Abstract
In vitro experiments demonstrate that P2X1 receptor activation is important for normal afferent arteriolar autoregulatory behavior, but direct in vivo evidence for this relationship occurring in the whole kidney is unavailable. Experiments were performed to test the hypothesis that P2X1 receptors are important for autoregulation of whole kidney blood flow. Renal blood flow (RBF) was measured in anesthetized male Sprague-Dawley rats before and during P2 receptor blockade with PPADS, P2X1 receptor blockade with IP5I, or A1 receptor blockade with DPCPX. Both P2X1 and A1 receptor stimulation with α,β-methylene ATP and CPA, respectively, caused dose-dependent decreases in RBF. Administration of either PPADS or IP5I significantly blocked P2X1 receptor stimulation. Likewise, administration of DPCPX significantly blocked A1 receptor activation to CPA. Autoregulatory behavior was assessed by measuring RBF responses to reductions in renal perfusion pressure. In vehicle-infused rats, as pressure was decreased from 120 to 100 mmHg, there was no decrease in RBF. However, in either PPADS- or IP5I-infused rats, each decrease in pressure resulted in a significant decrease in RBF, demonstrating loss of autoregulatory ability. In DPCPX-infused rats, reductions in pressure did not cause significant reductions in RBF over the pressure range of 100–120 mmHg, but the autoregulatory curve tended to be steeper than vehicle-infused rats over the range of 80–100 mmHg, suggesting that A1 receptors may influence RBF at lower pressures. These findings are consistent with in vitro data from afferent arterioles and support the hypothesis that P2X1 receptor activation is important for whole kidney autoregulation in vivo.
Keywords: P2 purinoceptors, P1 purinoceptors, hemodynamics, IP5I, DPCPX, PPADS
autoregulation defines the inherent maintenance of stable blood flow during fluctuations in arterial pressure. In the kidney, a relatively constant blood flow is maintained through the combined influences of at least two mechanisms: myogenic and tubuloglomerular feedback (TGF) (8). Both mechanisms have been extensively studied, with evidence strongly supporting ATP and adenosine as the probable signaling mediators (2, 14, 16, 42, 45).
Evidence suggests a role for ATP-sensitive P2X1 receptors in renal autoregulation (20). Activation of P2X1 receptors induces the ligand-gated influx of Ca2+ into vascular smooth muscle cells with subsequent depolarization and vasoconstriction (20, 23, 46). ATP and ATP analogs are potent activators of renal vascular tone in vivo (10, 31). Several studies demonstrate that genetic, pharmacological, or pathological interruption of P2X1 receptor signaling results in a decline in autoregulatory behavior in juxtamedullary afferent arterioles (19–22). A prior study demonstrated that afferent arteriolar autoregulatory behavior is inhibited by pharmacological inhibition of P2X1 receptors in vitro (19). Additional evidence demonstrated loss of autoregulation in juxtamedullary afferent arterioles from mice genetically deficient in P2X1 receptors (21). Importantly, the majority of studies supporting P2X1 receptors as mediators of renal autoregulation have focused on in vitro assessment of blood-perfused juxtamedullary afferent arterioles.
Other studies suggested that genetic removal of the adenosine A1 receptor inhibits autoregulatory afferent arteriolar vasoconstriction (42). Adenosine is formed via the breakdown of ATP by ecto-nucleotidases, exerting its effect through A1 receptors. These receptors are coupled to Go/Gi protein and cause a decrease in cAMP production, while stimulating phospholipase C activity and resulting in vasoconstriction (1, 15). Typically, studies supporting A1 receptors in regulating renal blood flow (RBF) have utilized micropuncture of superficial nephrons.
The objective of the current study was to address the question: are P2X1 receptors responsible for autoregulation of whole kidney blood flow in vivo? We postulated that P2X1 receptors play an essential role in autoregulation of whole kidney blood flow in vivo. The current work examines the mechanism by which whole kidney blood flow is regulated during alterations of renal perfusion pressure. Additionally, we demonstrate renal vascular responses to selective activation of P2X1 and A1 receptors, with and without pharmacological inhibition.
METHODS
Animals.
Experiments were performed in 10-wk-old male Sprague-Dawley rats (n = 58, 340–360 g; Charles River Laboratories, Wilmington, MA). Rats were allowed ad libitum access to standard rat chow (8656, Harlan Teklad Global Diets, Wilmington, DE) and tap water. Animals in this study were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia.
Surgical preparation.
Before experimentation, animals were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and placed on a servo-controlled heating table to maintain body temperature (36–37°C) as measured by a rectal probe (RET-3, Physitemp, Clifton, NJ). A tracheotomy (PE-210) was performed to ensure a patent airway. The femoral artery was cannulated for continuous recording of arterial pressure. Blood volume was maintained by infusion of 6% bovine serum albumin fraction V (Calbiochem, Darmstadt, Germany) in saline through a femoral vein catheter. Maintenance fluid was infused at 100 μl/min until a volume equal to 1% of body wt was given; then, an isotonic saline solution was infused at 25 μl/min. A subcostal midline laparoscopy was performed to expose the bladder, aorta, and left renal artery. A catheter was inserted into the bladder for continuous collection of urine. The portion of the aorta between the left and right renal arteries was partially separated from the surrounding connective tissue and vena cava. An adjustable vascular occluder was placed around the aorta to manually control left renal artery pressure as measured by the femoral artery catheter. An ultrasonic flow probe (MA1PRB, Transonic Systems, Ithaca, NY) was placed around the left renal artery to measure RBF. Arterial pressure, heart rate, RBF, and rectal temperature were recorded by an eight-channel Powerlab (model no. ML870/P, ADInstruments, Colorado Springs, CO). After surgical preparation was completed, the wound was lightly covered in moist gauze and parafilm to reduce evaporative fluid loss and to maintain stable body temperature. The animals were allowed to stabilize for 30 min before beginning experiments, after which point they were assigned to one of three protocols.
Series 1: arterial pressure and RBF responses to P1 and P2 receptor agonists.
