Abstract
Chronic kidney disease is a growing medical concern, with an estimated 25.6 million people in the United States exhibiting some degree of kidney injury and/or decline in kidney function. Animal models provide great insight into the study of the genetics of complex diseases. In particular, heterogeneous stock (HS) rats represent a unique genetic resource enabling rapid fine-mapping of complex traits. However, they have not been explored as a model to study renal phenotypes. To evaluate the usefulness of HS rats in the genetics of renal traits, a time course evaluation (weeks 8–40) was performed for several renal phenotypes. As expected, a large degree of variation was seen for most renal traits. By week 24, three (of 40) rats exhibited marked proteinuria that increased gradually until week 40 and ranged from 33.7 to 80.2 mg/24 h. Detailed histological analysis confirmed renal damage in these rats. In addition, several rats consistently exhibited significant hematuria (5/41). Interestingly, these rats were not the same rats that exhibited proteinuria, indicating that susceptibility to different types of kidney injury is likely segregating within the HS population. One HS rat exhibited unilateral renal agenesis (URA), which was accompanied by a significant degree of proteinuria and glomerular and tubulointerstitial injury. The parents of this HS rat were identified and bred further. Additional offspring of this pair were observed to exhibit URA at frequency between 40% and 60%. In summary, these novel data demonstrate that HS rats exhibit variation in proteinuria and other kidney-related traits, confirming that the model harbors susceptibility alleles for kidney injury and providing the basis for further genetic studies.
Keywords: HS rats, proteinuria, chronic kidney disease, renal injury
chronic kidney disease (CKD) affects 10–13% of adults in the United States (6). Those with CKD are at risk for end-stage renal disease (ESRD) as well as cardiovascular disease (30). Prevalence of ESRD has increased at unexpected rates, resulting in large numbers of patients needing dialysis or kidney transplantation (34). Familial aggregation studies have shown that genetics play a role in development of general measures of kidney function decline (9) and ESRD (21). Recently, human genomewide association studies have identified several genes that play a role in renal function (12, 18), and one gene has been identified for ESRD (17). It is clear, however, that many more genes involved in kidney disease are yet to be identified.
The rat is the animal model of choice for several physiological traits (20), including kidney disease, because several inbred rat strains exhibit kidney disease spontaneously (33) or have been bred specifically for the study of cardiovascular and renal disease (4, 8, 19, 28). Linkage analysis in these inbred models of kidney disease has proven useful in identifying large chromosomal regions associated with kidney injury including proteinuria and albuminuria (e.g., Refs. 10, 25, 31). One disadvantage of these studies, however, is that the region identified often contains hundreds of genes, and subsequent studies can take years to narrow the region and identify a causative gene. To date, only few genes have been identified for renal injury with animal models (1, 27). In contrast, the National Institutes of Health (NIH)-derived heterogeneous stock (HS) rat serves as a source of animals that produce a range of diverse phenotypes (13) as well as offering a unique genetic resource that enables the rapid fine-mapping of quantitative trait loci (QTLs) to small genomic regions (22, 35). The HS colony represents a genetically random mosaic of eight founding animals, with each individual animal being genetically unique. At each generation of breeding there is the potential of new recombinations that could help reduce the size of a QTL. After 50 generations of breeding, it is the estimated that the average distance between recombination events is approximately 2 cM (22), thus enabling the fine-mapping of QTL into subcentimorgan intervals. The success of this methodology has been demonstrated by Flint and colleagues (35), who successfully fine-mapped 843 QTLs for over 100 phenotypes, using HS mice. Furthermore, we have recently used the HS rat colony to fine-map a diabetes locus to only 2.4 Mb (32a).
The HS rat stock was derived from eight inbred strains including ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N and are maintained with a breeding paradigm designed to minimize inbreeding (13). Some of these strains have been evaluated for renal traits. In particular, two strains (M520 and ACI) exhibit renal lesions (33), and one strain (BUF/N) exhibits proteinuria and focal and segmental glomerulosclerosis (24). In addition, 5–15% of ACI rats exhibit unilateral renal agenesis (URA) (7, 32). Together, these findings provide evidence a priori that alleles associated with renal injury should segregate within the HS colony because of genetic contribution from M520, BUF, and ACI genomes (and perhaps the interaction between susceptibility alleles donated between these strains). However, the HS colony has not been systematically studied for renal and/or cardiovascular traits.
