Abstract
Large-conductance voltage- and calcium-activated potassium (BK) channels have been shown to play a role in detrusor overactivity (DO). The goal of this study was to determine whether bladder outlet obstruction-induced DO is associated with downregulation of BK channels and whether BK channels affect myosin light chain 20 (MLC20) phosphorylation in detrusor smooth muscle (DSM). Partial bladder outlet obstruction (PBOO) was surgically induced in male New Zealand White rabbits. The rabbit PBOO model shows decreased voided volumes and increased voiding frequency. DSM from PBOO rabbits also show enhanced spontaneous contractions compared with control. Both BK channel α- and β-subunits were significantly decreased in DSM from PBOO rabbits. Immunostaining shows BKβ mainly expressed in DSM, and its expression is much less in PBOO DSM compared with control DSM. Furthermore, a translational study was performed to see whether the finding discovered in the animal model can be translated to human patients. The urodynamic study demonstrates several overactive DSM contractions during the urine-filling stage in benign prostatic hyperplasia (BPH) patients with DO, while DSM is very quiet in BPH patients without DO. DSM biopsies revealed significantly less BK channel expression at both mRNA and protein levels. The degree of downregulation of the BK β-subunit was greater than that of the BK α-subunit, and the downregulation of BK was only associated with DO, not BPH. Finally, the small interference (si) RNA-mediated downregulation of the BK β-subunit was employed to study the effect of BK depletion on MLC20 phosphorylation. siRNA-mediated BK channel reduction was associated with an increased MLC20 phosphorylation level in cultured DSM cells. In summary, PBOO-induced DO is associated with downregulation of BK channel expression in the rabbit model, and this finding can be translated to human BPH patients with DO. Furthermore, downregulation of the BK channel may contribute to DO by increasing the basal level of MLC20 phosphorylation.
Keywords: benign prostatic hyperplasia, MLC phosphorylation
benign prostatic hyperplasia (BPH) with associated bladder outlet obstruction is a common urological condition in men that usually arises after the age of 40 years old. The enlarged prostate compresses the urethra causing partial bladder outlet obstruction (PBOO) (6, 24). In response to this PBOO, the detrusor smooth muscle (DSM) remodels in an effort to increase its force generating capability. A key aspect of this remodeling process is a 2- to 10-fold increase in the bladder mass, which is mediated by the hypertrophy of DSM cells (5). Surgically induced PBOO in rabbits has been used as an animal model of obstruction-induced detrusor remodeling. The rabbit model of PBOO reveals bladder dysfunction and alterations in DSM contractility, similar to those that occur in men with BPH (15). Previous studies show that changes in the expression and activity of DSM myosin and regulatory proteins may contribute to this altered detrusor contractile phenotype (4, 8, 23).
The large-conductance calcium-activated potassium channel (BK) in smooth muscle cells consists of a pore-forming α-subunit and an accessory β1-subunit (9, 19). The BK channel is thought to play a significant role in the regulation of smooth muscle contraction. BK channels work as negative feedback regulators to limit smooth muscle contraction by regulating cytosolic Ca2+ levels (12, 13), which is required for the generation of force in smooth muscle (7). BK channel knockout mice show enhanced detrusor contractility, increased bladder pressure, and urine leakage, suggesting that the absence of BK channel function leads to an overactive bladder (also known as detrusor overactivity; DO) and subsequent urinary incontinence (27). Another recent study shows that PBOO induces DO in rats, and this overactivity is associated with a decrease in the expression and activity of the BK channel (17). PBOO also alters the level of phosphorylated myosin light chain (MLC20), a prerequisite for force generation (21, 26). The basal level of MLC20 phosphorylation in DSM is high in obstructed dysfunctional bladders (26).
The goal of this study was to determine whether PBOO alters expression of BK channel protein and how this alteration contributes to DSM contractile dysfunction. We show that decreased expression of the BK channel is accompanied by an enhanced spontaneous contraction in rabbit bladders 2 wk after the surgical induction of PBOO. Additionally, we show that downregulation of the BK channel is associated with DO in human BPH patients. Furthermore, we determined that small interfering (si) RNA-mediated reduction of BK channel expression affects MLC20 phosphorylation, which is a key regulator of smooth muscle contraction (22). Thus these data suggest that BK channel downregulation affects the basal level of MLC20 phosphorylation and contributes to DO.
MATERIALS AND METHODS
PBOO.
All animal experimental protocols were approved by the University of Pennsylvania Institutional Animal Care Use Committee. The surgical procedure to create PBOO has been previously described (25, 28). Briefly, adult male New Zealand White rabbits weighing ∼3 kg were anesthetized with ketamine/xylazine (25 mg/10 mg im). Surgical anesthesia was maintained with pentobarbital sodium (25 mg/kg iv). Under sterile conditions, the urinary bladder was catheterized with an 8F Foley catheter, and the bladder was exposed through a midline incision. PBOO was induced by placing a 0-silk ligature around the catheterized urethra. The catheter was removed, and the incision was closed with 3-0 silk (25). After PBOO, rabbits were maintained for 2 wk and then killed for bladder tissue collection. One day before death, rabbits were placed in metabolic cages (Kent Scientific, Torrington, CT) with free access to food and water to monitor their voiding patterns. The frequency was determined as the number of voids per 24 h. The average voided volume is expressed as milliliter per void.
