Abstract
Contraction of detrusor smooth muscle (DSM) at short muscle lengths generates a stiffness component we termed adjustable passive stiffness (APS) that is retained in tissues incubated in a Ca2+-free solution, shifts the DSM length-passive tension curve up and to the left, and is softened by muscle strain and release (strain softened). In the present study, we tested the hypothesis that APS is due to slowly cycling actomyosin cross bridges. APS and active tension produced by the stimulus, KCl, displayed similar length dependencies with identical optimum length values. The myosin II inhibitor blebbistatin relaxed active tension maintained during a KCl-induced contraction and the passive tension maintained during stress-relaxation induced by muscle stretch in a Ca2+-free solution. Passive tension was attributed to tension maintaining rather than tension developing cross bridges because tension did not recover after a rapid 10% stretch and release as it did during a KCl-induced contraction. APS generated by a KCl-induced contraction in intact tissues was preserved in tissues permeabilized with Triton X-100. Blebbistatin and the actin polymerization inhibitor latrunculin-B reduced the degree of APS generated by a KCl-induced contraction. The degree of APS generated by KCl was inhibited to a greater degree than was the peak KCl-induced tension by rhoA kinase and cyclooxygenase inhibitors. These data support the hypothesis that APS is due to slowly cycling actomyosin cross bridges and suggest that cross bridges may play a novel role in DSM that uniquely serves to ensure proper contractile function over an extreme working length range.
Keywords: urinary bladder, muscle mechanics, Triton X-100 permeabilization, Rho-kinase
as in striated muscle, smooth muscle displays a somewhat parabolic active length-tension curve with ascending and descending limbs on either side of an optimum length at which tension is maximum (3, 15, 19, 44, 55). However, an early in vitro study on cat ileum concluded that although “…there is a definite optimum length at which the maximum tension is developed…,” this optimum length “…is less on lengthening than on shortening the same muscle…” (3). That is, in certain smooth muscle cells, the active length-tension curve is not static, but can shift its position on the length axis. Such acute adaptation of smooth muscle to length change can result in an apparent broad plateau of the active length-tension curve (54). Only more recently have studies proposed mechanisms for such relatively acute length history-dependent adaptation of active tension (termed length adaptation for simplicity). Because muscle cell active tension is due to the transmission through cytoskeletal adhesion junctions of tension generated by actomyosin cross bridges, the proposed mechanisms for length adaptation of active tension include changes in actin and myosin polymerization leading to addition and subtraction of sarcomeres, and sarcomere and cytoskeletal adhesion junction reorganization (13, 16, 17, 25). Although the most extensive work on length adaptation of active tension has been done on airway smooth muscle (12), there is also evidence of length adaptation of active tension in certain arteries (40, 51), rabbit bladder (45), and cat ileum (3).
Unlike active length-tension curves that are parabolic, passive length-tension curves increase approximately exponentially with increasing muscle lengths beyond slack length (27). Passive tension assists in the maintenance of organ shape and sarcomere homogeneity during muscle lengthening. Moreover, it is the total tension (passive plus active tensions) that is physiologically relevant during muscle contraction, and in detrusor smooth muscle (DSM), passive tension can contribute over 30% to total tension when the muscle is at its optimum muscle length for contraction (1). In a muscle that displays length adaptation of active tension, one might expect that the passive length-tension curve will also length adapt (i.e., shift along the length axis). This seems especially relevant in the urinary bladder, a hollow organ that can accommodate large volumes at low luminal pressures during filling by lengthening DSM cells over sevenfold (52), which is over twofold greater than in striated muscles (14).
In striated muscles, titin and proteins of the extracellular matrix provide the primary tension-bearing molecules responsible for generation of passive tension, and the relative contribution of titin and extracellular matrix proteins varies considerably among different striated muscle types (34). Given the apparent differences in the molecular structures responsible for active and passive tensions, it is not surprising that the mechanism of length adaptation of passive tension remains to be determined in those few smooth muscle tissues in which length adaptation of active tension has been identified. In an earlier study, we proposed that strain softenable crosslinks (10, 30) participate in length adaptation of passive tension in DSM (46), but the molecular identity of these crosslinks remains to be determined. We showed that the magnitudes of DSM passive tension and stiffness (that remaining when tissues are incubated in a Ca2+-free solution) are highly dependent on the histories of muscle activation and muscle length (1, 46–48). We attributed this phenomenon to an intracellular molecular entity or aggregate that has an intrinsic adjustable stiffness and named this measurable muscle property adjustable passive stiffness (APS). Although we often obtain an estimate of APS by measuring passive tension differences at particular muscle lengths following muscle loading and unloading, the term “stiffness” is used rather than “tension” because stiffness is a material property characterized by a particular length-tension relationship. DSM activation at short muscle lengths by KCl, a muscarinic receptor agonist and a prostaglandin receptor agonist, regenerates APS and thus passive tension at longer muscle lengths via a rhoA kinase (ROCK)-dependent mechanism (1, 46). Therefore, ROCK activation appears to add a dynamic passive tension component (i.e., APS) in parallel with a static passive tension component presumably due to extracellular matrix proteins (47).
The physiological significance of APS may be to permit length adaptation coupling of active and passive tensions during bladder filling, and thus, to maintain a constant passive-to-active tension ratio. For such coupling to occur, we propose that APS is due to an actin-myosin interaction similar but distinct from the actomyosin cross bridge responsible for active tension. The precedence for our hypothesis that an actin-myosin interaction is responsible for the APS component of passive tension is that a small number of actomyosin cross bridges have been shown to contribute along with titin and extracellular matrix proteins to passive tension in resting striated muscle (4, 5, 20, 35). Moreover, smooth muscle myosin II is a high-duty ratio motor protein that can form a long-lasting latchbridge (33, 39, 43) that may be ideally suited for maintenance of passive tension in DSM.
To test the hypothesis that APS in DSM is caused by formation of actomyosin cross bridges, the present study utilized blebbistatin, a selective uncompetitive inhibitor of myosin II (50). Blebbistatin stabilizes the ADP-Pi complex of the S1 myosin head that precedes the tension-generating step catalyzed by release of Pi, thus inhibiting myosin from entering into the cross-bridge cycle (24). Blebbistatin inhibits mammalian smooth muscle tension and actomyosin ATPase activity (9), does not inhibit myosin light chain phosphorylation in smooth or striated muscle (9, 49), and because it has no effect on action potentials or Ca2+ mobilization, it can selectively uncouple excitation-contraction coupling in cardiac muscle (11).
MATERIALS AND METHODS
Tissue Preparation and Mechanical Measurements
All experiments involving animals were conducted within the appropriate animal welfare regulations and guidelines and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Bladders were isolated from adult female New Zealand white rabbits, and longitudinal muscle strips were prepared as previously described (36, 41). In short, strips of DSM were isolated from underlying mucosa by microdissecting longitudinal muscle bundles clearly visible under a stereo microscope (Olympus SZX12). Tissue strips were ∼2–4 mm wide and ∼10–12 mm long. Tissues were stored in cold (4°C) modified physiological saline solution (PSS; in mM: 140 NaCl, 4.7 KCl, 1.2 MgSO4, 1.6 CaCl2, 1.2 NaHPO4, 2.0 MOPS adjusted to pH 7.4, 0.02 Na2ethylenediamine tetraacetic acid to chelate heavy metals, and 5.6 d-glucose made with high-purity, deionized water). Each muscle strip was secured between two tissue clips in an aerated muscle bath heated to 37°C such that the length of each tissue between the clips was 4 mm. One clip was attached to an isometric tension transducer (Radnoti Glass Technology, Monrovia, CA) or an electronic lever (Aurora Scientific Instruments, Aurora, Ontario, Canada) to introduce rapid and highly accurate length steps, and the other clip was attached to a micrometer for manual length adjustments. To induce active contraction, tissues were stimulated by addition of KCl (PSS in which 110 mM KCl was substituted iso-osmotically for NaCl). Strips of rabbit DSM contract strongly to KCl in the presence of Ca2+, but incubation for only 2 min in a Ca2+-free solution prevents KCl from causing a contraction (22).