Our objective here was to investigate the utility of HS rats for potential in fine-mapping of traits involved in kidney-related phenotypes. An evaluation (weeks 8–40) of proteinuria, along with several other renal traits, was performed in a panel of HS rats at 8, 16, 24, 32, and 40 wk of age. Urinalysis was performed at all time points to measure multiple parameters including hematuria, glucosuria, electrolyte excretion, and pH. Measures of renal function including serum creatinine, blood urea nitrogen (BUN), and creatinine clearance (CrCl) were also evaluated. In summary, we demonstrate that HS rats exhibit variation in multiple renal phenotypes and therefore will serve as an excellent model for rapidly fine-mapping genetic loci (to <5 Mb) controlling renal phenotypes.
MATERIALS AND METHODS
Animals
All experiments were approved by our Institutional Animal Care and Use Committee. Heterogeneous stock breeding pairs (25 pairs) were obtained from Dr. Eva Redei's colony at Northwestern University. In 2003, Dr. Redei obtained her colony from Dr. Carl Hansen at NIH. At the time we received the animals, the HS stock had been through 55 generations of breeding (50 at NIH and 5 at Northwestern University). The numbers of breeding pairs were increased to 46 at the Medical College of Wisconsin (MCW) and have been maintained with a rotational breeding strategy to minimize inbreeding (13). The official designation of these animals is NMcwi:HS, but they are referred to here as HS.
At 21 days of age, a group of 41 age-matched male HS progeny from the third generation of breeders set up at MCW were weaned to a standard rat chow diet (Purina 5010). The 41 animals came from 14 individual breeder pairs, representing an average family size of ∼3 rats. As a means of assessing phenotypic variability in HS animals, male Sprague-Dawley (SD; n = 15) rats were also evaluated. SD rats were purchased from Taconic and raised on the same diet. All HS animals were studied for several renal and cardiovascular traits from 8 to 40 wk of age. SD rats were studied only at week 40. Rats were killed by overdose of pentobarbital sodium. Body, heart, and kidney weights were measured. Kidney samples were processed for histological examination, and serum samples were obtained from cardiac puncture to measure blood parameters.
Phenotyping
Urine and blood parameters.
To collect urine, animals were kept in metabolism cages (Lab Products, Seaford, DE) for 24 h with free access to water at each time point: weeks 8, 12, 16, 20, 24, 32, and 40 (see Fig. 1A). A single urine collection was made at week 8. Subsequently, urine was collected twice for each rat at each time point to ensure accurate measurement of urine parameters, and the averages of the two measurements are given. For proteinuria, there was a significant correlation between the first and second measurements ranging from r = 0.84 to r = 0.98 (P < 0.0001). Total urine protein was determined as previously described (10, 11). No significant correlation was observed between proteinuria and body weight. Thus it was not necessary to normalize total urine protein for differences in body weight, and it is reported as milligrams per 24 hours. Quantitative measurement of creatinine, BUN, electrolytes (Na+, K+), and osmolality was determined by standard methods with an Alfa Wassermann ACE automated chemistry analyzer (and osmometer). Semiquantitative determination of blood (hematuria), glucose (glucosuria), ketone, leukocyte, nitrite, and pH was done with Chemstrip7 urine test strips (Roche).
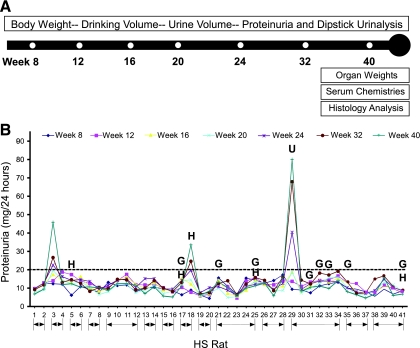
Fig. 1.
Evaluation of heterogeneous stock (HS) rats for proteinuria. A: schematic drawing of time course (weeks 8–40) and measured parameters. B: development of proteinuria in individual HS rats from 8 to 40 wk of age. Horizontal line represents the threshold for “proteinuria” (>20 mg/24 h) (10). Except for week 8, urine was collected twice at each time point, and the average is presented. Animals denoted by an H exhibited persistent hematuria (50–250 erythrocytes/μl) defined by ≥3 positive dipstick urine samples (i.e., weeks 24, 32, and 40). Animals denoted by a G exhibited persistent glucosuria also defined by ≥3 positive dipstick urine samples ranging from 50 to 100 mg/dl. U denotes the animal that exhibited unilateral renal agenesis (URA; observed on euthanasia). Line arrows (bottom) indicate HS rats from the same parents.
Glucose tolerance test.
We administered an intraperitoneal glucose tolerance test to a subset of 10 HS animals at week 40. Briefly, animals were food deprived for 16–18 h overnight. In the morning, a basal glucose sample was measured. We then injected the animals with 1 g/kg body wt of a glucose solution. Blood glucose levels were then measured at 15, 30, 60, 90, and 120 min after the injection. Blood glucose levels were measured with the Ascensia Elite system (Bayer, Elkhart, IN) as previously described (32a).