Bladder samples of human patients.
After obtaining Institutional Review Board approval (IRB 803645), we received frozen bladder tissue samples from Dr. C. M. Gomes of the University of São Paulo, São Paulo, Brazil (collected through an IRB protocol 811/04 at the University of São Paulo). The tissue samples were identified by age and disease only. Bladder tissue biopsies were collected from BPH patients who underwent preoperative urodynamic studies to evaluate their bladder function and the severity of obstruction. All BPH patients underwent preoperative multichannel urodynamics, including filling cystometry and pressure-flow voiding studies. Two 4F urodynamic catheters were transurethrally inserted into the bladder. Saline at room temperature was instilled through one of the catheters at a filling rate of 30 ml/min. Intravesical pressure was measured through the other catheter. The intra-abdominal pressure was obtained with a rectal balloon catheter. The detrusor pressure was electronically calculated. DO was defined as any involuntary detrusor contraction during the filling phase. The filling catheter was removed at the cystometric capacity, immediately before the voiding phase, and the patients were asked to void for the pressure/flow study. Bladder outlet obstruction was assessed using the bladder outlet obstruction index (BOOI). Patients were characterized as being obstructed when the BOOI >40 (1). All definitions conform to the standardized terminology of the International Continence Society (2, 11). BPH patients were divided into two groups: with or without detrusor overactivity. Control bladder samples were acquired from patients undergoing ureteral reimplantation and from nondiseased bladder tissue from bladder cancer patients undergoing cystectomy. Age-matched controls were used to compare with the BPH group.
Organ bath studies of DSM spontaneous contractions.
Strips of DSM from rabbits (∼50 mg) were longitudinally suspended in 15 ml of Tyrode buffer equilibrated with 95% O2-5% CO2 at 37°C. One end of each strip was connected to a glass rod at the bottom of the organ chamber while the other end was attached to a force displacement transducer connected to a Grass Model 7D Polygraph (Grass Instruments, Quincy, MA). After a 1-h equilibration, the length of optimal force development (Lo) was determined by increasing the length of each strip by 1.5-mm increments until a maximal contractile force to electrical field stimulation (80 V, 1 ms) was achieved. The muscle was allowed to equilibrate at Lo in Tyrode buffer for 30 min to allow stabilization at the resting level. The spontaneous contractions were recorded. A muscle strip contraction profile was measured by 125 mM KCl stimulation. The effect of BK channel opener NS1619 or isopimaric acid (Sigma) on detrusor muscle strip overactivity were also measured. We used 50 μM NS1619 or 50 μM isopiramic acid to inhibit spontaneous contraction, and muscle strips without treatment served as controls. Once recording was over, all muscle strips were snap frozen for two-dimensional gel electrophoresis.
RNA isolation and real-time PCR.
RNA was extracted from frozen bladder tissue using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Briefly, pieces of tissue (∼50 mg) were crushed to a fine powder using a liquid nitrogen-prechilled mortar and pestle. The specimens were mixed with 1 ml TRIzol reagent and then homogenized using a mini-electrical homogenizer. After a 5-min incubation at room temperature, 0.2 ml of chloroform was added, and the sample was vigorously vortexed to separate the phases. After 15 min of centrifugation at 12,000 g, 0.5 ml of isopropyl alcohol was added to the aqueous phase to precipitate the RNA. The RNA quality and quantity were measured by UV spectrophotometry: 1 μg of total RNA was incubated with 1 μl of oligo (dT)15 (Promega, Madison, WI) for 10 min at 70°C. After slow cooling, Moloney murine leukemia virus reverse transcriptase (Invitrogen) was added to synthesize cDNA in a 37°C water bath for 1 h. A TaqMan gene expression assay was used to determine the expression level of the gene using the ABI 7500 Fast Real-Time PCR System and Sequence Detection Software (Applied Biosystems, Foster City, CA). The comparative Ct method was used for quantification of transcripts, according to the manufacturer's protocol. Measurements were performed in triplicate. Assay ID Hs01119498_m1 was used to measure BKα; assay ID Hs00266938_m1 was used to measure BKβ; and human HRPT1 was used as an internal control.
Protein extraction and Western blot analysis.