Tissue Permeabilization
Tissues secured between two tissue clips in an aerated muscle bath at 20°C were permeabilized with Triton X-100 (40 μM) in a manner similar to that described previously when using β-escin (18). Triton X-100 was dissolved in a “relaxing solution” that contained 74.1 mM potassium methanesulphonate, 4.0 mM magnesium methanesulphonate, 4 mM Na2ATP, 4 mM EGTA, 5 mM creatine phosphate, 4 mM EGTA, and 30 mM PIPES, neutralized with 1 M KOH to pH 7.1 at 20°C. After permeabilization, tissues were washed to remove Triton X-100 and were incubated thereafter in the relaxing solution.
Drugs
GF-109203X, H-1152, latrunculin-B, and U-0126 were from EMD Biosciences (formerly Calbiochem, San Diego, CA). Forskolin and wortmannin were from Alexis. Indomethacin and blebbistatin were from Sigma. H-1152 was dissolved in distilled water; all other drugs were dissolved in DMSO. DMSO was added at a final concentration no greater than 0.1%, a concentration that had no effect on KCl-induced increases in tension. Drugs were added to tissue baths 10 min before and during KCl-induced contractions as shown in Fig. 1B (drug treatment).
Fig. 1.
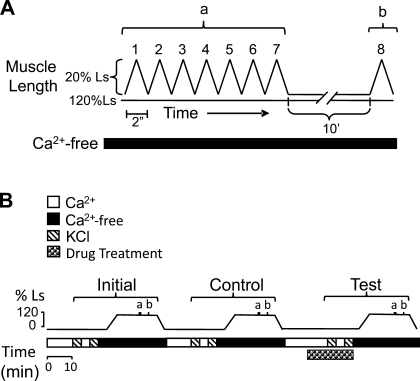
Protocol for the dynamic measurement of adjustable passive stiffness (APS) used to assess the ability of selective inhibitors to attenuate KCl-induced generation of APS. To measure APS, tissues were incubated in a Ca2+-free solution, stretched to 120% of slack length (Ls), and subjected to 7 sequential saw tooth loading and unloading cycles (“a” in A and B) followed 10 min (10′) later by a single (8th) loading and unloading cycle (“b” in A and B). Each cycle lasted 2 s (2″). B: to generate APS, tissues at Ls were twice contracted with KCl (hatched bars) during the 3 periods termed Initial, Control, and Test. The effect of an inhibitor (B, Drug Treatment) on a KCl-induced contraction and on generation of APS was assessed during the Test period.
Protocols
Estimate of APS.
Muscle strips were incubated in a Ca2+-free solution for at least 5 min to eliminate the activity of rapidly cycling cross bridges regulated by Ca2+-dependent processes (22) and then mechanically loaded (stretched) in a step-wise fashion from a length at which the muscle was just slack (Ls; defined as the length when tension was ∼0.49 mN) to 250% Ls and unloaded in a step-wise fashion from 250 to 120% Ls (see Fig. 1A). Each step change in length was performed manually using a micrometer (LS Starrett, Athol, MA; 20-mm range, 10-μm graduations). Tissues remained at each length for 5 min, at which time the pseudo steady-state tension was recorded. For each muscle length, the tension value produced during unloading (Tunload) was subtracted from that produced during loading (Tload). The resulting plot of Tload − Tunload vs. muscle length was presented as an estimate of length-dependent tension residing within the DSM that was due to the activity of an APS element.
Active tension protocol.
Muscle strips were loaded as in Estimate of APS, except that after ∼1 min at each muscle length tissues were strain softened by a rapid stretch to 250% Ls and releaseed back to the original length. Three minutes later, tissues were contracted with KCl until the peak contraction was achieved (∼30 s) and then washed twice with fresh PSS to cause complete muscle relaxation. Active tension produced by KCl as a function of muscle length (TKCl) was calculated as the total tension produced by KCl minus the passive tension measured before addition of KCl.
Stress-relaxation protocol.
Muscle strips were incubated in a Ca2+-free solution and preloaded by stretching rapidly from 120% Ls to 180% Ls using an electronic lever and then allowed to passively stress-relax for 60 min. At 30 min when stress-relaxation achieved a pseudo steady-state value, muscle strips were subjected to a single stretch-release of a magnitude that was 10% Ls (i.e., from 180 to 190% Ls) or were exposed to blebbistatin. Passive tension values taken just before the single stretch-release or addition of blebbistatin (f1) were compared with those taken at 60 min (30 min after the single stretch-release or addition of blebbistatin, f2). Control tissues were stretched from 120 to 180% Ls using an electronic lever and allowed to stress-relax for 60 min but were not subjected to either a single stretch-release or exposed to blebbistatin (see Fig. 3B, Control).
Fig. 3.
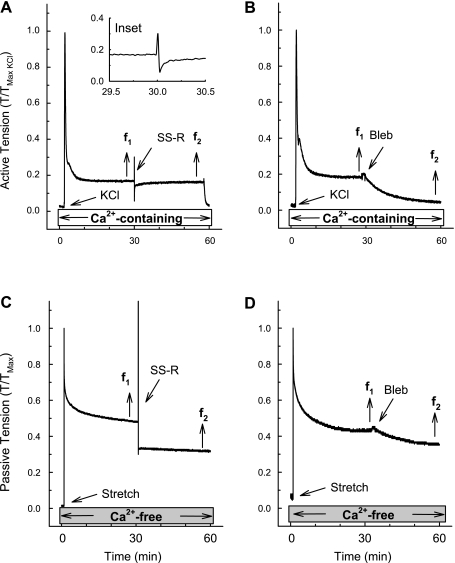
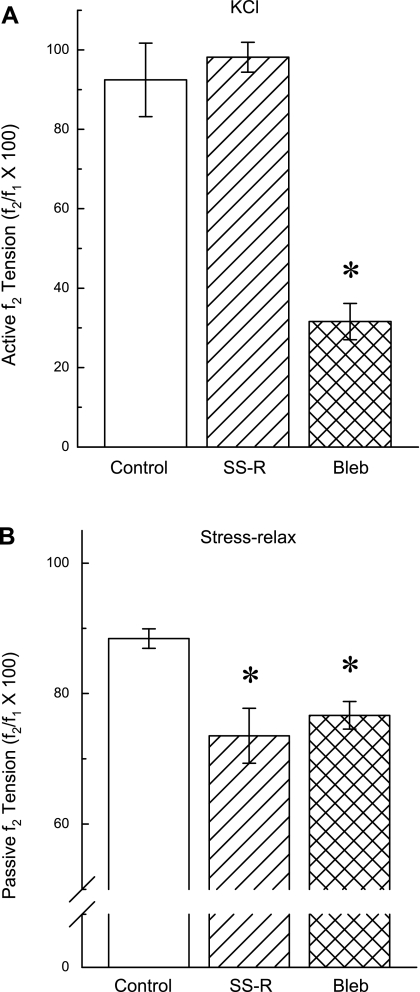
Myosin II inhibitor blebbistatin (Bleb) reduced both active (KCl-induced) and passive (stress-relaxation in a Ca2+-free solution) tension values. Examples of a tension tracings showing that there was full recovery of tension produced following a single stretch-release (SS-R) applied at the pseudo steady state (30 min) of a KCl-induced contraction (tension at data point f2 was equal to tension at data point f1; A). B: Bleb relaxed a KCl-induced contraction when applied at the pseudo steady-state period of tension maintenance (tension at data point f2 was less than tension at data point f1). C: degree of tension held during a passive stress-relaxation in a tissue incubated in a Ca2+-free solution was reduced by a SS-R applied at the pseudo steady-state period of stress-relaxation (tension at data point f2 was less than tension at data point f1). D: Bleb relaxed the degree of tension held during a passive stress-relaxation in a tissue incubated in a Ca2+-free solution when applied at the pseudo steady-state period of stress-relaxation (tension at data point f2 was less than tension at data point f1). To induce stress-relaxation, tissues were stretched rapidly from 120 to 180% Ls (Stretch, C and D). A, inset: zoomed tension tracing taken from 29.5 to 30.5 min. Control tissues for the tests shown in A and B were contracted for 60 min with KCl, and control tissues for the tests shown in C and D were stress-relaxed in a Ca2+-free solution for 60 min (data not shown but results are summarized in Fig 4).