Histology.
Kidney and heart tissue was fixed in zinc formalin and embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (H & E) and Masson's trichrome. Two or three central longitudinal sections from each kidney or transverse sections from each heart were examined in a blinded fashion. Kidney sections were evaluated in two stages. Initially, all 41 HS kidneys were evaluated microscopically for the presence of glomerular and/or tubulointerstitial injury (stage 1). A semiquantitative score was assigned by visual inspection of whole sections (10). Animals that exhibited a score above 0 (i.e., normal) were then evaluated more systematically (stage 2) by image capture as follows. For glomeruli, a minimum of 20 randomly selected images (H & E at ×40) were taken from each rat and assessed for degree of glomerulosclerosis and mesangial expansion as previously described (11, 26). Tubulointerstitial injury was evaluated separately on a semiquantitative scale from 0 (normal) to 4 (severe) with a minimum of 20 randomly selected images (Masson's trichrome at ×20) as follows: grade 0, no changes; grade 1, mild tubule atrophy/fibrosis involving <25%; grade 2, lesions affecting 25–50%; grade 3, lesions affecting 50–75%; and grade 4, lesions affecting >75%. Morphometric analysis was used to evaluate glomerular area (μm2) and vessel wall thickening and cellular proliferation. Vessel wall thickening (vessel media, μm2) was calculated by measuring the outer circumference of the vessel minus the inner circumference of the lumen (20 random images at ×40 per HS rat). The number of nuclei per vessel section was counted and is reported as the number of nuclei per 1,000 μm2 of vessel media. Images were captured with a Nikon 55i microscope with a DS-Fi1 5-Meg Color C digital camera (Nikon, Melville, NY) and analyzed with Nikon Elements image analysis software.
Ultrasound measurements.
To identify rats with one kidney, in vivo measurement of kidney number and size was performed with a General Electric Vivid 7 ultrasound system. Rats were anesthetized with isoflurane gas (2–4%) and maintained on a warming table at 37°C. The abdomen was shaved, scanning gel was applied liberally, and ultrasound measurements were done.
Statistical Analysis
Box plots and statistical analysis were performed with SPSS (Chicago, IL). Kidney size determined by ultrasound was evaluated by independent t-test. Data are presented as means ± SD for Table 1 and means ± SE for data reported in Fig. 6.
Table 1.
Body weight and urine parameters for HS and SD rats
| Body Wt, g |
Water Intake, ml |
Urine Volume, ml |
Urine Na+/K+, mmol |
Urine Osmolality, mosmol/kg H2O |
Urine pH |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | Group | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) |
| 8 | HS | 131 | 226 (30.1) | 29 | 13.6 (7.8) | 29 | 14.1 (7.3) | 0.65/1.2 | 1.2 (0.24)/2.6 (0.47) | 2,076 | 815 (459) | 2.8 | 6.5 (0.6) |
| 12 | HS | 153 | 311 (36.8) | 2 | 11.3 (6.8) | 29 | 12.4 (6.8) | ||||||
| 16 | HS | 182 | 356 (43.4) | 24 | 10.0 (5.7) | 19 | 10.9 (4.8) | 0.42/1.1 | 0.84 (0.19)/1.3 (0.31) | 1,677 | 1,019 (404) | 2.3 | 6.6 (0.6) |
| 20 | HS | 182 | 381 (46.6) | 24 | 8.3 (5.6) | 16 | 9.4 (4.2) | ||||||
| 24 | HS | 194 | 402 (50.3) | 18 | 6.3 (4.1) | 17 | 8.3 (3.4) | 0.28/0.99 | 0.56 (0.14)/1.1 (0.25) | 1,869 | 1,349 (487) | 2.5 | 6.6 (0.6) |
| 32 | HS | 212 | 430 (52.1) | 15 | 5.7 (4.2) | 17 | 7.8 (3.7) | 0.26/0.93 | 0.45 (0.12)/0.75 (0.22) | 1,759 | 1,375 (535) | 2.3 | 6.6 (0.7) |
| 40 | HS | 246 | 448 (55.9) | 16 | 5.8 (3.8) | 16 | 7.5 (3.8) | 0.29/0.97 | 0.51 (0.12)/1.0 (0.23) | 2,063 | 1,522 (621) | 2.4 | 6.6 (0.6) |
| 40 | SD | 150 | 540 (38.2) | 9 | 4.9 (2.6) | 12 | 10.8 (3.0) | 0.70/1.2 | 0.82 (0.27)/1.9 (0.41) | 1,005 | 1,543 (367) | 2.0 | 7.2 (1.0) |
HS, heterogeneous stock; SD, Sprague-Dawley.