Total protein was extracted from frozen bladder tissue that was free of mucosa and serosa (∼50 mg) using 1 ml of extraction buffer, which contained 50 mM Tris (pH 6.8), 20% glycerol, 1% SDS, and a protease inhibitors cocktail (Sigma, St. Louis, MO). The same extraction buffer was used to prepare protein extract from cultured human DSM cells. The protein concentration was determined using a DC protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (20 μg) were separated on 4–15% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane overnight at 30 V. After blocking in 5% fat-free milk in PBS for 1 h, the membranes were incubated overnight at 4°C with primary antibodies, including mouse anti-BKα (dilution 1:1,500; BD Biosciences, San Jose, CA); goat anti-BKβ (dilution 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-smooth muscle myosin heavy chain (dilution 1:10,000; Sigma); and mouse anti-α-actin (dilution 1:5,000; Sigma). The membrane was washed and incubated with secondary horseradish peroxidase-linked anti-mouse antibody (dilution 1:5,000) or anti-goat antibody (dilution 1:5,000) for 1 h at room temperature. Membranes were thoroughly washed with PBS containing 0.05% Tween 20 between incubations. Target proteins were detected using an enhanced chemiluminescence kit (Amersham Life Sciences, Arlington Heights, IL), followed by scanning on a Fujifilm Image Reader LAS-3000 (Fujifilm, Valhalla, NY). The amount of protein was determined by Optical Density scanning using Multi Gauge V 3.0 software (Fujifilm). Smooth muscle α-actin was used as an internal control to normalize the Western blot data.
Immunostaining.
Cross sections (5 μM) of the bladder wall (body) from rabbits that had undergone sham operations and PBOO were made from paraffin blocks. Tissue sections were placed in xylene and descending concentrations of alcohol (100, 95, 70, and 30%, and 1× PBS) to remove the paraffin and rehydrate the samples. After 30 min of blocking in a 1% BSA solution, the sections were incubated with BKβ antibody (described above) at 1:200 overnight at 4°C. Sections were washed three times in PBS and incubated with secondary anti-goat Cy3 antibody at 1:400 for 1 h. Sections were washed again three times with PBS and mounted onto slides with a drop of mounting medium containing 4,6-diamidino-2-phenylindole to stain the nuclei (Vector Laboratory, Burlington, ON). Sections were viewed under a Confocal Laser Scanning Biological Microscope (Olympus, Center Valley, PA). Images were captured using Fluoview FV1000 software. A negative control to confirm the specificity of the immunostaining was prepared using nonimmune goat serum in place of the primary antibody.
Transfection of human DSM cells with BKβ siRNA.
Primary cultures of human DSM cells were made from surgical samples and subcultured for 20 generations. These cells continued to express the smooth muscle phenotype and BK channel proteins. The cells were transfected with BKβ siRNA (Santa Cruz Biotechnology), according to the manufacturer's protocol. Briefly, DSM cells were plated to obtain 70% confluency in six-well plates and transfected with 200 nM of BK siRNA or control siRNA using Lipofectamine 2000 (Invitrogen,). After transfection for 12 h, fresh medium with 10% FBS was added to the plates. Cells were kept for 48 more hours and then harvested for protein preparation. The detrusor cells transfected with control siRNA were used as a negative control.
Two-dimensional gel electrophoresis.
Snap-frozen muscle strips or cultured cells were homogenized with isoelectric focusing (IEF) sample buffer [50 μl/10 mg tissue or 5 × 106 cells containing 9.5 M urea, 1.6% ampholyte (pH 5–7), 0.4% ampholyte (pH 3–10), 2% NP-40, and 5% -mercaptoethanol]. After centrifugation, ∼50 μl of the supernatant was applied to IEF cylindrical gels (1 × 65 mm), and IEF was performed at 350 V overnight. The IEF-separated proteins were subjected to SDS-PAGE (14%) and stained with Coomassie blue (14). Spots corresponding to the phosphorylated and unphosphorylated forms of MLC20 were scanned and analyzed using Bio-Rad GS-800 Calibrated Densitometry (Bio-Rad).
Statistical analysis.
All data are expressed as means ± SE. Student's t-test was used to compare two groups, and a one-way ANOVA was used to compare three groups. A P <0.05 was considered to be statistically significant. Each n refers to a set of rabbits (normal and obstructed rabbits with DO) or human patients (bladder cancer control, BPH with and without DO).
RESULTS
PBOO-induced DO in rabbits.