Single stretch-release protocol.
At the pseudo steady state of both a KCl-induced contraction and a stress-relaxation induced as described above, a single stretch-release was rapidly applied using an electronic lever as a mechanical probe to reveal the existence of cross-bridge activity in the form of tension recovery. The rapid release of an active muscle to a shorter muscle length will cause an immediate fall in tension due to shortening of the series elastic component following by tension recovery due to cross-bridge cycling (21). The rapid release of a preloaded but inactive (i.e., passive) muscle will likewise cause an immediate reduction in tension but strong tension recovery should subsequently not occur because cross bridges are not cycling (15). To ensure that cross bridges are detached after the quick release and to permit tension values before and after the length perturbation to be assessed at the same muscle length, we chose to apply a single stretch-release with amplitude of 10% Ls and rate of 1 mm/s rather than a simple quick-release. The 10% quick-stretch component of the single stretch-release should extend the series elastic component and detach any cross bridges that were attached (38, 53).
Loading-unloading protocol to estimate APS in permeabilized tissues.
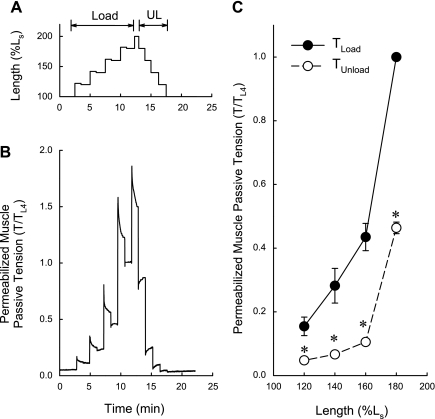
Muscle strips were strain softened by stretching from Ls to 200% Ls and releasing back to Ls. This procedure eliminates most of the APS present within the length range of Ls to 200% Ls (46–48) and essentially “sets” the level of APS within the working length range close to zero. Tissues at Ls were then contracted twice with KCl to reestablish APS (46) and then permeabilized with Triton X-100 and placed in a Ca2+-free relaxing solution. Tissues were then loaded by four incremental step increases in length (see Fig. 6A, Load) and unloaded by four incremental step releases (see Fig. 6A, UL). For each length step during loading, tissues were stretched 2% beyond the target length for 1 min and then released to the target length and passive tension was measured 1.5 min later. Although this protocol reduced slightly the passive tension that could be held at the target muscle length during the loading phase of the protocol, it reduced the time required to achieve the steady-state value because stress-relaxation following a stretch is very slow compared with tension redevelopment following a release (see especially the fourth loading tension values in Fig. 6B), and this allowed for relatively more symmetrical timing of loading and unloading. The differences between loading and unloading tension values were therefore a conservative underestimate of the full passive tension that could be held at the particular target length during loading.
Fig. 6.
TLoad is greater than TUnload in permeabilized detrusor smooth muscle (DSM). A: loading (Load) and unloading (UL) protocol used to determine the length dependencies of passive tension. B: example of a passive tension tracing of permeabilized DSM subjected to the protocol shown in A. C: summary data showing that for each length step, the tension values produced on muscle loading were greater than those produced on unloading. TL4, pseudo steady-state tension value at the 4th loading length. Data in C are means ± SE, n = 3. *P < 0.05 compared with TLoad, Student's t-test.
APS measured by dynamic length-tension work loops.
Muscle strips at Ls were strain softened by stretching to 140% Ls and releasing back to Ls to “set” the level of APS within the working length range close to zero, contracted twice at Ls with KCl to reestablish APS, incubated in a Ca2+-free solution, slowly stretched to 120% Ls over 10 min, and then, while continuously recording tension, subjected to seven sequential controlled saw tooth ramp stretches (loading) and releases (unloading) using an electronic lever. Ten minutes later an eighth saw tooth ramp stretch-release was imposed (Fig. 1A). The length-tension clockwise area enclosed by each saw tooth ramp stretch-release constitutes a work loop representing a loss of energy for the given length change. Work loop 1 minus work loop 8 represents APS (that amount of stiffness that can be induced by KCl when the muscle is at Ls), and work loop 8 minus work loop 7 represents viscoelastic stiffness (that amount of stiffness that spontaneously returns during the 10-min “rest” period between saw tooth 7 and saw tooth 8) (46).
Effects of Inhibitors on KCl-Induced Contraction and APS Assessed By Dynamic Length-Tension Work Loops
Muscle strips were subjected to the protocol shown in Fig. 1B, where “a” refers to seven saw tooth loading-unloading cycles and “b” refers to the eighth cycle produced 10 min later, as previously described and shown in Fig. 1A. In short, APS was induced by twice contracting tissues at Ls with KCl (Fig. 1B, cross-hatched bars). After complete relaxation, tissues were stretched to 120% Ls while incubated in a Ca2+-free solution and subjected to seven consecutive and a delayed eighth saw tooth loading-unloading cycles to measure APS. The value of APS was measured as the difference between work loop 1 and work loop 8. This sequence was repeated three times. The first was labeled “initial,” the second “control,” and the third “test” (Fig. 1B). An inhibitor was added during the KCl-induced contractions in the third sequence (Fig. 1B, Drug treatment). Inhibitors used for this experiment were the myosin light chain (MLC) kinase (MLCK) inhibitor, wortmannin (Wort; 3 μM), the ROCK inhibitor H-1152 (0.3 μM), the protein kinase C (PKC) inhibitor, GF-109203X (GF-109; 1 μM), the mitogen-activated protein kinase kinase (MEK) inhibitor U-0126 (20 μM), the actin polymerization inhibitor latrunculin-B (Latr-B; 0.1 μM), the nonselective inhibitor of cyclooxygenase indomethacin (INDO; 10 μM), the activator of adenylyl cyclase and thus of protein kinase A (PKA) forskolin (FSK; 10 μM), and the myosin II inhibitor blebbistatin [(−)Bleb; 30 μM] as well as the inactive isomer of blebbistatin [(+)Bleb; 30 μM].