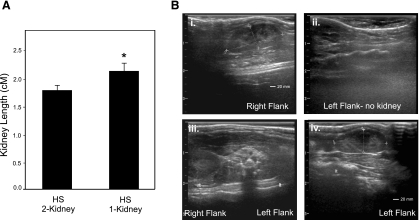
Fig. 6.
Measurement of kidney size between 2-kidney rats and breeding line exhibiting URA. A: kidney length based on ultrasound measurements. Error bars indicate SE. *P < 0.01. B: representative ultrasound images of 1-kidney (i–iii) and 2-kidney HS rats (iv) that originated from the same litter. Parents of HS29 were bred, and offspring (n = 9) underwent evaluation by ultrasound at 8 wk of age. Four rats were observed to have both kidneys, and 5 rats were observed to possess only a single kidney.
RESULTS
The HS population as a whole exhibited a large degree of variation in urinary protein levels (Fig. 1B). Despite variation in urinary protein between HS animals, the majority of individual HS rats examined showed little variation in urinary protein over the time course. Three (of 41) rats exhibited significant proteinuria, which increased gradually from week 8 through week 40 and ranged from 33.7 to 80.2 mg/24 h at week 40. Urinalysis was performed at all time points to measure multiple parameters including hematuria and glucosuria (Fig. 1B and Table 1). Several rats (5/40) consistently exhibited hematuria (weeks 24, 32, and 40), which was confirmed by the presence of red blood cells upon microscopic evaluation. Microscopic examination of bladders from these rats demonstrated normal histology (data not shown). The rats that demonstrated hematuria for the most part were not the same rats that exhibited proteinuria. Only one HS rat demonstrated both hematuria and proteinuria (HS18). Several rats demonstrated persistent glucosuria indicated by a positive result at more than three time points (Fig. 1B). These findings were supported by elevated blood glucose levels (defined as >200 mg/dl at 15 min after glucose injection) when the rats were subjected to a glucose tolerance test (data not shown).
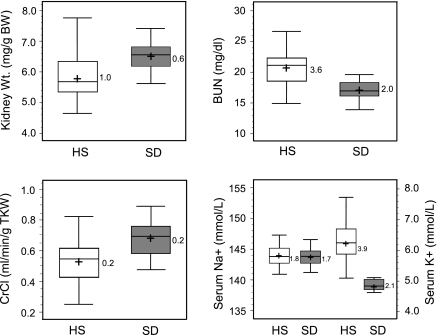
Figure 2 shows the variability of relevant renal and cardiovascular traits exhibited by HS rats and SD control rats at 40 wk of age. SD rats are genetically heterogeneous and exhibit greater phenotypic diversity compared with any given inbred rat. Thus SD rats provide a good comparison to evaluate the degree of variation attributable to the large amount of genetic diversity in the HS population. Overall, while SD rats were larger than age-matched HS rats (Table 1), HS rats exhibited more phenotypic variation in most measured traits, as demonstrated by a larger interquartile range (Fig. 2). More variation in kidney weight was exhibited in the HS rats compared with SD control rats (Fig. 2 and Supplemental Fig. S1).1 In contrast, there was less variation in heart weight across the HS panel and between strains (SD control). However, despite little variation in heart weight across the HS panel, a significant amount of cardiac fibrosis was observed compared with SD control rats (Supplemental Fig. S2).
Fig. 2.
Box plot comparing physical traits and renal parameters of HS and Sprague-Dawley (SD) rats. Open plots are HS data, and shaded plots are SD data. The box itself contains the middle 50% of the data. The upper quartile (UQ) indicates the 75th percentile of the data set, and the lower quartile (LQ) represents the 25th percentile. The interquartile range (IQR = UQ − LQ) represents a measure of variability and is displayed on right of each box plot. The line within the box indicates the median value of the data; + represents the mean value of the population. BW, body weight; BUN, blood urea nitrogen; CrCl, creatinine clearance.
HS rats exhibited more variability in BUN levels and overall exhibited higher values compared with SD control rats (Fig. 2). Similar variation in CrCl was observed between HS and SD rats, but HS rats exhibited lower CrCl in general. There was a large degree of variation in serum potassium, while serum sodium levels were nearly identical between HS and SD rats, as would be expected. Additional experimental parameters for HS and SD rats from week 8 to week 40 are shown in Table 1. Modest to large variation was observed for water intake, urine output, sodium and potassium excretion, and urine pH. In general, rats that exhibited low (or high) values of a given trait maintained that level throughout the time course.
