidase-blue reaction. I. Forebrain and upper brain stem. Exp Brain Res. 1980;39:377–388. doi: 10.1007/BF00239302. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. High-resolution paraventricular nucleus serial section model constructed within a traditional rat brain atlas. Neurosci Lett. 2008;438:85–89. doi: 10.1016/j.neulet.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Kasicki S. The frequency of rat's hippocampal theta rhythm is related to the speed of locomotion. Brain Res. 1998;796:327–331. doi: 10.1016/s0006-8993(98)00390-4. [DOI] [PubMed] [Google Scholar]
- Smith DA, Flynn JP. Afferent projections to affective attack sites in cat hypothalamus. Brain Res. 1980;194:41–51. doi: 10.1016/0006-8993(80)91317-7. [DOI] [PubMed] [Google Scholar]
- Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009;296:R512–R520. doi: 10.1152/ajpregu.90858.2008. [DOI] [PubMed] [Google Scholar]
- Solnitzky O. The hypothalamus and subthalamus of sus scrofa. J Comp Neurol. 1939;70:191–229. [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by 'theta' pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Steckler T, Inglis W, Winn P, Sahgal A. The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res Brain Res Rev. 1994;19:298–318. doi: 10.1016/0165-0173(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Bozhilova-Pastirova A, Ovtscharoff W. Distribution of GABA-immunoreactive nerve cells in the bed nucleus of the stria terminalis in male and female rats. Eur J Histochem. 1997;41:23–28. [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington's nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976;167:227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci. 2003a;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Architecture: Understanding the Basic Plan. 1st edition. Oxford University Press; 2003b. pp. 1–288. [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. 3rd edition. Academic Press; 2004. pp. 1–215. [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon's horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Lind RW. Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res. 1986;379:399–403. doi: 10.1016/0006-8993(86)90799-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain Res. 1981;228:1–34. doi: 10.1016/0165-0173(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Gerfen CR, Robinson P. Evidence for a projection from the lateral preoptic area and substantia innominata to the 'mesencephalic locomotor region' in the rat. Brain Res. 1984;295:161–178. doi: 10.1016/0006-8993(84)90827-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Simerly RB, Wu M. Anatomical and electrophysiological evidence for a projection from the medial preoptic area to the 'mesencephalic and subthalamic locomotor regions' in the rat. Brain Res. 1987;405:108–122. doi: 10.1016/0006-8993(87)90995-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Szentagothai J, Flerko B, Mess B, Halasz B. Hypothalamic Control of the Anterior Pituitary. An Experimental-Morphological Study. Budapest Akademiai Kiado. 1968 [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y. Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci. 2005;21:1659–1670. doi: 10.1111/j.1460-9568.2005.03980.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience. 1995;68:553–561. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol. 1998;55:225–256. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Taube JS, Bassett JP. Persistent neural activity in head direction cells. Cereb Cortex. 2003;13:1162–1172. doi: 10.1093/cercor/bhg102. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- ter Horst GJ, Luiten PG, Kuipers F. Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst. 1984;11:59–75. doi: 10.1016/0165-1838(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The Study of Instinct. Oxford: Oxford University Press; 1951. [Google Scholar]
- Trinh K, Storm DR. Detection of odorants through the main olfactory epithelium and vomeronasal organ of mice. Nutr Rev. 2004;62:S189–S192. doi: 10.1111/j.1753-4887.2004.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Tsubouchi K, Tsumori T, Yokota S, Okunishi H, Yasui Y. A disynaptic pathway from the central amygdaloid nucleus to the paraventricular hypothalamic nucleus via the parastrial nucleus in the rat. Neurosci Res. 2007;59:390–398. doi: 10.1016/j.neures.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Uchida N, Kepecs A, Mainen ZF. Seeing at a glance, smelling in a whiff: rapid forms of perceptual decision making. Nat Rev Neurosci. 2006;7:485–491. doi: 10.1038/nrn1933. [DOI] [PubMed] [Google Scholar]
- Uchida T, Adachi K, Fujita S, Lee J, Gionhaku N, Cools AR, Koshikawa N. Role of GABA(A) receptors in the retrorubral field and ventral pallidum in rat jaw movements elicited by dopaminergic stimulation of the nucleus accumbens shell. Eur J Pharmacol. 2005;510:39–47. doi: 10.1016/j.ejphar.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Kosboth M, Colflesh M, Miselis RR. Transneuronal labeling from the rat distal colon: anatomic evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J Comp Neurol. 2000;417:399–414. [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington's nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–143. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington's nucleus to the periaqueductal gray and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363:402–422. doi: 10.1002/cne.903630306. [DOI] [PubMed] [Google Scholar]