After the stabilization period, rats were randomly assigned to one of two groups. In one group (n = 6), the effect of the P2 purinoceptor agonist α,β-methylene ATP (0.25–1.0 μg bolus iv; Sigma, St. Louis, MO) on systolic pressure and RBF was evaluated. Changes in systolic blood pressure and RBF were compared with baseline systolic pressure and flow recordings. In a second group (n = 6), the effect of the selective A1 receptor agonist N6-cyclopentyl-adenosine (CPA; 0.025–0.250 μg iv bolus; Sigma) was assessed. The dose ranges of α,β-methylene ATP and CPA were chosen to optimize renal vascular effects without adversely affecting systemic hemodynamics. At the end of the experiment, the left kidney was excised, decapsulated, and weighed. RBF was normalized to left kidney weight.
Series 2: arterial pressure and RBF response to P1 and P2 receptor antagonists.
The effect of P2 receptor blockade on arterial pressure and RBF was determined by using the nonselective antagonist pyridoxal phosphate-6-azo-benzene-2,4-disulfonic acid (PPADS; 20 μmol/kg bolus iv repeated every 20 min, n = 6; Sigma) or the P2X1 receptor-specific antagonist P1,P5-Di-inosine-5′-pentaphosphate pentasodium salt (IP5I; 750 nmol·kg−1·min−1, n = 4; Sigma) followed by α,β-methylene ATP dose responses. The effect of adenosine A1 receptor blockade was determined by using the specific antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 33 nmol·kg−1·min−1 in DMSO 0.65 μl/min, n = 6; Sigma) followed by CPA dose responses. The doses of PPADS and DPCPX employed for receptor inhibition are based on literature reports of in vivo experiments (29, 30, 34, 43, 44). The dose of IP5I was established by demonstrating blockade of the renal vascular response to the P2X1 agonist, α,β-methylene ATP. Increasing doses of IP5I were administered until significant blockade of α,β-methylene ATP-induced renal responses occurred. At the end of the experiment, the left kidney was excised, decapsulated, and weighed. RBF was normalized to left kidney weight.
Series 3: in vivo autoregulation studies.
After postsurgical stabilization, rats were used for whole kidney autoregulation studies. Separate groups were used to evaluate the effects of PPADS (20 μmol/kg, n = 6), IP5I (1 μmol·kg−1·min−1, n = 6), and DPCPX (33 nmol·kg−1·min−1, n = 6) on renal vascular adjustments to acute changes in renal perfusion pressure. Separate vehicle controls were also performed to determine the effects of saline (25 μl/min, n = 6) or DMSO (0.65 μl/min, n = 6) on the autoregulatory response. An ultrasonic flow probe was placed on the left renal artery to continuously monitor RBF. Autoregulatory curves were generated by decreasing renal perfusion pressure from 120 to 70 mmHg in 10-mmHg decrements. Femoral artery pressure, indicative of renal artery pressure, was reduced by an adjustable occluder placed around the aorta just proximal to the left renal artery and held at a constant pressure for 2 min. Saline, DMSO, PPADS, IP5I, or DPCPX was infused for 20 min before two autoregulatory curves were obtained over the next 40–60 min. The two curves obtained were averaged together to determine an overall response. To quantitate the efficiency of RBF autoregulation over selected pressure ranges, the autoregulatory index (AI) was calculated as AI = [(RBF1 − RBF2)/(RBF1)]/[(AP1 − AP2)/(AP1)] where AP is arterial pressure, subscript 1 is the value before adjusting pressure, and subscript 2 is the value after the pressure change. Using this index, a value of zero indicates perfect autoregulation, with values greater than zero indicating less efficient autoregulation of RBF (37). At the end of the experiment, the left kidney was excised, decapsulated, and weighed. RBF was normalized to left kidney weight.
Statistics.
Values are reported as means ± SE. Two-way ANOVA with Holm-Sidak post hoc tests were used to evaluate statistical significance vs. the control group, as well as pairwise differences. All statistical analyses were performed using SigmaStat version 3.5 (Systat Software, Point Richmond, CA). Comparisons in which P < 0.05 were considered significantly different.
Pharmacological agents.
The P2 purinoceptor agonist α,β-methylene ATP, the selective A1 receptor agonist CPA, the nonselective P2 receptor antagonist PPADS, the P2X1 receptor-specific antagonist IP5I, and the A1 receptor-specific antagonist DPCPX were used in this study.
RESULTS
Arterial pressure response to α,β-methylene ATP.
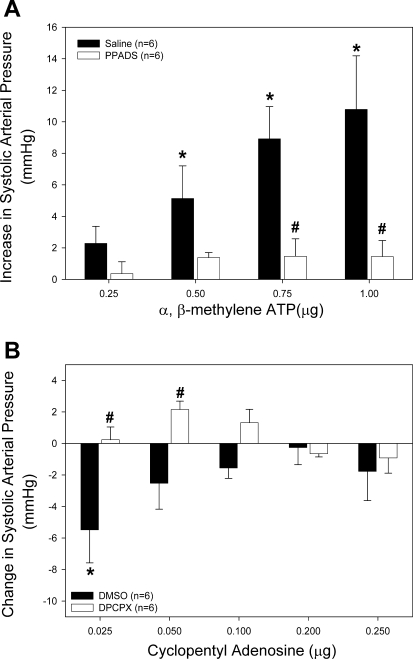
To determine the effect of P2 receptor blockade on systemic hemodynamics, we examined arterial pressure responses to P2X1 agonist α,β-methylene ATP before and during P2 receptor blockade with PPADS. Total body weight was not different between all groups and averaged 345 ± 5 g (n = 58). Left kidney weight was similar among all groups and averaged 1.29 ± 0.02 g. Arterial pressure responses to increasing doses of α,β-methylene ATP (0.25–1.0 μg iv) were measured in the presence of 20 μmol/kg PPADS (Fig. 1A). Baseline systolic arterial pressure was not different between PPADS-infused rats (142 ± 9 mmHg) and saline vehicle controls (143 ± 5 mmHg). In saline-infused rats, bolus intravenous injection of α,β-methylene ATP caused a dose-dependent increase in arterial pressure (Fig. 1A, filled bars). Systolic arterial pressure increased by 5 ± 3 mmHg (4 ± 2%) and 11 ± 3 mmHg (8 ± 3%) following bolus administration of 0.5 and 1.0 μg α,β-methylene ATP, respectively. P2 receptor blockade with 20 μmol/kg PPADS significantly reduced the pressor response to α,β-methylene ATP (Fig. 1A, open bars). When doses of α,β-methylene ATP exceeding 1.0 μg were administered to saline-infused rats, bradycardia and cardiac dyspnea-like respiratory distress occurred.