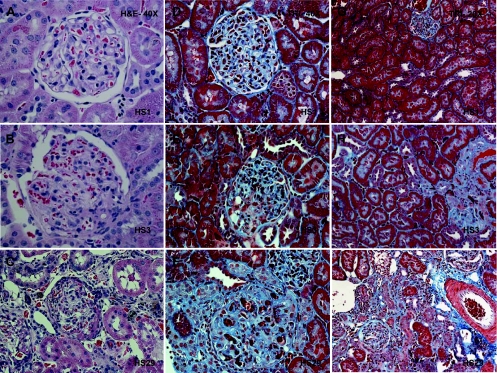
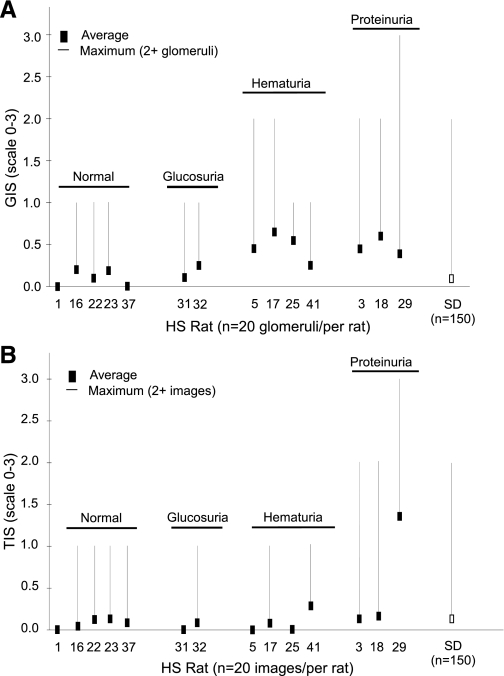
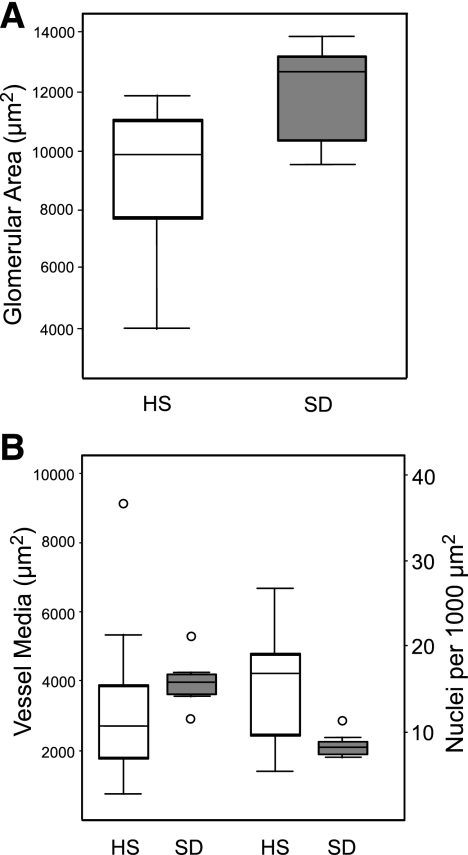
Histological evaluation of HS kidney sections demonstrated a range of renal injury, from normal to severe glomerulosclerosis and tubulointerstitial injury (Fig. 3). Most HS rats exhibited little to no detectable renal injury (e.g., HS1). However, rats with elevated proteinuria and/or persistent hematuria exhibited measurable renal damage (HS3 and HS20). Proteinuria values and histological scores based on the entire data set (n = 41) were significantly correlated (r = 0.64, P < 0.0001), as were those HS rats (n = 14) that exhibited an initial score above 0 and that underwent detailed histological evaluation (r = 0.86, P < 0.0001) as shown in Fig. 4. HS17 and HS18 exhibited the highest glomerular injury score (GIS) and despite HS29 demonstrating the most severe glomerulosclerosis (GIS = 3), overall it exhibited a modest GIS (Fig. 4A). The average GIS (n = 160 glomeruli) for SD control rats was relatively low, but a number of glomeruli did exhibit some injury (2 GIS >1). The most severe tubulointerstitial injury (and highest proteinuria) was exhibited by HS29, which upon euthanasia was observed to have only one kidney (i.e., URA). The tubulointerstitial injury score (TIS) was severalfold higher than that of the majority of the other HS rats evaluated (Fig. 4B). Similar variation was observed in glomerular size, but a high degree of variability in vessel media thickness and the number of nuclei (per 1,000 μm2 of vessel area) was observed in the HS rats, while a small amount of variation was found in SD rats (Fig. 5).
Fig. 3.