Statistics
The null hypothesis was examined using Student's unpaired t-test or a one-way ANOVA followed by the Newman-Keuls post hoc test (GraphPad Prism version 5, La Jolla, CA) to assess whether each “test” group was different than the control group. In all cases, the null hypothesis was rejected at P < 0.05. For each study described, the n value was equal to the number of bladders.
RESULTS
Comparison of the Length Dependencies of Active and Passive Tensions
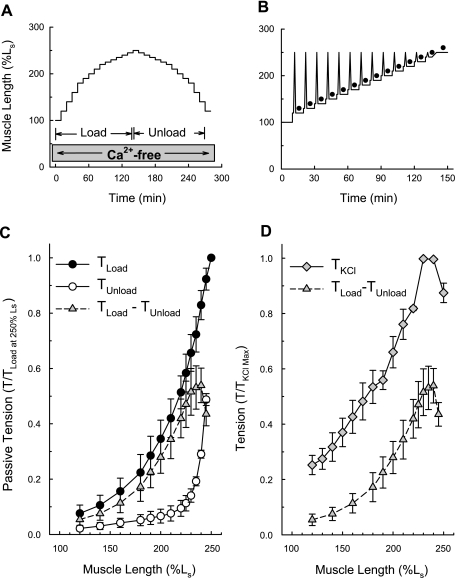
The muscle passive length-tension curve resulting from loading (Fig. 2A, Load) was significantly greater than that produced during unloading (Fig. 2A, Unload, and Fig. 2C, compare TLoad and TUnload). The tension difference between these two curves (Fig. 1C, TLoad − TUnload) represents an estimate of APS. This stiffness component is considered “passive” because tissues were bathed in a Ca2+-free solution (Fig. 2A, Ca2+-free) that abolishes the ability of KCl to cause contraction (22).
Fig. 2.
Length-tension curves generated for the stimulus KCl and for APS displayed a similar length dependency. A: loading and unloading protocol used to estimate the length dependency of APS. B: protocol used to generate an active length-tension curve. Tissues were strain softened before each period of stimulation with KCl (black dots) by briefly stretching to 250% of Ls to eliminate APS. C: tension values produced at each length step generated on muscle loading (TLoad) and unloading (TUnload). The difference between these two curves (TLoad − TUnload) is an estimate of APS. D: comparison of the active length-tension curve (TKCl) produced using the protocol shown in B with the estimated length-APS tension curve (TLoad − TUnload) generated as shown in A. Data in C and D are means ± SE, n = 3.
The protocol for generation of an active length-tension curve is shown in Fig. 2B (see materials and methods). When plotted on the same graph, the curve representing APS appeared to be a weaker version of the active length-tension curve calculated for tissues maximally contracted with KCl (Fig. 2D, compare curves TLoad − TUnload and TKCl). That is, both curves had ascending and descending limbs and maximum tension values within the same muscle length range. Because TKCl is due to the degree of actomyosin cross-bridge overlap, these data support the hypothesis that the TLoad − TUnload curve was also due to the degree of actomyosin cross-bridge overlap. The experiments described below investigate this hypothesis.
Effects of a Single Stretch-Release or Blebbistatin on KCl-Induced Active Tension and Passive Stress-Relaxation
As expected in tissues contracted with KCl for 60 min, a single stretch-release (Fig. 3A, SS-R) applied at 30 min caused a rapid increase and then decrease in tension to a nadir much lower than the prestretch tension followed by tension recovery (Fig. 3A and zoomed image in inset). The strength of tension recovery 30 min after the single stretch-release was over 90% (Fig. 3A, compare f2 and f1, and Fig. 4A, SS-R). Addition of 30 μM blebbistatin relaxed KCl-induced tonic tension (Fig. 3B) by ∼70% (Fig. 4A, Bleb). In control tissues that did not undergo a single stretch-release and were not exposed to blebbistatin, the tonic KCl-induced tension at 60 min (f2) was reduced by only ∼10% compared with the tension at 30 min (f1; Fig. 4A, Control). These data are consistent with the hypothesis that tonic tension maintenance of a KCl-induced contraction in DSM requires actively cycling actomyosin cross bridges.
Fig. 4.
Summary of the results obtained from the tests shown in Fig. 3, A and B (A) and C and D (B) compared with control data. Data are means ± SE, n = 3–8. *P < 0.05 compared with control, ANOVA/Newman-Keuls.
In tissues incubated in a Ca2+-free solution and subjected to a step-stretch to induce stress-relaxation, a subsequent single stretch-release at 30 min (Fig. 3C, SS-R) caused passive tension to fall to a level lower than that produced before the single stretch-release, and tension did not recover within 30 min (Fig. 3C, compare f2 with f1, and Fig. 4B, SS-R). Addition of blebbistatin also produced a significant reduction in passive tension (Figs. 3D and 4B, Bleb). The reduction in passive tension caused by blebbistatin was comparable to that induced by the single stretch-release (Fig. 4B). The passive tension remaining after blebbistatin or after the single stretch-release in tissues incubated in the Ca2+-free solution was likely due to extracellular matrix proteins. These data together suggest that actomyosin cross bridges contribute at least 15% (Fig. 4B) to the maintenance of passive tension in rabbit DSM when stretched one time from 120 to 180% of Ls and support the hypothesis that APS is due to actomyosin cross bridges.
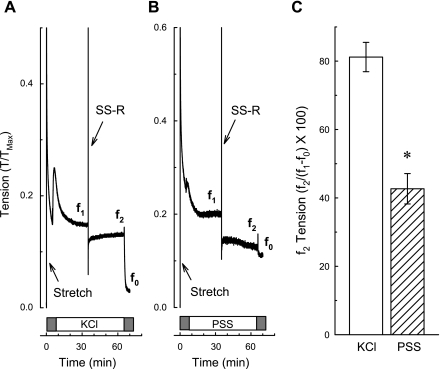
If cross bridges are active (i.e., cycling) even in the Ca2+-free bathing solution, then we would expect tension recovery after a single stretch-release, as was seen during a KCl-induced contraction. It is possible that the reason tension did not recover after a single stretch-release (see Fig. 3C) was because of the extra amount of total muscle strain imposed on the muscle (120 to 180% Ls plus an additional 10% Ls during the single stretch-release) compared with that imposed during a KCl-induced contraction (120% Ls plus an additional 10% Ls during the single stretch-release). To account for this possibility, tissues in a Ca2+-free solution were subjected to a step-increase in length from 120 to 180% Ls and allowed to stress-relax for 10 min and then contracted with KCl (in the presence of Ca2+) or exposed to a Ca2+-containing solution for 20 min, and finally subjected to a single stretch-release at 30 min and tension was recorded for an additional 30 min (Fig. 5). To identify the passive tension value near the time at which f2 tension was taken, tissues were exposed to a Ca2+-free solution at 60 min (Fig. 5, A and B, gray boxes). Under both conditions, tension fell immediately after completion of the single stretch-release (Fig. 5, A and B). Tension recovery to a level 80% of that just before the single stretch-release (Fig. 5C, KCl) occurred within 30 min in tissues contracted with KCl (Fig. 5A, compare f2 to f1). However, tissues exposed only to a Ca2+-containing solution did not appear to recover any tension, but maintained tone ∼40% above that induced when tissues were exposed to the Ca2+-free solution (Fig. 5B, compare f2 to f1, and Fig. 5C, PSS). The most important aspect of this experiment is that the greater tension induced by the single stretch-release applied to a muscle already at a long length did not prevent tension recovery on release during a KCl-induced contraction. In summary, the data showing that blebbistatin reduced the level of passive tension sustained during Ca2+-free stress-relaxation suggest that actomyosin cross bridges contribute to the maintenance of passive tension in rabbit DSM. Data showing that tension did not recover after a single stretch-release during Ca2+-free stress-relaxation imply that the Ca2+-free condition favors net cross-bridge detachment.