Fig. 1.
Effect of intravenous bolus infusion of α,β-methylene ATP or cyclopentyl adenosine (CPA) on systolic arterial pressure. A: changes in systolic pressure were measured in saline-infused (filled bars) and pyridoxal phosphate-6-azo-benzene-2,4-disulfonic acid (PPADS)-infused rats (20 μmol/kg, open bars) in response to α,β-methylene ATP infusion. B: changes in systolic pressure were measured in DMSO-infused (filled bars) and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX)-infused rats (33 nmol/kg, open bars) in response to CPA infusion. *P < 0.05 vs. baseline. #P < 0.05 between groups.
Arterial pressure response to CPA.
To determine the effect of A1 receptor blockade on systemic hemodynamics, we examined arterial pressure responses to the A1 agonist CPA before and during A1 receptor blockade. Selective A1 receptor activation with CPA caused a biphasic response in arterial pressure (Fig. 1B, filled bars). The lowest dose of CPA (0.025 μg) caused a 4 ± 2% decline in systolic arterial pressure from 127 ± 4 to 122 ± 4 mmHg. Baseline systolic arterial pressures did not differ between rats infused with DMSO and those receiving 33 nmol/kg DPCPX (127 ± 4 and 135 ± 1, respectively). Blockade of A1 receptors with DPCPX prevented the CPA-induced decrease in systolic pressure (Fig. 1B, open bars). Higher doses of CPA caused dose-dependent decreases in heart rate and arterial pressure. In three animals, 0.5 μg CPA caused a 30- to 50-mmHg decrease in arterial pressure and a drop in heart rate by 20–40 beats/min (data not shown).
RBF response to α,β-methylene ATP.
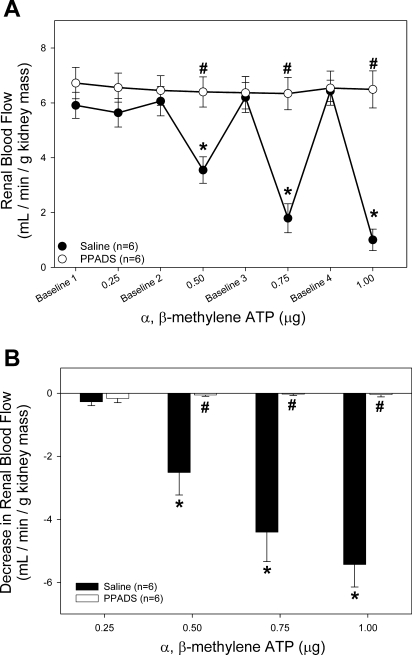
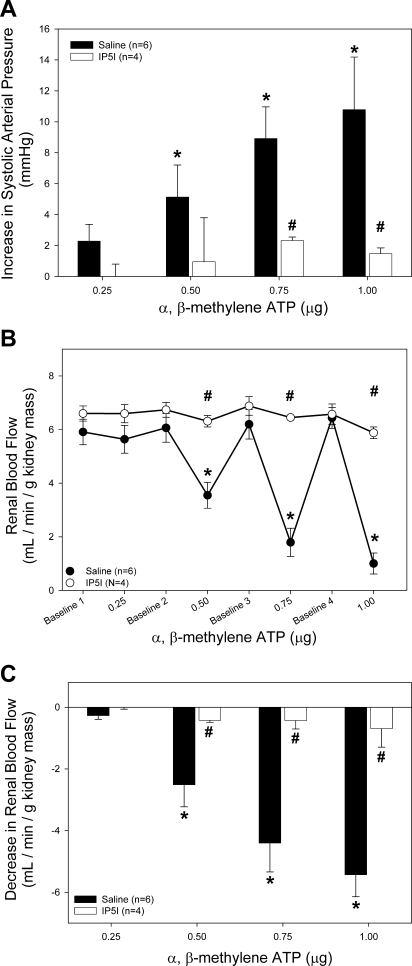
To determine the effectiveness of PPADS blockade of P2X1 receptors, RBF was measured in response to increasing doses of α,β-methylene ATP in the presence of PPADS (Fig. 2). A dose of 0.5 μg α,β-methylene ATP in saline-infused rats resulted in a 40 ± 9% decrease in RBF from 6.1 ± 0.5 to 3.5 ± 0.5 ml·min−1·g kidney mass−1 (Fig. 2A, ●). PPADS treatment completely abolished the decrease in RBF in response to 0.5 μg α,β-methylene ATP (from 6.5 ± 0.5 to 6.4 ± 0.5 ml·min−1·g−1 kidney mass, ○). This effect is emphasized in Fig. 2B. The decrease in RBF associated with α,β-methylene ATP administration is prevented with PPADS inhibition of P2 receptors. The ability of 0.5 μg α,β-methylene ATP to reduce RBF by 40% while increasing arterial pressure by only 4% indicates a highly specific role for P2X1 receptors in the regulation of RBF.
Fig. 2.
Effect of intravenous bolus infusion of α,β-methylene ATP on renal blood flow (RBF). A: average RBF in saline-infused (●) and PPADS-infused rats (20 μmol/kg, ○) in response to α,β-methylene ATP infusion. B: data are expressed as change from baseline RBF immediately preceding α,β-methylene ATP infusion. *P < 0.05 vs. baseline. #P < 0.05 between groups.
RBF response to CPA.