Representative light microscopy images of kidney from HS rats at 40 wk of age. A–F: images [×40 hematoxylin and eosin (H & E) or Masson's trichrome] of individual HS rats demonstrating normal to significant glomerular abnormalities including mesangial expansion, glomerulosclerosis, etc. G–I: images of the tubule-interstitial region (×20 Masson's trichrome) from individual HS rats demonstrating normal to severe tubule atrophy, immune cell infiltration, and/or fibrosis.
Fig. 4.
Semiquantitative histological evaluation of 14 HS rats. A: glomerular injury score (GIS). B: tubulointerstitial injury score (TIS). The black square denotes the average score, and the line represents the maximum score with at least 2 images (glomeruli or tubule/interstitium) exhibiting that degree of damage. For the SD comparison the average GIS is given (n = 15 rats) and denoted by the open box. A number of HS rats that exhibited no outward renal phenotype (denoted as normal) are provided as a comparison.
Fig. 5.
Morphometric analysis of HS and SD rat kidney structures. A: measurement of glomerular area. B: measurement of vessel media. Open plots are HS data, and shaded plots are SD data. IQR is similar between HS and SD for glomerular area, indicating little variability in glomerular size. However, a significant degree of variability is observed in vessel media and the number of nuclei (per 1,000 μm2 of vessel) between HS and SD. Outliers are denoted by open circles.
The unique observation that HS29 exhibited URA prompted further investigation to establish whether this was a rare developmental occurrence or an inheritable genetic trait within the HS population. The parents of HS29 were available, and additional breeding was performed. The litter resulted in nine offspring (6 female/3 male), and upon ultrasound investigation five rats (4 female/1 male) exhibited a single kidney. “One-kidney” rats had significantly larger kidney size (2.2 ± 0.13 cm) compared with “two-kidney” littermates (1.8 ± 0.08 cm) (Fig. 6). Further work will be required to develop this model and better characterize the genetic defect.
DISCUSSION
The purpose of this study was to evaluate phenotypic variation in renal traits within the HS population and establish whether the animal model would be conducive for future genetic studies. This study clearly demonstrates that there is large variation in baseline levels of proteinuria (and other renal measures) in the HS rat. That is, individual rats exhibit little change in urinary protein levels over time, but the HS population as a whole exhibits a wide range of urinary protein values. Three rats (HS3, HS18, and HS29) exhibited high proteinuria and concurrent renal injury. These data suggest that the HS population will be valuable for understanding both the genetic factors that contribute to baseline variation in proteinuria and those that play a role in renal injury and disease progression. Several rats consistently exhibited hematuria, and for the most part these rats were not the same rats that exhibited proteinuria, indicating that susceptibility to different types of kidney injury are likely segregating within the HS population. The HS animals also exhibited more variation in BUN levels, serum K+, kidney weight, vessel wall thickness and cellularity, and cardiac fibrosis compared with age-matched SD rats. Together, these results indicate that HS rats will be useful for dissecting the genetics of several kidney-related phenotypes.
The most striking renal phenotype observed was an HS rat (HS29) that exhibited URA. URA is a common developmental defect in humans and occurs in 1 in 500–1,000 births (3, 36, 38). It is often asymptomatic, but affected patients can exhibit a decline in renal function (38). The genetic basis is not well defined in humans, but there are a number of animal models with known defects in developmental genes that contribute to URA (29). Rats exhibiting URA had a significantly larger kidney compared with two-kidney HS littermates. In humans, renal hypertrophy of the sole kidney is observed prenatally and in children exhibiting URA (15). HS29 demonstrated the highest proteinuria and most severe tubulointerstitial injury of all the HS rats. It is not clear whether the severe renal injury in HS29 is due solely to URA or in combination with other renal susceptibility genes that the animal may also have inherited. This will need to be studied further. There are, however, a number of human-based studies demonstrating that patients with URA are at increased risk of hypertension, proteinuria, and renal failure as adults (reviewed in Ref. 14). Unfortunately, the prognosis for patients with URA is somewhat controversial because of limited long-term follow-up data.
From a genetic standpoint, while URA was observed in only one animal in the HS panel we studied, the trait was confirmed to be heritable by additional breeding and ranged from 40% to 60% in subsequent litters (data not shown). Past studies have shown that the ACI rat, a founder of the HS colony, also exhibits URA (32). However, the HS line exhibits a much higher incidence of URA compared with the ACI rat. It is not known whether the genetic defect leading to URA is the same in the HS line and ACI, but if the locus responsible is the same then the high penetrance may be explained by a more susceptible genetic background in the HS line compared with the ACI rat. Further breeding and genetic studies will be required to better characterize this new model and identify the gene defect leading to URA.
