Abstract
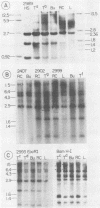
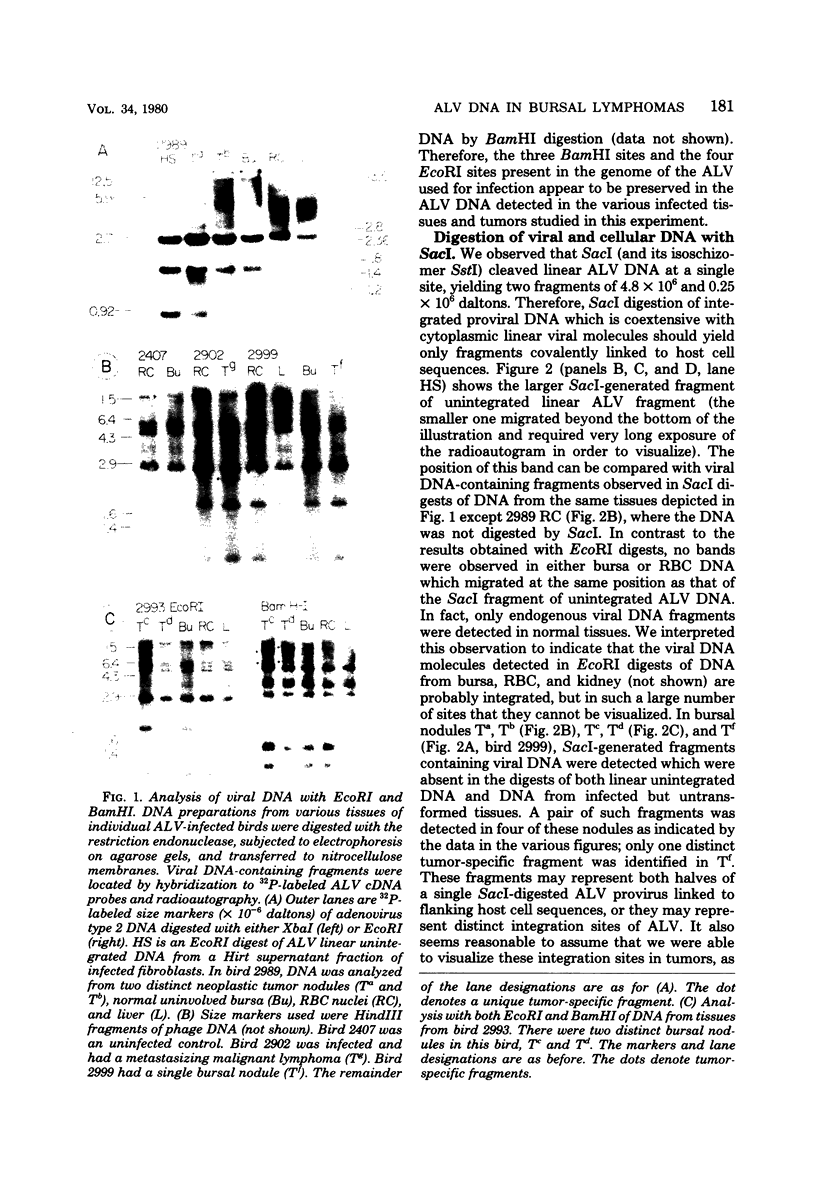
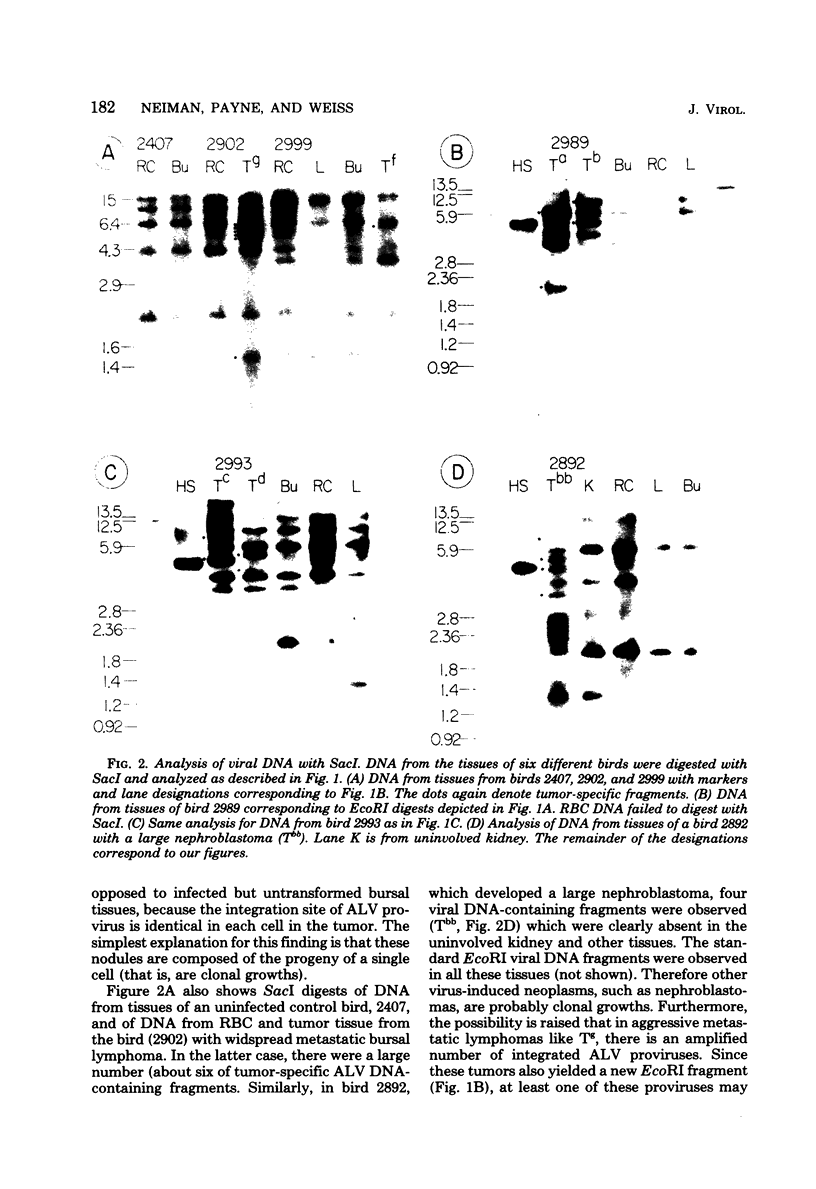
Avian leukosis viruses (ALV) induce malignant lymphoma of the bursa of Fabricius. Viral DNA in tumors and normal tissues from infected birds were analyzed by using restriction endonucleases. Viral DNA fragments diagnostic of the exogenous ALV were easily detected in tumors, uninvolved bursal tissue, kidney, and erythrocyte nuclei. Exogenous viral DNA was more difficult to detect in liver. Using a restriction endonuclease (SacI) which cleaves linear unintegrated ALV DNA in a single site to define integration sites in DNA from the various tissues, we were able to detect ALV DNA only in tumor tissue. We concluded that the proviral DNA detected in the various nontumor tissue must be integrated in multiple sites. The appearance of ALV integration sites uniquely in tumors suggests that they are clonal growths. Furthermore, the data suggested the presence of a single exogenous integration site for the ALV provirus in each of six early neoplastic bursal nodules. This provirus appeared to retain the organization of EcoRI and BamHI recognition sequences present in the genome of virus used to infect the birds. The ALV integration site appeared different in each of the tumors studied. In a widespread metastatic lymphoma, multiple ALV integration sites were found as well as structural alterations in at least some copies of the ALV provirus.