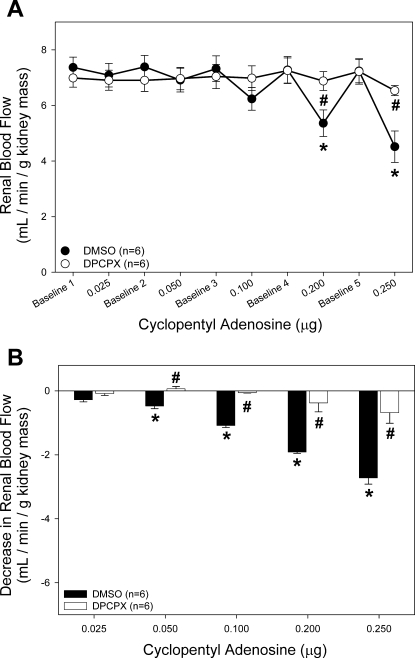
We also tested the effect of DPCPX on the A1 receptor-mediated reduction of RBF (Fig. 3). The highest dose of the A1 receptor agonist CPA (0.250 μg iv) reduced RBF by 39 ± 5% from 7.2 ± 0.4 to 4.5 ± 0.6 ml·min−1·g kidney mass−1 (Fig. 3A, ●). In contrast, during treatment with DPCPX, RBF only decreased by 9 ± 3% from 7.2 ± 0.5 to 6.5 ± 0.2 ml·min−1·g kidney mass−1 (○).
Fig. 3.
Effect of intravenous bolus infusion of CPA on RBF. A: average RBF in DMSO-infused (●) and DPCPX-infused rats (33 nmol/kg, ○) in response to CPA infusion. B: data are expressed as change from baseline RBF immediately preceding CPA infusion. *P < 0.05 vs. baseline. #P < 0.05 between groups.
Autoregulatory behavior during PPADS inhibition of P2 receptors.
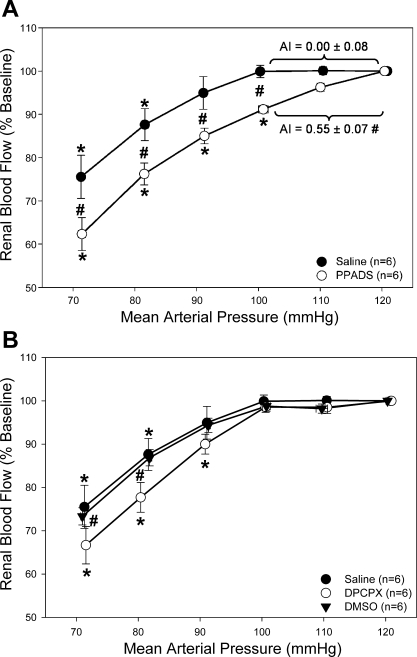
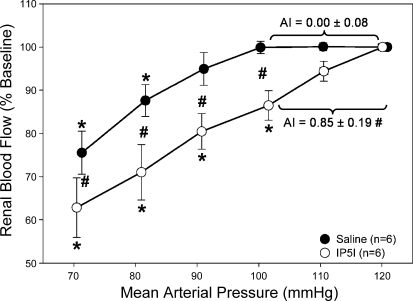
These studies were designed to test the hypothesis that P2 receptor blockade with PPADS would inhibit whole kidney autoregulatory behavior. Using 20 μmol/kg PPADS to block P2 receptors, we tested pressure-mediated autoregulation of RBF (Fig. 4A). Baseline mean arterial pressure (MAP) and RBF were not different between saline- and PPADS-infused rats. Saline-infused rats averaged a MAP of 126 ± 2 mmHg and a RBF of 8.09 ± 0.40 ml·min−1·g kidney mass−1, whereas MAP and RBF averaged 129 ± 3 mmHg and 8.01 ± 0.12 ml·min−1·g kidney mass−1, respectively, in rats infused with PPADS. In saline-infused rats, RBF remained stable as renal perfusion pressure was reduced from 120 to 100 mmHg (Fig. 4A, ●), consistent with properly maintained autoregulatory behavior. However, following administration of PPADS, RBF decreased with each stepwise reduction of renal perfusion pressure across a range of 120 to 100 mmHg (Fig. 4A, ○). Quantification of the AI revealed a value of 0.00 ± 0.08 over the 120- to 100-mmHg pressure range for the saline-infused group, consistent with proper autoregulatory control of RBF. In contrast, PPADS treatment resulted in an AI of 0.55 ± 0.07, indicating significant loss of autoregulation.
Fig. 4.
Percent change of baseline RBF in response to decreasing renal perfusion during P2 and A1 receptor antagonism. A: percent change from baseline RBF in response to decreasing renal perfusion pressure with (○) and without (●) P2 receptor antagonism by PPADS. B: percent change from baseline RBF in response to decreasing renal perfusion pressure with adenosine A1 receptor inhibition by DPCPX (○) and during DMSO vehicle infusion (▾). For comparison, saline vehicle experiments (●) are reproduced from A. *P < 0.05 vs. baseline. #P < 0.05 between saline vehicle.
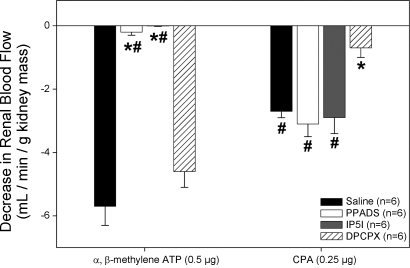
After autoregulatory curves were completed, RBF responses to 0.5 μg α,β-methylene ATP and 0.25 μg CPA were conducted to confirm receptor blockade. Saline-infused rats had an average decrease in RBF of 5.7 ± 0.6 and 2.7 ± 0.2 ml/min in response to α,β-methylene ATP and CPA, respectively (Fig. 5, filled bars). PPADS-infused rats had an average decrease in RBF of 0.2 ± 0.1 and 3.1 ± 0.4 ml/min in response to α,β-methylene ATP and CPA, respectively (Fig. 5, open bars). These data indicate the selective ability of PPADS to inhibit P2X1 receptor responses while leaving A1 receptor signaling intact.
Fig. 5.
Confirmation of P2X1 and A1 receptor blockade following autoregulatory curves. Change from baseline RBF immediately preceding α,β-methylene ATP and CPA injections in saline vehicle (filled bars)-, PPADS (open bars)-, P1,P5-Di-inosine-5′-pentaphosphate pentasodium salt (IP5I; gray bars)-, and DPCPX (cross-hatched bars)-infused rats. *P < 0.05 vs. vehicle-infused rats. #P < 0.05 vs. DPCPX-infused rats.
Autoregulatory behavior during DPCPX inhibition of A1 receptors.