In general, inbred rat models of kidney disease including the Dahl S, FHH, SBH, and MWF have served an important role in genetic mapping studies (5, 10, 31, 39). Linkage analysis has been particularly fruitful in identifying numerous chromosomal regions linked to proteinuria (and other measures of renal function) (12). Some genetic studies have been performed in the BUF and ACI strains, two strains used to derive the HS. For example, linkage analysis has identified a locus on rat chromosome 13 linked to proteinuria in the BUF (23) and rat chromosome 14 to URA in the ACI (32). The genes underlying these loci have yet to be definitively identified, but a recent report suggests that mutations in actin-related protein 3 (Arp3) may be causally linked to proteinuria in the BUF rat (2). Overall, the identification of the actual variants leading to proteinuria and/or renal injury has been limited because of the inherent difficulties of the conventional linkage analysis approach.
The HS model provides an alternative methodology to identify genes involved in kidney disease. The advantage of the HS for genetic analysis is that it allows for the identification of genomic regions linked to disease traits with higher resolution compared with the relatively broad localization of the QTL by conventional linkage approaches (22). This is accomplished by the large number of recombination events accumulated within the HS stock and enables the fine-mapping of QTL into small genomic interval (22). While the large number of animals required for fine-mapping most traits (>500 animals) precludes any mapping analysis from the present study, this approach has been validated in HS mice by successful fine-mapping of >100 different phenotypes to an average of only 2.8 Mb (35). To date, only two studies have used the HS rat model for fine-mapping. Our group has mapped a locus for glucose tolerance (32a), and Johannesson et al. (16) have mapped a locus for fear-related behavior. Importantly, while Johannesson et al. (16) measured multiple phenotypes for subsequent fine-mapping in the HS rat, the present work is the first to measure kidney-related traits in this resource.
One perceived limitation of using the HS model may be that the degree of renal injury is much less severe than what is observed in “disease” models. However, we have successfully fine-mapped a diabetic locus, using impaired glucose tolerance as the phenotype. None of the HS rats exhibited overt diabetes, but the population as a whole did exhibit significant variation in glucose tolerance (32a). In the case of renal phenotypes, we believe the model could be very useful in elucidating genetic factors that play a different role in disease onset and progression because the HS population exhibits variation in baseline levels of urinary protein levels as well as significant proteinuria and renal injury within the population.
In this study renal phenotypes were not measured in the eight inbred strains that were used to derive the HS. We reasoned that if significant variation is seen within the HS population, future genetic studies would be feasible, independent of the original founder phenotypes. Furthermore, in other work by our group, we have observed that variation for several metabolic phenotypes is more extreme in the HS rats than the variation found within any one inbred founder strain (unpublished observation). We did conduct measurements in SD rats with the aim of being able to make some conclusion regarding the degree of variation in the HS population. By comparing HS rats with SD rats we were able to assess the degree of phenotypic variation that exists in the HS population because SD rats are genetically heterogeneous and exhibit greater phenotypic diversity than would be expected in any given inbred rat. Blood pressure was not measured in our study; thus no firm conclusion regarding the possible interplay of blood pressure and renal injury can be addressed at this point. However, blood pressure has been evaluated in HS rats by another group (16). The study found that blood pressure overall for the HS population (n = 220 HS rats) was within the normal range (systolic BP <130 mmHg) and lower than that in most of the inbred strains (used to derived the HS) examined. In summary, the degree of phenotypic variation observed in the HS model firmly supports the utility of this resource for future genetic studies.
To date, the genetic methodology employed with the HS rat model has not been applied to study kidney-related traits. Our study now establishes that the HS model harbors significant variation in renal traits that will make the model useful for genetic studies and fine-mapping important traits related to kidney disease.
GRANTS
M. R. Garrett is supported by National Institutes of Health (NIH) Grant HL-094446 and funds from Advancing Healthier Wisconsin (AHW). K. R. Regner is also supported by funds from AHW. L. C. Solberg Woods is supported by funds from NIH Grant K01-DK-076977.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Leanne M. Harmann for performing ultrasound kidney measurements.
Footnotes
The online version of this manuscript contains supplemental material.