Fig. 5.
Tension recovered following a SS-R applied during a contraction induced by KCl which itself was applied during stress-relaxation. Tissues incubated in a Ca2+-free solution (gray boxes) were contracted with KCl (A) and washed in Ca2+-containing physiological saline solution (PSS) for 60 min (B), and a SS-R was applied at ∼30 min. Tension values were taken for analyses (C) at the times indicated by f1, f2, and f0. To induce stress-relaxation, tissues were stretched rapidly from 120 to 180% Ls (Stretch, A and B). Data in C are means ± SE, n = 3–4. *P < 0.05 compared with KCl, Student's t-test.
Triton X-100 Permeabilized Muscle
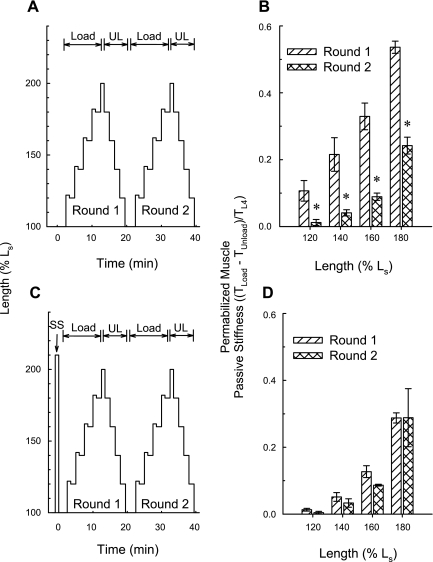
Intact tissues at Ls were strain softened by stretching to 210% Ls and releasing back to Ls to “set” the level of APS within the working length range close to zero. Tissues at Ls were then contracted twice with KCl to reestablish APS, permeabilized using Triton X-100, and placed in a Ca2+-free relaxing solution. As in intact tissues incubated in a Ca2+-free solution, the loading curve produced in permeabilized tissues incubated in a relaxing solution was stronger than the unloading curve (Fig. 6C). The difference between TLoad and TUnload represents APS only if a portion of the difference can be abolished by a prior strain-softening episode. To determine whether this was the case, tissues at Ls were permeabilized using Triton X-100, placed in a Ca2+-free relaxing solution, and subjected to two rounds of step-wise loading (from Ls to 200% Ls) and unloading (from 200% Ls to Ls) alone (Fig. 7A) or after undergoing a single step load and unload to 210% Ls (strain softening, Fig. 7C, SS). Also, the first round of step-wise loading and unloading served as strain softening for the second round. The calculated tension value, TLoad − TUnload, revealed by round 1 loading and unloading was significantly greater than that revealed by round 2 loading and unloading (Fig. 7B). Moreover, an initial strain softening to 210% (Fig. 7C) abolished this difference (Fig. 7D). These data indicate that the fraction of passive tension at a particular length in permeabilized DSM that was established by contraction of intact DSM at a shorter length was lost by strain softening the permeabilized tissue (i.e., by stretching the tissue to, and returning from, a longer length). Thus, these data support the hypothesis that APS is retained in permeabilized DSM.
Fig. 7.
APS is retained in permeabilized detrusor smooth muscle (DSM). When subjected to the protocol shown in A, for each muscle length, the tension value representing an estimate of APS (TLoad − TUnload) obtained during round 1 Load and UL was greater than that produced during round 2 (B). When subjected to the protocol shown in C, for each muscle length, the tension value representing an estimate of APS (TLoad − TUnload) obtained during round 1 Load and UL was equal to that produced during round 2 (D). SS, strain softening. Data in B and D are means ± SE, n = 3. *P < 0.05 compared with round 1, Student's t-test.
To determine whether blebbistatin can diminish the degree of passive tension retained in permeabilized muscle, intact tissues at Ls were strain softened as described above, incubated for 30 min with 30 μM active (−) and inactive (+) isomers of blebbistatin, and contracted twice at Ls with KCl. Upon washout of blebbistatin, these tissues were permeabilized with Triton X-100, placed in a Ca2+-free relaxing solution, and loaded by stretching to 200% Ls. Passive tension was recorded 5 min after stress-relaxation. Compared with tissues incubated with the inactive isomer [(+)blebbistatin], those incubated with the active myosin II inhibitor [(−)blebbistatin] produced about half the amount of active tension when stimulated with KCl [(+)blebbistatin: 0.87 ± 0.19 T/To; (−)blebbistatin: 0.41 ± 0.03 T/To; P < 0.05]. Likewise, tissues incubated with (−)blebbistatin developed about half the amount of passive tension compared with tissues incubated with (+)blebbistatin [(+)blebbistatin: 0.28 ± 0.05 T/To; (−)blebbistatin: 0.15 ± 0.03 T/To; P < 0.05]. These data support the hypothesis that the APS generated in intact tissues and preserved in Triton X-100-permeabilized tissues is caused by actomyosin cross bridges.
Comparison of Antagonists of Kinases, an Inhibitor of Actin Polymerization and a Relaxant Agent on APS Measured Dynamically and on KCl-Induced Active Tension
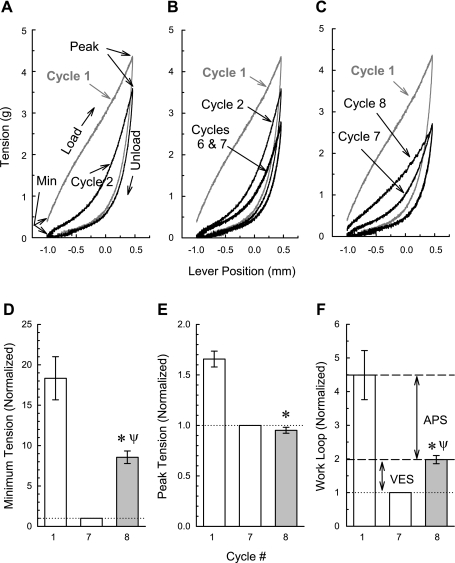
To assess the effects of selective inhibitors of contraction on the formation of APS, we adopted a technique previously used to dynamically measure APS (46) and described in materials and methods and in Fig. 1. The first of seven sequential saw tooth ramp length changes greatly reduces the amount of passive muscle stiffness because the area enclosed by a second loading and unloading curve is much smaller than that enclosed by the first loading and unloading curve (Fig. 8A, compare areas enclosed by loading and unloading curves of cycles 1 and 2). However, six consecutive saw tooth ramp length changes (see Fig. 1A) are required to reach steady state (note in Fig. 8B that the curves produced by cycles 6 and 7 are superimposable and less than that induced by cycle 2). An eighth saw tooth length change 10 min after the seventh reveals that some passive muscle stiffness softened by the seven consecutive length perturbations can return spontaneously with time (Fig. 8C, although both unloading curves are superimposable, the loading curve generated during cycle 8 is leftward shifted compared with that induced during cycle 7). Work loop 1 minus work loop 8 represents APS (that amount of stiffness that can be induced by KCl when the muscle is at Ls), and work loop 8 minus work loop 7 represents viscoelastic stiffness (that amount of stiffness that spontaneously returns during the 10-min “rest” period between saw tooth 7 and saw tooth 8). In the present study, we determined that APS and viscoelastic passive stiffness represent, respectively, 78 and 22% of the total dynamic stiffness softened by seven consecutive loading and unloading cycles (Fig. 8F). Interestingly, the minimum passive tension does (Fig. 8D), and peak passive tension does not (Fig. 8E), include viscoelastic stiffness.