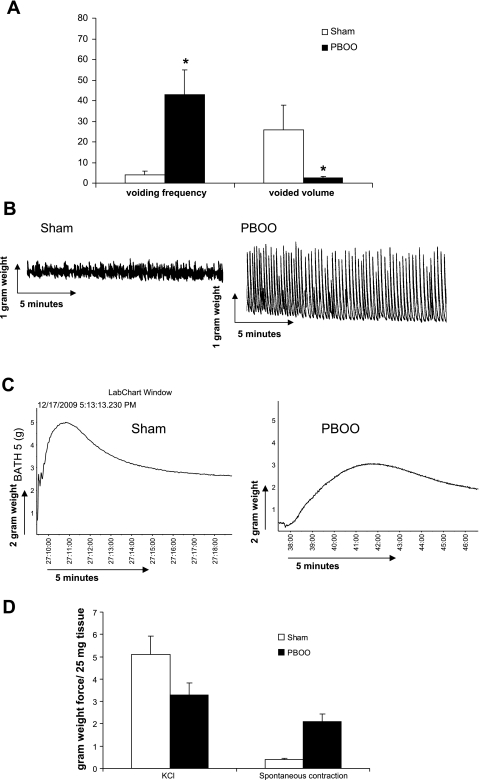
A PBOO rabbit model was used to study the effect of bladder outlet obstruction on bladder function and DSM contractility. We found that 2-wk PBOO caused significant detrusor hypertrophy. The average of bladder mass was increased from 2.1 ± 0.4 (sham) to 12.5 ± 3.3 g (PBOO). The voiding pattern of the rabbits also significantly changed following 2 wk of PBOO (Fig. 1A). For control rabbits, the average voiding frequency was 4 ± 3 times/day, and the average voided volume was 26 ± 16 ml. However, the voiding frequency is increased to 43 ± 13 voids/day after 2 wk of PBOO, and the volume per void is reduced to 2.5 ± 1.0 ml. In addition to the altered voiding pattern, DSM contractility was changed by PBOO. Muscle strips maintained in an organ bath showed a high degree of spontaneous contraction in the PBOO group, indicating DSM overactivity (Fig. 1B). PBOO also caused slow force generation in response to KCl stimulation and produced less maximal force (Fig. 1C). Figure 1D shows the analyzed data of PBOO induced force changes. The average amplitude of spontaneous contraction was about fourfold higher for the PBOO group (2.1 ± 0.4 g weight) compared with the sham control group (0.48 ± 0.11 g weight). The maximal force of KCl stimulation was decreased from 5.1 ± 0.83 g weight (sham control) to 3.3 ± 0.56 g weight (PBOO).
Fig. 1.
Partial bladder outlet obstruction (PBOO)-induced alteration in the voiding pattern and detrusor smooth muscle spontaneous contraction. A: analyzed data for urination frequency and void volume in sham and PBOO groups. B: representative organ bath recording (n = 5) of spontaneous contraction of detrusor muscle strips from sham and PBOO groups. Five small vertical boxes on the chart equal a 2-g force, and 5 small horizontal boxes equal 10 min. The amplitude of spontaneous contractions is ∼4-fold higher in the PBOO group than that for the sham control. C: representative recording of detrusor muscle strips force generation profile in response to 125 mM KCl. D: analyzed contractile data of detrusor muscle strips. Values are means ± SE. *Statistical significance, P < 0.05; n = 5.
Downregulation of BK channel protein expression in PBOO rabbit model.
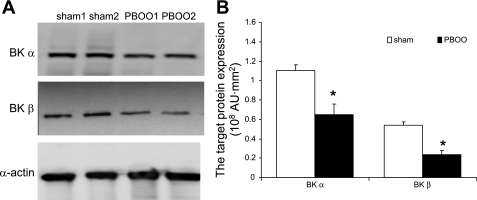
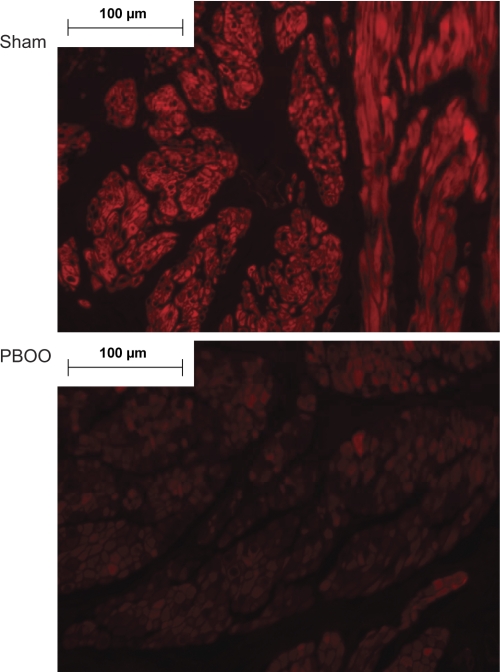
BK channel knockout mice showed increased urination frequency and enhanced detrusor contractility, similar to the detrusor isolated from PBOO rabbits. Therefore, we measured the effect of PBOO on the expression of the BK channel protein in our model system. We found that the expression of both BK α- and β-subunits was significantly downregulated by PBOO. Figure 2A is a representative Western blot of the BK α- and β-subunits, as well as α-actin, which served as an internal control. After arbitrarily setting the expression of the BK channel in the sham group to 100%, we analyzed Western blotting results from six animals from each group (Fig. 2B). In bladders from the PBOO group, the expression of BKα was reduced to 55 ± 14% and BKβ to 42 ± 11%. Moreover, immunostaining for BKβ was remarkably weak in sections from the PBOO group, confirming the downregulation of BKβ (Fig. 3). In addition, immunostaining showed that BKβ is localized to DSM cells in the bladder.