These studies were designed to test the hypothesis that A1 receptor blockade with DPCPX would inhibit whole kidney autoregulatory behavior. RBF was not significantly different in rats infused with DPCPX (8.55 ± 0.15) compared with either saline-infused rats (8.09 ± 0.04) or DMSO-infused rats (9.02 ± 0.13 ml·min−1·g kidney mass−1). Urinary flow rates were significantly increased in DPCPX-infused rats compared with saline-, DMSO-, and PPADS-infused rats (28.0 ± 4.7 vs. 11.9 ± 1.4, 11.7 ± 1.7, and 12.3 ± 2.6 μl/min, respectively). When autoregulatory curves were conducted in rats treated with DPCPX, no decrease in RBF was observed when renal perfusion pressure was reduced (Fig. 4B, ○). The AI in DPCPX-treated rats averaged 0.10 ± 0.08 and was not significantly different from saline-infused rats or rats infused with DMSO (0.04 ± 0.06). DPCPX was able to significantly inhibit a decrease in RBF in response to CPA without decreasing sensitivity to α,β-methylene ATP (0.7 ± 0.3 and 4.6 ± 0.5 ml/min decrease in RBF, respectively, Fig. 5, cross-hatched bars). These data suggest that inhibition of A1 receptors with DPCPX is insufficient to impair autoregulatory control of RBF and that DPCPX does not interfere with P2X1 receptor activation.
Renal vascular effects of selective P2X1 receptor inhibition with IP5I.
To determine the effect of P2X1 receptor blockade on systemic and renal hemodynamics, we examined arterial pressure and RBF responses to P2X1 agonist α,β-methylene ATP before and during P2X1 receptor blockade with IP5I. Since PPADS can have inhibitory effects on select P2X and P2Y receptors, the highly selective antagonist for P2X1 receptors, IP5I, was used to determine the P2X1 receptor-mediated response to α,β-methylene ATP (Fig. 6). IP5I significantly inhibited the increase in systolic arterial pressure observed with α,β-methylene ATP administration (Fig. 6A, open bars). Likewise, the renal vascular response to α,β-methylene ATP was significantly inhibited by IP5I (Fig. 6, B and C). The highest dose of α,β-methylene ATP elicited a marginal 9.5 ± 0.8% decrease in RBF from 6.6 ± 0.4 to 5.9 ± 0.2 ml·min−1·g kidney mass−1 (Fig. 6B).
Fig. 6.
Effect of intravenous bolus infusion of α,β-methylene ATP on systolic arterial pressure and RBF. A: changes in systolic pressure were measured in saline-infused (filled bars) and PPADS-infused rats (20 μmol/kg, open bars) in response to α,β-methylene ATP infusion. B: average RBF in saline-infused (●) and PPADS-infused rats (20 μmol/kg, ○) in response to α,β-methylene ATP infusion. C: data are expressed as change from baseline RBF immediately preceding α,β-methylene ATP infusion. For comparison, saline vehicle experiments (●) are reproduced from Figs. 1A, 2, A and B. *P < 0.05 vs. baseline. #P < 0.05 between groups.
Autoregulatory behavior during IP5I inhibition of P2X1 receptors.
These studies were designed to test the hypothesis that P2X1 receptor blockade with IP5I would inhibit whole kidney autoregulatory behavior. Inhibition of P2X1 receptors with IP5I caused significant impairment in autoregulatory efficiency (Fig. 7, ○, baseline RBF 8.66 ± 0.20 ml·min−1·g kidney mass−1) compared with saline-infused rats. Noticeably, the autoregulatory profiles were more passive for animals infused with IP5I than for animals treated with either control or PPADS. An AI of 0.85 ± 0.19 was obtained for rats infused with IP5I, demonstrating greatly diminished autoregulatory capacity. Decreasing MAP from 120 to 100 mmHg in IP5I-infused rats resulted in a 14 ± 3% decrease in RBF, compared with 9 ± 1 and 0 ± 0% in PPADS- and saline-infused rats, respectively. After autoregulatory curves were completed, RBF responses to 0.5 μg α,β-methylene ATP and 0.25 μg CPA were conducted to confirm receptor blockade (Fig. 5, gray bars). The decrease in RBF in response to α,β-methylene ATP was abolished in IP5I-infused rats (0.0 ± 0.0 vs. 5.7 ± 0.6 ml/min in saline-infused rats). Renal vascular responsiveness to CPA was unchanged in IP5I-infused rats (2.9 ± 0.5 vs. 2.7 ± 0.2 ml/min in saline). Urinary flow rates averaged 17.8 ± 6.2 μl/min and were not statistically different from the other groups. These data suggest that P2X1 receptor activation is essential for the proper maintenance of whole kidney autoregulation of blood flow.
Fig. 7.
Percent change from baseline RBF in response to decreasing renal perfusion pressure with (○) and without (●) P2X1 receptor antagonism by IP5I (1 μmol·kg−1·min−1. For comparison, saline vehicle experiments (●) are reproduced from Fig. 4A. *P < 0.05 vs. baseline. #P < 0.05 between groups.
In the analysis presented above, AIs were calculated for the pressure range from 120 to 100 mmHg. When the AIs were calculated over the pressure range from 100 to 80 mmHg, there were no statistical differences between any of the groups by one-way ANOVA (0.509). The AI averaged 0.86 ± 0.12, 0.93 ± 0.24, and 1.04 ± 0.10 in PPADS, IP5I, and DPCPX, respectively. Additionally, the saline- and DMSO-infused rats exhibited reduced autoregulatory efficiency at renal perfusion pressures below 100 mmHg. The AI averaged 0.68 ± 0.18 and 0.64 ± 0.11 in saline- and DMSO-infused rats, respectively. Similar outcomes were reached if the AIs were calculated over the range from 100 to 90 mmHg.
DISCUSSION
The aim of the present study was to evaluate the role of P2X1 receptors in the autoregulation of whole kidney RBF in vivo. The results establish that inhibition of P2X1 receptor activation with either IP5I or PPADS significantly inhibits autoregulatory control of whole kidney RBF in vivo, whereas A1 receptor blockade has no detectable effect. This is consistent with findings of previous in vitro experiments using the blood-perfused juxtamedullary nephron preparation in rats and mice showing a loss of pressure-induced autoregulatory vasoconstriction at the single afferent arteriolar level (20, 22). The in vitro model, however, has some limitations. First, it is an in vitro preparation. It is always important to demonstrate that in vitro findings are also readily demonstrated in vivo. Second, the in vitro data are somewhat limited due to the fact that these studies only visualize juxtamedullary vascular segments located at the innermost surface of the cortex which may not reflect the behavior of the entire nephron population. The in vivo whole kidney setting used in the current report provides a more physiological condition for assessment of whole kidney RBF autoregulatory control which reflects the entire nephron population.