Fig. 8.
Dynamic protocol for determining APS. Examples of the length-tension work loops for cycles 1 and 2 (A); 1, 2, 6, and 7 (B); and 1, 7, and 8 (C) in a control tissue, and summary values for cycles 1, 7, and 8 for minimum passive tension (D and see “Min” in A), peak passive tension (E and see “Peak” in A), and work loops (areas enclosed by the loading and unloading curve, F). APS is defined as the difference between cycles 1 and 8. Viscoelastic stiffness (VES) is defined as the difference between cycles 8 and 7. Data in D-F are means ± SE, n = 3. *P < 0.05 compared with cycle 1, ψP < 0.05 compared with cycle 7, ANOVA/Newman-Keuls.
If cross bridges play a role in APS, then certain agents that affect cross-bridge activation in response to a KCl-induced stimulation would likely also affect APS. To test this hypothesis, the degree of APS was assessed in DSM strips using the protocol shown in Fig. 1 (see materials and methods). The maximum active tension induced by KCl and the degree of APS measured during the “Initial” and “Control” periods (see Fig. 1B) were not significantly different [KCl maximum active tension (g): Initial: 3.90 ± 0.25; Control: 3.87 ± 0.25; P > 0.05; n = 27; APS (normalized to cycle 7): Initial: 2.15 ± 0.14; Control: 2.09 ± 0.14; P > 0.05; n = 27]. Thus, KCl-induced contractile strength and the generation and measurement of APS were sequentially repeatable. The effects of particular drugs on the subsequent ability of KCl to cause a contraction (see Fig. 1B, “Drug Treatment” bar in the “Test” period) and of KCl to regenerate APS were then examined. The “Test” data were normalized to the “Initial” data.
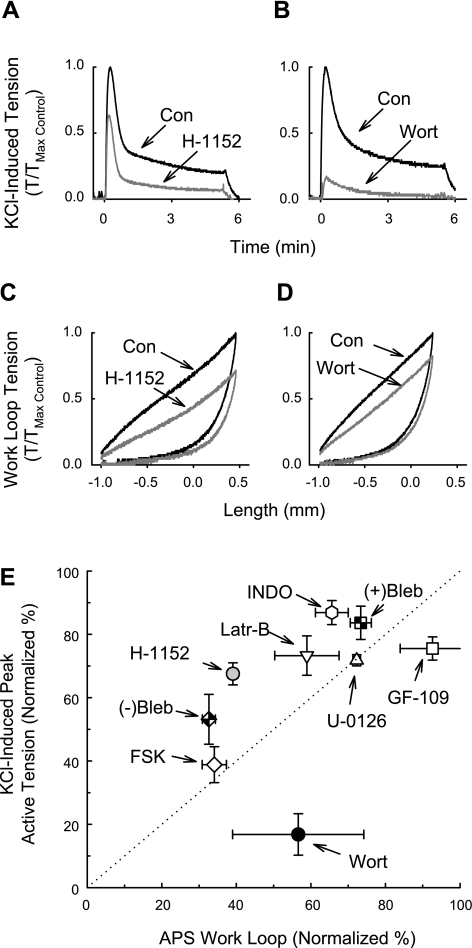
Examples of tension tracings for the effects of H-1152 and wortmannin are shown in Fig. 9, A–D. Note especially that wortmannin strongly inhibited a KCl-induced contraction (Fig. 9B) but the weak KCl-induced contraction was able to induce strong APS (Fig. 9D). All agents except GF-109203X produced some degree of inhibition of both active contraction and APS (Fig. 9E). The active isomer of blebbistatin [(−)Bleb] strongly inhibited both a KCl-induced contraction and APS, although APS was inhibited by a greater degree [∼70% compared with ∼50%; Fig. 9E, (−)Bleb]. By comparison, the inactive isomer of blebbistatin [(+)Bleb] produced only a weak inhibition [Fig. 9E, (+)Bleb]. Latrunculin-B also inhibited a KCl-induced contraction and APS, suggesting that actin polymerization plays a role in both processes (Fig. 9E, Latr-B). The dotted line in Fig. 9E indicates the line of equivalent inhibition of a KCl-induced contraction and APS. Interestingly, when considering the calculated average values, U-0126 (Fig. 9E) and forskolin (Fig. 9E, FSK) inhibited both a KCl-induced contraction and APS equally (the data fell on the dotted line), whereas wortmannin (Fig. 9E, Wort) and GF-109203X (Fig. 9E, GF-109) produced a greater inhibition of a KCl-induced contraction than of APS (the data fell to the right of the dotted line), and H-1152 (Fig. 9E) and indomethacin (Fig. 9E, INDO) produced a greater inhibition of APS than of a KCl-induced contraction (the data fell to the left of the dotted line). These data together support the notion that APS is due to actomyosin cross bridges and that one or more cyclooxygenase products and ROCK play a more important role compared with MLCK and PKC in regulation of APS formation.
Fig. 9.
Inhibition of KCl-induced contraction and the generation of APS. Examples of KCl-induced contractions in the absence (Con; A and B) and presence of the rhoA kinase (ROCK) inhibitor H-1152 (A) and the myosin light chain kinase (MLCK) inhibitor wortmannin (Wort; B). Examples of cycle 1 work loops (Fig. 8) during the control period (Con; C and D, and see Fig. 1B) and after the tissues were incubated with H-1152 (C) and Wort (D) when tissues were contracted with KCl during the test period (Fig. 1B). The work loop area representing APS was calculated as work loop 1 − work loop 8 as shown in Fig. 8F. Summary data in E are presented as % normalized to the values obtained during an Initial period (Fig. 1B). (+)Bleb and (−)Bleb are, respectively, the inactive and active isomers of the myosin II inhibitor blebbistatin (30 μM). GF-109 is the protein kinase C (PKC) inhibitor GF-109203X (1 μM). H-1152 was used at 0.3 μM. INDO is the nonselective cyclooxygenase inhibitor indomethacin (10 μM). Latr-B is the actin polymerization inhibitor latrunculin-B (0.1 μM). Wort was used at 3 μM. U-0126 (10 μM) is the MEK1 inhibitor and FSK is the protein kinase A activator forskolin (10 μM). Data in E are means ± SE, n = 3 for each drug.
DISCUSSION
We recently characterized a passive stiffness component of DSM that is generated by contractions at short muscle lengths, revealed by muscle loading and unloading when tissues are incubated in a Ca2+-free solution, and reduced in strength (i.e., strain softened) by the initial loading-unloading cycle (46–48). We termed this mechanical DSM component APS and proposed that it represents intracellular crosslinks (46). The aim of this study was to gain insight into the molecular identity of these crosslinks responsible for APS. The most important findings are that 1) the passive tension resulting from generation of APS was relaxed by addition of the selective myosin II inhibitor blebbistatin (Fig. 3D), 2) the generation of APS was greatly inhibited by blebbistatin (Fig. 9E), 3) APS was preserved in DSM permeabilized by Triton X-100 (Figs. 6 and 7), and 4) blebbistatin inhibited APS preserved in Triton X-100-permeabilized tissue. These data, along with the additional findings that the length-tension curves of APS-dependent tension and KCl-induced active tension displayed ascending and descending limbs and optimum tensions at an identical muscle length (Fig. 2D), and that the actin polymerization inhibitor latrunculin-B reduced the generation of APS (Fig. 9E), support the hypothesis that APS is caused by slowly cycling actomyosin cross bridges.