Fig. 2.
Downregulation of large-conductance calcium-activated potassium (BK) channel (α- and β-subunits) expression in bladders from rabbits with PBOO. A: representative Western blots for BKα, BKβ, and α-actin (used as an internal control). B: analyzed Western blot data with the quantified expression levels measured by the FUJIFILM Image Reader. Both BK α- and β-subunits are significantly downregulated in bladders from rabbits 2 wk after surgical induction of PBOO. Values are means ± SE. *Statistical significance, P < 0.05; n = 6.
Fig. 3.
Confocal images of BKβ immunostaining in paraffin sections of bladders from rabbits after sham surgery or PBOO. Red fluorescent signal (Cy3) was used to detect BKβ, and the signal is much stronger in the sham sample than in the PBOO sample. Confocal images also show that BKβ is localized to smooth muscle cells. Nuclei are stained with by 4,6-diamidino-2-phenylindole (blue). Representative images are shown; n = 3.
Effect of BK opener on PBOO-induced detrusor muscle strip spontaneous contraction and MLC20 phosphorylation.
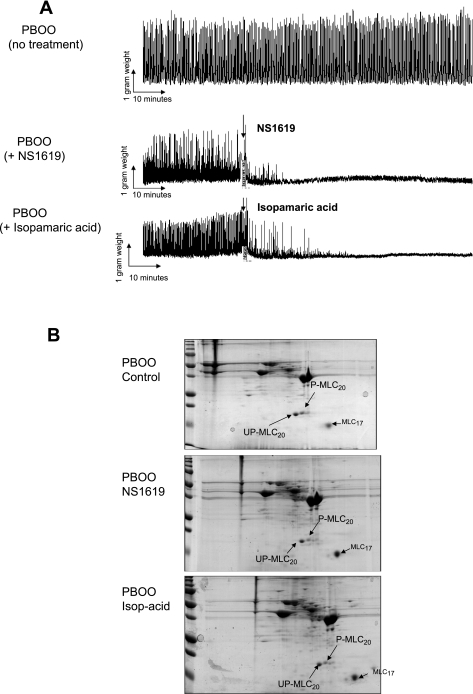
Taken together, results from Figs. 1–3 demonstrate that PBOO cause enhanced spontaneous contraction in detrusor muscle strips and decreased BK channel protein expression. Therefore, the next question is whether this increased detrusor spontaneous contraction is related to changes in the BK channel. Two specific BK channel openers, NS 1619 and isoparamic acid, were used to test whether they can reduce spontaneous contraction in the PBOO bladders. Figure 4A showed both frequency and amplitude of spontaneous contraction were reduced after addition of NS1619 and isoparamic acid, respectively. These BK openers need about 1–2 h to fully inhibit spontaneous contractions, and this inhibition can last several hours. Meanwhile, there was no change in spontaneous contractions in the control group. Figure 4B showed the effect of the BK opener on MLC20 phosphorylation. Both NS1619 and isoparamic acid reduced the phosphorylation level of MLC20. The phosphorylated MLC20 spot was bigger in the control group than the N1619- or isoparamic acid-treated sample.
Fig. 4.
Effect of BK opener on PBOO-induced spontaneous contraction and myosin light chain 20 (MLC20) phosphorylation. A: set of representative recordings of detrusor muscle strips after addition of BK opener NS1619 or isoparamic acid. Top tracing is no treatment as a control, middle tracing is after treatment with NS1619, and bottom tracing is after treatment with isoparamic acid. B: set of representative 2-dimensional gels for control and BK opener-treated muscle strips. Top gel is no treatment, middle tracing is after treatment with NS1619, and bottom tracing is after treatment with isoparamic acid. UP-MLC20, unphosphorylated MLC20; P-MLC20, phosphorylated MLC20.
Downregulation of BK channels in BPH patients with DO.