Both α,β-methylene ATP and CPA produce systemic effects on arterial pressure, heart rate, and respiratory rate when injected at doses exceeding 1 and 0.25 μg, respectively. Because we were interested specifically in the renal vascular effects, the treatment doses used in this study were optimized to minimize systemic effects. The first observed response to bolus intravenous injection of α,β-methylene ATP was a slight increase in arterial pressure. As the dose of α,β-methylene ATP was increased, a profound renal vasoconstriction was observed. The potency and relative selectivity of α,β-methylene ATP as a renal vasoconstrictor become apparent at higher doses. The highest dose of 1 μg resulted in a 62 ± 7% decrease in RBF with only a marginal 8 ± 2% increase in arterial pressure. This highlights the unique sensitivity of the renal circulation to P2X receptor activation and strengthens the contention that P2X1 receptors are strong regulators of renal hemodynamics.
Infusion of PPADS prevented α,β-methylene ATP-mediated vasoconstriction of the renal circulation and elevation in arterial pressure. The arterial pressure response to 1 μg α,β-methylene ATP following PPADS administration averaged <2 mmHg. PPADS abolished RBF responses to 1 μg α,β-methylene ATP, demonstrating the potent inhibitory effects of PPADS on P2X receptor activation.
In saline-infused rats, RBF remained constant within the arterial pressure range of 120 to 100 mmHg, consistent with properly maintained autoregulatory behavior. When pressure was reduced in rats infused with PPADS, a reduction in RBF occurred with each pressure reduction. Using the AI as a method for the quantification of autoregulatory behavior, we found a significant reduction in autoregulatory efficiency of 55%. These data illustrate the dominant role that P2X receptors play in autoregulatory control of RBF in vivo.
PPADS was originally considered a selective antagonist for P2X receptors (47); however, more recently, evidence suggests that PPADS blocks certain P2X and P2Y receptor subtypes (40). P2X1, P2X2, P2X3, and P2X5 receptors can be blocked by in vitro administration of PPADS (40). Additionally, P2Y1, P2Y2, P2Y4, and P2Y12 receptors are antagonized by PPADS administration in some vascular preparations (40). Confounding the interpretation of data generated using PPADS are reports that PPADS may also inhibit ecto-nucleotidase activity (7, 13). It could be argued that inhibition of ecto-nucleotidases prevents the catabolism of ATP to adenosine and therefore prevents activation of adenosine A1 receptors leading to loss of autoregulatory function. To investigate this possibility, we used DPCPX and IP5I to inhibit A1 and P2X1 receptors, respectively.
Previous studies indicate that the purine nucleoside adenosine plays an important role in the transmission of TGF (41). DPCPX is a potent and selective A1 receptor antagonist (6) that has been used extensively to characterize the renal vascular function of A1 receptors (28–30, 34, 48). Bolus administration of the A1 receptor agonist CPA reduced arterial pressure by ∼5 mmHg before any reductions in RBF. This drop in pressure coincided with a slight reduction in heart rate (data not shown). However, with increasing doses of CPA, the systemic response diminished while the renal vasoconstrictor response was enhanced. These data suggest a complex role for adenosine signaling in the regulation of arterial pressure. Infusion of DPCPX significantly reduced CPA-mediated renal vasoconstriction. Despite adequate pharmacological blockade of the A1 receptor, autoregulatory control of RBF was not affected by administration of DPCPX. This observation is supported by other studies showing a lack of prominent adenosine and A1 receptor involvement in in vivo autoregulation of RBF (18, 38) or steady-state RBF and glomerular filtration rate (GFR) (29).
Importantly, there are reports illustrating the involvement of A1 receptors in the regulation of GFR and RBF in vivo (5, 26, 34, 42, 44). While differences do exist in the conclusions of our study and others, there is still some qualitative agreement between the studies. Takenaka et al. (44) recently published a report that is most similar in design and intent to ours. In their study, Takenaka et al. conclude that “both adenosine and purinegic receptors contribute to glomerular autoregulation.” Our study is consistent with their finding that P2 receptors mediate autoregulatory behavior in vivo, and our study extends that work by strongly implicating P2X1 receptors as the primary receptor involved in autoregulatory resistance adjustments. However, there is some disagreement between the two studies regarding the role of adenosine A1 receptors in autoregulatory behavior; the previous report documents A1 receptor involvement, whereas our study does not find clear A1 receptor participation. There are several potential explanations for this difference. Our study was performed in Sprague-Dawley rats whose anesthetized blood pressure averaged ∼120 mmHg and pressure decrements were made in multiple 10-mmHg steps. In the Takenaka et al. study, Wistar-Kyoto rats, with anesthetized blood pressures of ∼103 mmHg, were used and renal perfusion pressure was reduced directly to ∼82 mmHg in one step. No data are provided to distinguish the plateau phase of a typical autoregulatory profile, the transition point, or the passive phase of the renal pressure flow relationship. Thus, there is a significant difference in the ambient blood pressure used to assess autoregulatory responses, and with just one pressure step, the actual transition point at which autoregulatory efficiency begins to decline cannot be determined.
In our hands, the transition point between efficient and less efficient autoregulation occurred at a renal perfusion pressure near 100 mmHg, whereas published reports indicate that the transition point for Wistar-Kyoto rats is ∼80 mmHg. This suggests that these two strains have different operational ranges and thus may yield qualitatively different results. Importantly, however, our data clearly show a strong, stable RBF over a pressure range of 120 to ∼100 mmHg and this was not influenced by A1 receptor blockade, indicating that A1 receptors are not involved in this range of whole kidney autoregulatory control.