Although this is a novel finding for DSM, it has precedence in earlier studies revealing that a small number of long-lived cross bridges participate in resting tension in skeletal, cardiac, and smooth muscles (4, 5, 20, 29, 42). The cross bridges proposed to participate in resting tension in striated muscle, like APS, can be strain softened. For this reason, we propose that APS reflects a population of cross bridges that function to sustain passive tension rather than to develop active tension. These cross bridges would fit the criteria of a “tension sensor” or “gate,” rather than as a “fast mover,” as described by Nyitrai and Geeves (33). If these cross bridges are attached during active tension development upon muscle stimulation by a contractile agent, then we would expect APS to contribute to total tension and to slow the rate of muscle shortening by imposing an internal load, as occurs in the latch-state (31). A major distinction between the cross bridges responsible for resting stiffness in DSM and striated muscle is their dependence on Ca2+. Resting stiffness is abolished in permeabilized striated muscle incubated in relaxing solution and is therefore Ca2+ dependent (29). The dependency of resting striated muscle stiffness on Ca2+ is one reason that a portion of resting tension is attributed to cross bridges. In the present and previous studies (1, 46–48), APS and the resulting passive tension in DSM are generated by contracting muscle strips at Ls with KCl, carbachol, or PGE2 in a Ca2+-containing solution, but then measured in tissues incubated for at least 2–5 min in a Ca2+-free solution, a duration sufficient to abolish a KCl-induced contraction (22). An early study indicated that smooth muscle displays a resistance to stretch when tissues are bathed in a Ca2+-containing medium that is absent when tissues are bathed in a Ca2+-free solution (42). In the present study, APS appeared to be retained for at least 30–60 min in tissues incubated in a Ca2+-free solution (Fig. 3D). Moreover, we found that APS was preserved in tissues incubated in a relaxing solution after permeabilization with Triton X-100. Although we did not directly compare the degree of passive stiffness retained by resting DSM exposed to Ca2+-containing and Ca2+-free solutions, it is likely that the level of stiffness would be considerably greater in tissues exposed to Ca2+.
Several Ca2+-independent kinases such as ROCK, zipper-interacting kinase (ZIPK), and intergrin-linked kinase (ILK) can “turn on” actomyosin cross bridges (2, 7, 32) largely by inhibition of MLC phosphatase activity (23). DSM displays high basal levels of MLC phosphorylation and incubation of tissues in a Ca2+-free solution reduces but does not abolish basal MLC phosphorylation (37). Thus, a fraction of the total cross-bridge pool could remain active in DSM incubated in a Ca2+-free solution. The strain-release cycle that softens passive stiffness may act to “turn off” these cross bridges by a purely mechanical mechanism, by altering the kinetics of ADP release or by activating a biochemical step. Although speculative at this time, the latter two models would suggest that strain softening does not physically break cross bridges, but instead, that the degree of strain acts as a sensor to biochemically regulate the number of attached cross bridges in resting DSM.
If APS reflects slowly cycling cross bridges generated by a contractile agonist, then it is possible that any agent that reduces an agonist induced contraction might also reduce APS generation. In fact, we found that all inhibitors of active tension generation except 1 μM GF-109203X inhibited APS. However, a KCl-induced contraction and APS were not necessarily attenuated equally by the same inhibitor. The PKC inhibitor GF-109203X when used at 1 μM significantly inhibited a KCl-induced contraction by ∼25% but did not reduce the degree of APS generated as a result of the KCl stimulation (Fig. 9E, GF-109). Moreover, despite the very strong (∼85%) inhibition of a KCl-induced contraction by wortmannin, a considerable degree of APS (>50%) could still be induced by the weak KCl contraction (Fig. 9, B, D, and E, Wort). H-1152 produced a greater inhibition of APS than of KCl-induced tension (Fig. 9, A, C, and E), suggesting that ROCK may play a more prominent role in regulation of APS than of contraction. These data do not rule out the possibility that other kinases, such as ZIPK and ILK, also participate in generation of APS. Cyclooxygenase inhibition by indomethacin produced a greater inhibition of APS than of a KCl-induced contraction (Fig. 9E). We recently showed that spontaneous rhythmic contraction in DSM can be abolished by cyclooxygenase inhibitors, suggesting that prostaglandins are basally released by detrusor (6). Thus, prostaglandin receptor activation may rather selectively activate cell signaling systems involved in regulating APS.
Together, these data support the hypothesis that APS and active tension are regulated by similar and distinct biochemical pathways. Both myosin II heavy chain and myosin II essential light chain are expressed as multiple isoforms in smooth muscles (8). Although speculative, one possible scenario envisioned is that “fast” and “slow” cross bridges are somewhat compartmentalized within the DSM cell such that they participate in, respectively, active tension and APS and are regulated largely by, respectively, MLCK and ROCK. Precedence for this can be found in a study showing that MLCK regulates cross bridges within the center of isolated porcine tracheal smooth muscle cells, whereas ROCK regulates those located at the cell periphery (28).
The notion that slowly cycling APS cross bridges can act to regulate the degree of resting muscle tension over a broad working muscle length range expands the functional role played by myosin II in muscles. We propose that APS cross bridges may participate in maintaining organ shape and sarcomere orientation throughout the sevenfold operational length range (52) during bladder filling. Moreover, we suggest that detachment of APS cross bridges is responsible for accommodation during slow bladder filling. By acting as an internal load during voiding, APS cross bridges may be expected to reduce the rate while enhancing the maximum power output (the product of muscle tension and muscle shortening velocity) of the voiding contraction (12). Based on these potential roles of APS, failure of this system may be expected to alter the degree of accommodation and lead to sarcomere in-homogeneities that would affect voiding. If APS provides an internal load, then reductions in APS might be expected to directly reduce bladder power output during voiding. In conclusion, data from this study support the hypothesis that APS is caused by slowly cycling cross bridges. APS cross bridges may represent a novel molecular target for research efforts focused on understanding and treating certain bladder contractile disorders.
GRANTS
This study was supported by a grant from the Edwin Beer Research Program in Urology and Urology Related Fields from the New York Academy of Medicine (to J. E. Speich) and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-59620 (to P. H. Ratz).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the superb technical contributions of A. S. Miner, L. Borgsmiller, and C. Dosier, and the support of Dr. H. P. Koo.