- Valverde F. Studies on the Piriform Lobe. Cambridge, Massachusetts: Harvard University Press; 1965. [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Van Erp AM, Kruk MR, Willekens-Bramer DC, Bressers WM, Roeling TA, Veening JG, Spruyt BM. Grooming induced by intrahypothalamic injection of ACTH in the rat: comparison with grooming induced by intrahypothalamic electrical stimulation and i.c.v. injection of ACTH. Brain Res. 1991;538:203–210. doi: 10.1016/0006-8993(91)90431-t. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Michael WJ. Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav Brain Res. 2002a;132:19–28. doi: 10.1016/s0166-4328(01)00390-4. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res. 2002b;136:329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154:483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol. 1995;358:584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 2002;116:85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract. J Neurosci. 2003;23:3506–3514. doi: 10.1523/JNEUROSCI.23-08-03506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vann SD, Honey RC, Aggleton JP. Lesions of the mammillothalamic tract impair the acquisition of spatial but not nonspatial contextual conditional discriminations. Eur J Neurosci. 2003;18:2413–2416. doi: 10.1046/j.1460-9568.2003.02959.x. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Cowan WM, Nieuwenhuys R, Geeraedts LM. The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J Comp Neurol. 1982;206:82–108. doi: 10.1002/cne.902060107. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Vertes RP. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol. 1981;46:1140–1159. doi: 10.1152/jn.1981.46.5.1140. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem generation of the hippocampal EEG. Prog Neurobiol. 1982;19:159–186. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Albo Z, Viana DP. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience. 2001;104:619–625. doi: 10.1016/s0306-4522(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Descending projections of the posterior nucleus of the hypothalamus: Phaseolus vulgaris leucoagglutinin analysis in the rat. J Comp Neurol. 1996;374:607–631. doi: 10.1002/(SICI)1096-9861(19961028)374:4<607::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. J Comp Neurol. 1995;359:90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana DP. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Viltart O, Sartor DM, Verberne AJ. Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull. 2006;71:51–59. doi: 10.1016/j.brainresbull.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Vincent SR. The ascending reticular activating system--from aminergic neurons to nitric oxide. J Chem Neuroanat. 2000;18:23–30. doi: 10.1016/s0891-0618(99)00048-4. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing A13 dopaminergic neurons: a PHA-L anterograde tract-tracing study in the rat. Brain Res. 1995;677:229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: A Phaseolus vulgaris leucoagglutinin study in rats. J Comp Neurol. 1999;415:341–367. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Nakai Y. The dorsal raphe: an important nucleus in pain modulation. Brain Res Bull. 1994;34:575–585. doi: 10.1016/0361-9230(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- Wang Z, Balet SC, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FJ. The hypothalamus of the opossum (Didelphis virginiana) J Nerv Ment Dis. 1929;70:485–501. [Google Scholar]
- Watts AG. Osmotic stimulation differentially affects cellular levels of corticotropinreleasing hormone and neurotensin/neuromedin N mRNAs in the lateral hypothalamic area and central nucleus of the amygdala. Brain Res. 1992;581:208–216. doi: 10.1016/0006-8993(92)90710-q. [DOI] [PubMed] [Google Scholar]
- Watts AG. Neuropeptides and the integration of motor responses to dehydration. Annu Rev Neurosci. 2001;24:357–384. doi: 10.1146/annurev.neuro.24.1.357. 357–384. [DOI] [PubMed] [Google Scholar]
- Watts AG, Kelly AB, Sanchez-Watts G. Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci. 1995;109:1146–1157. doi: 10.1037//0735-7044.109.6.1146. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19:6111–6121. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Christoph GR, Double BA, Leonzio RJ. Kainate and electrolytic lesions of the lateral habenula: effect on avoidance responses. Physiol Behav. 1986;36:413–417. doi: 10.1016/0031-9384(86)90307-0. [DOI] [PubMed] [Google Scholar]
- Winn P, Brown VJ, Inglis WL. On the relationships between the striatum and the pedunculopontine tegmental nucleus. Crit Rev Neurobiol. 1997;11:241–261. doi: 10.1615/critrevneurobiol.v11.i4.10. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Paden CM, Silverman AJ. The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology. 1982;35:429–438. doi: 10.1159/000123419. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen PA, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Witter MP, Ostendorf RH, Groenewegen HJ. Heterogeneity in the Dorsal Subiculum of the Rat. Distinct Neuronal Zones Project to Different Cortical and Subcortical Targets. Eur J Neurosci. 1990;2:718–725. doi: 10.1111/j.1460-9568.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodnorth MA, Kyd RJ, Logan BJ, Long MA, McNaughton N. Multiple hypothalamic sites control the frequency of hippocampal theta rhythm. Hippocampus. 2003;13:361–374. doi: 10.1002/hipo.10111. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Wray S, Hoffman G. A developmental study of the quantitative distribution of LHRH neurons within the central nervous system of postnatal male and female rats. J Comp Neurol. 1986;252:522–531. doi: 10.1002/cne.902520408. [DOI] [PubMed] [Google Scholar]
- Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol. 1981;199:495–512. doi: 10.1002/cne.901990405. [DOI] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yardley CP, Hilton SM. The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J Auton Nerv Syst. 1986;15:227–244. doi: 10.1016/0165-1838(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Gray TS. A direct neural projection from the nucleus of the solitary tract to the subfornical organ in the rat. Neurosci Lett. 1987;80:163–166. doi: 10.1016/0304-3940(87)90647-1. [DOI] [PubMed] [Google Scholar]