In the more passive pressure/flow portion of the autoregulatory curves between 100 and 80 mmHg, there may be indications of qualitative agreement. In the saline and DMSO control groups, the AI averaged ∼0.6 suggesting retention of some degree of autoregulatory control. This fraction was eliminated in the presence of IP5I (AI = 0.93), PPADS (AI = 0.86), or DPCPX (AI = 1.04). There were no statistically significant differences between any of the AIs over this pressure range, but the autoregulatory curve for the DPCPX group tended to be slightly steeper over this pressure range compared with the saline and DMSO controls. Accordingly, A1 receptors may provide some modest influence on renal vascular resistance in this pressure range, which is consistent with the pressure range employed by Takenaka and co-workers. Thus, some qualitative agreement implicating A1 receptor involvement may exist between the two studies. Consistent with this possibility is a recent study suggesting that A1 and A2 receptors may interact to modulate autoregulatory efficiency (11).
To clarify the issue of P2X function, a more selective antagonist for P2X1 receptors, IP5I (IC50 of 3.1 nM for P2X1) (27), was used to evaluate the renal vascular effects of α,β-methylene ATP. IP5I was first used to inhibit ATP-mediated contraction of guinea pig vas deferens (17). Studies have since illustrated the potency and selectivity of IP5I for P2X1 over P2X2–4 receptors (27). We reasoned that if proper pharmacological inhibition of P2X1 and A1 receptors could be obtained, it would provide a useful paradigm for ascertaining the role of P2X1 receptor activation in the autoregulatory control of whole kidney blood flow in vivo.
When IP5I was infused, it resulted in significant inhibition of the α,β-methylene ATP-mediated increase in arterial pressure. Likewise, significant blockade of α,β-methylene ATP-induced renal vasoconstriction occurred following systemic administration of IP5I. Inhibition of P2X1 receptors caused substantial impairment in autoregulatory efficiency. Importantly, during blockade of P2X1 receptors with either PPADS or IP5I, CPA-mediated renal vasoconstrictions were unchanged, demonstrating normal function of A1 receptors.
In the rat, RBF is relatively constant at MAPs above 90 mmHg. Although the exact range is not fully characterized, the general consensus is that the lower limit of autoregulation is between 90 and 100 mmHg in Sprague-Dawley kidneys. Our work is consistent with this observation, showing that when MAP is reduced below 100 mmHg, a marked fall in RBF begins, the slope of which is fairly consistent between all experimental groups. A remarkable difference is observed when examining responses within the autoregulatory range in renal perfusion pressures greater than 100 mmHg. In vehicle-infused rats, a reduction of MAP from 120 to 100 mmHg resulted in no change in RBF, consistent with properly maintained autoregulatory behavior. In both PPADS- and IP5I-infused rats, however, RBF declined significantly during stepwise decreases in MAP across the same pressure range. The finding that inhibition of P2X1 receptors blocks autoregulatory adjustments in RBF is consistent with previous in vitro and in vivo work (32, 44). Using the blood-perfused juxtamedullary nephron preparation, inhibition of P2 receptors by administration of 500 μM suramin or 50 μM PPADS markedly attenuated pressure-mediated afferent arteriolar vasoconstrictor responses (22). Use of a more selective P2X1 antagonist NF279 (9) caused a marked reduction in afferent arteriolar autoregulatory behavior (20), further supporting the involvement of P2X1 receptors in the proper maintenance of RBF.
A useful measure of autoregulatory efficiency is the determination of the AI. The average AI value of 0.00 ± 0.08 for saline-infused rats suggested properly maintained autoregulation. When rats were infused with PPADS, the calculated AI of 0.55 ± 0.07 suggested that ∼55% of the RBF change was directly dependent on MAP. When the AI was calculated for rats infused with the more potent P2X1 receptor inhibitor IP5I, a value of 0.85 ± 0.19 illustrated a markedly increased dependence on P2X1 receptors to affect autoregulatory adjustments in renal vascular resistance. Taken together with data from previous in vitro experiments (22), these data strongly support a role for P2X1 receptors in proper autoregulatory control of RBF.
Recent work examining dinucleoside polyphosphate-induced renal vasoconstriction suggests that ATP is not the only endogenous ligand for the P2X1 receptor. Notable candidates are Ap4A and Up4A, both of which decrease GFR at the level of the afferent arteriole (24, 25). Additional studies need to be conducted to determine whether either Ap4A or Up4A mediates pressure-induced vasoconstriction. Nevertheless, it is possible that dinucleoside polyphosphates could mediate autoregulation through activation of the P2X1 receptor.
Based on previous studies, the strongest argument can be made that P2X1 receptor activation mediates TGF responses (21), but the role of P2X1 receptors in the myogenic response is less clear. The main difference between the two mechanisms is the source of nucleotide release and the region of the afferent arteriole affected. Based on work by Peti-Peterdi (36), we postulate that TGF functions at the most terminal region of the afferent arteriole, very close to the glomerulus. The myogenic response contributes the majority of the autoregulatory resistance adjustments that traditionally have been attributed to the mid and early portions of the afferent arteriole and to a lesser degree the upstream arterial segments (33). The sources of ATP release for the myogenic and TGF mechanisms are thought to be the vascular smooth muscle and the macula densa, respectively (2, 39). Consistently, ATP mediates increases in afferent arteriolar resistance in response to increases in perfusion pressure or tubular NaCl delivery by activating P2X1 receptors. In vitro evidence argues that P2X1 receptors are involved in both responses. The whole kidney data presented in the current manuscript support the hypothesis that P2X1 receptors play an important role in renal autoregulatory resistance adjustments as a whole.
The importance of these data is derived from the critical role of autoregulation for preservation of glomerular capillary pressure, structure, and function. Transmission of elevated systemic arterial pressures into the glomerulus is a causative factor in the development of glomerular injury (4, 12). A recent review by Bidani et al. (3) discusses the importance of the myogenic response in the prevention of hypertensive renal injury. In situations where the myogenic response is impaired and there are fluctuations in arterial pressure, such as occurs even during mild hypertension, there is the possibility of developing glomerular structural injury. If structural injury progresses unhindered, the resultant loss of renal filtering ability could result in the exacerbation of hypertension and the development of end-organ damage. Given the recent finding that 29% of all adults in the United States are hypertensive and an additional 28% of the adult population are prehypertensive (35), there is sufficient reason to be concerned about preservation of autoregulatory behavior.