Full text
PDF


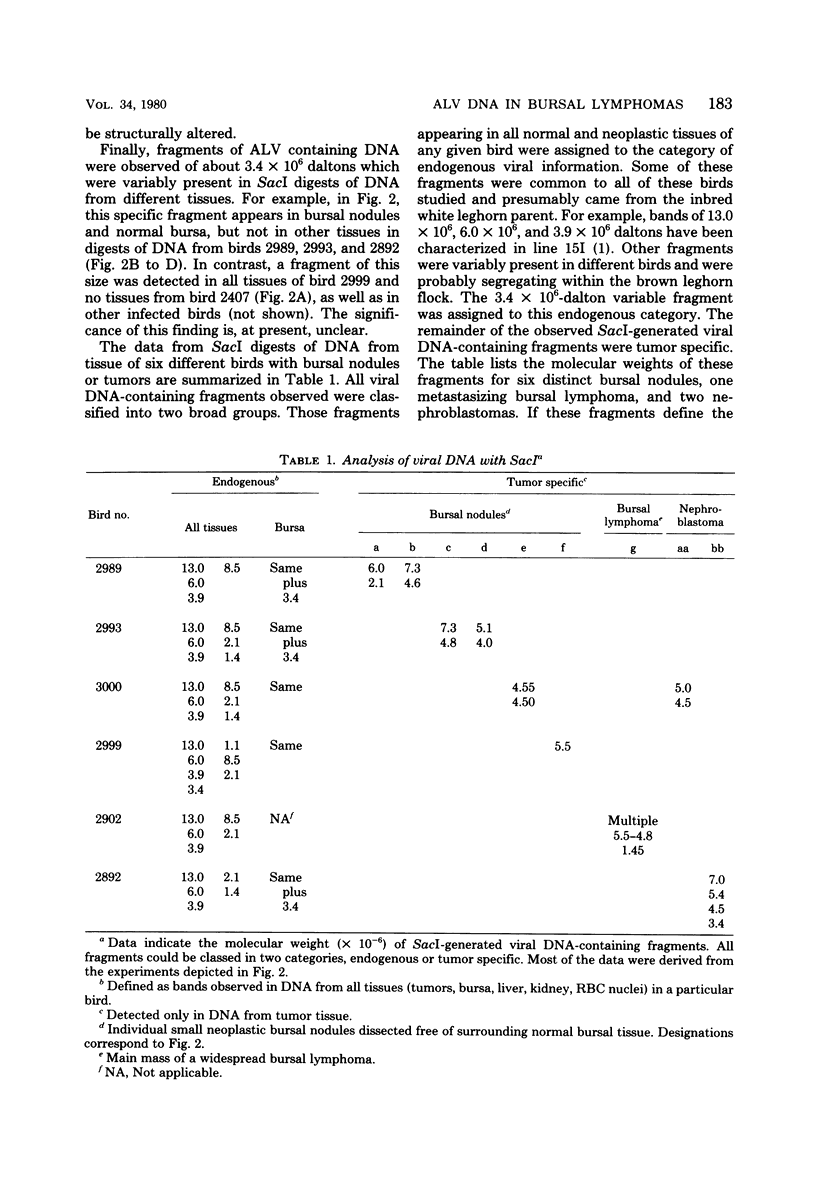
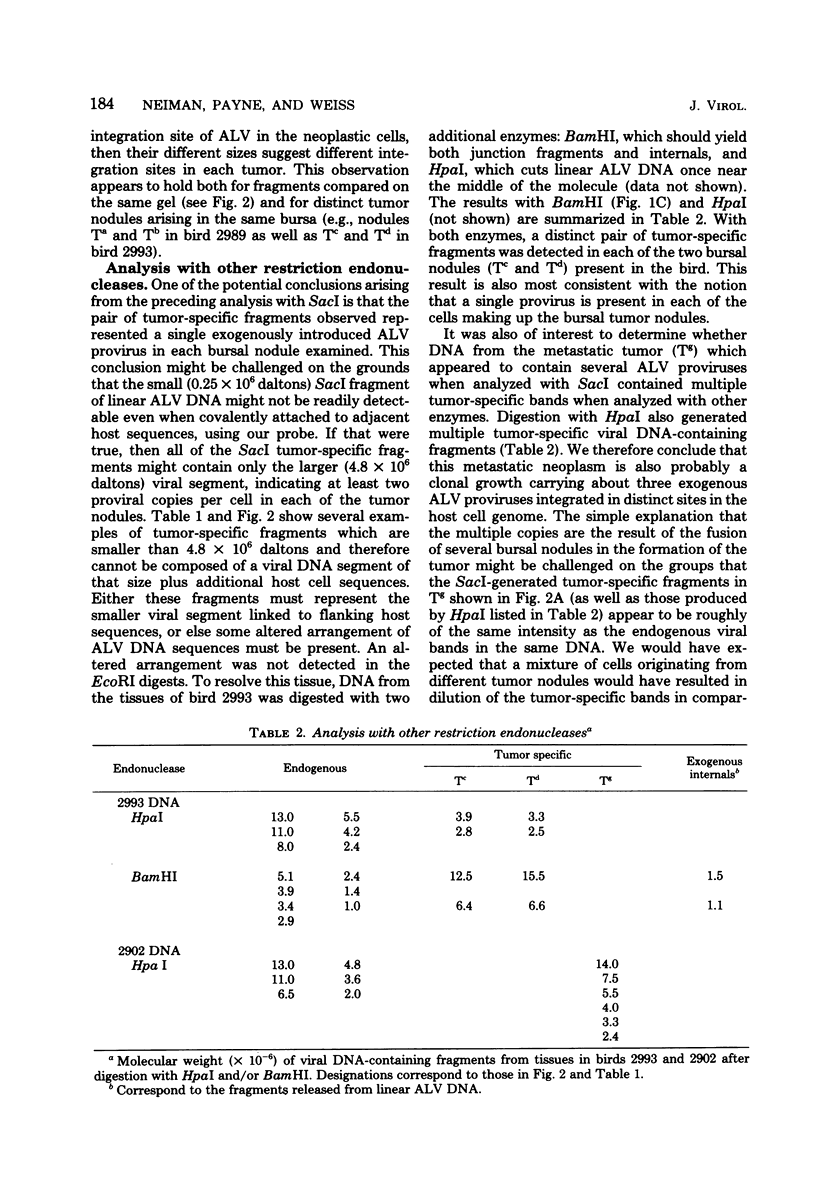


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Moloney leukemia virus gene expression and gene amplification in preleukemic and leukemic BALB/Mo mice. Virology. 1979 Feb;93(1):80–90. doi: 10.1016/0042-6822(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Das S., Macdonnell D., McMillin-Helsel C. Organization of shared and unshared sequences in the genomes of chicken endogenous and sarcoma viruses. Cell. 1977 Jun;11(2):321–329. doi: 10.1016/0092-8674(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Mapping by competitive hybridization of sequences which differ between endogenous and exogenous chicken leukosis viruses. Virology. 1978 Mar;85(1):9–16. doi: 10.1016/0042-6822(78)90407-5. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., McMillin-Helsel C., Cooper G. M. Specific restriction of avian sarcoma viruses by a line of transformed lymphoid cells. Virology. 1978 Sep;89(2):360–371. doi: 10.1016/0042-6822(78)90178-2. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- PETERSON R. D., BURMESTER B. R., FREDRICKSON T. N., PURCHASE H. G., GOOD R. A. EFFECT OF BURSECTOMY AND THYMECTOMY ON THE DEVELOPMENT OF VISCERAL LYMPHOMATOSIS IN THE CHICKEN. J Natl Cancer Inst. 1964 Jun;32:1343–1354. doi: 10.1093/jnci/32.6.1343. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]