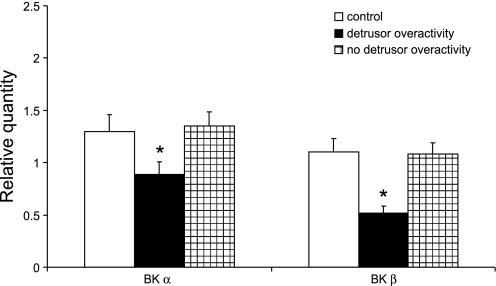
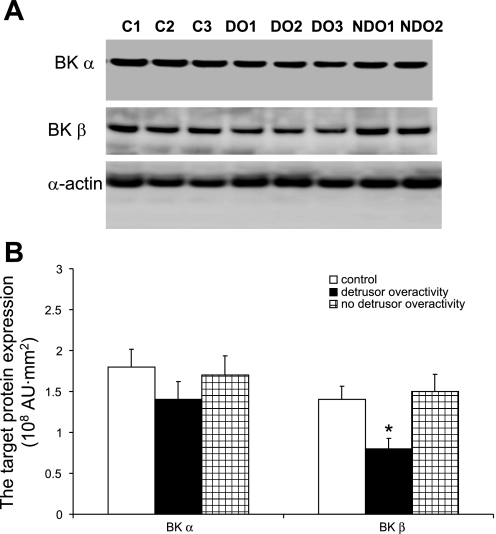
Since the rabbit model shows that PBOO-induced detrusor spontaneous contractions are associated with downregulation of the BK channel subunits, we assessed whether similar changes would be observed in men with BPH-induced DO. Table 1 provides the medical background and urodynamic findings for all 15 patients that participated in this study. Normal control patients included four men who underwent a radical cystectomy for treatment of bladder cancer (cancer-free tissue was used) and a man who underwent ureteral reimplantation and exhibited no pathological changes in his bladder. Five BPH patients had bladder outlet obstruction with DO, and the other five BPH patients had bladder outlet obstruction without DO. Supplemental Figs. A and B are representative urodynamic tracings to analyze bladder function in the presence and absence of DO (all supplementary material for this article are available on the journal web site). Based on urodynamic analysis, BPH patients did not differ in terms of the severity of their obstruction. There was no difference in the BOOI; DO patients have an average BOOI of 104.3 ± 41.7, and patients without DO have an average BOOI of 83.8 ± 28.9 (P = 0.415). Control patients were either asymptomatic or had mild voiding symptoms; however, no urodynamic data are available for this group to confirm this. The age of each group of patients is well matched. Real-time PCR and Western blot analyses were used to determine the expression of both BK α- and β-subunits in each group. Figure 5 shows a significant reduction in BK mRNA expression in those BPH patients with DO, and the fold-decrease in expression of the β-subunit is greater than that observed for the α-subunit. Figure 6 shows a significant reduction in BKβ protein expression in the group with BPH and DO. The protein expression of BKα also shows a trend toward a decrease, but it is not significant, which may be due to the small sample size.
Table 1.
Medical information about patients
| Patient | Pathology | Age, yr | Bladder Outlet Obstruction Index | Detrusor Overactivity |
|---|---|---|---|---|
| 1 | BPH | 76 | 161 | Yes |
| 2 | BPH | 82 | 60 | Yes |
| 3 | BPH | 60 | 84 | Yes |
| 4 | BPH | 77 | 80 | Yes |
| 5 | BPH | 66 | 132 | Yes |
| 6 | BPH | 72 | 126 | No |
| 7 | BPH | 80 | 89 | No |
| 8 | BPH | 61 | 74 | No |
| 9 | BPH | 77 | 84 | No |
| 10 | BPH | 73 | 46 | No |
| 11 | N (C) | 77 | Nonobstructed | No |
| 12 | N (U) | 60 | Nonobstructed | No |
| 13 | N (C) | 68 | Nonobstructed | No |
| 14 | N (C) | 75 | Nonobstructed | No |
| 15 | N (C) | 77 | Nonobstructed | No |
BPH, benign prostatic hyperplasia; N (C), normal region from cystectomy for bladder cancer; N (U), normal bladder tissue from ureteral reimplantation.
Fig. 5.
BK channel (α- and β-subunits) mRNA expression in bladders from benign prostatic hyperplasia (BPH) patients with and without detrusor overactivity (DO). Quantitative real-time PCR was performed on RNA isolated from bladder tissues from bladder cancer patients and BPH patients with and without DO. BK mRNA levels are expressed as relative quantity units. Values are means ± SE. *Statistical significance, P < 0.05; n = 5.
Fig. 6.
Western blot analysis of BK channel (α- and β-subunits) expression in bladders from BPH patients with and without DO. A: representative Western blots for BKα, BKβ, and α-actin (used as an internal control). B: analyzed Western blot data, with the expression levels measured by the FUJIFILM Image Reader. Values are means ± SE. *Statistical significance, P < 0.05; n = 3.
BKβ and MLC20 phosphorylation.