In summary, the present study extends previous in vitro findings by establishing that in vivo autoregulation of RBF is dependent on activation of renal vascular P2X1 receptors. Pharmacological blockade of P2X1 receptors results in marked inhibition of whole kidney autoregulatory behavior. Due to the dominant role of the kidney in the long-term maintenance of arterial pressure, loss of autoregulatory control has the potential to exacerbate hypertension and end-organ damage. These data suggest that a loss of P2X1 receptor signaling may contribute to some forms of renal injury.
GRANTS
D. A. Osmond is supported by Predoctoral Fellowship 0815156E from the Southeast Affiliate of the American Heart Association and the Cardiovascular Biology Training Grant NHLBI-00070 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH). E. W. Inscho is supported by DK-044628 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Akbar M, Okajima F, Tomura H, Shimegi S, Kondo Y. A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol Pharmacol 45: 1036–1042, 1994 [PubMed] [Google Scholar]
- 2. Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol 65: 481–500, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidani AK, Mitchell KD, Schwartz MM, Navar LG, Lewis EJ. Absence of glomerular injury or nephron loss in a normotensive rat remnant kidney model. Kidney Int 38: 28–38, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol 335: 59–63, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Chen BC, Lee CM, Lin WW. Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br J Pharmacol 119: 1628–1634, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, Rettinger J, Mutschler E, Lambrecht G. NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. Eur J Pharmacol 350: R5–R6, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Eppel GA, Ventura S, Evans RG. Regional vascular responses to ATP and ATP analogs in the rabbit kidney in vivo: roles for adenosine receptors and prostanoids. Br J Pharmacol 149: 523–531, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng MG, Navar LG. Adenosine A2 receptor activation attenuates afferent arteriolar autoregulation during adenosine receptor saturation in rats. Hypertension 50: 744–749, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Griffin KA, Picken M, Bakris GL, Bidani AK. Comparative effects of selective T- and L-type calcium channel blockers in the remnant kidney model. Hypertension 37: 1268–1272, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Grobben B, Claes P, Roymans D, Esmans EL, Van Onckelen H, Slegers H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br J Pharmacol 130: 139–145, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med (Maywood) 232: 715–726, 2007 [PubMed] [Google Scholar]
- 15. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol 290: F888–F891, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hoyle CH, Pintor J, Gualix J, Miras-Portugal MT. Antagonism of P2X receptors in guinea pig vas deferens by diinosine pentaphosphate. Eur J Pharmacol 333: R1–R2, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Ibarrola AM, Inscho EW, Vari RC, Navar LG. Influence of adenosine receptor blockade on renal function and renal autoregulation. J Am Soc Nephrol 2: 991–999, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol 280: F927–F944, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1077–F1085, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Inscho EW, LeBlanc EA, Pham BT, White SM, Imig JD. Purinoceptor-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension 33: 195–200, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Jankowski M, Angielski S, Szczepanska-Konkel M. Dissociation between the effects of P1, P4-diadenosine tetraphosphate (Ap4A) on renal haemodynamics and tubular function in anaesthetized rats. J Physiol Pharmacol 59: 129–137, 2008 [PubMed] [Google Scholar]
- 25. Jankowski V, Patzak A, Herget-Rosenthal S, Tran TN, Lai EY, Gunthner T, Buschmann I, Zidek W, Jankowski J. Uridine adenosine tetraphosphate acts as an autocrine hormone affecting glomerular filtration rate. J Mol Med 86: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489–F1500, 2007 [DOI] [PubMed] [Google Scholar]
- 27. King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol 128: 981–988, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knight RJ, Bowmer CJ, Yates MS. The diuretic action of 8-cyclopentyl-1,3-dipropylxanthine, a selective A1 adenosine receptor antagonist. Br J Pharmacol 109: 271–277, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuan CJ, Herzer WA, Jackson EK. Cardiovascular and renal effects of blocking A1 adenosine receptors. J Cardiovasc Pharmacol 21: 822–828, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Kuan CJ, Herzer WA, Jackson EK. An experimental paradigm for investigating the role of endogenous adenosine/A1 receptor interactions in vivo. J Pharmacol Exp Ther 263: 657–662, 1992 [PubMed] [Google Scholar]
- 31. Majid DS, Inscho EW, Navar LG. P2 purinoceptor saturation by adenosine triphosphate impairs renal autoregulation in dogs. J Am Soc Nephrol 10: 492–498, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Mitchell KD, Navar LG. Modulation of tubuloglomerular feedback responsiveness by extracellular ATP. Am J Physiol Renal Fluid Electrolyte Physiol 264: F458–F466, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Moore LC, Casellas D. Tubuloglomerular feedback dependence of autoregulation in rat juxtamedullary afferent arterioles. Kidney Int 37: 1402–1408, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Munger KA, Jackson EK. Effects of selective A1 receptor blockade on glomerular hemodynamics: involvement of renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol 267: F783–F790, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control–continued disparities in adults: United States, 2005–2006. NCHS Data Brief 3: 1–8, 2008. [PubMed] [Google Scholar]
- 36. Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol 288: F1079–F1083, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Pollock DM, Arendshorst WJ. Native tubular fluid attenuates ANF-induced inhibition of tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol 258: F189–F198, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Premen AJ, Hall JE, Mizelle HL, Cornell JE. Maintenance of renal autoregulation during infusion of aminophylline or adenosine. Am J Physiol Renal Fluid Electrolyte Physiol 248: F366–F373, 1985 [DOI] [PubMed] [Google Scholar]
- 39. Prosdocimo DA, Douglas DC, Romani AM, O'Neill WC, Dubyak GR. Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells. Am J Physiol Cell Physiol 296: C828–C839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 41. Schnermann J, Weihprecht H, Briggs JP. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol Renal Fluid Electrolyte Physiol 258: F553–F561, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 267: F879–F887, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol 294: R1–R11, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294: R1769–R1776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. White SM, Imig JD, Kim TT, Hauschild BC, Inscho EW. Calcium signaling pathways utilized by P2X receptors in freshly isolated preglomerular MVSMC. Am J Physiol Renal Physiol 280: F1054–F1061, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Ziganshin AU, Hoyle CH, Bo X, Lambrecht G, Mutschler E, Baumert HG, Burnstock G. PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br J Pharmacol 110: 1491–1495, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol 276: R790–R798, 1999 [DOI] [PubMed] [Google Scholar]