REFERENCES
- 1.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Morita H, Suetsugu S, Kuraba S, Numata Y, Yamamoto Y, Inui K, Ideura T, Wakisaka N, Nakano K, Oniki H, Takenawa T, Matsuyama M, Yoshimura A. Actin-related protein 3 (Arp3) is mutated in proteinuric BUF/Mna rats. Mamm Genome 19: 41–50, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Barakat AJ, Drougas JG. Occurrence of congenital abnormalities of kidney and urinary tract in 13,775 autopsies. Urology 38: 347–350, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ishay D, Saliternik R, Welner A. Separation of two strains of rats with inbred dissimilar sensitivity to Doca-salt hypertension. Experientia 28: 1321–1322, 1972 [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ. Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cramer DV, Gill TJ., 3rd Genetics of urogenital abnormalities in ACI inbred rats. Teratology 12: 27–32, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9: 1399–1406, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, Wilson PW, Levy D. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol 15: 2457–2461, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 14: 1175–1187, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Garrett MR, Gunning WT, Radecki T, Richard A. Dissection of a genetic locus influencing renal function in the rat and its concordance with kidney disease loci on human chromosome 1q21. Physiol Genomics 30: 322–334, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett MR, Pezzolesi MG, Korstanje R. Integrating human, rat, and mouse data to identify the genetic factors involved in chronic kidney disease. J Am Soc Nephrol 22: 222–223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8: 477–479, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Hegde S, Coulthard MG. Renal agenesis and unilateral nephrectomy: what are the risks of living with a single kidney? Pediatr Nephrol 24: 439–446, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hill LM, Nowak A, Hartle R, Tush B. Fetal compensatory renal hypertrophy with a unilateral functioning kidney. Ultrasound Obstet Gynecol 15: 191–193, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Johannesson M, Lopez-Aumatell R, Stridh P, Diez M, Tuncel J, Blazquez G, Martinez-Membrives E, Canete T, Vicens-Costa E, Graham D, Copley RR, Hernandez-Pliego P, Beyeen AD, Ockinger J, Fernandez-Santamaria C, Gulko PS, Brenner M, Tobena A, Guitart-Masip M, Gimenez-Llort L, Dominiczak A, Holmdahl R, Gauguier D, Olsson T, Mott R, Valdar W, Redei EE, Fernandez-Teruel A, Flint J. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res 19: 150–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Ida Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Pare G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 22: 222–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuijpers MH, Gruys E. Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65: 181–190, 1984 [PMC free article] [PubMed] [Google Scholar]
- 20.Lazar J, Moreno C, Jacob HJ, Kwitek AE. Impact of genomics on research in the rat. Genome Res 15: 1717–1728, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol 9: 1270–1276, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci USA 97: 12649–12654, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murayama S, Yagyu S, Higo K, Ye C, Mizuno T, Oyabu A, Ito M, Morita H, Maeda K, Serikawa T, Matsuyama M. A genetic locus susceptible to the overt proteinuria in BUF/Mna rat. Mamm Genome 9: 886–888, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Oite T, Shimizu F, Matsuyama M, Kazama T, Koda Y, Arakawa M. Sclerotic lesions in the glomeruli of Buffalo/Mna rats. Nephron 43: 50–55, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Nobrega MA, Solberg Woods LC, Fleming S, Jacob HJ. Distinct genetic regulation of the progression of diabetes and renal disease in the Goto-Kakizaki (GK) rat. Physiol Genomics 22: 222–223, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Packard M, Saad Y, Gunning WT, Gupta S, Shapiro J, Garrett MR. Investigating the effect of genetic background on proteinuria and renal injury using two hypertensive strains. Am J Physiol Renal Physiol 296: F839–F846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension 7: 340–349, 1985 [PubMed] [Google Scholar]
- 29.Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol 29: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Schulz A, Litfin A, Kossmehl P, Kreutz R. Genetic dissection of increased urinary albumin excretion in the Munich Wistar Frömter rat. J Am Soc Nephrol 13: 2706–2714, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Shull JD, Lachel CM, Strecker TE, Spady TJ, Tochacek M, Pennington KL, Murrin CR, Meza JL, Schaffer BS, Flood LA, Gould KA. Genetic bases of renal agenesis in the ACI rat: mapping of Renag1 to chromosome 14. Mamm Genome 17: 751–759, 2006 [DOI] [PubMed] [Google Scholar]
- 32a.Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol Genomics 41: 102–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solleveld HA, Boorman GA. Spontaneous renal lesions in five rat strains. Toxicol Pathol 14: 168–174, 1986 [DOI] [PubMed] [Google Scholar]
- 34.USRDS USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 35.Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, Taylor MS, Rawlins JN, Mott R, Flint J. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet 38: 879–887, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet 48: 131–144, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Woolf AS, Hillman KA. Unilateral renal agenesis and the congenital solitary functioning kidney: developmental, genetic and clinical perspectives. BJU Int 99: 17–21, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Yagil C, Sapojnikov M, Wechsler A, Korol A, Yagil Y. Genetic dissection of proteinuria in the Sabra rat. Physiol Genomics 25: 121–133, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.