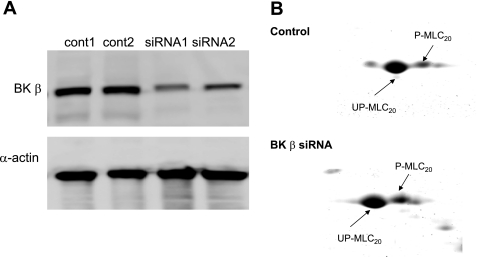
We showed that DO coincides with the downregulation of the BK channel in both PBOO-induced rabbits and BPH patients with DO. This result suggests that BK channel expression affects detrusor contractility. Furthermore, it is well established that spontaneous contraction of the detrusor is myogenic (10, 16). Based on the previous finding that the basal level of MLC20 phosphorylation is high in the DSM of obstructed bladders (26), we hypothesized that the BK channel may contribute to MLC20 phosphorylation. We tested the effect of downregulating BKβ on MLC20 phosphorylation using siRNA-mediated silencing of BK channel expression in cultured human DSM cells. This primary culture of human DSM cells still remains most of the smooth muscle phenotype. Hence, it is a simple model that we can use to test our hypothesis. Western blotting for BKβ and α-actin showed that transfection of BKβ siRNA resulted in an 80% reduction in BKβ protein expression in cultured human DSM cells (Fig. 7A). MLC20 phosphorylation is a key regulator of smooth muscle contraction. The level of MLC20 phosphorylation can be determined by calculating the ratio of the phosphorylated MLC20 to the total MLC20. In this study, two-dimensional gel electrophoresis was used to measure the levels of MLC20 phosphorylation in response to siRNA-mediated reduction of BKβ expression (Fig. 7B). We determined that the level of phosphorylated MLC20 was greater in cells transfected with BKβ siRNA. Since a high level of MLC20 phosphorylation favors muscle contraction, we conclude that depletion of BKβ contributes to enhanced muscle contraction by increasing the amount of MLC20 phosphorylation in DSM cells.
Fig. 7.
Increased MLC20 phosphorylation in cultured human DSM cells by small interference (si) RNA-mediated downregulation of BKβ. A: Western blot for BKβ, smooth muscle-specific myosin (marker for smooth muscle), and α-actin (internal control) to demonstrate the efficiency of BKβ siRNA. B: representative 2-dimensional gel for control and siRNA-treated cells.
DISCUSSION
BPH causes bladder outlet obstruction in men, and it is often associated with DO. (3) The rabbit model for PBOO, despite its acute occurrence, shows increased voiding frequency and decreased voided volume based on a metabolic cage study (Fig. 1A). These bladder functional changes are accompanied by detrusor hypertrophy (28) and enhanced spontaneous contraction of DSM in (Fig. 1B) in many cases. These pathological changes in PBOO rabbits are very similar to that seen in human BPH patients. BK channels are thought to play an important role in the overactivity of DSM in mice. Both the BK channel α-subunit and β-subunit contribute to DSM contraction. The deletion of BK α-subunit increased DSM contraction and urination frequency (18, 27). In vivo cystometry also showed higher bladder pressure and urine dripping in BK knockout mice (18, 27). In the absence of the BK channel β-subunit, mouse DSM also exhibited greater contraction (19).
In view of DO in BK channel knockout mice and a recent study showing downregulation of BKα in PBOO-induced rats with DO (17), we studied the role of the BK channel in PBOO rabbits. Because we lack a rabbit cystometry station, we used an organ bath study to select bladders with high detrusor spontaneous contraction as experimental samples. We found that a significantly decreased expression of BK channel protein was associated with greater detrusor spontaneous contraction (Figs. 2 and 3). The next question is whether the enhanced spontaneous contraction is related to downregulation of the BK channel. To answer this question, two different BK openers were used to examine their effect on PBOO-induced spontaneous contraction (Fig. 4A). Both NS1619 and isopimaric acid have been shown to activate the BK channel in artery smooth muscle. Our result clearly shows that a BK opener can inhibit spontaneous contraction for several hours in DSM strips. Therefore, we conclude that loss of the BK channel in PBOO rabbits contributes to detrusor spontaneous contraction.
Translation of findings made in animal models to the pathophysiology of humans is important to show the validity of the animal model. A major handicap in this regard is obtaining sufficient tissue from human patients to perform all the analyses needed to validate the animal model. We obtained bladder tissue samples from patients with BPH who were characterized as having or not having DO based on urodynamic analysis. The supplemental figure is a representative urodynamic tracing of BPH patients with and without DO, which shows several bladder contractions during the urine-filling period, while the bladder is quiet during filling in BPH patients without DO (see supplemental figure). Although the punch biopsies removed from patients are small, we were able to isolate enough RNA and protein to measure the expression of the BK channel in these bladder samples. Our data demonstrated a downregulation of BK channel expression at both the mRNA and protein levels in human BPH patients with DO (Figs. 5 and 6), which was similar to the downregulation of BK observed in our rabbit model of PBOO with high detrusor spontaneous contraction. Our data reveal that the finding of a relationship between DO and BK channel expression level in an animal model is translatable to human patients. We clearly show that the downregulation of the BK channel was only associated with DO, not BPH. The expression level of BK is similar between control patients and BPH patients without DO. Moreover, the degree of downregulation of the BK β-subunit is greater than that of the BK α-subunit.