REFERENCES
- 1. Almasri AM, Ratz PH, Bhatia H, Klausner AP, Speich JE. Rhythmic contraction generates adjustable passive stiffness in rabbit detrusor. J Appl Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Brocklehurst RJ. Studies on the physiology of plain muscle: the effect of alteration of initial length on the tension produced on contraction. J Physiol 61: 275–281, 1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell KS, Lakie M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J Physiol 510: 941–962, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell KS, Patel JR, Moss RL. Cycling cross-bridges increase myocardial stiffness at submaximal levels of Ca2+ activation. Biophys J 84: 3807–3815, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins C, Klausner AP, Herrick B, Koo HP, Miner AS, Henderson SC, Ratz PH. Potential for control of detrusor smooth muscle spontaneous rhythmic contraction by cyclooxygenase products released by interstitial cells of Cajal. J Cell Mol Med 13: 3236–3250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction: a novel function for integrin-linked kinase. J Biol Chem 276: 16365–16373, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Eddinger TJ, Meer DP. Myosin II isoforms in smooth muscle: heterogeneity and function. Am J Physiol Cell Physiol 293: C493–C508, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Eddinger TJ, Meer DP, Miner AS, Meehl J, Rovner AS, Ratz PH. Potent inhibition of arterial smooth muscle tonic contractions by the selective myosin II inhibitor, blebbistatin. J Pharmacol Exp Ther 320: 865–870, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Emery JL, Omens JH, McCulloch AD. Strain softening in rat left ventricular myocardium. J Biomech Eng 119: 6–12, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ford LE. Plasticity in airway smooth muscle: an update. Can J Physiol Pharmacol 83: 841–850, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Ford LE, Seow CY, Pratusevich VR. Plasticity in smooth muscle, a hypothesis. Can J Physiol Pharmacol 72: 1320–1324, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon AR, Siegman MJ. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol 221: 1243–1249, 1971 [DOI] [PubMed] [Google Scholar]
- 16. Gunst SJ, Meiss RA, Wu MF, Rowe M. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol Cell Physiol 268: C1267–C1276, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Gunst SJ, Wu MF. Selected contribution: plasticity of airway smooth muscle stiffness and extensibility: role of length-adaptive mechanisms. J Appl Physiol 90: 741–749, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Han SJ, Speich JE, Eddinger TJ, Berg KM, Miner AS, Call C, Ratz PH. Evidence for absence of latch-bridge formation in muscular saphenous arteries. Am J Physiol Heart Circ Physiol 291: H138–H146, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res 33: 257–283, 1973 [DOI] [PubMed] [Google Scholar]
- 20. Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol 199: 637–684, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huxley AF. Muscular contraction. J Physiol 243: 1–43, 1974 [PMC free article] [PubMed] [Google Scholar]
- 22. Jezior JR, Brady JD, Rosenstein DI, McCammon KA, Miner AS, Ratz PH. Dependency of detrusor contractions on calcium sensitization and calcium entry through LOE-908-sensitive channels. Br J Pharmacol 134: 78–87, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signaling pathways in health and disease. J Cell Mol Med 12: 2165–2180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kuo KH, Herrera AM, Wang L, Pare PD, Ford LE, Stephens NL, Seow CY. Structure-function correlation in airway smooth muscle adapted to different lengths. Am J Physiol Cell Physiol 285: C384–C390, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Labeit S, Lahmers S, Burkart C, Fong C, McNabb M, Witt S, Witt C, Labeit D, Granzier H. Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. J Mol Biol 362: 664–681, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science 230: 1280–1282, 1985 [DOI] [PubMed] [Google Scholar]
- 28. Miyazaki K, Yano T, Schmidt DJ, Tokui T, Shibata M, Lifshitz LM, Kimura S, Tuft RA, Ikebe M. Rho-dependent agonist-induced spatio-temporal change in myosin phosphorylation in smooth muscle cells. J Biol Chem 277: 725–734, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Moss RL, Sollins MR, Julian FJ. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature 260: 619–621, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Mullins L. Effect of stretching on the properties of rubber. J Rubber Res 16: 275–289, 1947. [Google Scholar]
- 31. Murphy R. Muscle cells of hollow organs. News Physiol Sci 3: 124–128, 1988 [Google Scholar]
- 32. Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem 276: 29567–29574, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Nyitrai M, Geeves MA. Adenosine diphosphate and strain sensitivity in myosin motors. Philos Trans R Soc Lond B Biol Sci 359: 1867–1877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126: 461–480, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil 20: 433–442, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Ratz PH. High α1-adrenergic receptor occupancy decreases relaxing potency of nifedipine by increasing myosin light chain phosphorylation. Circ Res 72: 1308–1316, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Ratz PH, Miner AS. Length-dependent regulation of basal myosin phosphorylation and force in detrusor smooth muscle. Am J Physiol Regul Integr Comp Physiol 284: R1063–R1070, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Ratz PH, Murphy RA. Contributions of intracellular and extracellular Ca2+ pools to activation of myosin phosphorylation and stress in swine carotid media. Circ Res 60: 410–421, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Ruegg C, Veigel C, Molloy JE, Schmitz S, Sparrow JC, Fink RH. Molecular motors: force and movement generated by single myosin II molecules. News Physiol Sci 17: 213–218, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Seow CY. Response of arterial smooth muscle to length perturbation. J Appl Physiol 89: 2065–2072, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Shenfeld OZ, Morgan CW, Ratz PH. Bethanechol activates a postreceptor negative feedback mechanism in rabbit urinary bladder smooth muscle. J Urol 159: 252–257, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Siegman MJ, Butler TM, Mooers SU, Davies RE. Cross bridge attachment, resistance to stretch, and viscoelasticity in resting mammalian smooth muscle. Science 191: 383–385, 1976 [DOI] [PubMed] [Google Scholar]
- 43. Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, Sheng S, Shao Z, Somlyo AP. Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci 359: 1921–1930, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Speden RN. The effect of initial strip length on the noradrenaline-induced contraction of arterial strips. J Physiol 154: 15–25, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Speich JE, Almasri AM, Bhatia H, Klausner AP, Ratz PH. Adaptation of the length-active tension relationship in rabbit detrusor. Am J Physiol Renal Physiol 297: F1119–F1128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Speich JE, Borgsmiller L, Call C, Mohr R, Ratz PH. ROK-induced cross-link formation stiffens passive muscle: reversible strain-induced stress softening in rabbit detrusor. Am J Physiol Cell Physiol 289: C12–C21, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Speich JE, Dosier C, Borgsmiller L, Quintero K, Koo HP, Ratz PH. Adjustable passive length-tension curve in rabbit detrusor smooth muscle. J Appl Physiol 102: 1746–1755, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Speich JE, Quintero K, Dosier C, Borgsmiller L, Koo HP, Ratz PH. A mechanical model for adjustable passive stiffness in rabbit detrusor. J Appl Physiol 101: 1189–1198, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Stewart M, Franks-Skiba K, Cooke R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J Muscle Res Cell Motil 30: 17–27, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299: 1743–1747, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Syyong H, Cheung C, Solomon D, Seow CY, Kuo KH. Adaptive response of pulmonary arterial smooth muscle to length change. J Appl Physiol 104: 1014–1020, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Uvelius B. Isometric and isotonic length-tension relations and variations in longitudinal smooth muscle from rabbit urinary bladder. Acta Physiol Scand 97: 1–12, 1976 [DOI] [PubMed] [Google Scholar]
- 53. Van Mastrigt R. Mechanical properties of (urinary bladder) smooth muscle. J Muscle Res Cell Motil 23: 53–57, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Wang L, Pare PD, Seow CY. Plasticity in skeletal, cardiac, and smooth muscle: selected contribution: effect of chronic passive length change on airway smooth muscle length-tension relationship. J Appl Physiol 90: 734–740, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Wingard CJ, Browne AK, Murphy RA. Dependence of force on length at constant cross-bridge phosphorylation in the swine carotid media. J Physiol 488: 729–739, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]