These findings in BPH patients with DO as well in the animal model for PBOO suggest that loss of BK channel expression contributes to DO. The correlation between downregulation of BK and DO led us to further study the mechanism of BK regulating detrusor contractility. We decided to study one of the subsequent effects in the downstream pathway, MLC20 phosphorylation. The reason we studied this event is that MLC20 phosphorylation is a prerequisite for muscle contraction (21, 22). We used two ways to study this relationship: 1) the effect of a BK opener on MLC20 phosphorylation and 2) the effect of BK siRNA on MLC20 phosphorylation. Figure 4B shows a decreased phosphorylation level caused by two types of a BK opener. This result indicates an inverse relationship between BK activity and the level of MLC20 phosphorylation. Thus downregulation of the BK channel will lead to higher MLC20 phosphorylation. We used an siRNA interference technique to eliminate BKβ expression in cultured human DSM cells. We found that knocking down the BK β-subunit increased MLC20 phosphorylation (Fig. 7) in cultured human DSM cells. We have previously shown upregulation of Rho-kinase pathway in the PBOO-affected bladder, which is considered to enhance basal MLC20 phosphorylation (4). However, the siRNA-mediated reduction in BK channel expression did not affect the expression of RhoA or Rho-kinase-α and -β in cultured human DSM cells (data not shown). Therefore, loss of the BK channel β-subunit directly increased MLC20 phosphorylation without altering Rho-kinase expression. There is evidence that a loss of BK leads to an increase in the basal calcium level (20). This rise in cytosolic calcium levels would be expected to increase MLC20 phosphorylation mediated by Ca2+-dependent MLC kinase. Hence, we have shown that downregulation of the BK channel might be another factor in causing a high basal level of MLC20 phosphorylation in detrusor from PBOO.
In summary, we have shown that PBOO-induced DO is associated with downregulation of BK channel expression in the rabbit model as well as in human BPH patients with DO. Furthermore, downregulation of the BK channel may contribute to DO by increasing the basal level of MLC20 phosphorylation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P50 DK52620.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int 84: 14–15, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van KP, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62: 3–10, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chacko S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol 285: F990–F997, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chacko S, Chang S, Hypolite J, Disanto M, Wein A. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand J Urol Nephrol Suppl: 26–36, 2004 [DOI] [PubMed] [Google Scholar]
- 6. de la Rosette JJ, Witjes WP, Schafer W, Abrams P, Donovan JL, Peters TJ, Millard RJ, Frimodt-Moller C, Kalomiris P. Relationships between lower urinary tract symptoms and bladder outlet obstruction: results from the ICS-“BPH” study. Neurourol Urodyn 17: 99–108, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211: 495–497, 1981 [DOI] [PubMed] [Google Scholar]
- 8. DiSanto ME, Stein R, Chang S, Hypolite JA, Zheng Y, Zderic S, Wein AJ, Chacko S. Alteration in expression of myosin isoforms in detrusor smooth muscle following bladder outlet obstruction. Am J Physiol Cell Physiol 285: C1397–C1410, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Calvo M, Knaus HG, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem 269: 676–682, 1994 [PubMed] [Google Scholar]
- 10. Geloso DA, Levin RM. Effect of partial outlet obstruction on the myogenic response to field stimulation. Gen Pharmacol 31: 291–295, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Griffiths D, Hofner K, van MR, Rollema HJ, Spangberg A, Gleason D. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. International Continence Society Subcommittee on Standardization of Terminology of Pressure-Flow Studies. Neurourol Urodyn 16: 1–18, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Hypolite JA, DiSanto ME, Zheng Y, Chang S, Wein AJ, Chacko S. Regional variation in myosin isoforms and phosphorylation at the resting tone in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C254–C264, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Levin RM, Longhurst PA, Monson FC, Kato K, Wein AJ. Effect of bladder outlet obstruction on the morphology, physiology, and pharmacology of the bladder. Prostate Suppl 3: 9–26, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Levin RM, Ruggieri MR, Velagapudi S, Gordon D, Altman B, Wein AJ. Relevance of spontaneous activity to urinary bladder function: an in vitro and in vivo study. J Urol 136: 517–521, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Li L, Jiang C, Song B, Yan J, Pan J. Altered expression of calcium-activated K and Cl channels in detrusor overactivity of rats with partial bladder outlet obstruction. BJU Int 101: 1588–1594, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Semenov I, Wang B, Herlihy JT, Brenner R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L802–L810, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Stanton MC, Delaney D, Zderic SA, Moreland RS. Partial bladder outlet obstruction abolishes the receptor- and G protein-dependent increase in calcium sensitivity in rabbit bladder smooth muscle. Am J Physiol Renal Physiol 287: F682–F689, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Steele GS, Sullivan MP, Sleep DJ, Yalla SV. Combination of symptom score, flow rate and prostate volume for predicting bladder outflow obstruction in men with lower urinary tract symptoms. J Urol 164: 344–348, 2000 [PubMed] [Google Scholar]
- 25. Stein R, Gong C, Hutcheson JC, Canning DA, Zderic SA. The decompensated detrusor III: impact of bladder outlet obstruction on sarcoplasmic endoplasmic reticulum protein and gene expression. J Urol 164: 1026–1030, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Zhang EY, Stein R, Chang S, Zheng Y, Zderic SA, Wein AJ, Chacko S. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of non-muscle caldesmon. Am J Pathol 164: 601–612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]